Abstract
Objectives:
To describe the incidence of antiretroviral treatment failure and associated factors in a pediatric clinical cohort within the East African International epidemiology Databases to Evaluate AIDS (EA-IeDEA) consortium.
Design:
A retrospective cohort study. Clinical treatment failure was defined as advancement in clinical WHO stage, or CDC class at least 24 weeks after initiation of treatment. Immunological failure was defined as developing or returning to the following age-related immunological thresholds after at least 24 weeks on treatment; CD4 count of <200 or CD4%<10% for children aged 2–5 years and CD4 count of < 100 for a child aged > 5years.
Setting:
The study utilized the electronic medical records of HIV-infected pediatric patients enrolled into the EA-IeDEA consortium clinics from January 2005 to August 2012.
Results:
A total of 5927 children were included in the analysis. The estimated cumulative incidence of clinical ART treatment failure at one year and four years post ART initiation was11.5% and 31% respectively, while that of immunological treatment failure was at 3% and 22.5% respectively. The main factors associated with clinical failure were advanced clinical stage at ART-initiation, year started ART and residing in a rural area. Factors associated with immunological failure were male gender and age of the child at ART-initiation. Only 6% of those identified as having clinical treatment failure were switched to second line treatment during the four years of follow-up.
Conclusion:
The probability of clinical and immunologic failure was relatively high and increased with time.
INTRODUCTION
Globally antiretroviral therapy (ART) services for treatment of HIV infections have been greatly scaled up.(1, 2)This in turn has been associated with reduced morbidity and mortality among HIV-infected persons (3–5).There is a currently shift from the initial focus on initiation of antiretroviral therapy to its long-term maintenance and management of the chronic disease. Treatment failure in the early days of ART rollout in sub-Saharan Africa was predominately identified using clinical and immunological criteria rather than virological criteria(6).
The numbers of children failing first line treatment and requiring second line will continue to rise over time. Timely identification of those failing and timely switch of regimen is important. It is also important to note that even with the evidence that immunologic criteria is a poor predictor of virological failure, many programs in limited resource settings continue to use this criteria to detect treatment failure(7).
Different national programs have reported on durability of 1st line ART among the various cohorts of patients. We studied the incidence and factors associated with first line antiretroviral treatment failure in a large cohort of children attending clinics affiliated to EA-IeDEA consortium.
MATERIALS AND METHODS
This retrospective cohort study utilized the electronic medical records of HIV-infected pediatric patients enrolled in clinics affiliated with the EA-IeDEA consortium from January 2005 to August 2012. All analysis was performed with de-identified data. The IeDEA protocol was approved by Institution Research Ethics Committee (IREC) of Moi University and Moi Teaching and referral Hospital, the IRB at Indiana University School of Medicine, and all other regulatory bodies affiliated with each participating site, and where applicable national regulatory bodies. Patient level consent was waived by all regulatory entities because the data were de-identified and all data were routinely collected as part of patient care.
A total of 32 sites were included in this study across six programs in Kenya, Uganda, and Tanzania. In Kenya, participating programs included the Academic Model Providing Access to Healthcare (AMPATH), the Family AIDS Care and Education Services (FACES), and the MTCT (Mother-to-Child Transmission)-Plus program in Kisumu. In Uganda, affiliated sites included the Makerere University-Johns Hopkins University (MU-JHU) collaboration at Mulago Hospital (MTCT-Plus program), St Francis Nsambya and the Masaka Regional Referral Hospital. Contributing sites in Tanzania included the Tumbi Regional Hospital in Kibaha, and the Morogoro Regional Hospital in Morogoro. The clinics were in urban, semi urban and rural settings.
Local data quality protocols are maintained at each site. On a bi-annual basis de-identified data were transferred to the EA-IeDEA Regional Data Center (RDC) where they underwent standardized data quality checks. All HIV-infected children aged less than or equal to14 years at ART initiation who had at least 24 weeks on ART prior to database closure, and who were attending one of the HIV clinics affiliated with EA-IeDEA were eligible for study inclusion. During the study period no site utilized routine viral load testing to monitor patients. AMPATH site utilized immunologic monitoring with confirmatory viral load testing during the analysis period.
