Abstract
Background
Limited studies have evaluated the joint influence of redox-related metals and genetic variation on metabolic pathways. We analyzed the association of 11 metals with metabolic patterns, and the interacting role of candidate genetic variants, in 1145 participants from the Hortega Study, a population-based sample from Spain.
Methods
Urine antimony (Sb), arsenic, barium (Ba), cadmium (Cd), chromium (Cr), cobalt (Co), molybdenum (Mo) and vanadium (V), and plasma copper (Cu), selenium (Se) and zinc (Zn) were measured by ICP-MS and AAS, respectively. We summarized 54 plasma metabolites, measured with targeted NMR, by estimating metabolic principal components (mPC). Redox-related SNPs (N = 291) were measured by oligo-ligation assay.
Results
In our study, the association with metabolic principal component (mPC) 1 (reflecting non-essential and essential amino acids, including branched chain, and bacterial co-metabolism versus fatty acids and VLDL subclasses) was positive for Se and Zn, but inverse for Cu, arsenobetaine-corrected arsenic (As) and Sb. The association with mPC2 (reflecting essential amino acids, including aromatic, and bacterial co-metabolism) was inverse for Se, Zn and Cd. The association with mPC3 (reflecting LDL subclasses) was positive for Cu, Se and Zn, but inverse for Co. The association for mPC4 (reflecting HDL subclasses) was positive for Sb, but inverse for plasma Zn. These associations were mainly driven by Cu and Sb for mPC1; Se, Zn and Cd for mPC2; Co, Se and Zn for mPC3; and Zn for mPC4. The most SNP-metal interacting genes were NOX1, GSR, GCLC, AGT and REN. Co and Zn showed the highest number of interactions with genetic variants associated to enriched endocrine, cardiovascular and neurological pathways.
Conclusions
Exposures to Co, Cu, Se, Zn, As, Cd and Sb were associated with several metabolic patterns involved in chronic disease. Carriers of redox-related variants may have differential susceptibility to metabolic alterations associated to excessive exposure to metals.
Keywords: Metals, Metabolomics, Oxidative stress, Candidate genes, Gene-environment interaction
Abbreviations: AAA, aromatic amino acids; AAS, atomic absorption spectrometry; AsB, arsenobetaine; BCAA, branched-chain amino acids; BKMR, Bayesian Kernel Machine Regression; CVD, cardiovascular disease; HDL, high-density lipoprotein; HWE, Hardy Weinberg equilibrium; ICPMS, inductively coupled-plasma mass spectrometry; LDL, low-density lipoprotein; LOD, limit of detection; MAF, minor allele frequency; mPC, metabolic principal component; NMR, Nuclear Magnetic Resonance; PCA, principal component analysis; ROS, reactive oxygen species; SNP, single nucleotide polymorphisms; SOD, superoxide dismutase; VLDL, very low-density lipoprotein
Graphical abstract
Highlights
-
•
In a population-based sample, cobalt, copper, selenium, zinc, arsenic, cadmium and antimony exposures were related to some metabolic patterns.
-
•
Carriers of redox-related variants displayed differential susceptibility to metabolic alterations associated to excessive metal exposures.
-
•
Cobalt and zinc showed a number of statistical interactions with variants from genes sharing biological pathways with a role in chronic diseases.
-
•
The metabolic impact of metals combined with variation in redox-related genes might be large in the population, given metals widespread exposure.
1. Introduction
Mounting evidence supports that exposure to trace elements -mostly metals and metalloids, hereinafter referred to as “metals” - can substantially influence human health [[1], [2], [3], [4], [5]]. In the human body, essential metals, such as copper (Cu), selenium (Se) and zinc (Zn), are key enzymatic components playing a fundamental role on several cellular processes, including redox balance maintenance [6,7]. Essential metals are also needed for human microbiota metabolic activities because they have a role as cofactors in bacterial enzymatic pathways [8]. While low concentrations of essential metals can be damaging for the optimal function of human and bacterial metabolism [9], excessive concentrations may have not only adverse metabolic outcomes, but also induce alteration of enzymes involved in structural functions [10,11]. In addition, non-essential metals, such as cadmium (Cd) or arsenic, with no physiological function, but well-established toxic effects, can act as competitors for enzymatic binding sites and interfere with several metabolic processes [7]. For instance, divalent toxic metals bind to sulfhydryl groups, which not only counteracts the antioxidant properties of glutathione and metallothioneins [12], but also interferes with glucose capture into cells [7], signal transduction pathways [6], and the one-carbon and citric acid metabolism [13].
Some studies have evaluated individual metals, mainly essential, in relation to specific metabolites subclasses, especially lipoproteins and fatty acids [[14], [15], [16], [17], [18]], but also inflammation markers and products of microbiota and microbiota composition [[19], [20], [21], [22], [23]]. However, limited epidemiologic studies have considered the joint influence of metal biomarkers on an extended panel of metabolites. A small pilot study in the Strong Heart Study participants (N = 145) found correlations between several urinary metals (including molybdenum [Mo], Se, Zn, inorganic arsenic and antimony [Sb]) and amino acids, fatty acids and lipid metabolism [24]. Larger epidemiologic studies are needed to confirm these findings.
The main objective of the current analysis was to evaluate the cross-sectional association of essential (urine cobalt [Co] and Mo, and plasma Cu, Se and Zn) and non-essential (urine barium [Ba], Cd, chromium [Cr], Sb, vanadium [V] and arsenic corrected for arsenobetaine [As]) metal exposure biomarkers with NMR-measured plasma metabolites (including amino acids, fatty acids, fluid balance, energy, bacterial co-metabolism, and lipoprotein subclasses) in the Hortega Study, a population-based sample of a general population from Spain. Omics technologies can give an expanded view of metabolites unbalance potentially exerted by metals. In this way, metabolomics coupled to advanced statistical methods, can provide a holistic view of how biological pathways are inter-related, and help to understand the overall impact of metals in cellular metabolism and health. In this study, we summarized correlated metabolites using variable-reduction methods based on principal components (PC). We subsequently evaluated the individual and joint influences of metals on metabolic profiles using traditional and Bayesian Kernel Machine Regression (BKMR) methods, which allow a flexible view of the highly dimensional non-linear inter-relationships among metals and the metabolic patterns represented by the estimated metabolic PCs (mPCs).
Finally, since altered redox metabolism has been postulated to be one of the main mechanisms for the detrimental health effects of metals [6,7,[25], [26], [27]] -indeed several metals were associated to oxidative stress biomarkers in our study population [26,28]-, we also explored candidate gene-environment interactions (i.e. whether carriers of genetic variants in redox-related genes show a differential association of metals with metabolic patterns) and conducted subsequent biological pathway analysis of genes annotated to relevant interacting genetic variants.
2. Materials and methods
2.1. Study population
The Hortega Cohort is a representative sample from beneficiaries of the Hospital Universitario Rio Hortega's catchment area. The examination phase was conducted in 2001–2003 resulting in 1502 adults recruited with ages between 15 and 85 years. The sampling scheme and methodology have been previously reported [29]. We excluded 299 participants missing metabolites, 55 participants missing metals, and 3 participants missing other variables of interest. A total of 1145 participants were included in the final analyses.
The Ethics Committee of the Hospital Universitario Rio Hortega approved the research protocol, and every participant provided informed consent.
2.2. Metals measures
Urine and plasma samples were collected at the 2001–2003 examination visit and kept frozen at <80° in the Hortega Study biorepository. In 2010, plasma Cu, Se and Zn, which are considered as biomarkers of essential metal status, were evaluated by atomic absorption spectrometry (AAS) with graphite furnace at Cerba international Laboratories Ltd. The limit of detection (LOD) (and corresponding coefficient variation [CV]) was 0.63 μg/dL (7.2%) for Cu, 29.9 μg/L (5.6%) for Se and 0.65 μg/dL (4.2%) for Zn; no individual had levels below the LOD for plasma metals. For plasma determinations, Scharlau Standard Solutions were used as reference material for accuracy. In 2013, total urine metals (arsenic, Ba, Cd, Cr, Co, Mo, Sb and V), which are considered as biomarkers of short-term exposure to most non-essential metals, and arsenobetaine (AsB), which was needed to distinguish organic arsenic from seafood, were measured using inductively coupled-plasma mass spectrometry (ICP-MS) and anion exchange high performance liquid chromatography (HPLC) coupled to ICP-MS, respectively, on a 7500 CE spectrometer with octapole reaction cell (Agilent Technologies, Tokyo, Japan). For urine metals, the LOD (and corresponding CV) were 0.024 μg/L (6.5%) for total arsenic, 0.0005 μg/L (1.9%) for Ba, 0.005 μg/L (5.2%) for Cd, 0.038 μg/L (4.3%) for Cr, 0.001 μg/L (3.0%) for Co, 0.01 μg/L (1.8%) for Mo, 0.003 μg/L (5.3%) for Sb and 0.008 μg/L (3.6%) for V. The percentage of individuals below the LOD was 0.07% for Cd, 0.14% for Co and 1.81% for Sb. For other urine metals there were no individuals with undetectable values. The corresponding LOD (CV) for urine AsB was 0.056 μg/L (9.7%), leaving 4.7% of participants with undetectable AsB values. ClinCheck Urine Control for AsB and for total trace elements at Level I and II (RECIPE) were used for accuracy. Reference materials for all metal determinations were traceable to the corresponding National Institute of Standards and Technology references. Quality control was additionally ensured corroborating internal controls. Appropriate analytical controls were within two standard deviations of reference means.
AsB is an organic arsenic specie without toxic effect in human body and it is predominantly found in seafood [30]. As seafood consumption in Spanish population is very high, we needed to consider the contribution of AsB to total arsenic levels. Because arsenic species concentrations, including AsB, were determined only in a randomized subsample of 295 individuals, an imputation strategy for missing data based on Markov Chain Monte Carlo (MCMC) by Gibbs sampling was performed following published methods [1]. For the present analysis, AsB values in the complete dataset after imputation were used to define a biomarker reflecting total urine arsenic concentrations not from seafood by regressing total urine arsenic on AsB [31]. Finally, the mean of total arsenic concentrations among participants with low AsB (defined as individuals with measured or imputed AsB levels below the second percentile of AsB distribution [4.72 μg/L]) was added to the residuals in order to obtain levels of inorganic As exposure meaningful for the population had seafood consumption been minimized. Urine biomarkers in μg/L were divided by urine creatinine in g/L to consider urine dilution.
2.3. Metabolites measures
Metabolomic profiles were detected by Nuclear Magnetic Resonance (NMR) Spectroscopy in non-fasting plasma. Eighty-two microliters of D2O were added to 418 μl of blood plasma and placed in a 5-mm NMR tube. 1H NMR spectra were recorded using a Bruker Avance DRX 600 spectrometer (Bruker GmbH, Rheinstetten, Germany). A single-pulse pre-saturation experiment was acquired in all samples. Measurements were performed at 37 °C. The spectra were referenced using the doublet of Alanine at 1,478 ppm. The aliphatic region of the spectra was investigated. To remove differences in metabolite total concentration, the spectra were normalized to total aliphatic spectral area. Signals belonging to selected metabolites were quantified using semi-automated in-house MATLAB 6.5 (The MathWorks Inc., Natick, Massachusetts) integration and peak-fitting routines. To identify and subsequently confirm the assessment of metabolites, we used Chenomx NMR Suite 4.5 software and two-dimensional NMR technology including homonuclear correlation spectroscopy and heteronuclear single quantum correlation spectroscopy. We used Human Metabolome Database [32] and 2D NMR experiments to aid the structural identification of relevant metabolites.
In addition, 500 μl of blood plasma samples were shipped on dry ice to Biosfer Teslab (Reus, Spain) for an advanced lipoprotein profiling by using the LIPOSCALE® test, a commercially available methodology based on 2-D diffusion-ordered 1H NMR spectroscopy [33]. The lipoprotein profile characterization included the lipid content and size of the main lipoprotein classes (very low-density lipoprotein [VLDL], low-density lipoprotein [LDL] and high-density lipoprotein [HDL]), and the particle concentration of its respective large, medium and small lipoprotein subclasses.
We adjusted all metabolites by fasting time (hours) to remove the potential variability introduced by fasting, and we recalibrated the distribution of resulting residuals to metabolites mean in individuals reporting fasting condition.
2.4. DNA isolation, SNP selection and genotyping
Using Chemagic System (Chemagen), we obtained DNA diluted in a concentration of 100 ng/mL from peripheral blood cells. The quality of DNA was considered with PicoGreen dsDNA Quantification Reagent (Invitrogen, Carlsbad, CA, USA). After a bibliography search and the use of SYSNPS program [34], a total number of 341 single nucleotide polymorphisms (SNPs) were identified from 79 oxidative stress-related candidate genes. Those genes encode proteins related to redox and mitochondrial respiratory chain reactions and other pathways involved in oxidative stress processes. Then, we performed an oligo-ligation assay (SNPlex, Applied Biosystems, Foster City, CA) to genotype the SNPs using the appropriate recommendations for the polymorphism nomenclature [35]. All these SNPs have been associated to functional nucleotides changes or health outcomes. Among the 341 selected SNPs, we excluded 33 SNPs because did not have exactly 2 alleles, and 17 SNPs because did not have exactly 3 genotypes, leaving a final number of 291 SNPs for the analyses. The mean coverage of the final 291 included SNPs was 96.9%. For most relevant SNPs, we reported the genotyping coverage, minor allele frequency (MAF) and Hardy Weinberg equilibrium (HWE) P value.
