Abstract
Although the pathogenesis of neurodegenerative diseases is still widely unclear, various mechanisms have been proposed and several pieces of evidence are supportive for an important role of mitochondrial dysfunction. The present review provides a comprehensive and up-to-date overview about the role of mitochondria in the two most common neurodegenerative disorders: Alzheimer’s disease (AD) and Parkinson’s disease (PD). Mitochondrial involvement in AD is supported by clinical features like reduced glucose and oxygen brain metabolism and by numerous microscopic and molecular findings, including altered mitochondrial morphology, impaired respiratory chain function, and altered mitochondrial DNA. Furthermore, amyloid pathology and mitochondrial dysfunction seem to be bi-directionally correlated. Mitochondria have an even more remarkable role in PD. Several hints show that respiratory chain activity, in particular complex I, is impaired in the disease. Mitochondrial DNA alterations, involving deletions, point mutations, depletion, and altered maintenance, have been described. Mutations in genes directly implicated in mitochondrial functioning (like Parkin and PINK1) are responsible for rare genetic forms of the disease. A close connection between alpha-synuclein accumulation and mitochondrial dysfunction has been observed. Finally, mitochondria are involved also in atypical parkinsonisms, in particular multiple system atrophy. The available knowledge is still not sufficient to clearly state whether mitochondrial dysfunction plays a primary role in the very initial stages of these diseases or is secondary to other phenomena. However, the presented data strongly support the hypothesis that whatever the initial cause of neurodegeneration is, mitochondrial impairment has a critical role in maintaining and fostering the neurodegenerative process.
Keywords: Neurodegeneration, Mitochondria, Alzheimer’s disease, Parkinson’s disease, Pathogenesis
Background
Neurodegenerative diseases represent one of the most important challenges which have to be faced by modem societies, with millions of patients affected worldwide [1].
Despite the devastating effects that neurodegenerative diseases cause to patients and despite the substantial rebound on families and on the entire community, the pathogenic mechanisms of these diseases remain widely unclear and only few therapeutic options are available.
Several molecular mechanisms have been proposed to be involved in the pathogenesis of these diseases, and mitochondria have often been considered as potential candidates implicated in the degenerative process.
Mitochondria are intracellular organelles which contribute to several metabolic pathways. They are complex structures composed of two membranes, an intermembrane space and a matrix. They contain their own DNA which encodes part of the proteins which are necessary for their functioning. In addition to many other important tasks, mitochondria play a crucial role in energy production, since they are responsible for some of the most important energetic pathways in the cell, including oxidative phosphorylation. [2]
Several diseases are characterized by a direct and primary involvement of mitochondria and are usually denoted as mitochondrial diseases [3–5]. However, considering the importance of these organelles, it is not surprising that they have often been implicated in apparently unrelated disorders, including neurodegenerative diseases.
The purpose of the present review is to highlight the importance of mitochondria in neurodegeneration by providing an up-to-date overview of their role in the two most common neurodegenerative diseases: Alzheimer’s disease (AD) and Parkinson’s disease (PD). The review examines most of the features related to this topic. However, a particular emphasis has been used to discuss the hints which suggest a primary or secondary role of mitochondria in neurodegeneration and the relationship between mitochondrial dysfunction and anomalous protein accumulation.
Alzheimer’s Disease
AD is the most common neurodegenerative disorder, with a prevalence of 10–30% and an incidence of 1–3% in the population over 65 years of age [6].
Dementia, intended as the combination of memory impairment and executive dysfunction which interfere with daily life, is the main clinical feature. However, atypical presentations characterized by the impairment of other domains, including language, visual, or executive functions, are also possible. [7]
Extracellular β-amyloid plaques and intracellular hyperphosphorylated tau protein neurofibrillary tangles are the main neuropathological hallmarks of the disease [8].
Although several progresses have been made in recent years, AD pathogenic mechanisms are still not completely clear. The most widely accepted hypothesis explaining the pathogenesis of the disease is represented by the amyloid hypothesis, which is supported by several important pieces of evidence. However, multiple mechanisms, including mitochondrial dysfunction, may be implicated and some investigators have even proposed a mitochondrial cascade hypothesis.
According to the amyloid hypothesis, the initial trigger of the disease is represented by the anomalous deposition of amyloid plaques in the extracellular environment of specific brain areas. In this perspective, the involvement of specific cellular compartments, including mitochondria, would represent a secondary phenomenon. The amyloid hypothesis is strongly supported by the fact that mutations in the genes encoding amyloid precursor protein (APP) and presenilins (involved in APP cleavage) are responsible for rare genetic forms of the disease [8, 9].
On the other hand, according to the mitochondrial cascade hypothesis, mitochondrial dysfunction would represent the initial trigger of most AD cases and the other pathological features should be considered as a secondary effect [10,11].
In both cases, mitochondria play a role, either as primary or secondary effectors (Fig. 1).
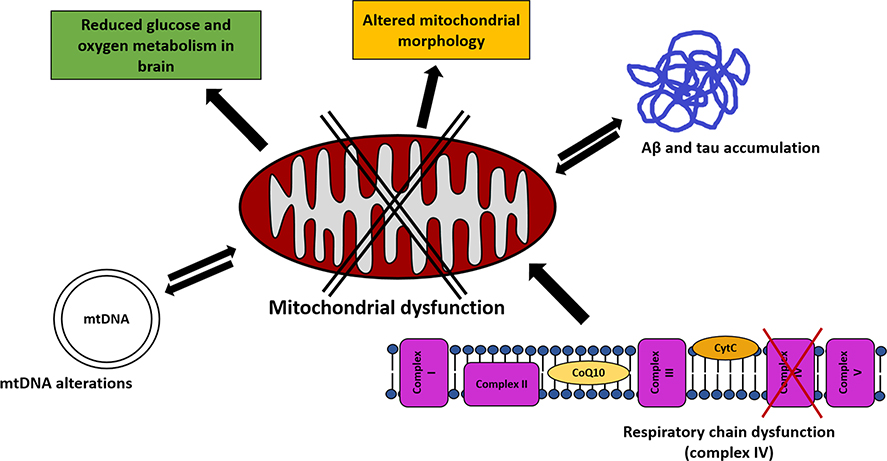
Fig. 1.
Mitochondrial dysfunction in Alzheimer’s disease. The figure summarizes the main mitochondria-related mechanisms which have been proposed to he involved in the pathogenesis of AD. A defective mitochondrial functioning is supported hy various findings, including altered mitochondrial morphology and reduced glucose and oxygen consumption in patients’ hrains. An impairment of respiratory chain activity, in particular complex IV, has been detected in the disease. Extracellular Aβ and intracellular tau protein accumulation, prominent neuropathological findings in AD, have been proposed to he bi-directionally finked to mitochondrial dysfunction. Mitochondrial DNA alterations have also been detected in the disease
Signs of Mitochondrial Involvement
The first, although indirect, evidence supporting the role of mitochondria in AD is represented by the finding of reduced glucose and oxygen metabolism in patients’ brains.
The evaluation of glucose utilization rate in a small cohort of patients and controls through 18-FDG positron emission tomography (PET) has demonstrated a reduction of metabolic activity in patients, which correlated with the degree of cognitive impairment [12]. A wider study, based on the same technique, has proposed that glucose metabolism alterations of specific brain regions correlate with the most prominent clinical features of each subject [13]. It has also been observed that the reduced 18-FDG uptake of AD brains is particularly striking in the temporoparietal cortex [14], although other groups have shown that the metabolic defect is common to several brain areas [15]. This topic has been investigated by various subsequent studies, and 18-FDG PET now represents one of the available diagnostic tools in the management of the disease [16].
In addition to reduced glucose metabolism, various studies have pointed out impaired oxygen consumption in patient brains, thus providing further evidence about bioenergetic dysfunction and mitochondrial impairment in the disease. Studies are consistent in detecting reduced oxygen metabolism in various brain regions in AD, while the finding of a reduced oxygen extraction fraction has not always been confirmed [17–20].
In addition to clinical features, mitochondrial involvement has been demonstrated by several descriptive studies detecting structural and functional abnormalities in these organelles.
Electron microscopy analyses have pointed out altered mitochondrial morphology in brains of AD patients, including decreased size, altered and broken cristae, osmophilic material accumulation, lipofuscin vacuoles, and elongated interconnected organelles [21–23].
Defective activity of mitochondrial enzymes, including pyruvate dehydrogenase and ketoglutarate dehydrogenase complexes [24–26], has been detected in patients. However, the most remarkably affected mitochondrial enzyme is cytochrome oxidase (respiratory chain complex IV), which has been widely studied in AD. An initial study, performed on a small number of subjects, has identified a reduction of cytochrome oxidase activity (but not of other electron transport chain enzymes) in platelets of patients [27] and the same finding has been confirmed in an independent study detecting reduced ATP levels, increased reactive oxygen species, and decreased cytochrome oxidase activity (but normal cytochrome oxidase subunits amount) in platelets of AD patients [28]. Cytochrome oxidase activity has been found to be reduced also in brain tissue: a first study, performed on 9 patients and 8 controls, has detected a generalized reduction of electron transport chain activity in AD, particularly striking for cytochrome oxidase, and the amount of cytochromes b, c1, and aa3 has not shown significant differences between groups [29]. A wider subsequent study, performed on brains of 19 patients and 30 controls, has detected a significant reduction of cytochrome oxidase activity in frontal and temporal cortices, but not in other brain areas [30].
Although only few studies have been performed in this field, also mitophagy has been proposed to be involved in AD. Indeed, an impairment of the mitophagic machinery has been detected in both human AD samples and experimental models and mitophagy stimulation has been found to improve neuropathological and clinical features in these models [31–34].
The role of mitochondrial DNA (mtDNA) alterations has been widely investigated in AD [35]. Several reports have pointed out a reduction of mtDNA content in brain tissue and cerebrospinal fluid of AD subjects [36–40], although a few groups have detected contrasting results [22]. Mitochondrial haplogroup U has been associated with increased (males) or decreased (females) risk of developing AD [41], and sub-haplogroup H5 has been proposed to be a risk factor for the disease [42], as well as mitochondrial clusters UK [43] and HV [44]; however, independent studies have not found an association between mitochondrial haplogroups and AD or early-onset AD [45–47]. AD has been associated with a mitochondrial haplotype characterized by modifications at positions 5633, 7476, and 15,812 [48], while a decreased risk of developing the disease has been associated with haplotypes H6A1A and H6A1B [49]. The tRNA(Gln) gene variant at nucleotide pair 4336 has been found to be more frequent in patients [50], and this finding has been confirmed by independent groups [51, 52], with some exceptions [53, 54]. The mtDNA 4977 deletion has been shown to be more common in brain cortex of young AD patients compared to age-matched controls [55] and subsequent analyses have detected a similar finding also in temporal cortex of elderly patients [56]; however, the same result has not been replicated in independent studies [57, 58]. An increased point mutation frequency has been detected in brains of AD subjects [57] and an analogous result has been found comparing elderly subjects and AD patients to young individuals [59]; however, this latter study does not explain whether the increased mutational burden is due to aging or to the disease. A wide study, focused on alterations of mtDNA control region (CR), has pointed out that the T414G mutation is detectable in 65% of AD brains, but not in controls [36]. Furthermore, a 63% increase of heteroplasmic CR mutations, including the T414C, T477C, T146C, T195C, and A189G mutations, has been found in patients [36]. Recent studies, performed on a remarkable number of samples, indicate that inherited polymorphisms and mtDNA heteroplasmy are not likely to play a significant role in AD pathogenesis [40, 60]. To conclude, several efforts have been dedicated to the comprehension of the role of mtDNA in AD, but, although several interesting hints have emerged, the elevated heterogeneity of the results does not allow drawing a definite hypothesis.
The findings listed above merely describe some mitochondrial abnormalities which can be detected in patients, but do not explain whether mitochondria play a primary or secondary role in the pathogenesis of the disease. However, various pieces of evidence have been provided to support both the hypotheses.
Evidence for Mitochondrial Primary Involvement
Since mtDNA inheritance is exclusively maternal, the finding of an increased risk to develop AD in case of maternal disease family history [61] is supportive for a mitochondrial primary role. Furthermore, subjects with maternal family history of AD display a decreased glucose metabolism in the brain [62], an extensive brain volume reduction at voxel-based morphometry [63], and an increase of Pittsburgh compound B (binding amyloid) at PET [64] when compared with subjects with no family history or paternal family history.
Moreover, various studies have suggested that amyloid and tau deposition may be the consequence of mitochondrial dysfunction. Treating rats with complex IV inhibitor sodium azide leads to nerve cell loss in the frontal cortical area, corkscrew-like dendrites, dendritic and axonal thickening, pyknotic nerve cells, and tau-positively-staining granules in the frontal cortical area [65]. Rats treated with complex I inhibitor rotenone show abnormally high levels of tau protein in the cytoplasm of neurons, oligodendrocytes and astrocytes, filamentous material staining positive for phosphorylated tau and cell bodies staining positive for thioflavin S, nitrotyrosine, and ubiquitin [66]. The administration of another complex I inhibitor, annonacin, to cultures of rat striatal neurons, is followed by redistribution of tau from axons to cell bodies, cell death, ATP level reduction, and mitochondria retrograde transport [67]. Inhibiting cytochrome oxidase in cells through sodium azide enhances the transformation of APP into amyloidogenic derivatives [68, 69]. APP/Ld mice display an increased burden of Aβ42 and amyloid plaques when crossed with the PolgA D257A mice (defective for mitochondrial DNA polymerase γ) [70]. The fusion of mitochondrially depleted cells with platelet-derived mitochondria of AD subjects (the AD “cybrids” model) leads to reduced complex IV activity and increased ROS production [71, 72], increased Aβ40 and Aβ42 secretion, increased intracellular Aβ40 level, and Congo red-positive Aβ deposits [73]. Mitochondrial impairment in AD cybrids, involving oxygen and glucose fluxes, bioenergetics regulatory mechanisms, and mitochondrial fission/fusion, has been recently confirmed in an independent study [74], although various concerns have been raised about the suitability of cybrids to model AD [75]. Finally, it has been suggested that oxidative stress, closely related to mitochondrial function, may play an important role in amyloid processing. [76]
Evidence for Mitochondrial Secondary Involvement
Aβ administration to cultured cells has been demonstrated to affect mitochondrial function in several ways: inhibiting respiratory chain (complexes I, III and, most of all, IV), depolarizing the mitochondrial membrane and decreasing oxygen consumption [77]. The administration of β-amyloid fragment 25–35 to rat-isolated mitochondria has been associated with a reduction of the activity of respiratory chain complex IV, but not complexes I, II–III or citrate synthase [78]. Furthermore, treating mitochondria with the Aβ42 peptide leads to a reduction of cytochrome c oxidase activity in a dose-dependent manner in the presence of Cu2+ [79]. The finding that Aβ-mediated damage cannot be observed in mitochondria-depleted (rho-0) cells has led to hypothesize that toxicity is mediated by electron transport chain [80]. A similar conclusion has been drawn by the authors of a study showing that transgenic mice knockout for COX10 and mutated in APP and PS1 display reduced Aβ42 protein level and reduced Aβ plaque accumulation [81].
