Abstract
Objective
In this study, we aimed to examine thiol/disulfide homeostasis and oxidative DNA damage in patients with OCD and compare them with healthy controls.
Methods
Thirty-five patients previously diagnosed with OCD in Van Yuzuncu Yil University Department of Psychiatry and thirty-three healthy volunteers were included in the study. The severity of the symptoms was measured using the Yale-Brown Obsessive-Compulsive Scale. Five μL of blood samples were taken from the patient and control groups. The samples were stored at appropriate conditions until use. Leukocyte DNA was isolated and the levels of 8-hydroxy-2-deoxyguanosine (8-OHdG) and deoxyguanosine were detected to assess the oxidative DNA damage. The level of oxidative DNA damage was expressed as 8-OHdG/106dG. Total thiol/native thiol levels were measured for thiol/disulfide homeostasis. The level of disulfide was determined by subtracting the native thiol value from the total thiol value and the result was divided by two. Results were given as percentages.
Results
The total and native thiol levels in patients with OCD were significantly lower, and the disulfide levels were significantly higher in patients with OCD than healthy control subjects. In addition, 8-OHdG, an indicator of DNA damage, was significantly lower in the control group compared to the patient group.
Conclusion
Increased levels of disulfide/native thiol and disulfide/total thiol in patients with OCD show that levels of oxidative stress were elevated and therefore, higher 8-OHdG levels in patients with OCD is a marker of oxidative DNA damage.
Keywords: Obsessive-compulsive disorder, Thiol disulfide, DNA damage
INTRODUCTION
While obsessions are involuntary, persistent, repetitive thoughts; impulses and images, compulsions are repetitive behaviors and mental actions which the person feels like he or she must comply and execute as the person feels obliged to perform in response to these obsessions [1]. It has been stated that genetic and environmental factors may affect the etiopathology of obsessive compulsive disorders (OCD), and patients with OCD suffer from functional damage in the orbital and frontal cortex of the brain, basal ganglion and thalamus, and due to this damage, functionality of some neurotransmitters have been impaired [2-5]. In addition, it has been reported that the etiology of OCD is affected by oxidative damage, and that the oxidative balance is impaired in other psychiatric diseases [6]. While the body produces free radicals, it also eliminates them by using various methods, which is a stable and balanced process [7]. Excessive free oxygen radicals can result in damage in cell membrane, degradation of DNA, protein carboxylation, lipid peroxidation, and cell death [8,9]. Tissues of central nervous system are highly prone to oxidative damage, and the metabolism has developed antioxidative mechanisms against oxidation [10]. The most significant enzymes for antioxidant mechanisms are superoxide dismutase, glutathione peroxidase, catalase and thiols [11].
Thiols can undergo oxidation and develop disulfide bonds. Free oxygen radicals attack the thiol groups of sulfur-containing amino acids, which result in oxidation, and this is the first observable change in protein oxidation [12]. The thiol group of the cysteine amino acid is highly prone to oxidative attack, and the thiol radical formed by thiol groups by different mechanisms lead to the formation of disulfide bonds in proteins [12]. Thiol/disulfide balance plays vital roles in detoxification, intracellular signal transmission, defense against oxidizing agents, regulation of enzyme activity, and programmed cell death [13,14].
Recently, a number of studies have stated that thiol/disulfide homeostasis has been impaired in schizophrenia, autistic spectrum disorders, as well as neurological diseases such as migraine, epilepsy, and internal diseases such as diabetic nephropathy, and thus may play a role in the pathogenesis of many disorders [15-17].
In order to make a true assessment of thiol / disulfide homeostasis, it is necessary to measure both thiol and total thiol levels. Thiol/disulfide homeostasis cannot be assessed by measuring thiol alone [18,19]. Free oxygen radicals formed in the body over time damage both intracellular and extracellular structures and disrupt genetic codes by making permanent changes in DNA [20]. 8-hydroxy-2-deoxyguanosine (8-OHdG) is a marker that is thought to cause degradation in mitochondria after oxidation [21]. Normally, the damaged DNA is repaired by a number of cellular mechanisms after oxidation reactions and 8-OHdG, a mitochondrial marker of oxidative damage, is removed from the body. Ultimately, endogenous 8-OHdG can be detected in both blood and urine and is evaluated as an indicator of DNA damage. Therefore, endogenous 8-OHdG has been stated as a biomarker of DNA damage in some diseases [22].
