Abstract
Prior single center or registry studies have shown that living donor liver transplantation (LDLT) decreases waitlist mortality and offers superior patient survival over deceased donor liver transplantation (DDLT). The aim of this study was to compare outcomes for adult LDLT and DDLT via systematic review. A meta-analysis was conducted to examine patient survival and graft survival, MELD, waiting time, technical complications, and postoperative infections. Out of 8600 abstracts, 19 international studies comparing adult LDLT and DDLT published between 1/2005–12/2017 were included. U.S. outcomes were analyzed using registry data. Overall, 4,571 LDLT and 66,826 DDLT patients were examined. LDLT was associated with lower mortality at 1, 3, and 5 years post-transplant [5-year HR 0.87 (95% CI 0.81–0.93), p<0.0001], similar graft survival, lower MELD at transplant (p<0.04), shorter waiting time (p<0.0001), and lower risk of rejection (p=0.02), with a higher risk of biliary complications (OR 2.14, p<0.0001). No differences were observed in rates of hepatic artery thrombosis. In meta-regression analysis, MELD difference was significantly associated with post-transplant survival (R2 0.56, p=0.02). In conclusion, LDLT is associated with improved patient survival, less waiting time, and lower MELD at LT, despite posing a higher risk of biliary complications that did not affect survival post-transplant.
Introduction
With an ongoing shortage of deceased donor organs, living donor liver transplantation (LDLT) has emerged as an option to reduce waitlist mortality and address the growing disparity between organ supply and demand. As programs have gained experience, LDLT has been shown to result in equivalent, and in some cases, superior recipient survival and long-term outcomes compared to deceased donor liver transplantation (DDLT), even following risk-adjustment 1,2. LDLT also conveys the benefits of decreased mortality on the waitlist, reduced waiting time, and potential for transplantation at a lower Model for End-Stage Liver Disease (MELD) score 1,3.
Despite the potential for good outcomes, LDLT has constituted less than 5% of all liver transplants performed in the U.S. and <30% of all liver transplants in the Americas and Europe 4,5. Concerns regarding donation-related complications and outcomes following living liver donation may have slowed the expansion of LDLT in the Western hemisphere. Long-term follow up of the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) cohort involving 740 donors showed that 40% experienced one or more complication, primarily Clavien-Dindo Grade 1 and 2, 95% of which resolved within the first-year post-donation 6. In a recent Scientific Registry for Transplant Recipients (SRTR) analysis, among 105 non-directed living liver donors, only 15% experienced a post-operative complication or needed hospital readmission after donation, further demonstrating that the risk for living donors is generally low 7.
In the early era of LDLT, technical complications including biliary stricture or leak, hepatic artery thrombosis (HAT), and small-for-size syndrome impacted post-transplant outcomes 8–11. More recently, these early post-LDLT complications, while recognized to be higher than DDLT, have largely been mitigated by center experience and patient selection 12–15. Generally, studies examining LDLT outcomes and complications, even in the contemporary era, have been limited to single-center and/or national registry studies and have recognized limitations including differences in center experience, transplant recipient demographics, and duration of follow-up 2,12,16.
Even in the contemporary era, the experience and outcomes of LDLT continues to be differentiated between lower volume, Western hemisphere countries and high-volume programs from the Middle East and Asia who rely on LDLT to overcome cultural and religious barriers to DDLT 2,12,16,17. Previous meta-analyses have compared outcomes of LDLT and DDLT as it relates to biliary complications or hepatocellular carcinoma (HCC), focusing on patient survival and risk of disease recurrence 18–22.
To date, a collective, global analysis of outcomes comparing LDLT and DDLT has not been completed. The aim of this study was to compare outcomes of LDLT to DDLT by performing a systematic review, meta-analysis, and meta-regression of patient survival, graft survival, and pre- and post-transplant outcomes.
Experimental Methods
Literature Search and Study Selection:
This systematic review was performed according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and as outlines in a predefined protocol (PROSPERO 2018: CRD42018104794) 23. A health sciences librarian developed the search strategy and searched the following databases on March 28, 2018: PubMed (coverage 1946 – Present), Embase and Embase Classic (coverage 1947 – Present), Cochrane Library (coverage 1898–present), Web of Science (coverage 1900-present), Clinicaltrials.gov, and Google Scholar. No filters were applied for date, study type, language, or any other limit. A combination of subject headings (when available) and keywords were used for the concepts living donor, deceased donor, and liver transplantation. See Supplemental Table 1 for full search strategies and database details. Duplicated citations were removed in EndNote x9.2 using the Bramer method 24. Cross-referencing and forward searches of articles fulfilling inclusion criteria were performed using Web of Science.
Study Selection:
Screening was independently performed by two authors. Any conflict regarding study inclusion was resolved by the senior author. Studies were included if they were published between January 2005 and December 2017, available in full text, compared LDLT and DDLT cohorts, studied transplant recipients ≥18 years of age, and reported on the primary outcome of overall patient survival at ≥1-year post-transplant. A study was excluded if it was limited to <10 patients, did not include DDLT as a reference group, did not differentiate pediatric recipients from adults, did not report patient demographical information or pre-transplant characteristics, or did not describe its methods of statistical analysis. Studies including multi-organ transplants, re-transplants, and those reporting only acute liver failure were also excluded.
At the outset, we anticipated that we would include A2ALL data. The most recent comprehensive analyses of A2ALL recipient outcomes include data from ~1000 LDLT and ~500 DDLT recipients from 11 U.S. centers and Toronto, performed between 1998–2010 25,26. While both studies reported primary outcomes of graft and patient survival, neither included the majority of the secondary outcomes formatted for meta-analysis. Based on the Cochrane Handbook for Systemic Review of Interventions, we ultimately excluded the A2ALL papers and other U.S. single center papers and instead performed a larger, more contemporary SRTR analysis to represent U.S. outcomes, with 2,750 LDLT and 58,120 DDLT performed between 2005–2017 27. Two studies from the Toronto collectively describing 193 LDLT and 273 DDLT patients transplanted between 2001–2014 were also included, which reported both primary outcome measures and data related to all secondary outcomes 28,29. Using this approach, we have captured all of the A2ALL centers in this meta-analysis.
SRTR:
A primary, up-to-date analysis of the U.S. SRTR registry data was completed to supplement what is presented in the annual data report, with the intent of including primary and secondary outcomes of interest 5. For details on the SRTR data and analysis, please refer to Supplemental Data. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Data Extraction and Outcome Measures:
Data extraction from eligible studies was independently conducted by two authors. For all studies, data regarding study design and characteristics (year of publication, first author, country), population characteristics (sample size for each patient cohort, recipients and donor demographics, MELD at transplant), and liver disease diagnosis were recorded when available. The primary study outcome was 1-, 3-, and 5-year patient survival. Secondary outcomes included 1-, 3-, and 5-year graft survival; pre-operative variables (MELD score and time on waiting list); and post-operative variables (biliary complications, HAT, infection, rejection, and length of stay).
Assessment of Risk Bias:
The assessment for risk of bias was independently carried out by two authors. The NIH Quality Assessment Tool for Case-Control Studies was adopted to evaluate the quality of each included study. Based on the overall score, each study was classified as good (scored 9 or higher), fair (scored between 5 and 8) or poor (lower than 5) (Supplemental Table 4).
Statistical Analysis:
For the meta-analysis, percentage and total numbers were used to report categorical variables and mean with standard deviation (SD) for continuous variables. When included studies reported median and interquartile range, mean and SD were estimated according to established methods 30. For pooled analyses, all variables reported in ≥5 studies were analyzed. Continuous variables were analyzed by Mean Difference (MD), whereas categorical variables were analyzed by Odds Ratio (OR), both with 95% Confidence Intervals (CI). Random-effects model was adopted to balance intrinsic heterogeneity and effect size.31 Heterogeneity was also assessed with chi-square statistic and I2 statistic with I2,>= 50% representing significant heterogeneity. The hazard ratio (HR) for time-to-event outcomes was estimated indirectly from other summary statistics or from data in published Kaplan-Meier curves 32. The derived observed minus expected number of cases (O-E) and the variance for the single studies were then used to calculate individual and overall HR with the fixed-effect model to give a pooled HR for survival analyses 33. Forest plots were created to display results. All data analyses were conducted using RevMan 5.3 according to published guidelines 27. A random effects meta-regression analysis was conducted to better understand potential sources of heterogeneity of the primary outcome, specifically 1-year overall patient survival. The selection of covariates to include as moderator in the meta-regression model was based on their clinically likelihood to modify the outcome of interest and possible statistically significant different distributions between LDLT and DDLT patients that resulted from the meta-analysis. Meta-regression analysis was conducted using Metafor-package for R studio (version 3.6.3).
