Abstract
Aim
This research was conducted to evaluate the mortality outcome of cancer patients with new-onset atrial fibrillation. We also aimed to assess if there was any confounding relation between the mortality of these patients and surgical intervention.
Materials and Methods
A systemic search was conducted from electronic databases (PubMed/Medline, Cochrane Library, and Google Scholar) from inception to 7 February 2022. All statistical analyses were conducted in Review Manager 5.4.1. Studies meeting inclusion criteria were selected. Only those studies that involved cancer patients without pre-existing atrial fibrillation were selected, and mortality rate was compared between the patients who developed atrial fibrillation and those who did not. A random-effect model was used when heterogeneity was seen to pool the studies, and the result was reported in the odds ratio (OR) and the corresponding 95% confidence interval (CI).
Results
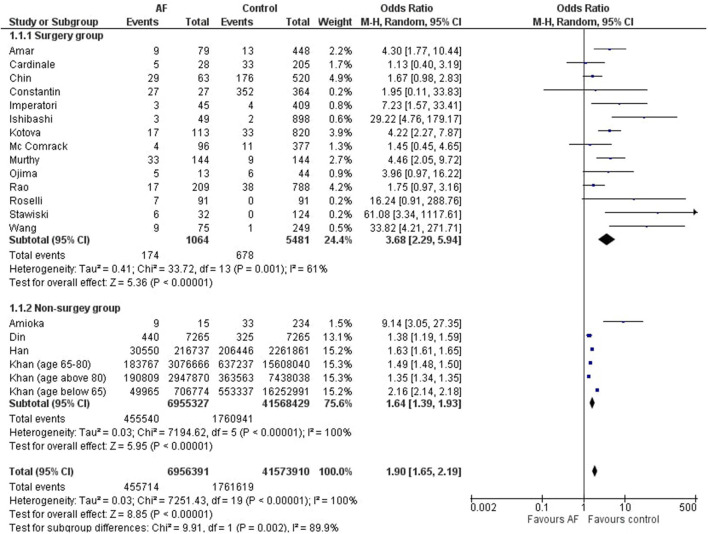
Eighteen studies were selected for meta-analysis. Statistical analysis showed that the cancer patients who subsequently developed atrial fibrillation had a significantly higher mortality rate as compared to those who did not (OR = 1.90 [1.65, 2.19]; p < 0.00001; I2 = 100%). We also separately analyzed the mortality risk in the surgery group and the non-surgery group. Statistical analysis showed that there was significantly higher mortality rate associated with new-onset atrial fibrillation in cancer patients in the surgery group (OR= 3.68 [2.29, 5.94]; p < 0.00001; I2 = 61%) as well as in the non-surgery group (OR = 1.64 [1.39, 1.93]; p < 0.00001; I2 = 100%).
Conclusion
Cancer patients, who subsequently developed atrial fibrillation, had a higher mortality rate as compared to those cancer patients who did not develop atrial fibrillation. A higher mortality rate was seen in both surgical and non-surgical subgroups. This implies that extra care and specific measures must be taken in the management of cancer patients with new-onset atrial fibrillation.
Keywords: new-onset atrial fibrillation, cancer, mortality, cardio-oncology, meta-analysis
Introduction
Cancer is among the most terrible diseases in the world, and its incidence is constantly on the rise (1). Considering the prevalence of known risk factors such as aging, family history, obesity, and radiation exposure, and increased adoption of unhealthy lifestyles such as tobacco smoking, alcohol, physical inactivity, and unhealthy diet, the rate of occurrence of almost all types of cancer is expected to further increase (2).
Lung cancer and breast cancer were found to be the most frequently diagnosed cancers and the leading cause of death among males and females, respectively, in 2012. Esophageal cancer notably varies in incidence rates internationally, and is found to be higher in men. Colorectal cancer is the third most frequently diagnosed cancer in males, and the second-most in females. Non-Hodgkin lymphoma is more common in more developed areas, with the highest incidence rates found in Australia, Western and Northern Europe, and Northern America (2).
Atrial fibrillation is the most frequently encountered cardiac arrhythmia that has great clinical importance. It causes significant morbidity and mortality by increasing the incidence of cardiomyopathies and their subsequent complications (3). It is well established that cancer patients undergoing surgery have an increased risk of developing atrial fibrillation in the perioperative and postoperative periods (4, 5).
In a meta-analysis conducted by Yuan et al., it was demonstrated that there is a higher risk of developing atrial fibrillation in cancer patients as compared to non-cancer patients. Cancer patients have a 47% higher risk of atrial fibrillation (6). The risk of atrial fibrillation varies in different types of cancer. For instance, colorectal cancer patients have a 54% higher risk and breast cancer patients have double the risk of developing atrial fibrillation as compared to people with no cancer (6).
Conversely, an increased risk of subsequent diagnosis of cancer in patients with atrial fibrillation has also been reported (7).
It has been found that cancer-related atrial fibrillation occurs more often after cancer surgery. Many risk factors have been implicated in the development of postoperative atrial fibrillation. These include advanced age, male gender, and advanced cancer stage (8). Moreover, a study reported 19.6% incidence rate of postoperative atrial fibrillation in patients undergoing an operation for malignant pulmonary disease, compared to the 3.1% incidence rate in those operated for benign pulmonary disease. This suggests that atrial fibrillation is not merely a complication of surgery, but it has a strong link with cancer itself (9).
