Highlights
-
•
2-HG is expected to be a useful biomarker for diagnosing and treating IDH-mutant tumors.
-
•
Both intratumoral and serum levels of 2-HG were significantly higher in IDH-mutant tumors.
-
•
Serum 2-HG levels were correlated with tumor volume and tumor progression.
-
•
MR spectroscopy (MRS) detected 2-HG peaks in a xenograft model of IDH-mutant chondrosarcoma.
-
•
In vivo MRS can be a useful tool for determining the therapeutic effect of mutant IDH inhibitors.
Keywords: isocitrate dehydrogenase (IDH), Chondrosarcoma, 2-Hydroxyglutarate (2-HG), Biomarker
Abbreviations: AML, acute myeloid leukemia; IDH, isocitrate dehydrogenase; MR, magnetic resonance; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NMR, nuclear magnetic resonance; NOD-SCID, NOD/ShiJic-scidJcl; ROI, region of interest; STEAM, stimulated echo acquisition mode; 2-HG, 2-hydroxyglutarate
Abstract
Background
Chondrosarcoma is a common form of malignant bone tumor with limited treatment options. Approximately half of chondrosarcomas harbor gain-of-function mutations in isocitrate dehydrogenase (IDH), and mutant IDH produces 2-hydroxyglutarate (2-HG), which is an oncometabolite that contributes to malignant transformation. Therefore, inhibiting 2-HG production is a novel and promising treatment for advanced chondrosarcoma. 2-HG is also expected to be a useful biomarker for the diagnosis and treatment of IDH-mutant tumors. However, few studies have confirmed this using chondrosarcoma clinical specimens. Non-invasive monitoring of 2-HG levels is useful to infer that mutant IDH inhibitors reach therapeutic targets and to confirm their therapeutic efficacy in clinical practice.
Methods
To evaluate the clinical utility of 2-HG as a surrogate biomarker for diagnosis and therapeutic efficacy, we measured intra-tumor and serum levels of 2-HG using frozen tissues and peripheral blood from patients with chondrosarcoma. We also developed a non-invasive method to detect intra-tumor 2-HG signals in vivo using magnetic resonance spectroscopy (MRS)
Results
Both intratumoral and serum 2-HG levels were significantly elevated in IDH-mutant tumors, and these levels correlated with decreased survival. Furthermore, we detected intratumoral 2-HG peaks using MR spectroscopy in a xenograft model of IDH-mutant chondrosarcoma, and observed that 2-HG peak signals disappeared after administering an inhibitor of mutant IDH1.
Conclusions
Our findings suggest that both intratumoral and serum 2-HG levels represent potentially useful biomarkers for IDH-mutant tumors and that the 2-HG signal in MR spectra has potential value as a non-invasive biomarker. Taken together, these findings may positively impact the clinical development of mutant IDH inhibitors for the treatment of advanced chondrosarcoma.
1. Introduction
Chondrosarcoma is the second most common primary malignant neoplasm of bone. It is composed of malignant chondrocytes that produce hyalin cartilage, myxoid cartilage, or fibromyxoid matrix, depending on their histological grade. Surgery with wide resection margins plays an important role in the treatment of chondrosarcoma, but few treatment options are available for recurrent and metastatic cases because chondrosarcoma is refractory to conventional chemotherapy and radiotherapy. Furthermore, 10–15% of central chondrosarcomas undergo dedifferentiation, and once low-grade chondrosarcoma dedifferentiates, patients have a dismal prognosis [1]. Therefore, there is an urgent need to develop novel targeted therapies for the treatment of advanced chondrosarcoma.
Mutations in genes encoding isocitrate dehydrogenase (IDH) are observed in acute myeloid leukemia (AML) and in solid malignancies such as glioma, cholangiocarcinoma, and chondrosarcoma [2], [3], [4], [5]. Among chondrosarcomas, IDH mutations are present in the primary central, secondary central, and dedifferentiated types, which together account for over 40% of all chondrosarcomas [5]. IDH mutations are considered to be gain-of-function mutations. That is, mutated IDH produces 2-hydroxyglutarate (2-HG) instead of α-ketoglutarate (α-KG), and 2-HG induces various epigenetic changes such as aberrant DNA and histone methylation [6], [7]. Therefore, mutant IDH and 2-HG are attractive therapeutic targets for IDH-mutant chondrosarcoma because 2-HG is considered an oncometabolite and is rarely produced in normal tissues containing wild-type (WT) IDH. Inhibiting 2-HG production, either by specific deletion of the mutant IDH1R132C allele or overexpression of dextro-rotatory 2-HG dehydrogenase, was shown to increase α-KG and related metabolites and to restore the activity of several α-KG–dependent dioxygenases [8]. In addition, it significantly suppressed anchorage-independent growth in vitro and tumor growth in a xenograft mouse model [8]. These results indicate that inhibiting 2-HG produced by mutant IDH may be a valid treatment strategy for chondrosarcoma. Based on these findings, we developed DS-1001b, a novel, orally available, selective mutant IDH1 inhibitor [6], [9]. This drug inhibits the growth of IDH1-mutant chondrosarcoma cells in vitro and in vivo, and simultaneously reduces 2-HG levels [6]. In addition, we showed that DS-1001b acts against IDH1-mutant chondrosarcoma by decreasing the levels of H3K4me3 and H3K9me3 and reversing aberrant histone modifications induced by 2-HG [6]. Several other inhibitors of mutant IDH are in development, some of which are currently in clinical trials for IDH-mutant tumors, including chondrosarcoma [10], [11], [12], [13].
Since IDH mutation is a somatic mutation that occurs only in malignant tumors, and 2-HG is rarely produced in normal tissues, 2-HG is expected to be a useful biomarker for diagnosing and treating IDH-mutant tumors. We reported that intracellular 2-HG levels were significantly higher in IDH-mutant chondrosarcoma cell lines than in those with WT IDH, and DS-1001b treatment markedly reduced 2-HG levels in tumors and serum in a preclinical model [6]. However, few studies have attempted to clarify the usefulness of 2-HG as a biomarker using clinical specimens of chondrosarcoma as opposed to glioma, which is another solid tumor that exhibits IDH mutations [14], [15], [16]. This may be due to the fact that IDH mutations are currently important for diagnosis and treatment decision-making in glioma, whereas this is not the case for chondrosarcoma, and therefore chondrosarcoma resection specimens are rarely tested for IDH mutations and 2-HG levels. Furthermore, establishing 2-HG as a biomarker is important to determine whether administration of mutant IDH inhibitors may be indicated in advanced chondrosarcoma, where tumor specimens are difficult to obtain, by estimating the presence of IDH mutations.
In this study, we measured intratumoral and serum 2-HG levels in chondrosarcoma patients with or without IDH mutations, and evaluated the clinical usefulness of 2-HG as a surrogate biomarker. Furthermore, non-invasive monitoring of 2-HG levels by imaging tests would be useful for confirming that drugs reach their therapeutic targets and for determining therapeutic effects, so we developed a non-invasive method to detect intra-tumor 2-HG signals in vivo using magnetic resonance spectroscopy (MRS).