Results
Systematic Review
The literature review is summarized in a PRISMA diagram (Figure 1). After removal of duplications, 5364 abstracts were screened and 374 were selected for full-text review. A total of 19 studies from countries including Canada, China, France, Germany, South Korea, Italy, and Saudi Arabia were included in this meta-analysis (summarized in Table 1). Seventeen studies were from single centers and two included multicenter data. All studies but one were retrospective, while three had a matched-pair design and one was prospective. No randomized controlled studies were identified. The quality risk assessment for these studies determined that all met criteria for fair or good quality, and none showed poor design (Supplemental Table 4).
Figure 1:
PRISMA diagram of Systematic Review.
Table 1.
Characteristics of included studies and patient populations stratified by donor type.
| Studies: Author, Year, Country | Study design | Arms | Sample size | Age, Years (mean ±SD) | Sex, No. Female (%) | MELD at Transplant (mean ± SD) | Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCC | NASH | HCV/ HBV | ALD | PSC/ PBC/ AIH | |||||||
| Barbas, 2017, Canada28 | Retrospective study | LDLT | 48 | 54.7±9.4 | 13(27) | 17.8±8.7 | 8 | 48 | - | - | - |
| DDLT | 128 | 56.7±9.3 | 41(32) | 21.8±10.3 | 42 | 128 | - | - | - | ||
| Reichman, 2013, Canada29 | Matched cohort study | LDLT | 145 | 54.2±7.5 | 28(19.3) | 14.4±3.8 | 55 | 4 | 99 | 26 | 16 |
| DDLT | 145 | 53.9±7.7 | 28(19.3) | 14±6.8 | 80 | 4 | 99 | 26 | 16 | ||
| Chen, 2015, China63 | Retrospective study | LDLT | 66 | 45.8±7.7 | 6(9.1) | 11.1±4.5 | 66 | ||||
| DDLT | 163 | 47.9±9.5 | 19(11.7) | 12±6.4 | 163 | ||||||
| Lei, 2013, China64 | Retrospective study | LDLT | 31 | 44.4±9.7 | 13(41.9) | 9.3±6.1 | 31 | 28 | |||
| DDLT | 52 | 44±8.2 | 21(40.4) | 9.1±5.8 | 52 | 45 | |||||
| Li, 2011, China34 | Retrospective study | LDLT | 128 | 43±8.6 | 20(15.6) | 19.5±10.7 | 116 | 2 | 1 | ||
| DDLT | 221 | 44.5±9.7 | 42(19) | 18.2±9.6 | 209 | 5 | 5 | ||||
| Chok, 2017, China65 * | Retrospective study | LDLT | 54 | 51±12 | 12(22.2) | 40±1.3 | 1 | 43 | 1 | ||
| DDLT | 40 | 51±10.8 | 6(15) | 39±1.3 | 3 | 36 | |||||
| Liu, 2006, China43 * | Prospective study | LDLT | 124 | 47.5±8.3 | 27(21.8) | 21±6.5 | 36 | 111 | 1 | 3 | |
| DDLT | 56 | 48±9.8 | 12(21.4) | 19±10.8 | 11 | 49 | 0 | 1 | |||
| Wan, 2014, China37 | Matched cohort study | LDLT | 40 | 48.6±9.7 | 6(15) | 40 | 39 | ||||
| DDLT | 80 | 49.5±8.9 | 12(15) | 80 | 77 | 1 | 1 | ||||
| Chen, 2014, China66 | Matched cohort study | LDLT | 47 | 3(6.4) | 47 | ||||||
| DDLT | 94 | 6(6.4) | 94 | ||||||||
| Hu et al, 2015, China38 | Multi-center Retrospective study | LDLT | 389 | 48.1±8.7 | 29(7.5) | 389 | |||||
| DDLT | 6471 | 50.1±9.4 | 652(10.1) | 6471 | |||||||
| Bhangui, 2011, France36 | Retrospective study | LDLT | 36 | 54±7 | 4(11.1) | 13.5±5.9 | 36 | 28 | 6 | ||
| DDLT | 120 | 56±8 | 20(14.7) | 14.5±5.9 | 120 | 88 | 26 | ||||
| Schmeding, 2007, Germany67 | Retrospective study | LDLT | 20 | 55.7±8.9 | 7(35) | 11 | 20 | ||||
| DDLT | 269 | 51.4±9.8 | 105(39) | 73 | 269 | ||||||
| Kim, 2014, Korea68 | Retrospective study | LDLT | 21 | 53.1±10.3 | 7(33.3) | 13.1±5.4 | 17 | 18 | 3 | 0 | |
| DDLT | 29 | 51.3±9.2 | 14(48.3) | 24.9±11.6 | 11 | 14 | 6 | 3 | |||
| E. Kim, 2017, Korea69 | Retrospective study | LDLT | 109 | 52±8.5 | 28(26.6) | 12.5±8.3 | 68 | 93 | 19 | 1 | |
| DDLT | 76 | 53.2±11 | 26(34.2) | 24.9±11.7 | 16 | 40 | 21 | 4 | |||
| J.M. Kim, 2017, Korea70 * | Multi-center retrospective study | LDLT | 146 | 57±6.3 | 42(28.8) | 15±5.7 | 73 | 146 | |||
| DDLT | 35 | 53±8.8 | 11(31.4) | 21±10.5 | 11 | 35 | |||||
| Lee, 2012, Korea41 | Retrospective study | LDLT | 48 | 50±7.8 | 8(16.7) | 24.5±4.4 | 12 | 42 | 4 | ||
| DDLT | 23 | 48±12.9 | 10(43.5) | 23±3 | 6 | 16 | 2 | ||||
| Vigano’, 2008, Italy71 | Retrospective study | LDLT | 77 | 24 | 57 | ||||||
| DDLT | 244 | 75 | 143 | ||||||||
| Al Sebayel, 2015, Saudi Arabia72 * | Retrospective Study | LDLT | 222 | 53±10.8 | 83(37.4) | 18 | 45 | 120 | 24 | ||
| DDLT | 269 | 52±10.2 | 116(52.3) | 16 | 48 | 139 | 32 | ||||
| Jiang, 2013, China73* | Retrospective study | LDLT | 70 | 40.3±8.2 | 8(11.4) | 23.9±11.1 | 70 | ||||
| DDLT | 191 | 44.1±9.3 | 29(15.2) | 21.7±9.9 | 191 | ||||||
| SRTR, 2017, USA | Retrospective study | LDLT | 2750 | 51.9±12.3 | 1200(43.6) | 15±5.3 | 340 | 611 | |||
| DDLT | 58120 | 54.8±9.6 | 18120(31.2) | 21±9.9 | 12163 | 15673 | |||||
Denotes median to mean conversion or calculated SD
Meta-Analysis
A total of 1,821 LDLT and 8,706 DDLT recipients were pooled from the published studies for inclusion in the meta-analysis; study and patient population characteristics are summarized in Table 1. When U.S. SRTR data were added, 4,571 LDLT and 66,826 DDLT recipients were analyzed. For the entire study population, the mean age was 54.0±9.9 years (51.2±11.4 for LDLT vs 54.2±9.7 for DDLT, p<0.001) and 29.6% were female (33.8% of LDLT vs 28.9% of DDLT, p<0.001). The most common etiology of liver disease was hepatocellular liver disease (autoimmune hepatitis, NASH or alcoholic liver disease; collectively 34.6%), followed by HCC (29.2%), viral hepatitis (26.3%), and cholestatic liver disease (7.7%).
Examination of our first primary outcome, patient survival, revealed superior overall patient survival for LDLT recipients when compared to the DDLT recipients (p<0.0001, Figure 2). Specifically, LDLT recipients had a 17% reduction [95% CI 10–24] in the risk of mortality at 1-year post-transplant when compared to the DDLT group [HR 0.83 [95% CI 0.76–0.90]; p<0.0001, Fig. 2A). The survival benefit for LDLT recipients was also observed at both 3- and 5-years post-transplant (3 year: HR 0.85 [95% CI 0.79–0.92] and 5 year: HR 0.87 [95% CI 0.81–0.93], p<0.0001 at both intervals, Fig. 2B and 2C). Graft survival was studied as a secondary outcome. At all time points, graft survival was comparable between LDLT and DDLT recipients (1 year: HR 0.94 [95% CI 0.84–1.02], p=0.14, 3-year: HR 0.96 [95% CI 0.89–1.03] p=0.25, and 5 year: HR 0.95 [95% CI 0.88–1.01], p=0.12] (Figure 3).