In the case of surgical patients, pre-operative cardiac symptoms and echocardiogram (ECG) abnormalities, operative parameters, and post-operative clinical findings may be responsible for the development of atrial fibrillation, but their causal role could not be demonstrated (9).
Cancer could not be demonstrated as an independent predictor of atrial fibrillation. However, the elevated levels of C-reactive protein (CRP) associated with cancer, and the remodeling of the atrial structure due to the presence of an inflammatory state suggest inflammation as the causal intermediary link between the two (10).
Increased incidence of atrial fibrillation in cancer patients may also be related to autonomic disturbances, atrial inflammation due to autoimmune paraneoplastic syndromes, or cancer therapy (10, 11).
The previous meta-analysis conducted by Yuan et al. determined the relationship between cancer and the risk of developing atrial fibrillation, but it did not evaluate the impact of atrial fibrillation on cancer mortality (6). There has been inconsistent evidence on whether the new-onset atrial fibrillation in cancer patients significantly affects mortality outcomes. Therefore, this systematic review and meta-analysis was conducted to establish a conclusive relationship between new-onset atrial fibrillation and mortality in cancer patients.
Materials and Methods
Data Sources and Search Strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (12). An electronic search from PubMed/Medline, Cochrane Library, and Google Scholar was conducted from their inception to 7 February 2022 (detailed strategy is provided in Table 1), with only English language-based literature, using the search string: (cancer* OR carcinoma* malignancy*) AND (atrial fibrillation OR AF OR a-fibrillation) AND (mortality OR death OR survival). In addition, we manually screened the cited articles of previous meta-analyses, cohort studies, and review articles to identify any relevant studies.
Table 1.
Search strategy.
| Search engine | Search strategy |
|---|---|
| Pubmed/medline | (“cancer*”[All Fields] OR (“carcinoma*”[All Fields] AND “malignanc*”[All Fields])) AND (“atrial fibrillation”[MeSH Terms] OR (“atrial”[All Fields] AND “fibrillation”[All Fields]) OR “atrial fibrillation”[All Fields] OR “AF”[All Fields] OR “a-fibrillation”[All Fields]) AND (“mortality”[MeSH Terms] OR “mortality”[All Fields] OR “mortalities”[All Fields] OR “mortality”[MeSH Subheading] OR (“death”[MeSH Terms] OR “death”[All Fields] OR “deaths”[All Fields]) OR (“mortality”[MeSH Subheading] OR “mortality”[All Fields] OR “survival”[All Fields] OR “survival”[MeSH Terms] OR “survivability”[All Fields] OR “survivable”[All Fields] OR “survivals”[All Fields] OR “survive”[All Fields] OR “survived”[All Fields] OR “survives”[All Fields] OR “surviving”[All Fields])) |
| Cochrane | (cancer* OR carcinoma* malignanc*) AND (atrial fibrillation OR AF OR a-fibrillation) AND (mortality OR death OR survival) |
| Google scholar | (cancer* OR carcinoma* malignanc*) AND (atrial fibrillation OR AF OR a-fibrillation) AND (mortality OR death OR survival) |
Study Selection
All studies were included, which met the following eligibility described as PECOS: (1) P (Population): cancer patients; (2) E (Exposure): atrial fibrillation; (3) C (Control): cancer patients without atrial fibrillation; (4) O (Outcome): mortality; (5) S (Studies): human-based randomized controlled trials and cohort studies published in English only.
Case series, case reports, literature reviews, editorials, and studies not meeting the inclusion criteria were excluded.
Data Extraction and Quality Assessment of Studies
Two reviewers independently searched electronic databases. Studies searched were exported to the EndNote Reference Library software version 20.0.1 (Clarivate Analytics), and duplicates were screened and removed.
Data extraction and quality assessment of included studies were done simultaneously and independently by two reviewers. Newcastle-Ottawa Scale (NOS) was used to assess the quality of the cohort studies. NOS score <6 was considered high risk for bias, 6–7 was moderate, and score >7 was considered low risk of bias (details of scoring is provided in Table 2). The modified Cochrane Collaboration's risk of bias tool for randomized controlled trials was used to assess the quality of published trials (details are provided in Table 3).
Table 2.
Quality assessment of cohorts using New Ottawa scale (NOS).
| Studies | Selection (maximum 4) | Comparability (maximum 2) | Outcome (maximum 3) | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Amar et al. (14) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Amioka et al. (15) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Cardinale et al. (16) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Chin et al. (17) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Constantin et al. (18) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Imperatori et al. (19) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ishibashi et al. (20) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Kotova et al. (21) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| McComrack et al. (22) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Murthy et al. (23) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Rao et al. (25) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Roselli et al. (26) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Stawicki et al. (27) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Wang et al. (28) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Ammad Ud Din et al. (29) | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 1 | 8 |
| Han et al. (30) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Zubair Khan et al. (31) | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Table 3.
