Abstract
Objective
This study aimed to evaluate whether somatic symptoms in adolescents with attention deficit hyperactivity disorder (ADHD) are associated with a dissociative pattern of functional connectivity (FC) within the default mode network (DMN) and whether methylphenidate administration can improve clinical and somatic symptoms. We also evaluated whether the improvement of somatic symptoms is associated with increased FC within the DMN in response to methylphenidate treatment.
Methods
Fifteen male adolescents with somatic symptoms of ADHD and 15 male adolescents with ADHD without somatic symptoms were included. At baseline and after 6 months of methylphenidate treatment, all adolescents were asked to complete questionnaires for the Korean version of the Dupaul’s ADHD rating scale, the symptom checklist-90- revised-somatization subscales, the Beck Depression Inventory, and the Beck Anxiety Inventory. Additionally, a resting-state functional magnetic resonance imaging scan was conducted.
Results
Methylphenidate treatment improved clinical and somatic symptoms in adolescents with ADHD. In addition, it increased brain FC within the DMN from the posterior cingulate cortex (posterior DMN) to the middle prefrontal cortex (anterior DMN). The improvement of somatic symptoms was associated with FC within the DMN from the posterior cingulate cortex to the middle prefrontal cortex in ADHD adolescents with somatic symptoms.
Conclusion
Methylphenidate increased brain FC between the anterior and posterior DMN. The improvement of somatic symptoms in adolescents with ADHD was associated with FC within the DMN. The DMN in adolescents with ADHD seems to be associated with the severity of the clinical and somatic symptoms of ADHD.
Keywords: Attention deficit hyperactivity disorder, Default mode network, Somatic symptoms, Methylphenidate
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a developmental disorder characterized by symptoms of inattention, distraction, hyperactivity, and impulsivity [1]. In addition, ADHD is mostly associated with poor health outcomes including hypertension, diabetes mellitus, obesity, smoking, substance abuse, injury due to accidents, insomnia, suicidality [2], and increased medical costs [3]. Although somatic symptoms are not included in the core symptoms of ADHD, subjective health (psychological and somatic) complaints and somatic symptoms are more frequently reported in children with ADHD [4-7]. In the data from a public health survey of children and adolescents in Scania, Sweden, it was documented that students with ADHD presented with subjective health complaints more frequently healthy students did [5]. In a multi-center, cross-sectional study of 6 to 18-year-old adolescents with ADHD, headache and associated comorbidities were three times more frequent in children with ADHD than in healthy children [6]. Furthermore, somatic complaints have been associated with emotional disorders in girls and disruptive behavior disorders in boys [4]. In a review of the public health care system for African American males, somatic symptoms including muscle pain, headaches, and stomach aches were associated with ADHD as well as oppositional defiant disorder [7]. Somatic symptoms in adults with ADHD result in poor compliance with pharmacotherapy [8]. Comorbid diseases including substance abuse are also impacted [9]. During abstinence, smokers with ADHD experience more severe withdrawal symptoms, including somatic symptoms, compared to that of smokers without ADHD [9].
Altered brain activity within the default mode network (DMN) has been reported in patients with ADHD. The DMN consists of several brain regions, including the ventromedial, anteromedial, and dorsal prefrontal cortex, posterior parietal cortex, middle temporal gyrus, precuneus, and posterior cingulate cortex (PCC) [10]. It is associated with internal thoughts and is more active when there is no task-related processing [10]. During rest, the DMN maintains strong brain activity; however, its activity is attenuated in response to external stimulation (tasks) [10]. The failure of attenuation within the DMN may predict errors or less accurate task output performance [11]. Several studies have suggested task-irrelevant processes and failure of DMN attenuation in patients with ADHD [12-14]. In studies on resting-state functional magnetic resonance imaging (fMRI), the brain connectivity between the anterior and posterior DMN was found to be diminished in patients with ADHD [15-17].
Abnormalities in the DMN have also been reported in patients with somatic symptoms. In a previous fMRI study using the fractional amplitude of low frequency fluctuations approach, patients with somatic symptom disorder showed abnormal functional connectivity (FC) in the bilateral superior medial prefrontal cortices and left precuneus, which are the major regions of the DMN [18]. Furthermore, the FC value of the bilateral superior medial prefrontal cortices was positively associated with the severity of somatization disorder [18]. In another study, abnormal network homogeneity in the left superior frontal gyrus and bilateral precuneus, which are major regions within the DMN, was observed in patients with somatization disorders [19]. Moreover, patients with chronic somatic pain were found to have increased FC from the DMN to the anterior cingulate cortex, left inferior parietal lobule, and insular cortex in another fMRI study [20]. These serial studies on patients with somatization disorder and chronic pain have shown a dissociation pattern of brain connectivity between the anterior and posterior sections of the DMN [18,21].
As a first-line medication for ADHD, methylphenidate is thought to improve the clinical symptoms of ADHD by inhibiting the reuptake of dopamine and norepinephrine [22,23]. In addition, methylphenidate improves somatic complaints in children with ADHD [24]. Several studies have suggested that methylphenidate increases brain FC within the DMN in patients with ADHD [15,17]. Twelve weeks of methylphenidate treatment was shown to increase brain activity in the left fronto-parietal area and left insular cortex within the DMN in children with ADHD [17]. In adults with ADHD, methylphenidate increased FC within the DMN [15].
Based on previous studies, we hypothesized that somatic symptoms in adolescents with ADHD would be associated with a dissociative pattern of FC within the DMN. We also theorized that methylphenidate would improve not only attentional and behavioral symptoms but also somatic symptoms in adolescents with ADHD. Finally, we assumed that the improvement of somatic symptoms may be associated with increased FC within the DMN in response to methylphenidate treatment.