Figure 2: Forest plot of Hazard Ratios for overall patient survival at 1 year (a), 3 years (b), and 5 years (c) post-transplant.
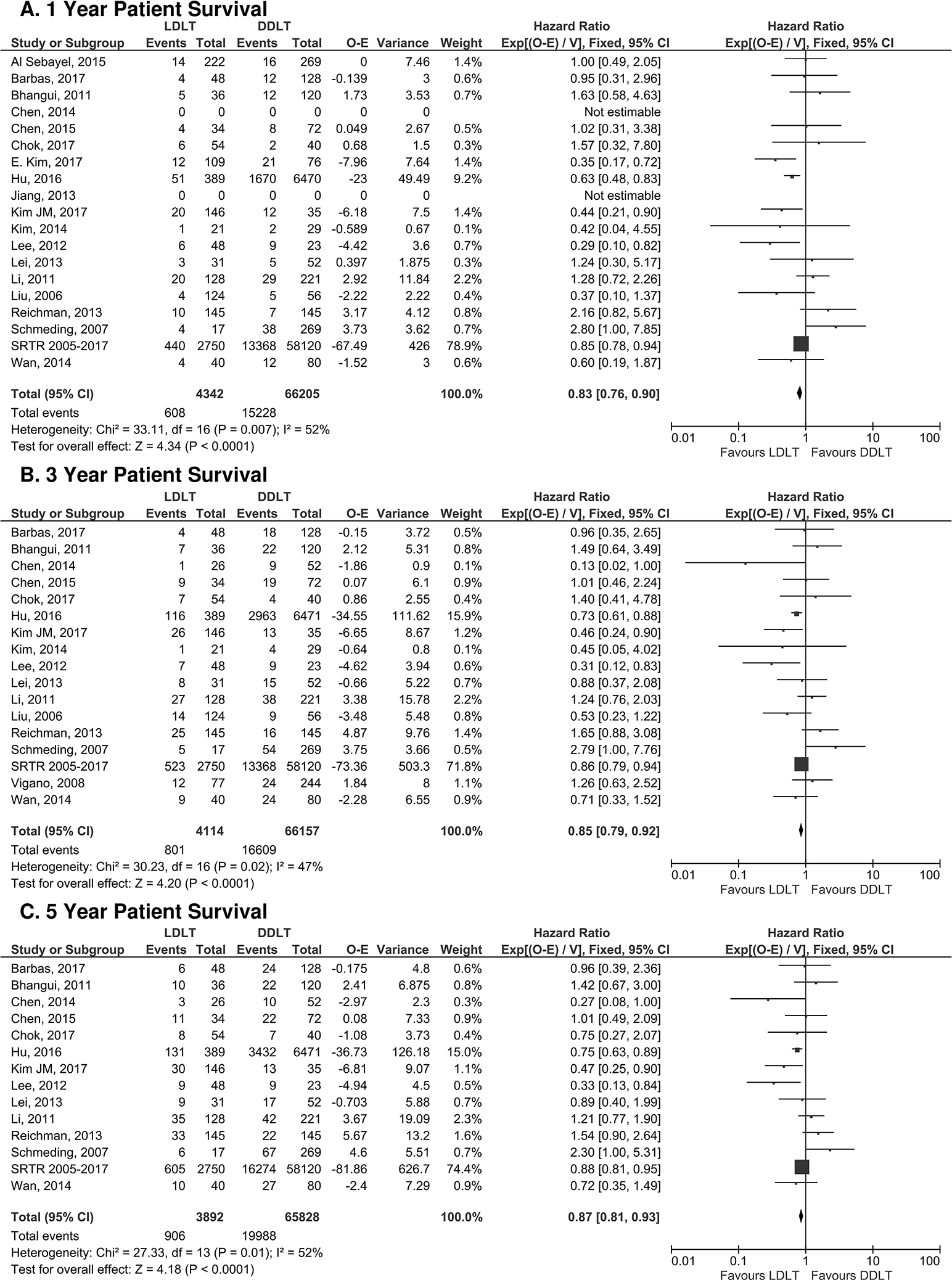
LDLT favored patient survival when compared to DDLT at all time points.
Figure 3: Forest plot of hazard ratios for overall graft survival at 1 year (a), 3 years (b), and 5 years (c) post-transplant.
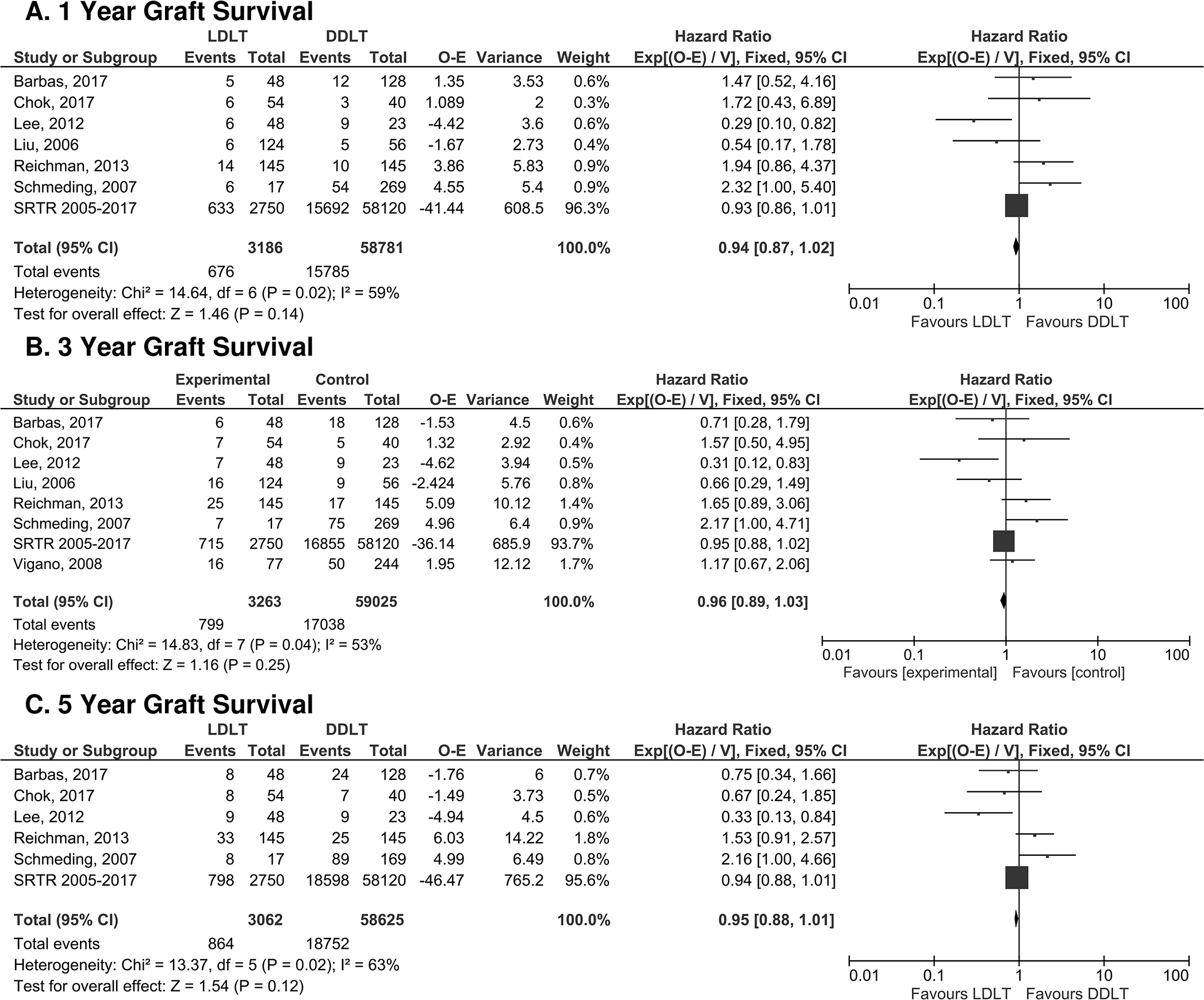
LDLT and DDLT had equivalent graft survival at 1-, 3- and 5-years post-transplant.
