Abstract
The aim of this study was to examine non-starch polysaccharide (NSP) degradation in the gastrointestinal tract of chickens fed a range of commercial-type diets supplemented with a commercial dose of xylanase, a double dose of xylanase or a cocktail of NSP – degrading enzymes. Cobb 500 broilers (n = 1,080) were fed 12 dietary treatments; 4 diets with differing primary grain sources (barley, corn, sorghum, and wheat) and three different enzyme treatments (commercial recommended dose of xylanase (16,000 BXU/kg), a double dose of xylanase (32,000 BXU/kg) or an NSP-degrading enzyme cocktail (xylanase, β-glucanase, cellulase, pectinase, mannanase, galactanase, and arabinofuranosidase at recommended commercial levels). There were 108 pens, approximately 10 birds per pen, 9 replicates per dietary treatment. The diets were fed as 3 phases, starter (d 0–12), grower (d 12–23), and finisher (d 23–35). On bird age d 12, 23, and 35, performance (total pen body weight, feed intake, and feed conversion ratio corrected for mortality [cFCR]), litter and excreta dry matter content, and ileal and total tract soluble and insoluble NSP degradability and free oligosaccharide digestibility was determined. On d 35, the quantity of NSP in the gizzard, jejunum, ileum and excreta was determined. Results from this study showed that the double xylanase dose and NSP-ase cocktail had positive impacts on starter phase performance in birds fed the corn- and wheat-based diets. In the grower phase in birds fed the barley-based diet, these enzyme treatments improved cFCR and increased litter dry matter content. The NSP-ase cocktail had a negative impact on finisher phase cFCR in birds fed the sorghum-based diet. The double xylanase dose induced a positive impact on NSP degradability and free oligosaccharide digestibility. In conclusion, there appears to be advantages to feeding broilers a double xylanase dose, but lack of consistency when using an NSP-ase cocktail containing many enzymes.
Key words: non-starch polysaccharide, xylanase, enzyme cocktail, grains
INTRODUCTION
Genetic selection for high production rates and feed intake has resulted in modern broiler chickens being fed very nutrient and energy dense diets. Consequently, formulation of these diets is almost entirely focused on meeting the bird's energy and amino acid requirements. However, broilers are very sensitive to dietary non-starch polysaccharide (NSP) content and composition, because NSP directly influences digesta passage rate, intestinal health and microbiome composition. Therefore, NSP somewhat dictates nutrient utilization and productive performance, suggesting it warrants consideration during feed formulation. Modern broiler diets contain approximately 10 to 12% total NSP (Bach Knudsen, 2001; Morgan et al., 2021). This NSP is categorised based on whether it is soluble or insoluble in water, with both inducing advantageous and detrimental effects in the bird's gastrointestinal tract. Soluble NSP has a high water holding capacity, causing increased digesta viscosity when in excess. The consequence of this is reduced accessibility of enzymes to substrates and reduced absorption of nutrients through the gastrointestinal wall, as well as increased water consumption and thus excreta moisture content (Choct et al., 2010; Morgan et al., 2018). However, a significant proportion of soluble NSP can be fermented by beneficial microbiota, resulting in production of short chain fatty acids and improved microbiota composition, alongside reduced competition between the host and microbiota for valuable nutrients (Bedford, 2000). Moderate levels of soluble NSP also ensure digesta transit rate is not too fast, providing opportunity for nutrients to be absorbed (Mateos et al., 2012). Insoluble NSP act as a nutrient diluent and physical barrier to digestive enzymes (Hetland et al., 2004). It can also stimulate gizzard and proventriculus function, increasing grinding of feed and peptic digestion in the gizzard, and maintains motility and digesta flow in the gastrointestinal tract through absorbing water and increasing digesta bulk (Yokhana et al., 2016). This is important for both maintaining consistency of the digesta and ensuring microbial fermentation occurs in the ceca and end of gastrointestinal tract, where there is greater abundance of beneficial microbiota species. This highlights the importance of considering dietary NSP levels and physiochemical properties of these NSP in broiler diets and grain sources, and applying strategies to gain the most benefits from these NSP. The main NSP present in all poultry diets are arabinoxylans (Kim et al., 2021a).
Xylanase application is now ubiquitous in commercial poultry diets, primarily as a tool to reduce the negative impacts of dietary xylan on digesta viscosity and litter quality (Aftab and Bedford, 2018). Xylanases cleave the internal glycosidic linkages in xylan and arabinoxylan, resulting in production of short-chain xylo-oligosaccharides (XOS) and arabinose-substituted xylo-oligosaccharides (AXOS) (Jommuengbout et al., 2009). These XOS and AXOS are selectively fermented by intestinal bacteria, resulting in production of short chain fatty acids (SCFA) which act as a source of energy, and induce a positive influence on the composition and activity of gastrointestinal microbiota and bird performance (De Maesschalck et al., 2015; Bautil et al., 2020). This suggests that the success of xylanase may be equally attributable to production of these prebiotic oligosaccharides, and the impact this has on microbiota composition, as it is to viscosity-reduction. One hypothesis of this study was that feeding more xylanase would increase xylan degradation further, resulting in more XOS production and consequently increased performance. This was based on a number of studies observing increased performance when feeding higher xylanase doses. For example, Nusairat and Wang (2021) saw improved BWG at d 1 to 42 when feeding 15 XU/g compared to 10 XU/g xylanase, Van Hoeck et al. (2021) saw reduced FCR at d 0 to 35 when feeding 90,000 BXU/kg compared to 30,000 BXU/kg xylanase, and Liu and Kim (2017) found body weight increased and FCR decreased linearly with increasing xylanase level ranging from 0 to 5,625 XU/kg xylanase at d 1 to 18. However, there are also conflicting studies presenting no effect or a poorer performance as a consequence of increasing xylanase dose (Olukosi et al., 2007; Rabello et al., 2021). This proposes that there is a need to better understand how dietary composition influences xylanase efficacy. Response to xylanase and XOS also varies with bird age and gastrointestinal development (Bautil et al., 2020). Consequently, one aim of this study was to examine how well NSP is utilized at different dietary phases as a result of feeding a higher dose of xylanase.
Recently there has been increased interest in application of NSP-ase cocktails into poultry diets, as a tool to increase the quantity and variety of prebiotic oligosaccharides generated, which can provide fuel for a range of beneficial probiotic bacteria species. However, results from feeding different NSP-ase combinations are inconsistent, for example, Stefanello et al. (2015) and Cowieson et al. (2010) saw no additive effect on performance when feeding amylase or glucanase, respectively, together with xylanase, whereas Jimoh and Atteh (2018) and Mathlouthi et al. (2002) observed better performance when supplementing β-glucanase with xylanase and Ko et al. (2021) found feeding a cocktail of mannanase, β-glucanase, and xylanase improved feed conversion. This highlights the need to select enzyme treatments based on the substrates present, and therefore necessity to elucidate the NSP levels and composition in diets. The dose of the enzymes in NSP-ase cocktails also warrants future consideration, as lack of response to NSP-ase cocktails may be attributable to suboptimal dose of one or more of the enzymes, eliminating the additive effects of these enzymes. A secondary aim of this study was to examine if an NSP-ase cocktail containing the most common NSP-ases used in poultry diets (β-glucanase, cellulase, pectinase, mannanase, galactanase, and arabinofuranosidase) at recommended doses, in combination with xylanase, could increase NSP degradation and bird performance in a range of diets.
In this study broilers were fed commercial-type meat chicken diets with differing primary grain sources, wheat, barley, corn, and sorghum, to represent diets fed in different states across Australia, and to provide a range of NSP concentrations and compositions as substrates. The dietary treatments were formulated to have similar protein and energy values. The aim of this study was to compare the effects of supplementing these diets with a single dose of xylanase, based on commercial recommendations, to supplementation with a double dose of xylanase or cocktail of NSP-degrading enzymes on productive performance, excreta and litter moisture content and NSP degradation at different dietary phases.