According to another line of investigation, Aβ influences mitochondrial function by altering the balance between fusion and fission. Reduced levels of Fis1 and increased levels of Drp1, Opa1, Mfn1, and Mfn2 have been detected in hippocampal tissue of AD patients [82] and Drp1 reduction has been confirmed also in fibroblasts of sporadic AD [83]. Overexpressing APP in cells has been shown to lead to Drp1 and Opa1 downregulation and Fis1 upregulation [84]. A more recent study has shown that both mRNA and protein levels of Drp1 and Fis1 are upregulated in AD brains while Mfn1, Mfn2, and Opa1 are downregulated. Moreover, an interaction between Drp 1 and Aβ has been observed in both AD brains and primary neurons of AβPP transgenic mice [85].
Another topic which has recently gained interest is represented by the effect of β-amyloid on mitochondrial proteostasis. In this perspective, it has been shown that the expression of mitochondrial unfolded protein response (UPRmt) genes is upregulated in brains of sporadic and familial AD cases [86] and that both UPRmt and mitophagy-related transcripts are increased in brains of patients with mild cognitive impairment, as well as with mild and moderate AD, while oxidative phosphorylation related genes have been found to be downregulated in these subjects [87]. This latter study [87] has also investigated mitochondrial proteostasis in the GMC101 worm model of Aβ proteotoxicity. Various mitochondria-related findings have been detected in these worms, including an upregulation of mitochondrial stress response ortholog genes, reduced respiratory capacity, increased expression of oxidative phosphorylation genes, and reduced mitochondrial mass. The overexpression of atfs-1, a transcription factor involved in mitochondrial function, has been shown to be associated with UPRmt induction and with an improvement of the phenotype in these worms. The authors have also observed an upregulation of genes involved in UPRmt and mitophagy, as well as an improvement in health and lifespan, when treating worms with doxycycline, when silencing the mitochondrial ribosomal protein mrps-5, and when administering NAD+-boosting compounds [87].
Furthermore, APP and Aβ have been described to localize also in mitochondria [88–90] and a mitochondrial targeting motif has been detected on AβPP [91, 92]. Various hypotheses have been proposed to explain the relationship between mitochondrial Aβ localization and mitochondrial dysfunction. For example, Aβ-binding alcohol dehydrogenase (ABAD) has been involved, since oxidative stress is ameliorated by blocking the interaction between ABAD and Aβ [93]. Cyclophilin D, located in the mitochondrial transition pore, is another protein putatively involved in this process. Indeed, knocking out cyclophilin D leads to reduced Aβ-induced apoptosis and improved cognitive performance in transgenic mice. [94] Finally, Aβ-mediated mitochondrial dysfunction may also involve calcium homeostasis alteration [95].
It is also important to highlight the role that tau pathology plays in this context. As already mentioned, tau neurofibrillary tangles represent one of AD neuropathological features and several studies have pointed out the role of tau in the pathogenesis of the disease [96]. Among other lines of investigation, it has been proposed that tau accumulation may contribute to the observed mitochondrial dysfunction, as supported by several findings in experimental models [97], and various hypotheses have been proposed [98,99]. In this perspective, it has been suggested that tau accumulation may impair the transport and distribution of mitochondria along neurons [100,101] and that tau may also interfere with the mitochondrial fission/fusion mechanisms [102,103].
Some studies have hypothesized that Aβ and tau may synergistically concur to determine mitochondrial dysfunction in the disease. For example, the investigation of a triple mutant mouse model (obtained by cross-breeding APPSW/PS2N141I mice and P301L-tau mice) has shown signs of mitochondrial dysfunction, including altered mitochondrial membrane potential, increased reactive oxygen species production, and impaired respiratory chain activity [104]. Many of these findings were more pronounced in triple transgenic mice than in APPsw/PS2N141I or P301L-tau mice [104]. The authors of the same study have also suggested that Aβ mainly causes a complex IV defect, while tau mainly affects complex I [104], Signs of mitochondrial dysfunction involving reduced enzymatic activity and increased oxidative stress have also been observed in another AD triple transgenic mouse model [105].
To conclude, several studies have pointed out that mitochondria play an important role in AD pathogenesis. It is still a matter of debate whether mitochondrial dysfunction only represents a consequence of amyloid deposition or also plays a direct role in the very initial steps of the disease. Amyloid pathology and mitochondrial dysfunction may also play a synergic role in the pathogenic pathway, as suggested by the results of various studies [70]. However, considering the relevance of the matter, further investigation is needed.
Parkinson’s Disease
PD is the second most common neurodegenerative disorder.
It is clinically characterized by variable combinations of bradykinesia, rigidity, rest tremor, and postural instability, accompanied by other motor and non-motor symptoms.
Although the neuropathological presentation of the disease is complex, the main feature is represented by the progressive degeneration of dopaminergic neurons in midbrain substantianigra (SN). Microscopically, PD is characterized by intracellular protein aggregates, mainly composed of alpha-synuclein (α-syn) and often denoted as Lewy bodies [106].
Although mitochondria have been found to be involved in many neurodegenerative diseases, their role is particularly striking in PD (Fig. 2), as supported by numerous biochemical and genetic findings [107–109].
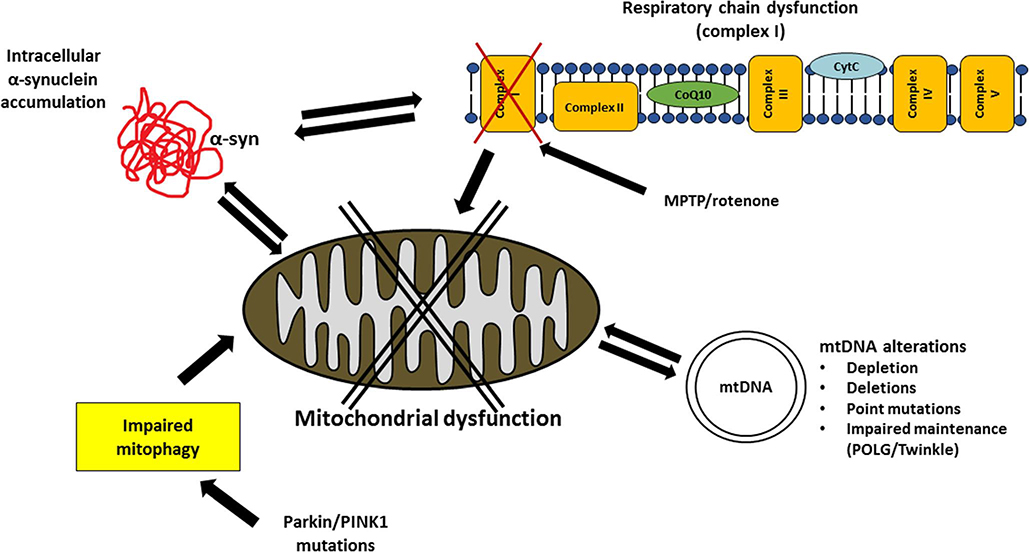
Fig. 2.
Mitochondrial dysfunction in Parkinson’s disease. The figure summarizes the main mitochondria-related mechanisms which have been proposed to he involved in the pathogenesis of PD. Respiratory chain activity (in particular complex I) is dysfunctional in the disease and mitochondrial inhibitors (MPTP and rotenone) cause clinical and neuropathological parkinsonian features. Intracellular alpha-synuclein accumulation is bi-directionally finked to mitochondrial dysfunction. Alterations of mitochondrial DNA, including depletion, deletions, point mutations and impaired maintenance, have been described. Mitophagy is also involved in the disease, as supported by rare familial PD cases due to mutations in Parkin and PINK1
The Involvement of Respiratory Chain
Several studies have investigated the presence of defects in the activity of mitochondrial respiratory chain. Interestingly, although a certain variability can be observed among individuals [107], various reports are consistent in describing a selective deficiency of respiratory chain complex I and this finding is particularly evident when the enzymatic activity is measured in patients’ SN [110–114]. The evaluation of complex I activity in other tissues has provided contrasting results. For example, some laboratories have reported a reduced complex I activity in skeletal muscle of PD patients [115–117], while others have not [112, 118]. Similarly, various studies have provided evidence for a reduced complex I activity in patients’ platelets [119–121], but, also in this case, with exceptions [122]. Contrasting results have been detected in patients’ leukocytes [122–124]. A deficiency of other respiratory chain complexes (namely complexes II + III or complex IV) has only sporadically been described [115–117, 119, 124]. Although the reason for the selective involvement of complex I has not been elucidated yet, two neuropathological studies, performed on patients’ SN and striatum and aimed at assessing the amount of respiratory chain complexes in these tissues, have described a reduction of complex I subunits in patients [125, 126]. Another piece of evidence supporting complex I dysfunction in PD is represented by “cybrids,” obtained by repopulating mtDNA-depleted human cells with mitochondria derived from platelets of patients or controls. PD cybrids are characterized by various pathological features, including reduced complex I activity, increased oxidative stress, and increased susceptibility to toxin-mediated apoptosis [127, 128].
The possible role of complex I in the pathogenesis of PD is further supported by the finding that the administration of complex I inhibitors to humans and animal models is accompanied by the onset of parkinsonism and striatonigral degeneration. In 1979, a 23-year-old man has been reported to have developed chronic parkinsonism after the intravenous injection of a meperidine analogue (4-propyloxy-4-phenyl-N-methylpiperidine). This subject has shown motor features persisting for more than 18 months and responding to dopamine receptors stimulation; moreover, neuropathological evaluation has pointed out a damage of SN aminergic neurons [129]. In 1983, another report [130] has described four subjects who have developed a severe form of parkinsonism after the intravenous injection of a drag which resulted positive for l-methyl-4-phenyl-l,2,5,6-tetrahydropyridine (MPTP) and, to a lesser extent, l-methyl-4-phenyl-propionoxy-piperidine (MPPP). Furthermore, it has been shown that the administration of MPTP to animal models is accompanied by a rapid onset of parkinsonism For example, the intravenous injection of this compound to rhesus monkeys induces parkinsonian clinical features like akinesia, tremor, and rigidity which are reversed by L-DOPA administration, as well as neuropathological alterations, including neuronal depletion in SN pars compacta, although classical Lewy bodies pathology has not been reported [131]. The parenteral administration of MPTP to mice is followed by analogous findings, including nerve cell loss in SN pars compacta [132]. Since these first descriptions, MPTP-based animal models have often been used to investigate the disease [133–135]. It is interesting to point out that MPTP exerts its toxicity by inhibiting mitochondrial respiratory chain complex I [136], and its mechanism of action, mainly mediated by the metabolite MPP+, has been widely investigated. It has been proposed that MPTP is oxidized to MPDP+ by monoamine oxidase B in glia and serotonergic neurons and is then converted to MPP+. The high affinity of MPP+ for dopamine transporters may contribute to explain the selectivity of MPTP toxicity for specific neuronal populations and the ability of this compound to determine a parkinsonian phenotype [137–139]. Several complex I inhibitors have been described [140], and MPTP is not the only one which has been associated with PD. Some of these compounds have been used as pesticides and, among them, a particular attention has been attributed to rotenone [141]. The administration of rotenone to rats has been associated with the onset of parkinsonian clinical features, including hypokinesia, unsteady movements, hunched posture, rigidity, and shaking. Moreover, degeneration of dopaminergic neurons and cytoplasmic aggregates containing α-syn and ubiquitin has been detected in these animals [142]. In addition, treating neuroblastoma cells with rotenone induces α-syn and ubiquitin accumulation, oxidative damage, and apoptosis upregulation [143]. However, not all the studies are consistent with the hypothesis of a selective involvement of SN dopaminergic neurons as a consequence of rotenone administration, since a more generalized neuronal loss also involving cholinergic, noradrenergic, and serotonergic neurons has been found in treated rats [144]. Moreover, some authors have questioned whether the detrimental effect of these toxins is actually mediated by complex I inhibition [145]. Annonacin is another complex I inhibitor whose administration to rats induces neurodegeneration: a significant loss of SN dopaminergic neurons and of striatal GABAergic and cholinergic neurons has been observed in these animals [146].
The Role of Mitochondrial DNA
Several pieces of evidence support the hypothesis that also mtDNA plays a role in the pathogenesis of PD [108]. The load of mtDNA deletions has been one of the first aspects to be analyzed, and conflicting results have emerged. Two old studies have proposed that the occurrence of mtDNA deletions, although physiologically detectable in elderly subjects, is accelerated in the striatum of PD patients [147,148]. However, other reports of the same period have not detected an increased amount of mtDNA deletions in the SN, putamen, and frontal cortex [149–151]. A subsequent investigation [152], based on long-extension polymerase chain reaction, has confirmed the initial hypothesis, detecting an increased level of mtDNA deletions/rearrangements in the SN and other brain areas of PD patients in comparison to healthy controls and subjects with other neurodegenerative disorders. A high mtDNA deletion burden has been observed in PD SN also by another group [153], and a significant increase of mtDNA deletions has been associated with the disease by evaluating DNA from SN individual neurons of patients and controls [154]. Therefore, although data remain conflicting, independent studies suggest that mtDNA deletions may be increased in the disease. In this perspective, since mtDNA deletions are also numerous in the SN of aged individuals [155], it would be crucial to distinguish between the load of deletions due to the disease and that due to aging.
