Abstract
Out of the wide range of calcium phosphate (CaP) biomaterials, calcium phosphate bone cements (CPCs) have attracted increased attention since their discovery in the 1980s due to their valuable properties such as bioactivity, osteoconductivity, injectability, hardening ability through a low-temperature setting reaction and moldability. Thereafter numerous researches have been performed to enhance the properties of CPCs. Nonetheless, low mechanical performance of CPCs limits their clinical application in load bearing regions of bone. Also, the in vivo resorption and replacement of CPC with new bone tissue is still controversial, thus further improvements of high clinical importance are required. Bioactive glasses (BGs) are biocompatible and able to bond to bone, stimulating new bone growth while dissolving over time. In the last decades extensive research has been performed analyzing the role of BGs in combination with different CaPs. Thus, the focal point of this review paper is to summarize the available research data on how injectable CPC properties could be improved or affected by the addition of BG as a secondary powder phase. It was found that despite the variances of setting time and compressive strength results, desirable injectable properties of bone cements can be achieved by the inclusion of BGs into CPCs. The published data also revealed that the degradation rate of CPCs is significantly improved by BG addition. Moreover, the presence of BG in CPCs improves the in vitro osteogenic differentiation and cell response as well as the tissue-material interaction in vivo.
Keywords: Calcium phosphate, Bioactive glass, Injectable bone cements, Bone regeneration, Calcium phosphate bone cements
Graphical abstract
Highlights
-
•
Properties of injectable calcium phosphate bone cements and bioactive glasses are discussed.
-
•
Benefits that BG addition to CPC could bring are highlighted.
-
•
Desirable injectable properties of bone cements can be achieved by the inclusion of BGs into CPCs.
-
•
The presence of BG in CPC advances in vitro and in vivo response of the composites.
-
•
Future research direction of BG containing injectable CPC composites are provided.
1. Introduction
The development of alternative biomaterials for bone augmentation has been highly crucial considering that autografts, allografts or xenografts exhibit several drawbacks. Generally, such problems can be listed as limited amount of available material and donor site morbidity for autograft applications and severe immune responses and infections for allografts and xenografts [1]. Thus, bone-like materials are considered to promote bone healing, to strengthen bone tissue, or to improve the bone function in diseased or injured bone.
Bone graft materials can be mainly classified into autografts, allografts, xenografts, synthetic materials, and combinations of them with cells and growth factors [2,3]. Among bone graft materials, calcium phosphate (CaP) ceramic-based bone grafts have attracted increasing attention because the chemical and biological characteristics of CaP show great resemblance to the bone mineral phase. Conventional therapies with CaP bioceramics for bone repair are carried out by implantation of bone grafts in the form of blocks or granules, which requires the prior knowledge of the size and shape of the defect, the shaping of corresponding bone substitutes and then their implantation via surgery. It leads to the problem that scaffolds cannot usually fit exactly to the defect area [4,5]. To overcome these limitations, injectable CaP bone cements (CPCs) could be used as alternatives to repair bone defects. Because of CPC intrinsic properties such as injectability and capability of setting in in vivo conditions, these CaP biomaterials offer the possibility of minimally invasive surgeries, eliminating the need for an open surgery, minimizing patient discomfort, the risk of infection, scar formation and the costs of the treatment. They also provide the optimal defect filling, implant fixation and ease of handling [5]. However, CPCs have also some drawbacks like the possibility of collapse under physiological conditions, poor degradability, lack of macroporosity, and weak mechanical properties [[6], [7], [8]]. Despite these drawbacks, favorable physical and chemical properties, such as being excellent adsorbents for many biomolecules as well as CPC bioactivity that favors their combination with growth factors, drugs and polymers [[9], [10], [11], [12], [13]], have brought CPC to the forefront of many other CaP based biomaterials. Liao et al. [14] reported an animal model to examine the bone regeneration ability of an injectable CaP cement formulation. Injectable CaP cement composed of (poly (lactic-co-glycolic acid) (PLGA), gelatin (GEL) or poly (trimethylene carbonate) (PTMC)) has been for example injected into rabbit femoral bone defect. The results indicated that bone response and degradation properties of CaP/PLGA composite cement were significantly better than those for the CaP/gelatin [14]. Yang et al. [15] reported the in vivo performance of injectable biphasic synthetic bone graft material composed of calcium sulfate and β‐tricalcium phosphate. Their results revealed that new bone formation was detected with an appropriate material resorption after 8 weeks into the sheep vertebral bone defect model [15]. An anti-osteoporotic drug combined injectable CaP formulation has been also implanted into a sheep bone model by Varron et al. [16]. Their results were found to be beneficial to support the bone content and microarchitectural properties of the trabecular bone surrounding the implant [16]. These in vivo studies exhibited promising results showing bone regeneration in the case of injectable CPC application.