The primary outcome of interest during our analysis was treatment failure. Clinical treatment failure was defined as appearance or re-appearance of a WHO stage 3 or 4 condition, an advancement in clinical stage (1/2 to 3/4 or 3 to 4) or an advancement in CDC class (N/A to B/C or B to C) at least 24 weeks after initiation of treatment. Immunologic failure was defined as developing or returning to the following age-related immunological thresholds after at least 24 weeks on treatment; CD4 count of <200 or CD4%<10% for children aged 2–5 years and CD4 count of < 100 for a child aged > 5years. WHO Child Growth Standards and WHO Reference 2007 data were used to calculate Z-scores for height-for age (HAZ; 0–19 years of age), weight-for-age (WAZ; 0–10 years of age) and weight-for-height(WHZ; 0–5 years of age). CDC Reference 2000 data was used to calculate weight-for-age for ages 10–19.Failure to thrive was defined as a WHZ of < −2 after 24 weeks of treatment.
Switch to second line treatment was a secondary outcome of interest and was defined as a complete switch (as opposed to just replacing one drug in the regimen) from first line regime to a second line regime with no record of drug toxicity. Information on death is passively collected by the clinical programs.
Data were analyzed using STATA version 13 (Stata Corporation, College Station, TX) and R (R Development Core Team 2011). To determine factors associated with treatment failure competing risk analysis was performed. The cumulative incidence function Fk(t)was used to estimate the probability of failing from cause k at a given time t rather than the standard Kaplan Meier approach. The Fine and Gray model was used to determine factors associated with treatment failure in the presence of competing risks(8). The analysis was performed using the crr function in the R library cmprsk.
RESULTS
A total of 5927 children met inclusion criteria for this analysis with 81.8% of the participants being from the AMPATH program (Table 1). The median age at ART initiation was 5.6 years (IQR: 2.9– 8.9) and 51.4% of the children were male. Within sites where orphan status was collected, only 46% of children had documentation that both parents were alive. Nearly half of the children had advanced HIV (WHO stage 3 or 4 disease) at initiation of ART (Table 1). The nutritional status of the children at ART initiation was suboptimal as indicated by a median WAZ score of −1.82 (IQR:−2.86, −0.92).
Table 1:
Socio-demographic and clinical characteristics of the participants
| Variable | Frequency (%) participants |
|---|---|
| East Africa IeDEA Site Name | n=5927 |
| AMPATH | 4848 (81.8) |
| FACES | 441 (7.4) |
| Kisumu (MTCT-plus) | 46 (0.8) |
| MU-JHU, Mulago (MTCT-plus) | 98 (1.7) |
| Masaka | 140 (2.4) |
| Morogoro | 246 (4.2) |
| St. Francis, Nsambya (MTCT-plus) | 42 (0.7) |
| Tumbi | 66 (1.1) |
| Gender | n=5927 |
| Female | 2882 (48.6) |
| Male | 3045 (51.4) |
| Orphan Status at start of | n=4848 |
| ART(AMPATH) | |
| Both parents Alive | 2259 (46.6) |
| Both parent deceased | 643 (13.3) |
| Mother deceased | 504 (10.4) |
| Father deceased | 598 (12.3) |
| Missing information | 844 (17.4) |
| WHO stage at ART-initiation | |
| WHO Stage 1 | 980 (16.5) |
| WHO Stage 2 | 1581 (26.7) |
| WHO Stage 3 | 1435 (24.2) |
| WHO Stage 4 | 1436 (24.2) |
| Missing information | 495 (8.4) |
| Year of ART-initiation | |
| 2005 | 462 (7.8) |
| 2006 | 822 (13.9) |
| 2007 | 930 (15.7) |
| 2008 | 878 (14.8) |
| 2009 | 1400 (23.6) |
| 2010 | 1365 (23.0) |
| 2011 | 70 (1.2) |
| Variable | Median (IQR) |
| Age in years at start of ART |
n=5927 5.6 (2.9,8.9) |
| CD4 at start of ART 1 | n=3255 351 (163, 645) |
| CD4 % at start of ART 2 | n=2910 13 (8,17) |
| WAZ at start of ART | n=5911 −1.82 (−2.86, −0.92) |
| WHZ at start of ART | n=2562 −0.29 (−1.42,0.71) |
CD4 available for children above 5 years
CD4% available for children below 5 years
Clinical Treatment Failure
The cumulative incidence of clinical failure at 12 and 48 months post ART-initiation was 11.5% (95%CI 10.6, 12.3), and 31.2% (95% CI: 21.1, 24.0), respectively. While the cumulative incidence of Death/LTFU at 12 and 48 months was 5.5% (95%CI 5, 6.2) and 22.5% (95%CI 21.1, 24.0), respectively. The competing risks of death/LTFU and clinical failure are displayed in Figure 1.