Another major field of investigation is represented by the finding of mtDNA point mutations. Old studies [50,156–159] have reported a high mutational load in PD. Furthermore, relatively more recent investigations have observed homoplasmic mutations in MT-ND1 and MT-ND2 genes, potentially contributing to neuronal vulnerability, in patients’ SN and platelets [160] as well as heteroplasmic mutations of MT-ND5 in PD SN [161]. However, the role of mtDNA mutations in PD has not always been confirmed and the issue remains controversial. For example, the analysis of the sequence of mitochondrial tRNA and complex I genes in five couples of monozygotic twins discordant for the disease has detected a certain amount of mtDNA mutations, but no differences have been observed between affected and unaffected siblings [162]. The analysis of the entire mtDNA sequence in the SN of 8 PD subjects and 9 healthy controls has not provided significant results: despite a remarkable mutational burden, no major differences have been observed between patients and controls [163]. A similar observation has emerged from another study aimed at sequencing several mtDNA-encoded genes in affected and unaffected individuals [164]. Recent reports, based on large cohorts of subjects, have not been useful to definitely elucidate the issue. The evaluation of brain samples from 180 PD patients and 40 controls has pointed out an increased mutational load in patients’ frontal cortex and SN [165], and another report has proposed that mtDNA mutations are particularly frequent in the SN of early PD patients [166]. On the other hand, a wide study [40] investigating mtDNA in brain samples of subjects affected by various neurodegenerative diseases (including 89 DLB-PD) and 351 controls has not observed an increased mutational burden in PD. Furthermore, mtDNA point mutational load has not been described to be increased also in an already mentioned study which has evaluated mtDNA in individual neurons of patients and controls [154].
It is also interesting to highlight that specific mitochondrial haplogroups have been associated with an increased or decreased risk of developing PD. A wide study [167] investigating 609 PD patients and 340 healthy controls has shown that subjects with haplogroups J or K are less susceptible to develop the disease than those carrying haplogroup H. The authors have also pointed out that this protective effect may be related to the single-nucleotide polymorphism 10398G. These findings have been confirmed by a subsequent investigation [168] which has analyzed 455 PD patients and 447 healthy controls and has observed an association between the UKJT haplogroup cluster and a reduced risk of developing the disease. Furthermore, the same report suggests that this protective effect is specific for PD, since analyses on AD patients have not provided similar results. Two independent studies have confirmed a reduced risk of developing the disease in patients harboring mitochondrial haplogroups K [169] and UK [170], respectively. The findings of these studies partially disagree with the description of an association between the mitochondrial polymorphism 4216C [171], strictly associated with haplogroups J and T, and an increased risk of developing PD. However, this latter finding has not been replicated by the same group when analyzing a cohort of young healthy controls. In a more recent two-stage association study, it has been confirmed that PD risk is decreased in subjects carrying haplogroups J, K, and T, while it is increased in subjects carrying the super-haplogroup HV [172].
It has been reported that also mtDNA amount may be altered in PD. The investigation of cell-free circulating mtDNA in cerebrospinal fluid of patients and age-matched controls has shown a reduction in patients [173]. Various studies have observed a reduced mtDNA content also in PD SN [154, 174, 175], but not in other brain areas [40, 175]. The report of the same finding also in blood samples [175] suggests a possible peripheral response to mitochondrial dysfunction.
Finally, another independent, although indirect, piece of evidence which suggests a correlation between mtDNA alterations and parkinsonism is represented by patients carrying mutations in genes involved in mtDNA maintenance. Polymerase γ is the polymerase involved in mtDNA replication and is encoded by POLG gene. POLG mutations have been described to be responsible for some cases of progressive external ophthalmoplegia (PEO) with mtDNA deletions [176]. A wide study [177] has assessed the incidence of parkinsonian features in PEO patients from seven families. Clinical evaluation has pointed out a co-segregation between POLG mutations and parkinsonism, and imaging studies have shown dopaminergic neuronal loss. The post-mortem evaluation of two of these subjects has confirmed a loss of pigmented neurons in the SN, although Lewy bodies have not been detected. Various other subsequent studies have described the presence of parkinsonian features in POLG-mutated subjects [178–185]; moreover, additional neuropathological studies have confirmed the finding of neuronal loss in the SN, although sometimes accompanied by AD-like pathological features [186, 187]. The recent evaluation of 11 POLG-mutated subjects has highlighted an involvement of the nigrostriatal pathway even if parkinsonian clinical features were not present: DAT-scan and PET analyses have shown nigral neuronal loss and nigrostriatal depletion, while the post-mortem assessment of SN has demonstrated a severe depletion of dopaminergic neurons, reduced complex I amount, and reduced mtDNA content [188]. The involvement of POLG mutations in idiopathic PD is debated [189–193]; furthermore, the investigation of the role of a trinucleotide CAG repeat located in POLG gene has provided conflicting results, since some groups have reported an association between the repeat length and PD [189, 194–196], while others have not [190, 197, 198]. Finally, it is interesting to highlight that POLG is not the only mtDNA maintenance-related gene to be associated with parkinsonism. Indeed, parkinsonian features have been detected in patients carrying mutations in Twinkle [199–201], a mitochondrial DNA helicase which has been associated with PEO [202].
To conclude, a remarkable amount of work has been done to understand the role of mtDNA in PD and several interesting results have emerged. However, as already mentioned when discussing the same topic in AD, the elevated heterogeneity of the results across different studies, often contradictory with one another, makes it difficult to draw clear and definite conclusions about this issue. Further investigation is therefore needed, and the availability of larger datasets will probably help in this direction.
Insights from Genetics: Parkin and PINK1
Another important hint which suggests a role of mitochondria in the pathogenesis of PD is provided by genetics. Indeed, some of the genes which have been found to cause familial cases of PD are directly involved in mitochondrial biology. The most prominent examples are represented by Parkin and PINK1, whose pathogenic mutations are responsible for autosomal recessive forms of early-onset PD [203, 204]. Both PINK1 and Parkin are involved in the mitophagic machinery, thus contributing to coordinate the directing of damaged mitochondria to degradation. PINK1 monitors mitochondrial status, detects damages, and recruits and activates Parkin. Parkin, once recruited, conjugates ubiquitin onto proteins of the outer mitochondrial membrane, thus fostering the pathway which leads to the engulfment of mitochondria into the autophagosome. This latter structure subsequently fuses with a lysosome and initiates the pathway which leads to mitochondrial degradation [205]. The synergic action of PINK1 and Parkin in directing mitochondria to degradation, with PINK1 acting upstream of Parkin, has been confirmed by investigating Drosophila mutants [206,207].
It is interesting to point out that Parkin and PINK1 have also been demonstrated to be involved in mitochondrial fission and fusion [208]. Studies performed on Drosophila models have shown an involvement of this pathway in Parkin or PINK1 mutants and have observed that the inhibition of mitochondrial fusion or the enhancement of mitochondrial fission is able to rescue the pathological phenotype in these flies [209, 210]. Interestingly, analogous findings have also been observed in models not directly related to Parkin or PINK1, since flies expressing human α-syn are characterized by enlarged mitochondria and decreased Drpl mitochondrial localization and show a behavioral and pathological improvement after Drpl overexpression [211]. However, contrasting results have been obtained by analyzing human cells. In this case, PINK1/Parkin silencing has been shown to be accompanied by mitochondrial fragmentation. Moreover, a rescue of the pathological phenotype has been observed by enhancing the fusion-related proteins Mln2 and Opal or by negatively modulating the fission-related protein Drpl [212]. Another study has also proposed to target the fission/fusion pathway as a possible therapeutic approach for PD and has shown that the inhibition of Drpl ameliorates the phenotype of PINK1-mutated mice and of the MPTP toxin-mediated murine model [213].
Finally, it must be acknowledged that a pathological phenotype, often involving mitochondria-related aspects, has been detected in various PINK1 or Parkin models: Drosophila models [214], mouse models [215], iPSC-based models [216–222], and other cellular models [223].
Mitochondria and Alpha-Synudein
Another field of investigation which is providing important insights about the role of mitochondria in PD is represented by the correlation between α-syn accumulation and mitochondrial dysfunction. As already reported, PD is neuropathologically considered as a synucleinopathy, that is, a disease characterized by the anomalous intracellular deposition of α-syn. α-Syn is a 14 KDa protein which is physiologically expressed in the human brain. Although it has been proposed to be involved in synaptic vesicle trafficking and neurotransmitter release, its physiological function is not completely clear [224–226]. However, the role of α-syn in the pathogenesis of PD is supported not only by the finding of its accumulation in neurons, but also by the fact that multiplications and point mutations of SNCA gene are responsible for rare genetic forms of the disease [227–230].
In this perspective, various studies have investigated the effect of α-syn accumulation on mitochondrial functioning. Only to provide some examples, signs of mitochondrial dysfunction have been observed in brain of mice overexpressing α-syn under the neuronal Thy-1 promoter [231], in primary neurons of SNCA A53T-mutated mice [232], in mouse-isolated mitochondria treated with soluble prefibrillar α-syn [233], in rat brain mitochondria incubated with recombinant α-syn [234, 235], and in rat dopaminergic neurons treated with preformed α-syn fibrils [236]. Some laboratories have specifically described a correlation between α-syn and complex I deficiency: a dysfunctional activity of this complex has been observed after overexpressing α-syn (both wild-type and mutated) in animals [237,238] and cellular models [239,240]. It has also been reported that the α-syn-mediated inhibitory effect on complex I is dose dependent in rat brain [241]. Furthermore, it has been proposed that the effect of specific parkinsonism-inducing toxins may be mediated, or at least facilitated, by α-syn. For example, the detrimental effect of MPTP has been found to be enhanced in mice which overexpress human α-syn [242, 243] and in SNCA A30P-mutated transgenic animals [244]. Moreover, α-syn-null mice display increased resistance to the MPTP-mediated degeneration of dopaminergic neurons [245,246] and an analogous resistance has been observed in α-syn knocked-down neuroblastoma cells [247]. Similar results have been obtained also with rote-none, since animals or cellular models overexpressing α-syn or carrying pathogenic mutations in SNCA gene have been shown to be more sensitive to the detrimental effects of this compound [248, 249]; instead, contrasting opinions exist about the protective effect of SNCA downregulation on rote-none toxicity [247, 250]. Although the issue remains widely unclear, a few groups have also tried to investigate the effect of mitochondrial inhibition on α-syn accumulation. Two already mentioned studies have reported that treating rats [249] or neuroblastoma cells [250] with rotenone induces α-syn accumulation; moreover, the continuous administration of MPTP to mice is accompanied by the formation of nigral inclusions positive for α-syn, while the same effect is not observed after the sporadic administration of the same compound [251]. Although most of these studies suggest an association between α-syn and complex I dysfunction, an interaction between this protein and other respiratory chain complexes, in particular ATP synthase, has also sporadically been described [252].
The exact mechanism which explains the relationship between α-syn accumulation and mitochondrial dysfunction is not completely clear. However, various hypotheses have been suggested. Among these, it is interesting to point out that a mitochondrial targeting motif has been detected in the N-terminal domain of human α-syn [239]. It has also been proposed that α-syn interacts with the mitochondrial protein import machinery. In particular, specific forms of α-syn have been shown to bind to the translocase of the outer membrane 20 (TOM20), thus inhibiting the interaction between TOM20 and TOM22. This finding, observed in both experimental models and in human post-mortem tissues, has been supposed to affect the efficiency of the mitochondrial protein import mechanisms and, consequently, the overall mitochondrial functioning [253]. Another group has reported that TOM40, another component of the mitochondrial import machinery, but not TOM20, is reduced in brains of PD patients. Furthermore, overexpressing wild-type or A53T-mutated, but not A30P-mutated α-syn in rat neuroblastoma cells, results in TOM40 reduction. Overexpressing TOM40 in PDGF-α-syn-transgenic mice leads to reduced oxidative stress, increased ATP levels, and reduced and redistributed α-syn in these animals [254], According to other studies, based on various experimental models, α-syn is responsible for an increased mitochondrial fragmentation [255,256]; one of these reports suggests that α-syn also inhibits mitochondrial fusion and that the α-syn-induced mitochondrial fragmentation is rescued by the co-expression of wild-type PINK1, Parkin, and DJ-1 [255]. An alternative recent hypothesis suggests that a portion of α-syn is not localized in mitochondria, but in mitochondria-associated endoplasmic reticulum membranes (MAM). Furthermore, according to the same study, SNCA point mutations reduce the amount of MAM-associated α-syn, impair MAM function, and trigger mitochondrial fragmentation through a Drpl-independent mechanism [257,258].
Mitochondria in Atypical Parkinsonisms: the Example of Multiple System Atrophy
It is interesting to observe that mitochondrial dysfunction has not been detected only in classical PD, but several hints suggest an involvement of these organelles also in atypical parkinsonisms, in particular multiple system atrophy (MSA). MSA is a severe neurodegenerative disease which is clinically characterized by variable combinations of parkinsonism, cerebellar ataxia, dysautonomia, and other motor and non-motor symptoms. Two clinical subtypes, MSA-P and MSA-C, have been described on the basis of the predominant symptomatology, parkinsonian or cerebellar respectively. Similarly to PD and dementia with Lewy bodies, MSA is neuropathologically characterized by the intracellular accumulation of α-syn. However, the peculiarity of MSA is that α-syn accumulates not only in neurons but also in oligodendrocytes. The causes of this aberrant protein localization have been extensively investigated, although a definite mechanism has not been demonstrated yet [259,260]. The role of mitochondria in MSA has been poorly considered for several years, and only a few studies [152, 261, 262] have been published until 2013, when a causative role of COQ2 gene mutations has been proposed in familial and sporadic cases of MSA [263]. COQ2 is one of the enzymes involved in the biosynthesis of Coenzyme Q10 (CoQlO), a molecule which plays several roles in cell biology and, prominently, in mitochondrial respiratory chain, being responsible for transferring electrons from complexes I and II to complex III. Mutations in genes encoding CoQlO biosynthesis enzymes, including COQ2, had already been found to cause rare multi-organ syndromes, often characterized by a severe neurological symptomatology, when inherited in an autosomal recessive fashion [264, 265], but the association with MSA had not been reported before. Therefore, several groups have tried to recapitulate the same genetic finding in independent cohorts of MSA patients and conflicting results have emerged [266–271]. Although the finding of COQ2 mutations in MSA remains controversial, further investigation has tried to address whether CoQlO may be anyway involved in the disease. Interestingly, a reduced CoQlO amount has been observed, independently from COQ2 mutational status, in MSA cerebellum [272, 273], cerebrospinal fluid [274], plasma [275], serum [276], and fibroblasts [277]. Also, CoQlO biosynthesis enzymes have been found to be altered in the disease, with reduced PDSS1 and COQ5 in patients’ brain [272], increased COQ5 and COQ7 in fibroblasts [277], and increased PDSS1, PDSS2, COQ4, and ADCK3 in iPSC-derived dopaminergic neurons [278]. Furthermore, it has recently been shown that iPSC-derived neurons from a COQ2-mutated MSA patient are characterized by mitochondrial dysfunction, reduced CoQlO level, and increased oxidative stress [279]. The role of mitochondria in MSA has been investigated in two recent extensive studies based on cellular models, already mentioned when discussing the CoQlO-related pathology [277,278]. In these reports, an impaired activity of respiratory chain complexes, in particular complex II, has been observed in MSA primary fibroblasts and iPSC-derived neurons. Fibroblasts’ analyses have also suggested an impaired mitophagic flow, while neurons’ evaluation has highlighted an alteration of the amount of respiratory chain complexes and an increased mitochondrial mass.
