Highlights
-
•
Vulvar cancer is rare, but the incidence is rising, and outcomes are poor in advanced disease.
-
•
Immune checkpoint inhibitors (ICIs) are a promising therapeutic option that have shown clinical benefit in certain patients.
-
•
The role of ICIs in vulvar cancer is mainly extrapolated from trials in cutaneous squamous cell and cervical cancers.
-
•
Further elucidation of the role of immunotherapy in the treatment of vulvar cancer is warranted.
Keywords: Vulvar cancer, Vulvar squamous cell carcinoma, Recurrent disease, Metastatic disease, Immunotherapy
Abstract
There are limited treatment options for patients with advanced vulvar cancer. However, several immune checkpoint inhibitors (ICIs) are FDA-approved or NCCN-Compendia–listed for qualified patients with advanced disease. In this case report, we present a patient with metastatic vulvar squamous cell carcinoma who was treated with pembrolizumab in the setting of disease progression following prior treatment with radiation and chemotherapy. Best response to immunotherapy was an unconfirmed partial response. We summarize the current role of ICIs in treating advanced vulvar cancer, which is largely extrapolated from the squamous cell skin cancer and cervical cancer guidelines. Additionally, we emphasize the need for more inclusive clinical trials and a better understanding of vulvar cancer molecular biology, as well as the identification of biomarkers to predict response to targeted therapy in patients with advanced vulvar cancer.
1. Introduction
Vulvar cancer represents only 4% of gynecologic cancers (Borella et al., 2021). Based on data from 2014 to 2018, the incidence is 2.6 new cases per 100,000 women per year, with a median age at diagnosis of 69 years (Borella et al., 2021). In the United States, the incidence is rising in older patients, whereas for younger patients the incidence is decreasing, likely because of vaccination against human papillomavirus (HPV) (National Cancer Instiute Surveillance, Epidemioligy, and End Results Program, 2022). Vulvar squamous cell carcinoma (VSCC) is the predominant histologic type of vulvar cancer, accounting for about 80% of cases. VSCC comprises three molecularly distinct subtypes: HPV-associated VSCC, HPV-independent VSCC with mutant p53 expression pattern (which is highly correlated with a TP53 mutation status), and HPV-independent VSCC with wild-type p53 expression pattern. HPV-associated VSCC is characterized by p16 overexpression, mostly arises in younger patients (aged 30–50 years) and has a better overall survival (OS) and longer disease-free survival (DFS) when compared to HPV-independent VSCC. However, HPV-associated VSCC accounts for only up to 40% of VSCC (Borella et al., 2021, Kortekaas et al., 2021, Woelber et al., 2021). The majority of VSCC cases are HPV-independent, often harbor TP53 mutations and typically present in post-menopausal patients. HPV-independent/p53-mutant VSCC has a higher rate of local recurrence after primary treatment and a lower 5-year OS (48%) compared to HPV-associated VSCC (83%) and HPV-independent/p53-wildtype (64%) (Kortekaas et al., 2021, Kortekaas et al., 2020). The oncogenesis of HPV-independent/p53-wildtype VSCC may be associated with NOTCH1 and HRAS mutations (Borella et al., 2021).
The immune system plays a crucial role in modulating the tumor microenvironment by exerting an antitumoral effect or contributing to tumor progression (Borella et al., 2021). The immune system can clear HPV infection. Consequently, immunosuppressed patients have a significantly increased risk of cutaneous squamous cell carcinoma (CSCC) (Migden et al., 2018). Immune checkpoint inhibitors (ICIs) target tumor mechanisms that would otherwise allow the tumor cells to evade the immune system. The response to ICIs can sometimes be predicted using intrinsic and extrinsic tumor factors, including programmed death ligand 1 (PD-L1) expression, microsatellite instability (MSI) status, tumor mutational burden (TMB) status, and the presence and type of T-cell infiltration into the tumor (Borella et al., 2021, Howitt et al., 2016).