2. Materials and methods
2.1. Case selection
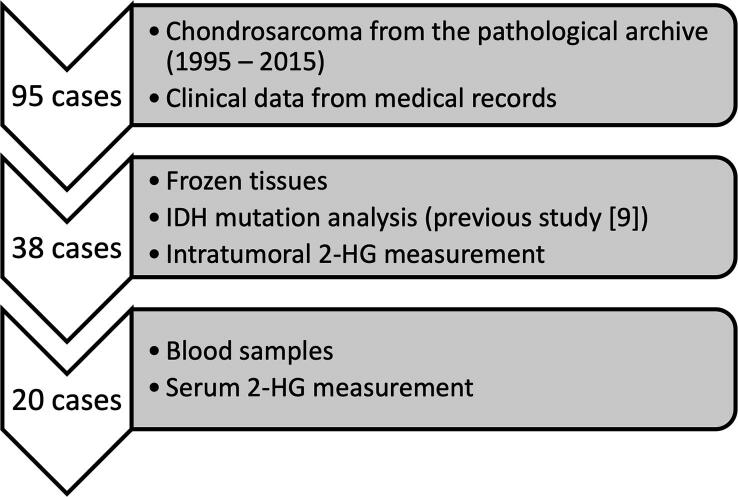
Ninety-five cases diagnosed with chondrosarcoma were retrieved from the pathology archive (1995–2015) of the National Cancer Center Hospital (Tokyo, Japan) after institutional review board approval (2014–236 and 2017–336). Cases of extraskeletal myxoid chondrosarcoma, clear cell chondrosarcoma, and mesenchymal chondrosarcoma were excluded. The study analyzed the 38 cases in which frozen tissue was obtained at the time of biopsy or surgery [17]. Tumor samples were collected from macroscopically tumor-rich areas immediately after surgical excision and were cryopreserved. Blood samples were collected before initial treatment in 20 of the 38 patients. Clinical data were extracted from medical records. The median age was 59 years (4–86 years), and the cohort contained an equal number of men and women (19 each) [17]. Sanger sequencing revealed that 15 cases (40%) of heterozygous IDH1 mutations and five cases (13%) of heterozygous IDH2 mutations; the remaining tumors were IDH WT [17]. The median follow-up period was 100 months (1–253 months). On the acquired magnetic resonance (MR) images, three orthogonal diameters (D1, D2, and D3) of the tumor were measured, and the tumor volume (TV) was calculated as follows: TV = D1 × D2 × D3 × π/6.
2.2. 2-HG measurement
For intratumoral 2-HG measurement, ethanol diluted with distilled water was added to tumor samples to a final ethanol concentration of 80%. After homogenization using a multi-bead shaker (MB701C; Yasui Kikai, Osaka, Japan) for 30 s at 2,500 rpm, the homogenates were incubated at room temperature for 30 min and at −80 °C for 15 min. After centrifugation at 15,000 rpm at 4 °C for 15 min, ethanol diluted with distilled water was added to the supernatants to a final ethanol concentration of 50%. 2-HG levels were analyzed in these purified samples using LC-MS/MS [18], and were corrected based on the variant allele of IDH mutations.
For measurement of serum 2-HG, ethanol diluted with distilled water was added to serum samples to a final ethanol concentration of 80%. Samples were incubated at −20 °C for more than 1 h, and then ethanol diluted with distilled water was added to the supernatants to a final ethanol concentration of 50%. After centrifugation, the supernatant was collected. 2-HG levels were analyzed in these purified samples using LC-MS/MS. Serum from healthy Japanese donors (22, 24, 26, 31, and 46 years old), used as a control, was purchased from Biopredic International (Rennes, France).
2.3. Compounds
The synthesis and characterization of DS-1001b (Daiichi Sankyo, Tokyo, Japan) are described in a Patent Cooperation Treaty application (publication number: WO2016052697 A) [9]. For in vivo administration, DS-1001b was mixed with sterilized pellet food (CRF-1; Oriental Yeast, Tokyo, Japan) and administered ad libitum.
2.4. Cell lines and culture
The chondrosarcoma cell lines JJ012 (IDH1R132G) (RRID:CVCL_D605) and NDCS-1 (IDH WT) (RRID:CVCL_EJ23) were kindly provided by Dr. Joel A. Block (Rush Medical College, Chicago, IL, USA) [19] and Dr. Akira Ogose (Niigata University, Niigata, Japan) [20], respectively. Both human cell lines were authenticated using STR profiling within the 3 years prior to this study. Cells were maintained in RPMI 1640 (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). Cells were grown at 37 °C in a humidified incubator in an atmosphere of 95% air and 5% CO2. All experiments were performed with mycoplasma-free cells as determined by testing with the e-Myco plus Mycoplasma PCR detection kit (iNtRON Biotechnology, Burlington, MA, USA).
2.5. In vivo xenograft studies
NOD/ShiJic-scidJcl (NOD-SCID) mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the National Cancer Center (Tokyo, Japan). Each experiment was conducted at the animal facility of the National Cancer Center in accordance with institutional guidelines and in a specific pathogen–free environment. JJ012 (IDH1R132G) and NDCS-1 (IDH WT) cells (5.0 × 106 each) were suspended in 100 μL of 50% Matrigel prepared in PBS and subcutaneously inoculated into the left flank of 6-week-old female mice. Two xenograft cell lines derived from patients with primary glioblastoma multiforme were kindly provided by Dr. Koichi Ichimura. A1074 (GB181 passage 7) harbors a heterozygous IDH1 mutation (IDH1R132H), while A1056 (GB138 passage 12) has WT IDH [9], [21]. Tumor fragments were implanted subcutaneously into the left flank of 6-week-old female mice, and the resultant tumors were passaged to other mice when their diameter reached 1 cm. Eight tumor-bearing JJ012 and A1074 mice were divided into two groups by stratified randomization, and treatment was initiated when the tumors became palpable at 7 and 8 weeks after JJ012 and A1074 cell inoculation, respectively. Before MRS, DS-1001b mixed with sterilized pellet food was administered ad libitum to JJ012 and A1074 mice for 3 weeks and 1 week, respectively [6], [9]. TVs were monitored at 10 and 9 weeks after transplantation to determine regions of interest (ROIs) by magnetic resonance imaging (MRI). Mice were sacrificed when treatments and MRS were completed, and tumors were collected for nuclear magnetic resonance (NMR) analysis and determination of intratumoral 2-HG levels by LC-MS/MS.
2.6. MRI
MRI was performed using a 9.4-T scanner (Biospec 94/20 USR; Bruker BioSpin, Ettlingen, Germany). Mice (n = 4 in each cell lines; two DS-1001b-treated and two control mice in JJ012 and A1074 cells) were anesthetized with a gas mixture of oxygen, nitrous oxide, and isoflurane (typically 1–2% concentration), and placed on an animal bed in the prone position. Subsequently, the tumor was inserted into a cryogenic radiofrequency probe (Bruker BioSpin). Transverse and coronal images of the subcutaneous tumors were acquired using a fast spin-echo sequence with the following parameters: repetition time, 2500 ms; echo time, 33 ms; echo train length, 8; field-of-view, 20 × 20 mm; matrix, 256 × 256; slice thickness, 0.5–1 mm; and number of excitations, 1.
2.7. MRS
Single-voxel MRS was performed in each mouse to acquire the proton MR spectrum of the chondrosarcoma xenograft. A single voxel of 3 × 3 × 3 mm3 was placed in the tumor with reference to T2-weighted MR images; great care was taken to ensure that the voxel did not include an area of intratumoral hemorrhage. FASTMAP was used to optimize the shim coil currents and thereby increase the local magnetic homogeneity [22]. The field homogeneity for a 27-mm3 voxel typically ranged from 20 to 25 Hz for the full width at the half maximum of the water resonance. Subsequently, MRS acquisitions were carried out using the stimulated echo acquisition mode (STEAM) sequence with the following parameters: TR, 1500 ms; TE, 3 ms; mixing time, 10 ms; and number of excitations, 512. A total of 4 K (4096) data points were acquired with a spectral width of 10 ppm (i.e., 400 Hz). The data were multiplied with a Gaussian window function (line broadening, −8 Hz; Gaussian max. position, 0.04) and Fourier-transformed to obtain MR spectra with a spectral resolution of 0.98 Hz. The chemical shift value of the N-methyl (NCH3) resonance of creatine was set to 3.03 ppm and used as the internal reference. Peak assignment in each MR spectrum for each tumor was performed with reference to the NMR spectrum of the corresponding tumor extract, as mentioned in the NMR section below. Chemical shift values of 2-hydroxyglutarate were determined with reference to the literature [23] and to the 1H NMR spectrum of 2-hydroxyglutarate (Sigma 94577; Sigma-Aldrich Co., St. Louis, MO, USA) dissolved in D2O.