MATERIALS AND METHODS
Birds and Husbandry
Cobb 500 mixed-sex broilers (n = 1,080 + 30 spare birds) were obtained from a commercial hatchery at day of hatch. The chicks were randomized by weight and placed in 0.85 m2 floor pens, 108 pens of approximately 10 birds per pen, 90 birds per treatment, resulting in 9 replicates of the 12 dietary treatments. Birds were bedded on clean wood shavings, and all received vaccination against Marek's disease, infectious bronchitis, and Newcastle disease at the hatchery under Australian code of practice for distribution of broiler chickens. Temperature settings followed Cobb 500 recommendations of 33 to 34°C on arrival, followed by a gradual decrease by approximately 0.5°C daily until a temperature of 21 to 22°C was reached by d 21. The lighting regimen used was 24 h light on d 1, with darkness increasing by 1 h a day until 6 h of darkness was reached, which was maintained throughout the remainder of the study. Mortality was recorded daily, and any birds culled or dead were weighed. Institutional and national guidelines for the care and use of animals were followed and all experimental procedures involving animals were approved by the Animal Ethics Committee at University of New England, New South Wales, Australia (AEC20-035).
Dietary Treatments
The dietary treatments replicated commercial diet formulations from different states across Australia, and were barley, corn, sorghum or wheat-based. Within each of these four treatments, the diets were supplemented with either a commercial recommended dose of xylanase (16,000 BXU/kg Econase XT 5P, AB Vista, Marlborough, UK), a double dose of xylanase (32,000 BXU/kg Econase XT 5P, AB Vista) or an NSP-ase cocktail (xylanase (16,000 U/kg Econase XT 5P, AB Vista,), β-glucanase (20,000 U/kg Econase GT, AB Vista), cellulase (2,000 U/kg Sigma-Aldrich Pty. Ltd, Castle Hill, Australia), pectinase (1,400 U/kg Deltagen Kilsyth, Australia), mannanase (250 U/kg Deltagen Kilsyth), galactanase (20 U/kg Deltagen Kilsyth), and arabinofuranosidase (10,000 U/kg Deltagen Kilsyth). This resulted in a total of 12 dietary treatments. All diets contained 0.5% titanium dioxide (TiO2) as a digestibility marker, and supplemental phytase at commercial level (Quantum Blue, AB Vista). Birds had ad libitum access to water and feed throughout the trial period. The diets were fed as starter from d 0 to 12, grower from d 12 to 23 and finisher from d 23 to 35. The diets were cold pelleted and fed as crumble (⌀0.1–0.2 mm) from d 0 to 7 and then pellet (⌀3 mm pellet) for the remainder of the trial period. The nutrient composition of the test diets is presented in Table 1.
Table 1.
Nutrient composition of dietary treatments.
| Barley |
Corn |
Sorghum |
Wheat |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredient (g/kg) | Starter | Grower | Finisher | Starter | Grower | Finisher | Starter | Grower | Finisher | Starter | Grower | Finisher |
| Wheat | 386.3 | 445.9 | 491.3 | 304.2 | 292.2 | 326.1 | 202.6 | 295.1 | 328.6 | 556.9 | 591.0 | 646.3 |
| Barley | 149.3 | 149.2 | 149.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Canola seed | 79.6 | 47.6 | 69.7 | 69.7 | 19.9 | 69.7 | 64.7 | 20.5 | 69.7 | 69.7 | 43.1 | 69.7 |
| Corn | 0.0 | 0.0 | 0.0 | 248.8 | 298.5 | 298.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sorghum | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 298.5 | 298.5 | 298.5 | 0.0 | 0.0 | 0.0 |
| Soybean meal | 289.7 | 257.4 | 202.2 | 300.4 | 292.7 | 222.7 | 282.1 | 284.2 | 214.5 | 290.4 | 262.9 | 215.7 |
| Canola meal | 29.9 | 29.8 | 20.7 | 29.9 | 29.8 | 29.9 | 79.6 | 29.8 | 29.9 | 24.9 | 29.8 | 5.0 |
| Canola oil | 23.3 | 28.5 | 29.9 | 5.2 | 23.7 | 17.2 | 23.7 | 28.8 | 22.7 | 15.8 | 29.8 | 26.3 |
| Limestone | 13.9 | 15.0 | 12.0 | 13.9 | 15.5 | 11.7 | 17.5 | 15.3 | 11.7 | 13.9 | 15.2 | 12.0 |
| Monocalcium phosphate | 7.8 | 7.0 | 5.8 | 8.0 | 7.7 | 5.8 | 11.1 | 7.6 | 5.8 | 7.8 | 7.5 | 5.8 |
| Sodium bicarbonate | 3.6 | 3.7 | 3.8 | 3.3 | 4.2 | 3.2 | 3.5 | 4.3 | 3.3 | 3.7 | 4.7 | 3.7 |
| DL-Methionine | 2.9 | 3.0 | 3.3 | 2.8 | 3.1 | 3.3 | 2.8 | 3.1 | 3.2 | 2.8 | 3.1 | 3.3 |
| L-Lysine HCl | 2.8 | 2.6 | 2.6 | 2.8 | 2.2 | 2.2 | 2.9 | 2.4 | 2.4 | 3.1 | 2.7 | 2.6 |
| Salt | 1.6 | 1.7 | 1.4 | 1.8 | 2.1 | 1.9 | 1.9 | 2.0 | 1.7 | 1.6 | 1.8 | 1.5 |
| L-Threonine | 1.3 | 1.3 | 1.0 | 1.2 | 1.1 | 0.8 | 1.1 | 1.1 | 0.9 | 1.4 | 1.3 | 1.0 |
| Copper sulphate | 0.4 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 |
| Choline chloride | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.1 | 0.0 |
| Phytase3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin premix2 | 1.0 | 0.8 | 0.8 | 1.0 | 0.8 | 0.8 | 1.0 | 0.8 | 0.8 | 1.0 | 0.8 | 0.8 |
| Mineral premix1 | 1.3 | 1.0 | 1.0 | 1.3 | 1.0 | 1.0 | 1.3 | 1.0 | 1.0 | 1.3 | 1.0 | 1.0 |
| Titanium Dioxide | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Salinomycin | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Probiotic4 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Analysed composition | ||||||||||||
| Dry matter (g/100g) | 90.53 | 89.30 | 88.20 | 88.73 | 88.07 | 87.49 | 90.46 | 89.50 | 88.25 | 89.70 | 89.02 | 88.55 |
| Protein (g/100g DM) | 23.91 | 23.27 | 21.36 | 23.69 | 22.47 | 21.14 | 23.92 | 22.64 | 21.50 | 25.24 | 23.48 | 22.72 |
| Energy (MJ/kg DM) | 17.53 | 17.10 | 17.00 | 16.80 | 16.48 | 16.59 | 17.21 | 16.78 | 16.85 | 17.08 | 16.93 | 17.00 |
| Soluble NSP (g/kg DM) | 19.09 | 22.67 | 21.06 | 19.01 | 17.91 | 16.59 | 19.65 | 17.51 | 15.71 | 21.30 | 20.73 | 23.39 |
| Insoluble NSP (g/kg DM) | 77.67 | 78.60 | 65.62 | 63.26 | 71.16 | 66.07 | 67.93 | 65.84 | 60.71 | 76.38 | 69.30 | 70.51 |
| Free OS (g/kg DM) | 41.72 | 38.31 | 40.14 | 41.58 | 39.85 | 38.41 | 38.99 | 36.83 | 36.05 | 36.13 | 41.54 | 35.32 |
Formulated to supply 23 mg copper, 1.79 mg iodine, 57 mg iron, 171 mg manganese, 0.43 mg selenium and 143 mg zinc per kg finished feed.
Formulated to supply 5,040 mg retinol, 17.5 mg cholecalciferol, 105 mg tocopheryl acetate, 4 mg menadione, 4 mg thiamine, 11 mg riboflavin, 77 mg niacin, 18 mg pantothenate, 7 mg pyridoxine, 0.35 mg biotin, 3.0 mg folate, 0.02 mg cyanocobalamin per kg of finished feed.
Quantum Blue, AB Vista.
Alterion, Adisseo.
Response Variables
Performance and Ileum and Excreta Collection
Total pen weight and feed intake (FI) was determined on arrival and on d 12, 23, and 35 post-hatch and used to calculate feed conversion ratio corrected for mortality (cFCR).
Fresh excreta samples (approximately 200 g fresh sample) were collected per pen on d 12, 23, and 35, by placing clean metal trays into the bottom of each pen and then monitoring the birds and immediately collecting the sample from the pen floor post-defecation. Litter samples were collected from 5 different points per pen and placed in a sealed plastic bag on d 12, 23, and 35.