Next, secondary outcomes were analyzed among sub-cohorts of studies that included the specified variables. Two pre-operative outcomes were studied: MELD score at transplant and waiting time (days). As shown in Figure 4A, MELD score at transplant was lower for LDLT recipients when compared to DDLT recipients [MD −2.54 [95% CI −5.02, −0.06] p=0.04]. LDLT recipients had a shorter waiting time when compared to DDLT recipients (MD −71.43 [95% CI −101.42, −41.44], p<0.0001, Figure 4B). Post-operative technical complications including HAT and biliary complications were analyzed. While there was no difference between the two groups in the risk of HAT (OR 2.07 [95% CI 0.84–5.09], p=0.11, Fig. 5A), LDLT recipients experienced an approximately two-fold increase in the risk of biliary complications [OR 2.14 [95% CI 1.76–2.59], p<0.001, Fig. 5B]. Pooled analysis for the risk of post-operative infection and length of hospital stay showed no difference between LDLT and DDLT recipients (OR 0.67 [95% CI 0.42–1.09], p=0.11 (Fig. 5C) and MD −3.80 [95% CI −8.36, 0.76], p=0.10 (Fig. 5D), respectively). Finally, LDLT recipients showed a lower risk of rejection when compared to DDLT recipients (OR 0.72 [ 95% CI 0.55–0.95], p=0.02, Fig. 5E).
Figure 4: Forest plot of pre-operative variables.
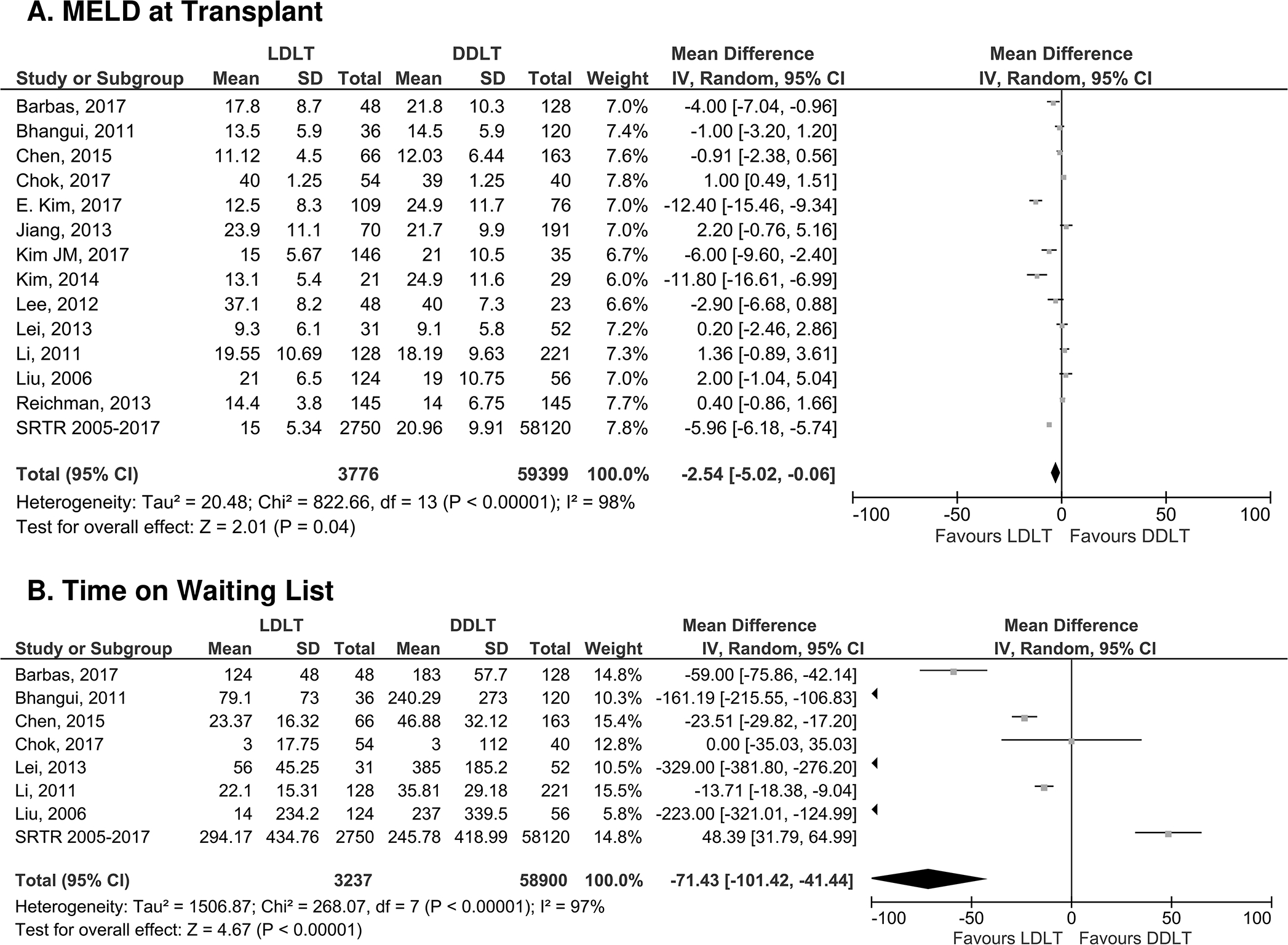
Panel A: MELD at transplant and Panel B: Time on Waiting List. LDLT favored lower MELD at transplant and less time on the waiting list.
Figure 5: Forest plot of post-operative variables.
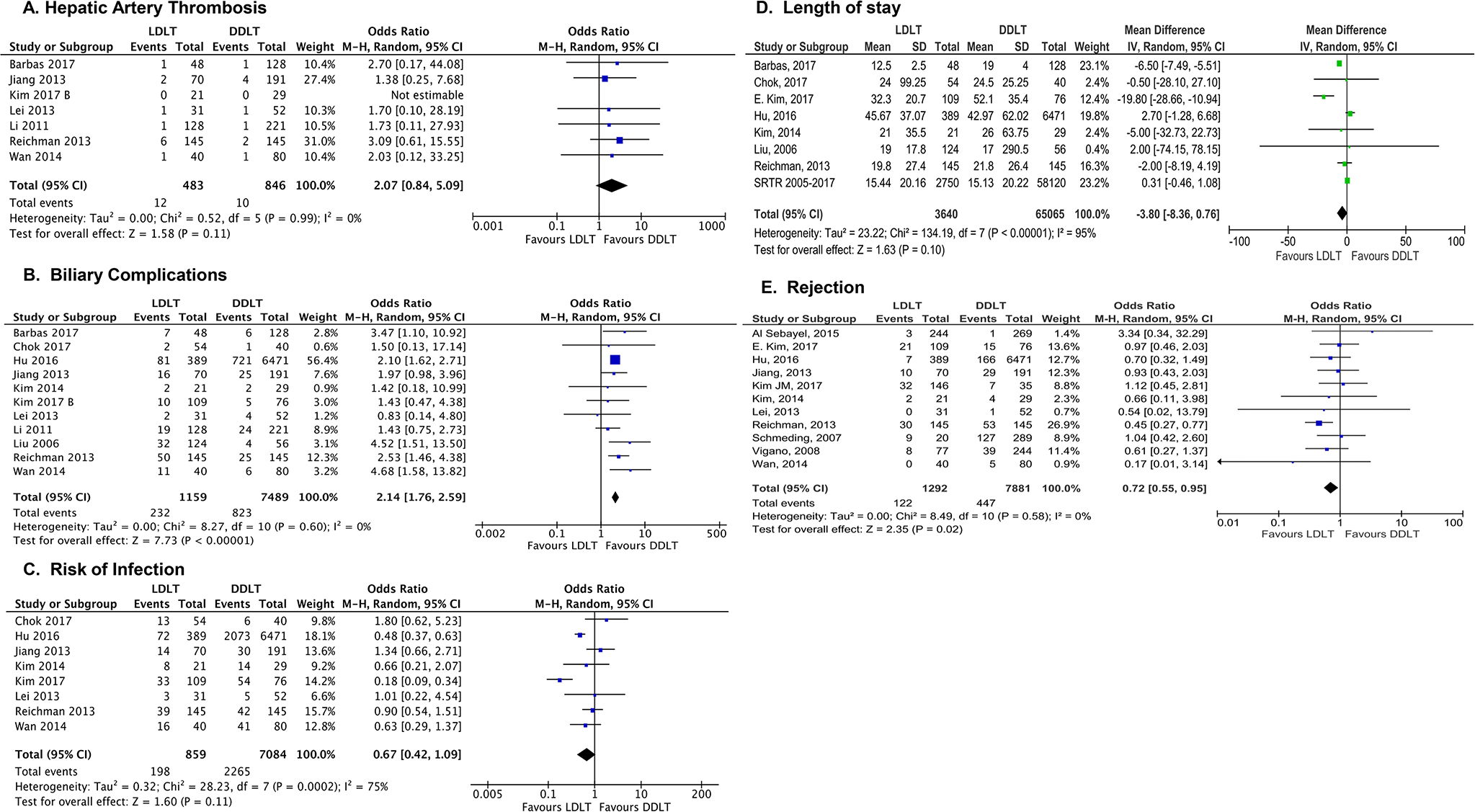
Panel A: Hepatic Artery Thrombosis, Panel B: Biliary Complications, Panel C: Risk of Infection, Panel D: Length of Stay, Panel E: Rejection rate. LDLT was equivalent to DDLT for rates of post-operative HAT (A), infections, and length of stay (D). LDLT were more likely to have biliary complications (B) and had a lower risk of rejection when compared to DDLT (E).