Some studies have suggested that the levels of markers of oxidative stress are increased and the oxidative balance is disturbed in patients with OCD [23,24]. Basal ganglia containing high levels of catecholamine may play a role in the etiopathogenesis of OCD. Moreover, it has been reported that free oxygen radicals highly affect basal ganglia [24]. Since catecholamine metabolism is the main source of free oxygen radicals in the brain, it has been reported that increased levels of catecholamine in the brain are associated with damage caused by free oxygen radicals [6].
A number of publications evaluating oxidative stress in patients with OCD exist in the literature [25]. However, to the best of our knowledge, this is the first study evaluating thiol/disulfide homeostasis and oxidative DNA damage in OCD patients. Further studies can be conducted to examine the levels of oxidative balance and markers of DNA damage in patients with OCD who receive a regular or irregular treatment and those who do not receive a treatment, or to examine the relationship between these markers and disease duration. In this context, our study will provide important information on the oxidant-antioxidant status and levels of oxidative DNA damage in patients with OCD.
METHODS
This study was conducted at Van Yuzuncu Yil University Medical Faculty, Department of Psychiatry between January and September 2020. The institutional ethics committee approved the study with reference number 07/18.12.2019. All participants were informed about the study and a written consent was obtained from all participants.
Thirty-five patients diagnosed with OCD by a psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders 5th edition diagnostic criteria at Van Yuzuncu Yil University Psychiatry Clinic were included in the study. Thirty-three volunteers consisted the control group.
The exclusion criteria were presence of metabolic, endocrine and neurological disorders, diabetes mellitus, pregnancy, obesity, smoking, alcohol and substance use disorders, other psychiatric pathologies, mental retarda-tions, psychosis, schizophrenia, mood disorders, and a previous history of antioxidant agent use. In addition, patients receiving electroconvulsive therapy were excluded.
Both groups were matched in terms of age, sex, smoking status, and other sociodemographic data. Bio-chemical tests and inflammatory markers were investi-gated in all participants, and those with out-of-range levels were not included in the study.
The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) was used to evaluate the severity of obsession and compulsion symptoms in patients. The Y-BOCS is a 10-item scale rated between 0−4, which is administered by a clinician [23].
Thiol/Disulfide Homeostasis
A colorimetric detection method was used to measure the total thiol/native thiol levels following the manufac-turer’s protocols (Rel Assay Diagnostic, Gaziantep, Turkey).
Detection of total thiol levels
Briefly, 10 μL of serum sample or standard and 10 μL of reagent 1 were mixed in a test tube. Following an incubation period for 100 seconds at room temperature, 110 μL of reagent 2 was added and incubated for 200 seconds at room temperature. The initial absorbance was detected at a wavelength of 415 nm. Then, 10 μL of reagent 3 was added to the mix and incubated for 300 seconds at room temperature. The final absorbance was detected at 415 nm. Total thiol levels were calculated based on the standard curve.
Detection of native thiol levels
Ten μL of serum sample or standard and 100 μL of reagent 1 were mixed in a test tube and incubated for 300 seconds at room temperature. Following the incubation period, the first absorbance was detected at a wavelength of 415 nm. Then, 10 μL of reagent 2 was added to the mix and incubated for another 300 seconds at room tempera-ture. Following the incubation, the final absorbance was determined at 415 nm. Native thiol levels were calculated based on the standard curve. Disulfide levels were calculated by subtracting the native thiol values from the total thiol values and dividing them by two. Disulfide/total thiol, disulfide/native thiol and native thiol/total thiol ratios were calculated and the results were given as percentages [27].