On d 12 and 23 birds were euthanized (n = 3 per pen at d 12 and n = 2 per pen at d 23) and ileum digesta samples were collected, for determination of NSP digestibility at each phase. Bird gender was noted during dissection. On d 35, one male and one female per pen were euthanized and gizzard, jejunum, and ileum digesta samples were collected and pooled per pen.
Analysis of Excreta and Litter Dry Matter
Dry matter content of the excreta and litter samples was determined; the entire litter sample or subsample of excreta sample was weighed into duplicate crucibles and oven dried at 105°C to a constant weight and then reweighed, and dry matter (%) was calculated. The remaining excreta sample was frozen at −20°C, freeze-dried to constant weight and then ground through a 0.5 mm screen. Samples of the diets were also ground through a 0.5 mm screen. Dry matter was determined in the digesta and diet samples using the method described above.
Sample Analysis
TiO2 marker was quantified in the digesta, excreta and diet samples by UV-spectroscopy at 410 nm (Cary 50 Bio UV-Visible spectrophotometer equipped with a Cary 50 MPR microplate reader, Varian Inc., Palo Alto, CA), using the method described by Short et al. (1996). The majority of the enzyme activities in the dietary treatments were measured using Megazyme assay kits (Megazyme International Ireland Ltd, Wicklow, Ireland); the kit codes were K-XylX6-2V for xylanase activity, K-CellG5-2V for cellulase activity, E-EXBGOS for beta-glucanase activity, T-MNZ for mannanase activity and S-AGALP for galactanase activity. Pectinase activity was measured using the Sigma Aldrich recommended method for P4716. Arabinofuranosidase activity was measured using the method described by Patel et al. (2015) with minor modifications. The xylanase activity ranged from 15,800 to 16,200 in diets supplemented with the single dose, and from 30,900 to 33,200 BXU/kg in diets supplemented with the double dose. All measured enzyme activities were within 6% of the predicted enzyme activity for all other supplemental enzymes. All enzymes were measured in all diets, with those not supplemented being undetectable by the assay.
Soluble and insoluble NSP and free oligosaccharide composition of the ingredients, diets, ileum digesta, and excreta samples at d 12, 23, and 35, and gizzard and jejunum digesta samples at d 35 was determined following the procedure of Englyst et al. (1994) with some modifications as described by Theander et al. (1995) and Morgan et al. (2018). Approximately 190 to 200 mg of samples was weighed accurately, and then fat extracted with 10 mL hexane; the sample was sonicated and centrifuged and the supernatant discarded. Free oligosaccharides were extracted from the resulting sample by heating the sample at 80°C with 5 mL of 80% ethanol. The starch in the residue was gelatinized by boiling the sample with 10 mL acetate buffer (pH 5). After 30 min, 50 µL of α-amylase was added and the samples were maintained at 95°C for 30 min, followed by 50 µL amyloglucosidase for 30 min at 55°C. The samples were then maintained at 55°C for 16 h. The prepared samples was then centrifuged at 2,000 × g for 30 min, and the resulting supernatant and residue used for the analysis of soluble and insoluble NSP, respectively. For the soluble NSP analysis, the sugars released by the enzymes were removed by addition of 16 mL 80% ethanol to 4 mL sample, centrifuging and discarding the supernatant, then repeating this process with 10 mL of 80% ethanol and then 10 mL of absolute ethanol; samples were maintained at 4°C during this entire process. The resulting residue was dried and then 1 mL of 2 M trifluoroacetic acid added and heated at 125°C for 60 min. For the insoluble NSP analysis, the glucose released from starch digestion was removed with 10 mL H2O, and then 2 mL acetone, centrifuging and removing the supernatant each time. The resulting supernatant was removed and the residue was dried. Following this, 1 mL of 12 M H2SO4 was added and the sample was heated to 35°C for 2 h, and then 11 mL H2O was added and the sample was heated to 100°C, cooled and then centrifuged at 3,000 × g for 15 min to sediment the insoluble materials. An aliquot of 0.8 mL of sample was combined with 0.2 mL 28% ammonium. For the free sugar analysis, the extracted sample was dried, hydrolysed with 3 ml 1M H2SO4 at 100°C for 2 h and centrifuged to sediment the insoluble material. An aliquot of 0.4 ml of sample was combined with 0.1 mL 28% ammonium. For all the resulting samples, 50 µL of internal standard was added (allose, 4 mg/mL) and the sample was evaporated to dryness. The dried samples were then re-dissolved in 0.2 mL H2O with slight alkalinity. Freshly prepared NaBH4 was then added (0.3 mL), the sample was incubated at 40°C for 60 min, and any excess NaBH4 was decomposed with 250 µL glacial acetic acid. 1-methylimidazole (0.5 mL) and C4H6O3 (5 mL) were added followed by 8 mL H2O. Three mL dichloromethane was then added, and the sample was centrifuged, and the bottom layer collected and dried. Finally, 1.3 mL ethyl acetate and 1.3 ml H2O were added, the sample was centrifuged, and the supernatant was analyzed by gas chromatography (Model CP3800, Varian Inc.). Rhamnose, fucose, ribose, arabinose, xylose, mannose, galactose, and glucose were measured, and added together to calculate the total NSP, accounting for the polymerization factor.
Degradability of soluble and insoluble NSP and free oligosaccharides in the ileum and excreta at d 12, 23, and 35 was determined using the following equation:
Disappearance of NSP along the gastrointestinal tract, in the gizzard, jejunum, ileum, and excreta, was calculated by the undigested dry matter (100 minus the dry matter digestibility) multiplied by the quantity of NSP, for the gizzard, jejunum, ileum, and excreta at d 35.
Data Analysis
All data was analyzed using IBM SPSS statistics version 25. Pen represented the replicate unit for statistical analysis. After Kolmogorov-Smirnov testing to confirm normality, ANOVA analysis was used to evaluate the impact of enzyme treatment within each of the four dietary treatments with differing primary grain sources. The percentage of male birds per pen was applied as a co-variate. Treatment means were separated using Tukey post-hoc test where appropriate. Correlations between dietary sNSP level and the measured parameters were investigated using Pearson product-moment correlation coefficient. Statistical significance was declared at P < 0.05.
RESULTS
Performance
Table 2 presents that in birds fed the barley-based diet at d 12 to 23, feed intake was greater in birds fed the single dose of xylanase compared to the NSP-ase cocktail (P = 0.045), and cFCR value was lower in birds fed the double dose of xylanase or NSP-ase cocktail compared to those fed the single dose of xylanase (P = 0.042). In birds fed the corn-based diet at d 0 to 12, BWG was higher in birds fed the double xylanase dose or NSP-ase cocktail compared to those fed the single xylanase dose (P = 0.011), and the cFCR value was lower in bird fed the double compared to single dose of xylanase (P = 0.004). In birds fed the sorghum-based diet at d 23 to 35, BWG was higher and cFCR value was lower in birds fed the single dose of xylanase compared to the NSP-ase cocktail (P = 0.030 and P = 0.009, respectively). In birds fed the wheat based diet at d 0 to 12, BWG was higher in birds fed the NSP-ase cocktail compared to the single dose of xylanase (P = 0.030).