Treatment and prognosis for VSCC depend on the tumor stage at initial diagnosis (3-year progression-free survival [PFS] rate of 35.2% and OS rate of 56.2% in patients with node-positive disease, compared to 75.2% and 90.2%, respectively, in those with node-negative disease) (Borella et al., 2021, Woelber et al., 2021). Treatment for early-stage disease consists of radical surgery. Postoperative radiotherapy with or without radiosensitizing chemotherapy is considered for those with higher-risk features. In more locally-advanced disease, an observed response following primary chemoradiation may facilitate a less radical surgery. (Kortekaas et al., 2021). Therapeutic options are extremely limited for locally advanced, recurrent, or metastatic disease not amenable to radiotherapy and/or radical surgery. There is poor prognosis in the metastatic setting, as reflected by a one-year survival of 15–30% (Woelber et al., 2021). Given the poor response rates to second-line chemotherapy after initial platinum-based regimens and the presence of frequent comorbidities in older patients, there is a clear need for new and less toxic therapeutic options (Borella et al., 2021, Han et al., 2012, Witteveen et al., 2009).
The molecular landscape underlying different subtypes of VSCC remains poorly understood, partly due to the rarity of disease (Woelber et al., 2021). Immunotherapy has transformed the management and prognosis of many solid tumor cancers and presents a promising treatment option for patients with VSCC (Borella et al., 2021). Herein, we report a case of a patient with metastatic VSCC who experienced a partial response with pembrolizumab. The aim of this report is to assist the clinician in the management of this rare disease by summarizing the relevant, current FDA-approved and National Comprehensive Cancer Network (NCCN)-Compendia–listed immunotherapeutic options.
2. Case presentation
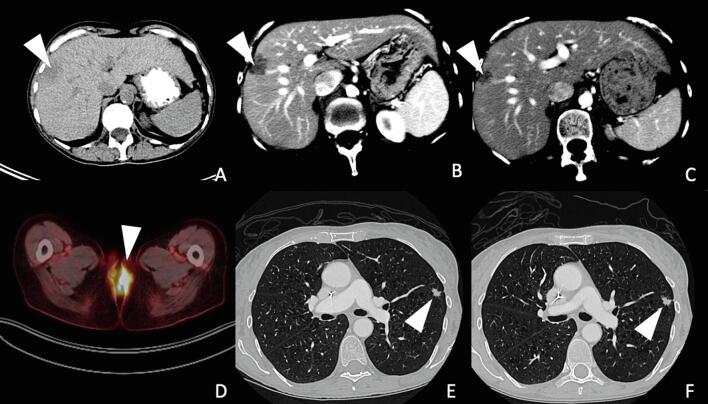
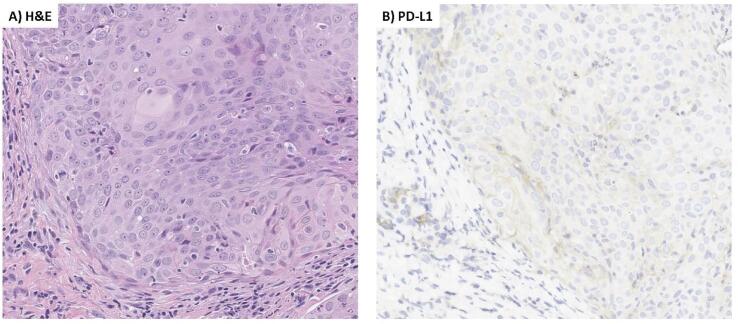
A 64-year-old G3P1 postmenopausal, female patient presented with a complaint of pain and vulvar irritation. Upon examination, the patient had an exophytic, fungating vulvar mass encompassing the posterior introitus, posterior vaginal wall, and anus. Vulvar biopsy revealed moderately differentiated squamous cell carcinoma, high-risk HPV-positive (in situ hybridization positive for HPV 16 mRNA), with overt (>1mm) stromal invasion, and associated high grade squamous intraepithelial lesion (HSIL, VIN3). PET scan was notable for metastatic disease, including an FDG-avid lingular lung nodule and a hepatic lesion (Fig. 1). The patient received palliative radiation to