Introduction:
According to the NCI’s surveillance epidemiology and end results program (SEER) brain and other nervous system tumors will account for an estimated 24,530 cancer diagnoses and 18,600 fatalities in 2021 (www.seer.cancer.gov). The average age of diagnosis for a CNS tumor is 60 years, however, this can vary widely with tumor type. Gliomas account for 25% of non-malignant and 81% of malignant CNS lesions1. Tumors of astrocytic origin account for the majority of all gliomas (76%), with glioblastoma (GBM), accounting for 57% of all malignant gliomas diagnosed1. According to the Central Brain Tumor Registry of the United States (CBTRUS) data compiled from 2012–2016, GBM carried the highest age adjusted annual incidence rate (AAAIR) of any malignant tumor at 3.22 per 100,000 individuals. Based on this incidence rate, there will be roughly 11,000 new cases of GBM diagnosed yearly in the United States. CBTRUS estimates the 5 year survival post GBM diagnosis at 6.8%, meaning roughly only 750 of those 11,000 individuals diagnosed this year will survive until 2026.
Prior to 2016, the World Health organization (WHO) classified gliomas based on histology alone (Table 1) to aid in therapeutic decision-making and predict prognosis. Highly proliferative high-grade gliomas (HGG) were associated with rapid disease progression as well as poor overall survival (OS), whereas slow growing low-grade gliomas (LGG) were associated with better prognosis, but eventual recurrence. However, clinical data demonstrates that despite exhibiting similar histological characteristics, there is significant variation within each tumor grade in terms of disease course, time to recurrence, response to therapy and overall survival2.
Table 1:
WHO grading of Glioma
| Grade | Characteristics | Tumor Types |
|---|---|---|
| I | Low proliferative potential | Pilocytic Astrocytoma |
| II | Low proliferative potential, but infiltrative. Often recur and can progress to higher grade tumors | Astrocytoma Oligodendroglioma Oligoastrocytoma |
| III | Demonstrate histological evidence of malignancy: 1. Hypercellularity 2. Nuclear atypia 3. Increased mitotic activity |
Anaplastic Astrocytoma Anaplastic Oligodendroglioma Anaplastic Oligoastrocytoma |
| IV | Demonstrate histological evidence of malignancy: 1. Hypercellularity 2. Nuclear atypia 3. Increased mitotic activity Plus vascular proliferation and presence of necrosis |
Glioblastoma |
Recent data suggests that the molecular classification of gliomas based on genetic and epigenetic changes is more predictive of clinical course than histological classification2,3. For example, a high grade tumor as defined by histology, such as GBM, that carries a molecular mutation in isocitrate dehydrogenase (IDH) has almost double the median overall survival of an IDH-wildtype (wt) tumor (15 months vs 31 months)4. Other molecular markers have been recognized for their ability to predict prognosis and distinguish tumors that deviate significantly from typical survival patterns. 1p/19q co-deletion typically co-occurs with IDH mutations and is indicative of a less aggressive tumor, regardless of histology5. O6-Methylguanine-DNA Methyltransferase (MGMT) gene methylation status has also emerged as a prognostic biomarker for the efficacy of alkylating therapies in the treatment of gliomas. In GBM specifically, MGMT promoter methylation increases survival from 15.3 months to 21.7 months6. Other therapeutic and prognostic markers important for glioma diagnosis are summarized in (Table 2). Consequently, in 2016 the WHO revised the classification of gliomas to incorporate these molecular biomarkers together with histological features thereby introducing the concept of “integrated diagnosis” as a means to better guide therapeutic strategy and predict patient outcomes2.
Table 2:
Molecular Markers of Glioma
| Molecular Markers | Identification | Primary Outcome Difference | Comments |
|---|---|---|---|
| IDH1/IDH2 Mutation | Histological Stain DNA sequencing IDH1 (Codon 132) IDH2 (Codon 172) |
Tumors harboring IDH mutations carry a much higher overall survival rate and may offer additional targeting or therapeutic strategies | The most common IDH mutation in glioma is IDH1 R132H |
| 1p/19q Co-Deletion | FISH NGS (with copy number information) |
1p/19q co-deletion is a defining feature of ogligodendroglial tumors and a predictor of favorable response among other gliomas | Almost always co-occurs with IDH mutations so disagreement between the two should raise concerns for false positives in deletion detection |
| ATRX Mutation | Histological Stain (loss of nuclear ATRX expression indicates presence of ATRX mutation) DNA Sequencing |
Typically used as a broad classifier for well differentiated astrocytic glioma No prognostic indication |
ATRX mutation closely correlates with IDH and TP53 mutations but is mutually exclusive with 1p/19q co-deletion |
| TP53 Mutation | Histological Staining (nuclear localization) DNA sequencing |
Supportive in diagnosis for IDH mutant astrocytoma No prognostic indication |
Nuclear localization of TP53 can reflect mutation of the gene but sequencing should still confirm |
| H3K27m Mutation | Histological Staining for specific mutation protein DNA Sequencing |
Prognostically can indicate poor survival in pediatric glioblastomas | Typically present in diffuse gliomas in midline locations such as DIPG |
| H3G34 Mutation | Histological Staining for specific mutation proteins NGS |
Associated with poor prognosis in mainly diffuse hemispheric gliomas in children and young adults | The mutations associated with H3G4 are missense and result in G34R or G34V alterations |
| BRAF Alteration | Histological Stain for mutation specific proteins (V600E Mutation) DNA Sequencing FISH (KIAA1549-BRAF fusion) |
BRAF V600E mutation typically correlates with increased risk of recurrence in pediatric gliomas | BRAF V600E mutational IHC histological stains can be difficult to interpret |
| RELA Fusion | FISH | RELA fusion clinically defines 70% of childhood supratentorial ependymomas No prognostic indication |
Fusion is between RELA and C19orf95 L1CAM expression on histology can correlate with presence of RELA fusion |
| YAP1 Fusion | FISH NGS |
Diagnostically characteristic of a subset of childhood supratentorial ependymomas No prognostic indication |
Fusion typically occurs with MAMLD1 but is not exclusive |
Clinical Presentation:
Patients with gliomas most commonly present with focal neurologic deficits that have progressed over days to weeks in patients with HGGs, or over longer periods in those with LGGs7. Patients may also experience headaches (50–60%) or seizures (20–50%), with seizures occurring as a presenting symptom more commonly in patients with LGGs harboring isocitrate dehydrogenase type 1 (IDH1) mutations7–9. Tumor location is the primary determinant of presenting signs and symptoms. Frontal tumors may present with weakness, those in the parietal lobe with numbness or hemineglect, and occipital tumors or masses along the optic radiations with visual field deficits. Gliomas within the temporal lobe, prefrontal cortex, or corpus callosum may present with less overt findings such as short-term memory deficits, personality changes, or mood disorders. Tumors within eloquent regions of the brain are likely to present at smaller size, whereas those in less eloquent areas, such as the frontal lobe, tend to be larger at presentation10. (Box 1)
Box 1: Clinical Evaluation for glioma.
