Abstract
Depression is one of the most important causes of disability and loss of useful life of people around the world. Acute respiratory infection caused a large number of severe illnesses and deaths of the world and most of these due to viral infections, which is estimated more than 80% of respiratory infections. Detection of viruses by immune pathogen recognition receptors activates the intracellular signaling cascade and eventually cause produces interferons. Inflammatory process begins with secretion of interferons and the expression of interferon-stimulated genes. One of the most important of these genes is indoleamine-pyrrole 2,3-dioxygenase (IDO), which plays a major role in tryptophan catabolism. IDO is an intracellular monomeric enzyme that is also responsible for breaking down and consuming tryptophan in the Kynurenine pathway. Increased inflammation has been linked to decrease tryptophan concentrations and increase kynurenine levels. We tried to explain the role of inflammation by viral respiratory infections in causing depression.
Keywords: Depression; Viral infection; Inflammation; Indoleamine-pyrrole 2,3-dioxygenase; Kynurenine
INTRODUCTION
Depression is one of the most important causes of disability and loss of useful life of people around the world [1] and it is predicted that mental disorders in 2030 will be the second factor that human suffer from [2] and it is estimated about 20.9% of American adults suffer from mental disorders [3], and risk of suicide is higher in people with a history of mental disorders [4,5]. Activation of inflammatory pathways of the immune system is known to be associated with depression, and it is possible that several factors such as physiological and external stressors or drug side effects are involved in this phenomenon [1]. The suicide rate in spring is high. This could coincide with the outbreak of viral infections of the respiratory system and the activation of related immune pathways during this time of year [5,6].
Recently, Dantzer and colleagues [7] developed the cytokine theory for depression, which suggests that proinflammatory cytokines may be an important factor in behavioral disorders, neuroendocrine, neurochemical, and depressive disorders [8]. Depressed patients have changes in the immune system, such as abnormal levels of cytokines in plasma and cerebrospinal fluid [8,9], so that treatment with proinflammatory cytokines in cancer patients or those with hepatitis C virus has induced depressive disorder. On the other hand, preventing proinflammatory cytokines or signaling pathways related to these cytokines improves mood and increases the response to normal treatment in depressed people. Sometimes depression seems to be an inflammatory disorder [8].
It is important to note that evidence of a link between respiratory infections and mental disorders dates back to the 19th century, when 18 previous cases of influenza with manic disorders and depression were admitted to Bethlehem Hospital in London, 1958 Harrison also describes 36 depressed people with a previous flu infection in Kent, England. Influenza virus has also been associated with symptoms of dementia in people without a history of mental illness [5]. In trying to understand the mechanism by which respiratory viruses such as influenza and corona can associate with mood disorders, two pathophysiological mechanisms can be considered (a) Viruses directly affect the brain and (b) the immune response to viruses affects the brain. In addition, it can be speculated that viruses or immune responses to viruses may make some people vulnerable to depression or suicide [5]. Detection of viruses by immune pathogen recognition receptors activates the intracellular signaling cascades and eventually produces interferon-producing transcription factors (IRF), These factors are transferred to the nucleus after activation and regulate antiviral and inflammatory processes [10].
Acute respiratory illnesses caused a large number of severe infections and deaths of the world, most of these acute respiratory infections are caused by viral infections, which is estimated more than 80% of respiratory infec-tions. Acute viral infections of the respiratory system caused by these viruses lead to the hospitalization of infants, children and adults in developed countries and they are also known as important cause of death in developing countries. Among different viruses, influenza, respiratory syncytial virus (RSV), coronavirus, adenovirus and rhinovirus play a more prominent role [11,12]. In recent years, several new respiratory viruses including influenza viruses H5N1, H7N7, H7N3, human metapneumovirus, SARA coronavirus, human coronavirus NL63 and HKU1, poliovirus WU/KI, parvovirus 4 and 5, Mimi virus, SARS coronavirus 2 have emerged and identified as causative agents of lower and upper respiratory tract infections [11]. Influenza viruses A, B, COVID-19 and RSV have a special place among viruses that cause respiratory infections with mortal and economic consequences. According to the first Atlas of Respiratory Infections, acute respiratory infec-tions are the cause of 4 million annual deaths in the world. Acute respiratory infections are the third leading cause of death in low- and middle-income countries. According to this atlas, respiratory infections cause at least 6% of disability and death, especially in poor countries [13].
In this review study, we tried to explain the role of inflammation by viral respiratory infections in causing depression and the indoleamine-pyrrole 2,3-dioxygenase (IDO) enzyme as a link between the immune and nervous systems.
VIRAL RESPIRATORY INFECTION
Respiratory tract virus infections that lead to rhinitis, pharyngitis, and laryngitis are more common among children and they are also occur several times during the year. In infants and young children, the prevalence of these viruses will be decreased with age. The main causes of respiratory disease in children and adults are influenza virus type A, B, C, parainfluenza viruses type 1, 2 and 3, RSV, adenovirus and the recent pandemic COVID-19. Other respiratory viruses, such as adenovirus, have a lower mortality rate and a very high incidence, which causes very high economic losses.
