Abstract
Objectives
The effectiveness of omega-3 fatty acids (PUFAs) in cardiovascular diseases (CVD) remains a matter of debate. The aim of this work was to evaluate PUFAs in the reduction of cardiovascular mortality in primary and secondary prevention of CVD to determine if further original studies are needed or the available data can be considered conclusive.
Methods
A meta-analysis was performed according to a dichotomous endpoint followed by a trial-sequential analysis (TSA). Clinical data were identified through a PubMed search based on the following keywords: omega-3 fatty acids; cardiovascular disease; death; and cardiovascular risk. The clinical trials identified by this procedure were subjected to standard meta-analysis and TSA.
Results and conclusions
A total of 11 randomised studies for 100 609 patients were analysed. Our meta-analysis showed a statistically significant reduction in mortality due to cardiovascular issues (RR=0.937; 95% CI: 0.88 to 0.98; P=0.018). The TSA indicated that no further trials are needed to better evaluate the efficacy of PUFAs in preventing death related to CVD.
Keywords: cardiac epidemiology, coronary heart disease, evidence-based medicine, nutrition & amp, dietetics, statistics & amp, research methods
Introduction
The effectiveness of omega-3 polyunsaturated fatty acids (PUFAs) in cardiovascular diseases (CVD) has been extensively investigated through both observational and randomised clinical trials (RCTs). In spite of this evidence, there is still disagreement about the benefits of PUFAs supplementation,1 particularly concerning their impact on hard endpoints in both primary and secondary prevention of CVD.2–7 At present, the main clinical effect of PUFAs seems to be restricted to their ability to decrease the triglycerides plasma levels.8
A recent meta-analysis by Aung et al9 assessed the efficacy of PUFAs in secondary prevention of coronary heart disease: the endpoint was represented by major vascular events. These negative conclusions are in agreement with a number of studies demonstrating no effects from omega-3 PUFA supplementation on oxidative stress, inflammatory parameters, and coagulation and metabolic status in patients with atherosclerotic vascular disease and type 2 diabetes mellitus.7 8 The meta-analysis by Aung et al,9 which has been widely cited worldwide, is the basis on which, in March 2019, the European Medicines Agency (EMA) concluded that omega-3 fatty acid medicines are not effective in preventing further heart and blood vessels problems in patients with previous heart attacks. This statement has had an immediate clinical impact on several guidelines and recommendations, but does not suggest whether or not further investigations into the effectiveness of PUFAs in primary and secondary prevention are required.10
Trial-sequential analysis (TSA) can represent a useful tool in filling this gap. The advantages of TSA are already recognised not only for handling superiority questions but also regarding non-inferiority ones. In fact, TSAs aim at classifying each meta-analysis into one of four mutually exclusive categories (superiority, inferiority, futility, inconclusive result).11
The aim of this work was to perform a meta-analysis to evaluate PUFAs in the reduction of cardiovascular mortality in primary and secondary prevention of CVD along. Then, we conducted a TSA to determine if further original studies were necessary or the available data could be considered conclusive.
Methods
This review was conducted in line with the statement on Preferred Reporting Items for Systematic Reviews and Meta-Analyses.12
Search strategy
PubMed, EMBASE and the Cochrane Library were searched for relevant studies from their date of inception through to September 2019. The search was limited to the English language and the following search strategy was adopted: ‘omega-3 AND cardiovascular disease’; filter: ‘randomised controlled trial’, ‘meta-analysis’; and ‘humans’. References cited in the included articles were examined to identify additional studies.
Inclusion criteria and exclusion criteria
Studies were included if they met the following criteria: RCTs; outcomes including mortality related to cardiovascular issues; and PUFAs supplementation at 1 g/daily dosage. For each trial, the information concerning mortality due to CVD was extracted for both the intervention and the control groups. We excluded the studies that did not match these inclusion criteria. Articles partially published or without a full text available were also excluded.
Study selection and data extraction
The PICO approach was employed to collect the characteristics (population, intervention, comparator, outcome) of the included studies. Two investigators (MFC and AR) carried out the assessments and independently performed the literature search. Data extraction was completed by a third reviewer (LA) and disagreements were resolved involving two other reviewers (MC and DM). They discussed controversial points with the co-authors in order to make a final decision.