| Clinical Evaluation for patient presenting with neurological deficits or seizures: |
|
| Initial Management |
Symptom Management
|
Imaging
Diagnosis and pre-operative planning:
Gadolinium-enhanced magnetic resonance imaging (MRI) remains the gold standard for the diagnosis of glioma. In adults, LGGs are typically non-enhancing on T1 and appear as hyperintense lesions on T2/FLAIR with an absence of vasogenic edema11,12. Alternatively, HGGs are hypointense masses and demonstrate heterogeneous contrast enhancement on T1-weighted sequences11,13. In contrast to LGGs, HGGs are associated with significant vasogenic edema, which appears hyperintense on T2/FLAIR11,13. Specifically, GBMs are characterized by a peripheral rim of enhancement surrounding a non-enhancing region of central necrosis11,13 (Figure 1). Gliomas with distinct molecular characteristics may demonstrate specific findings on imaging. For instance, compared to IDH-wt tumors, IDH-mutant gliomas demonstrate less contrast enhancement, larger tumor size and the presence of cystic components6. 1p/19q-codeleted tumors may be distinguished by the presence of calcifications and indistinct tumor margins6. Additionally, the T2/FLAIR mismatch sign, which describes homogeneous hyperintensity on T2-weighted imaging with a relatively hypointense signal on T2/FLAIR, is highly specific for IDH-mutant, 1p/19q non-co-deleted astrocytoma6.
Figure 1: MRI imaging showing differences between low and high grade gliomas:
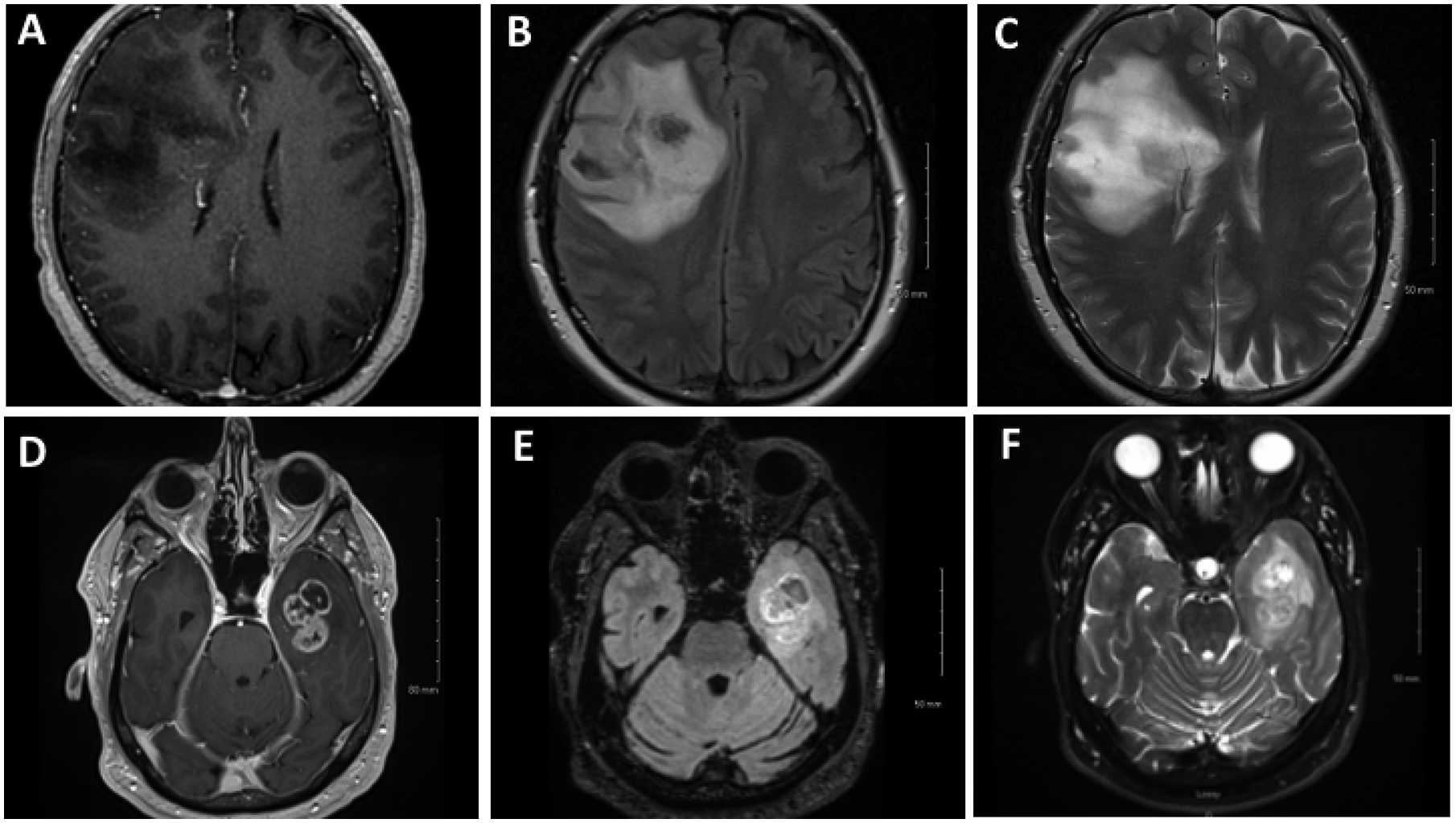
A) Axial T1 with contrast, B) axial FLAIR and C) axial T2 shows a non-enhancing right frontal lesion. D) Axial T1 with contrast, E) axial FLAIR and F) axial T2 shows a ring enhancing left temporal lesion.