Influenza is a febrile illness with cough, upper respiratory tract symptoms such as sore throat, runny and stuffy nose, and systemic symptoms such as headache, fatigue, and body aches that lead to hospitalization for a number of people of all ages. Due to the high mutations in viral glycoproteins, especially hemagglutinin (HA) and neuraminidase (NA), and despite the presence of vaccines and antiviral drugs, influenza pandemics cause 3 to 5 million severe infections and 250,000−500,000 deaths in developed countries and 300,000 hospitalizations and 35,000 deaths in the United State. Pneumonia due to influenza has a high mortality rate during epidemics, not only in immunocompromised individuals and patients with underlying disease, but also in young and healthy adults, which is usually takes 3 to 8 weeks. The cost of colds in the United States is estimated at 87 billion annually. On June 11, 2009, the World Health Organization announced phase 6 for the outbreak of the flu virus, and in July, 332 deaths were confirmed out of 77,000 infected in the world. The mortality rate for the new influenza virus is estimated at 0.4 to 0.7 percent. On August 11, 2010, the World Health Organization announced the end of the epidemic and the entrance into the post-epidemic period [11,14]. RSV-induced pneumonia causes approximately 1.4 to 1.8 million deaths in children under 5 years among the most common respiratory infections. Respiratory Syncytial virus is one of the leading causes of death among children under 1 year of age, with 20% annual hospitalization, 18% emergency department visits and 15% office visits attributed to this virus [15]. RNA of coronaviruses has been isolated from the brains of a number of MS patients [16]. In late December, 2019, a novel coronavirus was first reported in Wuhan, named as SARS-CoV-2 caused a series of acute atypical respiratory diseases. It is found that 41 patients were hospitalized with symptoms of fever, shortness of breath and cough were identified by laboratory tests as SARS-CoV-2 and approximately two-third of them were associated with the famous Huanan seafood market which is a major market for seafood and live animals [17]. By the end of 2020, virus has infected > 80 million people and more than 1,700,000 deaths that is estimated. Studies have shown that SARS coronavirus 2 has the ability to infect the central nervous system and can cause immunopathological damage to the brain. The neurological symptoms observed in covid positive patients confirm the neurotropic potential of this virus. Cytokine storms usually occur in patients who have longer and more severe symptoms after COVID, which is actually due to the immune system’s inflammatory response to the infec-tion. Recent data confirm that corona virus infection can lead to acute necrotic encephalopathy, which can be caused by a cytokine storm [18].
INFLAMMATION IN VIRAL RESPIRATORY INFECTION
Studies on different viral infections with different virions, receptors, and proliferative mechanisms in the respiratory system have shown similar clinical complications and pathology, and it appears that in respiratory infections the host defense mechanisms and immune response are the main cause of lung injury and clinical signs. Death of infected cells by host defense mechanisms causes damage to lung tissue. There are molecular structures on all cells in the body to identify pathogens called pathogen detection receptors. These structures actually identify molecular structures associated with pathogens which are different from host cell structures. Pattern recognition receptors for viruses include toll-like receptors, NOD-like receptors, retinoic acid-inducible gene-I, melanoma differentiation-associated gene 5. These diagnostic patterns are present in many cells especially in respiratory epithelial cells. Activation of these patterns following a viral infection activates the immune system and ultimately activates the innate and adaptive immune system. The intensity of the activity of pattern recognition receptors in the respiratory system following infection determines the activity of the immune system [19,20].
Many cellular sensors induce interferon production after virus detection. Interferons are the first antiviral response that play a key role in viral infections and induce antiviral activity in infected and adjacent cells. Interferons also appear to play a role in regulating innate and adaptive immune responses, cell growth and survival. Interferons are divided into several types based on binding receptors. Interferon type 1, which includes two groups of interferon (INF)-alpha and beta, and disrupt the processes of virus’s replication and propagation. Interferon type 2 or interferon gamma mediates Th1-related immune responses by natural killer cells, CD4 and CD8 T cells, and interferon type 3 or lambda. The secretion of interferons causes the expression of thousands of genes, the expressed genes following the secretion of interferon are called interferon-stimulated genes (ISGs) [20,21].
Intrinsic immune system antiviral responses begin primarily with type 1 interferon (interferon alpha and beta) and type 3 (lambda one interferon, lambda two, and lambda three interferons), after that type 2 interferon stimulates macrophage and differentiates Th1 cells [10].
Interferon type 1 binds to its specific receptor, IFNAR. interferon-inducing genes, or ISGs are expressed after binding interferon to the receptor and by activating the janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. The expressed genes prevent replication and propagation of viruses to uninfected cells primarily. They also activate immune cells either directly or by stimulating the production of various chemokines and cytokines [19,22]. Interferon 1 activates lymphocytes, which stimulate the production of interferon type 2 or interferon-gamma and in turn activates phagocytosis in macrophages and increases antigen presentation by dendritic cells [23]. Interferon type 1 increases the killing power of T and NK cells. Interferons stimulate the responses of the humoral immune system indirectly by stimulating T and dendritic (DC) cells, and also stimulate the production of antibodies by B cells directly [19,24]. Respiratory epithelial cells in addition to interferon produce large amounts of cytokines and chemokines, including interleukin-6, tumor necrosis factor (TNF)-alpha, granulocyte colony‐stimulating factor and granulocyte‐macrophage colony‐stimulating factor. Interleukin 6 and alpha necrosis factor are proinflammatory factors that regulate the activity of a number of immune cells. Interleukin 6 replaces innate immune responses with adaptive immune responses by decreasing neutrophil activity and increasing T cell proliferation, differentiation, and activity. High levels of interleukin-6 are associated with higher disease severity, while lower levels of interleukin cause uncontrolled viral infection and ultimately higher mortality. TNF-a disrupts virus replication by stimulating cytokine production and endothelial cell activity. Elevated TNF- levels have been associated with increasing symptoms in acute viral infections, while decreased levels have been associated with decreasing immune-induced pathology [19,25,26].
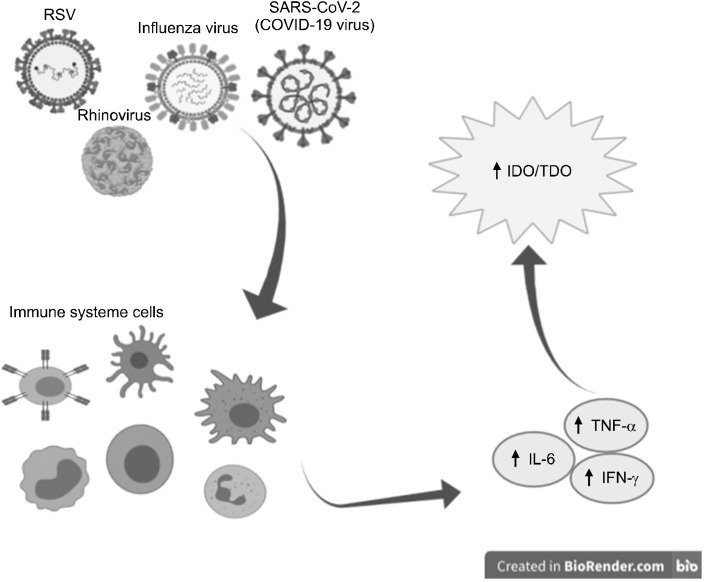
In general, inflammation is the main mechanism of the immune system’s fight against viral infections. The inflammatory process begins with the secretion of interferon and the expression of ISG genes. One of the most important of these genes is IDO, which plays a major role in tryptophan catabolism (Fig. 1).