2.8. NMR
Perchloric acid extraction was performed on each frozen sample of chondrosarcoma xenograft as previously described, with minor modifications [24]; briefly, we weighed each sample and pulverized it in liquid nitrogen using a cryogenic mill (Cryo-Press: Microtec Co., Ltd., Funahashi, Japan). Perchloric acid extraction was performed with 6.25% perchloric acid without addition of DL-valine-2,3-d2. Subsequently, the samples were centrifuged at 3000 rpm at 4 °C for 20 min to precipitate lipids and macromolecules, and the supernatant was recovered. After neutralization of the recovered sample by addition of KOH, the samples were centrifuged again to spin down KClO4 salt. The supernatant was recovered and stored at 4 °C overnight to further precipitate KClO4 salt. The next day, the samples were treated with ion-exchange resin (Chlex 100 Resin, 142–2832; Bio-Rad Laboratories, Hercules, CA, USA) and then filtered using a filter-funnel to remove these resin particles. The filtered fluid was lyophilized using a freeze-dry machine, and the lyophilized sample was reconstituted with 540 μL of D2O and 60 μL of 3-(trimethyl-silyl)-propionic-2,2,3,3,-d4 acid (TSP-d4, 269913; Isotec, Miamisburg, OH, USA) dissolved in D2O at the final amount of 5 μmol/sample and centrifuged at 12,000 rpm for 20 min to spin down undissolved KClO4 salt. The supernatant was recovered and placed in a 5-mm NMR tube. NMR measurement was performed on a 400-MHz NMR spectrometer (AVANCE III 400; Bruker BioSpin, Fällanden, Switzerland) using a single-pulse sequence with solvent suppression (zgpr; Bruker BioSpin) with the following parameters: 10-s delay; 90° flip angle; 20.6 ppm spectral width; and 16 acquisitions. A total of 64 K (65536) data points were acquired, and the data were Fourier-transformed with 0.3-Hz line broadening to obtain NMR spectra with a spectral resolution of 0.12 Hz. Spectra were automatically phased, and the baseline was flattened using the manufacturer’s software (TopSpin version 3.1; Bruker BioSpin). The chemical shift value of the trimethylsilyl resonance of TSP was set to 0 ppm, and the chemical shift values of each metabolite were determined relative to the TSP trimethylsilyl resonance.
2.9. Statistical analysis
Statistical significance was determined by the Mann–Whitney test using Prism 8 (GraphPad Software, San Diego, CA, USA). The Mantel–Cox log-rank test was used to determine the statistical significance of Kaplan–Meier analysis results. Data are expressed as mean values, with error bars representing the standard deviation (s.d.).
3. Results
3.1. Intratumoral 2-HG analysis
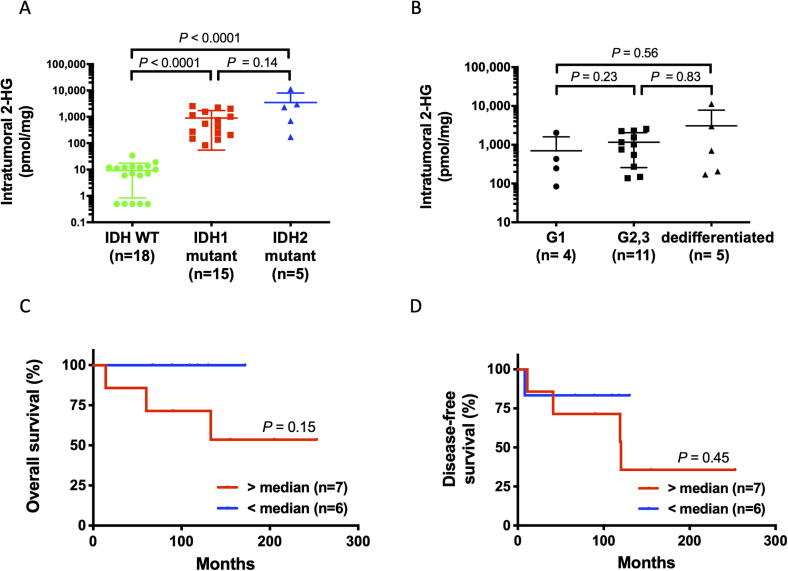
Fig. 1 shows the overview of our analysis using chondrosarcoma patients’ samples. Intratumoral 2-HG levels in 38 frozen chondrosarcoma tissue samples obtained at biopsy or surgery were measured by LC-MS/MS, and were significantly higher in IDH mutants than in WT IDH (IDH1 mutant vs. WT IDH, P < 0.0001; IDH2 mutant vs. WT IDH, P < 0.0001) (Fig. 2A). In contrast, 2-HG levels did not correlate significantly with either histological grade (Fig. 2B) or TV (R2 = 0.008, P = 0.71, data not shown), suggesting that even low-grade or low-volume tumors with IDH mutations can produce 2-HG. Although 2-HG is produced only in mutant IDH, there is conflicting evidence between IDH mutation status and prognosis in chondrosarcoma [5], [17], [25], [26]. Since the prognosis of chondrosarcoma in our cohort was significantly worse in the IDH mutant group (IDH1/2 Mut vs. IDH Wt, P = 0.006; IDH1 Mut vs. IDH Wt, P = 0.030; IDH2 Mut vs. IDH Wt, P < 0.0001) [17] and the recent meta-analysis also showed the negative impact of IDH mutations on patient overall survival [27], we examined the relationship between intratumoral 2-HG level and prognosis. The prognosis of patients with high-grade (G2, 3) chondrosarcoma without metastasis at initial presentation showed a negative trend in tumors with higher intratumoral 2-HG levels, although the difference was not significant (Fig. 2C, 2D). These results indicate that intratumoral 2-HG may have a potential to be a useful biomarker for IDH status and treatment.
Fig. 1.
A flow chart of the analysis using chondrosarcoma patients’ samples.
Fig. 2.
Intratumoral 2-HG is a potentially useful biomarker in IDH-mutant chondrosarcoma. A. Scatter plots showing intratumoral 2-HG levels in 38 patients, according to IDH mutation. B. Relationship between intratumoral and serum 2-HG levels in IDH-mutant chondrosarcoma. C. Kaplan–Meier overall survival curves of high-grade (grade 2, 3) IDH-mutant chondrosarcoma patients, according to intratumoral 2-HG levels. D. Kaplan–Meier disease-free survival curves of high-grade (grade 2, 3) IDH-mutant chondrosarcoma patients according to intratumoral 2-HG levels. C–D. Cases that were metastatic at initial diagnosis were excluded.
3.2. Analysis of circulating 2-HG
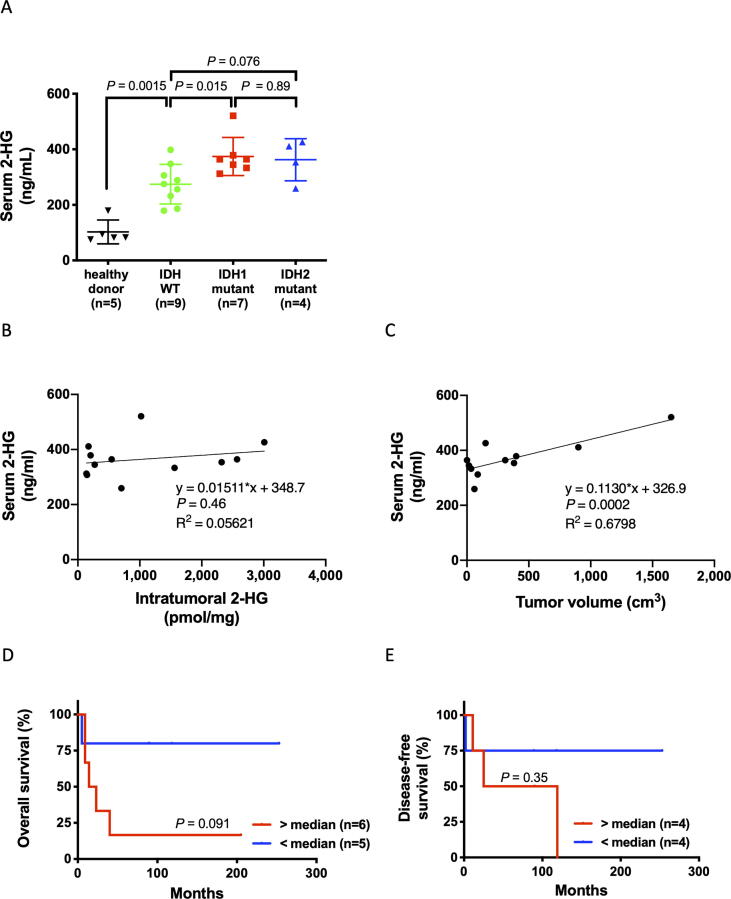
If 2-HG in peripheral blood proves to be a useful biomarker, non-invasively obtained samples can be used to diagnose IDH mutation and determine therapeutic efficacy. Monitoring 2-HG would also enable early detection of recurrence and metastasis. Therefore, to verify the usefulness of 2-HG in peripheral blood, we employed LC-MS/MS to analyze pre-treatment 2-HG levels in the sera of 20 patients. 2-HG levels were higher in IDH1-mutant samples (median, 364.1 ng/mL) than in IDH-WT samples (median, 275.3 ng/mL, P = 0.015) although the variability of them within each cohort was high with an overlap between IDH mutant samples and IDH-WT samples (Fig. 3A). Interestingly, 2-HG levels in IDH-WT samples were significantly higher than in those from healthy donors (median, 82.6 ng/mL; P = 0.0015) (Fig. 3A). Although there was no correlation between serum and intratumoral 2-HG levels (Fig. 3B), serum 2-HG levels correlated significantly with tumor volume in IDH-mutant specimens (R2 = 0.68, P = 0.0002) (Fig. 3C). The characteristics of IDH-mutant patients whose serum 2-HG levels were measured are summarized in Table 1. High serum 2-HG levels showed positive trends with higher tumor grade and poorer outcome, although not significantly so (Fig. 3D and 3E). Three of the four patients with the highest 2-HG levels had metastases at the time of measurement (Table 1). These results suggest that serum 2-HG, like intratumoral 2-HG, may have a potential to be a useful biomarker for tumor progression.