Table 2.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on individual feed intake (FI), body weight gain (BWG), and feed conversion ratio corrected for mortality (cFCR) in broilers at d 0–12, d 12–23 and d 23–35.
| D 0–12 |
D 12–23 |
D 23–35 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grain | Enzyme | FI (g) | BWG (g) | cFCR | FI (g) | BWG (g) | cFCR | FI | BWG (g) | cFCR |
| Barley | Single | 328 | 296 | 1.11 | 1396a | 1,019 | 1.37a | 1940 | 1244 | 1.56 |
| Double | 319 | 292 | 1.09 | 1352ab | 1,019 | 1.33b | 1949 | 1221 | 1.60 | |
| Cocktail | 318 | 296 | 1.08 | 1321b | 994 | 1.33b | 1800 | 1116 | 1.63 | |
| SEM | 3.27 | 2.93 | 0.01 | 12.68 | 9.43 | 0.01 | 43.67 | 33.19 | 0.02 | |
| P-value | 0.416 | 0.814 | 0.175 | 0.045 | 0.497 | 0.042 | 0.324 | 0.265 | 0.401 | |
| Corn | Single | 325 | 280b | 1.16a | 1,422 | 1,042 | 1.37 | 1,994 | 1251 | 1.60 |
| Double | 333 | 298a | 1.12b | 1,394 | 1,041 | 1.34 | 1,916 | 1184 | 1.63 | |
| Cocktail | 344 | 299a | 1.14ab | 1,391 | 1027 | 1.36 | 1,980 | 1225 | 1.62 | |
| SEM | 3.33 | 3.12 | 0.01 | 15.02 | 11.44 | 0.01 | 45.39 | 32.75 | 0.02 | |
| P-value | 0.216 | 0.011 | 0.004 | 0.677 | 0.845 | 0.171 | 0.775 | 0.725 | 0.758 | |
| Sorghum | Single | 311 | 287 | 1.08 | 1,359 | 1,000 | 1.36 | 1,915 | 1266a | 1.52b |
| Double | 320 | 293 | 1.09 | 1,357 | 1,012 | 1.34 | 2,000 | 1252ab | 1.60ab | |
| Cocktail | 315 | 289 | 1.09 | 1354 | 1,006 | 1.35 | 1,859 | 1101b | 1.70a | |
| SEM | 2.36 | 2.53 | 0.01 | 13.45 | 11.38 | 0.01 | 40.36 | 28.42 | 0.02 | |
| P-value | 0.278 | 0.637 | 0.672 | 0.989 | 0.915 | 0.370 | 0.385 | 0.030 | 0.009 | |
| Wheat | Single | 315 | 291b | 1.09 | 1,390 | 1027 | 1.35 | 1,899 | 1197 | 1.60 |
| Double | 321 | 299ab | 1.07 | 1,308 | 980 | 1.34 | 1,825 | 1148 | 1.59 | |
| Cocktail | 332 | 313a | 1.06 | 1,311 | 987 | 1.33 | 1,802 | 1151 | 1.57 | |
| SEM | 3.58 | 3.45 | 0.01 | 15.91 | 11.34 | 0.01 | 35.44 | 25.74 | 0.02 | |
| P-value | 0.127 | 0.030 | 0.476 | 0.058 | 0.201 | 0.146 | 0.537 | 0.709 | 0.831 | |
Means within the same column, within the same parameter, with no common subscript, differ significantly.
Excreta and Litter Dry Matter
Table 3 shows that in birds fed the barley-based diet at d 23, litter dry matter content was greater when supplementing diets with the double xylanase dose or NSP-ase cocktail compared to the single xylanase dose (P = 0.014). At d 35, in birds fed the wheat-based diet excreta dry matter was greater in birds fed the double dose of xylanase compared to the single dose of xylanase (P = 0.019).
Table 3.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on excreta and litter dry matter (DM).
| Litter DM (%) |
Excreta DM (%) |
||||||
|---|---|---|---|---|---|---|---|
| Grain | Enzyme | D 12 | D 23 | D 35 | D 12 | D 23 | D 35 |
| Barley | Single | 74.57 | 51.91b | 67.35 | 16.63 | 19.55 | 20.18 |
| Double | 75.89 | 60.31a | 70.69 | 16.41 | 18.34 | 22.70 | |
| Cocktail | 75.44 | 60.76a | 72.59 | 16.77 | 19.13 | 22.06 | |
| SEM | 1.43 | 1.43 | 1.26 | 0.29 | 0.19 | 0.39 | |
| P-value | 0.937 | 0.014 | 0.246 | 0.928 | 0.096 | 0.866 | |
| Corn | Single | 74.99 | 57.26 | 68.66 | 14.86 | 18.39 | 20.56 |
| Double | 77.15 | 54.80 | 71.08 | 17.61 | 17.71 | 21.77 | |
| Cocktail | 76.42 | 52.64 | 69.33 | 17.73 | 18.43 | 21.28 | |
| SEM | 1.60 | 1.58 | 1.85 | 0.46 | 0.16 | 0.47 | |
| P-value | 0.870 | 0.523 | 0.873 | 0.065 | 0.263 | 0.963 | |
| Sorghum | Single | 75.04 | 55.74 | 69.28 | 16.53 | 18.54 | 21.21 |
| Double | 77.25 | 56.01 | 70.98 | 18.25 | 18.17 | 22.13 | |
| Cocktail | 77.97 | 56.49 | 72.87 | 17.59 | 18.81 | 22.11 | |
| SEM | 1.08 | 1.36 | 1.13 | 0.30 | 0.28 | 0.24 | |
| P-value | 0.543 | 0.978 | 0.458 | 0.170 | 0.777 | 0.376 | |
| Wheat | Single | 75.03 | 53.00 | 68.63 | 18.30 | 18.90 | 20.02b |
| Double | 71.04 | 52.64 | 72.57 | 17.65 | 18.77 | 22.72a | |
| Cocktail | 75.29 | 51.51 | 63.36 | 16.80 | 18.66 | 21.23ab | |
| SEM | 1.14 | 1.63 | 1.65 | 0.39 | 0.25 | 0.33 | |
| P-value | 0.255 | 0.934 | 0.075 | 0.479 | 0.961 | 0.019 | |
Means within the same column, within the same parameter, with no common subscript, differ significantly.
Non-starch Polysaccharide Degradability and Free Oligosaccharide Digestibility
The enzyme treatments had no impact on ileal soluble NSP degradability at d 12 or d 23, insoluble NSP degradability at d 12 or d 35, or free oligosaccharide digestibility at d 23 or d 35 (P > 0.05). The enzyme treatments also had no effect on total tract soluble or insoluble NSP degradability at d 35, or free oligosaccharide digestibility at d 12 and d 23 (P > 0.05). Therefore, figures for these parameters are not presented.
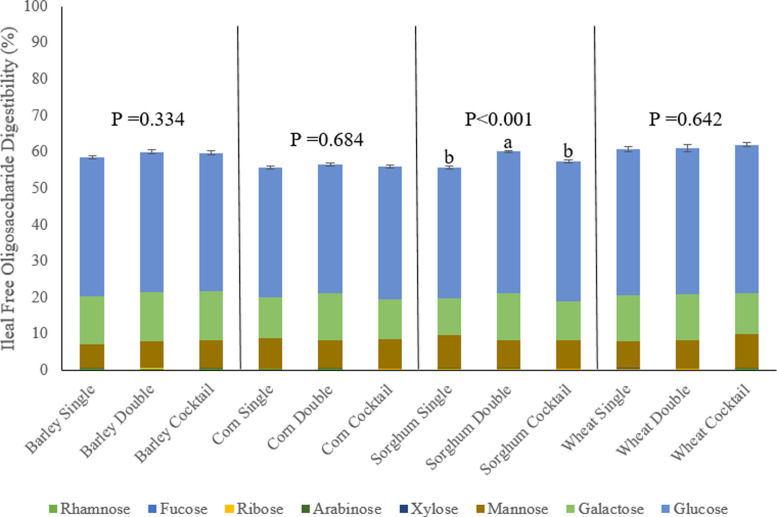
Figure 1 presents total tract degradability of soluble and insoluble NSP at d 12. In birds fed the barley-based diet total tract degradability of soluble NSP was greater when feeding the double compared to single dose of xylanase (P = 0.021). In birds fed the wheat-based diet total tract degradability of soluble NSP was greater when feeding the double dose of xylanase or NSP-ase cocktail compared to the single xylanase dose (P = 0.022). In birds fed the sorghum-based diet, total tract digestibility of insoluble NSP was higher in birds fed the double dose of xylanase compared to those fed the single xylanase dose or NSP-ase cocktail (P = 0.002). This was also true for free oligosaccharide digestibility at d 12, as seen in Figure 2 (P < 0.001).
Figure 1.
Effect of grain type and xylanase level (16,000 BXU/kg [Single) or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on total tract degradability of soluble and insoluble non-starch polysaccharides at d 12 (% DM).
Figure 2.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on ileal digestibility of free oligosaccharides at d 12 (% DM).