Data synthesis and analysis
Investigators carried out a meta-analysis of studies using the software OpenMeta Analyst. In the case of dichotomous data, the rate ratio (RR) was calculated for each trial with 95% CIs. Thereafter, a TSA was performed using the software developed by the Copenhagen Trial Unit (Centre for Clinical Intervention Research, www.ctu.dk/tsa). The result of TSA was expressed through the graph of cumulative Z-curve: the boundaries for concluding superiority, inferiority or futility were determined according to the O’Brien–Fleming alpha-spending function. To control the risk of type I error, we adjusted the thresholds for the Z-values with the use of the O'Brien–Fleming α-spending function, allowing the type I error risk to be set at the pre-determined maximum risk. Crossing the O'Brien–Fleming α-spending boundaries with a Z-curve indicates statistical significance. The risk of type II error was controlled with the use of the β-spending function and futility boundaries. Crossing the futility boundaries by the Z-curve indicates that the two interventions do not differ more than the anticipated effect. Our assumptions to perform the TSA included: two-sided testing, type-1 error 5% and power 80% – the effect of omega-3 fatty acids was set at relative risk reduction (RRR) of 10%, as already reported in the literature.9–16
Results
Search results and study characteristics
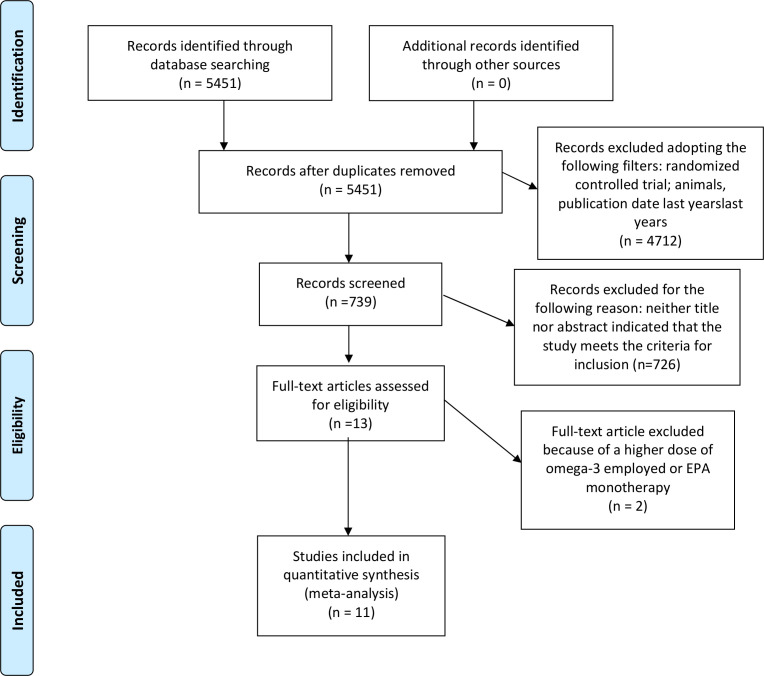
We initially identified 5451 records. A total of 4714 articles were excluded according to the inclusion/exclusion criteria and the search strategy. We finally excluded 726 records following scanning of the title and/or abstract. The full text of the remaining 13 references was then examined. Two studies, the JELIS trial13 and the REDUCE-IT trial,14 were not included because they employed eicosapentaenoic acid alone (1800mg/daily) or a higher dose of PUFAs (4 g daily), respectively. Therefore, we included 11 RCTs in our analysis for a total of 100 609 patients (table 1). The article search and screening process are described in the flow chart (figure 1). Apart from the JELIS and REDUCE-IT trials, the other studies were also reported in the meta-analysis by Aung et al.9 Moreover, we decided to include two other international trials: the Study of Cardiovascular Events in Diabetes, ASCEND15 and the VITamin D and OmegA-3 TriaL, VITAL.16
Table 1.