Detection of oxidative DNA damage
In order to detect the oxidative DNA damage, DNA was isolated from leukocytes and 8-OHdG and deoxyguano-sine (dG) levels were measured. Oxidative DNA damage was defined as 8-OHdG/106dG. Initially, DNA was isolated from whole blood samples taken from patients and healthy subjects by using commercial DNA isolation kits (Nucleo Spin Blood DNA, RNA and Protein Preparation, Macherey-Nagel, Germany). Isolated DNA samples were hydrolyzed with formic acid, as previously described by Kaur and Halliwell [28]. The concentrations of 8-OHdG and dG of the hydrolyzed DNA samples were determined using the previously described high pressure liquid chromatography (HPLC) method with electrochemical detector (ECD) and variable wavelength detector [29]. Prior to analysis by HPLC, hydrolyzed DNA samples were dissolved in HPLC eluent in a final volume of 1 μL. A total of 20 μL of final lysate was analyzed by HPLC-ECD. A reverse phase-C18 analytical column (250 mm 4.6 mm 4.0 mm; Phenomenex, Torrance, CA, USA) was used. The mobile phase contained 0.05 M potassium phosphate buffer, pH 5.5 and acetonitrile (97:3, v/v) and the flow rate was 1 μL/min. The dG concentration was monitored based on absorption at 245 nm, and the 8-OHdG level was determined based on the ECD reading (600 mV). The dG and 8-OHdG levels were measured using dG and 8-OHdG standards (Sigma Aldrich, St. Louis, MO, USA); The 8-OHdG level was described as 8-OHdG/106dG.
Statistical Analysis
The results were analyzed using IBM SPSS 20 package program. Shapiro-Wilks test was used to confirm normal distribution. Independent sample-t test and χ2 tests were used to compare the differences in both groups. Results were presented as mean ± standard deviation (SD). The rate of significance was determined as p < 0.05.
RESULTS
Thirty-five patients with OCD (mean age 27.6 ± 5.03 years) and thirty-three healthy subjects (mean age 27.7 ± 5.21 years) matching in terms of age, sex, and marital status participated in the study. None of the participants were smokers. The mean duration of disease in the patient group was 10.3 ± 8.71 years. According to the Y-BOCS scoring, three patients had mild, sixteen patients had moderate, twelve had severe, two had very severe OCD symptoms. Two patients were scored subclinical. The sociodemographic characteristics of the participants are summarized in Table 1.
Table 1.
Sociodemographic data of the participants
| Variable | OCD group (n = 35) | Healthy control group (n = 33) | 95 % CI | pvalue | |
|---|---|---|---|---|---|
|
| |||||
| Lower | Upper | ||||
| Sex | 0.808 | ||||
| Male | 16 (48.5) | 17 (51.5) | |||
| Female | 19 (54.3) | 16 (45.7) | |||
| Age (yr) | 27.6 ± 5.03 | 27.7 ± 5.21 | −2.49 | 2.47 | 0.995 |
| Education status | 0.875 | ||||
| Primary school | 4 (50) | 4 (50) | |||
| High school | 14 (48.3) | 15 (51.7) | |||
| University | 17 (54.8) | 14 (45.2) | |||
| Marital status | 0.246 | ||||
| Single | 10 (43.5) | 13 (56.5) | |||
| Married | 25 (55.6) | 20 (44.4) | |||
| BMI (kg/m2) | 23.7 ± 1.97 | 23.8 ± 2.27 | −1.16 | 0.88 | 0.788 |
Values are presented as number (%) or mean ± standard deviation.
OCD, obsessive compulsive disorder; CI, confidence interval; BMI, body mass index.