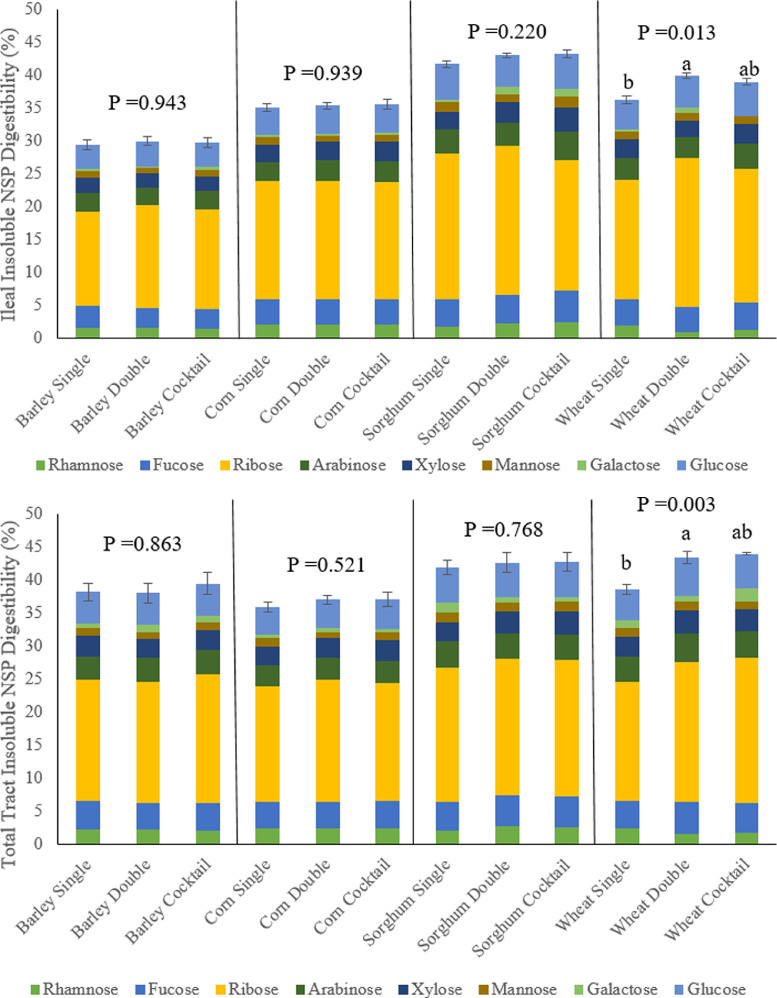
Figure 3 shows that in birds fed the wheat-based diet, total tract degradability of soluble NSP was greater in birds fed the double xylanase dose compared to the single xylanase dose or NSP-ase cocktail (P = 0.004). Furthermore, Figure 4 presents that in birds fed the wheat-based diet, ileal degradability of insoluble NSP was greater in birds fed the double compare to single dose of xylanase (P = 0.013), and total tract degradability of insoluble NSP was lower in birds fed the single dose of xylanase compared to the double xylanase dose or NSP-ase cocktail (P = 0.003).
Figure 3.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on total tract degradability of soluble non-starch polysaccharides at d23 (% DM).
Figure 4.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on ileal and total tract degradability of insoluble non-starch polysaccharides at d23 (% DM).
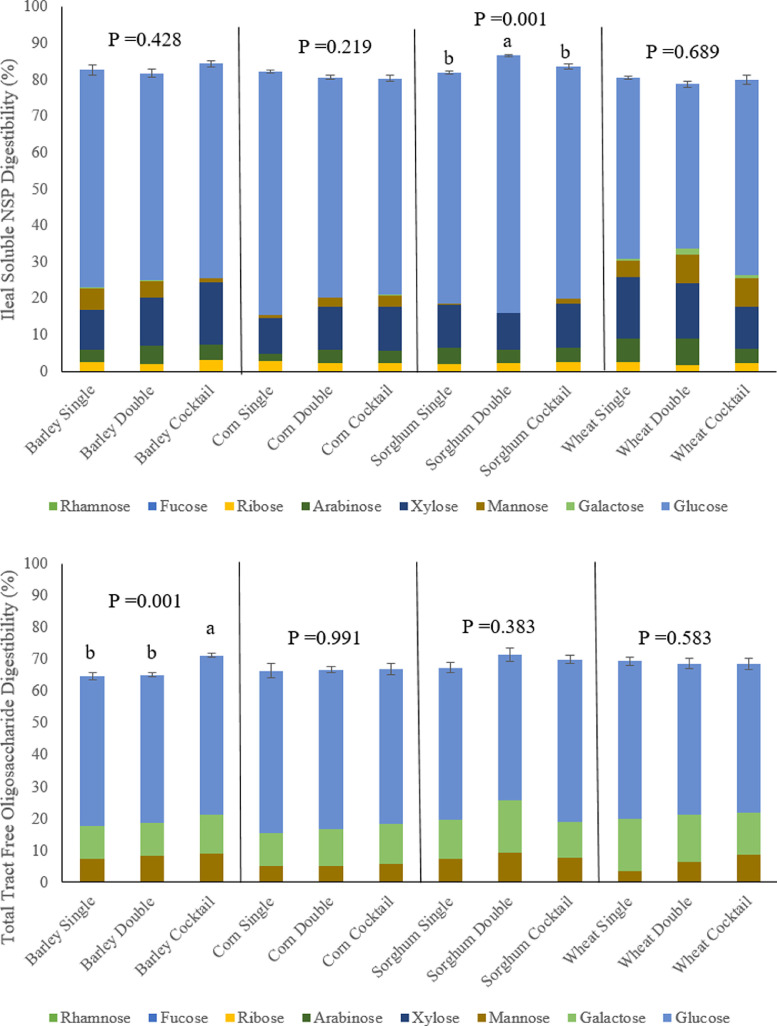
Figure 5 presents that in birds fed the sorghum-based diet, ileal degradability of soluble NSP was greater in birds fed the double xylanase dose compared to the single xylanase dose or NSP-ase cocktail (P = 0.001). In birds fed the barley-based diet, total tract free oligosaccharide digestibility was greater in birds fed the NSP-ase cocktail compared to the single or double dose of xylanase (P = 0.001).
Figure 5.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on ileal degradability of soluble non-starch polysaccharides and total tract digestibility of free oligosaccharides at d 35 (% DM).
Tables 4 and 5 presents the quantity of soluble and insoluble NSP and free oligosaccharides present in the gizzard, jejunum, ileum, and excreta at d 35, as an indicator of NSP disappearance along the gastrointestinal tract. The higher values in the gizzard compared to the diet present accumulation of NSP in the gizzard. As shown in Table 4, in birds fed the barley-based diet there was more insoluble NSP present in the gizzard in birds fed the single dose of xylanase compared to the double dose or NSP-ase cocktail (P = 0.010). Table 5 shows that in birds fed the sorghum-based diet, there was more soluble NSP present in the gizzard of bird fed the single dose of xylanase compared to the NSP-ase cocktail (P = 0.012), and less free oligosaccharides present in the jejunum of birds fed the NSP-ase cocktail compared to the single or double dose of xylanase (P = 0.001).
Table 4.
Effect of grain type and xylanase level (16,000 BXU/kg [Single] or 32,000 BXU/kg [Double]) or NSP-ase cocktail (Cocktail) on quantity of soluble non-starch polysaccharides (sNSP), insoluble non-starch polysaccharides (iNSP), and free oligosaccharides (FS) present along the gastrointestinal tract in broilers fed barley- and wheat-based diets at d35 (g/kg DM digesta).