2.5. Other relevant variables
Participants were interviewed by trained staff in order to collect self-reported sociodemographic and lifestyle data. Smoking and alcohol intake were classified as never, former and current status. Diet was assessed with semi-quantitative food frequency and two 24-h recall questionnaires. We collected physical activity information by asking about weekly amount of specific individual activities. First, we assessed the amount and frequency of dedicated time to practice physical activity per week -walking and physically active hobbies, sports, or exercises, including jogging or running, riding a bicycle or an exercise bicycle, swimming, aerobic dancing, other dancing, calisthenics or floor exercises, gardening or yard work, and weight lifting- and subsequently we estimated METs-minute/week following the equivalences in the Compendium of Physical activities [36] to obtain the METs-minute/week. Second, we added the METs-minute/week for all the reported activities for each participant. We categorized physical activity below and above 3000 METs minute/week, since it has been shown that lower risk for diabetes, stroke and other outcomes occurred especially above 3000 METs minute/week [37]. Urine cotinine was measured by enzyme-linked immunosorbent assay (ELISA) (“Analysis DRI® Cotinine” Kit, Ref. 0395 Microgenics laboratories), with concentrations below the LOD (34 ng/mL) in 77% of the participants. We defined diabetes as fasting plasma glucose ≥126 mg/dL, hemoglobin A1c (HbA1c) ≥ 6.5%, medical record of type 2 diabetes or medication use. High cholesterol was defined as a total cholesterol ≥200 mg/dL or lipid lowering medication. Body mass index (BMI) was calculated from the weight (kilograms) divided into height (meters) squared. Obesity was defined as a BMI equal or higher than 30 kg/m2. Urine and serum creatinine were measured by the modified kinetic Jaffé method by isotope dilution mass spectrometry on a Hitachi 917 analyzer (Rocher, Boheringer, Germany). Kidney function was assessed with the glomerular filtration rate (eGFR), estimated by CKD-EPI equation [38].
2.6. Statistical analysis
Descriptive analysis. We estimated median and interquartile range of plasma metabolites by participants characteristics. We used principal components analysis (PCA) to summarize correlated metabolites. First, we standardized plasma metabolites by calculating a z-score on the fasting-adjusted residuals. To maximize the variances of the factor loading across variables we calculated the varimax rotation. Subsequently, we described participants characteristics above and below median mPC scores.
Association analysis. The association between metals (independent variables) with mPCs (dependent variables) was evaluated by adjusted linear regression introducing first one metal at a time as independent variable in separate models (so called “single-metal” model). For each statistically significant metal from single-metal models, we also ran models further adjusted for other statistically significant metals (so-called “multiple-metals” model). Metal concentrations were modeled as log-transformed continuous variables, and the obtained regression coefficients were re-scaled to compare the 80th to the 20th percentiles of metal distributions. Statistical models were adjusted for age (years), sex (female, male), education (<high school, ≥high school), smoking status (never, former, current), urine cotinine levels (<34, 34–500, ≥500 ng/mL), cumulative smoking (packs-years), alcohol intake (gr/day), eGFR (mL/min/1.73 m2), lipid lowering medication treatment (yes, no), exercise (METs min/week) and total triglycerides (mg/dL). In preliminary analysis, we also adjusted for total energy, fat, carbohydrate, and protein intakes and BMI, with no substantial change in the results. We, thus, excluded these variables from subsequent analysis to avoid excluding additional participants with missing values.
Metal mixture analysis using BKMR. Due to the difficulties to apply regression models when considering multiple metals simultaneously in the models, including between-metal correlations, non-linear relationships and high order interactions within mixture components [39], we run flexible BKMR models separately for each of the estimated mPCs endpoints. Posterior inclusion probabilities (PIPs) obtained from the BKMR models gives a measure of how likely a compound within a the mixture in the model is driving the association of the full mixture [40]. BKMRs also enables the evaluation of the full-mixture dose response as well as specific dose responses for individual metals when all the others are fixed at a given percentile of their distribution.
Gene-environment interaction. To explore the potential interaction of metals with SNPs annotated to candidate genes involved in oxidative stress pathways, we included an interaction term in separate linear regression models for log-transformed metals by the individual SNPs. For each SNP we assumed an additive model (0, 1, or 2, minor allele dosage). We considered strongly suggestive statistical interactions if the P value associated to the interaction regression coefficient was ≤ 1 × 10−3. We also reported statistically significant interactions according to a Bonferroni P value cut-off of 1.7 × 10−4 (estimated as 0.05 divided by 291 SNPs).
Biological pathway analysis. In brief, we performed KEGG pathway enrichment and network analysis with genes annotated to SNPs with interaction P values≤ 1 × 10−3. The enrichment analysis aims to provide a global view of significant data, as it takes into account the accumulated biological knowledge of how genes work together, allowing the identification of predominant or enriched pathways. One of the most widely used databases for enrichment analysis is KEGG (Kyoto Encyclopedia of Genes and Genomes), which is a knowledge base for the systematic analysis of gene functions that links genomic information with high-level functions in the biological systems [41]. The significance threshold for KEGG pathway enrichment was set to a P value ≤ 0.05 based on a two-sided hypergeometric exact test. We conducted pathway enrichment analysis out of the union set of interacting genes annotated across the four mPCs (i.e. “overall pathway enrichment”) and, also, separately by specific mPCs (i.e. “mPC-specific pathway enrichment”). We also descriptively show pathways within the KEGG database that contain at least one of the genes annotated to statistically suggestive and significant interactions separately for specific mPC. A Kappa statistic, which is used to define KEGG terms interrelations (edges) and functional groups based on shared genes between terms, was estimated and set to 0.6 [42,43].
Statistical analysis was conducted with “survey” package in R software (version 4.0.4) to account for the complex survey design. For the joint analysis of metals, we used MCMC algorithm as conducted by the BKMR package in R software [39]. We conducted pathway enrichment and network analysis of genes that showed statistically significant and suggestive gene-metal interactions using Cytoscape (version.3.8.2) [44] with the ClueGO (version 2.5.8) and CluePedia (version 1.5.8) [42,43] plugins.
3. Results
3.1. Descriptive analysis
The geometric mean of urine metals were 0.25 μg/g for Co, 26.1 μg/g for Mo, 6.54 μg/g for As, 62.04 μg/g for Ba, 0.37 μg/g for Cd, 3.55 μg/g for Cr, 0.07 μg/g for Sb, 2.08 μg/g for V. The corresponding geometric means for plasma metals were 93.8 μg/dL for Cu, 83.7 μg/L for Se, 77.1 μg/dL for Zn. In PCA, four mPC with eigen values > 2 explained 40.3%, 16.3%, 12.5% and 9.2% respectively of the metabolites joint variability. Based on the estimated loadings, mPC1 mostly reflected non-essential and essential amino acids including branched-chain (BCAA) (leucine, isoleucine, and valine) and bacterial co-metabolism (trimethylamines, isobutyrate and phenylpropionate) versus fatty acids and VLDL subclasses; mPC2 reflected essential including aromatic amino acids (AAA) (tyrosine, tryptophan), fluid balance and bacterial co-metabolism (isopropanol and methanol); mPC3 reflected LDL and mPC4 HDL subclasses (Supplemental Fig. S1). Females, never smokers and drinkers, and participants without obesity and diabetes showed higher mPC1 and mPC2. On the contrary, participants with higher mPC3 overall showed an unhealthier cardiovascular profile (Table 1). Participants with higher mPC4 were more likely women, never smoker, but with lower physical activity levels. Correspondingly, while men and elder participants and participants with obesity and diabetes had lower plasma amino acids concentrations, they showed higher fatty acids and pro-atherogenic lipoprotein levels and unhealthy lifestyle (Supplemental Table S1).
Table 1.
Participant characteristics in subgroups defined by median metabolic principal component (mPC) levels in the Hortega Study (N = 1,145).
| mPC1 | mPC2 | mPC3 | mPC4 | |||||
|---|---|---|---|---|---|---|---|---|
| ≤0.27 | >0.27 | ≤0.03 | >0.03 | ≤-0.13 | >-0.13 | ≤-0.12 | >-0.12 | |
| Female %, (N) | 37.7 (224) | 64.36 (343) | 51.69 (283) | 50.33 (284) | 51.03 (274) | 50.99 (293) | 29.03 (158) | 73.07 (409) |
| Age (years), Mean (SE) | 52.26 (0.52) | 44.95 (0.49) | 49.73 (0.52) | 47.48 (0.52) | 44.04 (0.5) | 53.18 (0.51) | 45.73 (0.47) | 51.5 (0.49) |
| Never Smoker, % (N) | 39.94 (252) | 49.82 (282) | 45.21 (265) | 44.54 (269) | 44.4 (253) | 45.35 (281) | 38.96 (225) | 50.81 (309) |
| Former Smoker, % (N) | 31.07 (195) | 25.94 (148) | 30.37 (180) | 26.65 (163) | 27.64 (167) | 29.38 (176) | 31.03 (182) | 25.98 (161) |
| Current Smoker, % (N) | 28.99 (146) | 24.23 (122) | 24.43 (124) | 28.81 (144) | 27.95 (145) | 25.28 (123) | 30.00 (150) | 23.22 (118) |
| Alcohol intake (gr/day), Mean (SE) | 14.48 (1.08) | 8.99 (0.75) | 12.45 (1.06) | 11.03 (0.78) | 12.79 (0.93) | 10.69 (0.93) | 13.83 (0.94) | 9.65 (0.93) |
| Obesity, % (N) | 23.45 (141) | 10.31 (61) | 17.77 (106) | 16.13 (96) | 13.36 (79) | 20.58 (123) | 18.02 (105) | 15.91 (97) |
| Diabetes, % (N) | 10.26 (79) | 1.99 (15) | 6.73 (52) | 5.52 (42) | 6.37 (49) | 5.88 (45) | 7.08 (55) | 5.17 (39) |
| High cholesterol, % (N) | 60.53 (363) | 45.59 (255) | 63.73 (366) | 42.4 (252) | 23.98 (148) | 82.21 (470) | 48.49 (273) | 57.67 (345) |
| Total triglycerides (mg/dL), Mean (SE) | 238.2 (5.13) | 112.1 (1.45) | 197.1 (5.25) | 153.3 (3.44) | 162.9 (4.2) | 187.6 (4.78) | 181.5 (4.66) | 168.9 (4.4) |
| eGFR (mL/min/1.73m2), Mean (SE) | 90.3 (0.77) | 98.2 (0.7) | 92.4 (0.77) | 96.0 (0.72) | 98.0 (0.73) | 90.4 (0.75) | 96.1 (0.72) | 92.3 (0.75) |
| Exercise <3000 METs min/week, % (N) | 41.74 (245) | 38.88 (208) | 39.95 (223) | 40.67 (230) | 41.81 (234) | 38.81 (219) | 44.05 (247) | 36.57 (206) |
Abbreviations: SE: standard error; eGFR: estimated glomerular filtration rate.
3.2. Association analysis
In our study population, plasma Se and Zn were positively associated with mPC1 (mean difference [MD] [95%CI] 0.06 [0.01, 0.10] and 0.07 [0.01, 0.13], respectively), while plasma Cu, urine As and Sb were inversely associated with mPC1 (MD [95%CI] −0.10 [−0.16, −0.05], −0.05 [−0.09, 0.00] and −0.08 [−0.15, 0.00], respectively). Plasma Se and Zn and urine Cd were inversely associated with mPC2 (MD [95%CI] −0.20 [−0.35, −0.05], −0.15 [−0.27, −0.04] and −0.18 [−0.33, −0.04], respectively). The association with mPC3 was positive for plasma Cu, Se and Zn (MD [95%CI] 0.20 [0.10, 0.30], 0.16 [0.07, 0.26] and 0.19 [0.07, 0.30], respectively) and inverse for urine Co (MD [95%CI] −0.10 [−0.19, −0.02]). The association with mPC4 was inverse for Zn (MD [95%CI] −0.15 [−0.23, −0.06] and positive for Sb (0.15 [0.01, 0.30]) (Table 2). In multiple-metal models, the associations identified between metals and mPCs remained basically unchanged (Supplemental Table S2).
Table 2.