METHODS
Participants
The current study was conducted at the Chung-Ang University Hospital, Seoul, Korea, from May 2019 to December 2019. Thirty-nine male adolescents with ADHD who visited the Department of Psychiatry at this Hospital were screened for participation in the current study. Our definition of experiencing somatic symptoms was patients’ reporting a score greater than 20 for the symptom checklist-90-revised-somatization subscale (SCL-90R-SOM) [25]. Accordingly, the inclusion criteria for the ADHD+ SOM group were as follows: 1) diagnosis of ADHD based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) Disorders-Clinician Version (SCID-5-CV) [26]; 2) psychiatric drug naïve; and 3) presence of somatic symptoms based on an SCL-90R-SOM score of over 20 [25,27]. The inclusion criteria for the ADHD-SOM group were as follows: 1) diagnosis of ADHD based on the SCID-5-CV [26]; 2) psychiatric drug naïve; and 3) absence of somatic symptoms in the last 20 days (SCL-90R-SOM score less than 20). The exclusion criteria for both the ADHD+SOM and ADHD-SOM groups were as follows: 1) past or current diagnosis of any psychiatric disorder other than ADHD based on the SCID-5-CV or severe medical illnesses such as hepatic disease, renal impairment, malignancy, hematologic disease, or cardiovascular disease; 2) history of taking psychiatric medication; 3) past or current substance use disorder; 4) history of head trauma and/or epilepsy; and 5) suffering from claustrophobia. Among the 39 adolescents, 19 satisfied our definition of experiencing somatic symptoms (ADHD+SOM group), and the remaining 20 adolescents with ADHD did not satisfy the criteria of having somatic symptoms (ADHD-SOM group).
Among the 19 adolescents in the ADHD+SOM group, one adolescent was excluded because he was claustrophobic, which prevented him from undergoing fMRI. Two participants from the ADHD+SOM group did not complete the medication schedule without a reason. Among the 20 participants in the ADHD-SOM group, two did not complete the medication schedule without a reason, one was excluded due to severe depressive mood, and two were excluded due to medication related adverse effects, such as headache and sleep disturbances. Finally, 15 participants from the ADHD+SOM group and 15 from the ADHD-SOM group completed the study protocol.
Study Procedure
At baseline, all participants from the ADHD+SOM group and ADHD-SOM group were asked to complete clinical questionnaires including the Korean version of Dupaul’s ADHD rating scale (K-ARS) [28,29], SCL-90R-SOM [25], Beck Depression Inventory (BDI) [30], and Beck Anxiety Inventory (BAI) [31]; participants also underwent fMRI. After completing the clinical questionnaires, all participants from the ADHD+SOM and ADHD-SOM groups received methylphenidate for the treatment of clinical symptoms of ADHD. Beginning with an initial sustained- release methylphenidate dose (ConcertaⓇ 18 mg; Janssen- Cilag Limited, High Wycombe, United Kingdom or Metadate CDⓇ 20 mg; UCB Manufacturing Inc., Smyrna, GA, USA), which was a methylphenidate equivalent 10 mg dose, the maintenance dose for each participant was adjusted at the physician’s discretion (D.H.H. and S.M.K.) within the Ministry of Food and Drug’s safety-approved dosage range. The final maintained methylphenidate dose was 31.3 ± 11.5 mg/day for the ADHD+SOM group and 31.4 ± 10.3 mg/day for the ADHD-SOM group. After a treatment period of 6 months, all participants were asked to complete the clinical rating questionnaires again, and they also underwent fMRI.
Symptom Measurements
In this study, we used the SCL-90R-SOM to assess the severity of somatic symptoms [25]. The SCL-90R-SOM consists of 12 items with a 5-point Likert scale (1: ‘not at all’ to 5: ‘extremely’) reflecting distress due to autonomic system dysfunction and muscle pain [25]. In a previous study, SCL-90R-SOM attained a reliability coefficient of 0.82 [27]. The K-ARS was used to evaluate the severity of ADHD symptoms. It consists of nine items for measuring inattentive symptoms and nine items for measuring hyperactivity symptoms with a 4-point Likert scale (0: ‘never or rarely’ to 3: ‘very often’) [28,29]. The internal consistency of the K-ARS was reported to be in the range of 0.77−0.89 [29]. The BDI [30] and BAI [31] were administered to evaluate the levels of depressive symptoms and anxiety, respectively. Both the BDI and BAI are 21-item questionnaires with each item scored from 0 to 3. The internal consistency coefficient of the Korean version of the BDI is 0.92, and that of the BAI is 0.93 [30,32,33].
Brain Image Acquisition, Processing, and Analysis
Resting-state fMRI data of all participants were acquired using a 3.0 T Philips Achieva scanner (Philips Healthcare, Best, Netherlands). All participants were told to lie awake with their eyes closed. Each participant’s head was cushioned and taped to minimize head move-ment. fMRI images were obtained in the axial direction with an echo-planar imaging sequence using the following parameters: 3,000/40 ms TR/TE, 40 slices, 64 × 64 matrix, 90° flip angle, 230-mm field of view, and 3-mm section thickness without a gap. The fMRI scan was continued for 12 min, and 230 volumes were acquired for each participant [34].
Data preprocessing and processing were conducted using the Data Processing Assistant for Resting-State fMRI (DPARSF; http://www.restfmri.net), which is a plug-in software compatible with statistical parametric mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and the Resting-State fMRI Data Analysis Toolkit (REST; http://resting-fmri.sourceforge.net). Images were corrected for slice acquisition time differences, realigned, normalized, spatially smoothed with a 6-mm full width at half- maximum kernel, de-trended, and then band pass filtered to preserve frequencies of 0.01−0.08 Hz [35-37]. An independent component-based noise correction method was applied to regress physiological and additional sources of noise, including cerebrospinal fluid signals, white matter, and head motion-related covariates (translation in the x, y, or z direction greater than 3 mm or rotation greater than 2° around one of the axes).
To assess the FC among the groups at baseline and follow-up, seed-based FC analysis was performed using REST software before and after the treatments were administered. In preprocessing, Fisher-transformed correlation coefficients were calculated for each pair of regions of interest (ROIs) for individual participants. The FC between ROIs was measured using the CONN-fMRI functional connectivity toolbox (version 15; https://www.nitrc.org/projects/conn). Gaussian smoothing was performed with a 6-mm full width at half maximum to reduce the effects of spatial noise and registration errors after spatial normalization. The ROIs were derived based on the cluster of the t-map with a given threshold (false discovery rate [FDRp] < 0.05, k > 20). Using the ROIs from the previous step, FC analysis was performed to evaluate the seed-based correlations. Pearson’s correlation coefficients were calculated based on the averaged blood oxygenation level-dependent time course from the seed and every voxel. Fisher’s z-transformation was performed to convert the correlation coefficients to normally distributed z-scores. For seed-based analysis, the PCC was selected as the seed region. The PCC is thought to be a representative region of the DMN and has been frequently used for “seed” analysis in many seed-based analyses [10].