Fig. 3.
High serum 2-HG levels are associated with poor prognosis. A. Scatter plots showing serum 2-HG levels in 20 patients, according to IDH mutation. B. Relationship between intratumoral and serum 2-HG levels in IDH-mutant chondrosarcoma. C. Relationship between tumor volume and serum 2-HG levels in IDH-mutant chondrosarcoma. D. Kaplan–Meier overall survival curves of IDH-mutant chondrosarcoma patients, according to serum 2-HG levels. Serum 2-HG levels were measured in samples acquired before the start of treatment. E. Kaplan–Meier disease-free survival curves of IDH-mutant chondrosarcoma patients without metastasis at first presentation, according to serum 2-HG levels.
Table 1.
Characteristics of IDH-mutant patients with serum 2-HG measurements.
| Patient | Primary lesion |
Serum 2-HG (ng/mL) | Tumor volume (cm3) | IDH mutation | Tumor grade |
Time to metastasis (months) |
Clinical outcome |
|---|---|---|---|---|---|---|---|
| CS07 | Femur | 520.96 | 1,650 | IDH1 R132S | 3 | At diagnosis | DOD |
| CS11 | Femur | 426.24 | 150 | IDH2 R172M | Dediff | At diagnosis | DOD |
| CS12 | Humerus | 411.44 | 900 | IDH2 R172S | Dediff | 25 | DOD |
| CS33 | Pelvis | 378.88 | 399 | IDH1 R132L | Dediff | At diagnosis | DOD |
| CS01 | Humerus | 364.08 | 29 | IDH1 R132C | 1 | 119 | AWD |
| CS26 | Vertebrae | 364.08 | 310 | IDH1 R132L | 2 | DOD | |
| CS04 | Humerus | 353.72 | 11 | IDH2 R172M | 2 | NED | |
| CS32 | Fibula | 344.84 | 16 | IDH1 R132C | 2 | NED | |
| CS37 | Metatarsal | 333.00 | 34 | IDH1 R132C | 2 | NED | |
| CS05 | Femur | 312.28 | 88 | IDH1 R132G | 2 | NED | |
| CS10 | Femur | 259.00 | 60 | IDH2 R172S | Dediff | 2 | DOD |
Dediff: dedifferentiated.
3.3. In vivo MRS
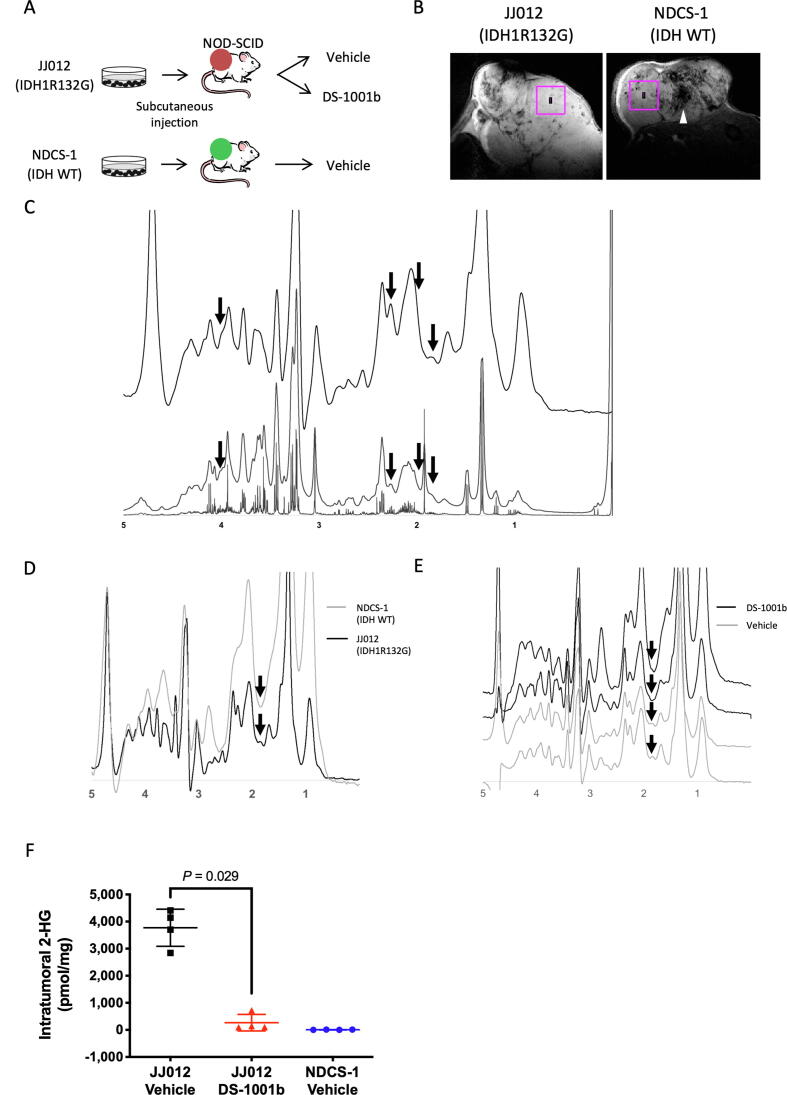
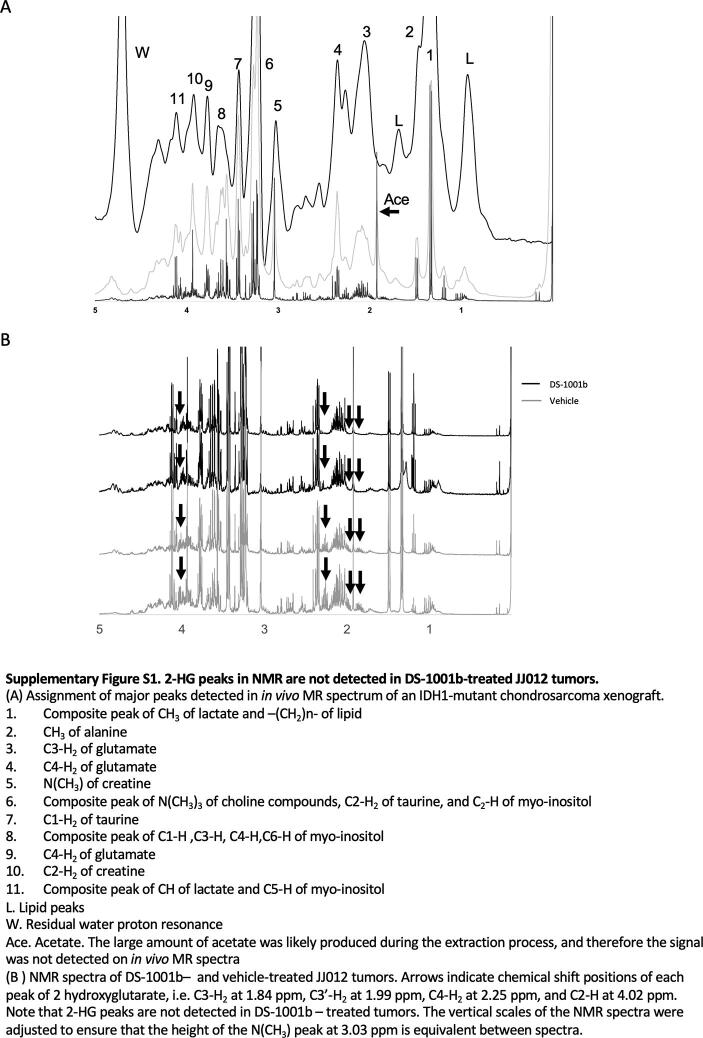
Finally, we investigated whether intratumoral 2-HG signals could be detected non-invasively in chondrosarcoma xenograft tumor models using an MRS pulse sequence called STEAM. Two chondrosarcoma cell lines, JJ012 (IDH1R132G) and NDCS-1 (IDH Wt), were implanted subcutaneously into the left flank of 6-week-old female NOD-SCID mice. Tumor-bearing JJ012 mice were randomized into two groups: treatment and vehicle. In the treatment group, DS-1001b, an IDH1-mutant inhibitor, was administered for 3 weeks before analysis (Fig. 4A). Fig. 4B shows representative MRI sections of JJ012 and NDCS-1 tumors. The squares in these MR images represent ROIs where we acquired MRS data, i.e., cross sections of an MRS voxel. Fig. 3C shows NMR spectra and a representative in vivo MR spectrum of vehicle-treated JJ012 tumor. NMR spectra of the vehicle-treated JJ012 tumors detected four 2-HG peaks, at 1.84, 1.99, 2.25, and 4.02 ppm, respectively (Fig. 4C, upper). The small 2-HG peaks were detectable on the spectrum with 10-Hz line broadening, even though they looked like the shoulders of adjacent peaks: C2-H3 of acetate, C3-H2 of glutamate, C4-H2 of glutamate, and C2-H2 of creatine (Fig. 4C, middle and bottom; peak assignments of metabolites other than 2-HG are shown in Supplemental Fig. S1A). The C3-H2 peaks of 2-HG were still detectable at 1.84 ppm on the in vivo MR spectra of vehicle-treated JJ012 tumors, but not on the spectra of NDCS-1 tumors (Fig. 4D), suggesting that C3-H2 peaks of 2-HG are likely to be detectable at 1.84 ppm in vivo. Moreover, we did not observe the peaks at 1.84 ppm in DS-1001b–treated JJ012 mice (Fig. 4E), further supporting the idea that reduced intratumoral 2-HG levels can be determined from the C3-H2 peaks of 2-HG in in vivo MR spectra. In addition, the 2-HG peak heights, including those of the C3-H2 peaks, were significantly reduced (to a level equivalent to noise) in extracts from DS-1001b–treated JJ012 tumors, whereas in extracts from vehicle-treated JJ012 tumors, the 2-HG peak heights remained