| Barley |
Wheat |
|||||||
|---|---|---|---|---|---|---|---|---|
| Single | Double | Cocktail | P-value | Single | Double | Cocktail | P-value | |
| sNSP | ||||||||
| Diet | 21.47 | 21.28 | 20.44 | 23.10 | 23.83 | 23.25 | ||
| Gizzard | 32.78 | 30.50 | 28.20 | 0.434 | 23.17 | 23.04 | 22.68 | 0.993 |
| Jejunum | 21.10 | 18.10 | 17.40 | 0.143 | 19.48 | 20.85 | 18.89 | 0.299 |
| Ileum | 15.67 | 15.44 | 14.99 | 0.962 | 16.68 | 17.67 | 16.12 | 0.806 |
| Excreta | 12.14 | 10.99 | 10.60 | 0.393 | 8.83 | 10.36 | 9.16 | 0.431 |
| iNSP | ||||||||
| Diet | 66.04 | 65.31 | 65.50 | 70.84 | 69.15 | 71.53 | ||
| Gizzard | 272.67a | 221.55b | 211.38b | 0.010 | 222.24 | 200.33 | 213.97 | 0.697 |
| Jejunum | 57.12 | 57.51 | 55.29 | 0.859 | 54.07 | 50.84 | 54.15 | 0.776 |
| Ileum | 54.29 | 55.22 | 52.26 | 0.768 | 46.03 | 46.04 | 47.32 | 0.933 |
| Excreta | 41.67 | 42.43 | 41.22 | 0.652 | 41.49 | 40.84 | 42.61 | 0.799 |
| FS | ||||||||
| Diet | 41.14 | 39.21 | 40.07 | 35.03 | 35.44 | 35.50 | ||
| Gizzard | 33.06 | 31.18 | 28.77 | 0.491 | 31.75 | 31.46 | 26.41 | 0.257 |
| Jejunum | 35.29 | 33.48 | 28.19 | 0.162 | 36.93 | 32.67 | 30.85 | 0.490 |
| Ileum | 26.34 | 27.65 | 24.59 | 0.829 | 27.51 | 26.27 | 25.22 | 0.915 |
| Excreta | 16.59 | 20.22 | 14.03 | 0.210 | 20.32 | 17.93 | 15.99 | 0.533 |
Means within the same row, within the same parameter, with no common subscript, differ significantly.
Table 5.
Effect of grain type and xylanase level (16,000 BXU/kg or 32,000 BXU/kg) or NSP-cocktail on quantity of soluble non-starch polysaccharides (sNSP), insoluble non-starch polysaccharides (iNSP), and free oligosaccharides (FS) present along the gastrointestinal tract in broilers fed corn- and sorghum-based diets at d35 (g/kg DM digesta).
| Corn |
Sorghum |
|||||||
|---|---|---|---|---|---|---|---|---|
| Single | Double | Cocktail | P-value | Single | Double | Cocktail | P-value | |
| sNSP | ||||||||
| Diet | 16.35 | 16.70 | 16.73 | 15.55 | 15.88 | 15.71 | ||
| Gizzard | 19.29 | 17.66 | 17.40 | 0.821 | 23.57 | 21.40 | 20.90 | 0.775 |
| Jejunum | 13.35 | 13.29 | 12.59 | 0.852 | 13.69a | 12.61ab | 11.21b | 0.012 |
| Ileum | 12.77 | 11.13 | 10.43 | 0.172 | 9.39 | 9.41 | 8.82 | 0.792 |
| Excreta | 7.09 | 7.11 | 6.48 | 0.634 | 6.67 | 6.40 | 6.01 | 0.609 |
| iNSP | ||||||||
| Diet | 66.00 | 66.64 | 65.58 | 60.60 | 61.79 | 59.73 | ||
| Gizzard | 217.95 | 205.57 | 199.30 | 0.622 | 202.53 | 194.29 | 166.76 | 0.125 |
| Jejunum | 59.69 | 57.71 | 54.68 | 0.560 | 51.60 | 52.27 | 46.88 | 0.138 |
| Ileum | 52.84 | 51.78 | 50.99 | 0.795 | 41.80 | 41.60 | 40.83 | 0.946 |
| Excreta | 41.73 | 40.64 | 39.54 | 0.801 | 38.03 | 38.59 | 36.15 | 0.350 |
| FS | ||||||||
| Diet | 38.43 | 39.08 | 37.71 | 36.08 | 35.65 | 36.41 | ||
| Gizzard | 27.32 | 27.07 | 25.24 | 0.847 | 28.82 | 25.30 | 22.01 | 0.540 |
| Jejunum | 33.69 | 34.42 | 35.13 | 0.952 | 31.80a | 33.56a | 18.85b | 0.001 |
| Ileum | 30.19 | 23.39 | 30.33 | 0.307 | 29.33 | 26.48 | 16.40 | 0.110 |
| Excreta | 15.38 | 12.78 | 15.71 | 0.594 | 16.22 | 15.61 | 14.40 | 0.765 |
Means within the same row, within the same parameter, with no common subscript, differ significantly.
DISCUSSION
This study examined if NSP hydrolysis in the gastrointestinal tract, and the advantages that come with NSP-degradation on bird performance and excreta moisture content, is enhanced by the presence of a double dose of xylanase or an NSP-ase cocktail, compared to feeding the current commercial recommended dose of xylanase.
Effect of Xylanase Dose and NSP-ase Cocktail in Barley-Based Diets
The double xylanase dose and NSP-ase cocktail improved feed conversion in birds fed the barley-based diet in the grower phase (d 12–23). This is in agreement with Hashemi et al. (2017) who found that supplementing an NSP-ase cocktail containing ß-glucanase, cellulase, α-amylase, xylanase, and protease, or just combined xylanase and ß-glucanase, to a barley-based diet resulted in reduced FCR in the grower period (d 15–28). Similarly Moftakharzadeh et al. (2017) and Shirzadi and Moravej (2009) found FCR values at d 11 to 28 and d 11 to 30, respectively, were consistently lower when supplementing an NSP-ase cocktail containing ß-glucanases, cellulase, and xylanase to barley-based diets Shirzadi and Moravej (2009). also observed increased litter dry matter content as a consequence of the NSP-ase cocktail supplementation, which is agreement with this study, as determined at bird age d 23. The barley used in this study contained 9.6% insoluble NSP, two thirds of which was arabinoxylan (AX). Analysis of AX degradability revealed that the NSP-ase cocktail and double xylanase were more successful at hydrolysing the AX in the barley compared to the single xylanase dose, likely resulting in reduced water holding capacity, increased accessibility to nutrients and heightened production of prebiotic XOS. It is also possible that these enzyme treatments partially solubilized the insoluble AX, and that the resulting soluble AX could be readily fermented by beneficial microbiota species (Svihus et al., 2013). This is illustrated by a numerical decrease in excreta sNSP concentration in birds fed the diets with double xylanase dose and NSP-ase cocktail compared to the single dose of xylanase; the lack of significance could be attributable to variability between samples, due to complexity of the polymers and thus challenges during sample preparation and analysis, and variation between birds and sample collection time. The observed reduced insoluble NSP present in the gizzard of the barley-fed birds fed the NSP-ase cocktail and double xylanase presents increased solubilization of NSP early in the tract, which may have influenced digesta passage rate (Sacranie et al., 2012). Additionally, it is predicted, based on the work of Bautil et al. (2019), that more xylan-degrading bacteria were established in the microbiota of young birds fed these dietary treatments, which enabled these birds to utilize the dietary xylan better when they reached the grower phase. This is justified by the heightened soluble NSP degradability at d 12 seen in the presence of double xylanase in birds fed the barley-based diets. The lack of effect of the enzyme treatments on finisher phase performance potentially suggests that the xylan-degrading microbiota was fully established by this point, so the enzymes could provide little additional impact on xylan utilization. The increased free oligosaccharide digestibility observed when the barley-based diet was supplemented with the NSP-ase cocktail illustrates the increased abundance and quantity of oligosaccharides produced as a consequence of providing a variety of enzymes to a diet containing a range of different polymers.
Effect of Xylanase Dose and NSP-ase Cocktail in Corn-Based Diets
The increased body weight gain and reduced FCR value seen in the starter phase of birds fed the corn-based diets as a result of applying the higher xylanase dose is in agreement with Nusairat and Wang (2021), who saw linear improvements in both parameters in the starter phase with increasing xylanase concentration in birds fed corn-based diets Van Hoeck et al. (2021). observed the same at age d 0 to 35. The NSP-ase cocktail also increased starter phase body weight gain in birds fed the corn-based diet, which is in agreement with Tang et al. (2014) who found that feeding an enzyme cocktail containing amylase, xylanase, glucanase, cellulase, mannanase, and pectinase improved body weight gain at d 1 to 21. Also, De Keyser et al. (2016) found feeding xylanase in combination with glucanase and pectinase enhanced body weight gain at d 0 to 14. This outcome is also in partial agreement with Ko et al. (2021), who observed that supplementing a corn-based diet with an NSP-ase cocktail containing mannanase, β-glucanase, and xylanase significantly improved feed conversion at d 0 to 35, but not specifically in the starter phase. This would suggest that the NSP-ase cocktail was successful at breaking down the cell wall matrix, particularly the insoluble components, which facilitated release of encapsulated nutrients and enhanced accessibility of digestive enzymes. However, this was not reflected by the analysis of NSP degradation in this study, which showed no effects of the enzyme treatments on NSP utilization in birds fed the corn-based diet. This may be a consequence of the method used to determine NSP degradability, which relied on using a titanium dioxide marker; there are concerns around if this technique can truly reflect passage rate of both the solid and liquid components of digesta (Morgan et al., 2014; Kolakshyapati et al., 2019), potentially questioning the accuracy of determination of flow of the insoluble NSP fractions. The lack of effect of the double xylanase dose or NSP-ase cocktail in the grower and finisher phase suggests that providing a high quantity and range of oligosaccharides is advantageous at stimulating and establishing AX fermentation capacity in birds fed AX-poor diets, but once this is founded the single dose is sufficient at maintaining AX degradation.