Statistics
Demographic data were analyzed using a Mann−Whitney U test. Changes in the clinical scores of K-ARS, SCL-90R-SOM, BAI, and BDI in each group were assessed using a Wilcoxon signed-rank test. In addition, the changes in the clinical scores of the K-ARS, SCL-90R-SOM, BAI, and BDI between the two groups were assessed using a repeated-measures analysis of variance (ANOVA). Brain fMRI analysis was performed as follows: first, the FC from the PCC to other brain regions in each group was assessed using a paired t test with SPM. In addition, the FC from the PCC to other brain regions was compared between the ADHD+SOM and ADHD-SOM groups using an analysis of covariance (ANCOVA) test, controlling for age, education, and sex. Second, changes in the FC from the PCC to other brain regions during the treatment period were assessed using a paired t test with SPM12. Moreover, the changes in the FC from the PCC to other brain regions during the treatment period were compared between the ADHD+SOM and ADHD-SOM groups using an ANCOVA test, controlling for age, education, and sex. Third, the changes in the FC from the PCC to other brain regions after the treatment period were compared between the ADHD+SOM and ADHD-SOM groups using a mixed- model ANOVA with SPM12. The final result map was thresholded using p < 0.05 FDR correction for multiple comparisons with a range of more than 20 consecutive voxels, and cluster analysis was performed (derived from an uncorrected p < 0.001 and 20 extended voxels). The significance threshold of the analysis set at FDRp < 0.05 was considered for correction of multiple comparisons. Finally, the correlation between changes in the clinical scale scores of ADHD, somatic symptoms, and FC from the PCC to the right superior frontal gyrus was assessed using Spearman correlation.
Ethics Statement
The Institutional Review Board of Chung-Ang University Hospital approved the protocol of the current study (approval number: 1911-003-356). Informed consent was obtained from all participants, in addition to consent from their parents and/or legal guardians. All clinical investigations were conducted in accordance with the 2013 Declaration of Helsinki and guidelines for good clinical practice [38]. The current study was registered at the Clinical Research Information Service (CRIS, http://cris.nih.go.kr; number KCT0003866).
RESULTS
Characteristics of Demographic Data and Symptom Scales
Patients were divided into two groups: those with ADHD with somatic symptoms (ADHD+SOM) and those with ADHD without somatic symptoms (ADHD-SOM). There were no significant differences in age, years of education at baseline, or methylphenidate maintenance dosage during the treatment period between the ADHD+ SOM group and ADHD-SOM group. However, the scores on the K-ARS, SCL-90R-SOM, BDI, and BAI for the ADHD+SOM group were significantly higher than those for the ADHD-SOM group (Table 1). In the ADHD+SOM group, SCL-90R-SOM scores were positively correlated with the K-ARS scores (r = 0.61, p = 0.02) and BDI scores (r = 0.67, p = 0.02).
Table 1.
Characteristics of demographic data and symptom measurements
| Variable | ADHD+SOM | ADHD-SOM | Statistics | |
|---|---|---|---|---|
| Age (yr) | 14.6 ± 1.1 | 14.8 ± 1.1 | z = −0.88, p = 0.38 | |
| Years of education | 7.4 ± 1.1 | 7.8 ± 1.1 | z = −0.90, p = 0.36 | |
| Methylphenidate (mg/day) | 31.3 ± 11.5 | 31.4 ± 10.3 | z = −0.23, p = 0.81 | |
| Clinical scales | ||||
| K-ARS | Baseline | 27.7 ± 5.3 | 22.4 ± 4.9 | z = 2.38, p < 0.05 |
| Follow-up | 17.1 ± 7.0 | 13.6 ± 4.9 | z = 1.27, p = 0.20 | |
| SCL-90R-SOM | Baseline | 35.5 ± 3.4 | 6.0 ± 2.0 | z = 4.67, p < 0.01 |
| Follow-up | 27.8 ± 7.3 | 6.4 ± 2.8 | z = 4.65, p < 0.01 | |
| BDI | Baseline | 13.4 ± 2.7 | 6.7 ± 2.3 | z = 4.26, p < 0.01 |
| Follow-up | 10.5 ± 3.7 | 5.3 ± 1.8 | z = 3.59, p < 0.01 | |
| BAI | Baseline | 12.0 ± 3.6 | 7.4 ± 3.3 | z = 3.10, p < 0.01 |
| Follow-up | 9.5 ± 4.2 | 7.3 ± 3.2 | z = 1.50, p = 0.13 | |
Values are presented as mean ± standard deviation.
ADHD+SOM, adolescents with attention deficit hyperactivity disorder (ADHD) with somatic symptoms; ADHD-SOM, adolescents with ADHD without somatic symptoms; K-ARS, Korean version of Dupaul’s ADHD rating scale; SCL-90R-SOM, symptom checklist-90-revised-somatization subscale; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory.
Patients in both groups were treated with methylpheni-date for 6 months. During the treatment period, the scores of the K-ARS (z = 3.10, p < 0.01; z = 3.61, p < 0.01) and the BDI (z = 2.63, p = 0.03; z = 2.13, p = 0.03) were improved in both the ADHD+SOM group and the ADHD- SOM group. However, there were no significant differences in the changes in the K-ARS (F = 0.79, p = 0.38) and the BDI (F = 2.15, p = 0.15) scores between the ADHD+ SOM group and the ADHD-SOM group. There was no significant change in the BAI scores in either the ADHD+ SOM group or the ADHD-SOM group during the treatment period. In the ADHD+SOM group, the SCL-90R-SOM scores were significantly improved (z = 3.32, p < 0.01). There was no change in the SCL-90R-SOM score in the ADHD-SOM group (z = 1.06, p = 0.29). There were significant differences in the changes in SCL-90R-SOM scores between the ADHD+SOM and ADHD-SOM groups (F = 14.6, p < 0.01).