Fig. 1.
IDO production in inflammatory virus response. IDO, indoleamine-pyrrole 2,3-dioxygenase; TDO, tryptophan 2,3‐dioxygenase; TNF, -6, tumor necrosis factor; IL, interleukin; INF, interferon; RSV, respiratory syncytial virus.
ROLE OF IDO IN TRYPTOPHAN CATABOLISM AND KYNURENINE PATHWAY
The Kynurenine pathway is the primary and rate limiting pathway that catalysis tryptophan, which produces metabolites such as Kynurenine, Kynurenic acid, 3-hydroxy Kynurenic acid, 3-hydroxy Anthranilic acid, Picolinic acid, and Quinolinic acid from tryptophan. The entry of tryptophan into the Kynurenine pathway can affect reactions related to cell growth and other important func-tions. An important enzyme that determines the pathway of Kynurenine is the enzyme indole amine 2 and 3 dioxygenase or IDO. IDO is an intracellular monomeric enzyme that is also responsible for breaking down and consuming tryptophan in the Kynurenine pathway [27].
IDO is a cytosolic enzyme that regulates the amount of tryptophan degradation in the Kynurenine pathway. There are two types of IDO enzymes, IDO1 and IDO2, both of them induce the conversion of tryptophan to Kynurenine, although the activity of these two enzymes is different. Another enzyme that catalyzes tryptophan is the enzyme tryptophan 2 and 3 dioxygenase or tryptophan 2,3‐dioxygenase, which has a different distribution compare to IDO and it is present in bacteria (Table 1) [27-31].
Table 1.
Feature of IDO1, IDO2, and TDO
| Main features | IDO1 | IDO2 | TDO |
|---|---|---|---|
| Distribution | Ubiquitously distributed throughout the body, including brain, lung, intestine, spleen, stomach, placenta, pancreas, cortex, medulla, adrenal ,urinary bladder, prostate [30] | Liver, testis, and thyroid, myeloid and plasmacytoid dendritic cells [30] | Liver and brain [30] |
| Enzyme inducer | Interferon-γ (IFN-γ), lipopolysaccharide (LPS) and tumor necrosis factor (TNF), interleukin (IL)-β, IL-6 [30] | Liver, kidney and epididymis in mice Dendritic cells (DCs) and monocytes [31] |
Tryptophan, glucocorticoids, corticosterone, catecholamines [30] |
| Enzyme inhibitor | 1-methyl-l-tryptophan, Epacadostat (INCB24360), Navoximod (GDC-0919) and D-1-methyl-tryptophan (D-1MT), α-cyclohexyl-5H-imidazo (NLG-919) and Linrodostat (BMS-986205) [30] |
D-1MT appears to be a more effective inhibitor of IDO2 than 1-Methyl-L-tryptophan (L-1-MT), 1-alkyl-tryptophan, tenatoprazole [30] | 6-Fluoro-3-[(1E)-2-(3-pyridinyl) ethanoyl)-1H-indole (680C91) Tryptamine, 5-hydroxytryptophan (5-HTP) and melatonin, 2-(3,4- Dihydroxyphenyl)-3,6,7-trihydroxy-2,3-dihydro-4H-chromen-4-one(NSC36398) [30] |
IDO, indoleamine-pyrrole 2,3-dioxygenase; TDO, tryptophan 2,3dioxygenase.
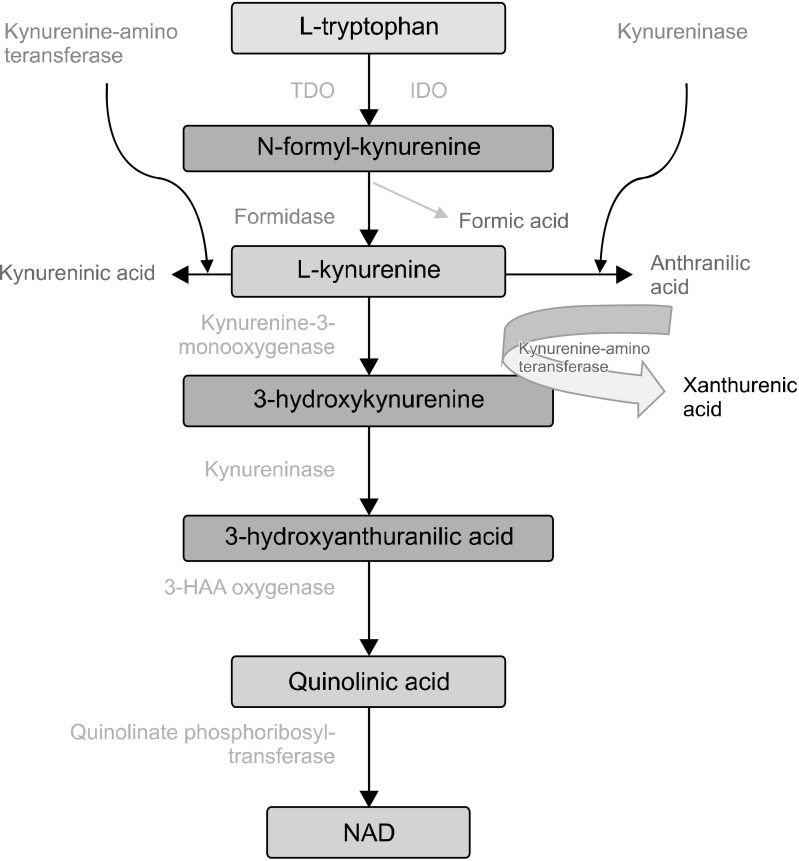
IDO catalyzes the oxidative catabolism phase of compounds containing the indole ring, such as tryptophan and 5-hydroxytryptophan (5-HT). In cells that express IDO, tryptophan is catalyzed in the Kynurenine pathway, and downstream IDO enzymes further break down Kynurenine and produce metabolites such as Kynurenic acid, 3-hydroxyanthranilic acid, niacin, and quinolinic acid (Fig. 2) [32].