the vulva and inguinal nodes, 4500 cGy over 25 fractions with a boost to 5940 cGy, and concurrent weekly cisplatin. This was followed by intravenous carboplatin (AUC 5), paclitaxel (175 mg/m2) and bevacizumab (15 mg/kg) once every 3 weeks for 5 cycles with initial radiographic response, followed by 6 additional cycles of bevacizumab maintenance. Follow-up imaging demonstrated progression of disease with increased hepatic and pulmonary metastases (Fig. 1). PD-L1 testing by immunohistochemical staining (IHC) was then performed on the original vulvar biopsy sample and demonstrated a combined positivity score (CPS) of > 1 (Fig. 2). The patient initiated second-line intravenous pembrolizumab (200 mg) once every 3 weeks. The treatment was well-tolerated with transient grade 1 diarrhea, mucositis, and rash during the fifth cycle. There were no treatment delays. Follow-up imaging after 3 months demonstrated an unconfirmed partial response (PR) and she continued pembrolizumab until imaging at 6 months showed disease progression.
Fig. 1.
Radiographs throughout patient’s clinical course. (A) CT scan at initial diagnosis, axial view of liver, lesion measuring 1.79 × 2.38 cm; (B) CT scan 1 year later at first recurrence, axial view of liver, lesion measuring 2.51 × 1.60 cm; (C) CT scan 3 months later following pembrolizumab, axial view of liver, lesion measuring 1.5 × 1.2 cm; (D) PET scan at initial diagnosis, axial view of vulva, lesion with FDG avidity, SUV 11.9; (E) CT scan 1 year later at first recurrence, axial view of lungs, left upper lobe lesion measuring 1.07 × 0.57 cm; (F) CT scan 3 months later following pembrolizumab, axial view of lungs, left upper lobe lesion unchanged, measuring 0.99 × 0.64 cm.
Fig. 2.
Hematoxylin & eosin stain, PD-L1 immunohistochemical stain. (A) H&E: Hematoxylin and eosin stain of patient’s vulvar squamous cell carcinoma; (B) PD-L1: Programmed death ligand 1 immunohistochemistry of the same tumor sample.
3. Discussion
There are limited treatment options for patients with metastatic or recurrent VSCC. The current NCCN recommendations are based on clinical trials that included only a handful of patients with VSCC. Many of the existing recommendations are extrapolated from the broader squamous cell and cervical cancer guidelines. The molecular landscape of VSCC is diverse and not well understood; however, immunotherapy is a promising option for patients with advanced disease, especially in the setting of progression following prior treatment. We summarize the ICIs that are currently FDA-approved or NCCN compendia-listed that are applicable to VSCC (Table 1).
Table 1.
Immunotherapy for vulvar cancer.