PICO characteristics of included studies
| Study | Year | Patients (n) | Dose of EPA/DHA (mg) | Population | Intervention | Comparator | Outcome |
| DOIT18 | 2010 | 563 | 1150/800 | Patients between 64 and 74-years-old, 74% of them without cardiovascular diseases | PUFAs | Placebo | All causes of mortality and cardiovascular disease |
| ARED-S19 | 2014 | 4203 | 650/350 | Individuals between 50 and 85-years- old, with intermediate or advanced age-related macular degeneration and with stable, existing CVD | PUFAs or lutein + zeaxanthin | Combination of the two, or matching placebos | Myocardial infarction, stroke and cardiovascular death |
| SU-FOL-OM320 | 2010 | 2501 | 400/200 | Patients with a history of myocardial infarction, unstable angina or ischaemic stroke | PUFAs | Vitamins or placebo | First major cardivascular events and cardiovascular death |
| Alpha Omega21 | 2010 | 4837 | 226/150 | Men and women, 60 to 80 years of age, who had had a clinically diagnosed myocardial infarction up to 10 years before randomisation |
PUFAs | Margarine | Major cardiovascular events, which comprised fatal and nonfatal cardiovascular disease and the cardiac interventions PCI and CABG |
| OMEGA22 | 2010 | 3818 | 460/380 | Patients with a minimum age of 18 years who were admitted to hospital for acute STEMI or non-STEMI | PUFAs | Olive oil | Sudden cardiac death |
| R&P23 | 2013 | 12 505 | 500/500 | Eligibility was based on one of the following: clinical evidence of atherosclerotic cardiovascular disease; multiple major cardiovascular risk factors; or other conditions putting the patient at high cardiovascular risk according to the GPs' judgement | PUFAs | Placebo | Death from cardiovascular causes |
| GISSI-HF24 | 2008 | 6975 | 850/950 | Patients were men and women aged 18 years or older, with clinical evidence of heart failure of any cause that was classified according to the European Society of Cardiology | PUFAs | Placebo | Time to death or time to admission to hospital for cardiovascular reasons |
| ORIGIN25 | 2012 | 12 536 | 465/375 | Patients with an age of at least 50 years; a diagnosis of diabetes, history of myocardial infarction, stroke or revascularisation; or angina, a ratio of urinary albumin to creatinine of more than 30 mg per gram | PUFAs | Placebo | Death from cardiovascular causes |
| GISSI-P26 | 1999 | 11 334 | 850/1700 | Patients with recent (3 months) myocardial infarction | PUFAs and VIT E | Placebo | Death |
| ASCEND15 | 2018 | 15 480 | 1 g (n-3 fatty acids) | Patients with diabetes but without evidence of atherosclerotic cardiovascular disease | n-3 fatty acids | Placebo | Serious vascular event |
| VITAL16 | 2019 | 25 871 | 1 g (n-3 fatty acids) | Men aged≥50 and women aged≥55 with no history of cancer or cardiovascular disease | Vitamin D3 and n-3 fatty acids | Vitamin D3+placebo or placebo + n-3 fatty acids | Major cardiovascular events |
CI, confidence interval; ctrl, controls; ev, events; PUFAs, polyunsaturated fatty acids.
Figure 1.
PRISMA diagram of literature screening: preferred reporting items for systematic reviews and meta-analyses.
Meta-analysis and trial-sequential analysis results
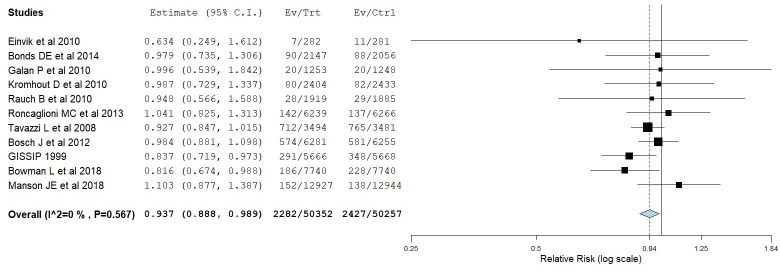
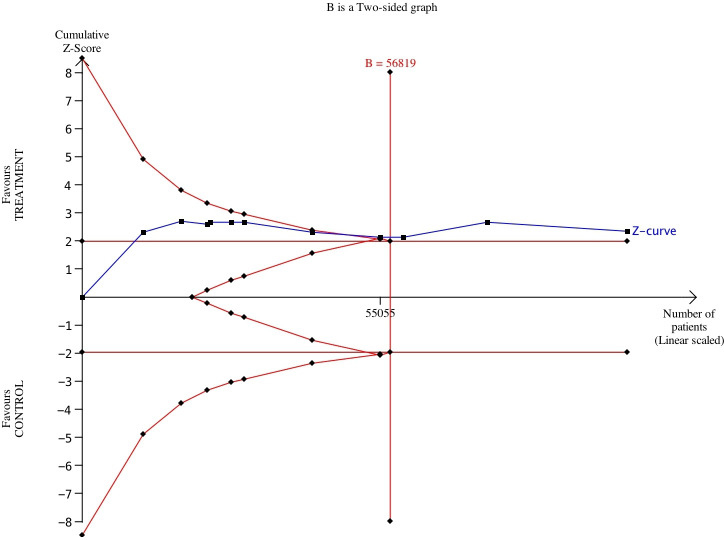
Meta-analysis results of the 11 studies showed a statistically significant reduction in mortality related to cardiovascular issues (RR=0.937; 95% CI: 0.88–0.98; P=0.018). The standard forest plot is depicted in figure 2. More interestingly, our TSA suggested that the superiority of PUFAs was demonstrated at a cumulative number of 56 819 patients (where the Z-line crossed the boundaries of superiority): on the other hand, the 11 trials reached a total number of 100 609 patients. The Z-curve graph is reported in figure 3. Therefore, these results support the conclusion that conducting further trials is unlikely to modify this scenario according to which the benefits of PUFAs are small, but statistically significant.