Figure 1:
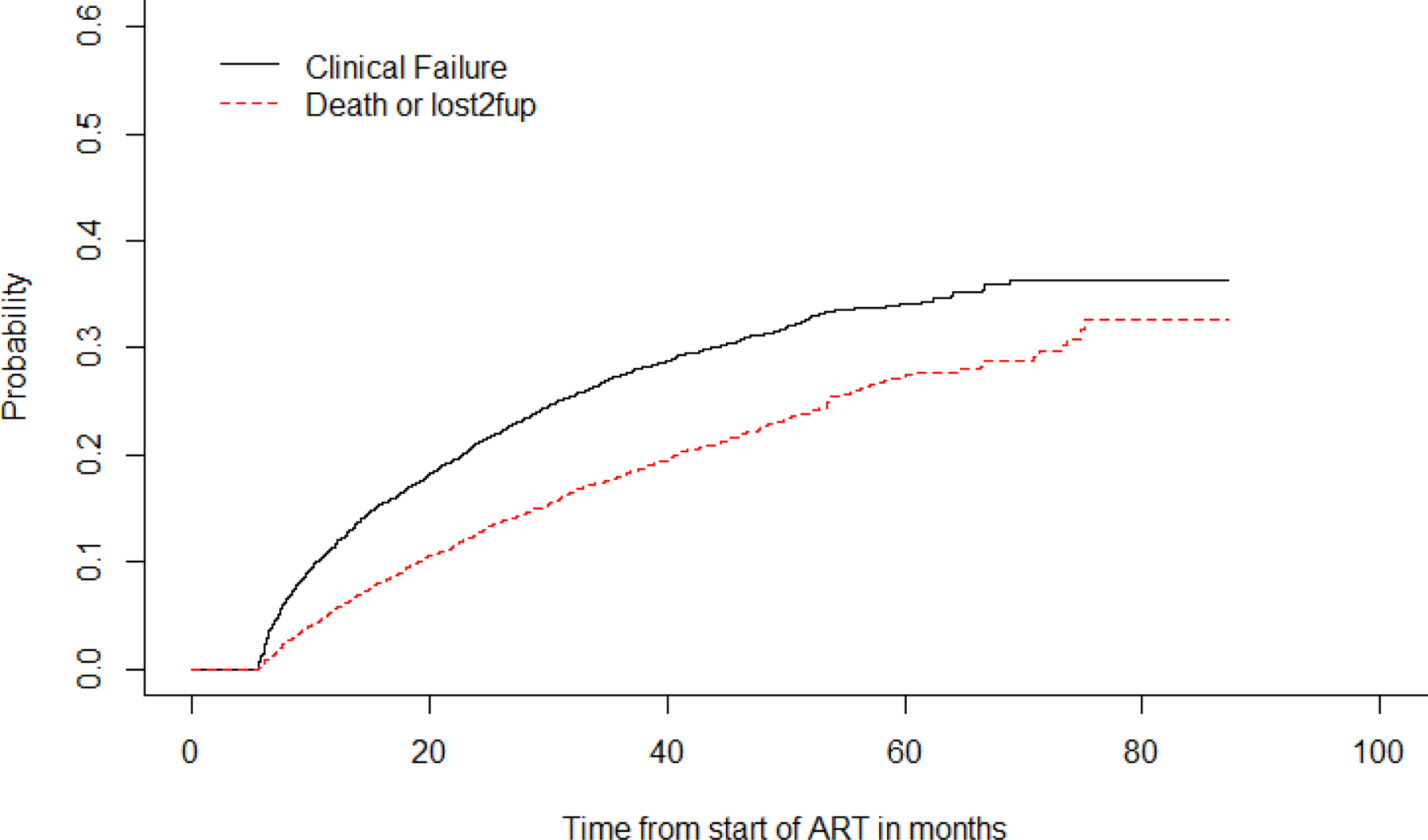
Time to first line ART clinical failure
A multivariate analysis showed that age and clinical stage (WHO) at ART-initiation were significantly associated with clinical failure. The incidence of clinical failure decreased with each subsequent year of ART initiation while patients with WHO stage 3and 4 at initiation of ART had a higher incidence of clinical failure as shown in table 2.