Fig. 2.
Kynurenine pathway. IDO, indoleamine-pyrrole 2,3-dioxygenase; TDO, tryptophan 2,3‐dioxygenase; 3-HAA, 3-hydroxyanthranilic acid; NAD, nicotine adenine dinucleotide.
In the human body, IDO is expressed in a variety of cells, such as endothelial, Antigen-presenting, fibroblasts, macrophages, and DCs.
IDO pathway activity has been observed in many pathological conditions such as infection, obesity, transplant rejection, autoimmunity and atherosclerosis. After inflammation by INF-g and TNF-a, these cells express the IDO enzyme. Cerebral blood vessel endothelial cells degrade tryptophan to Kynurenine using IDO. This reaction is necessary for the function of these cells. In dendritic cells, interferon-alpha, beta, gamma and tumor necrosis factor-alpha activate the JAK/STAT and IRF3 pathways, all of which induce IDO activity.
IDO2 is expressed in a small number of cells, such as liver and kidney tissue and in some immune cells, such as antigen presentation and dendritic cells. This enzyme (IDO2) rearranges peripheral blood mononuclear cells in inflammation and appears to be involved in the inflammatory process [27].
TRYPTOPHAN AND SEROTONIN METABOLISM
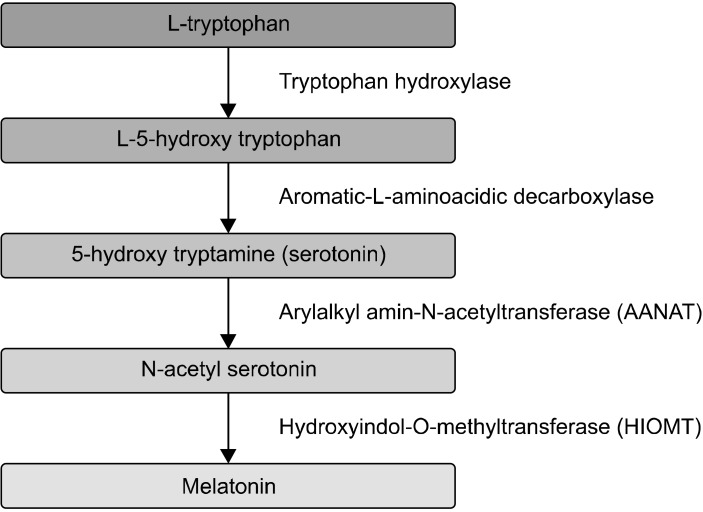
Tryptophan is one of the essential amino acids needed for cell survival, protein synthesis, and also as a precursor to the functional molecules serotonin, niacin, and melatonin in the brain [33]. Tryptophan organizes two different metabolic pathways, one for methoxide indole or 5-HT and the other for tryptophan catabolism. The main products of the methoxide indole pathway are serotonin and melatonin (Fig. 3). Tryptophan is converted to 5-HT by the enzyme tryptophan hydroxylase. Tryptophan catabolism is actually the breakdown and conversion to Kynurenine, and tryptophan catabolism has biological effects on both the central nervous system and peripheral organs [1]. Tryptophan is consumed either in the Kynurenine pathway or acts as a coenzyme for nicotine adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) [34]. Approximately 90% of tryptophan enters the Kynurenine pathway and converts to metabolites of this pathway and only one percent of tryptophan enters the serotonin synthesis pathway [35].
Fig. 3.
Serotonin and melatonin metabolism.
ROLE OF SEROTONIN IN DEPRESSION
The prevalence of depression is increasing and it seems to be one cause of disability over the world. More than 300 million people in different age groups suffer from depression [36].
There is evidence that tryptophan metabolism plays a role in the pathophysiology of depression. Activation of the kynurenine synthesis pathway and its related neurological metabolites has been associated with some psychiatric disorders such as depression, schizophrenia, and bipolar disorder. The two enzymes indole amine 2 and 3 dioxygenase and tryptophan 2 and 3 dioxygenases are involved in the first step and rate limitation of the n-formyl kynurenine catalysis from L-tryptophan [30].
Serotonin is one of the most important neurotransmitters that affects mental health. Most serotonin is distributed outside the central nervous system and affects a wide range of physiological processes in many organs. However, only 2% of CNS serotonin plays a major role in causing many mental disorders [37].
Serotonin is now considered as one of the most diffuse, effective, and most neurotransmitters. Currently, there is so much information about serotonin that it is possible to examine various aspects of its distribution, physiology, receptor subtypes, how it functions, and many other activi-ties. It seems that all psychiatric disorders linked with serotonin disorders. Drugs used to treat many psychological disorders affect the production and function of serotonin [38].
Many researchers believe that an imbalance in serotonin levels may affect individual mood lead to depression as a result. Possible problems include low production of serotonin in brain cells, lack of capable receptor sites of receiving serotonin, inability of serotonin to reach receptor sites, or lack of tryptophan (the chemical from which serotonin is made). Researchers believe that each one of these biochemical defects can lead to depression, obsessive-compulsive disorder, anxiety, panic and even excessive anger [39].