|
Inclusion Criteria |
Number of Patients with VC Studied | Clinical Efficacy in VC | Immunotherapy-Related Adverse Events (not specific to cancer type) | Reference Studies |
|---|---|---|---|---|
|
Pembrolizumab (a PD-1 inhibitor) Dose: 200 mg IV every 3 weeks or 400 mg IV every 6 weeks | ||||
| Disease progression on or after chemotherapy in patients whose tumors express PD-L1with CPS ≥ 1 as determined by a validated/FDA-approved test. | 18 patients with VSCC | mOS: 3.8 months, ORR: 6% |
Total AEs: 311 patients (66%); grade 3 or worse AEs: 67 patients (14%); treatment discontinued because of AEs: 17 patients (4%) | KEYNOTE-028 (Ott et al., 2019) |
| Unresectable or metastatic solid tumors with TMB-H, that have progressed following prior treatment and with no other satisfactory treatment options. | 71 patients with VSCC (12 with tTMB-high and 59 with non- tTMB-high) | For the tTMB-high VC group, mOS: 10.8 months (2·2–not assessable), ORR: 17% |
Total AEs: 67 patients (64%); grade 3 or worse AEs: 16 patients (15%); treatment discontinued because of AEs: 8 patients (8%); 1 patient died from treatment-related pneumonia | KEYNOTE-158 (Marabelle et al., 2020a) |
| MSI-H/dMMR tumors | 1 patient with VC | Not assessed in VC. Across all tumors, mOS: 23.5 months (95% CI, 13.5 months to not assessable), ORR: 34.3% (95% CI, 28.3 to 40.8) |
Total AEs: 151 patients (64.8%); grade 3 or worse AEs: 34 patients (14.6%); treatment discontinued because of AEs: 22 patients (9.4%); 1 patient died from treatment-related pneumonia |
KEYNOTE-158 (Marabelle et al., 2020b) |
| Recurrent or metastatic CSCC or locally advanced CSCC that is not curable by surgery or radiation | Unknown number of patients with VC, if any | Not assessed in VC. Across all tumors,mOS: not reached (95% CI, 10.7 months to not reached), ORR: 34.3% (95% CI, 25.3 to 44.2) |
Total AEs: 70 patients (66.7%); grade 3 or worse AEs: 6 patients (5.7%); treatment discontinued because of AEs: 5 patients (4.8%); 1 patient died of treatment-related cranial nerve neuropathy | KEYNOTE-629 (Grob et al., 2020) |
|
Nivolumab (a PD-1 inhibitor) Dose: 240 mg IV every 2 weeks or 480 mg IV every 4 weeks | ||||
| HPV-related advanced or recurrent/metastatic vulvar cancer | 5 patients with vaginal or vulvar cancer | ORR was 20.0% (95% CI, 0.5 to 71.6), 18-month OS: 20.0% |
Total AEs: 151 patients (64.8%); grade 3 or worse AEs: 34 patients (14.6%); treatment discontinued because of AEs: 22 patients (9.4%) | CheckMate 358 trial (Naumann et al., 2019) |
|
Cemiplimab (a PD-1 inhibitor) Dose: 350 mg IV every 3 weeks | ||||
| Locally advanced or metastatic CSCC in patients who are not candidates for curative surgery/radiation | 0 patients with VC | Not assessed in VC. Across all CSCC,estimated 12-month OS: 81% (95% CI, 68 to 89) ,ORR: 47% (95% CI, 34 to 61) |
Total AEs: 44 patients (74.6%);grade 3 or worse: 7 patients (11.9%); treatment discontinued because of AEs: 4 patients (7%) |
(Migden et al., 2018) |
PD-1, programmed death 1 receptor; PD-L1, programmed death ligand 1; VC, vulvar cancer; AEs, adverse events; TMB-H, tumor mutational burden-high (≥10 mutations/megabase, determined by an FDA-approved test); CPS, Combined Positive Score; MSI-H, high microsatellite instability; dMMR, deficient in DNA mismatch repair; VSCC, vulvar squamous cell carcinoma; CSCC, cutaneous squamous cell carcinoma; mOS, median overall survival; OOR, objective response rate (defined as confirmed complete response or partial response assessed per Response Evaluation Criteria in Solid Tumors (RECIST)); DVT, deep vein thrombosis; 95% CI, 95% confidence interval; IV, intravenous.
4. Pembrolizumab
4.1. Dose: 200 mg IV every 3 weeks or 400 mg IV every 6 weeks
KEYNOTE-028, a non-randomized, multicenter, multicohort phase Ib trial, investigated the use of single-agent pembrolizumab (a PD-1 inhibitor) in patients with PD-L1–positive advanced solid tumors, including 18 VSCCs (Ott et al., 2019). For patients with VSCC, the median PFS was 3.8 months, and the objective response rate (ORR) was 7%. At 6 and 12 months, OS rates were 42% and 28%, whereas the PFS rates were 20% and 7%, respectively. Although no patient with VSCC achieved a complete response (CR), 1 had a PR, 7 had stable disease (SD), and 6 experienced disease progression (PD). There was a significant correlation between the PD-L1 CPS, which was available for 8 patients with VSCC, and both ORR (p = 0.018) and PFS (p = 0.005).