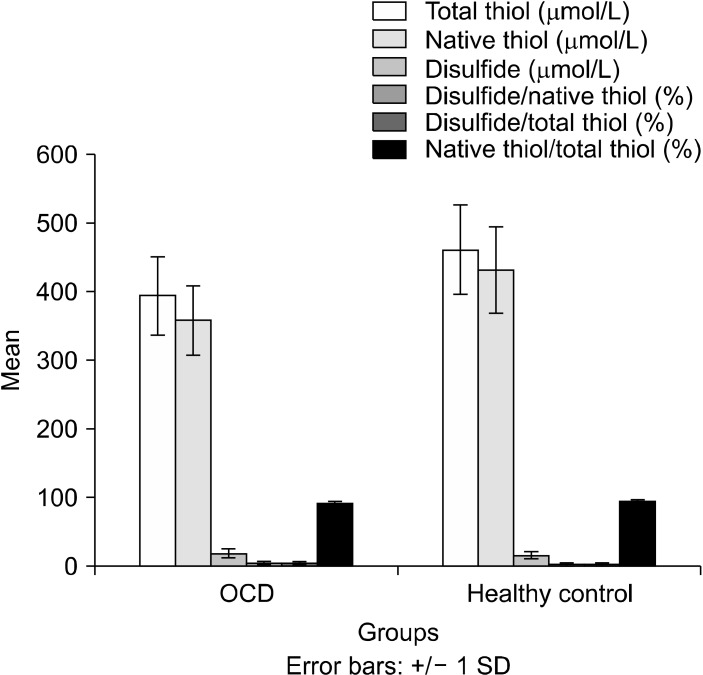
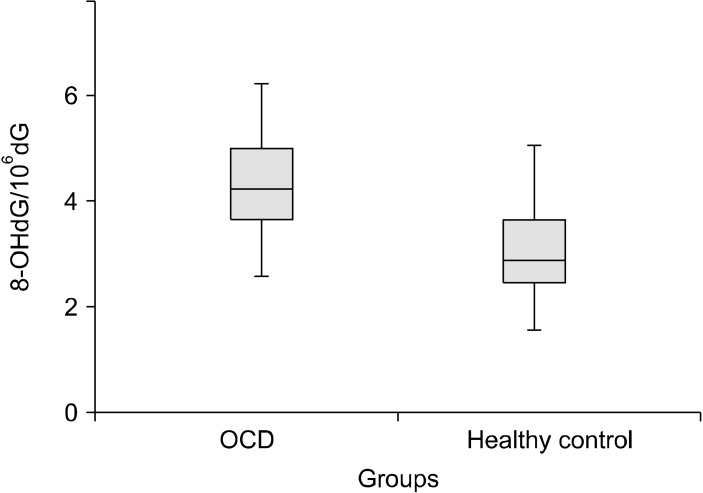
The total and native thiol levels were lower in patients with OCD than those in the control group (Fig. 1). On the contrary, disulfide levels were significantly higher in patients with OCD. The disulfide/total thiol and disulfide/native thiol levels were significantly higher in OCD patients. In addition, 8-OHdG/106dG levels, a marker of oxidative DNA damage, were significantly higher in OCD group than the control group (Fig. 2). All values are presented in Table 2.
Fig. 1.
Comparison of total thiol, native thiol, disulfide ratios of both groups. SD, standard deviation; OCD, obsessive compulsive disorder.
Fig. 2.
8-OHdG/106dG ratios of study and control group. OCD, obsessive compulsive disorder.
Table 2.
Total thiol, native thiol, disulfide, disulfide/native thiol, disulfide/total thiol, native thiol/total thiol and 8-OHdG/106dG ratios of the study and control groups
| Variable | OCD group (n = 35) | Healthy control group (n = 33) | 95 % CI | pvalue | |
|---|---|---|---|---|---|
|
| |||||
| Lower | Upper | ||||
| Total thiol (mmol/L) | 396.1 ± 58.8 | 464.6 ± 65.2 | −98.2 | −38.1 | 0.001 |
| Native thiol (mmol/L) | 358.9 ± 51.1 | 434.1 ± 60.4 | −72.1 | −16.1 | 0.003 |
| Disulfide (mmol/L) | 17.9 ± 6.87 | 14.1 ± 5.09 | 0.72 | 6.76 | 0.016 |
| Disulfide/native thiol (%) | 5.01 ± 1.84 | 3.28 ± 1.13 | 0.94 | 2.48 | 0.001 |
| Disulfide/total thiol (%) | 4.49 ± 1.52 | 3.06 ± 1.01 | 0.78 | 2.07 | 0.001 |
| Native thiol/total thiol (%) | 90.7 ± 3.44 | 93.8 ± 2.01 | −4.56 | −1.74 | 0.008 |
| 8-OHdG/106dG | 4.26 ± 1.11 | 2.74 ± 0.98 | 0.66 | 1.68 | 0.001 |
Values are presented as mean ± standard deviation.