MR spectroscopy (MRS), which measures metabolite concentrations within the tissues, can also be used to predict glioma grade and differentiate residual tumor infiltration from surrounding normal tissues14. In the setting of LGG, a study utilizing both intraoperative MRS (iMRS) and intraoperative MRI (iMRI) found that sensitivity of iMRS for identifying residual tumor was 85.7%, the specificity was 100%. Thus, this imaging modality may be used to limit unnecessary resections at the tumor border when gliomas are located in eloquent regions.
Functional MRI, and MR tractography are utilized for determining the localization of important functional regions and white matter tracts11. However, the accuracy of fMRI in localizing language and motor functions is highly variable across studies. Although fMRI can be very helpful for preoperative evaluation and surgical planning, the data does not yet support its use over intraoperative direct cortical stimulation (DCS) for functional mapping11. The largest study to evaluate the accuracy of fMRI in mapping language and motor functions in patients with focal masses adjacent to eloquent cortex (34 total, 28 glioma) reported an overall sensitivity of 83% and specificity of 82%15. Notably, the authors also found that sensitivity and specificity varied with respect to glioma grade14. Across the literature, accuracy is reported to range from 66%−100%11.
MR tractography utilizes diffusion tensor imaging (DTI) to visualize the anatomical location of white matter tracts11. An RCT comparing resection of gliomas involving the pyramidal tracts with and without preoperative DTI in 214 patients demonstrated that the use of DTI is associated with decreased postoperative motor deficits and improved 6-month Karnofsky performance score (KPS) in both LGG and HGG patients. Additionally, the use of DTI was associated with increased rate of complete resection (74.4% versus 33.3%, p < 0.001) and improved median survival (21.2 vs. 14.0 months, p=0.048) in patients undergoing resection for HGGs16. (Figure 2)
Figure 2: fMRI and DTI imaging:
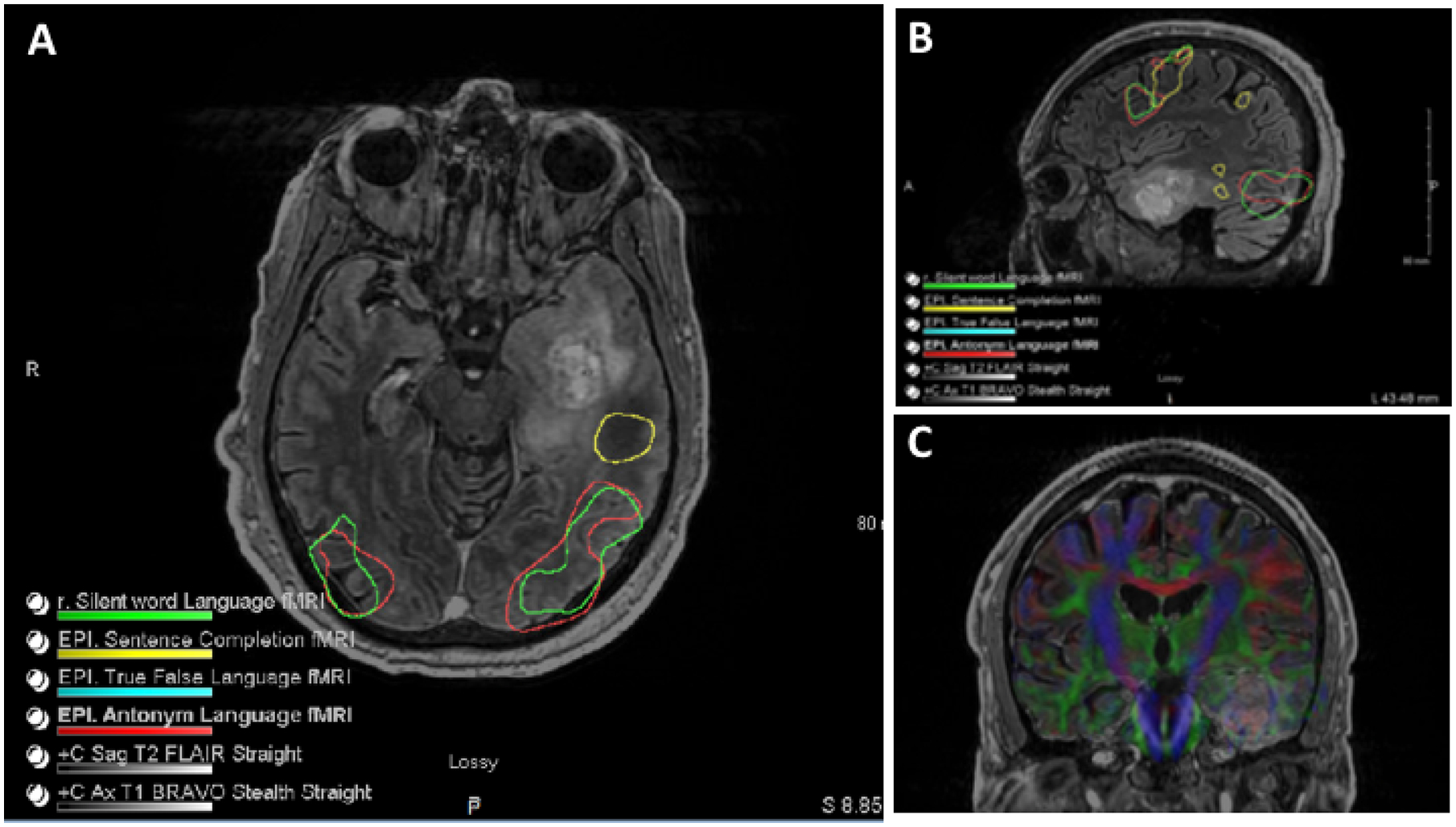
A) Axial & B) sagittal FLAIR sequencing demonstrating left temporal lesion with functional mapping. C) Tractography shows the relationship of major tracts in relation to the lesion.