Quality assessment of clinical trials using Cochrane Collaboration's risk of bias tool.
| Study | Adequate sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective outcome reporting | Free of other bias | Net risk |
|---|---|---|---|---|---|---|---|---|
| Ojima et al. (24) | High risk | Unclear risk | High risk | Unclear risk | Low risk | High risk | High risk | High risk |
Statistical Analysis
Review Manager (version 5.4.1; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2020) was used for all statistical analyses. The data from studies were pooled using a random-effects model. Analysis of results was done by calculating the odds ratio (OR) with respective 95% confidence intervals (CI). The chi-square test was performed to assess any differences between the subgroups. Sensitivity analysis was done to see if any individual study was driving the results and to implore reasons for high heterogeneity. As per Higgins et al., scale for heterogeneity was considered as follows: I2 = 25–60%–moderate; 50–90%–substantial; 75–100%–considerable heterogeneity, and p < 0.1 indicated significant heterogeneity (13). A value of p < 0.05 was considered significant for all analyses.
Results
Literature Search Results
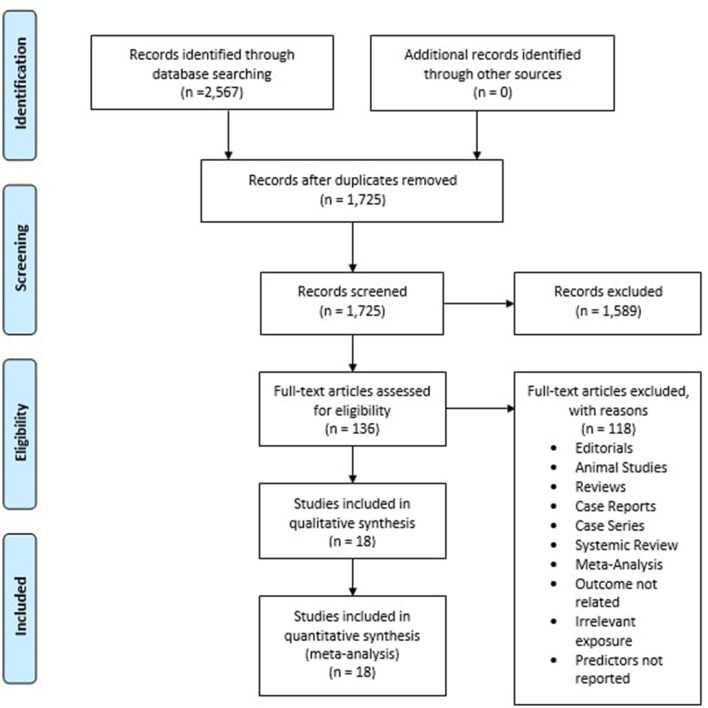
The initial search of the three electronic databases yielded 2,567 potential studies. After exclusions based on titles and abstracts, the full texts of 136 studies were read for possible inclusion. A total of 18 studies remained for quantitative analysis. Figure 1 summarizes the results of our literature search.
Figure 1.
Prisma flow chart summarizing the literature search.
Study Characteristics
Table 4 provides the basic characteristics of included studies (14–31). Our analysis included 18 published studies. Among these, there are 17 observational studies and 1 randomized controlled trial. The studies were conducted in different regions of the world, i.e., USA, Japan, Italy, Korea, Romania, Ireland, China, and the UK. A total of 6 studies evaluated lung cancer, 5 studies evaluated esophageal cancer, and 6 studies evaluated various other cancers including lymphoma, leukemia, colorectal cancer, prostate cancer, and breast cancer. The mean age of patients was 67.12 years.
Table 4.
Characteristics of included studies.
| Study | Year | Study design | Duration | Country | Total cancer patients (n) | Males (n) | Mean age (years) | Incident AF (n) | Cancer type | Mortality attestation | Major causes of death | Surgery or non-surgery group | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amar et al. (14) | 2002 | Cohort | 1990–1999 | USA | 527 | 325 | 68 (AF) 62 (non-AF) | 79 | Lung cancer, Esophageal cancer | Medical record | Surgery-related complications | Surgery | Moderate Risk |
| Amioka et al. (15) | 2016 | Cohort | 2009–2013 | Japan | 249 | 121 | 67 | 15 | Hodgkin, Non-Hodgkin lymphoma | Medical record | Primary disease, solid cancer, hepatitis, sepsis | Non-surgery | Low Risk |
| Cardinale et al. (16) | 1999 | Cohort | 1995–1997 | Italy | 233 | 170 | 59.3 | 28 | Lung cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Chin et al. (17) | 2016 | Cohort | 2005–2012 | Korea | 583 | 548 | 67 (AF) 62 (non-AF) | 63 | Esophageal cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Constantin al (18) | 2020 | Cohort | 2008–2017 | Romania | 391 | N/A* | N/A* | 27 | Colorectal cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Imperatori et al. (19) | 2012 | Cohort | 1996–2009 | Italy | 454 | 369 | 65.4 | 45 | Lung cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Ishibashi et al. (20) | 2020 | Cohort | 2010–2019 | Japan | 947 | 626 | 69.2 | 49 | Lung cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Kotova et al. (21) | 2017 | Cohort | 2005–2014 | USA | 933 | 426 | 72 (AF) 65.4 (non-AF) | 113 | Lung cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| McComrack et al. (22) | 2014 | Cohort | 2006–2013 | Ireland | 473 | 344 | 63 | 96 | Esophageal cancer, Junctional cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Murthy et al. (23) | 2003 | Cohort | 1982–2000 | China | 288 | 235 | 66.8 (AF) 67 (non-AF) | 144 | Esophageal cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Ojima et al. (24) | 2020 | RCT* | 2014–2016 | Japan | 57 | 77 | N/A* | 13 | Esophageal cancer | Medical record | Surgery-related complications | Surgery | High Risk |
| Rao et al. (25) | 2012 | Cohort | 1991–2009 | UK | 997 | 709 | 67 | 209 | Esophageal cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Roselli et al. (26) | 2005 | Cohort | 1998–2002 | USA | 183 | N/A* | N/A* | 91 | Lung cancer | Medical record | Surgery-related complications | Surgery | Moderate Risk |
| Stawicki et al. (27) | 2011 | Cohort | 1996–2007 | USA | 156 | 145 | 63.7 (AF) 59.8 (non-AF) | 32 | Esophageal cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Wang et al. (28) | 2021 | Cohort | 2013–2018 | China | 324 | 291 | 58.4 | 75 | Lung cancer | Medical record | Surgery-related complications | Surgery | Low Risk |
| Ammad Ud Din et al. (29) | 2021 | Cohort | 2009–2018 | USA | 14,530 | 8,569 | 81.80 (AF) 81.76 (non-AF) | 7,265 | chronic lymphocytic leukemia | Medical record | Acute Myocardial Infarction, heart failure, cardiac arrest, stroke | Non-surgery | Low Risk |
| Han et al. (30) | 2021 | Cohort | 2003–2014 | China | 2,478,598 | 1,104,903 | N/A | 216,737 | Various cancers | Medical record | Not specified | Non-surgery | Low Risk |
| Zubair Khan et al. (31) | 2021 | Cohort | 2005–2015 | USA | 46,030,380 | 7,673,063 | N/A | 6,731,310 | Various cancers | Medical record | Not specified | Non-surgery | Low Risk |
N/A*, Not available; RCT*, Randomized Controlled Trial.
Publication Bias and Quality Assessment
The visual inspection of the funnel plot (Figure 2) did indicate that there is publication bias in our meta-analysis.
Figure 2.
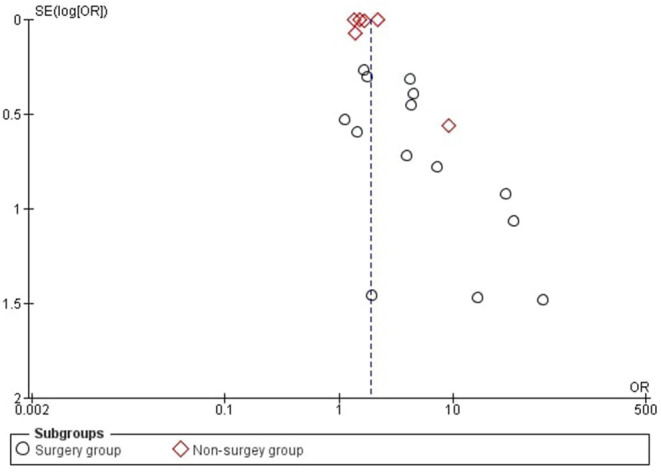
Funnel plot which is used to demonstrate publicaion bias.
Out of the 18 studies, 15 studies have a low risk of bias (15–23, 25, 27–31), two studies have a moderate risk of bias (14, 26), and one study has a high risk of bias (24).
Results of Meta-Analysis
Detailed forest plots, outlining the effect size of overall mortality outcome of atrial fibrillation in cancers, are shown in Figure 3. A forest plot outlining the effect size based on surgery and the non-surgery group is also presented in Figure 3.
Figure 3.
Forest plot showing effect size of mortality outcome of atrial fibrillation in different types of cancer and also its overall affect.
Overall Mortality Outcome of Atrial Fibrillation in Cancers
Eighteen studies evaluated the mortality outcome for cancer patients who develop atrial fibrillation. Table 5 provides further details of the studies selected for this objective. Pooled results (Figure 3) showed a significantly higher mortality rate in cancer patients who subsequently develop atrial fibrillation as compared to those in the control group (OR = 1.90 [1.65, 2.19]; p < 0.00001; I2 = 100%).
Table 5.
Analytical details of the studies that were selected.