EPIGENETICS FINDING
Depression is associated with many pathological processes and abnormalities in the brain and immune system. Studies have shown that Epigenetic and inflammatory mechanisms play key role in depression among animals.
It sounds that Inflammation is involved in the brain dysfunction in depressed people. Various studies have shown a direct relationship between elevated levels of inflammatory cytokines such as interleukin-6 and interferon-gamma and the development of depressive symptoms [40,41].
Methylation in CPG regions of the body of the IDO gene has been associated with mRNA expression of this gene. Studies also show a direct relationship between the mRNA expression level of genes involved in the inflammatory pathway (IFNG, STAT1, STAT2, JAK2, and IRF9) with methylation in the body of IDO gene and mRNA expression of this gene [42].
IDO gene expression (human chromosome 8p22) is stimulated by interferon. Interferon-responsive sites and gamma activation sequences have been identified in the IDO gene promoter [40].
There are few studies that have examined the epigenetic processes affecting inflammation and expression of IDO and how these pathways affect the symptoms of mental disorders, so the epigenetic interpretation of these pathways needs further investigation.
THE LINK BETWEEN INFLAMMATORY RESPONSE IN VIRAL RESPIRATORY INFECTION AND DEPRESSION
As mentioned in previous paragraphs, inflammation occurs in most viral infections due to the secretion of interferon and the other inflammatory factors, including tumor necrosis factor and interleukin-6. Today, many studies have shown that inflammation in the body or central nervous system combined with psychological stressors can lead to mood disorders (Table 2) [43-53]. Activity of the immune system, especially the cytokines IL-1b, IL-6, and TNF-a, is increased in some patients with major depressive disorders, and it appears that people with chronic inflammation are more likely to develop depressive symptoms [18].
Table 2.
Inflammation and depression
| Row | Brief summary of papers related linkage between viral infections with inflammation and depression |
|---|---|
| 1 | Markers of influenza A, B, and C infections were higher in people with mood disorders than in controls [43] |
| 2 | After one month of recovery in patients with COVID-19 more severe inflammation and symptoms of behavioral disorders were possible [44] |
| 3 | Incidence of depressive symptoms and behavioral disorders in patients with COVID-19 infection was higher than the control group [45] |
| 4 | Acute respiratory distress syndrome related influenza A (H1N1) in patients can cause anxiety and depression [46] |
| 5 | It seems the rate of depression in the human H7N9 bird flu infected patients is 36.40% after infection [47] |
| 6 | The risk of depression in patients with a previous flu infection was higher than in patients without a history of the flu so that it moderately increase [48] |
| 7 | Significant increase in plasma interleukin-6 levels was observed in people with depressive symptoms compared to those without depression [49] |
| 8 | Depressive symptoms were dramatically higher in flu infected patients who had symptomatic infection in the last six months compared to the asymptomatic group [50] |
| 9 | One year after recovery, symptoms such as fatigue and mental disorder are observed in patients who recovered from the MERS infection [40] |
| 10 | Symptoms such as pain, fatigue and depression have seen up to 6 months after recovery in COVID-19 patients [51] |
| 11 | Depression is more common among women compared to men after COVID-19 infection [52] |
| 12 | It seems that COVID-positive patients with more severe disease are more exposed to symptoms such as depression and anxiety [53] |
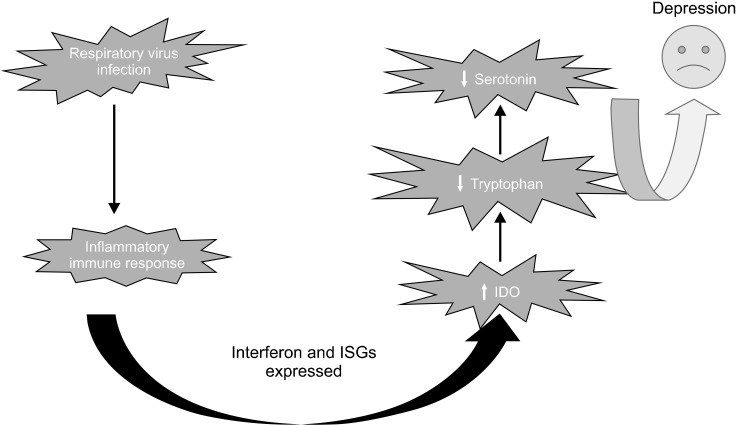
Increased inflammation has been linked to decrease tryptophan concentrations and increase kynurenine levels. Increased tryptophan catabolism following inflammation is caused by the secretion of the IDO enzyme. The decrease of tryptophan levels can cause some depressive symptoms, including decreased motivation, anorexia, pessimism, and fatigue, which induced by increasing catabolism of this amino acid. Over the past decade, proinflammatory cytokines have been repeatedly reported associated with mood and cognitive disorders (Fig. 4) [54].
Fig. 4.
Viral respiratory infection and depression. IDO, indoleamine-pyrrole 2,3-dioxy--genase; ISGs, interferon-stimulated genes.
Much laboratory evidence supports the effect of proinflammatory cytokines on the function of neurotransmitters and endocrine glands, which can cause symptoms called disease. Clinically, it seems that receiving cytokine treatments in patients can cause severe depression and psychological symptoms in more than 45% of patients. Immune cascades (pathways that activate the immune system) disrupt the metabolism of neurotransmitters such as serotonin, norepinephrine, and dopamine, which regulate behavioral and cognitive processes. These neurotransmitters are made from the amino acid precursors tryptophan and tyrosine in the brain. After activation of the immune system, changes in tryptophan metabolism occurs by activation of the pathway of the IDO enzyme.
The secretion of inflammatory factors (interferon-gamma) by the immune system leads to the activity of dendritic cells and macrophage monocytes, which increase the production and activity of IDO and ultimately induce more tryptophan catabolism [54,55].