Mean difference (MD) (95% CI) of metabolic principal components (mPC1 to mPC4) comparing the 80th to the 20th percentile of the urine metal distribution in adult participants from the Hortega Study (N = 1,145).
| mPC1 | mPC2 | mPC3 | mPC4 | |
|---|---|---|---|---|
| Essential metals | ||||
| Urine Co (μg/g) | ||||
| MD (95% CI) | −0.02 (−0.07, 0.03) | −0.05 (−0.13, 0.03) | −0.10 (−0.19, −0.02) | 0.05 (−0.02, 0.12) |
| P value | 0.43 | 0.20 | 0.02 | 0.18 |
| Plasma Cu (μg/dL) | ||||
| MD (95% CI) | −0.10 (−0.16, −0.05) | 0.03 (−0.07, 0.12) | 0.20 (0.10, 0.30) | 0.08 (−0.02, 0.18) |
| P value | <0.001 | 0.60 | <0.001 | 0.11 |
| Urine Mo (μg/g) | ||||
| MD (95% CI) | −0.01 (−0.05, 0.04) | −0.07 (−0.15, 0.02) | −0.08 (−0.16, 0.01) | 0.03 (−0.05, 0.11) |
| P value | 0.75 | 0.12 | 0.10 | 0.42 |
| Plasma Se (μg/L) | ||||
| MD (95% CI) | 0.06 (0.01, 0.10) | −0.20 (−0.35, −0.05)a | 0.16 (0.07, 0.26) | 0.02 (−0.06, 0.10) |
| P value | 0.03 | 0.01a | 0.001 | 0.61 |
| Plasma Zn (μg/dL) | ||||
| MD (95% CI) | 0.07 (0.01, 0.13) | −0.15 (−0.27, −0.04) | 0.19 (0.07, 0.30) | −0.15 (−0.23, −0.06) |
| P value | 0.02 | 0.007 | 0.001 | 0.001 |
| Non-essential metals | ||||
| Urine As (μg/g) | ||||
| MD (95% CI) | −0.05 (−0.09, 0.00) | −0.03 (−0.11, 0.06) | −0.02 (−0.11, 0.07) | 0.03 (−0.05, 0.11) |
| P value | 0.03 | 0.54 | 0.65 | 0.44 |
| Urine Ba (μg/g) | ||||
| MD (95% CI) | 0.00 (−0.04, 0.05) | −0.03 (−0.12, 0.06) | −0.07 (−0.16, 0.02) | −0.01 (−0.09, 0.07) |
| P value | 0.88 | 0.55 | 0.11 | 0.84 |
| Urine Cd (μg/g) | ||||
| MD (95% CI) | 0.01 (−0.04, 0.05) | −0.18 (−0.33, −0.04)a | −0.06 (−0.15, 0.03) | 0.02 (−0.06, 0.10) |
| P value | 0.76 | 0.01a | 0.17 | 0.66 |
| Urine Cr (μg/g) | ||||
| MD (95% CI) | −0.03 (−0.07, 0.02) | 0.01 (−0.08, 0.11) | −0.05 (−0.14, 0.04) | 0.05 (−0.04, 0.13) |
| P value | 0.26 | 0.83 | 0.26 | 0.30 |
| Urine Sb (μg/g) | ||||
| MD (95% CI) | −0.08 (−0.15, 0.00)a | 0.03 (−0.06, 0.11) | −0.03 (−0.11, 0.06) | 0.15 (0.01, 0.30)a |
| P value | 0.04a | 0.58 | 0.55 | 0.04a |
| Urine V (μg/g) | ||||
| MD (95% CI) | −0.04 (−0.08, 0.01) | 0.02 (−0.08, 0.11) | −0.05 (−0.14, 0.04) | 0.05 (−0.03, 0.13) |
| P value | 0.11 | 0.76 | 0.25 | 0.23 |
Model adjusted for age (years), sex (male and female), education (<high school, ≥high school), smoking status (never, former and current smoker), cotinine (<34, 34–500, ≥500 ng/mL), cumulative smoking (packs-years), alcohol intake (gr/day), eGFR (mL/min/1.73 m2), lipid lowering medication treatment (yes, no), exercise (METs min/week) and total triglycerides (mg/dL).
The 80th and 20th percentiles of essential metals biomarkers distributions were 0.63 and 0.12 μg/g for Co, 126.8 and 70.9 μg/dL for Cu, 58.6 and 10.7 μg/g for Mo, 103.6 and 68.8 μg/L for Se and 99.6 and 61.2 μg/dL for Zn; and for non-essential metals were 13.2 and 3.3 μg/g for As, 120.5 and 28.8 μg/g for Ba, 0.75 and 0.19 μg/g for Cd, 6.5 and 1.9 μg/g for Cr, 0.2 and 0.03 μg/g for Sb, and 3.8 and 1.2 μg/g for V.
Non-linear associations with corresponding metals modeled as restricted quadratic splines.
3.3. Bayesian Kernel Machine Regression analysis
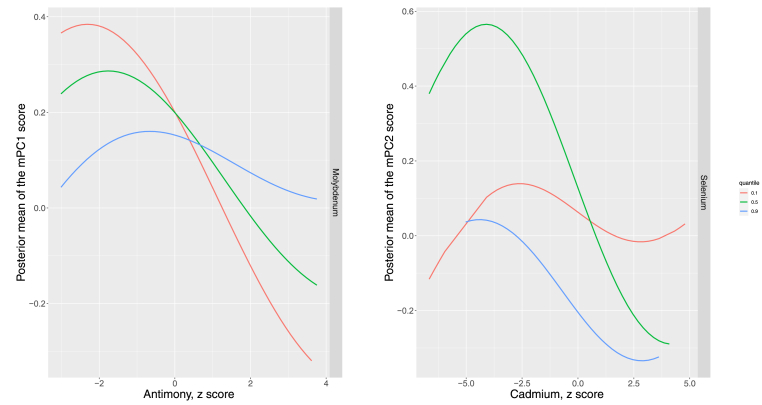
We ran BKMR models with variable selection to identify most relevant metals from our linear regression results based on the estimated PIPs. The highest PIPs in mPC1 models were 0.72 for Cu and 0.43 for Sb; the corresponding PIPs were 0.82 for Zn, 0.80 for Se and 0.35 for Cd in mPC2 models; 0.98 for Zn, 0.67 for Co and 0.33 for Se in mPC3 models, and 0.91 for Zn in mPC4 models (Supplemental Table S3). In addition, the full metals mixture was associated with mPCs 1 to 3, but not mPC4 (Supplemental Fig. S2). Metals within the mixture did not show relevant interactions for most mPCs, except a potential interaction of Sb by Mo with mPC1, and Cd by Se with mPC2 (Fig. 1). Supplemental Figs. S3–S6 show the bivariate-metal exposure response association (difference in the mPCs scores) for a given metal, considering a second metal fixed to the 25th, 50th and 75th percentiles.
Fig. 1.
Posterior mean of the difference in mPC1 and mPC2 scores by antimony and cadmium levels, when molybdenum and selenium, respectively, are fixed at its 10th, 50th and 90th percentiles.
Flexible dose responses were estimated form a Bayesian Kernel Machine Regression (BKMR) model adjusted for age (years, splines), sex (men and women education (<high school, ≥high school), smoking status (never, former and current smoker), cotinine (<34, 34–500, ≥500 ng/mL), cumulative smoking (packs-years), alcohol intake (gr/day), eGFR (mL/min/1.73m2), lipid lowering medication treatment (yes, no), exercise (METs min/week) and total triglycerides (mg/dL). The 10th, 50th, and 90th percentiles of urine metal distributions were, respectively, 7.33, 25.24 and 96.97 μg/g for Mo, and 61.27, 84.34 and 114.70 μg/L for Se. For example, the exposure-response association is interpreted as the posterior mean of the difference in mPC1 score by Sb concentrations (X axis) when Mo (Z axis) is fixed to the 10th, 50th and 90th percentiles. The lines for the exposure-response associations when the second metals fixed are not parallel, suggesting a potential interaction.
3.4. Candidate gene-metals interaction
In gene-environment interaction analyses between metals and redox-related SNPs with mPCs, 20 genetic variants showed interaction P values < 0.001 (6 of them also statistically significant at the Bonferroni significance threshold, including SNPs annotated to XDH, NDUFS6, COX6B1, NOX1, NOX5 and AGT) (Supplemental Table S4).
For essential metals, Co showed interactions with mPC1 by rs3730103 (REN), with mPC3 by rs139998 (TXN2), rs2071429 (G6PD), rs1014852 (GCLC) and rs1002149 (GSR), and with mPC4 by rs1926723 (AGT) and rs699 (AGT); Cu showed interactions with mPC3 by rs35404864 (NOX1); Mo showed interactions with mPC4 by rs35404864 (NOX1), rs1492078 (AGTR1) and rs35887529 (RAC1); Se showed interactions with mPC3 by rs1014852 (GCLC) and with mPC4 by rs35404864 (NOX1); Zn showed interactions with mPC1 by rs45564939 (XDH); with mPC2 by rs2647169 (SDHB) and rs4806187 (COX6B1) and with mPC3 by rs11122576 (AGT).
For non-essential metals, As showed interactions with mPC2 by rs2911678 (GSR), with mPC3 by rs3215332 (REN) and rs11571092 (REN), and with mPC4 by rs1133322 (COX5A); Ba showed interactions with mPC1 by rs1002149 (GSR) and rs972891 (NDUFS6) and with mPC4 by rs35404864 (NOX1), rs3749930 (NOX3), rs2036343 (NOX5) and rs2871 (NR3C2); Cd showed interactions with mPC1 by rs6849903 (NR3C2) and with mPC3 by rs4889657 (COX6A2); Cr showed interactions with mPC1 by rs35404864 (NOX1) and with mPC2 by rs12907196 (NOX5); Sb showed interactions with mPC2 by rs2071409 (LPO) and with mPC3 by rs12095517 (AGTRAP); and V showed interactions with mPC2 by rs12907196 (NOX5) and with mPC3 by rs35404864 (NOX1).
3.5. Biological pathway analysis of genes related to interacting SNPs
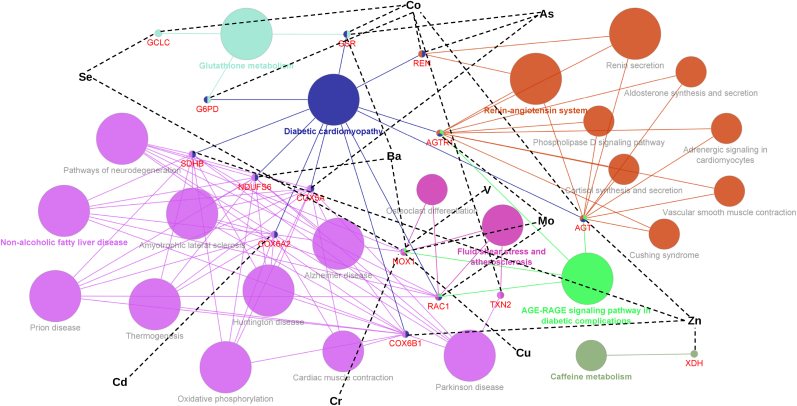
In overall KEGG enrichment analysis, “Diabetic cardiomyopathy” was the most enriched term among the union set of redox-related genes annotated to SNPs that showed relevant statistical interactions with metals across all mPCs (11 genes interacting with Co, Mo, Zn, As, Ba and Cd), followed by “Pathways of neurodegeneration” (7 genes interacting with Cu, Mo, Se, Zn, As, Ba, Cd, Cr and V), “Non-alcoholic fatty liver disease”, “Amyotrophic lateral sclerosis” and “Prion disease” (6 genes interacting with Mo, Zn, As, Ba and Cd); “Alzheimer disease” (6 genes interacting with Cu, Mo, Se, Zn, As, Ba, Cd, Cr, and V) and “Parkinson disease” (6 genes interacting with Co, Zn, As, Ba and Cd) (Fig. 2). NOX1, which interacted with 6 out of 11 metals (Cu, Mo, Se, Ba, Cr and V), was associated with other biological pathways such as “AGE-RAGE signaling pathway in diabetes complications” and “Fluid shear stress and atherosclerosis” (Fig. 2). Other relevant genes annotated to SNPs interacting with more than one metal were GSR (interactions with Co, As and Ba), AGT (interactions with Co and Zn), GCLC (interactions with Co and Se) and REN (interactions with Co and As) (Fig. 2). Supplemental Figs. S7–S10 descriptively show the connection of KEGG biological pathways that included at least one gene annotated to identified interacting SNPs, separately for mPC-specific subnetworks.
Fig. 2.
Enriched KEGG pathways out of the union set of redox-related genes from single nucleotide polymorphisms interacting with metals across all mPCs.