| Study name and year | Deaths in AF | Deaths in control | Odds ratio [95% CI] | P value | |||
|---|---|---|---|---|---|---|---|
| Events (n) | Total (n) | Events (n) | Total (n) | ||||
| Amar et al. (14) | 2002 | 9 | 79 | 13 | 448 | 4.30 [1.77, 10.44] | 0.0013 |
| Amioka et al. (15) | 2016 | 9 | 15 | 33 | 234 | 9.14 [3.05, 27.35] | 0.0001 |
| Cardinale et al. (16) | 1999 | 5 | 28 | 33 | 205 | 1.13 [0.40, 3.19] | 0.8132 |
| Chin et al. (17) | 2016 | 29 | 63 | 176 | 520 | 1.67 [0.98, 2.83] | 0.0576 |
| Constantin et al. (18) | 2020 | 27 | 27 | 352 | 364 | 1.95 [0.11, 33.83] | 0.6463 |
| Imperatori et al. (19) | 2012 | 3 | 45 | 4 | 409 | 7.23 [1.57, 33.41] | 0.0113 |
| Ishibashi et al. (20) | 2020 | 3 | 49 | 2 | 898 | 29.22 [4.76, 179.17] | 0.0003 |
| Kotova et al. (21) | 2017 | 17 | 113 | 33 | 820 | 4.22 [2.27, 7.87] | 0.0000 |
| McComrack et al. (22) | 2014 | 4 | 96 | 11 | 377 | 1.45 [0.45, 4.65] | 0.5352 |
| Murthy et al. (23) | 2003 | 33 | 144 | 9 | 144 | 4.46 [2.05, 9.72] | 0.0002 |
| Ojima et al. (24) | 2020 | 5 | 13 | 6 | 44 | 3.96 [0.97, 16.22] | 0.0559 |
| Rao et al. (25) | 2012 | 17 | 209 | 38 | 788 | 1.75 [0.97, 3.16] | 0.0652 |
| Roselli et al. (26) | 2005 | 7 | 91 | 0 | 91 | 16.24 [0.91, 288.76] | 0.0576 |
| Stawicki et al. (27) | 2011 | 6 | 32 | 0 | 124 | 61.08 [3.34, 1,117.61] | 0.0056 |
| Wang et al. (28) | 2021 | 9 | 75 | 1 | 249 | 33.82 [4.21, 271.71] | 0.0009 |
| Ammad Ud Din et al. (29) | 2021 | 440 | 7,265 | 325 | 7,265 | 1.38 [1.19, 1.59] | 0.0000 |
| Han et al. (30) | 2021 | 30,550 | 2,16,737 | 2,06,446 | 2,261,861 | 1.63 [1.61, 1.65] | 0.0000 |
| Zubair Khan et al. (31) (age 65-80) | 2021 | 1,83,767 | 30,76,666 | 6,37,237 | 15,608,040 | 1.49 [1.48, 1.50] | 0.0000 |
| Zubair Khan (31) (age below 65) | 2021 | 1,90,809 | 29,47,870 | 3,63,563 | 74,38,038 | 1.35 [1.34, 1.35] | 0.0000 |
| Zubair Khan (31) (age above 80) | 2021 | 49,965 | 7,06,774 | 5,53,337 | 1,62,52,991 | 2.16 [2.14, 2.18] | 0.0000 |
Surgery Group
Out of 18 studies, 14 studies reported data of patients undergoing cancer-related surgeries. Statistical analysis showed that there was a significantly higher mortality rate in cancer patients who subsequently develop atrial fibrillation as compared to those in the control group (OR = 3.68 [2.29, 5.94]; p < 0.00001; I2 = 61%).
Non-Surgery Group
Out of 18 studies, 4 studies reported data of non-surgical patients having cancer. Statistical analysis showed that there was a significantly higher mortality rate in cancer patients who subsequently develop atrial fibrillation as compared to those in the control group (OR = 1.64 [1.39, 1.93]; p < 0.00001; I2 = 100%).
Sensitivity Analysis
A sensitivity analysis was conducted to assess the influence of each study on the overall effect, by excluding one study at a time, followed by the generation of pooled OR for the rest of the studies. No significant change was observed after the exclusion of any individual study, suggesting that the results were robust.
We also removed the study by Amioka et al. (15) to check its effect on our results, as it was the only study with a high risk of bias, but there was no statistically significant change. The overall result was OR = 1.85 [1.61, 2.14]; p < 0.0001; I2= 100%.
Discussion
Our systematic review and meta-analysis of 18 published studies suggest that cancer patients who subsequently develop atrial fibrillation have an increased rate of mortality as compared to those who do not (OR = 1.90 [1.65, 2.19]; p < 0.00001; I2 = 100%). We also separately analyzed the mortality risk in the surgery group and the non-surgery group. This subgroup analysis shows that new-onset atrial fibrillation associated with cancer increases the mortality rate, irrespective of any surgical intervention. Mechanisms contributing to death included surgery-related complications, sepsis, hepatitis, pneumonia, heart failure, myocardial infarction, and stroke.
Previous studies have evaluated this relationship in specific cancer patients. However, the results are inconsistent, and the studies are limited by their sample size and geographical location. We have pooled data from studies performed in different regions of the world and with different cancer types to provide better and more reliable evidence.
Cancer continues to be one of the deadliest diseases worldwide. In the year 2020, an estimated 19.3 million new cancer cases and about 10 million cancer deaths occurred globally (32). Death in cancer patients may occur due to index cancer, non-index cancer, or non-cancer causes. Patients with cancers of the colorectum, prostate, breast, genitourinary tract, tonsils, melanoma, and lymphomas are more likely to die due to non-cancer causes. Heart disease is the most common non-cancer cause of death (33). Many mechanisms can lead to the death of cancer patients, including infections (36%), hemorrhagic and thromboembolic phenomena (18%), and respiratory failure (19%) (34).
It is well recognized that there is an increased risk of developing atrial fibrillation in cancer patients (6). Cancer itself is a high-risk and life-threatening condition. Concomitant development of atrial fibrillation brings additional risks including infections, stroke, and bleeding among others (35). Atrial fibrillation alone is associated with four times increased risk of all-cause mortality as compared to the general population (36). Atrial fibrillation has been demonstrated as a risk factor for stroke (37). A five-fold increased risk of stroke has been reported to be associated with chronic atrial fibrillation (38).