higher, and the integral of these peaks indicated that the tumors contained 3.6 and 4.3 µmol/g tissue of 2-HG, respectively (Supplemental Fig. S1B). At the same time, we confirmed that except for the C2-H3 peak of acetate at 1.9 ppm, no metabolite peak other than the C3-H2 peak of 2-HG resonated at ∼ 1.8 ppm in this JJ012 chondrosarcoma tumor. The acetate peak may have been artificially produced during the perchloric acid extraction process and is therefore unlikely to be detected in vivo. Although we noted peaks suggestive of the C4-H2 of 2-HG at ∼ 2.25 ppm in in vivo MR spectra, they were very likely to be composite peaks of the C4-H2 of 2-HG and –CH2-COO of lipid, which is well known to resonate at 2.25 ppm, as we were unable to observe a reduction in these peaks in vivo even in DS-1001b–treated JJ012 tumors (Fig. 4E). Because lipid molecules are completely removed from samples during the extraction process, this seems quite reasonable considering the fact that we detected lipid peaks only in in vivo MRS but not in the NMR of perchloric acid extracts, in which we observed a significant reduction of the C4-H2 of 2-HG in the tumor extract without the need to consider the –CH2-COO of lipid. Finally, LC-MS/MS after MRS showed that DS-1001b significantly decreased intratumoral 2-HG levels in JJ012 tumors to the same levels observed in NDCS-1 tumors (Fig. 4F). These results suggest that in vivo MRS is a useful tool for non-invasively detecting 2-HG signals and for determining the therapeutic effect of mutant IDH inhibitors.
Fig. 4.
The C3-H2 peak of 2-HG is detectable at 1.84 ppm in the in vivo MR spectrum of IDH1-mutant chondrosarcoma. A. Experimental schematic of the in vivo MRS study. NOD-SCID mice were transplanted subcutaneously with JJ012 (IDH1R132G) (n = 8) or NDCS-1 (IDH-WT) cells (n = 4), and continuously fed DS-1001b mixed with sterilized pellet food for 3 weeks after confirmation of tumor engraftment. B. Representative MR images of JJ012 and NDCS-1 tumors. Squares of 3 × 3 mm2 indicate voxels of interest (VOIs) in MRS measurements. The pictures on the left and right are representative transverse slices of an IDH-mutant (JJ012) and an IDH-WT (NDCS-1) tumor. Note that the IDH-WT tumor shows heterogeneity in the tumor interior and profoundly low signal areas suggestive of intratumoral hemorrhage (arrowhead). C. In vivo MR spectrum (upper) of a representative case of IDH1-mutant chondrosarcoma xenograft (JJ012). NMR spectra of the tumor extract with 10-Hz line broadening (middle) and 0.3-Hz line broadening (bottom). Arrows indicate chemical shift positions of each 2-HG peak: C3-H2 at 1.84, C3′-H2 at 1.99 ppm, C4-H2 at 2.25 ppm, and C2-H at 4.02 ppm. On the in vivo MR spectrum, peaks at 1.99 and 4.02 ppm are obscured because they produce composite peaks with adjacent metabolites. In addition, the C4-H2 peak likely overlaps with the –CH2-COO resonance of lipid at 2.25 ppm, which is detected in the in vivo MR spectrum but not in the NMR spectra. D. Overlays of representative MR spectra of IDH1-mutant (black) and IDH-WT (gray) tumors indicate that the tiny signal at 1.84 ppm, corresponding to the C3-H2 peak of 2-HG, is seen only in the IDH1-mutant tumor (arrow). The vertical scales of the two MR spectra were adjusted to ensure that the heights of the N(CH3) peaks at 3.03 ppm were equivalent. E. MR spectra of DS-1001b– and vehicle-treated JJ012 tumors. Note that the tiny signals at 1.84 ppm, corresponding to the C3-H2 peak of 2-HG (arrows), are detectable in vehicle-treated but not DS-1001b–treated tumors. The vertical scales of the four MR spectra were adjusted to ensure that the heights of the N(CH3) peaks at 3.03 ppm were equivalent. F. Scatter plots showing intratumoral 2-HG levels in NDCS-1 and JJ012 (vehicle- and DS-1001b–treated) tumors.