Depression is associated with disorders of the serotonin system and overstimulation of the hypothalamus, pituitary and adrenal glands. Proinflammatory cytokines such as interleukins 1, 2, and 6, interferon-gamma, and alpha-necrosis factor through IDO appear to induce decreased 5-HT production. IDO is an enzyme that induces kynurenine production from the amino acid tryptophan, which can lead to a decrease in tryptophan for 5-HT synthesis. Myint and Kim [56] report that increased IDO expression leads to decreased serum tryptophan levels and decreased 5-HT synthesis in the brain, all of which can eventually lead to depression. IDO activation has been associated with inflammation and symptoms of acute depression. In some studies, IDO enzyme inhibition leads to reduce depressive symptoms [8].
IDO appears to play a major role in the development of depressive symptoms when the immune system is activated. Stress, depression and inflammation are also linked. Wichers and Maes [57], showed that stress activates the activity of proinflammatory cytokines and their signals in the central and peripheral nervous systems. In humans, acute and chronic stress have been linked to increased production and secretion of inflammatory cytokines. Several studies have confirmed that changes in tryptophan metabolism, which induced by proinflammatory cytokines can cause depressive-like behavioral disorders [8].
Physical and physiological stressors activate the immune system in the peripheral and central nervous system, which triggers the release of inflammatory cytokines and ultimately changes in neurotransmitters and mood. Studies of brain and plasma specimens of depressed patients have shown higher levels of proinflammatory cytokines such as interferon-gamma, alpha-necrosis factor, and interleukin-1 beta. These inflammatory cytokines and bacterial endotoxin or lipopolysaccharide appear to cause depression and depressive-like behaviors such as lethargy, numbness, decreased sexual activity, and sleep disturbances [1,58].
In depressive disorders, stress and immune activity can activate the kynurenine pathway by two enzymes, IDO and TDO. When the immune system is activated, IDO is strongly triggered by several inflammatory cytokines in non-hepatic tissues, shifting extra-hepatic tryptophan metabolism to kynurenine. Interferon gamma is a strong inducer for IDO, and the production of this enzyme is weakly induced by interferon alpha. Anti-inflammatory cytokines such as interleukin-4 can inhibit the production of IDO, whereas proinflammatory cytokines such as interleukin-1 beta and alpha-necrotizing factor induces IDO production. Interleukin-6 and tumor necrosis factor have high serum levels in people with depression [30,59,60]. Activation of the kynurenine pathway in microglia leads to increase neurotoxic catabolism of kynurenine such as quinolinic acid (QA). Inflammatory cytokines may indirectly affect brain structure and neuronal activity through the enzyme IDO. Activation of IDO appears to have a compensatory and protective anti-inflammatory reflex system. Decreased plasma tryptophan and increased tryptophan metabolism following IDO production and activity may lower primary inflammatory immune responses [30,61,62]. There appears to be a relationship between acute depressive disorders and changes in tryptophan catabolism. This inhibition reflex may explain the fact that depression is sometimes a self-limiting disorder. There appears to be a relationship between acute depressive disorders and changes in tryptophan catabolism. IDO seems to play a role in the pathophysiology of depression caused by inflammation [30,63,64].
Depression in the body triggers the activity of pro-inflammatory cytokines in the body. Depression has been linked to increased expression of inflammatory mediators in the brain, high levels of the neurotoxic metabolite kynurenine and decreased levels of 5-HT. Increased IDO activity in patients with renal cell melanoma and carcinoma in response to interferon-alpha treatment is a major factor in the pathophysiology of interferon-induced de-pression. Interferon-alpha therapy has been shown to cause new depressive symptoms and exacerbate depressive symptoms in patients and the levels of kynurenine pathway metabolites in plasma and cerebrospinal fluid has also increased. Elevated kynurenine in the elderly is clearly associated with decreased motivation. Levels of quinolinic acid (a product of the metabolic pathway of kynurenine) increased in people who attempted suicide and remained stable for up to two years. A strong association has been identified between plasma and cerebrospinal fluid (CSF) metabolites of the kynurenine pathway. On the other hand, inflammation in plasma, especially TNF, was found to be associated with the kynurenine pathway [30].
Kynurenine induction focuses on the pathophysiology of depression and its role in the central nervous system. Introducing the kynurenine pathway as an intervening agent in depression in humans depends on understanding the relationship and complexity between the kynurenine pathway metabolites and inflammatory mediators in the peripheral nervous system and brain. There appears to be a positive and direct correlation between inflammatory plasma cytokines and kynurenine pathway metabolites [30,65].
Studies have shown that plasma and CSF kynurenine pathway metabolites in patients receiving interferon therapy were similar to those in people with neurological disorders.
Analyzes have shown that the concentration of kynurenine pathway metabolites in the body’s peripheral system is associated with immune system activity, and in particular the presence of TNF [30].
Viral and bacterial infections often cause immuno-pathology and tissue damage, not only because pathogen damage, but also because of the high activity of the immune system, which causes the destruction of host tissues. Indole amine 2 and 3 dioxygenase (IDO1) is an intracellular non-secretory enzyme that catalyzes kynurenine derivatives from the essential amino acid trypto-phan. Although interferon type 1 directly induces IDO1, maximal expression of this enzyme requires simultaneous induction of TNF alpha or lipopolysaccharide in addition to interferon. High activity of the IDO1 enzyme under the influence of interferon gamma causes a decrease in serum tryptophan. IDO1 expression plays an important role during viral infections such as HIV, influenza, Epstein-Barr virus, HBV and HCV [66].
IDO is produced under the influence of inflammatory cytokines and most interferons in monocytes derived from macrophages and microglia.
The kynurenine pathway has major effects on immune, inflammatory, and neurological processes; the balance between kynurenic acid (KA) and QA is an important factor associated with neurological inflammatory syndromes. High levels of QA have toxic effects on nerve cells and are associated with dementia, while high levels of KA modulate these harmful effects. Increased IDO activity in chronic inflammatory diseases appears to play a role in increasing pain, depression, and fatigue [32].