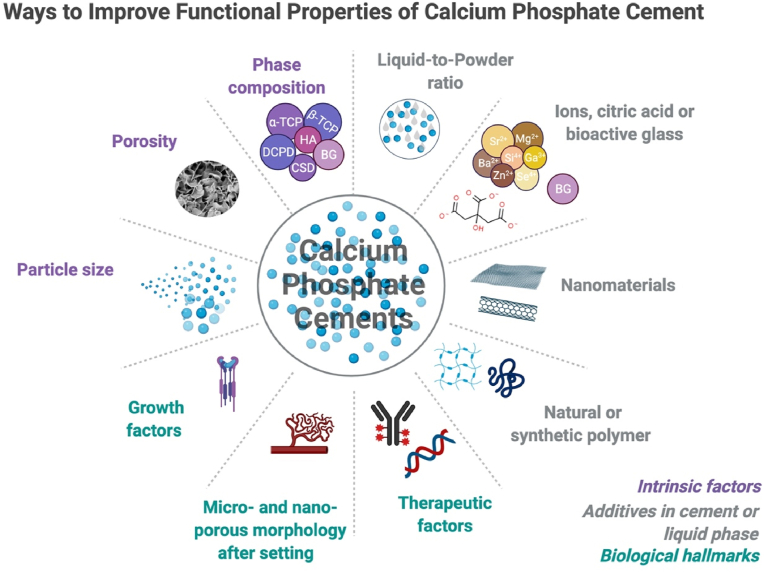
Augmentation of the bone is usually performed via surgery that may lead to infections, muscle retraction or post-operative pain. There is a need for treatment that could enhance the management options once bone fractures or disorders occur. Thus, to eliminate the side effects of bone augmentation, injectable bone cements have been developed and they are getting increasing attention due to their minimally invasive introduction approach. CaP are also known for their in vivo self-setting ability which can be utilized as an advantage for injectable materials for minimally invasive surgery [[17], [18], [19]]. Research done by Brown and Chow in 1983 pioneered a new injectable CaP formulation, which comprised tetracalcium phosphate (TTCP), dicalcium phosphate dihydrate (DCPD, CaHPO4–2H2O) and dicalcium phosphate anhydrous (DCPA, CaHPO4) [20,21]. Developed CPC exhibited such properties as self-setting ability, good injectability, moldability, increased reactivity and high feasibility for new drug delivery system development [18,22]. Since then, various alterations have been studied and applied to injectable CPCs to improve both their physicochemical and biological properties in clinical applications, as schematically visualized in Fig. 1.
Fig. 1.
Brief overview of the improvement approaches put forward for calcium phosphate cements, created by using BioRender.com.
The combination with polymeric solutions [14,23,24] and additives such as citric acid [25,26] has been investigated to mainly improve cohesiveness and injectability of CPC. Both natural and synthetic polymers have been incorporated as liquid phase to an injectable CPC in order to sustain a good cohesion and injectability but at the same time maintaining adequate setting time and mechanical properties [23,27,28]. Chitosan, an amino-polysaccharide, have been used as a natural additive liquid phase to alter such CPC physical properties as injectability, setting time and rheology as well as for enhancing in vivo bioactivity [29]. Sodium alginate [30,31], collagen [32,33], gelatin [34,35], hyaluronic acid [33,36] and cellulose derivatives (hydroxypropyl methylcellulose (HPMC), methylcellulose (MC), and carboxy methylcellulose (CMC)) [33,[37], [38], [39]], have been also utilized as a liquid phase for CPC formation. Commonly, the combination with biopolymers helps to adjust not only the cohesiveness and injectability of the obtained cement but also to improve the mechanical properties and degree of CPC bioactivity. Poly (lactic-co-glycolic acid) (PLGA) microparticles incorporated in CPC exhibited in situ macropore formation and resulted in high cement early strength, which were necessary for bone reconstruction [40]. If combined with citric acid, acting as a liquefier, injectability of CPC could be enhanced [41]. Some previous studies also have reported that citric acid helps to improve the CPC setting time and compressive strength depending on the additive concentration [42,43]. Moreover, there were also other attempts reported in the literature such as addition of glycerol [44], strontium carbonate [45], polyethylene glycol [46], foaming agent [47], and β-dicalcium silicate [48] to adjust the physicochemical and biological properties of CPC. In general, results have revealed that self-setting behavior, injectability, anti-washout ability and rheological properties were improved, bringing CPC formulations much closer to orthopedic clinical applications.
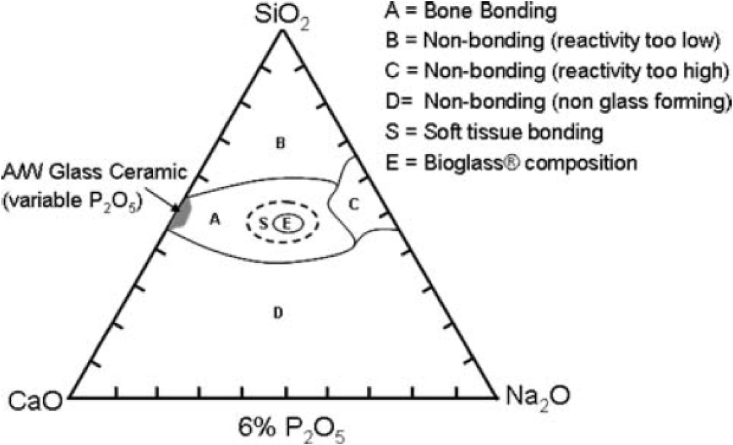
A more recent approach to advance the properties of CPCs is the combination of CaP containing cements with bioactive glasses (BGs). The first BG (45S5 Bioglass®) was synthesized in 1969 and was composed of silicon oxide (SiO2)-calcium oxide (CaO)-phosphorous oxide (P2O5)-disodium oxide (Na2O) [49]. BG is characterized for its ability to support bone growth by chemically bonding to bone [49,50]. Different BGs can be formed by varying the basic SiO2–CaO–P2O5–Na2O composition, as shown in Fig. 2 [49]. The bone-bonding process of BG initiates with the release of silica ions from the BG surface. The released ions form a silica gel layer on the surface, which is followed by the formation of an amorphous calcium phosphate precipitation. Precipitated amorphous calcium phosphate leads to the formation of a hydroxyapatite (HAp) layer, which further activates cell migration to trigger new bone formation [51]. Furthermore, a surface with negative charges due to the high density of silanol groups (Si–OH) on the silica gel layer is the key element to induce the nucleation of HAp [50].