Comparison of Functional Connectivity from the Posterior Cingulate Cortex Seed to Other Brain Regions between the Study Groups at Baseline
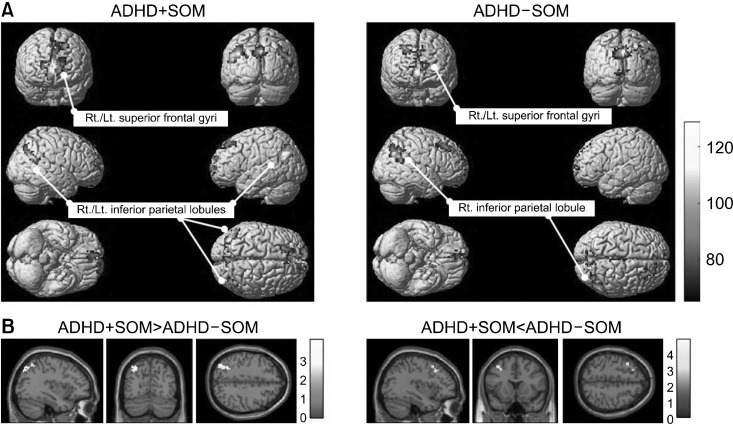
Resting-state fMRI was used to investigate differences in FC between the two groups. At baseline, the ADHD+ SOM group showed FC from the PCC seed to both inferior parietal lobules (posterior parietal cortex) (x, y, z: −51, −57, 24/x, y, z: 51, −57, 24, ke = 204, T = 22.77, FDRp < 0.01) and both superior frontal gyri (medial prefrontal cortex) (x, y, z: −7, 48, 12/x, y, z: 7, 48, 12, ke = 551, T = 16.62, FDRp < 0.01). The ADHD-SOM group showed FC from the PCC seed to the right inferior parietal lobule (posterior parietal cortex) (x, y, z: 54, −60, 39, ke = 322, T = 14.12, FDRp < 0.01) and both superior frontal gyri (medial prefrontal cortex) (x, y, z: −6, 57, 15/x, y, z: 6, 57, 15, ke = 517, T = 15.51, FDRp < 0.01) (Fig. 1A).
Fig. 1.
Comparison of the functional connectivity from the posterior cingulate cortex seed to other brain regions between study groups at baseline. (A) ADHD+SOM group showed FC from the posterior cingulate cortex PCC seed to both inferior parietal lobules (x, y, z: −51, −57, 24/x, y, z: 51, −57, 24, ke = 204, T = 22.77, FDRp < 0.01) and both superior frontal gyri (x, y, z: −7, 48, 12/x, y, z: 7, 48, 12, ke = 551, T = 16.62, FDRp < 0.01); the ADHD-SOM group showed FC from the PCC seed to the right inferior parietal lobule (x, y, z: 54, −60, 39, ke = 322, T = 14.12, FDRp < 0.01) and both superior frontal gyri (x, y, z: −6, 57, 15/x, y, z: 6, 57, 15, ke = 517, T = 15.51, FDRp < 0.01). (B) Compared to the ADHD-SOM group, the ADHD+SOM group showed decreased FC from the PCC seed to the left middle frontal gyrus (x, y, z: −36, 15, 51, ke = 47, T = 4.52, FDRp = 0.02) and increased FC from the PCC seed to the left inferior parietal lobule (x, y, z: −36, −72, 42, ke = 90, T = 3.53, FDRp = 0.03). ADHD+SOM, adolescents with attention deficit hyperactivity disorder (ADHD) with somatic symptoms; ADHD-SOM, adolescents with ADHD without somatic symptoms; FC, functional connectivity; PCC, posterior cingulate cortex.
Compared to the ADHD-SOM group, the ADHD+SOM group showed decreased FC from the PCC seed to the left middle frontal gyrus (lateral prefrontal cortex) (x, y, z: −36, 15, 51, ke = 47, T = 4.52, FDRp = 0.02), and increased FC from the PCC seed to the left inferior parietal lobule (posterior parietal cortex) (x, y, z: −36, −72, 42, ke = 90, T = 3.53, FDRp = 0.03) at baseline (Fig. 1B).
Changes in Functional Connectivity from the Posterior Cingulate Cortex Seed to Other Brain Regions in the Study Groups during the Treatment Period
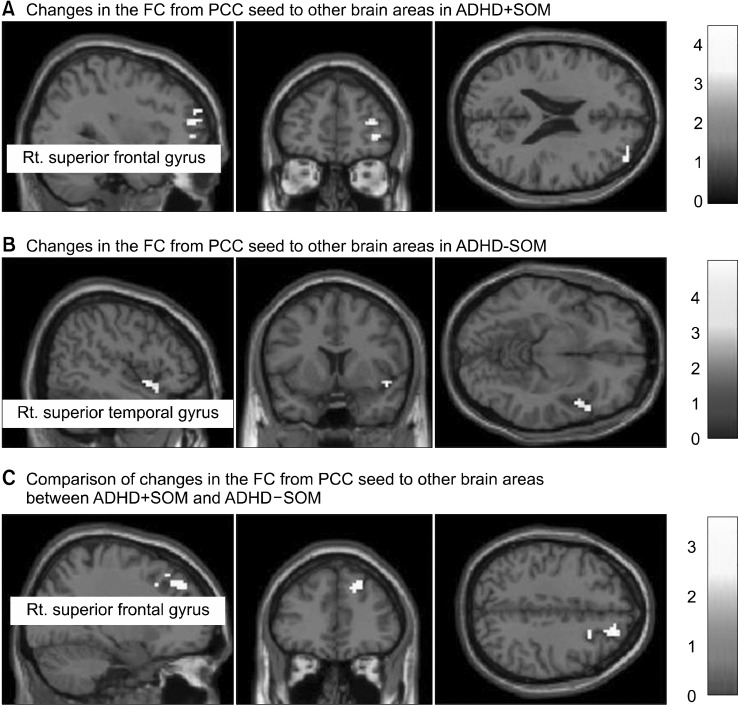
During the treatment period, the ADHD+SOM group showed increased FC from the PCC seed to the right superior frontal gyrus (x, y, z: 3, 54, −3, ke = 60, T = 4.32, FDRp = 0.04) (Fig. 2A). The ADHD-SOM group showed decreased FC from the PCC seed to the right superior temporal gyrus (x, y, z: 54, 18, −12, ke = 122, T = 3.99, FDRp = 0.03) (Fig. 2B).
Fig. 2.