Figure 2.
Meta-analysis. forest plot showing the efficacy of PUFAs on cardiovascular death vs placebo or no treatment. The P-value is statistically significant (P<0.05). In the figure, the P-value for heterogeneity is reported (P=0.567). The difference between patients receiving PUFAs and controls is statistically significant (P=0.018).
Figure 3.
Trial-sequential analysis of 11 RCTs comparing PUFAs vs no treatment or placebo for preventing cardiovascular death. The expected RRR was assumed to be 10%. in the Z-curve (blue line), Individual trials correspond to individual segments; trials are plotted in chronological order (from left to right). The x-axis indicates the cumulative number of patients. Red lines are the boundaries for superiority or inferiority; B, sample size or cumulative number of patients.
Discussion
Our analysis is an attempt to draw a conclusion in a landscape where the information remains contradictory. While our results confirmed the presence of a small benefit of PUFAs, the principal strength of our work is represented by the results of the TSA. They were strictly dependent on the initial assumptions that, however, are reasonable and, more importantly, reflect the current trends of the literature on this topic. The cumulative Z-curve (figure 3, blue curve) crossed the conventional boundary and demonstrated that PUFAs significantly reduced mortality related to cardiovascular issues as shown in our meta-analysis. The number of patients included in our meta-analysis was higher than the required information size (56 819 patients considering the two-sided graph).
Hence, our TSA confirmed that conducting other studies in this field cannot be recommended because they are very unlikely to change the current scenario. Unlike the EMA statement, we confirmed that PUFAs granted a small but significant benefit in these settings.
In our study, there are some limitations. First, we included only RCTs. Therefore, we may have missed real-world data or evidence from observational studies. In addition, the final number of studies included in the meta-analysis was small because of our inclusion criteria. For example, we decided to exclude the REDUCE-IT trial due to a different dose of PUFAs used in the experimental arm, and the JELIS trial because EPA monotherapy was employed.
Conclusion
Omega-3 supplementation confirms its small benefit in both primary and secondary prevention of CVDs. In this context, the main recommendation arising from our results is that no further trials are needed to better evaluate the efficacy of PUFAs. We demonstrated, through a TSA, that enough studies on this topic have already been conducted to reach a conclusion.
To our knowledge, this is the first meta-analysis combined with a TSA that has so far been conducted about this topic.
In the 1990 s, cumulative meta-analysis17 was proposed as a new methodological tool that described how the main result of a meta-analysis (eg, the pooled OR or the pooled RR) evolves as time (expressed as calendar years) passes. Thereafter, cumulative meta-analysis has found poor acceptance in the scientific community and has substantially be abandoned. In 2020, TSA can be seen as a similar methodological tool, but its performance is much better than that of cumulative meta-analysis.
What this paper adds.
What is already known on this subject
The effectiveness of omega-3 PUFAs in cardiovascular diseases has been extensively investigated through both observational trials and RCTs.
There is still disagreement about the benefits of PUFAs supplementation, particularly concerning their impact on hard endpoints in both primary and secondary prevention of CVD.
What this study adds
Meta-analysis showed a statistically significant reduction in mortality related to cardiovascular issues. TSA suggested that the superiority of PUFAs was demonstrated at a cumulative number of 56,819 patients
These results support the conclusion that conducting further trials is unlikely to modify this scenario according to which the benefits of PUFAs are small, but statistically significant.