KEYNOTE-158, a multi-cohort, single-arm, open-label, phase II study assessed pembrolizumab monotherapy in patients with incurable solid tumors. In this study, prospective biomarker analyses explored the association of high tissue tumor mutational burden (tTMB-high, defined as at least 10mt/Mb) and high microsatellite instability/deficiency in DNA mismatch repair (MSI-H/dMMR) with patient outcomes (Marabelle et al., 2020a, Marabelle et al., 2020b). The cohort that assessed tTMB status included 71 patients with vulvar cancer (12 with tTMB-high and 59 with non-tTMB-high). Two of 12 participants (17%) in the tTMB-high group, and 2 of 59 participants (3%) in the non-tTMB-high group had an objective response. Median OS for tTMB-high was 10.8 months, compared to 5.3 months for non-tTMB-high. These results supported a correlation between tTMB-high status and clinical benefit from pembrolizumab. MSI-H/dMMR (N = 223) was also explored as a predictive biomarker of response, but the cohort had only one patient with advanced vulvar cancer. Across all 27 tumor types represented, ORR was 34.3%, median PFS was 4.1 months, and median OS was 23.5 months. As a result, pembrolizumab monotherapy was FDA-approved for patients with unresectable or metastatic solid tumors that were either tTMB-high and/or MSI-H/dMMR and had progressed following prior chemotherapy with no other satisfactory treatment options. That said, high tTMB rates are very rarely reported in VSCC, and MSI-H/dMMR status is even less commonly reported.
KEYNOTE-629 was an open-label, single-arm, phase II study of pembrolizumab in patients with locally advanced or recurrent/metastatic CSCC (Grob et al., 2020). The study did not specify the anatomical origin of CSCC; thus, it is unclear whether any vulvar cases were included. The ORR was 34.3% (4 CR, 32 PR), and median PFS was 6.9 months. Median duration of response (DOR) and median OS were not reached.
The use of pembrolizumab in vulvar cancer is also extrapolated from cervical cancer data. A recent randomized controlled trial assessed pembrolizumab versus placebo in addition to platinum-based chemotherapy and, per investigator discretion, bevacizumab for persistent, recurrent, or metastatic cervical cancer (Colombo et al., 2021). In the intention-to-treat population of 617 patients, PFS was 10.4 months and 8.2 months, and OS at two years was 53% in the pembrolizumab group and 41.7% in the placebo group. Importantly, in subgroup analyses, PD-L1 CPS > 1 demonstrated improved survival for the patients receiving pembrolizumab.
In summary, pembrolizumab is an FDA-approved and NCCN-Compendia–listed treatment option for recurrent or metastatic VSCC, based on data showing efficacy of pembrolizumab in PD-L1–positive advanced solid and cervical tumors. This is in line with the patient in our case presentation, who had a PD-L1–positive tumor and has had an unconfirmed PR while on pembrolizumab. Rare cases of VSCC are TMB-H and MSI-H, for which treatment with pembrolizumab would also be considered. Yet, the activity of ICIs in these tumors needs to be better understood as these tumors further differ based on HPV-infection and p53-mutation status and might represent different disease entities.