Table 2:
Factors associated with clinical failure and Death/LTFU.
| Variable | Event | |||
|---|---|---|---|---|
| Clinical Failure | Death/LTFU | |||
| HR | 95% CI | HR | 95% CI | |
| Male | 1.004 | 0.897, 1.124 | 1.037 | 0.908, 1.185 |
| Age at ART start | 1.004 | 0.988, 1.020 | 0.968 | 0.949, 0.987 |
| NNRT vs NRTI | 0.918 | 0.776, 1.088 | 1.349 | 1.098, 1.658 |
| Year started ART | 0.877 | 0.842, 0.914 | 1.07 | 1.024, 1.117 |
| WHO 3 vs 1 | 1.297 | 1.111, 1.515 | 0.948 | 0.788, 1.14 |
| WHO 4vs 1 | 1.693 | 1.460, 1.964 | 1.148 | 0.965, 1.367 |
| Rural vs Urban | 1.012 | 0.795, 1.287 | 0.688 | 0.500, 0.948 |
| In between vs Urban | 1.103 | 0.980, 1.242 | 1.187 | 1.036, 1.359 |
Immunologic treatment failure
We observe that the cumulative incidence of immunologic failure at 12 and 48 months post ART initiation was 3% (95%CI 2.5,3.5) and 11.0% (95CI 10.0,12.1), respectively. While that for death or LTFU at 12 and 48 months was 2.1% (95% 1.7, 2.5) and 20.7 (95%CI 19.2, 22.2), respectively as shown in Figure 2.
Figure 2:
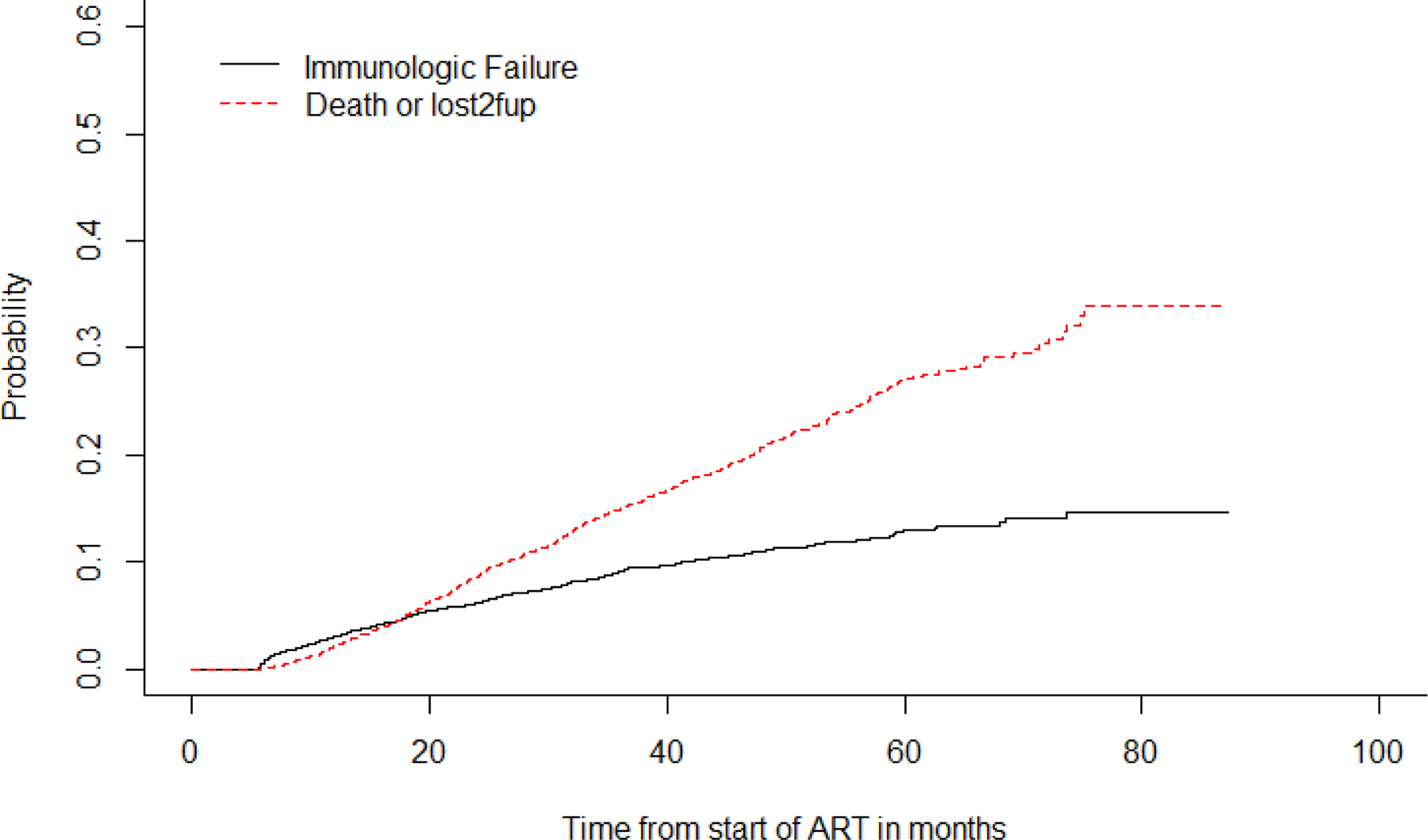
Time to first-line ART immunological failure