Pre-operative imaging plays a critical role not only for diagnosis but is also used routinely in the form of stereotactic navigation system for planning incision and help guide surgical resection. With respect to image-guided navigation system, T2/FLAIR imaging is the current standard for non-enhancing lesions, whereas contrast-enhanced T1-weighted sequences are preferred for enhancing tumors11.
Monitoring response to treatment and recurrence:
MRI is the modality of choice for monitoring response to treatment and tumor recurrence, but this is not without limitations. Post-contrast enhancement on T1-weighted imaging reflects nonspecific impairment of the blood brain barrier (BBB) and as such is not necessarily representative of active disease17. This is particularly important with respect to monitoring malignant progression of LGGs as the development or evolution of focal enhancement on imaging often precedes clinical changes17. While reduction or lack of enhancement can occur due to tumor shrinkage, it may also occur secondary to treatment with steroids or antiangiogenic therapy and the resultant vascular normalization in areas adjacent to tumor infiltration (pseudoresponse)17. Alternatively, pseudoprogression, an early subacute reaction to treatment (e.g., radiotherapy), is associated with findings seen in true progression such as contrast enhancement, edema, and mass effect. In some cases, associated clinical symptoms may also initially suggest tumor progression, but these subsequently resolve without any further treatment9,17. While T2 and FLAIR hyperintensity can be suggestive of tumor infiltration, it is also indicative of edema, ischemia, gliosis, demyelination, and inflammation. In particular, inflammation may mimic radiological features of tumor progression and this ambiguity can often delay the diagnosis of true disease progression10. To address the challenges of distinguishing treatment related changes from tumor progression, The Response Assessment in Neuro-Oncology (RANO) criteria were developed as an objective tool for radiologic assessment of treatment response for both LGG and HGG (TABLE 3)7,18–20.
Table 3:
RANO Criteria for evaluation of glioma recurrence
| RANO Guidelines | Response Criteria | Progression Criteria | |
|---|---|---|---|
| Complete | Partial | ||
| RANO-LGG PMID: 21474379 |
Requires all of the following: Complete disappearance of all T2/FLAIR disease for ≥4 weeks New or increased enhancement No new T2/FLAIR abnormalities other than what is attributable to treatment effects No more than physiological steroids, clinically stable or improved |
Requires all of the following: ≥50 % decrease in the sum of perpendicular diameters of T2/FLAIR disease for≥4 weeks; No new or increased enhancement, No new T2/FLAIR abnormalities other than what is attributable to treatment effects, No more steroids than at the time of the baseline scan, clinically stable or improved |
Defined by any of the following: Development of new lesions or increase of enhancement; ≥25 % increase in size of the T2/FLAIR abnormality on stable or increased doses of steroids and not attributable to radiation effect or comorbid events Definite clinical deterioration, death or loss to follow-up |
| RANO-HGG PMID: 20231676 |
Requires all of the following: Complete disappearance of all enhancing measurable and nonmeasurable disease sustained for at least 4 weeks No new lesions Stable or improved nonenhancing (T2/FLAIR) lesions Patients must be off corticosteroids (or on physiologic replacement doses only) And stable or improved clinically. Note: Patients with nonmeasurable disease only cannot have achieved CR; the best response possible is stable disease |
Requires all of the following: ≥50% decrease compared with baseline in the sum of products of perpendicular diameters of all measurable enhancing lesions sustained for at least 4 weeks No progression of nonmeasurable disease No new lesions; stable or improved nonenhancing (T2/FLAIR) lesions on same or lower dose of corticosteroids compared with baseline scan The corticosteroid dose at the time of scan evaluation should be no greater than the dose at time of baseline scan And stable or improved clinically |
Defined by any of the following: ≥25% increase in the sum of the products of perpendicular diameters of enhancing lesions (compared with baseline if no decrease) on stable or increasing doses of corticosteroids; Significant increase in T2/FLAIR nonenhancing lesions on stable or increasing doses of corticosteroids compared with the baseline scan or the best response after initiation of therapy, not due to comorbid events; The appearance of any new lesions; Clear progression of nonmeasurable lesions; or definite clinical deterioration not attributable to other causes apart from the tumor or to a decrease in the corticosteroid dose |
Management:
The management of glioma patients is best executed through a multi-disciplinary approach with the involvement of specialists from neurosurgery as well as neuro- and radiation oncology. Initial management should first address urgent clinical symptoms and establish a comprehensive molecular diagnosis. Although surgery is an essential component of management of gliomas, the highly infiltrative nature of these tumors means that surgical resection alone, even when complete, is not typically curative. Adjuvant therapy is therefore of the utmost importance in extending PFS and OS (Box 2).
Box 2: Standard of Care for Glioma.
| Standard of Care for High Grade Glioma for patients with good performance Scale: |
|
| Standard of Care for High Grade Glioma for patients with poor performance Scale: |
|
Surgical Management:
Indications for surgery include the need to obtain tissue for diagnosis, cytoreduction, relief of mass effect with potential for symptom improvement, and to improve survival and quality of life (QOL). In most patients, surgical intervention is warranted for the acquisition of tissue alone.
Biopsy:
Stereotactic needle biopsy is recommended in patients with deep lesions or those within eloquent structures where surgical resection carries an unacceptably high risk of morbidity or mortality21. Furthermore, as the degree of surgical resection may be dependent on the specific glioma subtype, patients may need to undergo a biopsy prior to more extensive surgical resection22. Needle biopsies are targeted, where possible, to the portion of the lesion that appears the highest grade on imaging – an enhancing area. Several biopsy specimens are obtained to allow for accurate diagnosis as a sampling bias can lead to non-diagnostic biopsies or biopsies of a lower grade2. Stereotactic navigation is required for this procedure and can be frame-based or non-frame-based. The use of frozen section analysis can help ensure the presence of lesioned tissue in the samples prior to completion of the procedure. Despite proper planning and surgical technique, the risk of catastrophic hemorrhage with sampling is 1% per procedure23.