The underlying mechanism leading to the development of atrial fibrillation in cancer patients has been a matter of concern. Atrial fibrillation may be a co-morbid state considering the common predisposing factors for both conditions, and it might involve specific etiologies (10).
Inflammation of the atria due to autoimmune paraneoplastic syndromes, the abnormal release of some hormones by cancer cells, and imbalances between the sympathetic and parasympathetic autonomic control may predispose the patient to atrial fibrillation (10). These mechanisms support our findings that new-onset atrial fibrillation can increase the risk of mortality in cancer patients.
Cancer therapy may be responsible for the development of atrial fibrillation. However, it has been observed that the risk of developing atrial fibrillation persists even if no cancer-specific treatment has been given (11).
Although the exact mechanism responsible for increased mortality in cancer patients with atrial fibrillation remains undetermined, it can be speculated from the following findings. In atrial fibrillation, the perfusion of tissues becomes inadequate, and due to this, oxidative injury can occur (38, 39). The hypoxic conditions not only favor tumor survival and growth but also cause resistance to radiation therapy (40, 41). Moreover, endothelial dysfunction, inflammatory conditions, and prothrombic state associated with atrial fibrillation may also be responsible for the worsened outcomes (42). The inflammatory state may be further aggravated by cancer surgery. The increase in in-hospital mortality can also be explained by the fact that patients with atrial fibrillation are susceptible to hospital-acquired pneumonia, which can become the cause of death in these patients (43).
Most of the studies included in our analysis enrolled patients undergoing cancer surgery. It is highly likely that the surgery may play an important role in the worsened outcomes. Various processes and mediators involved in surgical wound healing may accelerate tumor growth, invasion, and metastasis (44). Tumor outgrowth may be caused by activation of epithelial, endothelial, and inflammatory cells; platelets, and fibroblasts; and production of growth factors and cytokines during the healing of surgical wounds (45).
The efficacy of different prophylactic approaches to prevent atrial fibrillation after lung surgery was evaluated in a meta-analysis by Zhang et al. It was found that amiodarone was the most effective in preventing postoperative atrial fibrillation (46). The use of oral anticoagulants is associated with a lower risk of mortality in patients with atrial fibrillation (36). It has been demonstrated that the patients who have concomitant cancer and atrial fibrillation can be benefited from anticoagulation with nonvitamin K antagonist oral anticoagulants (also known as direct oral anticoagulants (DOACs)) as well as warfarin (47). However, DOACs have a safer profile and greater effectiveness as compared to vitamin K antagonists (VKAs) (48). Moreover, CHADS2 and CHA2DS2-VASc scores can be used as tools to predict the risk of stroke and mortality in patients with cancer and atrial fibrillation and guide in decision-making accordingly (49).
Although it is suggested that atrial fibrillation after cancer surgery tends to be transient with few clinical outcomes and does not require prolonged monitoring and intensive care (5), recent data suggest a relationship between atrial fibrillation and increased mortality in cancer patients. Cancer patients who subsequently develop atrial fibrillation may need closer surveillance during follow-up, and specific strategies to manage atrial fibrillation should be included in the cancer management plan.
Limitation
Our study is limited in several ways: (a) The majority of the studies were observational, the results of which can have some bias. (b) Most of the studies enrolled patients undergoing surgery, so our outcome, i.e., increased mortality, may not solely be due to the atrial fibrillation but there might be some role of the after-effects of surgery. (c) High heterogeneity was seen in our results because we pooled different studies containing different cancer types. These studies were pivotal in forming analysis, but more studies with the community and random controls should be conducted.
Conclusion
Cancer patients who subsequently developed atrial fibrillation had a higher mortality rate as compared to those cancer patients who did not develop atrial fibrillation. A higher mortality rate was seen in both surgical and non-surgical subgroups. This implies that cancer patients who subsequently develop atrial fibrillation need to be followed up with careful monitoring of the arrhythmia, and specific measures should be taken to minimize the adverse outcomes brought on by it.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MB worked alongside MM in all steps from literature search till manuscript preparation. JA reviewed and actively participated in the analysis, manuscript preparation and reviewing, and is designated as the senior author. LS and SB played role in final analysis and reviewing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Open Access publication fee has been funded by Liviu Ionut Serbanoiu (LS).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Roy PS, Saikia BJ. Cancer and cure: a critical analysis. Indian J Cancer. (2016) 53:441–42. 10.4103/0019-509X.200658 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. (2015) 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 3.Bhatt HV, Fischer GW. Atrial fibrillation: pathophysiology and therapeutic options. J Cardiothorac Vasc Anesth. (2015) 29:1333–40. 10.1053/j.jvca.2015.05.058 [DOI] [PubMed] [Google Scholar]