4. Discussion
In this study, both intratumoral and serum levels of 2-HG were significantly higher in IDH-mutant tumors than in IDH-WT tumors. In particular, the intratumoral levels of 2-HG were approximately 100 times higher in the IDH-mutant group than in the WT group, suggesting that 2-HG is a promising surrogate biomarker for the presence of IDH mutations. Serum 2-HG levels also differed significantly between IDH1-mutant and IDH-WT groups, but to a lesser degree than intratumoral 2-HG levels. Levels of peripheral blood 2-HG in tumors with IDH mutations did not differ significantly between IDH-mutant and IDH-WT groups in solid tumors such as glioma (median, 97 vs. 97.2 ng/mL, respectively) and cholangiocarcinoma (median, 343 vs. 55 ng/mL, respectively) as they did in AML, a blood cancer (median, 3,004 vs. 61 ng/mL, respectively); consequently, it remains controversial whether peripheral blood 2-HG can serve as a biomarker for IDH-mutant solid tumors [15], [16], [28]. Our analysis also showed some overlaps of serum 2-HG levels between IDH mutant group and IDH-WT group, suggesting that intratumoral 2-HG can be more reliable than serum 2-HG as a surrogate biomarker for IDH mutation status. Interestingly, the levels of serum 2-HG in IDH-mutant chondrosarcoma are higher than those in IDH-mutant glioma and similar to those in IDH-mutant cholangiocarcinoma (median: chondrosarcoma, 364.7 ng/mL; glioma, 97 ng/mL; cholangiocarcinoma, 343 ng/mL), but those in IDH-WT chondrosarcoma are higher than those in other solid tumors and in healthy donors (median: chondrosarcoma, 288.3 ng/mL; glioma, 97.2 ng/mL; cholangiocarcinoma, 55 ng/mL; healthy donor, 82.6 ng/mL) (Supplementary Table S1) [15], [16]. Ibosidenib (AG-120), an IDH1-mutant inhibitor, reduced plasma 2-HG levels to the levels of healthy donors in all patients throughout the phase I clinical trial [11], [13]. However, there are no reports comparing peripheral blood 2-HG levels in IDH-WT chondrosarcoma with those in healthy donors. Furthermore, it is difficult to directly compare these levels with those in other IDH-mutant solid tumors, due to differences in measurement methods and samples used. It is possible that 2-HG is produced in IDH-WT chondrosarcoma for some reason, such as inactivation of dextro-rotatory 2-HG dehydrogenase, which converts D-2-HG to α-KG. Our measurement of serum 2-HG has several limitations. First, we measured serum 2-HG of relatively younger healthy donors than that of chondrosarcoma patients (median age; 59 years). Serum 2-HG levels may be different between younger and older adults, which may possibly explain the higher serum 2-HG levels in IDH-WT chondrosarcoma. Also, this study analyzed relatively few samples, and it was not possible to compare serum 2-HG concentrations before and after treatment. Furthermore, due to technical difficulties, enantiomers such as D-2-HG and L-2-HG could not be separated. While further investigation of peripheral blood 2-HG is necessary in future clinical trials, our results indicate that it is a potential noninvasive surrogate biomarker for IDH-mutant tumors.
Clinically, antibodies against IDH1R132H, which is common in glioma, have been developed [29], but antibodies against IDH1R132C, which occurs frequently in chondrosarcoma, are technically difficult to develop and are not available for comparison. It is therefore impossible to accurately diagnose IDH-mutant chondrosarcomas by immunostaining, and the detection of 2-HG in IDH-mutant chondrosarcoma is thus important for the clinical application of novel mutant IDH inhibitors.
Our MRS data demonstrated that intratumoral 2-HG signals could be detected in a tumor model of IDH-mutant but not IDH-WT chondrosarcoma. MRS detection of 2-HG signals has not previously been reported in chondrosarcoma tumor models. More importantly, we also observed the disappearance of intratumoral 2-HG signals in IDH-mutant chondrosarcoma tumors after administration of the mutant IDH inhibitor DS-1001b. This indicates that the 2-HG signal in MR spectra may be a useful biomarker during treatment with mutant IDH inhibitor. We attribute our detection of 2-HG signals to the combination of a 9.4-T high-field MR scanner, a high-performance cryogenic radiofrequency coil, and short echo time (TE) STEAM pulse sequence, as these technologies yield a high signal-to-noise ratio, thereby permitting the detection of small metabolite peaks [30]. The use of a short TE STEAM sequence is particularly notable because our data demonstrate for the first time that this sequence could be a more realistic option for clearly detecting 2-HG peaks than various other MRS pulse sequences, including those that previous investigators preferred despite their rather long TE pulse sequences [31], [32]. Theoretically, a short TE pulse sequence is particularly beneficial for detecting metabolite peaks with coupled spin systems that exhibit J-modulation in vivo [33]. This is well-established in NMR studies of 2-HG in vitro; however, our MRS data suggest that this might be feasible even in vivo.
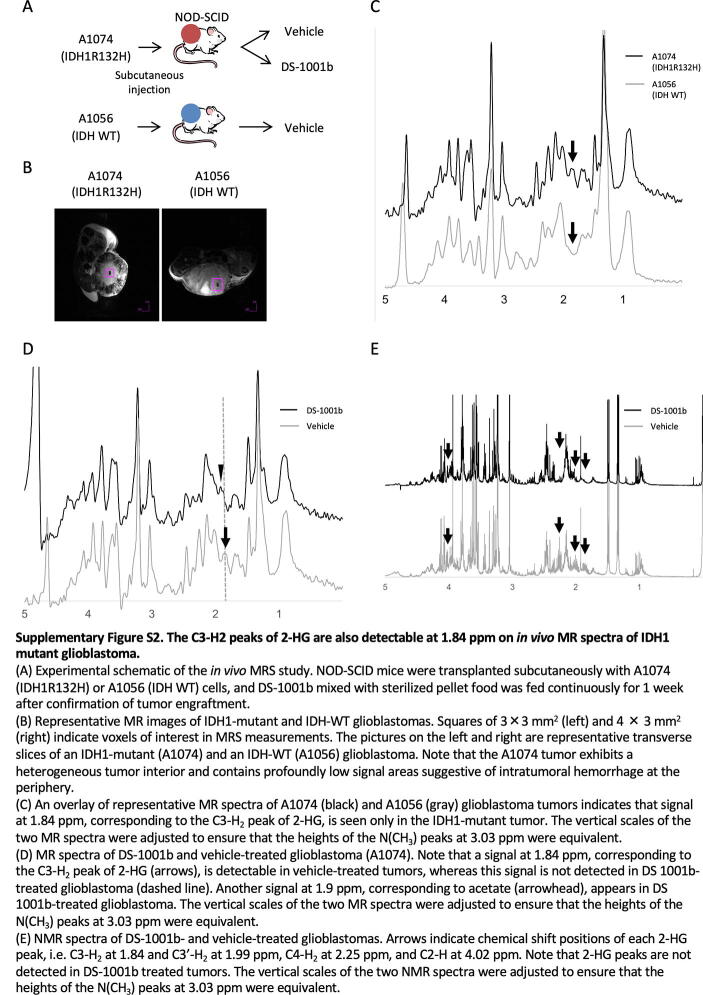
Among the four 2-HG peaks, only the C3-H2 peak at 1.84 ppm was detectable in in vivo MRS. The peaks at 1.99, 2.25, and 4.02 ppm were obscured because they were in close proximity to other metabolite peaks, such as C3-H2 of glutamate, –CH2-COO of lipid, and C2-H2 of creatine; consequently, these were composite peaks. Decomposition of 2-HG peaks from these adjacent metabolite peaks is quite challenging, particularly in vivo, due to line broadening caused by inherent shortening of T2* relaxation times of metabolites in vivo. Some decomposition techniques, including LC model analysis [34], may be useful for analyzing such composite peaks separately; however, these analyses are beyond the scope of this study, and further investigation is necessary to confirm the usefulness of such techniques for detecting 2-HG signals. At present, non-invasive investigation of intratumoral 2-HG in an IDH-mutant chondrosarcoma tumor is more realistic using the C3-H2 peak of 2-HG than the combination of all four peaks, as it is less likely to be obscured by other metabolite peaks. This contention is supported by the fact that the C3-H2 peak of 2-HG at 1.84 ppm is not accompanied by other metabolite peaks in the chemical shift range, as our NMR data show. Furthermore, the intratumoral 2-HG level, which was determined based on the C3-H2 peak in NMR, was consistent with that determined by LC-MS/MS. This was also true in an IDH1-mutant glioblastoma model, as we show in Supplementary Fig. S2A–S2E.