Surgical Resection:
For both LGG and HGG, the primary goals of surgery are the acquisition of tissue for accurate diagnostics and improving patient survival22. With respect to LGGs the resection threshold associated with improved survival is dependent on the specific subtype22,24. Data currently supports maximum total resection of enhancing tumor components for IDH-wt low-grade gliomas and both the enhancing and non-enhancing components in IDH-mutant astrocytomas22,25. Additionally, a recent meta-analysis of grade I/II gliomas suggests that extensive resection is associated with improved overall (OS) and progression free survival (PFS) at 2, 5, and 10 years compared to subtotal resection26. Maximum resection can also delay progression of LGGs to malignant tumors and is the preferred approach in the treatment of all LGG as well as diffuse gliomas regardless of grade27,28.
While achieving maximum resection is important for improving PFS and OS, this must be accomplished without compromising the integrity of the surrounding, normal tissue. Recent advances in technology have led to improvements in patient safety and outcomes while enabling the maximum tumor resection. The use of intraoperative MRI (iMRI) guides resection in “real time” by permitting evaluation of residual tumor volume while accounting for intraoperative anatomical changes and is associated with increased rate of complete tumor resection, without increased postoperative rates of new neurological deficits29,30. In patients where preservation of motor function is the primary objective motor evoked potentials may also be used to determine the integrity of the motor pathways, in lieu of performing an awake craniotomy31 (Figure 3).
Figure 3: Intraoperative monitoring:
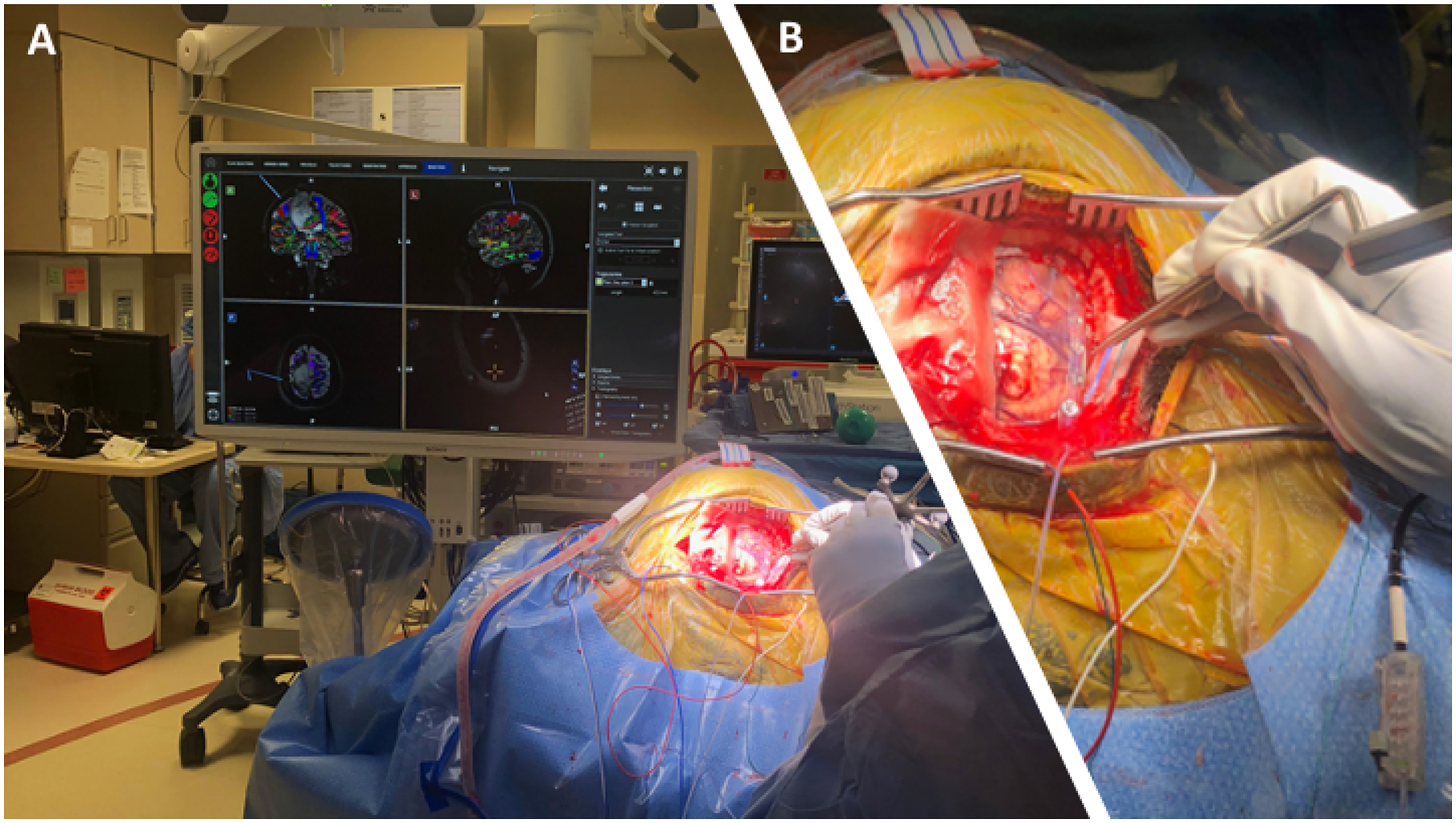
A) Intraoperative monitoring of motor evoked potential B) Intra-operative strip placement for motor mapping via DCS.
Awake craniotomies may be performed to allow for the protection of key motor and speech functions when tumors are located in eloquent regions32. Under these conditions, the patient is not intubated or placed under general anesthesia. He or she is then able to cooperate with an examiner during the procedure, which allows for early identification of any decrease in function and alerts the surgeon that they are approaching an area of functional importance30. Additionally, intraoperative neurophysiological monitoring with direct cortical stimulation (DCS) may also allow for maximal resection of tumors deemed inoperable on imaging by permitting accurate localization of functional areas intraoperatively28.