The kynurenine pathway has long been identified as the endogenous pathway for the synthesis of the enzyme cofactor NAD. However, the importance of this enzyme in the immune system became clear when the effect of interferon-gamma on the expression of this enzyme was discovered, and at the same time it was found that unconnected metabolites produced in the kynurenine pathway could play a biological role as specific molecular targets in the nervous system. About 95% of tryptophan is consumed in the kynurenine pathway. Any change in the activity of the kynurenine pathway causes a change in other pathways in which tryptophan is a precursor. Inflamma-tion appears to increase the rate of tryptophan catabolism in the kynurenine pathway, resulting in lower levels of tryptophan as a precursor in other pathways to make compounds such as serotonin, tryptamine, and melatonin [67].
CONCLUSION
Since interferons is the first and most important defense barrier of the immune system against viral infections, and especially in respiratory viral infections, activation of the immune system induces the production and secretion of interferon, inflammation and expression of interferon-de-pendent genes. Also, considering that IDO is known as one of the target genes of interferon, and previous studies have confirmed the essential role of this enzyme in the production of neurotransmitters and tryptophan catabolism, it seems that this enzyme can be used as a bridge between inflammation and depression in infection. However, the final conclusion in this regard requires further studies.
Footnotes
Funding
None.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: All authors contributed to the study conception and design. Data collection: Zeinab Karimi, Maryam Chenari, Shima Karimi, Najmeh Parhizgari. Super-vision: Talat Mokhtari-Azad. Writing−original draft: Zeinab Karimi, Farhad Rezaie, Maryam Chenari. Writing−review & editing: Talat Mokhtari-Azad. The final version of this manuscript was approved by all authors.
References
- 1.Chaves Filho AJM, Lima CNC, Vasconcelos SMM, de Lucena DF, Maes M, Macedo D. IDO chronic immune activation and tryptophan metabolic pathway: a potential pathophysiological link between depression and obesity. Prog Neuropsy-chopharmacol Biol Psychiatry. 2018;80(Pt C):234–249. doi: 10.1016/j.pnpbp.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002-2030. Ann Trop Med Parasitol. 2006;100:481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 5.Okusaga O, Yolken RH, Langenberg P, Lapidus M, Arling TA, Dickerson FB, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130:220–225. doi: 10.1016/j.jad.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postolache TT, Mortensen PB, Tonelli LH, Jiao X, Frangakis C, Soriano JJ, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121:88–93. doi: 10.1016/j.jad.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer R, Wollman E, Vitkovic L, Yirmiya R. Cytokines and depression: fortuitous or causative association? Mol Psychiatry. 1999;4:328–332. doi: 10.1038/sj.mp.4000572. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Sheng H, Xu Y, Liu Y, Lu J, Ni X. Swimming exercise ameliorates depression-like behavior in chronically stressed rats: relevant to proinflammatory cytokines and IDO activation. Behav Brain Res. 2013;242:110–116. doi: 10.1016/j.bbr.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troy NM, Bosco A. Respiratory viral infections and host responses; insights from genomics. Respir Res. 2016;17:156. doi: 10.1186/s12931-016-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahony JB, Petrich A, Smieja M. Molecular diagnosis of respiratory virus infections. Crit Rev Clin Lab Sci. 2011;48:217–249. doi: 10.3109/10408363.2011.640976. [DOI] [PubMed] [Google Scholar]
- 12.Zhang N, Wang L, Deng X, Liang R, Su M, He C, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infection Control Today, author. 4 million deaths caused annually by acute respiratory infections [Internet] Infection Control Today. [2020 Nov 11]. Available from: https://www.infectioncontroltoday.com/view/4-million-deaths-caused-annually-acute-respiratory-infections .
- 14.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 15.Meskill SD, O'Bryant SC. Respiratory virus co-infection in acute respiratory infections in children. Curr Infect Dis Rep. 2020;22:3. doi: 10.1007/s11908-020-0711-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray RS, MacMillan B, Burks JS. Detection of coronavirus RNA in CNS tissue of multiple sclerosis and control patients. In: Cavanagh D, Brown TDK, editors. Coronaviruses and their diseases. Springer US; Boston: 1990. pp. 505–510. [DOI] [PubMed] [Google Scholar]
- 17.Chenari M, Karimi Z, Niya MHK. SARS-CoV-2 as emerging disease: preventive instructions and review of literature. Int J Trop Med. 2020;15:103–108. [Google Scholar]
- 18.Bouças AP, Rheinheimer J, Lagopoulos J. Why severe COVID-19 patients are at greater risk of developing depression: a molecular perspective. Neuroscientist. 2020 doi: 10.1177/1073858420967892. doi: 10.1177/1073858420967892. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See H, Wark P. Innate immune response to viral infection of the lungs. Paediatr Respir Rev. 2008;9:243–250. doi: 10.1016/j.prrv.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 22.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 24.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 25.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 26.Teijaro JR. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Curr Top Microbiol Immunol. 2015;386:3–22. doi: 10.1007/82_2014_411. [DOI] [PubMed] [Google Scholar]
- 27.Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): only an enzyme or a checkpoint controller? J Oncol Sci. 2017;3:52–56. doi: 10.1016/j.jons.2017.04.001. [DOI] [Google Scholar]
- 28.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Panozzo C, Nawara M, Suski C, Kucharczyka R, Skoneczny M, Bécam AM, et al. Aerobic and anaerobic NAD+ metabolism in Saccharomyces cerevisiae. FEBS Lett. 2002;517:97–102. doi: 10.1016/S0014-5793(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 30.Qin Y, Wang N, Zhang X, Han X, Zhai X, Lu Y. IDO and TDO as a potential therapeutic target in different types of de-pression. Metab Brain Dis. 2018;33:1787–1800. doi: 10.1007/s11011-018-0290-7. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Yamasuge W, Imai S, Kunisawa K, Hoshi M, Fujigaki H, et al. Lipopolysaccharide shock reveals the immune function of indoleamine 2,3-dioxygenase 2 through the regulation of IL-6/stat3 signalling. Sci Rep. 2018;8:15917. doi: 10.1038/s41598-018-34166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellor AL, Lemos H, Huang L. Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aronson JK. Tryptophan. In: Aronson JK, editor. Meyler's side effects of drugs. 16th ed. Elsevier; Oxford: 2016. pp. 220–221. [DOI] [Google Scholar]