Changes in functional connectivity from the posterior cingulate cortex seed to other brain regions in study groups during the treatment period. (A) During the treatment period, the ADHD+SOM group showed increased FC from the PCC seed to the right superior frontal gyrus (x, y, z: 3, 54, −3, ke = 60, T = 4.32, FDRp = 0.04). (B) During the treatment period, the ADHD-SOM group showed decreased FC from the PCC seed to the right superior temporal gyrus (x, y, z: 54, 18, −12, ke = 122, T = 3.99, FDRp = 0.03). (C) Compared to the ADHD-SOM group, the ADHD+SOM group showed greater changes in FC from the PCC seed to the right superior frontal gyrus (x, y, z: 27, 18, 42, ke = 60, F = 3.13, FDRp = 0.04). ADHD+SOM, adolescents with attention deficit hyperactivity disorder (ADHD) with somatic symptoms; ADHD-SOM, adolescents with ADHD without somatic symptoms; FC, functional connectivity; PCC, posterior cingulate cortex.
Compared to the ADHD-SOM group, the ADHD+SOM group showed greater changes in the FC from the PCC seed to the right superior frontal gyrus (x, y, z: 27, 18, 42, ke = 60, F = 3.13, FDRp = 0.04) (Fig. 2C).
Correlations between Clinical Scale Scores and Brain Functional Connectivity
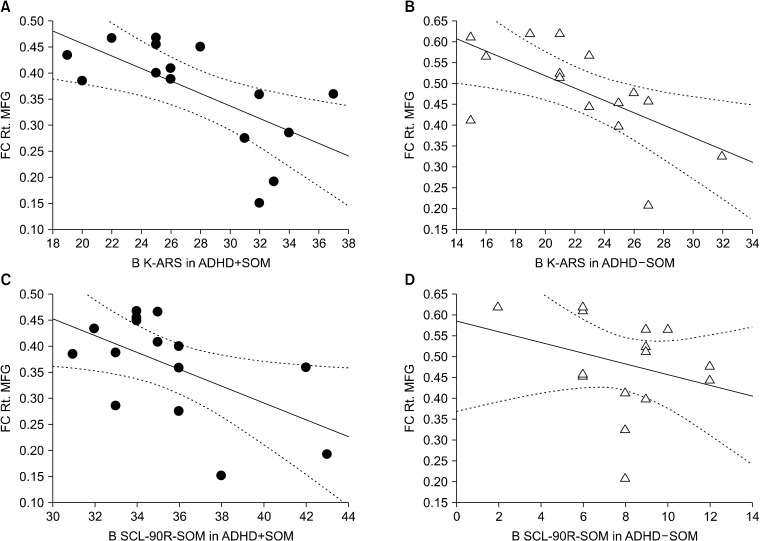
At baseline, the K-ARS scores in the ADHD+SOM group (r = −0.58, p = 0.03) and the K-ARS scores in the ADHD-SOM group (r = −0.61, p = 0.02) were negatively correlated with the FC from the PCC seed to the right middle frontal gyrus. At baseline, the SCL-90R-SOM scores in the ADHD+SOM group were negatively correlated with the FC from the PCC seed to the right middle frontal gyrus (r = −0.56, p = 0.03). However, the SCL-90R-SOM scores in the ADHD-SOM group were not correlated with the FC from the PCC seed to the right middle frontal gyrus (r = −0.11, p = 0.69; Fig. 3).
Fig. 3.
Correlations between clinical scale scores and brain functional connectivity. (A) At baseline, the correlation between the K-ARS scores and FC from the PCC seed to the right middle frontal gyrus in the ADHD+SOM group (r = −0.58, p = 0.03). (B) At baseline, the correlation between the K-ARS scores and FC from the PCC seed to the right middle frontal gyrus in the ADHD-SOM group (r = −0.61, p = 0.02). (C) At baseline, the correlation between the SCL-90R-SOM scores with FC from the PCC seed to the right middle frontal gyrus in the ADHD+SOM group (r = −0.56, p = 0.03). (D) At baseline, the correlation between the SCL-90R-SOM scores with FC from the PCC seed to the right middle frontal gyrus in the ADHD+SOM group (r = −0.11, p = 0.69). ADHD+SOM, adolescents with attention deficit hyperactivity disorder (ADHD) with somatic symptoms; ADHD-SOM, adolescents with ADHD without somatic symptoms; B K-ARS, baseline Korean version of Dupaul’s ADHD rating scale; B SCL-90R-SOM, baseline symptom checklist-90- revised-somatization subscale; FC Rt. MFC, functional connectivity (FC) from the posterior cingulate cortex (PCC) seed to the right middle frontal gyrus.
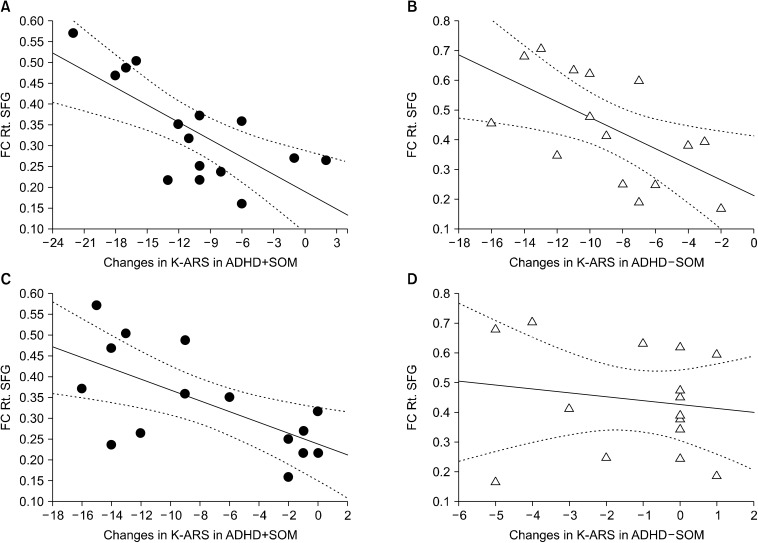
During the treatment period, the changes in the K-ARS scores in the ADHD+SOM group (r = −0.67, p < 0.01) and ADHD-SOM group (r = −0.60, p = 0.02) were negatively correlated with the FC from the PCC seed to the right superior frontal gyrus. During the treatment period, in the ADHD+SOM group, the change in the scores of the SCL-90R-SOM was negatively correlated with the FC from the PCC seed to the right superior frontal gyrus (r = −0.61, p = 0.02). In the ADHD-SOM group, the change in the scores of the SCL-90R-SOM was not correlated with the FC from the PCC seed to the right superior frontal gyrus (r = −0.09, p = 0.74) (Fig. 4).