In patients with HGGs, gross total resection (GTR) is associated with both a survival benefit and improved QOL33. As such achieving GTR is particularly important in these patients and several techniques can be employed for optimization. The use of fluorescent agents such as 5-aminolevulinic acid (5-ALA) and sodium fluorescein (SF) can assist the surgeon by allowing for improved visualization of the tumor11. 5-ALA is a non-fluorescent prodrug that causes an intracellular accumulation of fluorescent porphyrins and gives the tumor a pink appearance when visualized using a special filter on the operating microscope11. In clinical trials, the use of 5-ALA has been shown to improve rates of GTR of contrast-enhancing material and prolong 6-month PFS in patients with HGGs34. Alternatively, SF is a fluorescent dye that is injected intravenously during surgery and accumulates in malignant cells as a result of BBB disruption11. In contrast to 5-ALA, SF-fluorescent tumors appear yellow when visualized on the operating microscope11. Support for the use of SF comes from a prospective, multicenter phase II trial in which 82% of patients received GTR of contrast enhancement34. Results from biopsies collected from areas with and without fluorescence suggest the sensitivity and specificity of SF in identifying tumor containing tissues to be 80%34. While there has yet to be a prospective study comparing the efficacy of 5-ALA and SF, a recent retrospective study found no difference between the two, suggesting that SF may be a viable alternative to 5-ALA35.
Raman spectroscopy is another emerging technology that may be used to optimize GTR in glioma patients. This is a non-destructive vibrational spectroscopy technique that provides structural and chemical information based on the distinct composition and structure of specific samples36. Studies have shown that Raman spectroscopy is capable of differentiating between normal brain, brain tumors, and necrosis across a range of tissue preservation methods36. Additionally, when used intraoperatively, this technique demonstrates a high degree of accuracy (92%), sensitivity (93%) and specificity (91%) in distinguishing normal brain from both tumor bulk and tumor infiltration37.
Accessing and resecting HGGs located deep to critical cortical and subcortical structures has historically been associated with significant morbidity38. However, the use of a tubular retractor system with exoscopic visualization can provide access to these deep-seated tumors by displacing rather than disrupting the fibers of critical pathways38,39.
Surgical intervention also provides a direct means of delivering therapeutics in the resection cavity and bypasses blood brain barrier. The first-in-human, phase 1, dose-escalation trial of NSC-CRAd-S-pk7f, an engineered oncolytic adenovirus delivered by neural stem cells to the glioma resection cavity, was recently completed in patients with newly diagnosed HGGs40. There were no treatment-related deaths and the median PFS and OS were 9.1 and 18.4 months, respectively40. The intraoperative placement of GammaTile cesium-131 (131Cs), a permanent brachytherapy brain implant, allows for irradiation to begin immediately following tumor resection41. GammaTile cesium-131 (131Cs) has recently received FDA approval for the use outside of clinical trials and a multicenter observational study (NCT04427384) is currently recruiting patients who have undergone GammaTile placement to evaluate patient outcomes41. To optimize drug delivery for gliomas the use of ultrasound mediated disruption of the BBB through the physical interactions of ultrasonic waves and microbubbles that are administered systemically is also currently being evaluated for HGG42. There are several open clinical trials assessing the innovative role of surgery in glioma management (Table 4).
Table 4:
Innovative surgical clinical trials for glioma
| PI | Jian Campian | Michael Vogelbaum | Josh Rubin | John Boockvar | John Boockvar | Adam Sonabend |
| Identifier | NCT02311582 | NCT04608812 | NCT02372409 | NCT04222309 | NCT03630289 | NCT04528680 |
| Beginning Year | 2014 | 2020 | 2015 | 2020 | 2018 | 2020 |
| Estimated Completion | 2021 | 2021 | 2028 | 2021 | 2021 | 2024 |
| Trial Phase | Phase I/II | Phase I | Phase II | Phase I | Phase I | Phase I/II |
| Age Range | 18+ | 18+ | 3–21 | 18+ | 18+ | 18+ |
| Trial N | 58 | 24 | 12 | 10 | 10 | 39 |
| Primary Outcome Measure | Maximal Tolerated Dose of MK-3475 when combined with MRI guided laser ablation | Treatment emergent adverse events or dose limiting toxicities | Progression Free Survival | Proportion of patients reporting rapidly progressing disease | Proportion of patients reporting rapidly progressing disease | Dose limiting toxicity |
| Surgical Intervention | MRI guided laser ablation for BBB disruption to administer MK-3475 | Convection Enhanced Delivery for OS2966 | MRI guided laser ablation for BBB disruption and administration of doxorubicin or etoposide | Omental Free Tissue Autograft to Bypass BBB | Tissue Autograft of pericranial flap to bypass BBB | Sonication for opening of BBB to deliver paclitaxel |
Management of Recurrence / Progression:
Even with aggressive multi-modal treatment, most gliomas will recur or progress. Time to recurrence is significantly shorter for HGG compared to LGG and there is great variability in the management of recurrence.
Management of Recurrence for LGG:
Recurrence in LGG may be treated with repeat resection, radiation or re-irradiation depending on initial treatment and recurrence pattern. A study analyzing tumor recurrence pattern after surgical resection of LGG found that recurrence patterns differ based on molecular subtype of LGG with initial extent of resection < 90% however with extent of resection ≥ 90% no differences in recurrence characteristics were found between 3 molecularly defined groups of LGG. In addition, the study showed that early onset of recurrence, fast radiological progression, and non-local site of relapse has significant negative impact on OS and is often associated with malignant transformation43. Thus, surgical intervention in the setting of recurrence provides another opportunity to analyze the tissue for malignant transformation. However, a systematic review of recent literature on benefic of re-resection of LGG found insufficient evidence to make any specific recommendations. Rather, they recommended that individuals with recurrent LGGs be enrolled in a properly designed clinical trial to assess the role of surgery at recurrence44.