Fig. 2.
Diagram for the basic BG composition and its bone-bonding region [49] (Reprinted from the reference with permission).
It has been also recognized that ionic dissolution products of BGs stimulate gene expression of osteoblastic cells [52,53]. Moreover, recent researches have pointed out not only the angiogenesis stimulation capability of BGs [54], but also their antibacterial [55] and anti-inflammatory effects [56] both in vitro and in vivo. On this account, BGs are extensively utilized in hard tissue engineering on their own, or also as the inorganic phase in composites or hybrid materials [57].
Many bioactivity studies have been carried out on BGs in physiological fluids, revealing that porosity and specific surface area play an important role in the bioactivity of the developed materials. At the beginning of the bioactivity process of BG, an initial burst release of ions occurs when the BG surface becomes in a contact with body fluids. This can cause considerable increase of pH, sometimes even harmful for the surrounding cells and tissues [58]. For example, in a study where the kinetics and mechanism of BG conversion to hydroxyapatite in 0.02 M K2HPO4 solution at 37 °C with initial pH 7.0 were evaluated, it was shown that the pH of phosphate solution in case of silicate, borate and borosilicate glass increased to 11.5, 10.5 and 9.5, respectively [59]. Nevertheless, the value of this final pH can be controlled by the incorporation of other ions, changing their release rate and concentration in the solution. Furthermore, trace elements such as Sr, Zn, Cu or Co existing in the human body are well-known for their anabolic effect in bone regeneration [60,61]. Therefore, incorporation of these ions into different CaPs and BGs could lead to change in dissolution behavior and enhance the biological performance of these materials. In order to improve the in vitro and in vivo properties of the final injectable biomaterial and to overcome the drawbacks of both CPCs and BGs, the incorporation of BGs into CPCs appeared to be a practical approach to enhance the biological and mechanical properties of injectable bone cements, synergistically exploiting the positive features of BGs and CPCs.
Bellucci et al. [62] and Karadjian et al. [63] have comprehensively reviewed the existing literature for CaP and BG composites, focusing on their physiochemical and osteogenic properties. Briefly, Bellucci et al. [62] discussed the influence of BG addition as a sintering aid to HAp and tricalcium phosphate (TCP) in the form of powder or granules on the obtained composite processing, mechanical, and biological properties. Karadjian et al. [63] systematically reviewed CaP/BG composites, prepared by using CaP powder or granules sintered together with BG to form bioceramic scaffolds, based on their biomedical and osteogenic characteristics. However, to the authors knowledge, there is no previous systematic review discussing specifically the literature on BG containing injectable CaP bone cements. Thus, in this review paper, currently available scientific literature on injectable CPC modifications with bioactive glasses is analyzed and discussed, taking into the consideration the criteria for ideal injectable bone cements enriched by BG additions.
2. Properties of injectable CaP bone cements
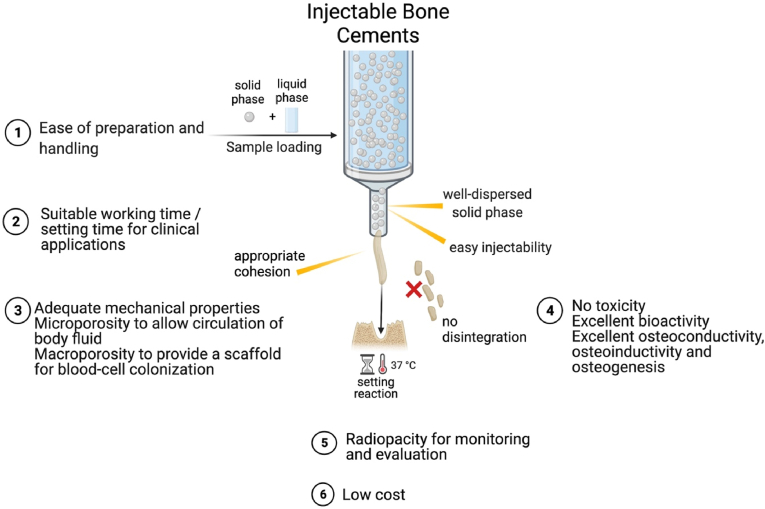
For better clinical performance, an injectable bone material needs to possess several desirable properties, however some drawbacks may be also present, as summarized in Table 1. For instance, injectable bone cements should fulfill such required properties like degree of injectability, rheology similar to the bone tissue, suitable setting time, adequate anti-washout properties when getting in contact with physiological body fluids, cohesiveness (suspension stability, anti-washout capacity) during operation, and radiopacity for detecting the injected material over time (see Fig. 3).
Table 1.
Advantages and disadvantages of CaP bone cements.
| Advantages of Injectable CaP Bone Cements |
| Application as minimally invasive surgery |
| Easy shaping – injectable, moldable and sets under physiological conditions |
| Bioactivity and Biocompatibility |
| Could be used as a local delivery vehicle for drugs/ions/therapeutic factors |
| Could be used as a fixative for implant to eliminate the risk of fracture displacement or implant loosening |
| Drawbacks of Injectable CaP Bone Cements |
| Low radiopacity – which makes it hard to detect in radiography fluoroscopy after surgery |
| Low mechanical properties – lead to brittle fractures or plastic deformations (which could further cause inflammation) |
| Rapid (for calcium sulfate-based cements) degradation – could cause local toxicity and mechanical instability at injection side (which could further cause insufficient or no bone ingrowth) |
| Relatively low bioactivity - could result in fibrous tissue formation |
Fig. 3.