5. Nivolumab
5.1. Dose: 240 mg IV every 2 weeks or 480 mg IV every 4 weeks
CheckMate 358 was a multicenter, open-label, multicohort phase I/II trial investigating nivolumab in patients with HPV-associated solid tumors in the neoadjuvant or recurrent/metastatic setting (Naumann et al., 2019). Vulvar cancer was grouped with vaginal cancer for a total of 5 cases, 2 of which were HPV-associated. In the vaginal/vulvar cohort, the ORR was 20.0%. The DOR was 5.0 months in the single responding patient (HPV-independent), and 3 patients had SD and 1 progressed. Twelve-month and 18-month OS rates were 40.0% and 20.0%, respectively, and 6-month PFS rate was 40.0%. Of the 5 responding patients in the cervical cancer cohort, 3 patients had HPV-associated tumors. The unexpectedly low rate of HPV-associated vulvar tumors could be due to different sensitivities of the various types of HPV assays used (in situ hybridization, real-time polymerase chain reaction, or immunohistochemistry) (Naumann et al., 2019). Extrapolating from the cervical cancer cohort, nivolumab is only NCCN-Compendia–listed to treat HPV-associated advanced or recurrent/metastatic VSCC, but that could evolve with further investigation.
6. Cemiplimab
6.1. Dose: 350 mg IV every 3 weeks
Lastly, cemiplimab is approved for patients with advanced CSCC, based on an open-label, multicenter, non-randomized phase I/II study that investigated cemiplimab in patients with advanced CSCC (Migden et al., 2018). For metastatic CSCC (N = 59), the ORR was 47%. Four patients had CR, 24 had PR, 9 had SD, and 11 had PD. Among the 28 patients who had a response, DOR exceeded 6 months in 57%, and 82% continued to respond on cemiplimab at the time of data cutoff. Although patients with primary vulvar cancer were not included, VSCC is considered a CSCC and therefore, is included as an indication for cemiplimab. However, these two cancers’ molecular signatures differ (e.g., ultra-violet light exposure), and CSCCs are almost never associated with HPV (Kortekaas et al., 2020).
In clinical practice, TMB score, PD-L1, and MSI/dMMR are commonly used biomarkers to help predict immunotherapy response. However, it is important to note that prescription of pembrolizumab and cemiplimab for advanced vulvar cancer does not require these additional tests because both ICIs are approved for treatment of CSCC, which includes VSCC. Nivolumab is NCCN-Compendia–listed only for HPV-associated vulvar cancer, relying on the results of the CheckMate 358 trial that showed a clinical benefit of nivolumab in virus-associated solid tumors; yet the trial included only 3 patients with vulvar cancer and the responder had an HPV-independent tumor.
As a monotherapy, ICIs demonstrated a manageable safety profile (Colombo et al., 2021, Grob et al., 2020, Marabelle et al., 2020b, Marabelle et al., 2020a, Naumann et al., 2019, Ott et al., 2019). Most common treatment-related adverse events were grade 1–2 and included fatigue, decreased appetite, nausea, asthenia, constipation, diarrhea, pruritis, rash, cough, and pneumonitis (Table 1). Two patients treated with pembrolizumab died because of treatment-related (1) pneumonia and (2) cranial nerve neuropathy. In the study that investigated cemiplimab for metastatic CSCC, there were 3 deaths due to non-treatment–related complications of (1) pneumonia, (2) hypercalcemia and DVT, and (3) unknown etiology. Overall, the safety profiles were similar to prior ICI reports.
With a manageable safety profile and demonstrated clinical benefit in VSCC treatment, checkpoint inhibition is a promising therapeutic option for recurrent or metastatic VSCC and warrants additional investigation. Currently, it is reasonable to consider ICIs for advanced, recurrent or metastatic VSCC, especially in tumors that are HPV-associated, PD-L1–positive and/or, in rarer instances, TMB-H, or MSI-H/dMMR. A more accurate usage of immunotherapies and improved patient outcomes may be promoted by a better understanding of the molecular landscape and underlying mechanisms of VSCC carcinogenesis, as well as careful inclusion of patients with VSCC in clinical trials.