Management of Recurrence for HGG:
For highly aggressive HGG local recurrence is an inevitable event with most patients experiencing recurrence after 6–9 months of initial treatment22. GBM recurrence often accompanies new neurological symptoms/deficits and in many cases, the new deficits are due to tumor infiltration into critical eloquent structure making the recurrence less conducive to further surgical or radiotherapy intervention. Even in the most optimistic studies, the rate of re-resection for GBM is less than 40%45. Although re-resection is of uncertain clinical benefit for a focal, local recurrence in patients with good performance status, repeat resection may be a reasonable option46.
As an alternative to surgery, laser interstitial thermal therapy (LITT) is an emerging technology that allows for laser ablation of tumors that could otherwise not be removed either due to deep location or involvement of an eloquent area. The laser fiber is passed to the tumor using stereotactic navigation. The laser is then used to heat the tissue surrounding the tip to a temperature causing cytolysis. One single institution study of using LITT for 8 newly diagnosed GBM and 13 recurrent GBM showed that in the setting of recurrent disease 5 patients showed response to LITT with radiographical shrinkage of the tumor which was not seen in any of the newly diagnosed patients. The study concluded that in carefully selected patients with recurrent GBM, LITT may be an effective alternative to surgery as a salvage treatment, however its role in the treatment of newly diagnosed, unresectable GBMs is not yet established47.
Regardless of operative management, following recurrence, additional adjuvant treatments must be considered to optimize clinical benefit. McBain et al., performed a network meta-analysis to evaluate the most effective treatment option for progressive or recurrent GBM after initial treatment with standard of care. They concluded that for treatment of first recurrence of GBM, lomustine appears to be the most effective chemotherapy treatment and other combination therapies tested had a higher risk of serious side effects. A second operation or radiotherapy, or both, may be of value in selected individuals. For second recurrence, radiotherapy with or without bevacizumab may have a role but more evidence is needed48. In a small, multi-institution, randomized clinical trial of involving 35 patients, Cloughesy et al., found that neoadjuvant administration of immune check-point PD-1 blockade followed by surgical re-resection for recurrent GBM enhances local and systemic antitumor immune response suggesting that timing of repeat surgery in combination with multi-modal innovative therapeutics will possibly direct future clinical trials in this arena49.
Summary:
Neurosurgical oncology plays a central role in the management of glioma patients by aiding with diagnosis, symptom control as well as providing an avenue for direct delivery of therapeutics that bypasses BBB. GTR is typically curative for grade I lesions, whereas more infiltrative gliomas (grade II-IV typically require maximal safe resection and potential adjuvant therapy with radiation and /or chemotherapy depending on the specific histological and molecular diagnosis. Grade IV gliomas carry a particularly dismal prognosis and significant research efforts are underway worldwide to improve patients outcomes.
Key Points:
Gliomas are the most common primary CNS malignancy in adults with no effective treatment options.
Surgical management of gliomas aids in tissue diagnosis, relieves mass effect, cytoreduction and improves overall survival.
Integrated histological and molecular tissue diagnosis is of utmost importance for the management of gliomas and clinical trial eligibility.
Modern surgical adjuncts such as functional mapping and improved intraoperative tumor visualization are critical to optimizing gross total resection without neurological deficits.
Surgical intervention plays a crucial role in innovative clinical trials involving intracavitary delivery of therapeutics, thus bypassing the limitations of BBB.
Synopsis:
Gliomas are the most common intrinsic brain tumor in adults. Although maximal tumor resection improves survival this must be balanced with preservation of neurological function. Technological advancements have greatly expanded our ability to safely maximize tumor resection and design innovative therapeutic trials that take advantage of intracavitary delivery of therapeutic agents following resection. In this chapter, we review the role of surgical intervention for both low- and high-grade gliomas and the innovations that are driving and expanding the role of surgery in this therapeutically challenging group of malignancies.
Clinics Care Points:
There is Class IIB evidence to suggest that ‘gross total resection’ in glioblastoma should be replaced by the more precise term ‘complete resection of enhancing tumor50
There is Class III evidence to suggest that supramaximal resection beyond enhancing tumor borders might be beneficial in terms of survival for HGG51
There is class II evidence to suggest that surgical resection in general is associated with improved survival in gliomas WHO grade 2 and 352,53
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Authors declare no conflict of Interest.
References:
- 1.Ostrom QT et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-oncology 21, v1–v100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis DN et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131, 803–820 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Network, C. G. A. R. et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. New Engl J Medicine 372, 2481–2498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H et al. IDH1 and IDH2 Mutations in Gliomas. New Engl J Medicine 360, 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao J et al. Human IDH mutant 1p/19q co-deleted gliomas have low tumor acidity as evidenced by molecular MRI and PET: a retrospective study. Sci Rep-uk 10, 11922 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegi ME et al. MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. New Engl J Medicine 352, 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Cairncross G et al. Phase III Trial of Chemoradiotherapy for Anaplastic Oligodendroglioma: Long-Term Results of RTOG 9402. J Clin Oncol 31, 337–343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Bent MJ et al. Adjuvant Procarbazine, Lomustine, and Vincristine Chemotherapy in Newly Diagnosed Anaplastic Oligodendroglioma: Long-Term Follow-Up of EORTC Brain Tumor Group Study 26951. J Clin Oncol 31, 344–350 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Liu X-Y et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol 124, 615–625 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Killela PJ et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc National Acad Sci 110, 6021–6026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verburg N & Hamer PC de W. State-of-the-art imaging for glioma surgery. Neurosurg Rev 44, 1331–1343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillevin R, Herpe G, Verdier M & Guillevin C Low-grade gliomas: The challenges of imaging. Diagn Interv Imag 95, 957–963 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Bai J, Varghese J & Jain R Adult Glioma WHO Classification Update, Genomics, and Imaging: What the Radiologists Need to Know. Top Magn Reson Imag 29, 71–82 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Pamir MN, Özduman K, Yıldız E, Sav A & Dinçer A