Fig. 4.
Correlations between changes in clinical scale scores and brain functional connectivity. (A) During the treatment period, there was a correlation between the change in the K-ARS scores and FC from the PCC seed to the right superior frontal gyrus in the ADHD+SOM group (r = −0.67, p < 0.01). (B) During the treatment period, there was a correlation between the change in the K-ARS and FC from the PCC seed to the right superior frontal gyrus in the ADHD-SOM group (r = −0.60, p = 0.02). (C) During the treatment period, there was a correlation between the change in the SCL-90R-SOM and FC from the PCC seed to the right superior frontal gyrus in the ADHD+SOM group (r = −0.61, p = 0.02). (D) During the treatment period, there was a correlation between the change in the scores of the SCL-90R-SOM and FC from the PCC seed to the right superior frontal gyrus in the ADHD-SOM group (r = −0.09, p = 0.74). ADHD+SOM, adolescents with attention deficit hyperactivity disorder (ADHD) with somatic symptoms; ADHD-SOM, adolescents with ADHD without somatic symptoms; K-ARS, Korean version of Dupaul’s ADHD rating scale; SCL-90R-SOM, symptom checklist-90-revised-somatization subscales; FC Rt. SFG, functional connectivity (FC) from the posterior cingulate cortex (PCC) seed to the right superior frontal gyrus.
DISCUSSION
The current results demonstrate that methylphenidate treatment may improve clinical as well as somatic symptoms in adolescents with ADHD and somatic symptoms. In addition, methylphenidate may increase brain FC within the DMN from the PCC to the medial and lateral prefrontal cortex. The improvement of somatic symptoms may be associated with the FC within the DMN from the PCC to the medial and lateral prefrontal cortex in adolescents with somatic symptoms of ADHD.
In response to methylphenidate treatment, ADHD symptoms measured by the K-ARS and depressive symptoms measured by the BDI in both the ADHD+SOM group and the ADHD-SOM group were improved. Somatic symptoms measured by the SCL-90R-SOM improved in the ADHD+SOM group. Previous studies on methylphenidate have suggested that it improves ADHD symptoms and mood symptoms in patients with ADHD [39]. Moreover, methylphenidate is administered to patients with major depressive disorder [40]. However, the effects of methylphenidate on anxiety reduction in patients with ADHD are controversial. Several studies have suggested that methylphenidate may improve anxiety in children with ADHD [41]. Kritchman et al. [42] suggested that methylphenidate delayed anxiety-provoking effects in children with ADHD.
At baseline, the level of ADHD symptoms was positively correlated with somatic symptoms in the ADHD+ SOM group. In addition, somatic symptoms improved in the ADHD+SOM group. Children with ADHD display sensory over-responsibility and impairments in the processing of stimuli, including somatic sensory stimuli [43,44]. In addition, the attentional mechanisms of pain processing including pain hypervigilance and pain catastrophizing may be associated with somatization [45]. In a study using single-photon emission computerized tomography, drug-naïve children with ADHD showed reduced regional cerebral blood flow in the right orbitofrontal cortex and middle temporal gyrus, while it was increased in the bilateral somatosensory area and dorsomedial prefrontal cortex [46]. After 4−5 weeks of treatment with methylphenidate, the improvement in ADHD symptoms was related to the normalization of the abnormally increased activity in the somatosensory area and abnormally reduced activity in the orbitofrontal cortex [46]. In agreement with these results, in an animal study with rats, methylphenidate enhanced noradrenergic transmission and suppressed the long-latency excitation evoked by sensory stimulation [47]. These effects seem to result from methylphenidate-induced enhancement of noradrenergic modulatory action on sensory systems [47]. Taken together, methylphenidate treatment may improve over-responsibility to irrelevant sensory stimuli and attentional processing of sensory information in patients with somatic symptoms of ADHD.
At baseline, both the ADHD+SOM group and the ADHD-SOM group showed similar brain FC from the PCC seed to the DMN (posterior parietal cortex and medial prefrontal cortex) in the current study. In addition, the K-ARS scores in both the ADHD+SOM and ADHD-SOM groups were negatively correlated with the FC between the anterior and posterior DMN (between the right middle frontal gyrus and the PCC). These results coincide with those of previous studies on resting-state fMRI that have reported decreased FC between the anterior and posterior DMN [15-17] as well as hyper-connectivity between the DMN and attention network [12-14] in patients with ADHD. Additionally, patients with ADHD have been reported to make frequent errors through task-irrelevant processes and exhibit failure of DMN attenuation [10,11].
In addition to the decreased FC between the anterior and posterior DMN in the ADHD+SOM group, the SCL-90R-SOM scores in this group were also negatively correlated with the FC between the anterior and posterior DMN (between the right middle frontal gyrus and the PCC). This finding is consistent with previous results suggesting that patients with somatic symptoms demonstrate a dissociative pattern of FC between the anterior and posterior sections of the DMN [18,21].
In response to methylphenidate treatment, the FC between the anterior and posterior DMN (between the right middle prefrontal cortex and the PCC) was increased in the ADHD+SOM group, while the FC between the DMN and the ventral attention network (between the PCC and the right superior temporal gyrus) was decreased in the ADHD-SOM group. Compared to the ADHD-SOM group, the ADHD+SOM group showed a greater increase in the FC between the anterior and posterior DMN (between the right middle prefrontal cortex and the PCC). Moreover, the improvement of somatic symptoms was positively correlated with the FC between the anterior and posterior DMN (between the right middle prefrontal cortex and the PCC) in the ADHD+SOM group. In a review of 11 articles demonstrating methylphenidate’s effects on the DMN, methylphenidate was thought to improve the DMN dysfunction observed in ADHD and other neuropsychiatric disorders, including narcolepsy and traumatic brain injury [48]. The main mechanism of improvement in DMN dysfunction may be deactivation of the DMN after methylphenidate administration [49,50].