Meta-Regression Analysis
A meta-regression analysis was completed to explore potential relationships between MELD at transplant, time on waitlist and biliary complications and 1-year patient survival (Table 2). MELD score and time on waitlist were expressed as weighted mean differences between LDLT and DDLT means, whereas biliary complications were expressed as difference of rate of occurrence in the LDLT versus DDLT. MELD score at LT was the sole variable that demonstrated a relationship with 1-year patient survival (Figure 6). These data indicate that as MELD score difference increased, survival at 1-year post-LT decreased. Time on waitlist and biliary complications had no impact on 1-year patient survival. The inclusion of MELD score as a moderator in the meta-regression of 1-year patient survival explained most of the observed heterogeneity in the relative risk of death (R2 0.56, p=0.02, Fig. 6).
Table 2: Results of meta-regression analysis of MELD difference, waiting time, and post-LT biliary complications on 1-year overall patient survival.
Residual τ2 indicates whether, after including each moderator, heterogeneity exists due to the covariate being examined.
| Outcome Measure | Relative Risk [95% CI] | Residual τ2 | p- value |
|---|---|---|---|
| 1-year patient survival | |||
| MELD difference -5.5 | 0.67 [0.51, 0.87] | 0.0515 | 0.02 |
| Time on Waitlist | 0.94 [0.55,1.61] | 0.0321 | 0.9 |
| Biliary complications | 0.83 [0.58,1.20] | 0.0976 | 0.21 |
Figure 6. Random effects meta-regression.
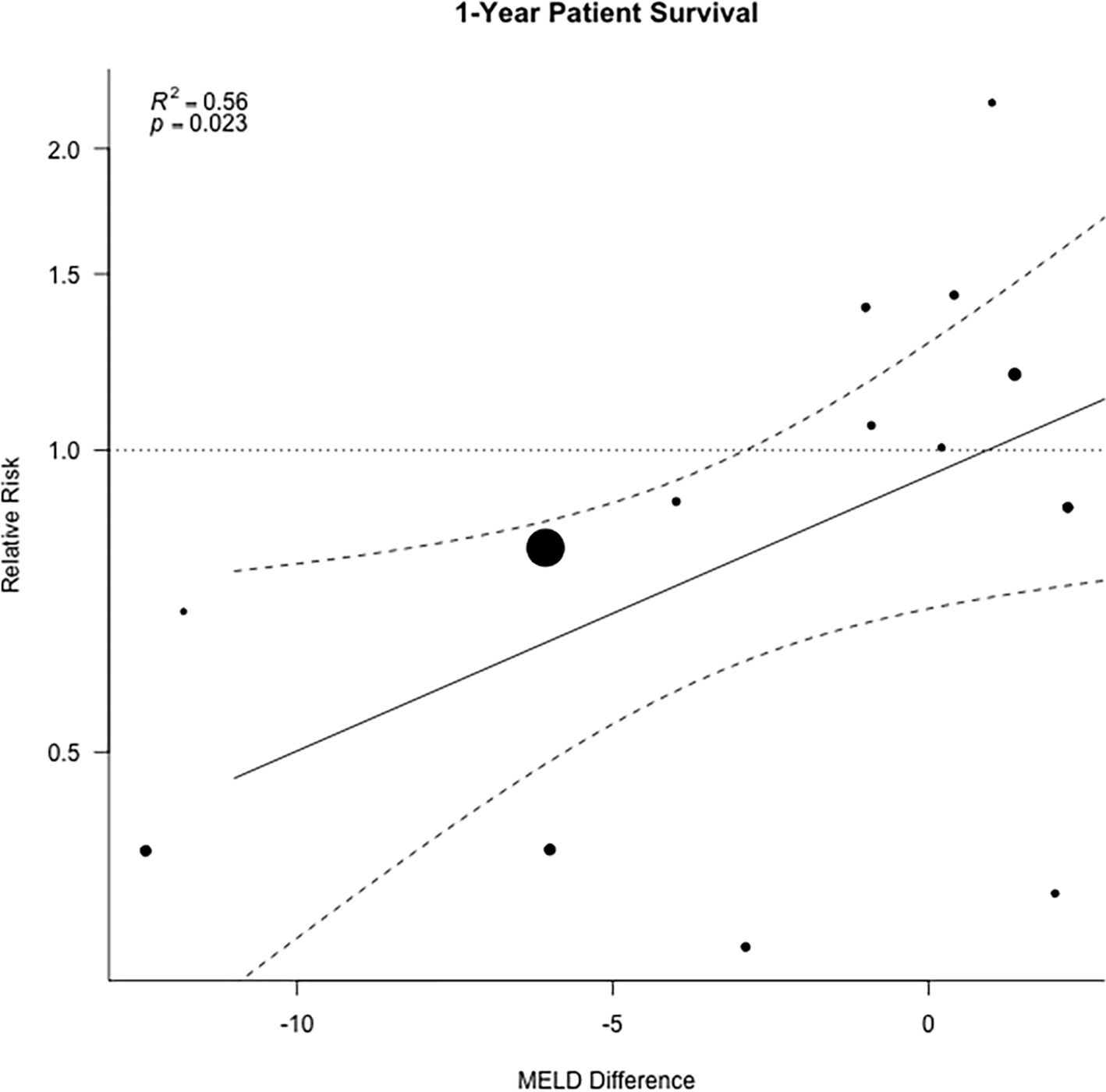
showing how results of meta-analysis examining 1-year patient survival are influenced by the difference in MELD score between LDLT and DDLT. Each dot represents an individual study, the solid line represents the regression prediction, and the dotted lines the 95% Confidence intervals.
Discussion
This meta-analysis identified and analyzed a global population of 4,571 LDLT and 66,826 DDLT recipients across a broad range of liver disease diagnoses, programs, and countries. The results confirm that LDLT recipients experience superior patient survival at 1-, 3-, and 5-years post-transplant when compared to DDLT recipients. LDLT resulted in equivalent graft survival when compared to DDLT at all time points. Pre-operative MELD and waiting time favored LDLT recipients, and lower MELD at transplant was strongly associated with post-transplant survival on meta-regression. Moreover, despite a higher rate of biliary complications, LDLT recipients had a similar rate of HAT, risk of postoperative infection, and overall length of hospital stay and less rejection when compared to DDLT. Collectively, these data suggest that LDLT can offer several advantages when compared to DDLT.
The primary outcome of this meta-analysis, overall patient survival, identified a reduced risk of mortality of 17%, 15% and 13% at 1-, 3- and 5-years post-transplant respectively for LDLT recipients (Fig. 2). Prior single center or consortium studies have also suggested that LDLT confers an overall survival advantage 34–38. This finding is likely multifactorial, as shown by analysis of secondary outcomes, specifically pre-operative variables indicating that LDLT recipients experience a shorter waiting time and are transplanted at a lower MELD (Fig. 4). Indeed, meta-regression examining the correlation between MELD at transplant and patient survival confirmed a strong relationship exists (Fig. 6). Other factors that likely contribute to superior outcomes for LDLT were not studied by this analysis. Generally, LDLT is an elective surgery and thus programs have the opportunity to screen and choose an ideal donor, schedule the procedure for the daytime with a highly specialized team, plan for anatomic variants, and optimize a recipient for surgery. Furthermore, a living donor allograft is not exposed to brain death, which may negatively affect both graft and patient survival 39,40.