The variables that were significantly associated with immunological failure were male gender and age at initiation of ART (Table 3).
Table 3:
Factors associated with Immunologic failure and Death/LTFU.
| Variable | Immunologic Failure | Death/LTFU | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Male | 1.44 | 1.17, 1.78 | 0.98 | 0.84, 1.14 |
| Age at start of ART | 1.09 | 1.06, 1.13 | 0.98 | 0.96, 1.00 |
| NNRTI vs NRT | 1.06 | 0.77, 1.45 | 1.24 | 0.97, 1.59 |
| Year started ART | 1.00 | 0.93, 1.09 | 1.09 | 1.03, 1.16 |
| WHO stage 3 vs 1 | 0.85 | 0.64, 1.13 | 1.22 | 0.99, 1.50 |
| WHO stage 4 vs 1 | 1.20 | 0.92, 1.55 | 1.42 | 1.16, 1.72 |
| Rural vs Urban | 1.02 | 0.68, 1.53 | 0.75 | 0.52, 1.06 |
| In between versus Urban | 0.84 | 0.67, 1.04 | 1.27 | 1.09, 1.48 |
Virological failure
A total of 495 patients had viral load measurements at AMPATH after being identified as having immunologic failure. Out of the 495, 69.5% were confirmed to have virological failure. Of whom only 47% were switched to second line regimen while only 6% of those with clinical failure were switched. Time to switch was longer for those with clinical failure compared to those with virological failure (Table 4).