OCD, obsessive compulsive disorder; CI, confidence interval.
DISCUSSION
Our study is the first study measuring the thiol disulfide balance in patients with OCD and healthy controls and evaluating them all together. We showed that total and native thiol levels were significantly low but disulfide levels were significantly high in patients with OCD compared to the control group. Another major outcome was that the disulfide/total thiol and disulfide/native thiol levels were significantly high in OCD patients but native thiol/total thiol levels were significantly high in control group. Apparently, thiol disulfide balance had shifted in favor of disulfide in patients with OCD.
The first target of reactive oxygen species (ROS) is the -SH group of the cysteine that is in structure of proteins. As these structures are attacked by ROS, -SH is oxidized to disulfide, and the function of the protein is lost. Thiol-containing compounds such as glutathione are important parts of the organism’s antioxidant defense system. In addition, they are important for apoptosis and stabilization of proteins. Determining the shift of thiol/disulfide balance can be useful for having knowledge about oxidative state of the organism. Ates et al. [30] have shown that thiol/disulfide homeostasis has been impaired in diabetic patients, and Altıparmak et al. [31] have demonstrated that this balance has been impaired in favor of disulfide in patients with coronary artery disease. In the oxidative balance study of Erzin et al. [27] which has been conducted in patients with bipolar disorder, it has been reported that levels of native thiol have decreased in manic attack group compared to the remission group. In another study of Erzin et al. [32], they have emphasized that thiol/disulfide balance has been impaired in favor of disulfide in patients with unipolar and bipolar depression. Unal et al. [33] have investigated the thiol/disulfide homeostasis in patients with schizophrenia and they have shown that native thiol and total thiol levels were significantly lower, but disulfide and disulfide/native thiol levels were significantly higher in schizophrenic patients. A number of studies have suggested that this balance has shifted towards disulfide in neurological disorders such as epilepsy and migraine [34,35]. Another study has suggested that this balance has changed in patients with anxiety disorder, another psychiatric disorder, and disulfide levels have increased compared to the control subjects [36].
In our study, levels of 8-OHdG were significantly high in the OCD group. Unlike previous studies, we did not detect 8-OHdG levels in serum, plasma or urine, but detected 8-OHdG levels in DNA in order to assess the DNA damage in patients with OCD, and to do this, we used a more sensitive method, that is HPLC method. Unless the base modifications caused by the attack of ROS are not corrected by DNA repair genes, they cause mutations in DNA, thus the function of proteins encoded by these genes are lost, which may lead to serious complications. A body of evidence has shown that the structure of lipids and proteins in cell membrane and DNA can be irreversibly damaged. Both nucleus and mitochondrial DNA are major indicators of 8-OHdG damage, which is constantly caused by oxygen radicals [20]. Post-mortem examinations have demonstrated that increased levels of 8-OHdG in cranial tissues samples and bodily fluids are associated with many degenerative diseases and carcinogenesis. Brain tissue, a major consumer of oxygen, is highly susceptible to oxidative damage [37]. In post-mortem studies, 8-OHdG has been determined as an indicator of oxidative DNA damage in psychiatric diseases such as schizophrenia, bipolar disorder, and major depression [37]. Post-mortem studies of schizophrenics have shown that serum levels of 8-OHdG have increased by 10-fold in the hippocampus [38]. High levels of serum 8-OHdG have also been reported in patients with major depression [39]. In addition, it has been reported that patients with recurrent depressive episodes have more oxidative DNA damage than patients with a single episode [40].