Intraoperative magnetic resonance spectroscopy for identification of residual tumor during low-grade glioma surgery: Clinical article. J Neurosurg 118, 1191–1198 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Bizzi A et al. Presurgical Functional MR Imaging of Language and Motor Functions: Validation with Intraoperative Electrocortical Mapping. Radiology 248, 579–589 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Wu J-S et al. Clinical Evaluation and Follow-Up Outcome Of Diffusion Tensor Imaging-Based Functional Neuronavigation: A Prospective, Controlled Study in Patients With Gliomas Involving Pyramidal Tracts. Neurosurgery 61, 935–949 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Upadhyay N & Waldman AD Conventional MRI evaluation of gliomas. Br J Radiology 84, S107–S111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chukwueke UN & Wen PY Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. Cns Oncol 8, CNS28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen PY et al. Updated Response Assessment Criteria for High-Grade Gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol 28, 1963–1972 (2010). [DOI] [PubMed] [Google Scholar]
- 20.van den Bent M et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12, 583–593 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Akshulakov SK et al. Current Trends for Improving Safety of Stereotactic Brain Biopsies: Advanced Optical Methods for Vessel Avoidance and Tumor Detection. Frontiers Oncol 9, 947 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupp R et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New Engl J Medicine 352, 987–996 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Kreth FW et al. The Risk of Haemorrhage after Image Guided Stereotactic Biopsy of Intra-Axial Brain Tumours – A Prospective Study. Acta Neurochir 143, 539–546 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Pope WB et al. MR imaging correlates of survival in patients with high-grade gliomas. Ajnr Am J Neuroradiol 26, 2466–74 (2005). [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen BK et al. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the Danish Neuro-Oncology Registry. J Neuro-oncol 135, 571–579 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Lapointe S, Perry A & Butowski NA Primary brain tumours in adults. Lancet 392, 432–446 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Kim M et al. Diffusion- and perfusion-weighted MRI radiomics model may predict isocitrate dehydrogenase (IDH) mutation and tumor aggressiveness in diffuse lower grade glioma. Eur Radiol 30, 2142–2151 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Qin J et al. Grading of Gliomas by Using Radiomic Features on Multiple Magnetic Resonance Imaging (MRI) Sequences. Medical Sci Monit Int Medical J Exp Clin Res 23, 2168–2178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fahlbusch R & Nimsky C Intraoperative MRI developments. Neurosurg Clin N Am 16, xi–xiii (2005). [DOI] [PubMed] [Google Scholar]
- 30.Duffau H et al. Usefulness of intraoperative electrical subcortical mapping during surgery for low-grade gliomas located within eloquent brain regions: functional results in a consecutive series of 103 patients. J Neurosurg 98, 764–778 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Zhou HH & Kelly PJ Transcranial Electrical Motor Evoked Potential Monitoring for Brain Tumor Resection. Neurosurgery 48, 1075 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Li Y-C et al. The Merits of Awake Craniotomy for Glioblastoma in the Left Hemispheric Eloquent Area: One Institution Experience. Clin Neurol Neurosur 200, 106343 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Brown PD et al. A Prospective Study of Quality of Life in Adults with Newly Diagnosed High-grade Gliomas: The Impact of the Extent of Resection on Quality of Life and Survival. Neurosurgery 57, 495–504 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Stummer W et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7, 392–401 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Hansen RW et al. Comparison of 5-aminolevulinic acid and sodium fluorescein for intraoperative tumor visualization in patients with high-grade gliomas: a single-center retrospective study. J Neurosurg 133, 1324–1331 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Livermore LJ et al. Raman spectroscopy to differentiate between fresh tissue samples of glioma and normal brain: a comparison with 5-ALA–induced fluorescence-guided surgery. J Neurosurg 1–11 (2020) doi: 10.3171/2020.5.jns20376. [DOI] [PubMed] [Google Scholar]
- 37.Jermyn M et al. Intraoperative brain cancer detection with Raman spectroscopy in humans. Sci Transl Med 7, 274ra19–274ra19 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Iyer R & Chaichana K Minimally Invasive Resection of Deep-seated High-grade Gliomas Using Tubular Retractors and Exoscopic Visualization. J Neurological Surg Part Central European Neurosurg 79, 330–336 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Gassie K, Wijesekera O & Chaichana KL Minimally invasive tubular retractor-assisted biopsy and resection of subcortical intra-axial gliomas and other neoplasms. J Neurosurg Sci 62, (2018). [DOI] [PubMed] [Google Scholar]
- 40.Fares J et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol 22, 1103–1114 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreira C et al. First clinical implementation of GammaTile permanent brain implants after FDA clearance. Brachytherapy 20, 673–685 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Zhang DY et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin Cancer Res 26, 477–486 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuya Y et al. Tumor recurrence patterns after surgical resection of intracranial low-grade gliomas. J Neuro-oncol 144, 519–528 (2019). [DOI] [PubMed] [Google Scholar]
- 44.Nahed BV et al. Management of patients with recurrence of diffuse low grade glioma. J Neuro-oncol 125, 609–630 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Chaichana KL et al. Multiple resections for patients with glioblastoma: prolonging survival: Clinical article. J Neurosurg 118, 812–820 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel M et al. Clinical Uncertainty and Equipoise in the Management of Recurrent Glioblastoma. Am J Clin Oncol 44, 258–263 (2021). [DOI] [PubMed] [Google Scholar]
- 47.Thomas JG, Rao G, Kew Y & Prabhu SS Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus 41, E12 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Lawrie TA et al. Treatment options for recurrent glioblastoma: a network meta-analysis. Cochrane Db Syst Rev (2020) doi: 10.1002/14651858.cd013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cloughesy TF et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature medicine 25, 477–486 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Group A-GS et al. Extent of Resection and Survival in Glioblastoma Multiforme: Identification of and Adjustment for Bias. Neurosurgery 62, 564–576 (2008). [DOI] [PubMed] [Google Scholar]
- 51.Eyüpoglu IY et al. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget 7, 25755–25768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakola AS et al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol 28, 1942–1948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roelz R et al. Residual Tumor Volume as Best Outcome Predictor in Low Grade Glioma – A Nine-Years Near-Randomized Survey of Surgery vs. Biopsy. Sci Rep-uk 6, 32286 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