Table 4:
Switch to second line regimen.
| Type of failure | Proportion Fail | Proportion switch (of those that had failure) | Median time to switch from failure in months (IQR) |
|---|---|---|---|
| Clinical Failure (n=5927) | 24.1% | 6.0% | 15.8 (3.0, 32.0) |
| Immunologic failure (n=4853) | 9.0 % | 21.2% | 8.9 (3.6, 18.6) |
| VL failure (n=495) | 69.5% | 47.0% | 4.4 (2.8, 7.6) |
| Failure to thrive (n=5916) | 73.03% | 3.5% | 23.3 (11.6, 37.3) |
DISCUSSION
This study identified a relatively high cumulative incidence of clinical failure one year post ART initiation of 11.5%. However this was relatively low compared to similar studies in similar settings with findings ranging from 34–38% (10, 11).The difference may be attributable to varied durations of follow up, use of different criteria for treatment failure and different socio-demographic characteristics of the study participants.
More recent studies have found Viral suppression rates of between 70–84% and also a poor predictive value of immunological parameters for virological failure(29,30).This is however short of the 2014 united nations treatment target of 90-90-90 by 2020(28).
The cumulative incidence of death/LTFU was 5.5% over 12 months.This was low compared to finding in other studies conducted in Ethiopia and Kenya that found death incidence of 12.4% and 8.4%respectively(31,32). The differences may be attributed to the differences in the clinical and socio-demographic characteristics of the study population. Also the different in the duration of follow up.
Year of ART initiation and advanced WHO stage at start of ART were noted to be significantly associated with ART clinical failure. Peri-urban dwelling was significantly associated with immunologic treatment failure. These findings were similar to findings from other studies in the region that found CD4 count, WHO stage and viral load at initiation of ART to be significantly associated with treatment failure. Other studies have shown age to have a significant influence on immunological treatment failure (9, 13, 14,15). Some other studies have however found that patients who started ART treatment earlier in life are less likely to have clinical failure, but have a higher rate of loss to follow up or death (18, 20–24).There is likelihood that those starting treatment later are more immunosuppressed and hence are likely to be in advanced stage of the disease and therefore may have problems with immune recovery and hence continue to have clinical events.
CDC classification B and C and WHO stage 3 and 4 at ART initiation were associated with clinical failure. This has similarly been reported in other studies (17, 18).Nutrition status was significantly associated with treatment failure with higher WAZ score, having a lower likelihood of treatment failure. A study by Bacha et al., 2012 showed that patients who had height for age less than the 3rdpercentile at initiation of ART had a higher probability of treatment failure(19). Good nutrition status plays an important role in prevention of opportunistic infections.
The one year cumulative incidence for immunological failure was 3%. This was however computed for only one site (AMPATH program). The factors associated with immunological failure were age and gender. The observed increased likelihood of immunological failure for males and older children at ART initiation is similar to observations made in other studies (21–24). The CD4 count at ART initiation was not significantly associated with either clinical or immunological failure. This is unlike the findings of other studies that reported a significant association between baseline CD4 count and treatment failure (25).
In all categories of treatment failure, less than 50% of those identified as having failed treatment had a regimen switch. Similar findings were noted in a study of a wider IeDEA pediatric cohort (27), with clinical treatment failure having the least likelihood of being switched to second line treatment and a more than 15month time to switch. Clinical and immunological treatment failure may not always signify virological failure and this may have informed the decision to delay the switch. This emphasizes the importance of virological monitoring of patients which has currently been scaled up in most of the sites.
Study limitations
This being a retrospective electronic data review the likelihood of missing data was high. However, the large number of patients assessed in this study likely mitigates some of the impact of missing data. It was not possible to assess true rates of ART failure as the study sites did not have the capacity to do viral loads routinely.
Conclusion
A high cumulative incidence of first line ART clinical and immunologic failure was identified in this cohort of children at four years following ART-initiation. A marked delay in switching to second line treatment following clinical failure and immunologic failure was identified when compared to switches prompted by virological failure. Given these findings, we believe that optimal treatment of children with HIV will require readily available viral load testing.