The comprehension of the specific role of mitochondria in MSA still needs further investigation. Also in this case, it will be important to understand whether these organelles play a primary role in the very initial stages of the disease, as suggested by the still controversial “COQ2 hypothesis,” or their dysfunction is just secondary to other phenomena, in particular protein accumulation. Differently from PD, no studies have so far been performed to understand the relationship between α-syn accumulation and mitochondrial dysfunction in MSA. However, the administration of the mitochondrial inhibitor 3-nitroproprionic acid to transgenic MSA mouse models overexpressing human α-syn in oligodendrocytes causes a worsening of both clinical and neuropathological features of these animals, which suggests that mitochondrial dysfunction and α-syn accumulation may play a synergic effect in the pathogenesis of the disease [280,281].
The Role of Aging
Another topic which has to be highlighted is the link which connects neurodegeneration, mitochondrial dysfunction, and aging. Indeed, the onset of neurodegenerative diseases most often occurs at an advanced age and aging has been demonstrated to be an independent risk factor itself [282]. Therefore, although this is not the main topic of the present review, a few hints are here provided.
Aging is a complex process which involves several pathways, including proteostasis, telomere loss, and DNA damage [283]. In this intricate scenario, mitochondria play an important role as well [109,284–286]. A progressive accumulation of mitochondrial defects has been described to occur during lifespan. For example, an increased load of mtDNA mutations and deletions [287–289] and a reduced respiratory chain activity [290, 291] have been observed in tissues of elderly individuals and experimental models. However, not only signs of mitochondrial dysfunction can be observed in aged subjects, but the impairment of mitochondrial functions seems to be directly implicated in the aging process. In this perspective, various mechanisms have been proposed. For example, mitochondrial defects have been suggested to contribute to the age-related inflammatory status by releasing specific molecules, including mtDNA, which activate the inflammatory cascade [284]. Another possibility is that mitochondrial dysfunction interferes with stem cell biology [285]. It has also been proposed that the mitophagic machinery becomes defective at advanced age [292], thus not allowing to properly eliminate old and malfunctioning mitochondria and contributing to the dysfunction of these organelles. The hypothesis which suggests a mitochondria-mediated oxidative stress which contributes to the aging process has been widely investigated [293] and remains suggestive, although some possible criticisms have emerged [284], The link between aging and mitochondria is also supported by experimental models. For example, a mouse model with defective mitochondrial DNA polymerase, which accumulates a high amount of mtDNA mutations, is characterized by signs of precocious aging, including reduced lifespan, hair loss, and kyphosis [294, 295]. Further investigation is needed, but, considering the direct role of mitochondrial dysfunction in both aging and neurodegenerative diseases, it is intriguing to hypothesize a possible link. In this perspective, age-related mitochondrial dysfunction may contribute to trigger the neurodegenerative process and, on the other hand, mitochondrial damages spontaneously occurring during life or triggered by specific agents (e.g., toxins) may contribute to the aging of specific tissues and concur to neurodegeneration.
Conclusions
Considering the remarkable amount of data which have been described so far, it is rational to conclude that mitochondria play an important role in the pathogenesis of Alzheimer’s disease and Parkinson’s disease. These results are also suggestive for a more general role of these organelles in neurodegeneration.
A critical question which arises is whether mitochondrial dysfunction directly contributes to the initial steps of neurodegeneration, therefore being primarily responsible for triggering the pathogenic process, or it only represents a secondary phenomenon caused by other mechanisms, in particular anomalous protein accumulation, or a general response to cell suffering. The issue is controversial and several pieces of evidence have been provided in favor of both the hypotheses. As far as AD is concerned, the finding that mutations in APP and presenilins’ genes are responsible for genetically inherited forms of the disease is the most direct hint which supports the primary role of Aβ deposition in the pathogenic cascade. The finding that Aβ administration is followed by mitochondrial dysfunction in experimental models further supports this hypothesis. On the other hand, it must be acknowledged that the administration of specific mitochondrial toxins contributes to amyloid pathology in animals and cells and that other hints, including the increased risk of developing the disease in the presence of maternal family history, are also supportive for a primary contribution of mitochondria. When considering PD, the issue is even more intricate. The fact that multiplications and point mutations in SNCA gene cause familial cases of PD is suggestive for a primary role of α-syn accumulation; the finding of mitochondrial dysfunction in several experimental models treated with α-syn is also supportive in this direction. However, the pieces of evidence which support the role of mitochondria in the very initial stages of PD are even stronger than in AD. Not only the administration of mitochondrial toxins, in particular MPTP and rotenone, are able to induce a clinical and neuropathological parkinsonian phenotype in humans and animal models (despite the already mentioned limitations), but mutations in genes directly related to mitochondria have also been found to be associated with the disease. Indeed, mutations in Parkin and PINK1, involved in the mitophagic machinery, are responsible for rare young-onset familial PD cases and an association between parkinsonism and mutations in POLG gene, which encodes the mitochondrial polymerase, has been described (Table 1). Another aspect which should be considered when discussing this topic and which applies to both AD and PD is that classical mitochondrial diseases, reliable examples of primary mitochondrial dysfunction because directly caused by mutations affecting the mitochondrial machinery, are often characterized by the involvement of multiple organs and systems, including the nervous system, the muscle, the eye, the kidney, and the gastrointestinal system [3, 296], while only specific neuronal populations are affected in AD and PD. However, it must also be acknowledged that the clinical presentation of mitochondrial diseases is highly variable and that different mutations cause remarkably different clinical phenotypes, thus suggesting that each cellular population may be selectively vulnerable to specific mitochondrial defects. Moreover, it has been proposed that specific neuronal subtypes, in particular dopaminergic neurons, are particularly sensitive to energetic dysfunction [297]. Furthermore, hypothesizing a primary mitochondrial role in neurodegeneration does not necessarily rule out the involvement of other co-occurring factors, also acting as primary effectors, which may be more specific for the affected neuronal types. On the basis of all these considerations, it is not possible to provide a definite answer about the primary hit which triggers the neurodegenerative process. However, it is interesting to observe that both Aβ and α-syn have been found to trigger mitochondrial dysfunction and that, on the other hand, mitochondrial inhibition has been found to trigger the accumulation of pathological proteins. Therefore, it is suggestive to hypothesize that, once the neurodegenerative process has started, whatever the initial cause is, mitochondrial dysfunction contributes to the progression of the disease by fostering a self-propagating loop which involves protein accumulation and mitochondrial dysfunction and which contributes to neuronal suffering and, ultimately, neurodegeneration (Fig. 3).
Table 1.
Hints suggesting a primary or secondary role of mitochondria in the pathogenesis of AD and PD
| Primary role | AD | Mitochondrial toxins contribute to amyloid pathology in animals and cells |
| Maternal family history increases the risk of developing the disease | ||
| PD | Mitochondrial toxins (MPTP and rotenone) induce clinical and neuropathological parkinsonian features in humans and animal models | |
| Mutations in Parkin and PINK1 genes (directly involved in mitophagy) cause rare young-onset familial cases of the disease | ||
| Mutations in POLG gene, encoding the mitochondrial polymerase, are associated with parkinsonian features | ||
| Secondary role | AD | Aβ administration is followed by mitochondrial dysfunction in experimental models |
| Mutations in APP and presenilins’ genes are responsible for genetically inherited forms of the disease | ||
| PD | Mitochondrial dysfunction is enhanced by α-syn administration in experimental models | |
| SNCA gene multiplications and point mutations are responsible for genetically inherited forms of the disease |
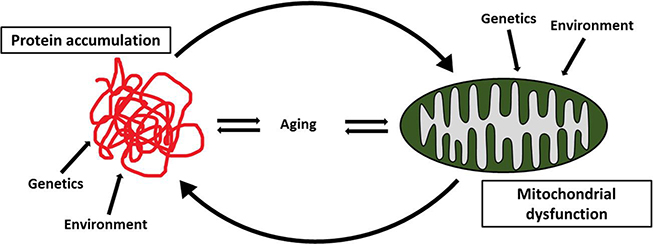
Fig. 3.
Anomalous protein accumulation and mitochondrial dysfunction are involved in the pathogenesis of neurodegenerative diseases. Both genetic and environmental factors are implicated and aging plays a role as well. Although the identification of the initial causative mechanism is still a matter of dehate, it is interesting to observe that mitochondrial dysfunction triggers anomalous protein accumulation and, vice versa, anomalous protein accumulation contributes to mitochondrial dysfimction. Therefore, it is suggestive to hypothesize that whatever the initial causative mechanism is, once the neurodegenerative process has started, mitochondrial dysfunction and anomalous protein accumulation form a self-propagating loop which contributes to the maintenance and progression of the disease
Another interesting aspect which emerges from this review is that, although mitochondrial dysfunction appears to be a common motif in neurodegeneration, it differently manifests in different diseases. In this perspective, it is particularly informative to analyze the topic of respiratory chain dysfunction. Respiratory chain is composed of five complexes and is responsible for oxidative phosphorylation. Although a generic dysfunction has been observed in several neurodegenerative disorders, each disease is characterized by a more pronounced involvement of certain complexes. For example, complex IV has been shown to be particularly affected in AD, while plenty of studies have been performed to elucidate the involvement of complex I in PD. The same can be said about other neurodegenerative diseases which have not been described in this review: for example, Huntington’s disease is prominently characterized by the involvement of complex II [298]. Another hint which suggests a disease-specificity is provided by the genetic factors which point toward mitochondrial dysfunction in neurodegenerative diseases. Indeed, some of these factors, such as mtDNA alterations (depletion, deletions, point mutations), although very controversial, have been described in both AD and PD, while other, like Parkin and PINK1 mutations in PD, are specific for a single disease. These specificities are interesting because they may help to understand why, despite the common finding of mitochondrial dysfunction, clinical and neuropathological features are so different in distinct diseases. However, the available knowledge is not sufficient to provide a solid and comprehensive hypothesis and further investigation is needed.
Finally, it must be acknowledged that the finding of a so prominent involvement of mitochondria in neurodegenerative diseases lays the foundations to investigate novel pharmacological approaches. Various studies have already been performed or are in progress and have targeted, at a preclinical or clinical level, multiple mitochondria-related aspects, including oxidative stress, mitochondrial dynamics, mitochondrial biogenesis, and mitochondrial proteostasis [87,213,216, 299–302], not always with positive results [303]. However, as shown in the present review, the mitochondrial targets which may be worth taking into consideration are numerous. The paucity of effective available therapeutic tools for neurodegenerative diseases urges a far more intense effort and the investigation of new mitochondria-targeting compounds, in addition to therapies acting on different pathways, is compelling.
To conclude, the present review summarizes the remarkable amount of data which have been collected during the last decades about the role of mitochondria in AD and PD and, in general, in neurodegenerative diseases. The emerging evidence suggests an important role of these organelles during the neurodegenerative process, and the understanding of these diseases has been strongly improved by the results of these studies. However, as described in detail in the review, various topics are still controversial and several questions are still waiting for an answer. Therefore, further investigation will be crucial in this field. Among other issues, it will be relevant to better elucidate the relationship between mitochondrial dysfunction and anomalous protein accumulation, to investigate the correlation between mitochondrial dysfunction and neuronal suffering, to understand the specificity of each neurodegenerative disease and, in the meantime, to understand whether common pathways can be detected across different disorders. Finally, as already mentioned, it will be important to understand whether this considerable amount of basic science data can be translated into clinical practice by investigating novel therapeutic approaches targeting these pathways.
Acknowledgments
The support from Associazione Amici del Centro Dino Ferrari is gratefully acknowledged.
Funding information The financial support was received from the Fresco Institute for Parkinson’s and Movement Disorders.