Overall integrative network showing the statistically significant KEGG pathways from hypergeometric enrichment analysis (P value ≤ 0.05), which was conducted out of the union set of genes annotated to SNPs interacting with metals considering all 4 mPCs. KEGG pathways are represented as large nodes and the node size is directly proportional to the term enrichment -log10 P value (larger nodes reflect higher statistical significance). Nodes with the same colors reflect they belong to the same clustering group according to an estimated Kappa score. The nodes with colored letters represent the most significant pathways per clustering group. The equally sized smallest nodes with annotated red gene symbols can be filled with more than one color indicating that they are contributing to different pathways clustered in different groups. The metals are indicated using black characters, and the corresponding black dashed line indicates that the interaction term in gene-environment interaction regression analysis was associated with a P value < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
In our population-based study, Co, Cu, Se, Zn, As, Cd and Sb exposure biomarkers were related to specific metabolic patterns. Particularly, Cu, Se, Zn, As, and Sb showed associations with a metabolic pattern (mPC1) reflecting non-essential and essential amino acids -including BCAA-, and bacterial co-metabolism, versus fatty acids and VLDL. Se, Zn and Cd showed associations with a metabolic pattern (mPC2) reflecting other essential amino acids -including AAA-, and bacterial co-metabolism. Co, Cu, Se and Zn showed associations with a metabolic pattern (mPC3) reflecting LDL. Zn and Sb showed associations with a metabolic pattern (mPC4) reflecting HDL. We identified gene-metal interactions in our data, which pointed to shared biological pathways with a role in endocrine, cardiometabolic and neurological diseases. Co and Zn showed, overall, the highest number of statistical interactions annotated to genes from enriched biological pathways.
Metal exposure and exposure biomarkers. For this study we used metal exposure biomarkers that integrate all exposure sources including food, water and air [45]. Particularly, we measured some essential metals, such as Se [46] and Zn [47], in plasma, which is an accepted biomarker of current status. While plasma is also considered a biomarker of nutritional status in Cu-deficient populations, it is unclear whether in Cu-repleted populations plasma Cu reflects exposure or, rather, Cu endogenous metabolic capacity [48]. For the rest of metals, total urine biomarkers mostly reflect recent exposure due to short half-life [49], except for Cd, with a longer half-life component, which is also interpreted as long term presence in the body [50], and As, which in our study was corrected for AsB values to reflect inorganic As exposure not from seafood following well-accepted methods [51] because seafood consumption is substantial in Spain. Interestingly, under continuous and maintained exposure conditions, urine could also be a good indicator for long-term exposure to metals, as it has been shown for inorganic As [52]. Metal exposure biomarkers are widely used in the field of environmental health sciences. Indeed, variation in metal exposure biomarkers has been related to multiple health endpoints in human populations, including samples of general populations such as our study population [1,3,4,53] and the US National Health and Nutrition Examination Survey [[54], [55], [56]], which showed relatively low metal exposure levels.
Metals and metabolomics. Some studies have evaluated the role of metabolomic profiling as biomarkers in pathologies such as cancer [[57], [58], [59]], renal [60,61], neurological [62], and mainly, cardiometabolic diseases [63,64]. However, few studies have explored the potential role of joint metals exposure as determinants of metabolites levels, which is relevant because it implies that metabolomic changes may mediate well-established associations of metals with health outcomes, including neurological [65], bone [66], kidney disease [67], cancer [[68], [69], [70]] and CVD [71]. In our data, the reported associations of Sb and Co with metabolic patterns are novel, except for two experimental studies that evaluated the role of Co on lipoprotein metabolism with heterogenous conclusions [72,73]. For other relevant metals, we will next review the consistency of our findings with available epidemiologic and mechanistic studies.
Amino acids. Amino acids are the basic protein structure, which are generally needed for homeostasis, nutrition and immune system regulation [74]. Among them, dysfunctional levels of BCAA (isoleucine, leucine and valine), which are essential amino acids, have been mainly related to cardiometabolic and vascular outcomes [75,76]. On the other side, AAA (tyrosine and tryptophan) are precursors of catecholamines, dopamine, melatonin and serotonin. While AAA deficiency has been related to neurological [77] and cancer conditions [78], disproportionate AAA levels are also related to metabolic and cardiovascular disease (CVD) [79].
In our data, mPC1 mainly reflected increased non-essential and essential amino acids including BCAA, whereas metabolic pattern mPC2 mainly reflected other amino acids including AAA. A body of evidence has reported the potential impact of metal exposure in amino acid levels [24,[80], [81], [82]] but there is still a major research gap. Consistent with our results, a cross-sectional study in children (N = 155) found that serum Zn was positively correlated to valine concentrations [77], and an experimental study with chickens reported that Se supplementation increased leucine and alanine and decreased cysteine and tyrosine levels [82]. Inconsistently to our results, a small study from the Strong Heart Study (N = 145) reported that urine Se and As were positively associated with plasma tryptophan and proline, respectively [24]. Nevertheless, plasma Se, a better-established biomarker of Se status compared to urine, was not available for a direct comparison with our results. Our results are not either consistent with those found in a small Mexican study, in which urine As was positively associated with proline and inversely with tyrosine [81]. In addition, an experimental study with chickens found that Cd supplementation in diet decreased cysteine, tyrosine and alanine, and increased leucine, as measured in pectoral muscles [82]. The exposure route and dose may not be extrapolated to our study population, where one of the main Cd exposure sources was smoking. In general, previous epidemiologic studies were small, so random chance cannot be discarded as the most likely explanation for the findings, or results were not directly comparable because the exposure biomarkers or sources were different. Interestingly, in our study we observed potential interactions of Sb by Mo for mPC1 and Cd by Se for mPC2. There are no available experimental studies supporting the Sb by Mo interaction. Interestingly, a study in mice suggested that Cd accumulation in several tissues depended on Se levels [83].
Bacterial co-metabolism. The microbiota hosted in humans stimulates immune system and contributes to metabolism [84]. Consequently, microbiome imbalance could have a role on the progress of cardiometabolic [85], infectious and inflammatory disease [86]. Some studies have pointed to a potential role of trimethylamines (TMAO), products of gut metabolism, in the pathogenesis of cardiometabolic conditions [85] including incident cardio-cerebrovascular events and all-cause mortality [87]. In our study, mPC1 (partly reflecting isobutyrate, but also, trimethylamines, phenylpropionate and O-phosphoethanolamine) models showed positive associations for Se and Zn and inverse associations for Cu, As and Sb; and mPC2 (partly reflecting isopropanol and methanol) models showed positive associations for Se, Zn and Cd. Associations of low serum Se and Zn levels and changes in microbiota composition have been reported in cross-sectional studies in Korean adults [88] and Polish children [89]. In a Chinese study, participants with inflammatory bowel disease, which had lower microbial diversity compared with healthy participants, reported lower Se consumption [23]. However, the directionality of these associations is not fully understood as Se modifies microbiota expression, but microbiota can also change Se levels in oxidative stress conditions, because Se is consumed by the bacteria [[90], [91], [92], [93]]. Some experimental studies demonstrate variations in microbiota diversity and composition after metal exposure [21,[94], [95], [96], [97], [98]]. For instance, an experimental study in mice detected changes in gut microbiome after Cd supplementation [94]. Excess Zn supplementation in mice [95] and piglets [97], but also, deficiency dietary Zn in chicken [96] and mice [21], have been related to a decline in gut microbiota diversity, and also genetic expression [98], possibly due to Zn-induced redox changes and competition with other metals for protein binding sites [99]. Additionally, many anaerobic gut bacteria produce acetone during fermentation [100] that can be transformed to pyruvate and acetate, acting as fuel for both glycolysis and ketone bodies metabolism [101], thus, providing a link of human energy metabolism to gut microbiota co-metabolism. In our study, mPC1 and mPC2, which reflected increased microbiota co-metabolism, also reflected increased ketone bodies.
Fatty acids and lipid metabolism. Fatty acids, partly reflected by mPC1 in our data, come from catabolism of dietary lipids and are an energy source with structural and regulatory function, commonly mobilized from the liver to other tissues by VLDL [102]. Lipoproteins, including VLDL, LDL and HDL, which were also partly reflected by metabolic patterns mPC1, mPC3 and mPC4, respectively, are essential for lipids transport and have a well-known role on atherosclerotic, cardiometabolic and inflammatory disease [103].
In our study, Cu exposure was inversely associated with mPC1, reflecting in turn a positive association with VLDL and fatty acids metabolites. Only one cross-sectional study reported statistically significant correlations between Cu exposure with some fatty acids metabolites in plasma [22], but models were not adjusted by dietary factors. Although epidemiological studies have evaluated the role of Cu levels in altering the lipoprotein profile [16,104,105], few of them have included VLDL biomarkers, except for a small Turkish study (N = 177), which did not find a statistically significant correlation between Cu and VLDL [80].
In addition, Se levels were positively associated with mPC1, reflecting in turn an inverse association with VLDL, and mPC3, reflecting a positive association with LDL. A meta-analysis of 3 randomized controlled trials conducted in healthy participants concluded, consistently with our findings, that Se supplementation was inversely related to VLDL cholesterol [106]. However, a previous meta-analysis of five clinical trials did not appreciate differences in VLDL after Se supplementation [107]. The inclusion criteria were limited, however, to studies including only participants with metabolic syndrome. Moreover, our results are largely consistent with a number of cross-sectional studies reporting a positive association between serum Se and LDL cholesterol, particularly in Se-repleted populations [14,18,[108], [109], [110]], but also in studies using other Se biomarkers such as urine Se [111]. Alternatively, two meta-analysis (one of eight cross-sectional studies and another of five clinical trials) did not find an association between Se supplementation and LDL levels [106,107]. Conversely, a Mendelian Randomization study from Europe found that genetically elevated blood Se levels were associated with lower LDL cholesterol [112,113], which suggests that genetically determined Se may have different influence on metabolism compared to Se determined by exposure.
Alternatively, Zn exposure was positively associated with mPC1 (which in turns reflected inverse relation with VLDL) and mPC3 (which reflected a positive relation with LDL), and inversely associated with mPC4 (which reflected an inverse relation with HDL). Although evidence about Zn and VLDL levels is scarce, our results are consistent with two randomized clinical trials [114,115] and one experimental study in mice [116] that reported an inverse association between Zn supplementation and VLDL cholesterol. While some studies did not find statistical significant associations between Zn exposure and LDL [110,117,118] or HDL [119,120], our results are consistent with two cross-sectional studies [109,121] that reported a positive association between Zn and LDL cholesterol and with three cross-sectional studies [117,121,122] and one meta-analysis of clinical trials [118] that reported an inverse association between Zn supplementation and HDL cholesterol in healthy participants younger than 40. Opposite to our results, two small cross-sectional studies [N = 202 and 255 [123,124]] and one meta-analysis of clinical trials evaluating Zn supplementation in patients with diabetes [125] reported inverse association between Zn and LDL metabolism and positive association between Zn and HDL metabolism. However, the heterogeneous study populations, limited sample sizes, and the wide range of Zn dosage or supplementation across studies makes that these results are not directly comparable with ours.
Finally, in our population As was inversely associated with mPC1, thus reflecting a positive association with VLDL. This finding is consistent with one study that found a positive association between serum As and VLDL [126]. Additionally, we did not observe any association between As levels with mPCs reflecting other lipoproteins, such as HDL and LDL. However, previous literature, including a recent meta-analysis of five studies, found that As exposure measured as water intake and urine levels was positively associated with LDL and inversely associated with HDL [127]. More studies are needed to confirm and understand the potential role of these metals on lipid metabolism.
Candidate gene-metal interaction and associated biological pathways. Gene-environment interactions and subsequent pathway enrichment analysis can potentially point to additional biological mechanisms by which the evaluated metals may induce chronic diseases in the population. While the observed gene-metal interactions need validation, the findings are biologically plausible and hypothesis-generating. For instance, a rare variant associated to NOX1, which encodes NADPH oxidase, releases reactive oxygen species (ROS) [128] and is involved in dysfunctional immune and inflammation response [129], showed statistical interactions with most of the metals. While this finding must be taken with caution given the low minor allele frequency, an in vitro study found that As and Cd stimulate NADPH oxidase activity leading to cell proliferation, essential mechanism in cancer progression [130]. Interestingly, the minor allele frequency of other SNPs was not low. For instance, relevant statistical interactions in our data were observed for GSR (Co and Se-interacting SNPs) and GLCL (Co, As and Ba-interacting SNPs), which have a key role in glutathione metabolism [131]. Remarkably, in our study population, Se [28] and Ba [26] exposures were related to the ratio of oxidized to reduced glutathione. As and Co exposure have also been related to glutathione metabolism in other studies [132,133]. Other interacting SNPs were annotated to REN (interacting with Co and As) and AGT (interacting with Co and Zn), which encode renin and angiotensinogen, respectively, with a well-known role on blood pressure regulation. Interestingly, an in vitro study reported that cells exposed to As secreted higher angiotensinogen [134], which is consistent with the results of a meta-analysis supporting As exposure as a risk factor for hypertension [135]. Alternatively, some in vitro studies with macrophages [136] and lung epithelial cells [137], showed that exposure to Co, the metal mostly interacting with candidate SNPs in our data, increased ROS production, and also increased the DNA damage [138]. Zn also showed a good number of statistical interactions with candidate SNPs (annotated to XDH and COX6B1, in addition to AGT), which is widely consistent with the well-established redox role of Zn. For instance, Zn stimulates the production of metallothioneins and reduced glutathione, and binds to sulfhydryl groups counteracting oxidative activity [139]. Moreover, Zn deficiency has been related to decreased Cu–Zn superoxide dismutase (SOD1), an enzyme with a high antioxidant activity [140].