CRediT authorship contribution statement
Aaron Praiss: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Anastasia Navitski: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Seth Cohen: Writing – review & editing. Basile Tessier-Cloutier: Writing – review & editing. Vance Broach: Writing – review & editing. Roisin E. O'Cearbhaill: Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Praiss declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this report. Dr. Navitski declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this report. Dr. Cohen declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this report. Dr. Tessier-Cloutier declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this report. Dr. Broach declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this report. Dr. O'Cearbhaill reports personal fees from Tesaro/GSK, personal fees from Regeneron, personal fees from Seattle Genetics, meal from AstraZeneca Pharmaceuticals, personal fees from Fresenius Kabi, personal fees from Gynecologic Oncology Foundation, personal fees from Bayer, personal fees from Immunogen, personal fees from Curio, travel for meeting from Hitech Health, personal fees from MJH, outside the submitted work. She is on the physician strategic advisory board for GSK, outside the submitted work. She is a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies and non-compensated advisor for Carina Biotech. Her institute receives funding for clinical research from Bayer/Celgene/Juno, Tesaro/GSK, Merck, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, Gynecologic Oncology Foundation. She is an NRG representative for the ComboMATCH study.
Acknowledgments
Acknowledgments
Informed Consent
Informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the consent documentation is available for review by the Editor-in-Chief of this journal on request.
Funding Statement
This study was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
References
- Borella, F., Preti, M., Bertero, L., Collemi, G., Castellano, I., Cassoni, P., Cosma, S., Carosso, A.R., Bevilacqua, F., Gallio, N., Benedetto, C., Micheletti, L., 2021. Is there a place for immune checkpoint inhibitors in vulvar neoplasms? A state of the art review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22010190. [DOI] [PMC free article] [PubMed]
- Colombo N., Dubot C., Lorusso D., Caceres M.V., Hasegawa K., Shapira-Frommer R., Tewari K.S., Salman P., Hoyos Usta E., Yañez E., Gümüş M., Olivera Hurtado de Mendoza M., Samouëlian V., Castonguay V., Arkhipov A., Toker S., Li K., Keefe S.M., Monk B.J. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021;385(20):1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- Grob J.-J., Gonzalez R., Basset-Seguin N., Vornicova O., Schachter J., Joshi A., Meyer N., Grange F., Piulats J.M., Bauman J.R., Zhang P., Gumuscu B., Swaby R.F., Hughes B.G.M. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: A single-arm phase II trial (KEYNOTE-629) J. Clin. Oncol. 2020;38(25):2916–2925. doi: 10.1200/JCO.19.03054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.N., Vergote I., Amant F. Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. Int. J. Gynecological Cancer. 2012;22(5):865–868. doi: 10.1097/IGC.0b013e31824b4058. [DOI] [PubMed] [Google Scholar]
- Howitt B.E., Sun H.H., Roemer M.G.M., Kelley A., Chapuy B., Aviki E., Pak C., Connelly C., Gjini E., Shi Y., Lee L., Viswanathan A., Horowitz N., Neuberg D., Crum C.P., Lindeman N.L., Kuo F., Ligon A.H., Freeman G.J., Hodi F.S., Shipp M.A., Rodig S.J. Genetic basis for PD-L1 expression in squamous cell carcinomas of the cervix and vulva. JAMA Oncol. 2016;2(4):518. doi: 10.1001/jamaoncol.2015.6326. [DOI] [PubMed] [Google Scholar]