In our study, there was a negative correlation between oxidative DNA damage, total and native thiol, and levels of 8-OHdG/106dG had increased but levels of total and native thiol had decreased. The decline in levels of antioxidative parameters indicates that this may damage the DNA structure. A previous study has stated that there was no significant relationship between oxidative stress parameters, levels of oxidative stress and DNA damage in patients with schizophrenia [41]. In another study investigating the relationship between oxidative stress and DNA damage in patients with depression, it has been stated that oxidative DNA damage has increased in patients with depression [42]. A recent study has investigated the relationship between the levels of DNA damage and oxidative stress in child and adolescent victims of sexual abuse with or without post-traumatic stress order and no difference between the two groups has been reported [43]. Significantly high levels of oxidative stress and oxidative DNA damage in patients with OCD have led us to think that stress related DNA damage can accelerate aging. Pharmacological treatments, cognitive therapies, treatment of sleep problems, regular exercise or administration of natural antioxidants in the early stages of treatment of the OCD patients may help regulate the thiol/disulfide balance and prevent oxidative DNA damage.
Limitations of the study: Since our study was a cross- sectional study, it was difficult generalize the results. Although we tried to homogenize the groups, the number of patients in each group was low, and we were not able to divide the groups in sub-groups in terms of medication as there was a large spectrum of drugs used by the pa-tients. Furthermore, due to the low number of patients in the OCD group, the patients could not be classified according to the severity of the disease. In addition, we were unable to eliminate factors that may affect levels of oxidative stress such as family medical histories, diet and sleep patterns, exercise status, and working conditions of the participants.
To conclude, we showed that the thiol/disulfide balance has shifted in favor of disulfide in patients with OCD compared to healthy controls and that 8-OHdG levels, indicators of DNA damage, were higher in OCD patients than the control subjects. Inflammation has been reported in cortico-striatal circuitry in patients with OCD [44], and this may be associated with increased levels of oxidative stress. Oxidative stress is particularly and closely related to inflammatory pathways. Pro-inflammatory cytokines are produced in response to oxidative stress, and oxidative stress enhances the inflammatory response [45]. Regulation of oxidative stress balance in OCD patients would be helpful in treatment, and in reducing morbidity.
Based on these results, OCD patients who are treated regularly or irregularly and those who do not receive any treatment should be monitored closely and measurements should be performed. In addition, patients who have been diagnosed a long time ago and recently should be compared. The patients should be examined in terms of how disease duration, treatment status and levels of oxidative stress affect them.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Design the study and Writing−protocol: Faruk Kurhan. Data acquisition: Faruk Kurhan, Yavuz Selim Atan. Statistical analysis: Hamit Hakan Alp, Faruk Kurhan. Design of the protocol: Hamit Hakan Alp. Writing−original draft: Mesut Işık. All authors interpreted the results and helped revise the manuscript.
References
- 1.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Pub; Arlington: 2013. [DOI] [Google Scholar]