Properties of an ideal injectable bone cement, created by using BioRender.com.
Injectability can be defined as the ability of a paste to extrude through the syringe. During injection, the extruded paste has to keep its homogeneity. This is a key element for the development of injectable bone cements. In literature, there is no unified standard procedure to measure the injectability. Usually, it is identified by the force required for the complete extraction or quantification of extruded material during a certain period. The study showing the correlation between objective and subjective measures of injectability done by Rabinson et al. [56] pointed out that materials are considered as easily injectable, if for 5 ml of sample the applied force is less than 12 N. Considerable effort is needed for 5 ml material, if force is between 12 N and 38 N and great effort is needed to inject the material, if force is between 38 N and 64 N. Finally, materials are considered as non-injectable, if the required force is more than 64 N. The graph of force (N)-extrusion (mm) curve can be evaluated as the maximum force and the plateau force. The graph of force (N)-extrusion (mm) curve can be evaluated as the maximum force and the plateau force. While the maximum force is determined objectively by recording the data from the outputs, the plateau force can be more representative of the injectability characteristics of the materials. The plateau force can also contain large fluctuations, meaning that the air bubbles or particles in the material are producing intermittent changes during extrusion, or that the instrument precision for small force measurements is low [64]. Additionally, if the curve presents more fluctuations, while the force is increasing, indicates that phase separation has occurred during the extrusion process of the paste [65,66]. Although this method is a usual approach to quantify the injectability, the injection speed and force can vary based on the material viscosity, injection side or injection angle [64]. Thus, the injectability of the viscous paste can be modified and strongly depends on the particle size, solid to liquid phase ratio, additives used, diameter of cannula/syringe and injection force applied [65,66].
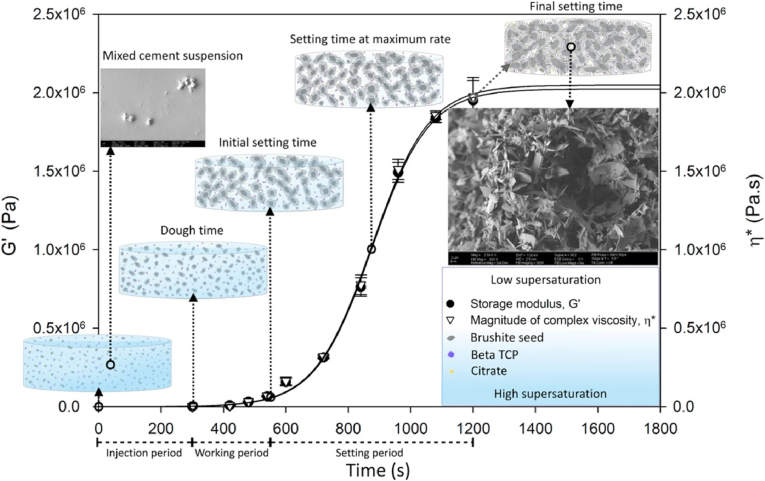
Understanding the rheological characteristics of injectable bone cements is truly important to quantitatively define the flow and injection process as well as the setting and hardening mechanisms. By examining the rheological properties of CPC, the intrinsic knowledge behind the cement paste can be revealed to further enhance such cement properties as cohesion, injectability, viscosity and mechanical properties. By using oscillatory shear conditions, the elastic and viscous behavior of the cement paste can be determined and information about such cement solid particle conditions in the paste as dispersity or flocculation can be evaluated [67,68]. Qualitative viscosity ranges of CaP pastes are reported between 100 and 1000 Pa s for 10 min due to the rheological investigation limitations of hardening reaction of CaP pastes at the early stages [69]. Sohrabi et al. [70] determined rheological properties of injectable biocomposites from hyaluronic acid and BG nano-particles by measuring the shear stress (τ) and viscosity (η) of the prepared paste by varying shear rate (γ) under rotational status (through the steady state mode). By evaluating these parameters, they were able to conclude on the content of liquid phase within the cement particles and additionally, how the surface area of these particles affects the viscosity of the cement [70,71]. Sahin and Kalyon [72] analyzed the setting kinetics and characterized the changes in the rheological properties of brushite-forming cement (see Fig. 4). Their study revealed that the setting reaction can be controlled by increasing the oscillation at a constant strain amplitude in the linear viscoelastic region. They pointed out that the cement paste can maintain its injectability and workability for a longer time if the strain amplitude is increased above the linear viscoelastic range [72]. These findings could be beneficial for orthopedic surgeons as a certain strain amplitude could be applied to the paste in order to gain the necessary operation time during the surgery. Also, the proposed technique can be easily adapted for other CaP cements to characterize the kinetics and stability of the cement setting process. More recently, Wu et al. [73] reported, how the particle size distribution affects the rheological properties of injectable borosilicate BG containing bone cement. They performed rheological measurement using both rotation and oscillation mode applying a controlled-stress rheometer. Samples were characterized in the linear viscoelastic region. The results revealed that the thixotropic level correlated with the particle size distribution. The authors concluded that this method could solve the primary problem of phase separation by understanding the flow behavior of the cement during the setting process. This combination of findings provides some support for the conceptual assumption that rheological measurements of injectable bone cements supply essential information regarding the paste's flow behavior and cement setting mechanism, which further could improve their performance not only during the cement preparation process at room temperature but also for injection in the in vivo environment.