Findings from the integrative biological pathway analysis are consistent with accumulated evidence supporting that non-essential metal exposures could play a role in the pathogenesis of several conditions, including CVD for Sb [5,49], As [71] and Cd [71], cancer and renal dysfunction for Cd [67,141,142] and As [67,[143], [144], [145]] and neurological disorders for As [146,147] and Cd [148]. However, the evidence assessing the potential health-effects of essential metals is less clear [2,4,71,[149], [150], [151]]. While tightly regulated levels are needed for physiological functions, overload and deficiency of these metals could lead to pathological conditions. For instance, extreme Cu and Zn levels have been associated to CVD [2,71], metabolic [149] and neurodegenerative diseases [150,151], and Se levels have been related to diabetes [152] and cardiometabolic risk [153,154], consistently with our data. Overall, the displayed interconnections of enriched pathways attributed to the genes interacting with metals is consistent with the hypothesis that metals can directly and indirectly influence redox-related pathways in common for human chronic diseases.
Limitations and strengths. Our study has several limitations. For instance, the interpretation of our findings requires some precaution because diet may modify metabolomic profiles and microbiota composition beyond host cardiometabolic conditions [155]. While we cannot discard residual confounding by dietary intake before the serum collection, we conducted several sensitivity analyses including adjustment for total energy, fat, carbohydrate and protein intake from 24 h recall questionnaires, with essentially similar findings, suggesting that potential confounding by dietary factors likely is not relevant in our data. Importantly, we used well-established metal exposure biomarkers [45] that integrate all exposure sources, not only diet. We cannot discard, however, non-differential measurement error in metal determinations potentially introduced by storage and other laboratory-related issues, which could bias the reported associations towards the null.
Another limitation relates to the cross-sectional design, which cannot address reverse causation and may be especially problematic for some of the metals and metabolites. For instance, in our study, mPC1 partly reflected albumin, which plays a main role in fluid balance regulation. It is well-known that some of the evaluated metals have a high affinity for albumin, particularly divalent metals such as Co, Cu, Zn and Cd, that can compete with each other for the albumin binding sites [156]. Also, mPC2 partly reflected plasma creatinine, a by-product of muscle metabolism whose levels are increased in kidney dysfunction [157]. Decreased glomerular filtration might result in lower urine metal excretion levels under prevalent renal disease. Alternatively, genetic variation is not expected to be affected by the cross-sectional design since SNPs are randomly assigned at conception given the Mendel law of independent assortment [158]. While, given the moderate sample size, we cannot discard that some of the available SNPs may be unbalanced by chance depending on metal and relevant confounders levels, we used fully adjusted gene-metal interaction models as an attempt to address potential confounding. Replication studies, however, are needed to confirm our gene-metal interaction results. In addition, the power was limited to detect gene-metal interactions after Bonferroni correction given the number of genetic variants. Thus, we could have missed relevant gene-metal interactions.
Importantly, the metal-metabolic pattern associations were widely consistent in BKMR analysis, which simultaneously assessed all the metals at a time and is not subject to the multiple comparisons problem, thus providing robustness to our main results. Other strengths of this study include the complex survey design, which makes our results representative of a general population from a region in Spain. Importantly, our study population was exposed to relatively low metal concentrations. Thus, these results may be relevant for other general populations with chronic-low exposure. Moreover, the unique availability of a considerable panel of metabolites and multiple redox-related metals and candidate SNPs measured with high quality procedures are additional study strengths.
5. Conclusions
In conclusion, increased Co, Cu, Se, Zn, As, Cd and Sb exposure biomarkers were related to metabolic patterns that have been traditionally linked to the development of multiple chronic conditions. While our findings should be confirmed in additional studies, including studies with metabolites measured longitudinally, intensified preventive interventions to avoid adverse health consequences of inadequate metal exposure at the individual level may be needed in carriers of specific redox-related genetic variants. Most importantly, our results add to the mounting evidence base in favor of a role of metals in altering human health at exposure levels that are relevant to general populations [1,[3], [4], [5],[53], [54], [55], [56],[65], [66], [67],[69], [70], [71],71,159,160], thus, supporting that public health interventions to prevent uncontrolled exposure to these metals may contribute to decrease the burden associated to chronic diseases.
Funding
This work was supported by the Strategic Action for Research in Health sciences [CP12/03080, PI15/00071, PI10/0082, PI13/01848, PI14/00874, PI16/01402, PI21/00506 and PI11/00726], CIBER Fisiopatología Obesidad y Nutrición (CIBEROBN) (CIBER-02-08-2009, CB06/03 and CB12/03/30,016), the State Agency for Research (PID2019-108973RB- C21 and C22), the Valencia Government (GRUPOS 03/101; PROM- ETEO/2009/029 and ACOMP/2013/039, IDIFEDER/2021/072 and GRISOLIAP/2021/119), the Castilla-Leon Government (GRS/279/A/08) and European Network of Excellence Ingenious Hypercare (EPSS- 037093) from the European Commission. The Strategic Action for Research in Health sciences, CIBERDEM and CIBEROBN are initiatives from Carlos III Health Institute Madrid and co-funded with European Funds for Regional Development (FEDER). The State Agency for Research and Carlos III Health Institute belong to the Spanish Ministry of Science and Innovation. ADR received the support of a fellowship from “la Caixa” Foundation (ID 100010434) (fellowship code “LCF/BQ/DR19/11740016”). MGP received the support of a fellowship from “la Caixa” Foundation (ID 100010434, fellowship code LCFLCF/BQ/DI18/11660001). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nuria Amigo reports a relationship with Biosfer Tesla that includes: board membership.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102314.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Grau-Perez M., Navas-Acien A., Galan-Chilet I., Briongos-Figuero L.S., Morchon-Simon D., Bermudez J.D., et al. Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ. Pollut. 2018;235:948–955. doi: 10.1016/j.envpol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domingo-Relloso A., Grau-Perez M., Briongos-Figuero L., Gomez-Ariza J.L., Garcia-Barrera T., Dueñas-Laita A., et al. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega Follow-Up Study. Int. J. Epidemiol. 2019;48(6):1839–1849. doi: 10.1093/ije/dyz061. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galvez-Fernandez M., Grau-Perez M., Garcia-Barrera T., Ramirez-Acosta S., Gomez-Ariza J.L., Perez-Gomez B., et al. Arsenic, cadmium, and selenium exposures and bone mineral density-related endpoints: the HORTEGA study. Free Radic. Biol. Med. 2021;162:392–400. doi: 10.1016/j.freeradbiomed.2020.10.318. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galan-Chilet I., Grau-Perez M., De Marco G., Guallar E., Martin-Escudero J.C., Dominguez-Lucas A., et al. A gene-environment interaction analysis of plasma selenium with prevalent and incident diabetes: the Hortega study. Redox Biol. 2017;12(April):798–805. doi: 10.1016/j.redox.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grau-Perez M., Caballero-Mateos M.J., Domingo-Relloso A., Navas-Acien A., Gomez-Ariza J.L., Garcia-Barrera T., et al. Toxic metals and subclinical atherosclerosis in carotid, femoral, and coronary vascular territories: the aragon workers health study. Arterioscler. Thromb. Vasc. Biol. 2021;42(1):87–99. doi: 10.1161/ATVBAHA.121.316358. Jan. [DOI] [PubMed] [Google Scholar]
- 6.Valko M., Jomova K., Rhodes C.J., Kuča K., Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016;90(1):1–37. doi: 10.1007/s00204-015-1579-5. Jan. [DOI] [PubMed] [Google Scholar]
- 7.Jomova K., Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. May 10. [DOI] [PubMed] [Google Scholar]
- 8.Lopez C.A., Skaar E.P. The impact of dietary transition metals on host-bacterial interactions. Cell Host Microbe. 2018;23(6):737–748. doi: 10.1016/j.chom.2018.05.008. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraga C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Aspect. Med. 2005;26(4–5):235–244. doi: 10.1016/j.mam.2005.07.013. Aug. [DOI] [PubMed] [Google Scholar]
- 10.Guo Q., Majeed S., Xu R., Zhang K., Kakade A., Khan A., et al. Heavy metals interact with the microbial community and affect biogas production in anaerobic digestion: a review. J. Environ. Manag. 2019;240:266–272. doi: 10.1016/j.jenvman.2019.03.104. Jun 15. [DOI] [PubMed] [Google Scholar]
- 11.Tinkov A.A., Gritsenko V.A., Skalnaya M.G., Cherkasov S.V., Aaseth J., Skalny A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018;235:429–434. doi: 10.1016/j.envpol.2017.12.114. Apr. [DOI] [PubMed] [Google Scholar]
- 12.Ballatori N. Transport of toxic metals by molecular mimicry. Environ. Health Perspect. 2002;110(Suppl 5):689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz-Hernandez A., Kuo C.C., Rentero-Garrido P., Tang W.Y., Redon J., Ordovas J.M., et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin. Epigenet. 2015;7(1):55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.González-Estecha M., Palazón-Bru I., Bodas-Pinedo A., Trasobares E., Palazón-Bru A., Fuentes M., et al. Relationship between serum selenium, sociodemographic variables, other trace elements and lipid profile in an adult Spanish population. J. Trace Elem. Med. Biol. 2017;43:93–105. doi: 10.1016/j.jtemb.2016.12.002. Sep. [DOI] [PubMed] [Google Scholar]
- 15.Disilvestro R.A., Joseph E.L., Zhang W., Raimo A.E., Kim Y.M. A randomized trial of copper supplementation effects on blood copper enzyme activities and parameters related to cardiovascular health. Metab., Clin. Exp. 2012;61(9):1242–1246. doi: 10.1016/j.metabol.2012.02.002. Sep. [DOI] [PubMed] [Google Scholar]
- 16.Song X., Wang W., Li Z., Zhang D. Association between serum copper and serum lipids in adults. Ann. Nutr. Metabol. 2018;73(4):282–289. doi: 10.1159/000494032. Dec. [DOI] [PubMed] [Google Scholar]
- 17.Stranges S., Tabák A.G., Guallar E., Rayman M.P., Akbaraly T.N., Laclaustra M., et al. Selenium status and blood lipids: the cardiovascular risk in young finns study. J. Intern. Med. 2011;270(5):469–477. doi: 10.1111/j.1365-2796.2011.02398.x. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen J., Mei J., Xia L., Zhe L., Guanrui W., Xiaofeng F., et al. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018;37(5):1512–1517. doi: 10.1016/j.clnu.2017.08.025. Oct. [DOI] [PubMed] [Google Scholar]
- 19.Zackular J.P., Moore J.L., Jordan A.T., Juttukonda L.J., Noto M.J., Nicholson M.R., et al. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat. Med. 2016;22(11):1330–1334. doi: 10.1038/nm.4174. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan Y., Wu C., Guo X., Xu Z., Xing C., Cao H., et al. High doses of copper and mercury changed cecal microbiota in female mice. Biol. Trace Elem. Res. 2019;189(1):134–144. doi: 10.1007/s12011-018-1456-1. [DOI] [PubMed] [Google Scholar]
- 21.Sauer A.K., Grabrucker A.M. Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front. Neurosci. 2019;13:1–16. doi: 10.3389/fnins.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X., Huang Y., Xing Y., Hu C., Zhang W., Tang Y., et al. Association of urinary cadmium, circulating fatty acids, and risk of gestational diabetes mellitus: a nested case-control study in China. Environ. Int. 2020;137 doi: 10.1016/j.envint.2020.105527. Apr. [DOI] [PubMed] [Google Scholar]