Abbreviations
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- APP
amyloid precursor protein
- PET
positron emission tomography
- mtDNA
mitochondrial DNA
- CR
control region
- ABAD
Aβ-binding alcohol dehydrogenase
- UPRmt
mitochondrial unfolded protein response
- SN
substantia nigra
- α-syn
alpha-synuclein
- MPTP
l-methyl-4-phenyl-1,2,5,6-tetrahydropyridine
- PEO
progressive external ophthalmoplegia
- TOM20
translocase of the outer membrane 20
- MAM
mitochondria-associated endoplasmic reticulum membranes
- MSA
multiple system atrophy
- CoQlO
coenzyme Q10
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no competing interests.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol 2018; 10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman JR, Nunnari J (2014) Mitochondrial form and function. Nature. 505(7483):335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348(26):2656–2668 [DOI] [PubMed] [Google Scholar]
- 4.Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorhurn DR et al. (2016) Mitochondrial diseases. Nat Rev Dis Primers 2:16080. [DOI] [PubMed] [Google Scholar]
- 5.Wallace DC (1999) Mitochondrial diseases in man and mouse. Science. 283(5407): 1482–1488 [DOI] [PubMed] [Google Scholar]
- 6.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1: 15056. [DOI] [PubMed] [Google Scholar]
- 7.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM (2016) Alzheimer’s disease. Lancet. 388(10043):505–517 [DOI] [PubMed] [Google Scholar]
- 8.Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766 [DOI] [PubMed] [Google Scholar]
- 9.Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 297(5580):353–356 [DOI] [PubMed] [Google Scholar]
- 10.Swerdlow RH (2018) Mitochondria and mitochondrial cascades in Alzheimer’s disease. J Alzheimers Dis 62(3): 1403–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swerdlow RH, Bums JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis 20(Suppl 2):S265–S279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris SH, de Leon MJ, Wolf AP, Farkas T, Christman DR, Reisberg B, Fowler JS, Macgregor R et al. (1980) Positron emission tomography in the study of aging and senile dementia. Neurobiol Aging 1(2): 127–131 [DOI] [PubMed] [Google Scholar]
- 13.Foster NL, Chase TN, Fedio P, Patronas NJ, Brooks RA, Di Chiro G (1983) Alzheimer’s disease: focal cortical changes shown by positron emission tomography. Neurology. 33(8):961–965 [DOI] [PubMed] [Google Scholar]
- 14.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, Ober BA, Huesman RH et al. (1983) Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr 7(4):590–598 [DOI] [PubMed] [Google Scholar]
- 15.de Leon MJ, Ferris SH, George AE, Christman DR, Fowler JS, Gentes C, Reisberg B, Gee B et al. (1983) Positron emission tomographic studies of aging and Alzheimer disease. AJNR Am J Neuroradiol 4(3):568–571 [PMC free article] [PubMed] [Google Scholar]
- 16.Shokouhi S, Claassen D, Riddle W (2014) Imaging Brain Metabolism and Pathology in Alzheimer’s Disease with Positron Emission Tomography. J Alzheimers Dis Parkinsonism 4(2): 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frackowiak RS, Pozzilli C, Legg NJ, Du Boulay GH, Marshall J, Lenzi GL, Jones T (1981) Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 104(Pt 4):753–778 [DOI] [PubMed] [Google Scholar]
- 18.Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J (1994) Altered cerebral energy metabolism in Alzheimer’s disease: a PET study. J Nucl Med 35(1): 1–6 [PubMed] [Google Scholar]
- 19.Ishii K, Kitagaki H, Kono M, Mori E (1996) Decreased medial temporal oxygen metabolism in Alzheimer’s disease shown by PET. J Nucl Med 37(7): 1159–1165 [PubMed] [Google Scholar]
- 20.Tohgi H, Yonezawa H, Takahashi S, Sato N, Kato E, Kudo M, Hatano K, Sasaki T (1998) Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 40(3): 131–137 [DOI] [PubMed] [Google Scholar]
- 21.Baloyannis SJ (2006) Mitochondrial alterations in Alzheimer’s disease. J Alzheimers Dis 9(2):119–126 [DOI] [PubMed] [Google Scholar]
- 22.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y et al. (2001) Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci 21(9):3017–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Trushin S, Christensen TA, Bachmeier BV, Gateno B, Schroeder A, Yao J, Itoh K et al. (2016) Altered brain energetics induces mitochondrial fission arrest in Alzheimer’s disease. Sci Rep 6:18725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gihson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P (1988) Reduced activities of thiamine-dependent enzymes in the hrains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol 45(8): 836–840 [DOI] [PubMed] [Google Scholar]
- 25.Perry EK, Perry RH, Tomlinson BE, Blessed G, Gihson PH (1980) Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neurosci Lett 18(1):105–110 [DOI] [PubMed] [Google Scholar]
- 26.Sorhi S, Bird ED, Blass JP (1983) Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer hrain. Ann Neurol 13(l):72–78 [DOI] [PubMed] [Google Scholar]
- 27.Parker WD Jr, Filley CM, Parks JK (1990) Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 40(8): 1302–1303 [DOI] [PubMed] [Google Scholar]
- 28.Cardoso SM, Proemja MT, Santos S, Santana I, Oliveira CR (2004) Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurohiol Aging 25(1):105–110 [DOI] [PubMed] [Google Scholar]
- 29.Parker WD Jr, Parks J, Filley CM, Kleinschmidt-DeMasters BK (1994) Electron transport chain defects in Alzheimer’s disease hrain. Neurology. 44(6): 1090–1096 [DOI] [PubMed] [Google Scholar]
- 30.Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefono LM et al. (1992) Brain cytochrome oxidase in Alzheimer’s disease. J Neurochem 59(2): 776–779 [DOI] [PubMed] [Google Scholar]
- 31.Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM et al. (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22(3):401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, Fang EF (2017) Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci 40(3): 151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Maestro P, Gargini RA, Sproul A, García E, Antón LC, Noggle S, Arancio O, Avila J et al. (2017) Mitophagy failure in fibroblasts and iPSC-derived neurons of Alzheimer’s disease-associated presenilin 1 Mutation. Front Mol Neurosci 10:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, Sun X, Starovoytov V, Cai Q (2015) Parkin-mediated mitophagy in mutant hAPP neurons and Alzheimer’s disease patient hrains. Hum Mol Genet 24(10):2938–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keogh MJ, Chinnery PF (2015) Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta 1847(11): 1401–1411 [DOI] [PubMed] [Google Scholar]
- 36.Coskun PE, Beal MF, Wallace DC (2004) Alzheimer’s hrains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci USA 101(29):10726–10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW et al. (2010) Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis 20(Suppl 2):S293–S310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Podlesniy P, Figueiro-Silva J, Llado A, Antonell A, Sanchez-Valle R, Alcolea D, Lleo A, Molinuevo JL et al. (2013) Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol 74(5):655–668 [DOI] [PubMed] [Google Scholar]
- 39.Rice AC, Keeney PM, Algarzae NK, Ladd AC, Thomas RR, Bennett JP Jr (2014) Mitochondrial DNA copy numbers in pyramidal neurons are decreased and mitochondrial biogenesis transcriptome signaling is disrupted in Alzheimer’s disease hippocampi. J Alzheimers Dis 40(2):319–330 [DOI] [PubMed] [Google Scholar]
- 40.Wei W, Keogh MJ, Wilson I, Coxhead J, Ryan S, Rollinson S, Griffin H, Kurzawa-Akinibi M et al. (2017) Mitochondrial DNA point mutations and relative copy number in 1363 disease and control human hrains. Acta Neuropathol Commun 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, Welsh-Bohmer KA, Saunders AM et al. (2004) Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neurosci Lett 365(l):28–32 [DOI] [PubMed] [Google Scholar]
- 42.Santoro A, Balbi V, Balducci E, Pirazzini C, Rosini F, Tavano F, Achilli A, Siviero P et al. (2010) Evidence for sub-haplogroup h5 of mitochondrial DNA as a risk factor for late onset Alzheimer’s disease. PLoS One 5(8):el2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G et al. (2010) Alzheimer’s disease neuroimaging initiative. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurohiol Aging 31(8): 1355–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maruszak A, Canter JA, Styczyńska M, Zekanowski C, Barcikowska M (2009) Mitochondrial haplogroup H and Alzheimer’s disease-is there a connection? Neurohiol Aging 30(11): 1749–1755 [DOI] [PubMed] [Google Scholar]
- 45.Elson JL, Herrnstadt C, Preston G, Thai L, Morris CM, Edwardson JA, Beal MF, Turnbull DM et al. (2006) Does the mitochondrial genome play a role in the etiology of Alzheimer’s disease? Hum Genet 119β):241–254 [DOI] [PubMed] [Google Scholar]
- 46.Krüger J, Hinttala R, Majamaa K, Remes AM (2010) Mitochondrial DNA haplogroups in early-onset Alzheimer’s disease and frontotemporal lobar degeneration. Mol Neurodegener 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancuso M, Nardini M, Micheli D, Rocchi A, Nesti C, Giglioli NJ, Petrozzi L, Rossi C et al. (2007) Lack of association between mtDNA haplogroups and Alzheimer’s disease in Tuscany. Neurol Sci 28(3): 142–147 [DOI] [PubMed] [Google Scholar]
- 48.Chagnon P, Gee M, Filion M, Robitaille Y, Belouchi M, Gauvreau D (1999) Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. Am J Med Genet 85(l):20–30 [DOI] [PubMed] [Google Scholar]
- 49.Ridge PG, Maxwell TJ, Corcoran CD, Norton MC, Tschanz JT, O’Brien E, Kerber RA, Cawthon RM et al. (2012) Mitochondrial genomic analysis of late onset Alzheimer’s disease reveals protective haplogroups H6A1A/H6A1B: the Cache County study on memory in aging. PLoS One 7(9):e45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoflner JM, Brown MD, Torroni A, Lott MT, Cabell MF, Mirra SS, Beal MF, Yang CC et al. (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics. 17(1): 171–184 [DOI] [PubMed] [Google Scholar]
- 51.Egensperger R, Kosel S, Schnopp NM, Mehraein P, Graeber MB (1997) Association of the mitochondrial tRNA(A4336G) mutation with Alzheimer’s and Parkinson’s diseases. Neuropathol Appl Neurohiol 23(4):315–321 [PubMed] [Google Scholar]
- 52.Hutchin T, Cortopassi G (1995) A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc Natl Acad Sci U S A 92(15):6892–6895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Lozano JR, Mir P, Alberca R, Aguilera L, Gil Néciga E, Fernández-López O, Cayuela A, Núñez-Roldan A (2002) Mitochondrial DNA A4336G mutation in Alzheimer’s and Parkinson’s diseases. Eur Neurol 48(l):34–36 [DOI] [PubMed] [Google Scholar]
- 54.Wragg MA, Talbot CJ, Morris JC, Lendon CL, Goate AM (1995) No association found between Alzheimer’s disease and a mitochondrial tRNA glutamine gene variant. Neurosci Lett 201(2): 107–110 [DOI] [PubMed] [Google Scholar]
- 55.Corral-Debrinski M, Horton T, Lott MT, Shoflner JM, McKee AC, Beal MF, Graham BH, Wallace DC (1994) Marked changes in mitochondrial DNA deletion levels in Alzheimer hrains. Genomics 23(2):471–476 [DOI] [PubMed] [Google Scholar]
- 56.Hamblet NS, Castora FJ (1997) Elevated levels of the Keams-Sayre syndrome mitochondrial DNA deletion in temporal cortex of Alzheimer’s patients. Mutat Res 379(2):253–262 [DOI] [PubMed] [Google Scholar]
- 57.Chang SW, Zhang D, Chung HD, Zassenhaus HP (2000) The frequency of point mutations in mitochondrial DNA is elevated in the Alzheimer’s hrain. Biochem Biophys Res Commun 273(1): 203–208 [DOI] [PubMed] [Google Scholar]
- 58.Mawrin C, Kirches E, Krause G, Schneider-Stock R, Bogerts B, Vorwerk CK, Dietzmann K (2004) Region-specific analysis of mitochondrial DNA deletions in neurodegenerative disorders in humans. Neurosci Lett 357(2): 111–114 [DOI] [PubMed] [Google Scholar]
- 59.Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF (2002) High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease hrain. Hum Mol Genet 11(2): 133–145 [DOI] [PubMed] [Google Scholar]
- 60.Hudson G, Sims R, Harold D, Chapman J, Hollingworth P, Gerrish A, Russo G, Hamshere M et al. (2012) GERAD1 consortium. No consistent evidence for association between mtDNA variants and Alzheimer disease. Neurology. 78(14):1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC (1996) Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 47(l):254–256 [DOI] [PubMed] [Google Scholar]
- 62.Mosconi L, Brys M, Switalski R, Mistur R, Glodzik L, Pirraglia E, Tsui W, De Santi S et al. (2007) Maternal family history of Alzheimer’s disease predisposes to reduced hrain glucose metabolism. Proc Natl Acad Sci U S A 104(48): 19067–19072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Honea RA, Swerdlow RH, Vidoni ED, Goodwin J, Bums JM (2010) Reduced gray matter volume in normal adults with a maternal family history of Alzheimer disease. Neurology. 74(2): 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mosconi L, Rinne JO, Tsui WH, Berti V, Li Y, Wang H, Murray J, Scheinin N et al. (2010) Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer’s. Proc Natl Acad Sci U S A 107(13):5949–5954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szahados T, Dul C, Majtényi K, Hargitai J, Pénzes Z, Urbanics R (2004) A chronic Alzheimer’s model evoked by mitochondrial poison sodium azide for pharmacological investigations. Behav Brain Res 154(1):31–10 [DOI] [PubMed] [Google Scholar]
- 66.Höglinger GU, Lannuzel A, Khondiker ME, Michel PP, Duyckaerts C, Féger J, Champy P, Prigent A et al. (2005) The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. JNeurochem 95(4):930–939 [DOI] [PubMed] [Google Scholar]
- 67.Escobar-Khondiker M, Hollerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES et al. (2007) Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci 27(29):7827–7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabuzda D, Busciglio J, Chen LB, Matsudaira P, Yankner BA (1994) Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. J Biol Chem 269(18):13623–13628 [PubMed] [Google Scholar]
- 69.Gasparini L, Racchi M, Benussi L, Curti D, Binetti G, Bianchetti A, Trahucchi M, Govoni S (1997) Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neurosci Lett 231(2): 113–117 [DOI] [PubMed] [Google Scholar]
- 70.Kukreja L, Kujoth GC, Prolla TA, Van Leuven F, Vassar R (2014) Increased mtDNA mutations with aging promotes amyloid accumulation and hrain atrophy in the APP/Ld transgenic mouse model of Alzheimer’s disease. Mol Neurodegener 9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheehan JP, Swerdlow RH, Miller SW, Davis RE, Parks JK, Parker WD, Tuttle JB (1997) Calcium homeostasis and reactive oxygen species production in cells transformed by mitochondria from individuals with sporadic Alzheimer’s disease. J Neurosci 17(12):4612–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP Jr, Davis RE, Parker WD Jr (1997) Cyhrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 49(4):918–925 [DOI] [PubMed] [Google Scholar]
- 73.Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC et al. (2000) Alzheimer’s disease cyhrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol 48(2): 148–155 [PubMed] [Google Scholar]