References
- 1.UNICEF. Pediatric Treatment and Care. http://data.unicef.org/hiv-aids/paediatric.htmlAccessed Jan 18, 2017.
- 2.UNAIDS. FACTS SHEET 2016: Global Statistics. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf Accessed 10 June 2016
- 3.Reddi A, Leeper SC, Grobler AC, Geddes R, France KH, Dorse GL, Vlok WJ, Mntambo M, Thomas M, Nixon K, Holst HL, Karim QA, Rollins NC, Coovadia HM, Giddy J. Preliminary outcomes of a paediatric highly active antiretroviral therapy cohort from KwaZulu-Natal, South Africa. BMC pediatrics. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musoke PM, Mudiope P, Barlow-Mosha LN, Ajuna P, Bagenda D, Mubiru MM, Tylleskar T, Fowler MG. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: a prospective cohort study. BMC pediatrics. 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puthanakit T, Oberdorfer A, Akarathum N, Kanjanavanit S, Wannarit P, Sirisanthana T, Sirisanthana V. Efficacy of highly active antiretroviral therapy in HIV-infected children participating in Thailand’s National Access to Antiretroviral Program. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41(1):100–7. [DOI] [PubMed] [Google Scholar]
- 6.Manual on Paediatric HIV Care and Treatment for District Hospitals: Addendum to the Pocket Book of Hospital Care of Children. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: 2011. [PubMed] [Google Scholar]
- 7.Mee P, Fielding KL, Charalambous S, Churchyard GJ, Grant AD. Evaluation of the WHO criteria for antiretroviral treatment failure among adults in South Africa. Aids. 2008;22(15):1971–7. [DOI] [PubMed] [Google Scholar]
- 8.Fine J, Gray R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 9.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, Doro Altan A, Zimba Ida V, San Lio MM, De Luca A, Program D. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48(1):115–22. [DOI] [PubMed] [Google Scholar]
- 10.Bunupuradah T, Puthanakit T, Kosalaraksa P, Kerr S, Boonrak P, Prasitsuebsai W, Lumbiganon P, Mengthaisong T, Phasomsap C, Pancharoen C, Ruxrungtham K, Ananworanich J. Immunologic and virologic failure after first-line NNRTI-based antiretroviral therapy in Thai HIV-infected children. AIDS Res Ther. 2011;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving antiretroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. The Pediatric infectious disease journal. 2011;30(1):52–6. [DOI] [PubMed] [Google Scholar]
- 12.Chaiwarith R, Wachirakaphan C, Kotarathititum W, Praparatanaphan J, Sirisanthana T, Supparatpinyo K. Sensitivity and specificity of using CD4+ measurement and clinical evaluation to determine antiretroviral treatment failure in Thailand. Int J Infect Dis. 2007;11(5):413–6. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, Hirschel B, Ledergerber B, Vernazza P, Bernasconi E, Rickenbach M, Egger M, Battegay M, Swiss HIVCSG. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–95. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez F, Padilla S, Masia M, Iribarren JA, Moreno S, Viciana P, Hernandez-Quero J, Aleman R, Vidal F, Salavert M, Blanco JR, Leal M, Dronda F, Perez Hoyos S, del Amo J, Co RM. Patients’ characteristics and clinical implications of suboptimal CD4 T-cell gains after 1 year of successful antiretroviral therapy. Curr HIV Res. 2008;6(2):100–7. [DOI] [PubMed] [Google Scholar]
- 15.Rajasuriar R, Gouillou M, Spelman T, Read T, Hoy J, Law M, Cameron PU, Petoumenos K, Lewin SR. Clinical predictors of immune reconstitution following combination antiretroviral therapy in patients from the Australian HIV Observational Database. PloS one. 2011;6(6):e20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrogoua DP, Kablan BJ, Kamenan BA, Aulagner G, N’Guessan K, Zohore C. Assessment of the impact of adherence and other predictors during HAART on various CD4 cell responses in resource-limited settings. Patient Prefer Adherence. 2012;6:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, Sinkala M, Kankasa C, Wilson CM, Wilfert CM, Mwango A, Levy J, Abrams EJ, Bulterys M, Stringer JS. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. Jama. 2007;298(16):1888–99. [DOI] [PubMed] [Google Scholar]