Analysis of the first secondary outcome, overall graft survival, demonstrated that graft survival is comparable between LDLT and DDLT for all time points (Fig. 3). This is an important finding, as it suggests that the risk for early graft loss for DDLT and LDLT are equivalent. That being said, the risk profile for each type of donor is different. LDLT is a highly technical procedure, and as a consequence, poses a greater risk for procedure-related complications including vascular complications, biliary stricture or leak, early allograft dysfunction, or ultimately early graft loss requiring re-transplant. In countries with a predominant LDLT experience and thus lower rate overall rate of technical complications, such as Japan or Korea, national registry data have shown that 1-year graft survival modestly favors DDLT over LDLT 12,16. Prior studies have reported variable outcomes for graft survival, ranging from equivalence between LDLT and DDLT, to improved graft survival for LDLT when compared to DDLT 13,29,38,41–43.
Our analysis established that LDLT recipients had a lower MELD at transplant when compared to DDLT recipients, and this was associated with improved survival rates on meta-regression. This is consistent with the North American A2ALL cohort, which reported a lower MELD at transplant for LDLT recipients, with only 16% of LDLT recipients with MELD >20 at the time of transplant compared to 43% of DDLT recipients 2. While LDLT candidates benefit from being transplanted at a lower MELD, studies have reported acceptable outcomes following LDLT even for higher MELD patients. A prior study comparing LDLT and DDLT with MELD >30 showed an improved overall patients survival for LDLT, even for patients with hepatorenal syndrome 41. Similarly, single center studies from Taiwan and India have demonstrated that 5-year overall survival for LDLT with MELD >30 is comparable to the outcome in patients with MELD <30 44,45.
A second pre-operative variable that may influence patient survival is time on the waiting list. Even when including U.S. data, which showed a modestly longer waiting time for LDLT recipients, our comprehensive meta-analysis confirmed an overall shorter waiting time for LDLT recipients, which was not associated with overall survival on meta-regression (Fig. 4 and Table 2). Specific factors contributing to longer waiting time for LDLT recipients in the U.S. were beyond the scope of our study, but it is likely that variable local access to LDLT in different states and additional time for LDLT referral and donor evaluation are involved. Shorter waiting time for LDLT recipients may specifically benefit patient populations that may be disadvantaged in current allocation schemes: children, women, and patients with HCC 46–48.
LDLT was associated with an increased incidence of arterial complications in the early era 49,50. However, in this meta-analysis, no difference in risk of HAT was observed between LDLT and DDLT recipients. Studies from high volume centers have confirmed this finding, as the rate of vascular complications has decreased over time, presumably as surgeons have gained experience and in some cases considered microvascular reconstruction 13,15,51–53. A single center analysis of risk factors associated with HAT identified prolonged anastomosis time, perioperative blood transfusion, and graft to recipient weight ratio >1.15% as risk factors for early HAT 54. One shortcoming of our analysis was the inability to effectively track HAT in the SRTR, and thus U.S. data was not included in examination of this variable.
Even with experience, early biliary complications are the recognized ‘Achille’s heel’ in LDLT. Our meta-analysis confirmed that the risk of biliary complication was approximately two-fold higher in the LDLT group; however, there was no difference in graft survival between LDLT and DDLT and biliary complications did not negatively impact survival on meta-regression. A recent study from an experienced Japanese program reported a rate of biliary complications in LDLT of 17.3% and observed that multiple bile duct anastomoses and recurrent cholangitis prior to transplant were risk factors for biliary stricture or leak 9. Our results are supported by a prior systematic review of biliary complications following LT, which identified MELD ≥35, multiple bile ducts, prolonged cold ischemic time, post-operative bile leak, and HAT as risk factors for biliary stricture for LDLT recipients on multivariable analysis 19.
Post-operative infections and length of stay were similar among LDLT and DDLT in this meta-analysis. Prior single center studies have reported a higher incidence of bacterial infection in DDLT when compared to LDLT 37,38,55. A Korean study identified receipt of a deceased donor allograft as an independent risk factor for post-operative infection (OR 5.5 [95% CI: 2.4–12.3]) 56. Length of stay is a difficult metric to study across different geographic regions, as practice patterns vary considerably. Even with regional variation, LDLT has been reported to be associated with a shorter length of stay in Canada (19 vs. 22 days), the U.S. (11 vs. 13 days), and China (42 vs. 45 days) 13,29,38.
This meta-analysis confirms that LDLT recipients have a lower risk of rejection when compared to DDLT (Fig. 5E). Single center studies have shown that LDLT recipients experience a lower rate of biopsy proven rejection at 24-months post-LT compared to DDLT recipients 57,58. It has been postulated that prolonged cold ischemic time and exposure to the physiology of brain death can lead to inflammatory cell recruitment into the allograft, thereby disrupting liver immune homeostasis, a phenomenon that is reduced in LDLT 59. A more recent study analyzing both A2ALL and OPTN data reported a lower risk of biopsy-proven acute rejection among biologically related LDLT when compared to nonbiologically related LDLT and DDLT recipients, and more importantly, acute rejection was associated with increased risk of graft failure and death 60. Thus, an additional factor that may relate to superior patient survival over time following LDLT is the lower rate of rejection episodes.
There are limitations to our study. By design, we required that eligible studies included a comparison cohort. As a consequence, studies from centers that exclusively performed either LDLT or DDLT were not included. While all available studies reporting outcomes of LDLT versus DDLT were included, data were screened by center to exclude studies that may have contained overlapping patient cohorts. The majority of the included studies were retrospective, and no randomized controlled studies were available. While 20 studies representing four continents were included, the U.S. data represents >50% of the LDLT and DDLT cohorts, which may have impacted some of the results. There were also inherent differences between LDLT and DDLT recipients in terms of age, sex, and etiology of underlying liver disease, that may have impacted our findings. Neither the SRTR analysis nor all studies examined reported on each of the secondary outcomes, potentially introducing bias and affecting the analysis. In particular, rejection, biliary and vascular complication are not consistently reported in the SRTR, limiting the possibility of including those data on analysis of secondary outcomes in this study. Additionally, there was heterogeneity among the studies, reflecting the differences in practice, protocols, and possibly in outcomes. Lastly, per our study design, some outcomes were not considered, such as graft size or volume, technical details including anatomic variants, or the recurrence of disease and its impact on patient outcome.
Conclusion
In summary, this meta-analysis and meta-regression confirms that LDLT provides superior overall patient survival when compared to DDLT, regardless of region of practice, spanning patients from both the East and the West. LDLT recipients are usually transplanted with a lower MELD, spend less time on the waiting list, have a lower risk of rejection, and have a comparable risk of postoperative vascular complications and infections with an equivalent length of stay when compared to DDLT. LDLT is associated with a higher rate of biliary complications, but this does not impact overall survival.
Recently, there has been renewed interest and growth in LDLT in the U.S. However, the overall proportion continues to be well below 10% of all adult LT, and only 20 states had LDLT activity in 2019 61. As the proportion of financially vulnerable LT candidates continues to grow, a greater proportion of patients will be covered by public health insurance, which can further limit ability to travel to an out-of-state LDLT center 62. This meta-analysis supports the continued expansion of LDLT for patients with end-stage liver disease who have access to a suitable living donor, even in regions where DDLT predominates, as LDLT allows for transplant at a lower MELD score, in patients with less deteriorated health condition, and can optimize post-transplant outcomes.
Supplementary Material
Acknowledgements/ Funding:
No financial support was received for this study.
Abbreviations:
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation Study
- C.I.