Effect of Xylanase Dose and NSP-ase Cocktail in Sorghum-Based Diets
Performance at d 23 to 35 was worse in birds fed the sorghum-based diet when the NSP-ase cocktail was supplemented, compared to the single xylanase dose. This may be because the lack of fermentable fibre in these diets reduced the fermentation capacity of the microbiota (Ribeiro et al., 2018). It may be that the single xylanase dose induced production of sufficient soluble xylan for these bacteria, but the NSP-ase cocktail produced too much, which had a detrimental impact on microbiota balance. The additional oligosaccharides produced by application of the NSP-ase cocktail may have had detrimental effects, possibly because the probiotic bacteria that could utilize them were not present, so they were instead used as fuel by pathogenic bacteria species. This then may have resulted in competition between the host and bacteria for nutrients such as amino acids, thus, causing reduced performance. Also, the oligosaccharides produced may have stimulated the NSP-degrading bacteria, but the lack of substrate available in the low-NSP diets meant there was insufficient fuel for them. Another explanation may be that the excess oligosaccharides increased osmotic stress along the gastrointestinal tract, although this was not reflected in the excreta or litter dry matter values in this study. The greater total tract soluble NSP degradability seen at d 35 in birds fed the double xylanase compared to the NSP-ase cocktail reinforces this idea, by suggesting that hydrolysis of additional polymers was not advantageous at enhancing xylan utilization. This suggests that in older birds the small amounts of xylan available for fermentation are fully degraded or absorbed by the ileum, so it is unlikely that they will make it to the ceca to be used by the beneficial microbiota (González-Ortiz et al., 2020).
Effect of Xylanase Dose and NSP-ase Cocktail in Wheat-Based Diets
In birds fed the wheat-based diet, starter phase body weight gain and total tract soluble NSP degradability was greater with application of the NSP-ase cocktail compared to the single xylanase. This is in agreement with Smeets et al. (2018) who found that supplementing a wheat-based diet with NSP-ase cocktail containing ß-glucanase, cellulase, α-amylase, xylanase increased body weight at d 12. However, in contrast, Smeets et al. (2018) did not see any improvements in water-extractable NSP digestibility with this enzyme treatment. The outputs from this study suggest that the NSP-ase cocktail increased accessibility to soluble NSP, which was then readily utilised by beneficial microbiota, inducing positive effects on nutrient utilization and establishing a microbiome that was effective at exploiting dietary xylan. It appears that once this microbiome was established no additional benefits could be gained from supplying the NSP-ase cocktail in the grower and finisher phase, as shown by lack of performance response; the free oligosaccharides present in the diet and those generated from single xylanase likely provided sufficient fuel for the maintaining the microbiota. Supplementing the double xylanase dose into the wheat-based diet increased total tract degradability of soluble NSP at d 12 and d 23, which appears to have contributed toward reduced excreta dry matter content at d 35. Again, it may be that these birds developed a microbiome that was better adapted to utilizing soluble xylan (Bautil et al., 2019), so when the bird was older there were reduced issues associated with soluble NSP on digesta viscosity, and thus water consumption and retention, meaning less water was excreted. Insoluble NSP degradation was also increased with the double xylanase and NSP-ase cocktail; insoluble NSP can also absorb water and increase digesta bulk and thus influences excreta consistency. Moreover, hydrolysis of these NSP results in increased nutrient absorption, meaning there are less nutrients, particularly unabsorbed sugars, present in the gastrointestinal tract to cause osmosis of water into the gut lumen (Kim et al., 2021b).
Conclusions
In conclusion, the outputs from this study demonstrate that supplementing a double xylanase dose was generally advantageous over a single xylanase dose or NSP-ase cocktail, in terms of NSP degradation, bird performance in the starter phase and excreta moisture content. In this study the NSP-ase cocktail induced differing effects depending on the NSP composition of the diets, such as negative effects on performance when applied to the sorghum-based diet, but positive effects on some parameters in birds fed the wheat- and barley-based diets. This highlights the need to use a tailored approach when using enzyme cocktails, targeting the specific polymers in the diet.
ACKNOWLEDGMENTS
The authors wish to thank Agrifutures Chicken Meat Program for funding this study.
DISCLOSURES
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- Aftab U., Bedford M.R. The use of NSP enzymes in poultry nutrition: myths and realities. World. Poult. Sci. J. 2018;74:277–286. [Google Scholar]
- Bach Knudsen K.E. The nutritional significance of dietary fibre analysis. Anim. Feed. Sci. Tech. 2001;90:3–20. [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Age-related arabinoxylan hydrolysis and fermentation in the gastrointestinal tract of broilers fed wheat-based diets. Poult. Sci. 2019;98:4606–4621. doi: 10.3382/ps/pez159. [DOI] [PubMed] [Google Scholar]
- Bautil A., Verspreet J., Buyse J., Goos P., Bedford M.R., Courtin C.M. Arabinoxylan-oligosaccharides kick-start arabinoxylan digestion in the aging broiler. Poult. Sci. 2020;98:4606–4621. doi: 10.1016/j.psj.2019.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford M.R. Exogenous enzymes in monogastric nutrition-their current value and future benefits. Anim. Feed Sci. Tech. 2000;86:1–13. [Google Scholar]
- Choct M., Dersjant-Li Y., McLeish J., Peisker M. Soy oligosaccharides and soluble non-starch polysaccharides: a review of digestion, nutritive and anti-nutritive effects in pigs and poultry. Asian-Aust. J. Anim. Sci. 2010;23:1386–1398. [Google Scholar]
- Cowieson A.J., Bedford M.R., Ravindran V. Interaction between xylanase and glucanase in maize-soy-based diets for broilers. Br. Poult Sci. 2010;51:246–257. doi: 10.1080/00071661003789347. [DOI] [PubMed] [Google Scholar]
- De Keyser K., Kuterna L., Kaczmarek S., Rutkowski A., Vanderbeke E. High dosing NSP enzymes for total protein and digestible amino acid reformulation in a wheat/corn/soybean meal diet in broilers. J. Appl. Poult. Res. 2016;25:239–246. [Google Scholar]
- De Maesschalck C.D., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., De Baere S., Croubels S., Daube G., Dewulf J., Haesebrouck F., Ducatelle R., Taminau B., Immerseel F.Van. Effects of xylo-oligosaccharides on performance and microbiota in broiler chickens. Appl. Enviro. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englyst H.N., Quigley M.E., Hudson G.J. Determination of dietary fibre as non-starch polysaccharides with gas–liquid chromatographic, high-performance liquid chromatographic or spectrophotometric measurement of constituent sugars. Analyst. 1994;119:1497–1509. doi: 10.1039/an9941901497. [DOI] [PubMed] [Google Scholar]
- González-Ortiz G., Olukosi O.A., Jurgens G., Apajalahti J., Bedford M.R. Short-chain fatty acids and ceca microbiota profiles in broilers and turkeys in response to diets supplemented with phytase at varying concentrations, with or without xylanase. Poult. Sci. 2020;99:2068–2077. doi: 10.1016/j.psj.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi M., Seidavi A., Javandel F., Gamboa S. Influence of non-starch polysaccharide-degrading enzymes on growth performance, blood parameters, and carcass quality of broilers fed corn or wheat/barley-based diets. Rev. Colom. Cienc. Pecua. 2017;30:286–298. [Google Scholar]