There were several limitations to the current study. First, owing to the small number of participants, the study results cannot be generalized. In the analyses of the follow-up brain data, we could not obtain multiple comparison correction data because of the small number of participants. Future studies must recruit a sufficiently large number of participants. Second, the results and interpretations of previous DMN studies are diverse and controversial. However, there are few studies on FC in patients with somatic symptoms. In a previous study, patients with somatic symptom disorders showed increased FC within the DMN, as well as increased FC between the sensorimotor network and DMN [51]. This is not consistent with the results of the current study, which showed decreased FC between the anterior and the posterior DMN as well as increased FC between the DMN and the attention network in adolescents with somatic symptoms of ADHD. This may be due to differences in the primary diagnosis or differences in the duration or repeatability of illnesses. In a study of resting state fMRI with 1,300 patients with major depressive disorder and 1,128 healthy controls, decreased FC in patients with recurrent depression was noted, in addition to increased FC in first-episode patients with depression [52]. Future studies are required to better understand the FC of the DMN and the mechanism of action of methylphenidate with regard to somatic symptoms. Finally, there is a potential danger that the dissociation patterns between the anterior and posterior DMN might be due to differences in mood and anxiety between the ADHD+SOM and ADHD-SOM groups, rather than due to somatic symptoms. Similar risks often exist in studies on somatic symptom disorder because depression and anxiety often coexist in patients with the disorder [53,54]. Therefore, a careful interpretation of the results is required. However, in this study, only the level of somatic symptoms, not the level of depression and anxiety, showed a significant difference between the ADHD+SOM and ADHD-SOM groups during the treatment period. In addition, during the treatment period, in the ADHD+SOM group, the change in the level of somatic symptoms was negatively correlated with FC within the DMN, which was not the case in the ADHD-SOM group. Therefore, we cautiously suggest that the dissociation pattern between the anterior and posterior DMN might be due to somatic symptoms rather than mood and anxiety.
The current results demonstrated that methylphenidate treatment may increase brain FC between the anterior and posterior DMN. The improvement of somatic symptoms in adolescents with ADHD may be associated with the FC within the DMN. DMN in adolescents with ADHD may be associated with the severity of clinical and somatic symptoms.
Footnotes
Funding
This research was funded by the Whan In Pharm., Republic of Korea. The sponsor had no role in the study design, data collection, data analysis, interpretation of data, manuscript preparation, or the decision to submit this article for publication.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Kyung Joon Min, Doug Hyun Han. Data acquisition: Sun Mi Kim, Doug Hyun Han. Formal analysis: Doug Hyun Han. Supervision: Kyung Joon Min. Writing−original draft: Sun Mi Kim, Doug Hyun Han. Writing−review and editing: Kyung Joon Min. Approval of the final manuscript: all authors
References
- 1.Beyens I, Valkenburg PM, Piotrowski JT. Screen media use and ADHD-related behaviors: four decades of research. Proc Natl Acad Sci U S A. 2018;115:9875–9881. doi: 10.1073/pnas.1611611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh Y, Yoon HJ, Kim JH, Joung YS. Trait anxiety as a mediator of the association between attention deficit hyperactivity disorder symptom severity and functional impairment. Clin Psychopharmacol Neurosci. 2018;16:407–414. doi: 10.9758/cpn.2018.16.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Secnik K, Swensen A, Lage MJ. Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. Pharmacoeconomics. 2005;23:93–102. doi: 10.2165/00019053-200523010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Egger HL, Costello EJ, Erkanli A, Angold A. Somatic complaints and psychopathology in children and adolescents: stomach aches, musculoskeletal pains, and headaches. J Am Acad Child Adolesc Psychiatry. 1999;38:852–860. doi: 10.1097/00004583-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Fridh M, Köhler M, Modén B, Lindström M, Rosvall M. Subjective health complaints and exposure to peer victimization among disabled and non-disabled adolescents: a population-based study in Sweden. Scand J Public Health. 2018;46:262–271. doi: 10.1177/1403494817705558. [DOI] [PubMed] [Google Scholar]
- 6.Kutuk MO, Tufan AE, Guler G, Yalin OO, Altintas E, Bag HG, et al. Migraine and associated comorbidities are three times more frequent in children with ADHD and their mothers. Brain Dev. 2018;40:857–864. doi: 10.1016/j.braindev.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Scott LD, Jr, McCoy H. Correlates of somatic symptoms among African American males transitioning from a public system of care. Am J Mens Health. 2018;12:274–282. doi: 10.1177/1557988316630304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torgersen T, Gjervan B, Lensing MB, Rasmussen K. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79–87. doi: 10.2147/NDT.S59271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClernon FJ, Van Voorhees EE, English J, Hallyburton M, Holdaway A, Kollins SH. Smoking withdrawal symptoms are more severe among smokers with ADHD and independent of ADHD symptom change: results from a 12-day contingency- managed abstinence trial. Nicotine Tob Res. 2011;13:784–792. doi: 10.1093/ntr/ntr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves PN, Foulon C, Karolis V, Bzdok D, Margulies DS, Volle E, et al. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun Biol. 2019;2:370. doi: 10.1038/s42003-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 12.Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methyl-phenidate. J Child Psychol Psychiatry. 2011;52:761–771. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS Comput Biol. 2012;8:e1002374. doi: 10.1371/journal.pcbi.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picon FA, Sato JR, Anés M, Vedolin LM, Mazzola AA, Valentini BB, et al. Methylphenidate alters functional connectivity of default mode network in drug-naive male adults with ADHD. J Atten Disord. 2020;24:447–455. doi: 10.1177/1087054718816822. [DOI] [PubMed] [Google Scholar]
- 16.Tao J, Jiang X, Wang X, Liu H, Qian A, Yang C, et al. Disrupted control-related functional brain networks in drug-naive children with attention-deficit/hyperactivity disorder. Front Psychiatry. 2017;8:246. doi: 10.3389/fpsyt.2017.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo JH, Kim D, Choi J, Jeong B. Treatment effect of methylphenidate on intrinsic functional brain network in medication-naïve ADHD children: a multivariate analysis. Brain Imaging Behav. 2018;12:518–531. doi: 10.1007/s11682-017-9713-z. [DOI] [PubMed] [Google Scholar]