Confidence Interval
- DDLT
Deceased Donor Liver Transplantation
- HAT
Hepatic Artery Thrombosis
- HCC
Hepatocellular Carcinoma
- HR
Hazard Ratio
- LDLT
Living Donor Liver Transplantation
- MELD
Model for End-Stage Liver Disease
- MD
Mean Difference
- OR
Odds Ratio
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Disclosure: The authors have of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
Supporting information statement: Additional supporting information may be found online in the Supporting Information section at the end of the article.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Goldberg DS, French B, Abt PL, Olthoff K, Shaked A. Superior survival using living donors and donor-recipient matching using a novel living donor risk index. Hepatology. 2014;60(5 PG-1717–1726):1717–1726. doi: 10.1002/hep.27307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olthoff KM, Smith AR, Abecassis M, et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann Surg. 2015;262(3 PG-465–475; discussion 473–465):465–475; discussion 473. doi: 10.1097/sla.0000000000001383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abt PL, Mange KC, Olthoff KM, Markmann JF, Reddy KR, Shaked A. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transpl. 2004;4(8 PG-1302–1307):1302–1307. doi: 10.1111/j.1600-6143.2004.00522.x [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. GENERAL INFORMATION OF THE COUNTRY. Global Observatory on Donation and Transplantation. http://www.transplant-observatory.org/. Published 2020. Accessed March 3, 2020. [Google Scholar]
- 5.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant. 2019;19:184–283. doi: 10.1111/ajt.15276 [DOI] [PubMed] [Google Scholar]
- 6.Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy--a comprehensive report. Am J Transplant. 2012;12(5):1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raza MH, Aziz H, Kaur N, et al. Global experience and perspective on anonymous nondirected live donation in living donor liver transplantation. Clin Transplant. March 2020:e13836. doi: 10.1111/ctr.13836 [DOI] [PubMed] [Google Scholar]
- 8.Moy BT, Birk JW. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J Clin Transl Hepatol. 2019;7(1):61–71. doi: 10.14218/JCTH.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyagi S, Kakizaki Y, Shimizu K, et al. Arterial and biliary complications after living donor liver transplantation: a single-center retrospective study and literature review. Surg Today. 2018;48:131–139. doi: 10.1007/s00595-017-1515-9 [DOI] [PubMed] [Google Scholar]
- 10.Lee SG. A complete treatment of adult living donor liver transplantation: A review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15(1):17–38. doi: 10.1111/ajt.12907 [DOI] [PubMed] [Google Scholar]
- 11.Goldaracena N, Echeverri J, Selzner M. Small-for-size syndrome in live donor liver transplantation—Pathways of injury and therapeutic strategies. Clin Transplant. 2017;31(2). doi: 10.1111/ctr.12885 [DOI] [PubMed] [Google Scholar]
- 12.Yoo S, Jang EJ, Yi NJ, et al. Effect of Institutional Case Volume on In-hospital Mortality after Living Donor Liver Transplantation: Analysis of 7073 Cases between 2007 and 2016 in Korea. Transplantation. 2020;103(5):952–958. doi: 10.1097/TP.0000000000002394 [DOI] [PubMed] [Google Scholar]
- 13.Humar A, Ganesh S, Jorgensen D, et al. Adult Living Donor Versus Deceased Donor Liver Transplant (LDLT Versus DDLT) at a Single Center: Time to Change Our Paradigm for Liver Transplant. Ann Surg. 2019;270(3):444–451. doi: 10.1097/SLA.0000000000003463 [DOI] [PubMed] [Google Scholar]
- 14.Gruessner RWG, Gruessner AC. Solid-organ Transplants From Living Donors: Cumulative United States Experience on 140,156 Living Donor Transplants Over 28 Years. Transplant Proc. 2018;50(10):3025–3035. doi: 10.1016/j.transproceed.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 15.Rather SA, Nayeem MA, Agarwal S, Goyal N, Gupta S. Vascular complications in living donor liver transplantation at a high-volume center: Evolving protocols and trends observed over 10 years. Liver Transplant. 2017;23(4):457–464. doi: 10.1002/lt.24682 [DOI] [PubMed] [Google Scholar]
- 16.Umeshita K, Eguchi S, Egawa H, et al. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res. 2019. doi: 10.1111/hepr.13364 [DOI] [PubMed] [Google Scholar]
- 17.de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist. 2007;12(11 PG-1321–1331):1321–1331. doi: 10.1634/theoncologist.12-11-1321 [DOI] [PubMed] [Google Scholar]
- 18.Sneiders D, Houwen T, Pengel LHM, Polak WG, Dor FJMF, Hartog H. Systematic Review and Meta-Analysis of Posttransplant Hepatic Artery and Biliary Complications in Patients Treated with Transarterial Chemoembolization before Liver Transplantation. Transplantation. 2018;102(1):88–96. doi: 10.1097/TP.0000000000001936 [DOI] [PubMed] [Google Scholar]
- 19.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24(4):379–392. doi: 10.1111/j.1432-2277.2010.01202.x [DOI] [PubMed] [Google Scholar]
- 20.Zhu B, Wang J, Li H, Chen X, Zeng Y. Living or deceased organ donors in liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. HPB. 2019;21(2):133–147. doi: 10.1016/j.hpb.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 21.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27(1):140–147. doi: 10.1111/ctr.12031 [DOI] [PubMed] [Google Scholar]
- 22.Zhang HM, Shi YX, Sun LY, Zhu ZJ. Hepatocellular carcinoma recurrence in living and deceased donor liver transplantation: A systematic review and meta-analysis. Chin Med J (Engl). 2019;132(13):1599–1609. doi: 10.1097/CM9.0000000000000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535–b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olthoff KM, Smith AR, Abecassis M, et al. Defining long-term outcomes with living donor liver transplantation in North America. Ann Surg. 2015;262(3):465–475; discussion 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samstein B, Smith AR, Freise CE, et al. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings from the A2ALL Cohort Study. Am J Transplant. 2016;16(2):594–602. doi: 10.1111/ajt.13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training. Version 6.0 (updated July 2019) Cochrane. [Google Scholar]
- 28.Barbas AS, Goldaracena N, Dib MJ, et al. Early Intervention With Live Donor Liver Transplantation Reduces Resource Utilization in NASH. Transplant Direct. 2017;3(6):e158. doi: 10.1097/txd.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichman TW, Katchman H, Tanaka T, et al. Living donor versus deceased donor liver transplantation: A surgeon-matched comparison of recipient morbidity and outcomes. Transpl Int. 2013;26(8):780–787. doi: 10.1111/tri.12127 [DOI] [PubMed] [Google Scholar]
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. 2005. doi: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borenstein M, Hedges LVHP and RH. Introduction to Meta-Analysis. New York: John Wiley & Sons Inc; 2009. [Google Scholar]
- 32.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta Blockade During and After Myocardial Infarction: An Overview of the Randomized Trials. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Mi K, Wen T fu, et al. Outcomes of Patients with Benign Liver Diseases Undergoing Living Donor versus Deceased Donor Liver Transplantation. Fung J, ed. PLoS One. 2011;6(11):e27366. doi: 10.1371/journal.pone.0027366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azoulay D, Audureau E, Bhangui P, et al. Living or Brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma. Ann Surg. 2017;266(6):1035–1044. doi: 10.1097/SLA.0000000000001986 [DOI] [PubMed] [Google Scholar]
- 36.Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: Living versus deceased donor transplantation. Hepatology. 2011;53(5):1570–1579. doi: 10.1002/hep.24231 [DOI] [PubMed] [Google Scholar]
- 37.Wan P, Zhang JJ, Li QG, et al. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: A case-matched comparison. World J Gastroenterol. 2014;20(15):4393–4400. doi: 10.3748/wjg.v20.i15.4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Z, Qian Z, Wu J, et al. ScienceDirect Clinical outcomes and risk factors of hepatocellular carcinoma treated by liver transplantation: A multi-centre comparison of living donor and deceased donor transplantation. Clin Res Hepatol Gastroenterol. 2016;40:315–326. doi: 10.1016/j.clinre.2015.08.003 [DOI] [PubMed] [Google Scholar]
- 39.Kling CE, Perkins JD, Reyes JD, Montenovo MI. Living Donation Versus Donation After Circulatory Death Liver Transplantation for Low Model for End-Stage Liver Disease Recipients. Liver Transplant. 2019;25(4):580–587. doi: 10.1002/lt.25073 [DOI] [PubMed] [Google Scholar]