- 18.Tadios Y, Davey G. Antiretroviral treatment adherence and its correlates in Addis Ababa, Ethiopia. Ethiop Med J. 2006;44(3):237–44. [PubMed] [Google Scholar]
- 19.Bacha T, Tilahun B, Worku A. Predictors of treatment failure and time to detection and switching in HIV-infected Ethiopian children receiving first line anti-retroviral therapy. BMC Infect Dis. 2012;12:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puthanakit T, Kerr S, Ananworanich J, Bunupuradah T, Boonrak P, Sirisanthana V. Pattern and predictors of immunologic recovery in human immunodeficiency virus-infected children receiving non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy. The Pediatric infectious disease journal. 2009;28(6):488–92. [DOI] [PubMed] [Google Scholar]
- 21.Davies MA, Moultrie H, Eley B, Rabie H, Van Cutsem G, Giddy J, Wood R, Technau K, Keiser O, Egger M, Boulle A, International Epidemiologic Databases to Evaluate ASAC. Virologic failure and second-line antiretroviral therapy in children in South Africa--the IeDEA Southern Africa collaboration. Journal of acquired immune deficiency syndromes. 2011;56(3):270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–89. [DOI] [PubMed] [Google Scholar]
- 23.Iroha E, Esezobor CI, Ezeaka C, Temiye EO, Akinsulie A. Adherence to antiretroviral therapy among HIV-infected children attending a donor-funded clinic at a tertiary hospital in Nigeria. Afr J AIDS Res. 2010;9(1):25–30. [DOI] [PubMed] [Google Scholar]
- 24.Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. BMC pediatrics. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lange CG, Lederman MM, Medvik K, Asaad R, Wild M, Kalayjian R, Valdez H. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. Aids. 2003;17(14):2015–23. [DOI] [PubMed] [Google Scholar]
- 26.Kara Wools-Kaloustian Irene Marete, Ayaya Samuel, Sohn Annette H., Lam Van Nguyen Shanshan Li, Leroy Valériane, Musick Beverly S., Newman Jamie E., Edmonds Andrew, Davies Mary-Ann, François Tanoh Eboua Marie-Thérèse Obama, Yotebieng Marcel, Sawry Shobna, Mofenson Lynne M., Yiannoutsos Constantin T. (2018) Time to First-Line ART Failure and Time to Second-Line ART Switch in the IeDEA Pediatric Cohort, JAIDS Journal of Acquired Immune Deficiency Syndromes, p. 1, url, doi: 10.1097/QAI.0000000000001667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World health organisation Global health sector response to HiV,2000–2015 :focus on Innovations in Africa: progress report [Internet].2015. [cited 2016 September 5]http://www.who.int/hiv/pub/progressreport/2015-progress-report/en/
- 28.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90–90–90: an ambitious treatment target to help end the AIDS epidemics. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf. Accessed Oct 2014.
- 29.Fokam J, Sosso SM, Yagai B et al. Viral suppression in adults, adolescents and children receiving antiretroviral therapy in Cameroon: adolescents at high risk of virological failure in the era of “test and treat”. AIDS Res Ther 16, 36 (2019). 10.1186/s12981-019-0252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nega J, Taye S, Million Y et al. Antiretroviral treatment failure and associated factors among HIV patients on first-line antiretroviral treatment in Sekota, northeast Ethiopia. AIDS Res Ther 17, 39 (2020). 10.1186/s12981-020-00294-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulugeta A, Assefa H, Tewelde T, Dube L. Determinants of survival among HIV positive children on antiretroviral therapy in public hospitals, Addis Ababa, Ethiopia. Qual Prim Care. 2017;25(4):235–41 [Google Scholar]
- 32.Wamalwa DC, Obimbo EM, Farquhar C, Richardson BA, Mbori-Ngacha DA, Inwani I, Benki-Nugent S, John-Stewart G. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]