- 23.Weng Y.J., Gan H.Y., Li X., Huang Y., Li Z.C., Deng H.M., et al. Correlation of diet, microbiota and metabolite networks in inflammatory bowel disease. Journal of Digestive Diseases. 2019;20(9):447–459. doi: 10.1111/1751-2980.12795. Jun. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez T.R., Hu X., Zhao J., Tran V.L., Loiacono N., Go Y.-M.M., et al. An atlas of metallome and metabolome interactions and associations with incident diabetes in the Strong Heart Family Study. Environ. Int. 2021;157 doi: 10.1016/j.envint.2021.106810. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galhardi C.M., Diniz Y.S., Rodrigues H.G., Faine L.A., Burneiko B.C., Ribas B.O., et al. Beneficial effects of dietary copper supplementation on serum lipids and antioxidant defenses in rats. Ann. Nutr. Metabol. 2005;49(5):283–288. doi: 10.1159/000087294. [DOI] [PubMed] [Google Scholar]
- 26.Domingo-Relloso A., Grau-Perez M., Galan-Chilet I., Garrido-Martinez M.J., Tormos C., Navas-Acien A., et al. Urinary metals and metal mixtures and oxidative stress biomarkers in an adult population from Spain: the Hortega Study. Environ. Int. 2019;123:171–180. doi: 10.1016/j.envint.2018.11.055. Feb. [DOI] [PubMed] [Google Scholar]
- 27.García-Sevillano M.A., García-Barrera T., Navarro F., Gómez-Ariza J.L. Analysis of the biological response of mouse liver (Mus musculus) exposed to As2O3 based on integrated -omics approaches. Metallomics. 2013;5(12):1644–1655. doi: 10.1039/c3mt00186e. Dec. [DOI] [PubMed] [Google Scholar]
- 28.Galan-Chilet I., Tellez-Plaza M., Guallar E., Marco G De, Lopez-Izquierdo R., Gonzalez-Manzano I., et al. Plasma selenium levels and oxidative stress biomarkers : a gene – environment interaction population-based study. Free Radic. Biol. Med. 2014;74:229–236. doi: 10.1016/j.freeradbiomed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Tellez-Plaza M., Briongos-Figuero L., Pichler G., Dominguez-Lucas A., Simal-Blanco F., Mena-Martin F.J., et al. Cohort profile: the Hortega Study for the evaluation of non-traditional risk factors of cardiometabolic and other chronic diseases in a general population from Spain. BMJ Open. 2019;9(6):1–10. doi: 10.1136/bmjopen-2018-024073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mozaffarian D., Rimm E.B. Fish intake, contaminants, and human health evaluating the risks and the benefits. JAMA. 2006;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. Oct. [DOI] [PubMed] [Google Scholar]
- 31.Jones M.R., Tellez-Plaza M., Vaidya D., Grau M., Francesconi K.A., Goessler W., et al. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: evidence from MESA and NHANES. Am. J. Epidemiol. 2016;184(8):590–602. doi: 10.1093/aje/kww097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wishart D.S., Guo A., Oler E., Wang F., Anjum A., Peters H., et al. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res. 2022;50(D1):D622–D631. doi: 10.1093/nar/gkab1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mallol R., Amigó N., Rodríguez M.A., Heras M., Vinaixa M., Plana N., et al. Liposcale: a novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. JLR (J. Lipid Res.) 2015;56(3):737–746. doi: 10.1194/jlr.D050120. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorente-Galdos B., Medina I., Morcillo-Suarez C., Heredia T., Carreño-Torres Á., Sangrós R., et al. Select your SNPs (SYSNPs): a web tool for automatic and massive selection of SNPs. Int. J. Data Min. Bioinf. 2012;6(3):324–334. doi: 10.1504/ijdmb.2012.049249. [DOI] [PubMed] [Google Scholar]
- 35.den Dunnen J.T., Antonarakis S. Nomenclature for the description of human sequence variations. Hum. Genet. 2001;109(1):121–124. doi: 10.1007/s004390100505. Feb. [DOI] [PubMed] [Google Scholar]
- 36.Ainsworth B.E., Haskell W.L., Herrmann S.D., Meckes N., Bassett D.R., Tudor-Locke C., et al. Compendium of physical activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. 2011 Aug. [DOI] [PubMed] [Google Scholar]
- 37.Kyu H.H., Bachman V.F., Alexander L.T., Mumford J.E., Afshin A., Estep K., et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857. doi: 10.1136/bmj.i3857. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y., Castro A.F., Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150(9):604. doi: 10.7326/0003-4819-150-9-200905050-00006. Lucy) May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bobb J.F., Valeri L., Claus Henn B., Christiani D.C., Wright R.O., Mazumdar M., et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. doi: 10.1093/biostatistics/kxu058. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsangarides C.G. A bayesian approach to model uncertainty. Int. Monetary Fund Working Paper. 2004:3–19. [Google Scholar]
- 41.Kanehisa M., Goto S. KEGG: kyoto Encyclopedia of genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. Jan 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bindea G., Mlecnik B., Hackl H., Charoentong P., Tosolini M., Kirilovsky A., et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25(8):1091–1093. doi: 10.1093/bioinformatics/btp101. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bindea G., Galon J., Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013;29(5):661–663. doi: 10.1093/bioinformatics/btt019. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., et al. Cytoscape: a software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordberg G., Fowler B.A., Nordberg M., Friberg Lars T., editors. Handbook on the Toxicology of Metals. third ed. Academic Press; Amsterdam ; Boston: 2007. [Google Scholar]
- 46.Högberg J., Alexander J. In: Handbook on the Toxicology of Metals. third ed. Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. 2007. Selenium; pp. 783–807. [Google Scholar]
- 47.Lowe N.M., Fekete K., Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89(6):2040S–2051S. doi: 10.3945/ajcn.2009.27230G. Jun. [DOI] [PubMed] [Google Scholar]
- 48.Harvey L.J., Ashton K., Hooper L., Casgrain A., Fairweather-Tait S.J. Methods of assessment of copper status in humans: a systematic review. Am. J. Clin. Nutr. 2009;89(6):2009S–2024S. doi: 10.3945/ajcn.2009.27230E. Jun. [DOI] [PubMed] [Google Scholar]
- 49.Nigra A.E., Ruiz-Hernandez A., Redon J., Navas-Acien A., Tellez-Plaza M. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Current Environ. Health Rep. 2016;3(4):416–433. doi: 10.1007/s40572-016-0117-9. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordberg G.F., Nogawa K., Nordberg M., Friberg L.T. In: Handbook on the Toxicology of Metals. third ed. Nordberg G.F., Fowler B.A., Nordberg M., Friberg L.T., editors. Elsevier; 2007. Cadmium; pp. 445–486. [Google Scholar]
- 51.Jones M.R., Tellez-Plaza M., Vaidya D., Grau M., Francesconi K.A., Goessler W., et al. Estimation of inorganic arsenic exposure in populations with frequent seafood intake: evidence from MESA and NHANES. Am. J. Epidemiol. 2016;184(8):590–602. doi: 10.1093/aje/kww097. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navas-Acien A., Umans J.G., Howard B.V., Goessler W., Francesconi K.A., Crainiceanu C.M., et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ. Health Perspect. 2009;117(9):1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Domingo-Relloso A., Grau-Perez M., Briongos-Figuero L., Gomez-Ariza J.L., Garcia-Barrera T., Dueñas-Laita A., et al. The association of urine metals and metal mixtures with cardiovascular incidence in an adult population from Spain: the Hortega Follow-Up Study. Int. J. Epidemiol. 2019;48(6):1839–1849. doi: 10.1093/ije/dyz061. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruiz-Hernandez A., Navas-Acien A., Pastor-Barriuso R., Crainiceanu C.M., Redon J., Guallar E., et al. Declining exposures to lead and cadmium contribute to explaining the reduction of cardiovascular mortality in the US population, 1988–2004. Int. J. Epidemiol. 2017;46(6):1903–1912. doi: 10.1093/ije/dyx176. Dec 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Everson T.M., Niedzwiecki M.M., Toth D., Tellez-Plaza M., Liu H., Barr D.B., et al. Metal biomarker mixtures and blood pressure in the United States: cross-sectional findings from the 1999-2006 National Health and Nutrition Examination Survey (NHANES) Environ. Health. 2021;20(1):15. doi: 10.1186/s12940-021-00695-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laclaustra M., Stranges S., Navas-Acien A., Ordovas J.M., Guallar E. Serum selenium and serum lipids in US adults: national health and nutrition examination survey (NHANES) 2003–2004. Atherosclerosis. 2010;210(2):643–648. doi: 10.1016/j.atherosclerosis.2010.01.005. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee K.B., Ang L., Yau W.P., Seow W.J. Association between metabolites and the risk of lung cancer: a systematic literature review and meta-analysis of observational studies. Metabolites. 2020;10(9):1–30. doi: 10.3390/metabo10090362. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long N.P., Heo D., Kim H.Y., Kim T.H., Shin J.G., Lee A., et al. Metabolomics-guided global pathway analysis reveals better insights into the metabolic alterations of breast cancer. J. Pharmaceut. Biomed. Anal. 2021:202. doi: 10.1016/j.jpba.2021.114134. Aug. [DOI] [PubMed] [Google Scholar]
- 59.Goveia J., Pircher A., Conradi L., Kalucka J., Lagani V., Dewerchin M., et al. Meta‐analysis of clinical metabolic profiling studies in cancer: challenges and opportunities. EMBO Mol. Med. 2016;8(10):1134–1142. doi: 10.15252/emmm.201606798. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tofte N., Vogelzangs N., Mook-Kanamori D., Brahimaj A., Nano J., Ahmadizar F., et al. Plasma metabolomics identifies markers of impaired renal function: a meta-analysis of 3089 persons with type 2 diabetes. J. Clin. Endocrinol. Metab. 2020;105(7):2275–2287. doi: 10.1210/clinem/dgaa173. Jul. [DOI] [PubMed] [Google Scholar]
- 61.Lerink L.J.S., Kok M.J.C., Mulvey J.F., Le Dévédec S.E., Markovski A.A., Wüst R.C.I., et al. Preclinical models versus clinical renal ischemia reperfusion injury: a systematic review based on metabolic signatures. Am. J. Transplant. 2021:1–27. doi: 10.1111/ajt.16868. Oct;00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholefield M., Unwin R.D., Cooper G.J.S. Shared perturbations in the metallome and metabolome of Alzheimer’s, Parkinson’s, Huntington’s, and dementia with Lewy bodies: a systematic review. Ageing Res. Rev. 2020;63 doi: 10.1016/j.arr.2020.101152. Nov. [DOI] [PubMed] [Google Scholar]
- 63.Marrachelli V.G., Rentero P., Mansego M.L., Morales J.M., Galan I., Pardo-Tendero M., et al. Genomic and metabolomic profile associated to clustering of cardio-metabolic risk factors. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0160656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGranaghan P., Saxena A., Rubens M., Radenkovic J., Bach D., Schleußner L., et al. Predictive value of metabolomic biomarkers for cardiovascular disease risk: a systematic review and meta-analysis. Biomarkers. 2020;25(2):101–111. doi: 10.1080/1354750X.2020.1716073. Jan. [DOI] [PubMed] [Google Scholar]
- 65.Wright R.O., Baccarelli A. Metals and neurotoxicology. J. Nutr. 2007;137(12):2809–2813. doi: 10.1093/jn/137.12.2809. [DOI] [PubMed] [Google Scholar]
- 66.Jalili C., Kazemi M., Taheri E., Mohammadi H., Boozari B., Hadi A., et al. Exposure to heavy metals and the risk of osteopenia or osteoporosis: a systematic review and meta-analysis. Osteoporos. Int. 2020;31(9):1671–1682. doi: 10.1007/s00198-020-05429-6. May. [DOI] [PubMed] [Google Scholar]
- 67.Jalili C., Kazemi M., Cheng H., Mohammadi H., Babaei A., Taheri E., et al. Associations between exposure to heavy metals and the risk of chronic kidney disease: a systematic review and meta-analysis. Crit. Rev. Toxicol. 2021;51(2):165–182. doi: 10.1080/10408444.2021.1891196. Feb. [DOI] [PubMed] [Google Scholar]
- 68.Feng Y., Zeng J.W., Ma Q., Zhang S., Tang J., Feng J.F. Serum copper and zinc levels in breast cancer: a meta-analysis. J. Trace Elem. Med. Biol. 2020;62 doi: 10.1016/j.jtemb.2020.126629. Dec. [DOI] [PubMed] [Google Scholar]
- 69.Fernández-Martínez N.F., Ching-López A., Olry de Labry Lima A., Salamanca-Fernández E., Pérez-Gómez B., Jiménez-Moleón J.J., et al. Relationship between exposure to mixtures of persistent, bioaccumulative, and toxic chemicals and cancer risk: a systematic review. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109787. Sep. [DOI] [PubMed] [Google Scholar]