- 34.Rodriguez Cetina Biefer H, Vasudevan A, Elkhal A. Aspects of tryptophan and nicotinamide adenine dinucleotide in immunity: a new twist in an old tale. Int J Tryptophan Res. 2017;10:1178646917713491. doi: 10.1177/1178646917713491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard DM, Dawes MA, Mathias CW, Acheson A, Hill- Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/IJTR.S2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, author. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet . 2015;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin SH, Lee LT, Yang YK. Serotonin and mental disorders: a concise review on molecular neuroimaging evidence. Clin Psychopharmacol Neurosci. 2014;12:196–202. doi: 10.9758/cpn.2014.12.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marazziti D. Understanding the role of serotonin in psychiatric diseases. F1000Res. 2017;6:180. doi: 10.12688/f1000research.10094.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bouchez C. Serotonin: 9 questions and answers [Internet] WebMD LLC. 2011 Available from: https://www.webmd.com/depression/features/serotonin . [Google Scholar]
- 40.Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, et al. Depression as a mediator of chronic fatigue and post-traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16:59–64. doi: 10.30773/pi.2018.10.22.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9:477. doi: 10.1038/s41467-017-02794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sailer V, Sailer U, Bawden EG, Zarbl R, Wiek C, Vogt TJ, et al. DNA methylation of indoleamine 2,3-dioxygenase 1 (IDO1) in head and neck squamous cell carcinomas correlates with IDO1 expression, HPV status, patients' survival, immune cell infiltrates, mutational load, and interferon g signature. EBioMedicine. 2019;48:341–352. doi: 10.1016/j.ebiom.2019.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakofsky JJ, Dunlop BW. Nothing to sneeze at: upper respiratory infections and mood disorders. Curr Psychiatry. 2019;18:29–34. [Google Scholar]
- 44.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Q, Zheng Y, Shi J, Wang J, Li G, Li C, et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed- method study. Brain Behav Immun. 2020;88:17–27. doi: 10.1016/j.bbi.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. 2012;34:7. doi: 10.1007/BF03391675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang R, Jiang T, Li N, Wang Z, Liu B, Fang L, et al. [The negative psychology for the public in Zhejiang province during the epidemic of human H7N9 avian influenza] Zhonghua Yu Fang Yi Xue Za Zhi. 2015;49:1073–1079. Chinese. [PubMed] [Google Scholar]
- 48.Bornand D, Toovey S, Jick SS, Meier CR. The risk of new onset depression in association with influenza--a population-based observational study. Brain Behav Immun. 2016;53:131–137. doi: 10.1016/j.bbi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 50.Malhotra S, Kaur N, Kumar P, Bhatia M, Hans C. Viral infections and depression. Delhi Psychiatry J. 2012;15:188–195. [Google Scholar]
- 51.Carenzo L, Protti A, Dalla Corte F, Aceto R, Iapichino G, Milani A, et al. Short-term health-related quality of life, physical function and psychological consequences of severe COVID-19. Ann Intensive Care. 2021;11:91. doi: 10.1186/s13613-021-00881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurissi E, Abu-jabir E, Mohammed A, Mahnashi M, Alharbi S, Alharbi A, et al. Assessment of new-onset depression and anxiety associated with COVID-19. Middle East Curr Psychiatry. 2021;28:33. doi: 10.1186/s43045-021-00112-w. [DOI] [Google Scholar]
- 53.Ismael F, Bizario JCS, Battagin T, Zaramella B, Leal FE, Torales J, et al. Post-infection depressive, anxiety and post-traumatic stress symptoms: a prospective cohort study in patients with mild COVID-19. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110341. doi: 10.1016/j.pnpbp.2021.110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myint AM, Kim YK. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses. 2003;61:519–525. doi: 10.1016/S0306-9877(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 57.Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29:11–17. [PMC free article] [PubMed] [Google Scholar]
- 58.Koo JW, Duman RS. IL-1b is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hughes MM, Connor TJ, Harkin A. Stress-related immune markers in depression: implications for treatment. Int J Neuropsychopharmacol. 2016;19:pyw001. doi: 10.1093/ijnp/pyw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams M, Zhang Z, Nance E, Drewes JL, Lesniak WG, Singh S, et al. Maternal inflammation results in altered tryptophan metabolism in rabbit placenta and fetal brain. Dev Neurosci. 2017;39:399–412. doi: 10.1159/000471509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, et al. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012;10:66. doi: 10.1186/1741-7015-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayhew J, Beart PM, Walker FR. Astrocyte and microglial control of glutamatergic signalling: a primer on understanding the disruptive role of chronic stress. J Neuroendocrinol. 2015;27:498–506. doi: 10.1111/jne.12273. [DOI] [PubMed] [Google Scholar]
- 63.Maes M. Depression is an inflammatory disease, but cell- mediated immune activation is the key component of depres-sion. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, et al. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–210. doi: 10.1016/j.psyneuen.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 65.Kruse JL, Cho JH, Olmstead R, Hwang L, Faull K, Eisenberger NI, et al. Kynurenine metabolism and inflammation-induced depressed mood: a human experimental study. Psychoneuro-endocrinology. 2019;109:104371. doi: 10.1016/j.psyneuen.2019.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fritsch SD, Weichhart T. Effects of interferons and viruses on metabolism. Front Immunol. 2016;7:630. doi: 10.3389/fimmu.2016.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogbechi J, Clanchy FI, Huang YS, Topping LM, Stone TW, Williams RO. IDO activation, inflammation and musculoskeletal disease. Exp Gerontol. 2020;131:110820. doi: 10.1016/j.exger.2019.110820. [DOI] [PubMed] [Google Scholar]