- 74.Silva DF, Selfridge JE, Lu J, E L, Roy N, Hutfles L, Bums JM, Michaelis EK, Yan S, Cardoso SM, Swerdlow RH. Bioenergetic flux, mitochondrial mass and mitochondrial morphology dynamics in AD and MCI cyhrid cell lines. Hum Mol Genet 2013;22(19):3931–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schon EA, Shouhridge EA, Moraes CT (1998) Cyhrids in Alzheimer’s disease: a cellular model of the disease? Neurology. 51(1):326–327 [DOI] [PubMed] [Google Scholar]
- 76.Tamagno E, Bardini P, Obbili A, Vitali A, Borghi R, Zaccheo D, Pronzato MA, Danni O et al. (2002) Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol Dis 10(3):279–288 [DOI] [PubMed] [Google Scholar]
- 77.Pereira C, Santos MS, Oliveira C (1998) Mitochondrial function impairment induced by amyloid heta-peptide on PC12 cells. Neuroreport. 9(8):1749–1755 [DOI] [PubMed] [Google Scholar]
- 78.Canevari L, Clark JB, Bates TE (1999) Beta-amyloid fragment 25–35 selectively decreases complex IV activity in isolated mitochondria FEBS Lett 457(1): 131–134 [DOI] [PubMed] [Google Scholar]
- 79.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Bamham KJ, Curtain CC, Cherny RA et al. (2005) Copper-dependent inhibition of human cytochrome c oxidase by a dimeric confbrmer of amyloid-betal-42. J Neurosci 25β):672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cardoso SM, Santos S, Swerdlow RH, Oliveira CR (2001) Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J 15(8):1439–1441 [DOI] [PubMed] [Google Scholar]
- 81.Fukui H, Diaz F, Garcia S, Moraes CT (2007) Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 104(35): 14163–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, Zhu X (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer’s disease. J Neurosci 29(28):9090–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Su B, Fujioka H, Zhu X (2008) Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. Am J Pathol 173(2):470–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A 105(49): 19318–19323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manczak M, Calkins MJ, Reddy PH (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drpl in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum Mol Genet 20(13):2495–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beck JS, Mufson EJ, Counts SE (2016) Evidence for mitochondrial UPR gene activation in familial and sporadic Alzheimer’s disease. Curr Alzheimer Res 13(6):610–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sorrentino V, Romani M, Mouchiroud L, Beck JS, Zhang H, D’Amico D, Moullan N, Potenza F et al. (2017) Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 552(7684): 187–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stem D et al. (2005) Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J 19(14):2040–2041 [DOI] [PubMed] [Google Scholar]
- 89.Chen JX, Yan SS (2010) Role of mitochondrial amyloid-beta in Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2):S569–S578 [DOI] [PubMed] [Google Scholar]
- 90.Devi L, Prahhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK (2006) Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease hrain is associated with mitochondrial dysfunction. J Neurosci 26β5):9057–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anandatheerthavarada HK, Biswas G, Robin MA, Avadhani NG (2003) Mitochondrial targeting and a novel transmembrane arrest of Alzheimer’s amyloid precursor protein impairs mitochondrial function in neuronal cells. J Cell Biol 161(l):41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pagani L, Eckert A (2011) Amyloid-beta interaction with mitochondria. Int J Alzheimers Dis 2011:925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X et al. (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 304(5669):448–452 [DOI] [PubMed] [Google Scholar]
- 94.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C et al. (2008) Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med 14(10): 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Supnet C, Bezprozvanny I (2010) Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. J Alzheimers Dis 20(Suppl 2):S487–S498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballatore C, Lee VM, Trojanowski JQ (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat RevNeurosci 8(9):663–672 [DOI] [PubMed] [Google Scholar]
- 97.David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Dröse S et al. (2005) Proteomic and functional analyses reveal a mitochondrial dysfimction inP301L tau transgenic mice. J Biol Chem 280(25):23802–23814 [DOI] [PubMed] [Google Scholar]
- 98.Eckert A, Nisbet R, Grimm A, Gotz J (2014) March separate, strike together—role of phosphorylated TAU in mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta 1842(8): 1258–1266 [DOI] [PubMed] [Google Scholar]
- 99.Cheng Y, Bai F (2018) The Association of tau with mitochondrial dysunction in Alzheimer’s disease. Front Neurosci 12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E (1998) Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. J Cell Biol 143(3):777–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kopeikina KJ, Carlson GA, Pitstick R, Ludvigson AE, Peters A, Luebke JI, Koffie RM, Frosch MP et al. (2011) Tau accumulation causes mitochondrial distribution deficits in neurons in a mouse model of tauopathy and in human Alzheimer’s disease brain. Am J Pathol 179(4):2071–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manczak M, Reddy PH (2012) Abnormal interaction between the mitochondrial fission protein Drpl and hyperphosphoiylated tau in Alzheimer’s disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet 21(11): 2538–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li XC, Hu Y, Wang ZH, Luo Y, Zhang Y, Liu X, Feng Q, Wang Q et al. (2016) Human wild-type lull-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci Rep 6:24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H et al. (2009) Amylois-beta and tau synergistically impair the oxidative phosphoiylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A 106(47):20057–20062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A 106(34): 14670–14675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang E (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. [DOI] [PubMed] [Google Scholar]
- 107.Schapira AH (2008) Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 7(1):97–109 [DOI] [PubMed] [Google Scholar]
- 108.Giannoccaro MP, La Morgia C, Rizzo G, Carelli V (2017) Mitochondrial DNA and primary mitochondrial dysfunction in Parkinson’s disease. Mov Disord 32β):346–363 [DOI] [PubMed] [Google Scholar]
- 109.Rango M, Bresolin N. Brain mitochondria, Aging, and Parkinson’s Disease. Genes (Basel). 2018;9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1(8649): 1269. [DOI] [PubMed] [Google Scholar]
- 111.Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQl reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55(6):2142–2145 [DOI] [PubMed] [Google Scholar]
- 112.Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AH, Marsden CD (1992) Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson’s disease. Brain. 115(Pt 2):333–342 [DOI] [PubMed] [Google Scholar]
- 113.Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zochling R, Boissl KW et al. (1994) Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett 169(1–2):126–128 [DOI] [PubMed] [Google Scholar]
- 114.Mann VM, Cooper JM, Daniel SE, Srai K, Jenner P, Marsden CD, Schapira AH (1994) Complex I, iron, and ferritin in Parkinson’s disease substantia nigra. Ann Neurol 36(6):876–881 [DOI] [PubMed] [Google Scholar]
- 115.Cardellach F, Martí MJ, Femández-Solá J, Marín C, Hoek JB, Tolosa E, Urbano-Márquez A (1993) Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology. 43(11):2258–2262 [DOI] [PubMed] [Google Scholar]
- 116.Shoflner JM, Watts RL, Juncos JL, Torroni A, Wallace DC (1991) Mitochondrial oxidative phosphorylation defects in Parkinson’s disease. Ann Neurol 30(3):332–339 [DOI] [PubMed] [Google Scholar]
- 117.Winkler-Stuck K, Kirches E, Mawrin C, Dietzmann K, Lins H, Wallesch CW, Kunz WS, Wiedemann FR (2005) Re-evaluation of the dysfunction of mitochondrial respiratory chain in skeletal muscle of patients with Parkinson’s disease. J Neural Transm (Vienna) 112(4):499–518 [DOI] [PubMed] [Google Scholar]
- 118.DiDonato S, Zeviani M, Giovannini P, Savarese N, Rimoldi M, Mariotti C, Girotti F, Caraceni T (1993) Respiratory chain and mitochondrial DNA in muscle and brain in Parkinson’s disease patients. Neurology. 43(11):2262–2268 [DOI] [PubMed] [Google Scholar]
- 119.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW (1995) Low platelet mitochondrial complex I and complex II/IE activity in early untreated Parkinson’s disease. Ann Neurol 37(6):714–722 [DOI] [PubMed] [Google Scholar]
- 120.Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH (1992) Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson disease research group. Ann Neurol 32(6):782–788 [DOI] [PubMed] [Google Scholar]
- 121.Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26(6):719–723 [DOI] [PubMed] [Google Scholar]
- 122.Martín MA, Molina JA, Jiménez-Jiménez FJ, Benito-León J, Ortí-Pareja M, Campos Y, Arenas J (1996) Respiratory-chain enzyme activities in isolated mitochondria of lymphocytes from untreated Parkinson’s disease patients. Grupo-Centro de Trastomos del Movimento Neurology 46(5):1343–1346 [DOI] [PubMed] [Google Scholar]
- 123.Müftüoglu M, Elibol B, Dalmizrak O, Ercan A, Kulaksiz G, Ogüs H, Dalkara T, Ozer N (2004) Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord 19(5):544–548 [DOI] [PubMed] [Google Scholar]
- 124.Yoshino H, Nakagawa-Hattori Y, Kondo T, Mizuno Y (1992) Mitochondrial complex I and II activities of lymphocytes and platelets in Parkinson’s disease. J Neural Transm Park Dis Dement Sect 4(l):27–34 [DOI] [PubMed] [Google Scholar]
- 125.Hattori N, Tanaka M, Ozawa T, Mizuno Y (1991) Immunohistochemical studies on complexes I, n, HI, and IV of mitochondria in Parkinson’s disease. Ann Neurol 30(4):563–571 [DOI] [PubMed] [Google Scholar]
- 126.Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T et al. (1989) Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun 163β):1450–1455 [DOI] [PubMed] [Google Scholar]
- 127.Gu M, Cooper JM, Taanman JW, Schapira AH (1998) Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol 44(2): 177–186 [DOI] [PubMed] [Google Scholar]
- 128.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP Jr, Davis RE et al. (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol 40(4):663–671 [DOI] [PubMed] [Google Scholar]
- 129.Davis GC, Williams AC, Markey SP, Ehert MH, Caine ED, Reichert CM, Kopin IJ (1979) Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res lβ):249–254 [DOI] [PubMed] [Google Scholar]
- 130.Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic parkinsonism in humans due to a product of meperidine -analog synthesis. Science. 219(4587):979–980 [DOI] [PubMed] [Google Scholar]
- 131.Bums RS, Chiueh CC, Markey SP, Ehert MH, Jacohowitz DM, Kopin U (1983) A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra hy N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A 80(14):4546–4550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heikkila RE, Hess A, Duvoisin RC (1984) Dopaminergic neurotoxicity of l-methyl-4-phenyl-l,2,5,6-tetrahydropyridine in mice. Science 224(4656): 1451–1453 [DOI] [PubMed] [Google Scholar]
- 133.Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2(5):325–334 [DOI] [PubMed] [Google Scholar]
- 134.Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Park Dis 1(1): 19–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Porras G, Li Q, Bezard E (2012) Modeling Parkinson’s disease in Primates: the MPTP model. Cold Spring Harh Perspect Med 2(3): a009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicklas WJ, Vyas I, Heikkila RE (1985) Inhibition of NADH-linked oxidation in brain mitochondria hy 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, l-methyl-4-phenyl-l,2,5,6-tetrahydropyridine. Life Sci 36(26):2503–2508 [DOI] [PubMed] [Google Scholar]
- 137.Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron. 39(6):889–909 [DOI] [PubMed] [Google Scholar]
- 138.Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH (1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-l,2,3,6 - tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine hy dopamine neurons explains selective toxicity. Proc Nad Acad Sri U S A 82(7):2173–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Przedborski S, Tieu K, Perier C, Vila M (2004) MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr 36(4):375–379 [DOI] [PubMed] [Google Scholar]
- 140.Degli EM (1998) Inhibitors of NADH-ubiquinone reductase: an overview. Biochim Biophys Acta 1364(2):222–235 [DOI] [PubMed] [Google Scholar]
- 141.Jenner P (2001) Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci 24(5):245–247 [DOI] [PubMed] [Google Scholar]
- 142.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12): 1301–1306 [DOI] [PubMed] [Google Scholar]
- 143.Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22(16):7006–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Höglinger GU, Féger J, Prigent A, Michel PP, Parain K, Champy P, Ruherg M, Oertel WH et al. (2003) Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem 84β):491–502 [DOI] [PubMed] [Google Scholar]
- 145.Choi WS, Kruse SE, Palmiter RD, Xia Z (2008) Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced hy rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A 105(39): 15136–15141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Champy P, Höglinger GU, Féger J, Gleye C, Hocquemiller R, Laurens A, Guérineau V, Laprévote O et al. (2004) Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: possible relevance for atypical parkinsonism in Guadeloupe. J Neurochem 88(l):63–69 [DOI] [PubMed] [Google Scholar]
- 147.Ikehe S, Tanaka M, Ohno K, Sato W, Hattori K, Kondo T, Mizuno Y, Ozawa T (1990) Increase of deleted mitochondrial DNA in the striatum in Parkinson’s disease and senescence. Biochem Biophys Res Commun 170(3): 1044–1048 [DOI] [PubMed] [Google Scholar]
- 148.Ozawa T, Tanaka M, Ikehe S, Ohno K, Kondo T, Mizuno Y (1990) Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum hy a kinetic PCR analysis. Biochem Biophys Res Commun 172(2):483–489 [DOI] [PubMed] [Google Scholar]
- 149.Lestienne P, Nelson J, Riederer P, Jellinger K, Reichmann H (1990) Normal mitochondrial genome in brain from patients with Parkinson’s disease and complex I defect. J Neurochem 55(5): 1810–1812 [DOI] [PubMed] [Google Scholar]
- 150.Mann VM, Cooper JM, Schapira AH (1992) Quantitation of a mitochondrial DNA deletion in Parkinson’s disease. FEBS Lett 299(3):218–222 [DOI] [PubMed] [Google Scholar]
- 151.Schapira AH, Holt IJ, Sweeney M, Harding AE, Jenner P, Marsden CD (1990) Mitochondrial DNA analysis in Parkinson’s disease. Mov Disord 5(4):294–297 [DOI] [PubMed] [Google Scholar]
- 152.Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J (2002) Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol 61(7):634–639 [DOI] [PubMed] [Google Scholar]
- 153.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5):515–517 [DOI] [PubMed] [Google Scholar]
- 154.Dölle C, Flønes I, Nido GS, Miletic H, Osuagwu N, Kristoflfersen S, Lilleng PK, Larsen JP et al. (2016) Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nat Commun 7:13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38(5):518–520 [DOI] [PubMed] [Google Scholar]