- 4.Higuchi S, Kabeya Y, Matsushita K, Arai N, Tachibana K, Tanaka R, et al. Incidence and complications of perioperative atrial fibrillation after non-cardiac surgery for malignancy. PLoS ONE. (2019) 14:e0216239. 10.1371/journal.pone.0216239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbs HR, Swafford J, Nguyen HD, Ewer MS, Ali MK. Postoperative atrial fibrillation in cancer surgery: preoperative risks and clinical outcome. J Surg Oncol. (1992) 50:224–27. 10.1002/jso.2930500405 [DOI] [PubMed] [Google Scholar]
- 6.Yuan M, Zhang Z, Tse G, Feng X, Korantzopoulos P, Letsas KP, et al. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol Res Pract. (2019) 2019:8985273. 10.1155/2019/8985273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lateef N, Kapoor V, Ahsan MJ, Latif A, Ahmed U, Mirza M, et al. Atrial fibrillation and cancer: understanding the mysterious relationship through a systematic review. J Commun Hosp Intern Med Perspect. (2020) 10:127–32. 10.1080/20009666.2020.1726571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. (2014) 63:945–53. 10.1016/j.jacc.2013.11.026 [DOI] [PubMed] [Google Scholar]
- 9.Beck-Nielsen J, Sorensen HR, Alstrup P. Atrial fibrillation following thoracotomy for non-cardiac diseases, in particular cancer of the lung. Acta Med Scand. (1973) 193:425–29. 10.1111/j.0954-6820.1973.tb10604.x [DOI] [PubMed] [Google Scholar]
- 10.Guzzetti S, Costantino G, Fundarò C. Systemic inflammation, atrial fibrillation, and cancer. Circulation. (2002) 106:e40. 10.1161/01.cir.0000028399.42411.13 [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick T, Carrier M, Le Gal G. Cancer, atrial fibrillation, and stroke. Thromb Res. (2017) 155:101–05. 10.1016/j.thromres.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amar D, Zhang H, Leung DH, Roistacher N, Kadish AH. Older age is the strongest predictor of postoperative atrial fibrillation. Anesthesiology. (2002) 96:352–56. 10.1097/00000542-200202000-00021 [DOI] [PubMed] [Google Scholar]
- 15.Amioka M, Sairaku A, Ochi T, Okada T, Asaoku H, Kyo T, et al. Prognostic significance of new-onset atrial fibrillation in patients with non-Hodgkin's lymphoma treated with anthracyclines. Am J Cardiol. (2016) 118:1386–389. 10.1016/j.amjcard.2016.07.049 [DOI] [PubMed] [Google Scholar]
- 16.Cardinale D, Martinoni A, Cipolla CM, Civelli M, Lamantia G, Fiorentini C, et al. Atrial fibrillation after operation for lung cancer: clinical and prognostic significance. Ann Thorac Surg. (1999) 68:1827–31. 10.1016/s0003-4975(99)00712-2 [DOI] [PubMed] [Google Scholar]
- 17.Chin JH, Moon YJ, Jo JY, Han YA, Kim HR, Lee EH, et al. Association between postoperatively developed atrial fibrillation and long-term mortality after esophagectomy in esophageal cancer patients: an observational study. PLoS ONE. (2016) 11:e0154931. 10.1371/journal.pone.0154931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantin GB, Firescu D, Voicu D, tefănescu B, Serban RMC, Berbece S, et al. Analysis of prognostic factors in complicated colorectal cancer operated in emergency. Chirurgia (Bucur). (2020) 115:23–38. 10.21614/chirurgia.115.1.23 [DOI] [PubMed] [Google Scholar]
- 19.Imperatori A, Mariscalco G, Riganti G, Rotolo N, Conti V, Dominioni L. Atrial fibrillation after pulmonary lobectomy for lung cancer affects long-term survival in a prospective single-center study. J Cardiothorac Surg. (2012) 7:4. 10.1186/1749-8090-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi H, Wakejima R, Asakawa A, Baba S, Nakashima Y, Seto K, et al. Postoperative atrial fibrillation in lung cancer lobectomy-analysis of risk factors and prognosis. World J Surg. (2020) 44:3952–59. 10.1007/s00268-020-05694-w [DOI] [PubMed] [Google Scholar]
- 21.Kotova S, Wang M, Lothrop K, Grunkemeier G, Merry HE, Handy JR. CHADS2 score predicts postoperative atrial fibrillation in patients undergoing elective pulmonary lobectomy. Ann Thorac Surg. (2017) 103:1566–72. 10.1016/j.athoracsur.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 22.Mc Cormack O, Zaborowski A, King S, Healy L, Daly C, O'Farrell N, et al. New-onset atrial fibrillation post-surgery for esophageal and junctional cancer: incidence, management, and impact on short- and long-term outcomes. Ann Surg. (2014) 260:772–8. 10.1097/SLA.0000000000000960 [DOI] [PubMed] [Google Scholar]
- 23.Murthy SC, Law S, Whooley BP, Alexandrou A, Chu KM, Wong J. Atrial fibrillation after esophagectomy is a marker for postoperative morbidity and mortality. J Thorac Cardiovasc Surg. (2003) 126:1162–67. 10.1016/s0022-5223(03)00974-7 [DOI] [PubMed] [Google Scholar]
- 24.Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Nakamori M, et al. Postoperative atrial fibrillation does not impact on overall survival after esophagectomy in patients with thoracic esophageal cancer: results from a randomized, double-blind, placebo-controlled trial. Oncotarget. (2020) 11:2414–23. 10.18632/oncotarget.27643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao VP, Addae-Boateng E, Barua A, Martin-Ucar AE, Duffy JP. Age and neo-adjuvant chemotherapy increase the risk of atrial fibrillation following oesophagectomy. Eur J Cardiothorac Surg. (2012) 42:438–43. 10.1093/ejcts/ezs085 [DOI] [PubMed] [Google Scholar]