Fig. 4.
Time-dependent development of linear viscoelastic properties of brushite-forming cement [72] (Reprinted from the reference with permission).
All variables (injectability, rheology, powder to liquid phase ratio, particle size of the powder phase, pH of the liquid phase) strongly influence each other, and then, they alter the setting reaction necessary to build a clinically suitable injectable CaP cement. For clinical applications, the final setting time should be less than 15 min [5] as injectable CaP cement must set slow enough to provide sufficient time to a clinician for injection and it should be fast enough to avoid the washout and leakage of the injected paste. The Gillmore and Vicat devices are the most commonly used equipments to examine the setting time of CaP cement pastes [65].
As CPC can be easily washout after their contact with the body fluids, cohesion has a pivotal role in the maintenance of the structural integrity of the cement paste and is described as ability of a paste to set in a fluid without disintegration [23]. If the cement is not enough cohesive, cement particles can go into the blood stream and that can cause detrimental effect such as vascular blockage to thrombus and pulmonary embolism [65]. Also, disintegrated particles can result in inflammatory response and death of the surrounding cells at the injection site. To avoid unsatisfactory cohesion of injectable CaP paste, the latter forces between the cement particles should be strong enough against the surrounding liquid to sustain the paste integrity [74]. The anti-washout capability and cohesion of CaP cements can be examined both qualitatively and quantitatively. In qualitative analysis, CaP cement paste is visually observed just after its extrusion into a liquid. Disintegration of paste and the turbidity of the incubated liquid can be recorded by capturing images [75]. Quantitative measurement of washout mass loss rate is calculated after extrusion into a liquid and then container with the extruded paste and liquid is shaken at a certain rpm in a predetermined time period [76]. Then, the mass of extruded samples and decayed samples after shaking are measured and the washout mass loss rate is calculated regarding their ratios. Bohner et al. [77] investigated a theoretical and experimental model to examine the effect of various parameters on cohesion of the cement paste and the results have highlighted numerous factors (utilizing smaller particle size, increasing P/L ratio, changing the composition of liquid phase) that are associated with the setting time reduction and can be correlated with the strength of CaP particle interaction, leading to augmented cohesion.
Radiopacity is an important factor that should be considered in the development of bone regenerative biomaterials, and plays a key role in visualization of biomaterials during and after surgical procedures. In this way, extruded injectable bone cement can be correctly positioned at the defect site, can be easily detected after setting as well as possible failure can be predicted by later monitoring. CPCs have a certain level of intrinsic radiopacity, however, this could be not enough when a surgeon requires a close fluoroscopic monitoring. In addition, it can also be difficult to distinguish CPC biomaterial from the surrounding bone during the surgery to understand whether cement leakage occurs or not [65]. Therefore, addition of radiopacifying agents into injectable CPC is required. Specifically, the radiopacifier amount should be 25–35% of the total mass of the cement powder as the visualization is essential for vertebroplasty and kyphoplasty surgeries [78]. Inorganic radiopaque agents such as barium sulfate, strontium carbonate, bismuth salicylate basic and zirconium dioxide are usually incorporated into CaP cements, yet this incorporation could cause changes in the physiochemical properties [45,[79], [80], [81]]. Overall, several systematic researches on radiopacity evaluation of injectable CPCs have been undertaken [[82], [83], [84], [85]]. These studies highlighted the need for examining the radiopacity of an injectable bone cement. Overall it was concluded that the evaluation parameters for radiopacity should be similar with those of the clinical procedures which are 60–130 kV and 3.1–10 mAs [83].
Apart from the desirable injectable properties of CPC composites, mechanical properties, degradation profile, and porosity after setting are essential parameters to examine. During the setting reaction of CPC (when CaP powder contacts the liquid phase), the sequential growth and entanglement of newly formed crystals result in the final hardening of the paste so that a microporous structure with nano or submicron size is formed [86]. During this process, the mechanical strength of CPC is gained; however, CPC mechanical properties cannot fulfill the required mechanical performance for load-bearing applications. Their mechanical properties, mainly examined in compressive mode, are comparable with those of trabecular bone (4–12 MPa). More particularly, up to 80 MPa compressive strength has been reported for apatite-forming CPC, while up to 52 MPa compressive strength has been found for brushite forming CPC [87]. Nevertheless, their inherent brittleness still limits their clinical application to non-load-bearing applications. Therefore, many attempts have been made to improve the mechanical properties of CPCs. One of these attempts is to change the porosity of CPCs since a less porous structure leads to a stronger cement matrix. In order to reduce the porosity, the powder-to-liquid ratio can be increased [88], particle size of starting powder can be decreased [89], or liquefiers such as glycolic acid, citric acid can be utilized [90,91]. Another approach to increase the mechanical properties of CPCs is to introduce polymers or fibers [92,93] within the cement matrix. More detailed description of these approaches can be found elsewhere [86,92,93]. While reducing the porosity, the parameter that should be considered is that the reduction of porosity affects the biological properties of CPC, especially the in vitro and in vivo degradation rates. That should match the requirements of the bone regeneration rate. Therefore, all desirable injectable properties, mechanical properties, porosity, and degradation should be in harmony with the clinical needs.