- 156.Brown MD, Shoffner JM, Kim YL, Jun AS, Graham BH, Cabell MF, Gurley DS, Wallace DC (1996) Mitochondrial DNA sequence analysis of four Alzheimer’s and Parkinson’s disease patients. Am J Med Genet 61(3):283–289 [DOI] [PubMed] [Google Scholar]
- 157.Ikehe S, Tanaka M, Ozawa T (1995) Point mutations of mitochondrial genome in Parkinson’s disease. Brain Res Mol Brain Res 28(2):281–295 [DOI] [PubMed] [Google Scholar]
- 158.Kapsa RM, Jean-Francois MJ, Lertrit P, Weng S, Siregar N, Ojaimi J, Donnan G, Masters C et al. (1996) Mitochondrial DNA polymorphism in substantia nigra. J Neurol Sci 144(l–2):204–211 [DOI] [PubMed] [Google Scholar]
- 159.Kösel S, Grasbon-Frodl EM, Mautsch U, Egensperger R, von Eitzen U, Frishman D, Hofmann S, Gerbitz KD et al. (1998) Novel mutations of mitochondrial complex I in pathologically proven Parkinson disease. Neurogenetics. 1(3): 197–204 [DOI] [PubMed] [Google Scholar]
- 160.Richter G, Sonnenschein A, Griinewald T, Reichmann H, Janetzky B (2002) Novel mitochondrial DNA mutations in Parkinson’s disease. J Neural Transm (Vienna) 109(5–6):721–729 [DOI] [PubMed] [Google Scholar]
- 161.Parker WD Jr, Parks JK (2005) Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem Biophys Res Commun 326(3):667–669 [DOI] [PubMed] [Google Scholar]
- 162.Kösel S, Grasbon-Frodl EM, Hagenah JM, Graeber MB, Vieregge P (2000) Parkinson disease: analysis of mitochondrial DNA in monozygotic twins. Neurogenetics. 2(4):227–230 [DOI] [PubMed] [Google Scholar]
- 163.Vives-Bauza C, Andreu AL, Manfredi G, Beal MF, Janetzky B, Gruenewald TH, Lin MT (2002) Sequence analysis of the entire mitochondrial genome in Parkinson’s disease. Biochem Biophys Res Commun 290(5): 1593–1601 [DOI] [PubMed] [Google Scholar]
- 164.Simon DK, Mayeux R, Marder K, Kowall NW, Beal MF, Johns DR (2000) Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson’s disease. Neurology. 54(3):703–709 [DOI] [PubMed] [Google Scholar]
- 165.Coxhead J, Kurzawa-Akanhi M, Hussain R, Pyle A, Chinnery P, Hudson G. Somatic mtDNA variation is an important component ofParkinson’s disease. Neurohiol Aging. 2016;38:217.el–217.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzherger T, Lees AJ, Betensky RA et al. (2012) Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann Neurol 71(6):850–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Huhhle JP, Haines JL et al. (2003) Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet 72(4): 804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pyle A, Foltynie T, Tiangyou W, Lambert C, Keers SM, Allcock LM, Davison J, Lewis SJ et al. (2005) Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol 57(4):564–567 [DOI] [PubMed] [Google Scholar]
- 169.Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P et al. (2005) Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur J Hum Genet 13(6):748–752 [DOI] [PubMed] [Google Scholar]
- 170.Khusnutdinova E, Gilyazova I, Ruiz-Pesini E, Derbeneva O, Khusainova R, Khidiyatova I, Magzhanov R, Wallace DC (2008) A mitochondrial etiology of neurodegenerative diseases: evidence from Parkinson’s disease. Ann N Y Acad Sci 1147:1–20 [DOI] [PubMed] [Google Scholar]
- 171.Ross OA, McCormack R, Maxwell LD, Duguid RA, Quinn DJ, Barnett YA, Rea IM, El-Agnaf OM et al. (2003) mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson’s disease in the Irish. Exp Gerontol 38(4):397–405 [DOI] [PubMed] [Google Scholar]
- 172.Hudson G, Nalls M, Evans JR, Breen DP, Winder-Rhodes S, Morrison KE, Morris HR, Williams-Gray CH et al. (2013) Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology. 80(22):2042–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pyle A, Brennan R, Kurzawa-Akanhi M, Yamall A, Thouin A, Mollenhauer B, Bum D, Chinneiy PF et al. (2015) Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson’s disease. Ann Neurol 78(6):1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Griinewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM (2016) Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol 79β): 366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pyle A, Anugrha H, Kurzawa-Akanhi M, Yamall A, Bum D, Hudson G (2016) Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurohiol Aging 38:216.e7–216.el0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Van Goethem G, Dermaut B, Löfgren A, Martin JJ, Van Broeckhoven C (2001) Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat Genet 28(3):211–212 [DOI] [PubMed] [Google Scholar]
- 177.Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I et al. (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet. 364(9437):875–882 [DOI] [PubMed] [Google Scholar]
- 178.Mancuso M, Filosto M, Oh SJ, DiMauro S (2004) A novel polymerase gamma mutation in a family with ophthalmoplegia, neuropathy, and parkinsonism. Arch Neurol 61(ll):1777–1779 [DOI] [PubMed] [Google Scholar]
- 179.Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Bum DJ et al. (2007) Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and parkinsonism. Arch Neurol 64(4):553–557 [DOI] [PubMed] [Google Scholar]
- 180.Invemizzi F, Varanese S, Thomas A, Carrara F, Onofij M, Zeviani M (2008) Two novel POLG1 mutations in a patient with progressive external ophthalmoplegia, levodopa-responsive pseudo-orthostatic tremor and parkinsonism. Neuromuscul Disord 18(6):460–464 [DOI] [PubMed] [Google Scholar]
- 181.Dolhun R, Presant EM, Hedera P (2013) Novel polymerase gamma (POLG1) gene mutation in the linker domain associated with parkinsonism. BMC Neurol 13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miguel R, Gago MF, Martins J, Barros P, Vale J, Rosas MJ (2014) POLGl-related levodopa-responsive parkinsonism. Clin Neurol Neurosurg 126:47–54 [DOI] [PubMed] [Google Scholar]
- 183.Remes AM, Hinttala R, Kärppä M, Soini H, Takalo R, Uusimaa J, Majamaa K (2008) Parkinsonism associated with the homozygous W748S mutation in the POLG1 gene. Parkinsonism Relat Disord 14(8):652–654 [DOI] [PubMed] [Google Scholar]
- 184.Synofzik M, Asmus F, Reimold M, Schöls L, Berg D (2010) Sustained dopaminergic response of parkinsonism and depression in POLG-associated parkinsonism. Mov Disord 25(2):243–245 [DOI] [PubMed] [Google Scholar]
- 185.Davidzon G, Greene P, Mancuso M, Klos KJ, Ahlskog JE, Hirano M, DiMauro S (2006) Early-onset familial parkinsonism due to POLG mutations. Ann Neurol 59(5):859–862 [DOI] [PubMed] [Google Scholar]
- 186.Betts-Henderson J, Jaros E, Krishnan KJ, Perry RH, Reeve AK, Schaefer AM, Taylor RW, Turnbull DM (2009) Alpha-synuclein pathology and parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurohiol 35(1): 120–124 [DOI] [PubMed] [Google Scholar]
- 187.Melberg A, Nennesmo I, Moslemi AR, Kollberg G, Luoma P, Suomalainen A, Holme E, Oldfors A (2005) Alzheimer pathology associated with POLG1 mutation, multiple mtDNA deletions, and APOE4/4: premature ageing or just coincidence? Acta Neuropathol 110(3):315–316 [DOI] [PubMed] [Google Scholar]
- 188.Tzoulis C, Tran GT, Schwarzhnüller T, Specht K, Haugarvoll K, Balafkan N, Lilleng PK, Miletic H et al. (2013) Severe nigrostriatal degeneration without clinical parkinsonism in patients with polymerase gamma mutations. Brain. 136(Pt 8):2393–2404 [DOI] [PubMed] [Google Scholar]
- 189.Gui YX, Xu ZP, Lv W, Liu HM, Zhao JJ, Hu XY (2012) Association of mitochondrial DNA polymerase y gene POLG1 polymorphisms with parkinsonism in Chinese populations. PLoS One 7(12):e50086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hudson G, Tiangyou W, Stutt A, Eccles M, Robinson L, Bum DJ, Chinnery PF (2009) No association between common POLG1 variants and sporadic idiopathic Parkinson’s disease. Mov Disord 24(7):1092–1094 [DOI] [PubMed] [Google Scholar]
- 191.Luoma PT, Eerola J, Ahola S, Hakonen AH, Hellström O, Kivistö KT, Tienari PJ, Suomalainen A (2007) Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 69(11): 1152–1159 [DOI] [PubMed] [Google Scholar]
- 192.Tiangyou W, Hudson G, Ghezzi D, Ferrari G, Zeviani M, Bum DJ, Chinnery PF (2006) POLG1 in idiopathic Parkinson disease. Neurology. 67(9): 1698–1700 [DOI] [PubMed] [Google Scholar]
- 193.Ylönen S, Ylikotila P, Siitonen A, Finnilä S, Autere J, Majamaa K (2013) Variations of mitochondrial DNA polymerase γ in patients with Parkinson’s disease. J Neurol 260(12):3144–3149 [DOI] [PubMed] [Google Scholar]
- 194.Anvret A, Westerlund M, Sydow O, Willows T, Lind C, Gaiter D, Belin AC (2010) Variations of the CAG trinucleotide repeat in DNA polymerase γ (POLG1) is associated with Parkinson’s disease in Sweden. Neurosci Lett 485(2): 117–120 [DOI] [PubMed] [Google Scholar]
- 195.Balafkan N, Tzoulis C, Muller B, Haugarvoll K, Tysnes OB, Larsen JP, Bindoff LA (2012) Number of CAG repeats in POLG1 and its association with Parkinson disease in the Norwegian population. Mitochondrion. 12(6):640–643 [DOI] [PubMed] [Google Scholar]
- 196.Eerola J, Luoma PT, Peuralinna T, Scholz S, Paisan-Ruiz C, Suomalainen A, Singleton AB, Tienari PJ (2010) POLG1 polyglutamine tract variants associated with Parkinson’s disease. Neurosci Lett 477(1): 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bentley SR, Shan J, Todorovic M, Wood SA, Mellick GD (2014) Rare POLG1 CAG variants do not influence Parkinson’s disease or polymerase gamma function. Mitochondrion. 15:65–68 [DOI] [PubMed] [Google Scholar]
- 198.Taanman JW, Schapira AH (2005) Analysis of the trinucleotide CAG repeat from the DNA polymerase gamma gene (POLG) in patients with Parkinson’s disease. Neurosci Lett 376(l):56–59 [DOI] [PubMed] [Google Scholar]
- 199.Baloh RH, Salavaggione E, Milbrandt J, Pestronk A (2007) Familial parkinsonism and ophthalmoplegia from a mutation in the mitochondrial DNA helicase twinkle. Arch Neurol 64(7):998–1000 [DOI] [PubMed] [Google Scholar]
- 200.Kiferle L, Orsucci D, Mancuso M, Lo Gerfo A, Petrozzi L, Siciliano G, Ceravolo R, Bonuccelli U (2013) Twinkle mutation in an Italian family with external progressive ophthalmoplegia and parkinsonism: a case report and an update on the state of art. Neurosci Lett 556:1–4 [DOI] [PubMed] [Google Scholar]
- 201.Vandenberghe W, Van Laere K, Debruyne F, Van Broeckhoven C, Van Goethem G (2009) Neurodegenerative parkinsonism and progressive external ophthalmoplegia with a twinkle mutation. Mov Disord 24(2):308–309 [DOI] [PubMed] [Google Scholar]
- 202.Spelbrink JN, Li FY, Tiranti V, Nikali K, Yuan QP, Tariq M, Wanrooij S, Garrido N et al. (2001) Human mitochondrial DNA deletions associated with mutations in the gene encoding twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat Genet 28(3):223–231 [DOI] [PubMed] [Google Scholar]
- 203.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y et al. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 392(6676):605–608 [DOI] [PubMed] [Google Scholar]
- 204.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D et al. (2004) Hereditaiy early-onset Parkinson’s disease caused by mutations in PINK1. Science. 304(5674): 1158–1160 [DOI] [PubMed] [Google Scholar]
- 205.Nguyen TN, Padman BS, Lazarou M (2016) Deciphering the molecular signals of PINKl/Parkin mitophagy. Trends Cell Biol 26(10):733–744 [DOI] [PubMed] [Google Scholar]
- 206.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA et al. (2006) Drosophila pinkl is required for mitochondrial function and interacts genetically with parkin. Nature. 441(7097): 1162–1166 [DOI] [PubMed] [Google Scholar]
- 207.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J et al. (2006) Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 441(7097):1157–1161 [DOI] [PubMed] [Google Scholar]
- 208.Charan RA, LaVoie MJ (2015) Pathologic and therapeutic implications for the cell biology of parkin. Mol Cell Neurosci 66(Pt A): 62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008) Pinkl regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sri U S A 105(19):7070–7075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Deng H, Dodson MW, Huang H, Guo M (2008) The Parkinson’s disease genes pinkl and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A 105(38): 14503–14508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ordonez DG, Lee MK, Feany MB (2018) α-synuclein induces mitochondrial dysfunction through spectrin and the actin cytoskeleton. Neuron 97(l):108–124.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lutz AK, Exner N, Fett ME, Schlehe JS, Kloos K, Lämmermann K, Brunner B, Kurz-Drexler A et al. (2009) Loss of parkin or PINK1 function increases Drpl-dependent mitochondrial fragmentation. J Biol Chem 284β4):22938–22951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rappold PM, Cui M, Grima JC, Fan RZ, de Mesy-Bentley KL, Chen L, Zhuang X, Bowers WJ et al. (2014) Drpl inhibition attenuates neurotoxicity and dopamine release deficits in vivo. Nat Commun 5:5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279(18): 18614–18622 [DOI] [PubMed] [Google Scholar]
- 215.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ (2003) Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A 100(7):4078–4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cooper O, Seo H, Andrabi S, Guardia-Laguarta C, Graziotto J, Sundberg M, McLean JR, Carrillo-Reid L et al. (2012) Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med 4(141):141ra90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Imaizumi Y, Okada Y, Akamatsu W, Koike M, Kuzumaki N, Hayakawa H, Nihira T, Kobayashi T et al. (2012) Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol Brain 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jiang H, Ren Y, Yuen EY, Zhong P, Ghaedi M, Hu Z, Azabdaflari G, Nakaso K et al. (2012) Parkin controls dopamine utilization in human midbrain dopaminergic neurons derived from induced phiripotent stem cells. Nat Commun 3:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sribler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D (2011) Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci 31(16):5970–5976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shaltouki A, Sivapatham R, Pei Y, Gerencser AA, Momcilovic O, Rao MS, Zeng X (2015) Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports 4(5):847–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Suzuki S, Akamatsu W, Kisa F, Sone T, Ishikawa KI, Kuzumaki N, Katayama H, Miyawaki A et al. (2017) Efficient induction of dopaminergic neuron differentiation from induced pluripotent stem cells reveals impaired mitophagy in PARK2 neurons. Biochem Biophys Res Commun 483(l):88–93 [DOI] [PubMed] [Google Scholar]
- 222.Tabata Y, Imaizumi Y, Sugawara M, Andoh-Noda T, Banno S, Chai M, Sone T, Yamazaki K et al. (2018) T-type calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Reports 11(5): 1171–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D et al. (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27(45): 12413–12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature. 388(6645):839–840 [DOI] [PubMed] [Google Scholar]
- 225.Bendor JT, Logan TP, Edwards RH (2013) The function of α-synuclein. Neuron. 79(6): 1044–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14(1):38–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kacheigus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646): 841. [DOI] [PubMed] [Google Scholar]