- 26.Roselli EE, Murthy SC, Rice TW, Houghtaling PL, Pierce CD, Karchmer DP, et al. Atrial fibrillation complicating lung cancer resection. J Thorac Cardiovasc Surg. (2005) 130:438–44. 10.1016/j.jtcvs.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 27.Stawicki SP, Prosciak MP, Gerlach AT, Bloomston M, Davido HT, Lindsey DE, et al. Atrial fibrillation after esophagectomy: an indicator of postoperative morbidity. Gen Thorac Cardiovasc Surg. (2011) 59:399–405. 10.1007/s11748-010-0713-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Wang Z, Zhou M, Chen J, Yao F, Zhao L, et al. Postoperative atrial fibrillation in pneumonectomy for primary lung cancer. J Thorac Dis. (2021) 13:789–802. 10.21037/jtd-20-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ammad Ud Din M, Thakkar S, Patel H, Saeed H, Hussain SA, Liaqat H, et al. The impact of atrial fibrillation on hospitalization outcomes for patients with chronic lymphocytic leukemia using the national inpatient sample database. Clin Lymphoma Myeloma Leuk. (2022) 22:98–104. 10.1016/j.clml.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 30.Han H, Chen L, Lin Z, Wei X, Guo W, Yu Y, et al. Prevalence, trends, and outcomes of atrial fibrillation in hospitalized patients with metastatic cancer: findings from a national sample. Cancer Med. (2021) 10:5661–70. 10.1002/cam4.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zubair Khan M, Gupta A, Patel K, Abraham A, Franklin S, Kim DY, et al. Association of atrial fibrillation and various cancer subtypes. J Arrhythm. (2021) 3:1205–14. 10.1002/joa3.12589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 33.Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. (2017) 28:400–07. 10.1093/annonc/mdw604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ambrus JL, Ambrus CM, Mink IB, Pickren JW. Causes of death in cancer patients. J Med. (1975) 6:61–4. [PubMed] [Google Scholar]
- 35.Fauchier L, Villejoubert O, Clementy N, Bernard A, Pierre B, Angoulvant D, et al. Causes of seath and influencing factors in patients with atrial fibrillation. Am J Med. (2016) 129:1278–87. 10.1016/j.amjmed.2016.06.045 [DOI] [PubMed] [Google Scholar]
- 36.Manolio TA, Kronmal RA, Burke GL, O'Leary DH, Price TR. Short-term predictors of incident stroke in older adults. The cardiovascular health study. Stroke. (1996) 27:1479–86. 10.1161/01.str.27.9.1479 [DOI] [PubMed] [Google Scholar]
- 37.Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. (1978) 28:973–77. 10.1212/wnl.28.10.973 [DOI] [PubMed] [Google Scholar]
- 38.Tu HT, Campbell BC, Christensen S, Desmond PM, De Silva DA, Parsons MW, et al. EPITHET-DEFUSE Investigators. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. (2015) 10:534–40. 10.1111/ijs.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guazzi M, Berti M, Belletti S, Reina G, Guazzi MD. Exercise metaboreflex activation and endothelial function impairment in atrial fibrillation. Am J Physiol Heart Circ Physiol. (2006) 291:H2396–402. 10.1152/ajpheart.00437.2006 [DOI] [PubMed] [Google Scholar]
- 40.Harris AL. Hypoxia–a key regulatory factor in tumour growth. Nat Rev Cancer. (2002) 2:38–47. 10.1038/nrc704 [DOI] [PubMed] [Google Scholar]
- 41.Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ. Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol. (2012) 3:21. 10.3389/fimmu.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60:2263–270. 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 43.Zhu J, Zhang X, Shi G, Yi K, Tan X. Atrial fibrillation is an independent risk factor for hospital-acquired pneumonia. PLoS ONE. (2015) 10:e0131782. 10.1371/journal.pone.0131782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ceelen W, Pattyn P, Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit Rev Oncol Hematol. (2014) 89:16–26. 10.1016/j.critrevonc.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 45.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. (1999) 25:231–43. 10.1053/ejso.1998.0634 [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Gao S. Systematic Review and Meta-analysis of Atrial Fibrillation Prophylaxis After Lung Surgery. J Cardiovasc Pharmacol. (2016) 67:351–57. 10.1097/FJC.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Mao M, Chang J, Yan J, Yang T, Liu Y, et al. Safety and efficacy of new oral anticoagulants compared to those of warfarin in AF patients with cancer: a meta-analysis of randomized clinical trials and observational studies. Eur J Clin Pharmacol. (2021) 77:849–57. 10.1007/s00228-021-03132-x [DOI] [PubMed] [Google Scholar]
- 48.Liu F, Xu Z, Luo J, Yu P, Ma J, Yuan P, et al. Effectiveness and safety of DOACs vs. VKAs in AF patients with cancer: evidence from randomized clinical trials and observational studies. Front Cardiovasc Med. (2021) 8:766377. 10.3389/fcvm.2021.766377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patell R, Gutierrez A, Rybicki L, Khorana AA. Usefulness of CHADS2 and CHA2DS2-VASc scores for stroke prediction in patients with cancer and atrial fibrillation. Am J Cardiol. (2017) 120:2182–186. 10.1016/j.amjcard.2017.08.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.