- 40.De Jonge J, Kurian S, Shaked A, et al. Unique early gene expression patterns in human adult-to-adult living donor liver grafts compared to deceased donor grafts. Am J Transplant. 2009;9(4):758–772. doi: 10.1111/j.1600-6143.2009.02557.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JP, Kwon HY, Park JI, et al. Clinical outcomes of patients with hepatorenal syndrome after living donor liver transplantation. Liver Transplant. 2012;18(10):1237–1243. doi: 10.1002/lt.23493 [DOI] [PubMed] [Google Scholar]
- 42.Gavriilidis P, Tobias A, Sutcliffe RP, Roberts KJ. Survival following right lobe split graft, living- and deceased-donor liver transplantation in adult patients: a systematic review and network meta-analysis. Transpl Int. 2018;31(10):1071–1082. doi: 10.1111/tri.13317 [DOI] [PubMed] [Google Scholar]
- 43.Liu CL, Fan ST, Lo CM, et al. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg. 2006;243(3 PG-404–410):404–410. doi: 10.1097/01.sla.0000201544.36473.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon KS, Chen TH, Jeng LB, et al. A high model for end-stage liver disease score should not be considered a contraindication to living donor liver transplantation. Transplant Proc. 2012;44(2):316–319. doi: 10.1016/j.transproceed.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 45.Yadav SK, Saraf N, Saigal S, et al. High MELD score does not adversely affect outcome of living donor liver transplantation: Experience in 1000 recipients. Clin Transplant. 2017. doi: 10.1111/ctr.13006 [DOI] [PubMed] [Google Scholar]
- 46.Cullaro G, Sarkar M, Lai JC. Sex-based disparities in delisting for being “too sick” for liver transplantation. Am J Transplant. 2018. doi: 10.1111/ajt.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldaracena N, Gorgen A, Doyle A, et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol. 2019. doi: 10.1016/j.jhep.2018.12.029 [DOI] [PubMed] [Google Scholar]
- 48.de Ville de Goyet Prof J, Grimaldi C, Tuzzolino F, di Francesco F. A paradigm shift in the intention-to-transplant children with biliary atresia: Outcomes of 101 cases and a review of the literature. Pediatr Transplant. 2019. doi: 10.1111/petr.13569 [DOI] [PubMed] [Google Scholar]
- 49.Salvalaggio PR, Modanlou KA, Edwards EB, Harper AM, Abecassis MM. Hepatic Artery Thrombosis After Adult Living Donor Liver Transplantation: The Effect of Center Volume. Transplantation. 2007;84(7):926–928. doi: 10.1097/01.tp.0000281554.00247.92 [DOI] [PubMed] [Google Scholar]
- 50.Ikegami T, Hashikura Y, Nakazawa Y, et al. Risk factors contributing to hepatic artery thrombosis following living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13(2):105–109. doi: 10.1007/s00534-005-1015-y [DOI] [PubMed] [Google Scholar]
- 51.Iida T, Kaido T, Yagi S, et al. Hepatic arterial complications in adult living donor liver transplant recipients: A single-center experience of 673 cases. Clin Transplant. 2014. doi: 10.1111/ctr.12412 [DOI] [PubMed] [Google Scholar]
- 52.Li PC, Thorat A, Bin Jeng L, et al. Hepatic artery reconstruction in living donor liver transplantation using surgical loupes: Achieving low rate of hepatic arterial thrombosis in 741 consecutive recipients—tips and tricks to overcome the poor hepatic arterial flow. Liver Transplant. 2017. doi: 10.1002/lt.24775 [DOI] [PubMed] [Google Scholar]
- 53.Lee CF, Lu JCY, Zidan A, et al. Microscope-assisted hepatic artery reconstruction in adult living donor liver transplantation—A review of 325 consecutive cases in a single center. Clin Transplant. 2017. doi: 10.1111/ctr.12879 [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Zhao JC, Yan LN, et al. Risk factors associated with early and late HAT after adult liver transplantation. World J Gastroenterol. 2014;20(30):10545–10552. doi: 10.3748/wjg.v20.i30.10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varghese J, Gomathy N, Rajashekhar P, et al. Perioperative Bacterial Infections in Deceased Donor and Living Donor Liver Transplant Recipients. J Clin Exp Hepatol. 2012;2 1(1):35–41. doi: 10.1016/S0973-6883(12)60081-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim S, Kim EJ, Lee TB, et al. Predictors of postoperative infectious complications in liver transplant recipients: Experience of 185 consecutive cases. Korean J Intern Med. 2018;33(4):798–806. doi: 10.3904/kjim.2017.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss S, Kotsch K, Francuski M, et al. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7(6):1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x [DOI] [PubMed] [Google Scholar]
- 58.Yankol Y, Fernandez LA, Kanmaz T, et al. Results of pediatric living donor compared to deceased donor liver transplantation in the PELD/MELD era: Experience from two centers on two different continents. Pediatr Transplant. 2016;20(1):72–82. doi: 10.1111/petr.12641 [DOI] [PubMed] [Google Scholar]
- 59.Hann A, Osei-Bordom DC, Neil DAH, Ronca V, Warner S, Perera MTPR. The Human Immune Response to Cadaveric and Living Donor Liver Allografts. Front Immunol. 2020;11. doi: 10.3389/fimmu.2020.01227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levitsky J, Goldberg D, Smith AR, et al. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clin Gastroenterol Hepatol. 2017;15(4):584–593.e2. doi: 10.1016/j.cgh.2016.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Organ Procurement and Transplantation Network (OPTN) data. https://optn.transplant.hrsa.gov/data/view-data-reports/. Accessed January 1, 2020. [Google Scholar]
- 62.Tumin D, Hayes D, Washburn WK, Tobias JD, Black SM. Medicaid enrollment after liver transplantation: Effects of medicaid expansion. Liver Transplant. 2016;22(8):1075–1084. doi: 10.1002/lt.24480 [DOI] [PubMed] [Google Scholar]
- 63.Chen LP, Li C, Wen TF, Yan LN, Li B, Yang JY. Can living donor liver transplantation offer similar outcomes to deceased donor liver transplantation using expanded selection criteria for hepatocellular carcinoma? Pakistan J Med Sci. 2015;31(4):763–769. doi: 10.12669/pjms.314.7523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lei J, Yan L, Wang W. Comparison of the outcomes of patients who underwent deceased-donor or living-donor liver transplantation after successful downstaging therapy. Eur J Gastroenterol Hepatol. 2013;25(11):1340–1346. doi: 10.1097/MEG.0b013e3283622743 [DOI] [PubMed] [Google Scholar]
- 65.Chok KSH, Fung JYY, Chan ACY, et al. Comparable short-and long-term outcomes in living donor and deceased donor liver transplantations for patients with model for end-stage liver disease scores ≥35 in a hepatitis-B endemic area. In: Annals of Surgery. Vol 265. Lippincott Williams and Wilkins; 2017:173–177. doi: 10.1097/SLA.0000000000001671 [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Xu X, Wu J, et al. The stratifying value of hangzhou criteria in liver transplantation for hepatocellular carcinoma. PLoS One. 2014;9(3). doi: 10.1371/journal.pone.0093128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmeding M, Neumann UP, Puhl G, Bahra M, Neuhaus R, Neuhaus P. Hepatitis C recurrence and fibrosis progression are not increased after living donor liver transplantation: A single-center study of 289 patients. Liver Transplant. 2007;13(5):687–692. doi: 10.1002/lt.21138 [DOI] [PubMed] [Google Scholar]
- 68.Kim DS, Yu YD, Jung SW, et al. Balanced approach can help initial outcomes: Analysis of initial 50 cases of a new liver transplantation program in East Asia. Ann Surg Treat Res. 2014;87(1):22–27. doi: 10.4174/astr.2014.87.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim EJ, Lim S, Chu CW, et al. Clinical impacts of donor types of living vs. deceased donors: Predictors of One-year mortality in patients with liver transplantation. J Korean Med Sci. 2017;32(8):1258–1262. doi: 10.3346/jkms.2017.32.8.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim JM, Lee KW, Song GW, et al. Increased survival in hepatitis c patients who underwent living donor liver transplant: A case-control study with propensity score matching. Ann Surg Treat Res. 2017;93(6):293–299. doi: 10.4174/astr.2017.93.6.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Viganò J, Gruttadauria S, Mandalà L, et al. The Role of Basiliximab Induction Therapy in Adult-to-Adult Living-Related Transplantation and Deceased Donor Liver Transplantation: A Comparative Retrospective Analysis of a Single-Center Series. Transplant Proc. 2008;40(6):1953–1955. doi: 10.1016/j.transproceed.2008.05.062 [DOI] [PubMed] [Google Scholar]
- 72.Al Sebayel M, Abaalkhail F, Hashim A, et al. Living donor liver transplant versus cadaveric liver transplant survival in relation to model for end-stage liver disease score. In: Transplantation Proceedings. Vol 47. Elsevier USA; 2015:1211–1213. doi: 10.1016/j.transproceed.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 73.Jiang L, Yan L, Tan Y, et al. Adult-to-adult right-lobe living donor liver transplantation in high model for end-stage liver disease score recipients with hepatitis B virus-related benign liver diseases. Surg Today. 2013;43(9):1039–1048. doi: 10.1007/s00595-013-0539-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