- 70.Callejón-Leblic B., Rodríguez-Moro G., Arias-Borrego A., Pereira-Vega A., Gómez-Ariza J.L., García-Barrera T. Absolute quantification of selenoproteins and selenometabolites in lung cancer human serum by column switching coupled to triple quadrupole inductively coupled plasma mass spectrometry. J. Chromatogr. A. 2020;1619 doi: 10.1016/j.chroma.2020.460919. May. [DOI] [PubMed] [Google Scholar]
- 71.Chowdhury R., Ramond A., O’Keeffe L.M., Shahzad S., Kunutsor S.K., Muka T., et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310. Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vladov I., Petrova E., Pavlova E., Tinkov A.A., Ajsuvakova O.P., Skalny A.V., et al. Alterations in blood metabolic parameters of immature mice after subchronic exposure to cobalt chloride. Biol. Trace Elem. Res. 2020;199:588–593. doi: 10.1007/s12011-020-02161-4. May. [DOI] [PubMed] [Google Scholar]
- 73.Kawakami T., Hanao N., Nishiyama K., Kadota Y., Inoue M., Sato M., et al. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 2012;258(1):32–42. doi: 10.1016/j.taap.2011.10.004. Jan. [DOI] [PubMed] [Google Scholar]
- 74.Durante W. Advances in Experimental Medicine and Biology. Springer; Cham: 2020. Amino acids in circulatory function and health; pp. 39–56. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Z.-Y., Monleon D., Verhamme P., Staessen J.A. Branched-chain amino acids as critical switches in health and disease. Hypertension. 2018;72(5):1012–1022. doi: 10.1161/HYPERTENSIONAHA.118.10919. [DOI] [PubMed] [Google Scholar]
- 76.Wang T.J., Larson M.G., Vasan R.S., Cheng S., Rhee E.P., McCabe E., et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tinkov A.A., Skalnaya M.G., Skalny A.V. Serum trace element and amino acid profile in children with cerebral palsy. J. Trace Elem. Med. Biol. 2021;64 doi: 10.1016/j.jtemb.2020.126685. Mar. [DOI] [PubMed] [Google Scholar]
- 78.Wiggins T., Kumar S., Markar S.R., Antonowicz S., Hanna G.B. Tyrosine, phenylalanine, and tryptophan in gastroesophageal malignancy: a systematic review. Cancer Epidemiol. Biomark. Prev. 2015;24(1):32–38. doi: 10.1158/1055-9965.EPI-14-0980. Jan. [DOI] [PubMed] [Google Scholar]
- 79.Haslam D.E., Liang L., Wang D.D., Kelly R.S., Wittenbecher C., Pérez C.M., et al. Associations of network-derived metabolite clusters with prevalent type 2 diabetes among adults of Puerto Rican descent. BMJ Open Diabetes Res. Care. 2021;9(1) doi: 10.1136/bmjdrc-2021-002298. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samadi A., Isikhan S.Y., Tinkov A.A., Lay I., Doşa M.D., Skalny A.V., et al. Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clin. Nutr. 2020;39(6):1849–1856. doi: 10.1016/j.clnu.2019.07.026. Jun. [DOI] [PubMed] [Google Scholar]
- 81.Martin E., González-Horta C., Rager J., Bailey K.A., Sánchez-Ramírez B., Ballinas-Casarrubias L., et al. Metabolomic characteristics of arsenic-associated diabetes in a prospective cohort in Chihuahua, Mexico. Toxicol. Sci. 2015;144(2):338–346. doi: 10.1093/toxsci/kfu318. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qu K.C., Li H.Q., Tang K.K., Wang Z.Y., Fan R.F. Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles. Biol. Trace Elem. Res. 2020;193(1):234–240. doi: 10.1007/s12011-019-01682-x. Jan. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez-Moro G., Roldán F.N., Baya-Arenas R., Arias-Borrego A., Callejón-Leblic B., Gómez-Ariza J.L., et al. Metabolic impairments, metal traffic, and dyshomeostasis caused by the antagonistic interaction of cadmium and selenium using organic and inorganic mass spectrometry. Environ. Sci. Pollut. Control Ser. 2020;27(2):1762–1775. doi: 10.1007/s11356-019-06573-1. Jan. [DOI] [PubMed] [Google Scholar]
- 84.Adak A., Khan M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019;76(3):473–493. doi: 10.1007/s00018-018-2943-4. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abbasalizad Farhangi M., Vajdi M. Gut microbiota–associated trimethylamine N-oxide and increased cardiometabolic risk in adults: a systematic review and dose-response meta-analysis. Nutr. Rev. 2020;79(9):1022–1042. doi: 10.1093/nutrit/nuaa111. Sep. [DOI] [PubMed] [Google Scholar]
- 86.Axelrad J.E., Cadwell K.H., Colombel J.F., Shah S.C. The role of gastrointestinal pathogens in inflammatory bowel disease: a systematic review. Therapeutic Adv. Gastroenterol. 2021;14 doi: 10.1177/17562848211004493. 17562848211004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schiattarella G.G., Sannino A., Toscano E., Giugliano G., Gargiulo G., Franzone A., et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur. Heart J. 2017;38(39):2948–2956. doi: 10.1093/eurheartj/ehx342. Jul. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Han J. Branched-chain amino acid transaminase 1 (BCAT1) promotes the growth of breast cancer cells through improving mTOR-mediated mitochondrial biogenesis and function. Biochem. Biophys. Res. Commun. 2017;486(2):224–231. doi: 10.1016/j.bbrc.2017.02.101. Apr. [DOI] [PubMed] [Google Scholar]
- 89.Stochel-Gaudyn A., Fyderek K., Kościelniak P. Serum trace elements profile in the pediatric inflammatory bowel disease progress evaluation. J. Trace Elem. Med. Biol. 2019;55:121–126. doi: 10.1016/j.jtemb.2019.06.016. Sep. [DOI] [PubMed] [Google Scholar]
- 90.Kasaikina M.V., Kravtsova M.A., Lee B.C., Seravalli J., Peterson D.A., Walter J., et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. Faseb. J. 2011;25(7):2492–2499. doi: 10.1096/fj.11-181990. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhai Q., Cen S., Li P., Tian F., Zhao J., Zhang H., et al. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ. Sci. Technol. Lett. 2018;5(12):724–730. Nov. [Google Scholar]
- 92.Chi X., Liu Z., Wang H., Wang Y., Xu B., Wei W. Biological Trace Element Research; 2021. Regulation of a New Type of Selenium-Rich Royal Jelly on Gut Microbiota Profile in Mice. [DOI] [PubMed] [Google Scholar]
- 93.Hrdina J., Banning A., Kipp A., Loh G., Blaut M., Brigelius-Flohé R. The gastrointestinal microbiota affects the selenium status and selenoprotein expression in mice. J. Nutr. Biochem. 2009;20(8):638–648. doi: 10.1016/j.jnutbio.2008.06.009. Aug. [DOI] [PubMed] [Google Scholar]
- 94.Li X., Brejnrod A.D., Ernst M., Rykær M., Herschend J., Olsen N.M.C., et al. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ. Int. 2019;126:454–467. doi: 10.1016/j.envint.2019.02.048. May. [DOI] [PubMed] [Google Scholar]
- 95.Podany A., Rauchut J., Wu T., Kawasawa Y.I., Wright J., Lamendella R., et al. Excess dietary zinc intake in neonatal mice causes oxidative stress and alters intestinal host–microbe interactions. Mol. Nutr. Food Res. 2019;63(3) doi: 10.1002/mnfr.201800947. Dec. [DOI] [PubMed] [Google Scholar]
- 96.Reed S., Neuman H., Moscovich S., Glahn R.P., Koren O., Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7(12):9768–9784. doi: 10.3390/nu7125497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Starke I.C., Pieper R., Neumann K., Zentek J., Vahjen W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Ecol. 2014;87(2):416–427. doi: 10.1111/1574-6941.12233. [DOI] [PubMed] [Google Scholar]
- 98.Zackular J.P., Skaar E.P. The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microb. 2018;9(5):469–476. doi: 10.1080/19490976.2018.1448354. Sep. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barwinska-Sendra A., Waldron K.J. Advances in Microbial Physiology. Academic Press; 2017. The role of intermetal competition and mis-metalation in metal toxicity; pp. 315–379. [DOI] [PubMed] [Google Scholar]
- 100.Dürre P., Fischer R.-J., Kuhn A., Lorenz K., Schreiber W., Stürzenhofecker B., et al. Solventogenic enzymes of Clostridium acetobutylicum : catalytic properties, genetic organization, and transcriptional regulation. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Rev. 1995;17(3):251–262. doi: 10.1111/j.1574-6976.1995.tb00209.x. Oct. [DOI] [PubMed] [Google Scholar]
- 101.Gavino V.C., Somma J., Philbert L., David F., Garneau M., Bélair J., et al. Production of acetone and conversion of acetone to acetate in the perfused rat liver. J. Biol. Chem. 1987;262(14):6735–6740. May. [PubMed] [Google Scholar]
- 102.Calder P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr. 2015;39(1 Suppl):18S–32S. doi: 10.1177/0148607115595980. Sep. [DOI] [PubMed] [Google Scholar]
- 103.Li J., Guasch-Ferré M., Chung W., Ruiz-Canela M., Toledo E., Corella D., et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020;41(28):2645–2656. doi: 10.1093/eurheartj/ehaa209. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zang X., Huang H., Zhuang Z., Chen R., Xie Z., Xu C., et al. The association between serum copper concentrations and cardiovascular disease risk factors in children and adolescents in NHANES. Environ. Sci. Pollut. Control Ser. 2018;25(17):16951–16958. doi: 10.1007/s11356-018-1816-6. Jun. [DOI] [PubMed] [Google Scholar]
- 105.Chen J., Lan C., An H., Jin Y., Li Q., Ge S., et al. Potential interference on the lipid metabolisms by serum copper in a women population: a repeated measurement study. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143375. Mar. [DOI] [PubMed] [Google Scholar]
- 106.Hasani M., Djalalinia S., Sharifi F., Varmaghani M., Zarei M., Abdar M.E., et al. Effect of selenium supplementation on lipid profile: a systematic review and meta-analysis. Horm. Metab. Res. 2018;50(10):715–727. doi: 10.1055/a-0749-6655. Oct. [DOI] [PubMed] [Google Scholar]
- 107.Tabrizi R., Akbari M., Moosazadeh M., Lankarani K.B., Heydari S.T., Kolahdooz F., et al. The effects of selenium supplementation on glucose metabolism and lipid profiles among patients with metabolic diseases: a systematic review and meta-analysis of randomized controlled trials. Horm. Metab. Res. 2017;49(11):826–830. doi: 10.1055/s-0043-119544. Nov. [DOI] [PubMed] [Google Scholar]
- 108.Laclaustra M., Stranges S., Navas-Acien A., Ordovas J.M., Guallar E. Serum selenium and serum lipids in US adults: national health and nutrition examination survey (NHANES) 2003-2004. Atherosclerosis. 2010;210(2):643–648. doi: 10.1016/j.atherosclerosis.2010.01.005. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fan Y., Zhang C., Bu J. Relationship between selected serum metallic elements and obesity in children and adolescent in the. U.S. Nutrients. 2017;9(2):104. doi: 10.3390/nu9020104. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Obeid O., Elfakhani M., Hlais S., Iskandar M., Batal M., Mouneimne Y., et al. Plasma copper, zinc, and selenium levels and correlates with metabolic syndrome components of Lebanese adults. Biol. Trace Elem. Res. 2008;123:58–65. doi: 10.1007/s12011-008-8112-0. Jun. [DOI] [PubMed] [Google Scholar]
- 111.Urbano T., Filippini T., Lasagni D., De Luca T., Sucato S., Polledri E., et al. Associations between urinary and dietary selenium and blood metabolic parameters in a healthy northern Italy population. Antioxidants. 2021;10(8):1193. doi: 10.3390/antiox10081193. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu Q., Sun X., Chen Q., Zhang X., Zhu Y. Genetically predicted selenium is negatively associated with serum TC, LDL-C and positively associated with HbA1C levels. J. Trace Elem. Med. Biol. 2021;67 doi: 10.1016/j.jtemb.2021.126785. Sep. [DOI] [PubMed] [Google Scholar]
- 113.Rath A.A., Lam H.S., Schooling C.M. Effects of selenium on coronary artery disease, type 2 diabetes and their risk factors: a Mendelian randomization study. Eur. J. Clin. Nutr. 2021;75(11):1668–1678. doi: 10.1038/s41430-021-00882-w. Nov. [DOI] [PubMed] [Google Scholar]
- 114.Foroozanfard F., Jamilian M., Jafari Z., Khassaf A., Hosseini A., Khorammian H., et al. Effects of zinc supplementation on markers of insulin resistance and lipid profiles in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Exp. Clin. Endocrinol. Diabetes. 2015;123(4):215–220. doi: 10.1055/s-0035-1548790. Apr 1. [DOI] [PubMed] [Google Scholar]
- 115.Karamali M., Heidarzadeh Z., Seifati S.M., Samimi M., Tabassi Z., Hajijafari M., et al. Zinc supplementation and the effects on metabolic status in gestational diabetes: a randomized, double-blind, placebo-controlled trial. J. Diabetes Complicat. 2015;29(8):1314–1319. doi: 10.1016/j.jdiacomp.2015.07.001. Nov. [DOI] [PubMed] [Google Scholar]