3. BG modified injectable CaP bone cements (CPCs)
3.1. Overview and principles behind BG incorporated CPC composites for bone tissue applications
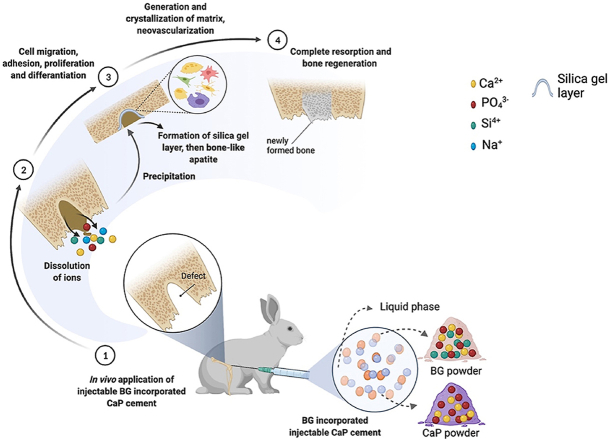
The impact of CPCs on bone bonding and bone tissue growth into scaffolds has been examined in many studies, identifying the slow or fast resorption of CPCs as challenging points [94,95]. While slow resorption rate results in a suppressed osteointegration, fast resorption rate may lead the to wash-out of the CPC paste from the defect site [62,96]. In order to slow down the fast CPC resorption rate, combinations of different CaP have been proposed (e.g. resorption rate of tricalcium phosphate (TCP) can be controlled by using biphasic CaP cements [97,98]).
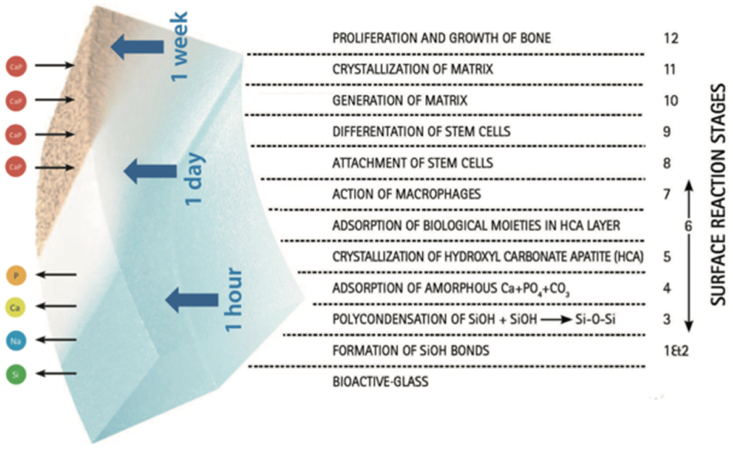
Various formulations of CPCs have led to different biological responses based on the CPC chemical composition, crystallinity, stoichiometry, dissolution/precipitation behavior, surface chemistry, and porosity [62]. Despite the good osteoconductivity of CPCs, their effect on osteogenic differentiation is limited because of their relatively low surface reactivity [63,99]. The indicator of “bioactivity” in the context of bone contacting materials is the formation of a HAp surface layer when the material is immersed in simulated body fluid (SBF) in vitro. However, although in vitro studies of dicalcium phosphate dehydrate have shown the formation of an HAp surface layer in SBF, in vivo studies with this material showed no direct bone bonding [[100], [101], [102]]. A similar contradiction has been found in a studies using β-TCP, indicating that its immersion in SBF does not always lead to the formation of HAp layer in spite of its well-known bone bonding ability [[103], [104], [105]]. On the other hand, BGs are well recognized for their direct bone bonding ability and can be used as an alternative to CPCs. BGs and their combination with CaP cements has emerged lately exploiting the BG ability of direct bonding to bone and surrounding tissues [49]. The direct bond between BG and bone occurs via the formation of HAp-like layer on the surface of the BG material (see Fig. 5). The first five steps that occur on the surface of the material were explained as resulting from a quick ion exchange of Na+ with H+ and H3O+ followed by a polycondensation reaction of surface silanols to form a high-surface area silica gel. This is followed by the nucleation and crystallization of a hydroxyl carbonate apatite (HCA) layer. This apatite layer is similar to the mineral phase of bone, so that osteoblasts can proliferate and differentiate on this layer to produce their own extracellular matrix. Therefore, the inclusion of BGs into CPCs is a promising approach to maintain bioactivity both in vitro and in vivo [101]. Additionally, the initial burst of pH increase that occurs due to the release of a high concentration of Na ions from BG can be controlled by the addition of a CaP phase [62,63]. This is an important issue which needs to be considered because osteoclasts’ activity is diminished by an alkaline environment and osseous regeneration, where osteoclasts play an essential role, could be restricted by an uncontrolled pH increase.
Fig. 5.
Interfacial reactions involved in forming a bond between BG and bone [106] (Reprinted from the reference with permission).