From a clinical viewpoint, monitoring intratumoral 2-HG is important to confirm that mutant IDH inhibitors are reaching their therapeutic target lesions and to evaluate treatment effects, especially in inoperable and metastatic cases. Furthermore, because IDH mutations are not usually examined when diagnosing chondrosarcoma, monitoring of intratumoral 2-HG is likely to play a role in determining whether the tumor has an IDH mutation. Before MRS techniques can be applied to actual chondrosarcoma patients, further technological improvements are needed, including those that detect 2-HG peaks with greater sensitivity and thus ensure accurate quantification of 2-HG signals. However, our preclinical data strongly suggest that MRS could become a useful, non-invasive tool for measuring intratumoral 2-HG levels when mutant IDH inhibitors are applied clinically. We also confirmed the intratumor 2-HG peaks in an IDH1-mutant glioblastoma model with IDH1R132H mutations other than IDH1R132G detected in JJ012 cells, indicating that MRS is useful for malignant tumors with IDH mutations other than chondrosarcoma. These results are encouraging for the future clinical application of MRS across various IDH mutant solid tumors.
In summary, both intratumoral and serum 2-HG levels represent potentially useful biomarkers for IDH-mutant tumors, which will positively impact the clinical development of mutant IDH inhibitors for treatment of advanced chondrosarcoma.
CRediT authorship contribution statement
Makoto Nakagawa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. Masayuki Yamaguchi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. Makoto Endo: Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. Yukino Machida: Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing. Ayuna Hattori: Data curation, Writing – review & editing. Fumie Tanzawa: Data curation, Formal analysis, Writing – review & editing. Shinji Tsutsumi: Data curation, Formal analysis, Writing – review & editing. Issay Kitabayashi: Funding acquisition, Supervision, Writing – review & editing. Akira Kawai: Supervision, Writing – review & editing. Fumihiko Nakatani: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: I.K. received a research grant from Daiichi Sankyo Co., Ltd. F.T. and S.T. are employees of Daiichi Sankyo, Co., Ltd. The other authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgments
We thank Dr. Joel A. Block, Dr. Akira Ogose, and Dr. Koichi Ichimura for providing important cell lines.
Ethics statement
This study was approved by the ethical committee of the National Cancer Center Hospital (Tokyo, Japan) (2014-236 and 2017-336), and written informed consent was obtained from the patients. All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the National Cancer Center.
Financial support
This work was supported in part by Acceleration of Transformative Research for Medical Innovation from the Japan Agency for Medical Research and Development under grant numbers JP20ck0106523 (M.N.) and JP16ck0106088 (I.K.), by the National Cancer Center Research and Development Fund (I.K.), and by the Foundation for Promotion of Cancer Research in Japan (F.N.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jbo.2022.100430.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
References
- 1.Mitchell A.D., Ayoub K., Mangham D.C., Grimer R.J., Carter S.R., Tillman R.M. Experience in the treatment of dedifferentiated chondrosarcoma. J. Bone Joint Surg. Br. 2000;82(1):55–61. doi: 10.1302/0301-620x.82b1.9020. [DOI] [PubMed] [Google Scholar]
- 2.Mardis E.R., Ding L., Dooling D.J., Larson D.E., McLellan M.D., Chen K., Koboldt D.C., Fulton R.S., Delehaunty K.D., McGrath S.D., Fulton L.A., Locke D.P., Magrini V.J., Abbott R.M., Vickery T.L., Reed J.S., Robinson J.S., Wylie T., Smith S.M., Carmichael L., Eldred J.M., Harris C.C., Walker J., Peck J.B., Du F., Dukes A.F., Sanderson G.E., Brummett A.M., Clark E., McMichael J.F., Meyer R.J., Schindler J.K., Pohl C.S., Wallis J.W., Shi X., Lin L., Schmidt H., Tang Y., Haipek C., Wiechert M.E., Ivy J.V., Kalicki J., Elliott G., Ries R.E., Payton J.E., Westervelt P., Tomasson M.H., Watson M.A., Baty J., Heath S., Shannon W.D., Nagarajan R., Link D.C., Walter M.J., Graubert T.A., DiPersio J.F., Wilson R.K., Ley T.J. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsons D.W., Jones S., Zhang X., Lin J.C., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., Olivi A., McLendon R., Rasheed B.A., Keir S., Nikolskaya T., Nikolsky Y., Busam D.A., Tekleab H., Diaz L.A., Jr., Hartigan J., Smith D.R., Strausberg R.L., Marie S.K., Shinjo S.M., Yan H., Riggins G.J., Bigner D.D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V.E., Kinzler K.W. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borger D.R., Tanabe K.K., Fan K.C., Lopez H.U., Fantin V.R., Straley K.S., Schenkein D.P., Hezel A.F., Ancukiewicz M., Liebman H.M., Kwak E.L., Clark J.W., Ryan D.P., Deshpande V., Dias-Santagata D., Ellisen L.W., Zhu A.X., Iafrate A.J. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–79. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amary M.F., Bacsi K., Maggiani F., Damato S., Halai D., Berisha F., Pollock R., O'Donnell P., Grigoriadis A., Diss T., Eskandarpour M., Presneau N., Hogendoorn P.C., Futreal A., Tirabosco R., Flanagan A.M. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011;224(3):334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M., Nakatani F., Matsunaga H., Seki T., Endo M., Ogawara Y., Machida Y., Katsumoto T., Yamagata K., Hattori A., Fujita S., Aikawa Y., Ishikawa T., Soga T., Kawai A., Chuman H., Yokoyama N., Fukushima S., Yahiro K., Kimura A., Shimada E., Hirose T., Fujiwara T., Setsu N., Matsumoto Y., Iwamoto Y., Nakashima Y., Kitabayashi I. Selective inhibition of mutant IDH1 by DS-1001b ameliorates aberrant histone modifications and impairs tumor activity in chondrosarcoma. Oncogene. 2019;38(42):6835–6849. doi: 10.1038/s41388-019-0929-9. [DOI] [PubMed] [Google Scholar]
- 7.Nicolle R., Ayadi M., Gomez-Brouchet A., Armenoult L., Banneau G., Elarouci N., Tallegas M., Decouvelaere A.V., Aubert S., Redini F., Marie B., Labit-Bouvier C., Reina N., Karanian M., le Nail L.R., Anract P., Gouin F., Larousserie F., de Reynies A., de Pinieux G. Integrated molecular characterization of chondrosarcoma reveals critical determinants of disease progression. Nat. Commun. 2019;10(1):4622. doi: 10.1038/s41467-019-12525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma S., Jiang B., Deng W., Gu Z.K., Wu F.Z., Li T., Xia Y., Yang H., Ye D., Xiong Y., Guan K.L. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015;6(11):8606–8620. doi: 10.18632/oncotarget.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machida Y., Nakagawa M., Matsunaga H., Yamaguchi M., Ogawara Y., Shima Y., Yamagata K., Katsumoto T., Hattori A., Itoh M., Seki T., Nishiya Y., Nakamura K., Suzuki K., Imaoka T., Baba D., Suzuki M., Sampetrean O., Saya H., Ichimura K., Kitabayashi I. A potent blood-brain barrier-permeable mutant IDH1 inhibitor suppresses the growth of glioblastoma with IDH1 mutation in a patient-derived orthotopic xenograft model. Mol. Cancer Ther. 2020;19(2):375–383. doi: 10.1158/1535-7163.MCT-18-1349. [DOI] [PubMed] [Google Scholar]
- 10.Polychronidou G., Karavasilis V., Pollack S.M., Huang P.H., Lee A., Jones R.L. Novel therapeutic approaches in chondrosarcoma. Future Oncol. 2017;13(7):637–648. doi: 10.2217/fon-2016-0226. [DOI] [PubMed] [Google Scholar]