- 228.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 276(5321):2045–2047 [DOI] [PubMed] [Google Scholar]
- 229.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet 18(2): 106–108 [DOI] [PubMed] [Google Scholar]
- 230.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J et al. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55(2):164–173 [DOI] [PubMed] [Google Scholar]
- 231.Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF (2014) Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis 70:204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li L, Nadanaciva S, Berger Z, Shen W, Paumier K, Schwartz J, Mou K, Loos P et al. (2013) Human A53T α-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons. PLoS One 8(12):e85815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ (2014) Soluble, prefibrillar α-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem 289β1):21490–21507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Banerjee K, Sinha M, Pham Cle L, Jana S, Chanda D, Cappai R, Chakrabarti S (2010) Alpha-synuclein induced membrane depolarization and loss of phosphoiylation capacity of isolated rat brain mitochondria: implications in Parkinson’s disease. FEBS Lett 584(8): 1571–1576 [DOI] [PubMed] [Google Scholar]
- 235.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65(7–8): 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Tapias V, Hu X, Luk KC, Sanders LH, Lee VM, Greenamyre JT (2017) Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci 74(15):2851–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chinta SJ, Mallajosyula JK, Rane A, Andersen JK (2010) Mitochondrial α-synuclein accumulation impairs complex I firnction in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett 486(3):235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Loeb V, Yakunin E, Saada A, Sharon R (2010) The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J Biol Chem 285(10): 7334–7343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK (2008) Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem 283(14):9089–9100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Reeve AK, Ludtmann MH, Angelova PR, Simcox EM, Horrocks MH, Klenerman D, Gandhi S, Turnbull DM et al. (2015) Aggregated α-synuclein and complex I deficiency: exploration of their relationship in differentiated neurons. Cell Death Dis 6: el 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K et al. (2009) alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett 454(3):187–192 [DOI] [PubMed] [Google Scholar]
- 242.Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E (2004) Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186(2):158–172 [DOI] [PubMed] [Google Scholar]
- 243.Song LK, Ma KL, Yuan YH, Mu Z, Song XY, Niu F, Han N, Chen NH (2015) Targeted overexpression of α-Synuclein by rAAV2/l vectors induces progressive nigrostriatal degeneration and increases vulnerability to MPTP in mouse. PLoS One 10(6): e0131281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nieto M, Gil-Bea FJ, Dalfó E, Cuadrado M, Cabodevilla F, Sánchez B, Catena S, Sesma T et al. (2006) Increased sensitivity to MPTP in human alpha-synuclein A30P transgenic mice. Neurobiol Aging 27(6):848–856 [DOI] [PubMed] [Google Scholar]
- 245.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K et al. (2002) Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A 99(22): 14524–14529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW et al. (2006) Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis 21(3):541–548 [DOI] [PubMed] [Google Scholar]
- 247.Fountaine TM, Wade-Martins R (2007) RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res 85(2):351–363 [DOI] [PubMed] [Google Scholar]
- 248.Cannon JR, Geghman KD, Tapias V, Sew T, Dail MK, Li C, Greenamyre JT (2013) Expression of human E46K-mutated α-synuclein in BAC-transgenic rats replicates early-stage Parkinson’s disease features and enhances vulnerability to mitochondrial impairment. Exp Neurol 240:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Shavali S, Brown-Borg HM, Ebadi M, Porter J (2008) Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett 439(2): 125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zharikov AD, Cannon JR, Tapias V, Bai Q, Horowitz MP, Shah V, El Ayadi A, Hastings TG et al. (2015) shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J Clin Invest 125(7):2721–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fomai F, Schlüter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL et al. (2005) Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A 102(9):3413–3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ludtmann MH, Angelova PR, Ninkina NN, Gandhi S, Buchman VL, Abramov AY (2016) Monomeric alpha-synuclein exerts a physiological role on brain ATP synthase. J Neurosci 36(41): 10510–10521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Di Maio R, Barrett PJ, Hoffinan EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J et al. (2016) α-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med 8β42):342ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, Laub C, Mueller S et al. (2013) TOM40 mediates mitochondrial dysfunction induced by α-synuclein accumulation in Parkinson’s disease. PLoS One 8(4):e62277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 255.Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T et al. (2010) Inhibition of mitochondrial fusion by α-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J 29(20):3571–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J et al. (2011) Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem 286(23):20710–20726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Guardia-Laguarta C, Area-Gomez E, Rüb C, Liu Y, Magrané J, Becker D, Voos W, Schon EA et al. (2014) α-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34(1): 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S (2015) A new role for α-synuclein in Parkinson’s disease: alteration of ER-mitochondrial communication. Mov Disord 30(8): 1026–1033 [DOI] [PubMed] [Google Scholar]
- 259.Ubhi K, Low P, Masliah E (2011) Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci 34(11):581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Monzio Compagnoni G, Di Fonzo A (2019) Understanding the pathogenesis of multiple system atrophy: state of the art and future perspectives. Acta Neuropathol Commun 7(1): 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Blin O, Desnuelle C, Rascol O, Borg M, Peyro Saint Paul H, Azulay JP, Billé F, Figarella D et al. (1994) Mitochondrial respiratoiy failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J Neurol Sci 125(1):95–101 [DOI] [PubMed] [Google Scholar]
- 262.Gu M, Gash MT, Cooper JM, Wenning GK, Daniel SE, Quinn NP, Marsden CD, Schapira AH (1997) Mitochondrial respiratory chain function in multiple system atrophy. Mov Disord 12(3): 418–422 [DOI] [PubMed] [Google Scholar]
- 263.Multiple-System Atrophy Research Collaboration (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369(3):233–244 [DOI] [PubMed] [Google Scholar]
- 264.Quinzii CM, Hirano M (2011) Primary and secondary CoQ(10) deficiencies in humans. Biofoctors. 37(5):361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Quinzii C, Naini A, Salviati L, Trevisson E, Navas P, Dimauro S, Hirano M (2006) A mutation in para-hydroxyhenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet 78(2):345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lin CH, Tan EK, Yang CC, Yi Z, Wu RM (2015) COQ2 gene variants associate with cerebellar subtype of multiple system atrophy in Chinese. Mov Disord 30(3):436–437 [DOI] [PubMed] [Google Scholar]
- 267.Ogaki K, Fujioka S, Heckman MG, Rayaprolu S, Soto-Ortolaza AI, Labbe C, Walton RL, Lorenzo-Betancor O et al. (2014) Analysis of COQ2 gene in multiple system atrophy. Mol Neurodegener 9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ronchi D, Di Biase E, Franco G, Melzi V, Del Sorbo F, Elia A, Barzaghi C, Garavagha B, Bergamini C, Fato R, Mora G, Del Bo R, Fortunato F, BoreUini L, Trezzi I, Compagnoni GM, Monlfini E, Frattini E, Bonato S, Cogiamanian F, Ardolino G, Priori A, Bresolin N, Corti S, Comi GP, Di Fonzo A. Mutational analysis of COQ2 in patients with MSA in Italy. Neurobiol Aging. 2016;45:213.el–213.e2. [DOI] [PubMed] [Google Scholar]
- 269.Schottlaender LV, Houlden H (2014) Multiple-System Atrophy (MSA) Brain hank collaboration. Mutant COQ2 in multiple-system atrophy. N Engl J Med 371(1):81. [DOI] [PubMed] [Google Scholar]
- 270.Sharma M, Wenning G, Kruger R; European multiple-system atrophy study group (EMSA-SG). Mutant COQ2 in multiple-system atrophy. N Engl J Med 2014;371(1): 80–81. [DOI] [PubMed] [Google Scholar]
- 271.Zhao Q, Yang X, Tian S, An R, Zheng J, Xu Y (2016) Association of the COQ2 V393A variant with risk of multiple system atrophy in East Asians: a case-control study and meta-analysis of the literature. Neurol Sci 37(3):423–430 [DOI] [PubMed] [Google Scholar]
- 272.Barca E, Kleiner G, T ang G, Ziosi M, T adesse S, Masliah E, Louis ED, Faust P et al. (2016) Decreased coenzyme Q10 levels in multiple system atrophy cerebellum J Neuropathol Exp Neurol 75(7): 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schottlaender LV, Bettencourt C, Kiely AP, Chalasani A, Neergheen V, Holton JL, Hargreaves I, Houlden H (2016) Coenzyme Q10 levels are decreased in the cerebellum of multiple-system atrophy patients. PLoS One ll(2):e0149557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Compta Y, Giraldo DM, Munoz E, Antonelli F, Fernandez M, Bravo P, Soto M, Camara A et al. (2018) Catalan MSA Registry (CMSAR). Cerebrospinal fluid levels of coenzyme Q10 are reduced in multiple system atrophy. Parkinsonism Relat Disord 46:16–23 [DOI] [PubMed] [Google Scholar]
- 275.Mitsui J, Matsukawa T, Yasuda T, Ishiura H, Tsuji S (2016) Plasma coenzyme Q10 levels in patients with multiple system atrophy. JAMA Neurol 73(8):977–980 [DOI] [PubMed] [Google Scholar]
- 276.Kasai T, Tokuda T, Ohmichi T, Ishii R, Tatebe H, Nakagawa M, Mizuno T (2016) Serum levels of coenzyme Q10 in patients with multiple system atrophy. PLoS One 1 l(l):e0147574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Monzio Compagnoni G, Kleiner G, Bordoni A, Fortunato F, Ronchi D, Salani S, Guida M, Corti C et al. (2018) Mitochondrial dysfunction in fibroblasts of multiple system atrophy. Biochim Biophys Acta Mol basis Dis 1864(12):3588–3597 [DOI] [PubMed] [Google Scholar]
- 278.Monzio Compagnoni G, Kleiner G, Samarani M, Aureli M, Faustini G, Bellucci A, Ronchi D, Bordoni A et al. (2018) Mitochondrial dysregulation and impaired autophagy in iPSC-derived dopaminergic neurons of multiple system atrophy. Stem Cell Reports 11(5): 1185–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Nakamoto FK, Okamoto S, Mitsui J, Sone T, Ishikawa M, Yamamoto Y, Kanegae Y, Nakatake Y et al. (2018) The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci Rep 8(1): 14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stefenova N, Reindl M, Neumann M, Haass C, Poewe W, Kahle PJ, Wenning GK (2005) Oxidative stress in transgenic mice with oligodendroglial alpha-synuclein overexpression replicates the characteristic neuropathology of multiple system atrophy. Am J Pathol 166(3):869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ubhi K, Lee PH, Adame A, Inglis C, Mante M, Rockenstein E, Stefenova N, Wenning GK et al. (2009) Mitochondrial inhibitor 3-nitropropionic acid enhances oxidative modification of alpha-synuclein in a transgenic mouse model of multiple system atrophy. J Neurosci Res 87(12):2728–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Collier TJ, Kanaan NM, Kordower JH (2011) Ageing as aprimaiy risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci 12(6):359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kirkwood TB (2005) Understanding the odd science of aging. Cell 120(4):437–447 [DOI] [PubMed] [Google Scholar]
- 284.JangJY BlumA, LiuJ FinkelT (2018) The role of mitochondria in aging. J Clin Invest 128(9):3662–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sun N, Youle RJ, Finkel T (2016) The mitochondrial basis of aging. Mol Cell 61(5):654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Theurey P, Pizzo P. The Aging Mitochondria. Genes (Basel). 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Corral-Debrinski M, Horton T, Lott MT, Shoflner JM, Beal MF, Wallace DC (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet 2(4):324–329 [DOI] [PubMed] [Google Scholar]
- 288.Khaidakov M, Heflich RH, Manjanatha MG, Myers MB, Aidoo A (2003) Accumulation of point mutations in mitochondrial DNA of aging mice. Mutat Res 526(1–2): 1–7 [DOI] [PubMed] [Google Scholar]
- 289.Schwarze SR, Lee CM, Chung SS, Roecker EB, Weindruch R, Aiken JM (1995) High levels of mitochondrial DNA deletions in skeletal muscle of old rhesus monkeys. Mech Ageing Dev 83(2): 91–101 [DOI] [PubMed] [Google Scholar]
- 290.Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byme E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev 1999; 11 l(l):39–47. [DOI] [PubMed] [Google Scholar]
- 291.Preston CC, Oberlin AS, Holmuhamedov EL, Gupta A, Sagar S, Syed RH, Siddiqui SA, Raghavakaimal S et al. (2008) Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech Ageing Dev 129(6):304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sun N, Yun J, Liu J, Malide D, Liu C, Rovira n, Holmstrom KM, Fergusson MM et al. (2015) Measuring in vivo mitophagy. Mol Cell 60(4):685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Harman D (1956) Aging: a theoiy based on free radical and radiation chemistry. J Gerontol 11(3):298–300 [DOI] [PubMed] [Google Scholar]
- 294.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S et al. (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 429(6990):417–423 [DOI] [PubMed] [Google Scholar]
- 295.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY et al. (2005) Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 309(5733)481–484 [DOI] [PubMed] [Google Scholar]
- 296.DiMauro S (2004) Mitochondrial diseases. Biochim Biophys Acta 1658(l-2):80–88 [DOI] [PubMed] [Google Scholar]
- 297.Pacelli C, Giguere N, Bourque M, Levesque M, Ruth SS, Trudeau L (2015) Elevated mitochondrial hioenergetics and axonal Arborization size are key contributors to the vulnerability of dopamine neurons. Curr Biol 25:2349–2360 [DOI] [PubMed] [Google Scholar]
- 298.Damiano M, Galvan L, Deglon N, Brouillet E (2010) Mitochondria in Huntington’s disease. Biochim Biophys Acta 1802(1):52–61 [DOI] [PubMed] [Google Scholar]
- 299.Elfewy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci 218:165–184 [DOI] [PubMed] [Google Scholar]
- 300.Wang J, Chen GJ (2016) Mitochondria as a therapeutic target in Alzheimer’s disease. Genes Dis 3(3):220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cenini G, Voos W (2019) Mitochondria as potential targets in Alzheimer disease therapy: an update. Front Pharmacol 10:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Thomas B, Beal MF (2010) Mitochondrial therapies for Parkinson’s disease. Mov Disord 25(Suppl 1):S155–S160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Parkinson Study Group QE3 Investigators (2014) A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit JAMA Neurol 71(5):543–552 [DOI] [PubMed] [Google Scholar]