A comparative study done by Campion et al. [107] has proved that the bioactivity of silicate substituted HAp materials is higher than that of commercially available β-TCP bone graft substitutes. Their findings confirmed that fewer crystals showing the octa-calcium phosphate (OCP) characteristic were formed on the β-TCP surface, while a thick continuous layer of hydroxycarbonate apatite (HCA) crystals was deposited on the surface of silicate substituted HAp materials [107]. In addition, one of the main reasons for the combination of CaP cements with BG is that ions released from the BG can induce increased angiogenesis and osteogenic differentiation [108,109]. Angiogenesis and osteogenic differentiation ability of BGs also highly depends on the BG composition. BGs with composition that falls within the region A (indicated in Fig. 2) are bioactive and exhibit bone bonding ability, whereas BG compositions within the region B show bioinert property [110]. Additionally, 3D scaffolds of both CPC and BG alone have low mechanical properties, which can be considered as a drawback [111,112]. High reactivity of BG is also problematic as it can cause the initial burst release of ions which drastically increases the pH of the surrounding environment [58]. If BG is used together with CPC, the initial burst release can be controlled and adjusted to the necessary extent. Apart from these possible advantages when combining BGs and CPC, both of them have been employed as drug carriers [113,114]. Thus, injectable BG incorporated CPCs can be designed to present a dual drug/ion delivery capability.
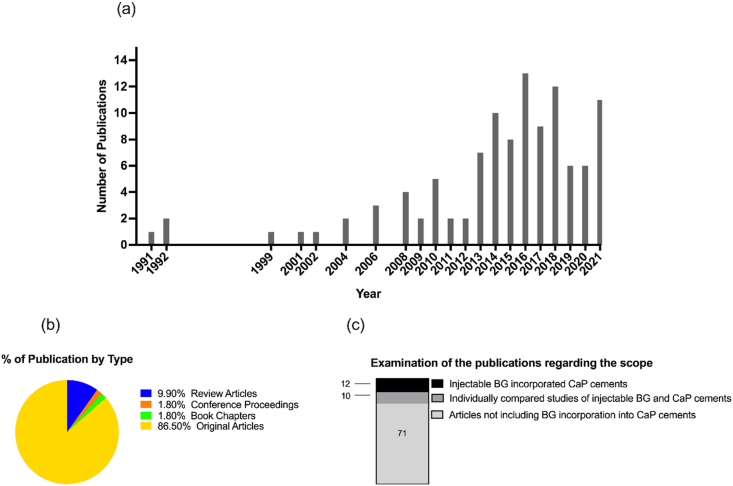
In recent years, there has been an increased amount of scientific literature on BG incorporated CPCs describing how this combination improves the drawbacks of both materials and their osteogenic properties. Bellucci et al. [62] discussed the influence of the BG addition to HAp and TCP on material processing, mechanical properties and biological implications. Karadjian et al. [63] systematically reviewed the CaP/BG composites based on their biomedical and osteogenic characteristics. Although these two review articles give clear evidence that BG alters positively the physicochemical, mechanical, and biological properties, when combined with CaP, there is a lack of information on how BG incorporation influences the desirable features of injectable bone cements. In this context, keywords as “calcium phosphate cements” or “calcium phosphate’, “bioactive glass” or “bioglass” and “injectable” were selected and a search of the literature was carried out in the databases of Scopus, PubMed and Web of Science. 111 articles were found with these keywords and the overview of the research database output is given in Fig. 6. However, only 12 of the studies were devoted to injectable BG modified CaP bone cements. The main outcomes of these articles as an injectable bone cement containing BG were summarized in Table 2. The output of these publications were examined regarding the injectable BG/CPC setting mechanisms, mechanical properties, in vitro performance as well as cellular and in vivo response.
Fig. 6.
(a) The number of publications performed until January 2022 with the keywords “calcium phosphate cements” or “calcium phosphate’, “bioactive glass” or “bioglass” and “injectable”, (b) % of type of publication found with these keywords and (c) distribution of the publications regarding the scope on Scopus, PubMed, and Web of Science databases.
Table 2.
Injectable BG incorporated CaP cement composites. Their physicochemical analysis results, in vitro and in vivo results, and highlights of the findings.
| Year | CaP Type | BG content | Liquid phase | P/L ratio | Injectability | Setting time | Mechanical properties | Radiopacity | In vitro response | In vivo response | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | teOCP | 58S BG nanoparticles | 5 wt% alginate, 2 wt% chitosan solution and DMEM | 0.39 | – | – | – | Yes | – | Bone ingrowth in femoral cavity of OVXed rat model | [115] |
| 2013 | 85% α -TCP 10% DCPA 5% HA |
Biosilicate® parent glass, up to 50 wt% | 2% Na2HPO4 | – | – | Around 18min for 50% BG composite | – | – | BG incorporation, in CPC/PLGA composites; increased degradation rate, rapidly transformation in PBS with interconnected pores and macroporosity | – | [116] |