- 11.Fan B., Mellinghoff I.K., Wen P.Y., Lowery M.A., Goyal L., Tap W.D., Pandya S.S., Manyak E., Jiang L., Liu G., Nimkar T., Gliser C., Prahl Judge M., Agresta S., Yang H., Dai D. Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest. New Drugs. 2020;38(2):433–444. doi: 10.1007/s10637-019-00771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Alfa G.K., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V., Borad M.J., Bridgewater J., Harris W.P., Murphy A.G., Oh D.-Y., Whisenant J., Lowery M.A., Goyal L., Shroff R.T., El-Khoueiry A.B., Fan B., Wu B., Chamberlain C.X., Jiang L., Gliser C., Pandya S.S., Valle J.W., Zhu A.X. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807. doi: 10.1016/S1470-2045(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tap W.D., Villalobos V.M., Cote G.M., Burris H., Janku F., Mir O., Beeram M., Wagner A.J., Jiang L., Wu B., Choe S., Yen K., Gliser C., Fan B., Agresta S., Pandya S.S., Trent J.C. Phase I study of the mutant IDH1 inhibitor ivosidenib: safety and clinical activity in patients with advanced chondrosarcoma. J. Clin. Oncol. 2020;38(15):1693–1701. doi: 10.1200/JCO.19.02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad N., Wong D., Lum A., Lin J., Ho J., Lee C.H., Yip S. Characterisation of isocitrate dehydrogenase 1/isocitrate dehydrogenase 2 gene mutation and the d-2-hydroxyglutarate oncometabolite level in dedifferentiated chondrosarcoma. Histopathology. 2020;76(5):722–730. doi: 10.1111/his.14018. [DOI] [PubMed] [Google Scholar]
- 15.Lombardi G., Corona G., Bellu L., Della Puppa A., Pambuku A., Fiduccia P., Bertorelle R., Gardiman M.P., D'Avella D., Toffoli G., Zagonel V. Diagnostic value of plasma and urinary 2-hydroxyglutarate to identify patients with isocitrate dehydrogenase-mutated glioma. Oncologist. 2015;20(5):562–567. doi: 10.1634/theoncologist.2014-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borger D.R., Goyal L., Yau T., Poon R.T., Ancukiewicz M., Deshpande V., Christiani D.C., Liebman H.M., Yang H., Kim H., Yen K., Faris J.E., Iafrate A.J., Kwak E.L., Clark J.W., Allen J.N., Blaszkowsky L.S., Murphy J.E., Saha S.K., Hong T.S., Wo J.Y., Ferrone C.R., Tanabe K.K., Bardeesy N., Straley K.S., Agresta S., Schenkein D.P., Ellisen L.W., Ryan D.P., Zhu A.X. Circulating oncometabolite 2-hydroxyglutarate is a potential surrogate biomarker in patients with isocitrate dehydrogenase-mutant intrahepatic cholangiocarcinoma. Clin. Cancer Res. 2014;20(7):1884–1890. doi: 10.1158/1078-0432.CCR-13-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa M., Sekimizu M., Endo M., Kobayashi E., Iwata S., Fukushima S., Yoshida A., Kitabayashi I., Ichikawa H., Kawai A., Nakatani F. Prognostic impact of IDH mutations in chondrosarcoma. J. Orthop. Sci. 2021 doi: 10.1016/j.jos.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Matsunaga H., Futakuchi-Tsuchida A., Takahashi M., Ishikawa T., Tsuji M., Ando O. IDH1 and IDH2 have critical roles in 2-hydroxyglutarate production in D-2-hydroxyglutarate dehydrogenase depleted cells. Biochem. Biophys. Res. Commun. 2012;423(3):553–556. doi: 10.1016/j.bbrc.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Ghert M.A., Jung S.T., Qi W., Harrelson J.M., Erickson H.P., Block J.A., Scully S.P. The clinical significance of tenascin-C splice variant expression in chondrosarcoma. Oncology. 2001;61(4):306–314. doi: 10.1159/000055338. [DOI] [PubMed] [Google Scholar]
- 20.Kudo N., Ogose A., Hotta T., Kawashima H., Gu W., Umezu H., Toyama T., Endo N. Establishment of novel human dedifferentiated chondrosarcoma cell line with osteoblastic differentiation. Virchows Arch. 2007;451(3):691–699. doi: 10.1007/s00428-007-0426-3. [DOI] [PubMed] [Google Scholar]
- 21.Goike H.M., Asplund A.C., Pettersson E.H., Liu L., Ichimura K., Collins V.P. Cryopreservation of viable human glioblastoma xenografts. Neuropathol. Appl. Neurobiol. 2000;26(2):172–176. doi: 10.1046/j.1365-2990.2000.026002172.x. [DOI] [PubMed] [Google Scholar]
- 22.Gruetter R. Automatic, localized in Vivo adjustment of all first-and second-order shim coils. Magn. Reson. Med. 1993;29(6):804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 23.Bal D., Gryff-Keller A. 1H and13C NMR study of 2-hydroxyglutaric acid and its lactone. Magn. Reson. Chem. 2002;40(8):533–536. [Google Scholar]
- 24.Nakagami R., Yamaguchi M., Ezawa K., Kimura S., Hamamichi S., Sekine N., Furukawa A., Niitsu M., Fujii H. Recovery correction technique for NMR spectroscopy of perchloric acid extracts using DL-valine-2,3–d2: validation and application to 5-fluorouracil-induced brain damage. Magn. Reson. Med. Sci. 2014;13(3):145–153. doi: 10.2463/mrms.2013-0089. [DOI] [PubMed] [Google Scholar]
- 25.Zhu G.G., Nafa K., Agaram N., Zehir A., Benayed R., Sadowska J., Borsu L., Kelly C., Tap W.D., Fabbri N., Athanasian E., Boland P.J., Healey J.H., Berger M.F., Ladanyi M., Hameed M. Genomic profiling identifies association of IDH1/IDH2 mutation with longer relapse-free and metastasis-free survival in high-grade chondrosarcoma. Clin. Cancer Res. 2020;26(2):419–427. doi: 10.1158/1078-0432.CCR-18-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugowska I., Teterycz P., Mikula M., Kulecka M., Kluska A., Balabas A., Piatkowska M., Wagrodzki M., Pienkowski A., Rutkowski P., Ostrowski J. IDH1/2 mutations predict shorter survival in chondrosarcoma. J Cancer. 2018;9(6):998–1005. doi: 10.7150/jca.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vuong H.G., Ngo T.N.M., Dunn I.F. Prognostic importance of IDH mutations in chondrosarcoma: An individual patient data meta-analysis. Cancer Med. 2021;10(13):4415–4423. doi: 10.1002/cam4.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiNardo C.D., Propert K.J., Loren A.W., Paietta E., Sun Z., Levine R.L., Straley K.S., Yen K., Patel J.P., Agresta S., Abdel-Wahab O., Perl A.E., Litzow M.R., Rowe J.M., Lazarus H.M., Fernandez H.F., Margolis D.J., Tallman M.S., Luger S.M., Carroll M. Serum 2-hydroxyglutarate levels predict isocitrate dehydrogenase mutations and clinical outcome in acute myeloid leukemia. Blood. 2013;121(24):4917–4924. doi: 10.1182/blood-2013-03-493197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capper D., Weissert S., Balss J., Habel A., Meyer J., Jager D., Ackermann U., Tessmer C., Korshunov A., Zentgraf H., Hartmann C., von Deimling A. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tkác I., Starcuk Z., Choi I.Y., Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn. Reson. Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 31.Andronesi O.C., Kim G.S., Gerstner E., Batchelor T., Tzika A.A., Fantin V.R., Vander Heiden M.G., Sorensen A.G. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 2012;4(116):116ra4. doi: 10.1126/scitranslmed.3002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi C., Ganji S.K., DeBerardinis R.J., Hatanpaa K.J., Rakheja D., Kovacs Z., Yang X.L., Mashimo T., Raisanen J.M., Marin-Valencia I., Pascual J.M., Madden C.J., Mickey B.E., Malloy C.R., Bachoo R.M., Maher E.A. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012;18(4):624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi M., Mitsumori F., Watanabe H., Takaya N., Minami M. In vivo localized 1H MR spectroscopy of rat testes: stimulated echo acquisition mode (STEAM) combined with short TI inversion recovery (STIR) improves the detection of metabolite signals. Magn. Reson. Med. 2006;55(4):749–754. doi: 10.1002/mrm.20829. [DOI] [PubMed] [Google Scholar]
- 34.Provencher S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.