- 2.International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC); OCD Collaborative Genetics Association Studies (OCGAS), author Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23:1181–1188. doi: 10.1038/mp.2017.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boedhoe PS, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta- and mega-analysis. Am J Psychiatry. 2017;174:60–69. doi: 10.1176/appi.ajp.2016.16020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brander G, Pérez-Vigil A, Larsson H, Mataix-Cols D. Systematic review of environmental risk factors for Obsessive-Compulsive Disorder: a proposed roadmap from association to causation. Neurosci Biobehav Rev. 2016;65:36–62. doi: 10.1016/j.neubiorev.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Bokor G, Anderson PD. Obsessive-compulsive disorder. J Pharm Pract. 2014;27:116–130. doi: 10.1177/0897190014521996. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty S, Singh OP, Dasgupta A, Mandal N, Nath Das H. Correlation between lipid peroxidation-induced TBARS level and disease severity in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:363–366. doi: 10.1016/j.pnpbp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Altan N, Dinçel A, Koca C. Diabetes mellitus and oxidative stress. Turk J Biochem. 2006;31:51–56. [Google Scholar]
- 8.Berg D, Youdim MB, Riederer P. Redox imbalance. Cell Tissue Res. 2004;318:201–213. doi: 10.1007/s00441-004-0976-5. [DOI] [PubMed] [Google Scholar]
- 9.Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepi-topes. Acta Psychiatr Scand. 2013;127:344–354. doi: 10.1111/j.1600-0447.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- 10.Ciobica A, Padurariu M, Dobrin I, Stefanescu C, Dobrin R. Oxidative stress in schizophrenia- focusing on the main markers. Psychiatr Danub. 2011;23:237–245. [PubMed] [Google Scholar]
- 11.Pavlović D, Tamburić V, Stojanović I, Kocić G, Jevtović T, Đorđević V. Oxidative stress as marker of positive symptoms in schizophrenia. Facta Univ. 2002;9:157–161. [Google Scholar]
- 12.Kayalı R, Çakatay U. Protein oksidasyonunun ana mekanizmaları. Cerrahpaş. a J Med. 2004;35:83–89. doi: 10.1016/j.physb.2004.05.015. [DOI] [Google Scholar]
- 13.Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Circu μL, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milenkovic D, Jude B, Morand C. miRNA as molecular target of polyphenols underlying their biological effects. Free Radic Biol Med. 2013;64:40–51. doi: 10.1016/j.freeradbiomed.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Topcuoglu C, Bakirhan A, Yilmaz FM, Neselioglu S, Erel O, Sahiner SY. Thiol/disulfide homeostasis in untreated schizophrenia patients. Psychiatry Res. 2017;251:212–216. doi: 10.1016/j.psychres.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Eren MA, Koyuncu İ, İncebıyık H, Karakaş H, Erel Ö, Sabuncu T. The evaluation of thiol/disulphide homeostasis in diabetic nephropathy. Diabetes Res Clin Pract. 2019;148:249–253. doi: 10.1016/j.diabres.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Erel O, Neselioglu S. A novel and automated assay for thiol/disulphide homeostasis. Clin Biochem. 2014;47:326–332. doi: 10.1016/j.clinbiochem.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Vural G, Gumusyayla S, Bektas H, Deniz O, Alisik M, Erel O. Impairment of dynamic thiol-disulphide homeostasis in patients with idiopathic Parkinson's disease and its relationship with clinical stage of disease. Clin Neurol Neurosurg. 2017;153:50–55. doi: 10.1016/j.clineuro.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Long JD, Matson WR, Juhl AR, Leavitt BR Paulsen JS; PREDICT-HD Investigators and Coordinators of the Huntington Study Group, author. 8OHdG as a marker for Huntington disease progression. Neurobiol Dis. 2012;46:625–634. doi: 10.1016/j.nbd.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokuş B, Çakir DÜ. Biomarker of invivo oxidative DNA damage; 8-Hydroxy-2'-deoxyguanosine. Turkiye Klinikleri J Med Sci. 2002;22:535–543. [Google Scholar]
- 23.Kuloglu M, Atmaca M, Tezcan E, Gecici O, Tunckol H, Ustundag B. Antioxidant enzyme activities and malondialdehyde levels in patients with obsessive-compulsive disorder. Neuropsychobiology. 2002;46:27–32. doi: 10.1159/000063573. [DOI] [PubMed] [Google Scholar]
- 24.Ersan S, Bakir S, Erdal Ersan E, Dogan O. Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive-compulsive disorder. Prog Neuro-psychopharmacol Biol Psychiatry. 2006;30:1039–1042. doi: 10.1016/j.pnpbp.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Alici D, Bulbul F, Virit O, Unal A, Altindag A, Alpak G, et al. Evaluation of oxidative metabolism and oxidative DNA damage in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 2016;70:109–115. doi: 10.1111/pcn.12362. [DOI] [PubMed] [Google Scholar]