- Kortekaas K.E., Bastiaannet E., van Doorn H.C., de Vos van Steenwijk P.J., Ewing-Graham P.C., Creutzberg C.L., Akdeniz K., Nooij L.S., van der Burg S.H., Bosse T., van Poelgeest M.I.E. Vulvar cancer subclassification by HPV and p53 status results in three clinically distinct subtypes. Gynecol. Oncol. 2020;159(3):649–656. doi: 10.1016/j.ygyno.2020.09.024. [DOI] [PubMed] [Google Scholar]
- Kortekaas K.E., Santegoets S.J., Tas L., Ehsan I., Charoentong P., van Doorn H.C., van Poelgeest M.I.E., Mustafa D.A.M., van der Burg S.H. Primary vulvar squamous cell carcinomas with high T cell infiltration and active immune signaling are potential candidates for neoadjuvant PD-1/PD-L1 immunotherapy. J. Immunother. Cancer. 2021;9(10):e003671. doi: 10.1136/jitc-2021-003671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., Chung H.C., Kindler H.L., Lopez-Martin J.A., Miller W.H., Italiano A., Kao S., Piha-Paul S.A., Delord J.-P., McWilliams R.R., Fabrizio D.A., Aurora-Garg D., Xu L., Jin F., Norwood K., Bang Y.-J. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- Marabelle A., Le D.T., Ascierto P.A., Di Giacomo A.M., De Jesus-Acosta A., Delord J.-P., Geva R., Gottfried M., Penel N., Hansen A.R., Piha-Paul S.A., Doi T., Gao B.o., Chung H.C., Lopez-Martin J., Bang Y.-J., Frommer R.S., Shah M., Ghori R., Joe A.K., Pruitt S.K., Diaz Jr L.A. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/ mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migden M.R., Rischin D., Schmults C.D., Guminski A., Hauschild A., Lewis K.D., Chung C.H., Hernandez-Aya L., Lim A.M., Chang A.L.S., Rabinowits G., Thai A.A., Dunn L.A., Hughes B.G.M., Khushalani N.I., Modi B., Schadendorf D., Gao B.o., Seebach F., Li S., Li J., Mathias M., Booth J., Mohan K., Stankevich E., Babiker H.M., Brana I., Gil-Martin M., Homsi J., Johnson M.L., Moreno V., Niu J., Owonikoko T.K., Papadopoulos K.P., Yancopoulos G.D., Lowy I., Fury M.G. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- Naumann R.W., Hollebecque A., Meyer T., Devlin M.-J., Oaknin A., Kerger J., López-Picazo J.M., Machiels J.-P., Delord J.-P., Evans T.R.J., Boni V., Calvo E., Topalian S.L., Chen T., Soumaoro I., Li B., Gu J., Zwirtes R., Moore K.N. Safety and efficacy of nivolumab monotherapy in recurrent or metastatic cervical, vaginal, or vulvar carcinoma: Results from the phase I/II CheckMate 358 trial. J. Clin. Oncol. 2019;37(31):2825–2834. doi: 10.1200/JCO.19.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott P.A., Bang Y.-J., Piha-Paul S.A., Razak A.R.A., Bennouna J., Soria J.-C., Rugo H.S., Cohen R.B., O’Neil B.H., Mehnert J.M., Lopez J., Doi T., van Brummelen E.M.J., Cristescu R., Yang P., Emancipator K., Stein K., Ayers M., Joe A.K., Lunceford J.K. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019;37(4):318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- Vulva. Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2018 [WWW Document], 2022. . Surveillance, Epidemiol. End Results Progr. URL https://seer.cancer.gov/explorer/application.html?site=63&data_type=1&graph_type=2&compareBy=age_range&chk_age_range_1=1&chk_age_range_62=62&chk_age_range_122=122&chk_age_range_160=160&hdn_rate_type=1&hdn_sex=3&race=1&stage=101&advopt_precision=1&advopt_s (accessed 2.9.22).
- Witteveen P.O., van der Velden J., Vergote I., Guerra C., Scarabeli C., Coens C., Demonty G., Reed N. Phase II study on paclitaxel in patients with recurrent, metastatic or locally advanced vulvar cancer not amenable to surgery or radiotherapy: A study of the EORTC-GCG (European Organisation for Research and Treatment of Cancer - Gynaecological Cancer Gro. Ann. Oncol. 2009 doi: 10.1093/annonc/mdp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelber L., Mathey S., Prieske K., Kuerti S., Hillen C., Burandt E., Coym A., Mueller V., Schmalfeldt B., Jaeger A. Targeted therapeutic approaches in Vulvar Squamous Cell Cancer (VSCC): Case series and review of the literature. Oncol. Res. 2021;28(6):645–659. doi: 10.3727/096504020X16076861118243. [DOI] [PMC free article] [PubMed] [Google Scholar]