- Hetland H., Choct M., Svihus B. Role of insoluble non-starch polysaccharides in poultry nutrition. World. Poult. Sci. J. 2004;60:415–422. [Google Scholar]
- Jimoh A., Atteh J.O. Improving the metabolisable energy value of brewers’ dried grains with enzyme cocktails in poultry nutrition. J. Ag. Sci. 2018;63:409–419. [Google Scholar]
- Jommuengbout P., Pinitgland S., Kyu K.L., Ratankhanokchai K. Substrate-binding site of Family 11 xylanase form Bacillus firmus K-1 by molecular docking. Biosci. Biotech. Biochem. 2009;73:833–883. doi: 10.1271/bbb.80731. [DOI] [PubMed] [Google Scholar]
- Kim E., Morgan N.K., Moss A.F., Li L., Ader P., Choct M. Characterisation of undigested components throughout the gastrointestinal tract of broiler chickens fed either a wheat- or maize-based diet. Anim. Nutr. 2021;8:153–159. doi: 10.1016/j.aninu.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Morgan N.K., Moss A.F., Li L., Ader P., Choct M. The flow of non-starch polysaccharides along the gastrointestinal tract of broiler chickens fed either a wheat- or maize-based diet. Anim. Nutr. 2021 doi: 10.1016/j.aninu.2021.11.004. accessed date- April 2022. (Ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H., Ku Kang H., Moturi J., Laxman Ingale S., Kim J. Supplementation of enzyme cocktail in chickens diet is an effective approach to increase the utilization of nutrient in wheat-based diets. J. Anim. Sci. Tech. 2021;63:69–76. doi: 10.5187/jast.2021.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakshyapati M., Bailey C., Sibanda T.Z., Morgan N., Ruhnke I. Determination of gastrointestinal passage rate using three different markers in laying hens. J. Anim. Phys. Anim. Nutr. 2019;103:1427–1436. doi: 10.1111/jpn.13145. [DOI] [PubMed] [Google Scholar]
- Lui W-C., Kim I-H. Effects of dietary xylanase supplementation on performance and functional digestive parameters in broilers fed wheat-based diets. Poult. Sci. 2017;96:566–573. doi: 10.3382/ps/pew258. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Jiménez-Moreno E., Serrano M.P., Lázaro R.P. Poultry response to high levels of dietary fiber sources varying in physical and chemical characteristics. J. Appl. Poult. Sci. 2012;21:156–174. [Google Scholar]
- Mathlouthi N., Saulnier L., Quemener B., Larbier M. Xylanase, β-glucanase and other side enzymatic activities have greater effects on the viscosity of several feedstuffs than xylanase and β-glucanase used alone or in combination. J. Ag. Food. Chem. 2002;50:5121–5127. doi: 10.1021/jf011507b. [DOI] [PubMed] [Google Scholar]
- Moftakharzadeh S.A., Moravej H., Shivazad M. Effect of using the matrix values for NSP-degrading enzymes on performance, water intake, litter moisture and jejunal digesta viscosity of broilers fed barley-based diet. Acta. Sci. Anim. Sci. 2017;39:65–72. [Google Scholar]
- Morgan N., Bhuiyan M.M., Nguyen T.N.A., Middlebrook T., Hopcroft R. Dietary soluble non-starch polysaccharide level and composition influences grower and finisher phase performance, excreta moisture content and total tract nutrient digestibility in broilers. Br. Poult. Sci. 2021;100 doi: 10.1080/00071668.2021.1919995. [DOI] [PubMed] [Google Scholar]
- Morgan N., Scholey D., Burton E.J. A comparison of two methods for determining titanium dioxide marker content in broiler digestibility studies. Animal. 2014;8:1–5. doi: 10.1017/S1751731114000068. [DOI] [PubMed] [Google Scholar]
- Morgan N.K., Choct M., Toghyani M., Wu S-B. Effects of dietary insoluble and soluble non-starch polysaccharides on performance and ileal and excreta moisture contents in broilers. Pages 34–37 in Proceedings of 29th Annual Australian Poultry Science Symposium; Sydney, Australia; University of Sydney; 2018. [Google Scholar]
- Nusairat B., Wang J-J. The effect of a modified GH11 xylanase on live performance, gut health, and clostridium perfringens excretion of broilers fed corn-soy diets. Front. Vet. Med. 2021;8:523. doi: 10.3389/fvets.2021.678536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukosi O.A., Bedford M.R., Adeola O. Xylanase in diets for growing pigs and broiler chicks. Can. J. Anim. Sci. 2007;87:227–235. [Google Scholar]
- Patel H., Chapla D., Divecha J., Shah A. Improved yield of α-L-arabinofuranosidase by newly isolated Aspergillus niger ADH-11 and synergistic effect of crude enzyme on saccharification of maize stover. Bioresour. Bioprocess. 2015;2 [Google Scholar]
- Rabello C.B.V., Costa M.J., Nogueira W.C.L., Guilherme Barbosa J., Carlose Rios-Alva J., Wyatt C.L., York T.W., Serrano M.P., Orlando Oviedo-Rondón E. Effects of graded levels of exogenous xylanase in corn-soy diets with two amino acid density and fat levels post-pellet in broiler chickens: live performance, energy utilization, digestibility, and carcass characteristics. Poult. Sci. 2021;100:820–834. doi: 10.1016/j.psj.2020.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro T., Cardoso V., Ferreira L.M.A., Lordelo M.M.S., Coelho E., Moreira A.S.P., Domingues M.R.M., Coimbra M.A., Bedford M.R., Fontes C.M.G.A. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult. Sci. 2018;97:4330–4341. doi: 10.3382/ps/pey336. [DOI] [PubMed] [Google Scholar]
- Sacranie A.B.Svihus, Denstadli V., Moen B., Iji P.A., Choct M. The effect of insoluble fiber and intermittent feeding on gizzard development, gut motility, and performance of broiler chickens. Poult. Sci. 2012;91:693–700. doi: 10.3382/ps.2011-01790. [DOI] [PubMed] [Google Scholar]
- Shirzadi H., Moravej H. Comparison of the effects of different kinds of NSP enzymes on the performance, water intake, litter moisture and jejunal digesta viscosity of broilers fed barley-based diet. J. Food. Ag. Env. 2009;7:615–619. [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed. Sci. Tech. 1996;59:215–221. [Google Scholar]
- Smeets N., Nuyens F., Van Campenhout L., Delezie E., Niewold T.A. Interactions between the concentration of non-starch polysaccharides in wheat and the addition of an enzyme mixture in a broiler digestibility and performance trial. Poult. Sci. 2018;97:2064–2070. doi: 10.3382/ps/pey038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanello C., Vieira S.L., Santiago G.O., Kindlein L., Sorbara J.O.B., Cowieson A.J. Starch digestibility, energy utilisation, and growth performance of broilers fed corn-soybean based diets supplemented with enzymes. Poult. Sci. 2015;94:2472–2479. doi: 10.3382/ps/pev244. [DOI] [PubMed] [Google Scholar]
- Svihus B., Choct M., Classen H.L. Function and nutritional roles of the avian caeca: a review. World. Poult. Sci. J. 2013;69:249–264. [Google Scholar]
- Tang D., Hao S., Liu G., Nian F., YR Effects of maize source and complex enzymes on performance and nutrient utilization of broilers. Asian-Australas. J Anim Sci. 2014;27:1755–1762. doi: 10.5713/ajas.2014.14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander O., Åman P., Westerlund E., Andersson R., Pettersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J. AOAC Int. 1995;78:1030–1044. [PubMed] [Google Scholar]
- Van Hoeck V., Wu D., Somers I., Wealleans A., Vasanthakumari B.L., Gonzalez Sanchez A.L., Morisset D. Xylanase impact beyond performance: a prebiotic approach in broiler chickens. J. Appl. Poult. Sci. 2021;30 [Google Scholar]
- Yokhana J., Parkinson G., Frankel T. Effects of insoluble fibe supplementation applied at different ages on digestive organ weight and digestive enzymes of layer-strain poultry. Poult. Sci. 2016;95:550–559. doi: 10.3382/ps/pev336. [DOI] [PMC free article] [PubMed] [Google Scholar]