- 26.Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 27.Erzin G, Kotan VO, Topçuoğlu C, Özkaya G, Erel Ö, Yüksel RN, et al. Thiol/disulphide homeostasis in bipolar disorder. Psychiatry Res. 2018;261:237–242. doi: 10.1016/j.psychres.2017.12.062. [DOI] [PubMed] [Google Scholar]
- 28.Kaur H, Halliwell B. Measurement of oxidized and methylated DNA bases by HPLC with electrochemical detection. Biochem J. 1996;318(Pt 1):21–23. doi: 10.1042/bj3180021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigenaga MK, Aboujaoude EN, Chen Q, Ames BN. Assays of oxidative DNA damage biomarkers 8-oxo-2'-deoxyguanosine and 8-oxoguanine in nuclear DNA and biological fluids by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1994;234:16–33. doi: 10.1016/0076-6879(94)34073-0. [DOI] [PubMed] [Google Scholar]
- 30.Ates I, Kaplan M, Yuksel M, Mese D, Alisik M, Erel Ö, et al. Determination of thiol/disulphide homeostasis in type 1 diabetes mellitus and the factors associated with thiol oxidation. Endocrine. 2016;51:47–51. doi: 10.1007/s12020-015-0784-6. [DOI] [PubMed] [Google Scholar]
- 31.Altıparmak IH, Erkuş ME, Sezen H, Demirbag R, Gunebakmaz O, Kaya Z, et al. The relation of serum thiol levels and thiol/disulphide homeostasis with the severity of coronary artery disease. Kardiol Pol. 2016;74:1346–1353. doi: 10.5603/KP.a2016.0085. [DOI] [PubMed] [Google Scholar]
- 32.Erzin G, Özkaya G, Topçuoğlu C, Yüksel RN, Erel Ö, Yurt EF, et al. Thiol/disulfide homeostasis in bipolar and unipolar depression. Clin Psychopharmacol Neurosci. 2020;18:395–401. doi: 10.9758/cpn.2020.18.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ünal K, Erzin G, Yüksel RN, Alisik M, Erel Ö. Thiol/disulphide homeostasis in schizophrenia patients with positive symptoms. Nord J Psychiatry. 2018;72:281–284. doi: 10.1080/08039488.2018.1441906. [DOI] [PubMed] [Google Scholar]
- 34.Kösem A, Yücel Ç, Titiz AP, Sezer S, Neşelioğlu S, Erel Ö, et al. Evaluation of serum thiol-disulphide homeostasis parameters as oxidative stress markers in epilepsy patients. Acta Neurol Belg. 2020 doi: 10.1007/s13760-020-01410-6. doi: 10.1007/s13760-020-01410-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Alagöz AN, Tasdemir SS, Yazar H, Aras YG, Can NU. Thiol disulfide homeostasis as oxidative stress marker in migraine patients. J Exp Clin Med. 2019;36:1–8. [Google Scholar]
- 36.Asoglu M, Kiliçaslan F, Beginoğlu O, Fedai U, Akıl Ö, Çelik H, et al. Thiol/disulphide homeostasis as a new oxidative stress marker in untreated patients with generalized anxiety disorder. Anadolu Psikiyatri Derg. 2018;19:143–149. [Google Scholar]
- 37.Che Y, Wang JF, Shao L, Young T. Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci. 2010;35:296–302. doi: 10.1503/jpn.090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12:167–175. doi: 10.1097/00019442-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2'-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 40.Ceprnja M, Derek L, Unić A, Blazev M, Fistonić M, Kozarić-Kovacić D, et al. Oxidative stress markers in patients with post-traumatic stress disorder. Coll Antropol. 2011;35:1155–1160. [PubMed] [Google Scholar]
- 41.Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology. 2016;73:92–97. doi: 10.1159/000444488. [DOI] [PubMed] [Google Scholar]
- 42.Ahmadimanesh M, Abbaszadegan MR, Morshedi Rad D, Moallem SA, Mohammadpour AH, Ghahremani MH, et al. Effects of selective serotonin reuptake inhibitors on DNA damage in patients with depression. J Psychopharmacol. 2019;33:1364–1376. doi: 10.1177/0269881119874461. [DOI] [PubMed] [Google Scholar]
- 43.Şimşek Ş, Yüksel T, Kaplan İ, Uysal C, Aktaş H. The levels of cortisol and oxidative stress and DNA damage in child and adolescent victims of sexual abuse with or without post-traumatic stress disorder. Psychiatry Investig. 2016;13:616–621. doi: 10.4306/pi.2016.13.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Privitera AP, Distefano R, Wefer HA, Ferro A, Pulvirenti A, Giugno R. OCDB: a database collecting genes, miRNAs and drugs for obsessive-compulsive disorder. Database (Oxford) 2015;2015:bav069. doi: 10.1093/database/bav069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]