- 18.Su Q, Yao D, Jiang M, Liu F, Jiang J, Xu C, et al. Dissociation of regional activity in default mode network in medication- naive, first-episode somatization disorder. PLoS One. 2014;9:e99273. doi: 10.1371/journal.pone.0099273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei S, Su Q, Jiang M, Liu F, Yao D, Dai Y, et al. Abnormal default-mode network homogeneity and its correlations with personality in drug-naive somatization disorder at rest. J Affect Disord. 2016;193:81–88. doi: 10.1016/j.jad.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 20.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gündel H, Valet M, Sorg C, Huber D, Zimmer C, Sprenger T, et al. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–421. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Ackerman PT, Dykman RA, Holcomb PJ, McCray DS. Methyl-phenidate effects on cognitive style and reaction time in four groups of children. Psychiatry Res. 1982;7:199–213. doi: 10.1016/0165-1781(92)90093-I. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih HH, Shang CY, Gau SS. Comparative efficacy of methylphenidate and atomoxetine on emotional and behavioral problems in youths with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2019;29:9–19. doi: 10.1089/cap.2018.0076. [DOI] [PubMed] [Google Scholar]
- 25.Derogatis LR. SCL-90-R: administration, scoring & procedures manual-II for the R(evised) version and other instruments of the psychopathology rating scale series. Clinical Psychometric Research; Towson: 1992. [Google Scholar]
- 26.First M, Williams J, Karg R, Spitzer R. User's guide for the SCID-5-CV structured clinical interview for DSM-5 disorders: clinician version. American Psychiatric Association; Arlington: 2015. [Google Scholar]
- 27.Mak WW, Zane NW. The phenomenon of somatization among community Chinese Americans. Soc Psychiatry Psychiatr Epidemiol. 2004;39:967–974. doi: 10.1007/s00127-004-0827-4. [DOI] [PubMed] [Google Scholar]
- 28.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating scale- IV: checklists, norms, and clinical interpretation. Guilford Publications; New York: 1998. [DOI] [Google Scholar]
- 29.So YK, Noh JS, Kim YS, Ko SG, Koh YJ. The reliability and validity of Korean Parent and Teacher ADHD Rating Scale. J Korean Neuropsychiatr Assoc. 2002;41:283–289. [Google Scholar]
- 30.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 32.Huh HJ, Kim KH, Lee HK, Chae JH. The relationship between childhood trauma and the severity of adulthood depression and anxiety symptoms in a clinical sample: the mediating role of cognitive emotion regulation strategies. J Affect Disord. 2017;213:44–50. doi: 10.1016/j.jad.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Lim YJ, Yu BH, Kim JH. Korean Anxiety Sensitivity Index- Revised: its factor structure, reliability, and validity in clinical and nonclinical samples. Depress Anxiety. 2007;24:331–341. doi: 10.1002/da.20210. [DOI] [PubMed] [Google Scholar]
- 34.Kwak KH, Hwang HC, Kim SM, Han DH. Comparison of behavioral changes and brain activity between adolescents with internet gaming disorder and student pro-gamers. Int J Environ Res Public Health. 2020;17:441. doi: 10.3390/ijerph17020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 36.Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. Anti-correlations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Medical Association, author. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 39.Golubchik P, Rapaport M, Weizman A. The effect of methylphenidate on anxiety and depression symptoms in patients with Asperger syndrome and comorbid attention deficit/hyper-activity disorder. Int Clin Psychopharmacol. 2017;32:289–293. doi: 10.1097/YIC.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 40.Cao B, Zhu J, Zuckerman H, Rosenblat JD, Brietzke E, Pan Z, et al. Pharmacological interventions targeting anhedonia in patients with major depressive disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:109–117. doi: 10.1016/j.pnpbp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Faraone SV. The pharmacology of amphetamine and methylphenidate: relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comor-bidities. Neurosci Biobehav Rev. 2018;87:255–270. doi: 10.1016/j.neubiorev.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kritchman M, Koubi M, Mimouni Bloch A, Bloch Y. Effect of methylphenidate on state anxiety in children with ADHD-A single dose, placebo controlled, crossover study. Front Behav Neurosci. 2019;13:106. doi: 10.3389/fnbeh.2019.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds S, Lane SJ. Sensory overresponsivity and anxiety in children with ADHD. Am J Occup Ther. 2009;63:433–440. doi: 10.5014/ajot.63.4.433. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu VT, Bueno OF, Miranda MC. Sensory processing abilities of children with ADHD. Braz J Phys Ther. 2014;18:343–352. doi: 10.1590/bjpt-rbf.2014.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huber C, Kunz M, Artelt C, Lautenbacher S. Attentional and emotional mechanisms of pain processing and their related factors: a structural equations approach. Pain Res Manag. 2010;15:229–237. doi: 10.1155/2010/516176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JS, Kim BN, Kang E, Lee DS, Kim YK, Chung JK, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24:157–164. doi: 10.1002/hbm.20067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drouin C, Page M, Waterhouse B. Methylphenidate enhances noradrenergic transmission and suppresses mid- and long-latency sensory responses in the primary somatosensory cortex of awake rats. J Neurophysiol. 2006;96:622–632. doi: 10.1152/jn.01310.2005. [DOI] [PubMed] [Google Scholar]
- 48.Santos PH, Goncalves R, Pedroso S. How does methylphenidate affect default mode network? A systematic review. Rev Neurol. 2019;68:417–425. doi: 10.33588/rn.6810.2018487. [DOI] [PubMed] [Google Scholar]
- 49.Querne L, Fall S, Le Moing AG, Bourel-Ponchel E, Delignières A, Simonnot A, et al. Effects of methylphenidate on default- mode network/task-positive network synchronization in children with ADHD. J Atten Disord. 2017;21:1208–1220. doi: 10.1177/1087054713517542. [DOI] [PubMed] [Google Scholar]
- 50.Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, et al. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One. 2008;3:e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SM, Hong JS, Min KJ, Han DH. Brain functional connectivity in patients with somatic symptom disorder. Psychosom Med. 2019;81:313–318. doi: 10.1097/PSY.0000000000000681. [DOI] [PubMed] [Google Scholar]
- 52.Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci U S A. 2019;116:9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiong N, Zhang Y, Wei J, Leonhart R, Fritzsche K, Mewes R, et al. Operationalization of diagnostic criteria of DSM-5 somatic symptom disorders. BMC Psychiatry. 2017;17:361. doi: 10.1186/s12888-017-1526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Vroege L, Timmermans A, Kop WJ, van der Feltz-Cornelis CM. Neurocognitive dysfunctioning and the impact of comorbid depression and anxiety in patients with somatic symptom and related disorders: a cross-sectional clinical study. Psychol Med. 2018;48:1803–1813. doi: 10.1017/S0033291717003300. [DOI] [PubMed] [Google Scholar]