- 116.Reiterer G., MacDonald R., Browning J.D., Morrow J., Matveev S.V., Daugherty A., et al. Zinc deficiency increases plasma lipids and atherosclerotic markers in LDL-receptor-deficient mice. J. Nutr. 2005;135(9):2114–2118. doi: 10.1093/jn/135.9.2114. [DOI] [PubMed] [Google Scholar]
- 117.Ahn B.I., Kim M.J., Koo H.S., Seo N., Joo N.S., Kim Y.S. Serum zinc concentration is inversely associated with insulin resistance but not related with metabolic syndrome in nondiabetic Korean adults. Biol. Trace Elem. Res. 2014;160(2):169–175. doi: 10.1007/s12011-014-0045-1. Aug. [DOI] [PubMed] [Google Scholar]
- 118.Foster M., Petocz P., Samman S. Effects of zinc on plasma lipoprotein cholesterol concentrations in humans: a meta-analysis of randomised controlled trials. Atherosclerosis. 2010;210(2):344–352. doi: 10.1016/j.atherosclerosis.2009.11.038. Jun. [DOI] [PubMed] [Google Scholar]
- 119.Seo J.-A., Song S.-W., Han K., Lee K.-J., Kim H.-N. The associations between serum zinc levels and metabolic syndrome in the Korean population: findings from the 2010 Korean national health and nutrition examination survey. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105990. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Neggers Y.H., Bindon J.R., Dressler W.W. The relationship between zinc and copper status and lipid levels in African-Americans. Biol. Trace Elem. Res. 2001;79(1):1–13. doi: 10.1385/BTER:79:1:01. [DOI] [PubMed] [Google Scholar]
- 121.Zhao J., Fan B., Wu Z., Xu M., Luo Y. Serum zinc is associated with plasma leptin and Cu-Zn SOD in elite male basketball athletes. J. Trace Elem. Med. Biol. 2015;30:49–53. doi: 10.1016/j.jtemb.2014.10.005. Apr. [DOI] [PubMed] [Google Scholar]
- 122.Yary T., Virtanen J.K., Ruusunen A., Tuomainen T.P., Voutilainen S. Association between serum zinc and later development of metabolic syndrome in middle aged and older men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Nutrition. 2017;37:43–47. doi: 10.1016/j.nut.2016.09.004. May. [DOI] [PubMed] [Google Scholar]
- 123.Tsuboi A., Terazawa Watanabe M., Kazumi T., Fukuo K. Serum copper, zinc and risk factors for cardiovascular disease in community-living Japanese elderly women. Asia Pac. J. Clin. Nutr. 2014;23(2):239–245. doi: 10.6133/apjcn.2014.23.2.04. [DOI] [PubMed] [Google Scholar]
- 124.Sales M.C., de Oliveira L.P., de Araújo Cabral N.L., de Sousa S.E.S., das Graças Almeida M., Lemos T.M.A.M., et al. Plasma zinc in institutionalized elderly individuals: relation with immune and cardiometabolic biomarkers. J. Trace Elem. Med. Biol. 2018;50:615–621. doi: 10.1016/j.jtemb.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 125.Jafarnejad S., Mahboobi S., McFarland L.V., Taghizadeh M., Rahimi F. Meta-analysis: effects of zinc supplementation alone or with multi-nutrients, on glucose control and lipid levels in patients with type 2 diabetes. Preventive Nutrition Food Sci. 2019;24(1):8–23. doi: 10.3746/pnf.2019.24.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dudka I., Kossowska B., Senhadri H., Latajka R., Hajek J., Andrzejak R., et al. Metabonomic analysis of serum of workers occupationally exposed to arsenic, cadmium and lead for biomarker research: a preliminary study. Environ. Int. 2014;68:71–81. doi: 10.1016/j.envint.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y., Li M., Tian X., Xie J., Liu P., Ying X., et al. Effects of arsenic exposure on lipid metabolism: a systematic review and meta-analysis. Toxicol. Mech. Methods. 2021;31(3):188–196. doi: 10.1080/15376516.2020.1864537. [DOI] [PubMed] [Google Scholar]
- 128.Gianni D., Diaz B., Taulet N., Fowler B., Courtneidge S.A., Bokoch G.M. Novel p47 phox -related organizers regulate localized NADPH oxidase 1 (Nox1) activity. Sci. Signal. 2009;2(88) doi: 10.1126/scisignal.2000370. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sacco K.A., Smith M.J., Bahna S.L., Buchbinder D., Burkhardt J., Cooper M.A., et al. NAPDH oxidase-specific flow cytometry allows for rapid genetic triage and classification of novel variants in chronic granulomatous disease. J. Clin. Immunol. 2020;40(1):191–202. doi: 10.1007/s10875-019-00712-6. Jan. [DOI] [PubMed] [Google Scholar]
- 130.Mohammadi-Bardbori A., Rannug A. Arsenic, cadmium, mercury and nickel stimulate cell growth via NADPH oxidase activation. Chem. Biol. Interact. 2014;224:183–188. doi: 10.1016/j.cbi.2014.10.034. Dec. [DOI] [PubMed] [Google Scholar]
- 131.Pannala V.R., Bazil J.N., Camara A.K.S., Dash R.K. A biophysically based mathematical model for the catalytic mechanism of glutathione reductase. Free Radic. Biol. Med. 2013;65:1385–1397. doi: 10.1016/j.freeradbiomed.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Santos A. de SE., Hauser-Davis R.A., Rocha R.C.C., Saint’Pierre T.D., Meyer A. Metal exposure and oxidative stress biomarkers in a Brazilian agricultural community. Arch. Environ. Occup. Health. 2021:1–10. doi: 10.1080/19338244.2021.1980759. [DOI] [PubMed] [Google Scholar]
- 133.Kubrak O.I., Husak V.V., Rovenko B.M., Storey J.M., Storey K.B., Lushchak V.I. Cobalt-induced oxidative stress in brain, liver and kidney of goldfish Carassius auratus. Chemosphere. 2011;85(6):983–989. doi: 10.1016/j.chemosphere.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 134.Xu X., Liu S., Aodengqimuge Wang H., Hu M., Xing C., et al. Arsenite induces vascular endothelial cell dysfunction by activating IRE1α/XBP1s/HIF1α-dependent ANGII signaling. Toxicol. Sci. 2017;160(2):315–328. doi: 10.1093/toxsci/kfx184. Dec. [DOI] [PubMed] [Google Scholar]
- 135.Zhao J., Li A., Mei Y., Zhou Q., Li Y., Li K., et al. The association of arsenic exposure with hypertension and blood pressure: a systematic review and dose-response meta-analysis. Environ. Pollut. 2021 doi: 10.1016/j.envpol.2021.117914. 289. [DOI] [PubMed] [Google Scholar]
- 136.Salloum Z., Lehoux E.A., Harper M.-E., Catelas I. Effects of cobalt and chromium ions on oxidative stress and energy metabolism in macrophages in vitro. J. Orthop. Res. 2018;36(12):3178–3187. doi: 10.1002/jor.24130. Dec. [DOI] [PubMed] [Google Scholar]
- 137.Patel E., Lynch C., Ruff V., Reynolds M. Co-exposure to nickel and cobalt chloride enhances cytotoxicity and oxidative stress in human lung epithelial cells. Toxicol. Appl. Pharmacol. 2012;258(3):367–375. doi: 10.1016/j.taap.2011.11.019. Feb. [DOI] [PubMed] [Google Scholar]
- 138.Lison D. Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup. Environ. Med. 2001;58(10):619–625. doi: 10.1136/oem.58.10.619. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choi S., Liu X., Pan Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018;39(7):1120–1132. doi: 10.1038/aps.2018.25. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Olechnowicz J., Tinkov A., Skalny A., Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018;68(1):19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Andersson E.M., Sandsveden M., Forsgard N., Sallsten G., Manjer J., Engström G., et al. Is cadmium a risk factor for breast cancer – results from a nested case–control study using data from the Malmö diet and cancer study. Cancer Epidemiol. Biomark. Prev. 2021;30(9):1744–1752. doi: 10.1158/1055-9965.EPI-21-0181. Sep. [DOI] [PubMed] [Google Scholar]
- 142.Vijayakumar V., Abern M.R., Jagai J.S., Kajdacsy‐balla A. Observational study of the association between air cadmium exposure and prostate cancer aggressiveness at diagnosis among a nationwide retrospective cohort of 230,540 patients in the United States. Int. J. Environ. Res. Publ. Health. 2021;18(16):8333. doi: 10.3390/ijerph18168333. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Straif K., Benbrahim-Tallaa L., Baan R., Grosse Y., Secretan B., El Ghissassi F., et al. A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol. 2009;10(5):453–454. doi: 10.1016/s1470-2045(09)70134-2. May. [DOI] [PubMed] [Google Scholar]
- 144.Celik I., Gallicchio L., Boyd K., Lam T.K., Matanoski G., Tao X., et al. Arsenic in drinking water and lung cancer: a systematic review. Environ. Res. 2008;108(1):48–55. doi: 10.1016/j.envres.2008.04.001. Sep. [DOI] [PubMed] [Google Scholar]
- 145.Benbrahim-Tallaa L., Waalkes M.P. Inorganic arsenic and human prostate cancer. Environ. Health Perspect. 2008;116(2):158–164. doi: 10.1289/ehp.10423. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodríguez-Barranco M., Lacasaña M., Aguilar-Garduño C., Alguacil J., Gil F., González-Alzaga B., et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci. Total Environ. 2013;454–455:562–577. doi: 10.1016/j.scitotenv.2013.03.047. Jun. [DOI] [PubMed] [Google Scholar]
- 147.Sugiyama T., Ishii N., Ebihara Y., Shiomi K., Mochizuki H. Detailed analysis of neurological symptoms and sensory disturbances due to chronic arsenic exposure in Toroku, Japan. Int. J. Environ. Res. Publ. Health. 2021;18(20):10749. doi: 10.3390/ijerph182010749. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciesielski T., Weuve J., Bellinger D.C., Schwartz J., Lanphear B., Wright R.O. Cadmium exposure and neurodevelopmental outcomes in U.S. Children. Environ. Health Perspect. 2012;120(5):758–763. doi: 10.1289/ehp.1104152. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma J., Zhou Y., Wang D., Guo Y., Wang B., Xu Y., et al. Associations between essential metals exposure and metabolic syndrome (MetS): exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020;140 doi: 10.1016/j.envint.2020.105802. Jul. [DOI] [PubMed] [Google Scholar]
- 150.Aaseth J., Skalny A.V., Roos P.M., Alexander J., Aschner M., Tinkov A.A. Copper, iron, selenium and lipo-glycemic dysmetabolism in alzheimer’s disease. Int. J. Mol. Sci. 2021;22(17):9461. doi: 10.3390/ijms22179461. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sikora J., Ouagazzal A.M. Synaptic zinc: an emerging player in Parkinson’s disease. Int. J. Mol. Sci. 2021;22(9):4724. doi: 10.3390/ijms22094724. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vinceti M., Filippini T., Wise L.A., Rothman K.J. A systematic review and dose-response meta-analysis of exposure to environmental selenium and the risk of type 2 diabetes in nonexperimental studies. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111210. Jun. [DOI] [PubMed] [Google Scholar]
- 153.Rayman M.P., Winther K.H., Pastor-Barriuso R., Cold F., Thvilum M., Stranges S., et al. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic. Biol. Med. 2018;127:46–54. doi: 10.1016/j.freeradbiomed.2018.02.015. Nov. [DOI] [PubMed] [Google Scholar]
- 154.Bleys J., Navas-Acien A., Laclaustra M., Pastor-Barriuso R., Menke A., Ordovas J., et al. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003-2004. Am. J. Epidemiol. 2009;169(8):996–1003. doi: 10.1093/aje/kwn414. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Akbaraly T., Würtz P., Singh-Manoux A., Shipley M.J., Haapakoski R., Lehto M., et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: analysis of two cohort studies. Sci. Rep. 2018;8(1):8620. doi: 10.1038/s41598-018-26441-1. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bal W., Sokołowska M., Kurowska E., Faller P. vol. 1830. Biochimica et Biophysica Acta - General Subjects; 2013. pp. 5444–5455. (Binding of Transition Metal Ions to Albumin: Sites, Affinities and Rates). [DOI] [PubMed] [Google Scholar]
- 157.Stevens L.A., Coresh J., Greene T., Levey A.S. Assessing kidney function — measured and estimated glomerular filtration rate. N. Engl. J. Med. 2006;354(23):2473–2483. doi: 10.1056/NEJMra054415. Jun. [DOI] [PubMed] [Google Scholar]
- 158.Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23(R1):R89–R98. doi: 10.1093/hmg/ddu328. Sep. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nigra A.E., Ruiz-Hernandez A., Redon J., Navas-Acien A., Tellez-Plaza M. Environmental metals and cardiovascular disease in adults: a systematic review beyond lead and cadmium. Curr Envir Health Rpt. 2016;3(4):416–433. doi: 10.1007/s40572-016-0117-9. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.García-Esquinas E., Pollan M., Tellez-Plaza M., Francesconi K.A., Goessler W., Guallar E., et al. Cadmium exposure and cancer mortality in a prospective cohort: the Strong heart study. Environ. Health Perspect. 2014;122(4):363–370. doi: 10.1289/ehp.1306587. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.