Acknowledgments
We thank Professor Andrea Messori for his support and advice.
Footnotes
MFC and AR contributed equally.
Contributors: The five authors equally contributed to the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Tenenbaum A, Fisman EZ. Omega-3 polyunsaturated fatty acids supplementation in patients with diabetes and cardiovascular disease risk: does dose really matter? Cardiovasc Diabetol 2018;17:119. 10.1186/s12933-018-0766-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavie CJ, Milani RV, Mehra MR, et al. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol 2009;54:585–94. 10.1016/j.jacc.2009.02.084 [DOI] [PubMed] [Google Scholar]
- 3. Tobin D, Brevik-Andersen M, Qin Y, et al. Evaluation of a high concentrate omega-3 for correcting the omega-3 fatty acid nutritional deficiency in non-alcoholic fatty liver disease (CONDIN). Nutrients 2018;10. 10.3390/nu10081126. [Epub ahead of print: 20 Aug 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tessier A-J, Chevalier S. An update on protein, leucine, omega-3 fatty acids, and vitamin D in the prevention and treatment of sarcopenia and functional decline. Nutrients 2018;10:pii:E1099. 10.3390/nu10081099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeromson S, Gallagher IJ, Galloway SDR, et al. Omega-3 fatty acids and skeletal muscle health. Mar Drugs 2015;13:6977–7004. 10.3390/md13116977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 2014;141:272–82. 10.1016/j.pharmthera.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 7. Koh AS, Pan A, Wang R, et al. The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese Health Study. Eur J Prev Cardiol 2015;22:364–72. 10.1177/2047487313517576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev 2018;11:CD003177. 10.1002/14651858.CD003177.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aung T, Halsey J, Kromhout D, et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol 2018;3:225–34. 10.1001/jamacardio.2017.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Medicines Agency (EMA) . European Medicines Agency. EMA confirms omega-3 fatty acid medicines are not effective in preventing further heart problems after a heart attack, 29 March 2019. Available: https://www.ema.europa.eu/en/documents/referral/omega-3-fatty-acid-medicines-ema-confirms-omega-3-fatty-acid-medicines-are-not-effective-preventing_en.pdf
- 11. Messori A, Fadda V, Maratea D, et al. Omega-3 polyunsaturated fatty acids for patients at risk of sudden cardiac death and ventricular arrhythmias: trial sequential analysis. Heart Lung 2013;42:391–2. 10.1016/j.hrtlng.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 13. Yokoyama M, Origasa H, Matsuzaki M, et al. Japan EPA Lipid Intervention Study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- 14. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 15. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–50. 10.1056/NEJMoa1804989 [DOI] [PubMed] [Google Scholar]
- 16. Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau J, Antman EM, Jimenez-Silva J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med 1992;327:248–54. 10.1056/NEJM199207233270406 [DOI] [PubMed] [Google Scholar]
- 18. Einvik G, Klemsdal TO, Sandvik L, et al. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil 2010;17:588–92. 10.1097/HJR.0b013e328339cc70 [DOI] [PubMed] [Google Scholar]
- 19. Bonds DE, Harrington M, Writing Group for the AREDS2 Research Group, et al. Effect of long-chain ω-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA Intern Med 2014;174:763–71. 10.1001/jamainternmed.2014.328 [DOI] [PubMed] [Google Scholar]
- 20. Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. 10.1136/bmj.c6273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kromhout D, Giltay EJ, Geleijnse JM, et al. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–26. 10.1056/NEJMoa1003603 [DOI] [PubMed] [Google Scholar]
- 22. Rauch B, Schiele R, Schneider S, et al. Omega, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–9. 10.1161/CIRCULATIONAHA.110.948562 [DOI] [PubMed] [Google Scholar]
- 23. Risk and Prevention Study Collaborative Group . N-3 fatty acids in patients with multiple cardiovascular risk factors. Eur J Prev Cardiol 2016;23:947–55.26525065 [Google Scholar]
- 24. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223–30. 10.1016/S0140-6736(08)61239-8 [DOI] [PubMed] [Google Scholar]
- 25. Bosch J, Gerstein HC, et al. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–18. 10.1056/NEJMoa1203859 [DOI] [PubMed] [Google Scholar]
- 26. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available upon reasonable request.