| 2013 | Equal molar ratio of TECP and DCPA | Bioglass 45S5; 10 and 20 wt% | Potassium phosphate buffer (pH 7.0) | 2.0 (g/ml) | 10–20% improved injectability | 21 and 25 min | BG wt. 20% reached 26 MPa and 40 MPa after soaking in SBF for 1 and 7days | – | Bone like apatite structure detected after soaking in SBF for 7 days. Increased degradation rate. No cytotoxicity and promotion of cell viability. |
Higher bone forming efficiency. | [117] |
| 2014 | TTCP, DCPD, CSD | BG; 0%, 10%, 20% and 30% | 2% chitosan, 4% HPMC and 10% citric acid | 1.8/1 | – | 24.67 ± 2.08 min for 30% BG | 15.04 ± 2.4 MPa for 30% BG | – | Increased apatite formation. Increased cell proliferation Higher expression of BSF with increased BG content |
Higher concentration of bone volume for 30% BG incorporated samples | [118] |
| 2015 | α-TCP | Mesoporous BG; up to 10% | 2.5 wt% Na2HPO4 | 1.54 (g/ml) | higher injectability compared to pure CPC | 25 min for 10% BG | 24 MPa for 10%BG after soaking in SBF for 7days | – | Nanotopology similar to that of pure CPC after soaking in SBF for 7 days Higher protein adsorption capacity of BG incorporated samples |
Increased bone formation with BG incorporation | [119] |
| 2016 | CPC | 45S5 bioactive glass; 20 wt% | 1 M dipotassium phosphate and 1 M monopotassium phosphate solutions | 2a | – | – | – | – | Improved cell adhesion, proliferation and differentiation of osteoblast from BG incorporated composites | – | [120] |
| 2017 | α-CSH | Mesoporous BG; SiO2/CaO: 80/20 mol% Glass-ceramic particles; SiO2/CaO/Na2O/ZrO2, 57/30/6/7 mol% |
Distilled water | 2.5 (g/ml) | ‘Suitable injectability’ | 1 h at RT 40 min at 37οC |
18.1 ± 0.8 MPa | Adequate | Uniform HA crystals on the surface after 1 week 89.3 ± 7.8% viability for 24 h |
– | [121] |
| 2018 | β-TCP/MCPM | mesoporous silica particles; 5 wt% | PEG 400/H2O | 2.5a | 50% increased injectability (time dependent) | 30 min | 2.81 ± 0.45 MPa | – | Homogenous layer of apatite formation No cytotoxicity |
– | [122] |
| 2019 | β-TCP/MCPM | 45S5 BG powder; 5 to 50 (v/v) | 0.5 M citrate ion solution |
0.5 (v/w) | – | Ranged from 5 to 12 min | ∼10 MPa | – | No cytotoxicity Increased ALP expression with BG Incorporation Faster degradation and apatite formation with BG incorporation |
Increased bone formation with BG incorporation Formation of new blood vessel with BG incorporation |
[123] |
| 2019 | MPC | Borosilicate glass (21.5–50%) | Distilled water | 5.0 (g/ml) | – | Increased from 6 min to 16 min | Decreased from 13.5 MPa to 11 MPa | Yes | Reduced wash-out with BG incorporation No cytotoxicity |
Increased of new bone formation with BG incorporated sample | [124] |
| 2021 | α-TCP/gypsum | BG powder (75% SiO2 and 25% CaO (75S25C)); 5 to 20 wt% | 10 wt% γ-PGA | 1.67 (g/ml) | ∼90% | Ranged from 13.2 to 17.3 min for initial setting time | Ranged from 30.17 to 16.75 MPa | – | Increased apatite formation No cytotoxicity |
– | [125] |
| 2021 | β-TCP | Mg and Sr doped BG nanospheres (0–70%) | 0.25 M Na2HPO4 | 0.3 (ml/g) | – | For 50% BG incorporated samples; initial setting time: 8.3 ± 0.6 final setting time: 15.2 ± 0.7 min | Ranged from 2.91 MPa to 8.83 MPa | – | Reduced wash-out Increased apatite formation No cytotoxicity |
– | [126] |
This number does not have the unit provided in the article, teOCP- trace elements-multidoped octacalcium phosphate, BG-bioactive glass, DCPA-dicalcium phosphate anhydrous, β-TCP- β-tricalcium phosphate, CPC – calcium phosphate cement, MCPM-monocalcium phosphate monohydrate, BVF- bone volume fraction, RMVF- remaining material volume fraction, α-TCP- α-tricalcium phosphate, TECP- tetra calcium phosphate, γ-PGA-poly-γ-glutamic acid, α-CSH - α-calcium sulfate hemihydrate, C3S - tricalcium silicate, CMC – carboxy methyl cellulose, K2HPO4 - dipotassium phosphate, SBF – simulated body fluid, A-V-C3S – amine-grafted and vaterite-contained tricalcium silicate, MPC – magnesium phosphate cement.
3.2. BG impact on CPC setting time
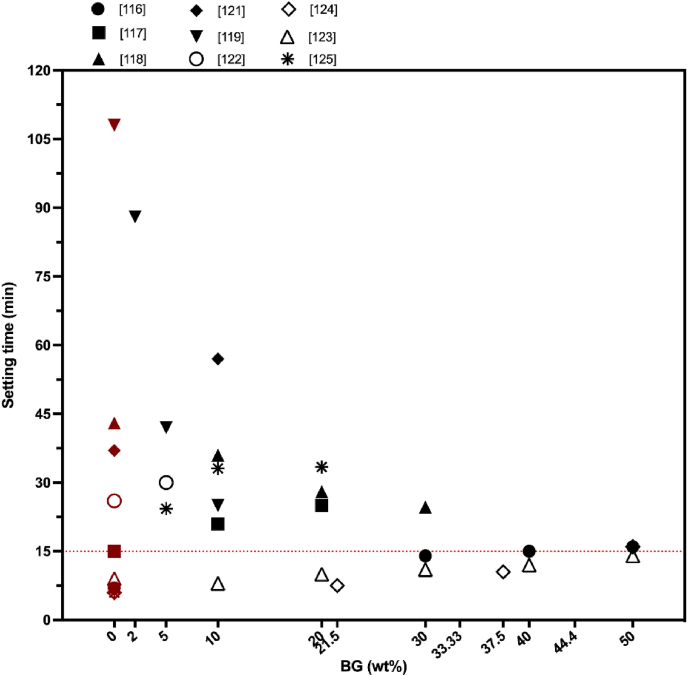
From the biomaterial science point of view, the setting time characteristics, and the physicochemical, and mechanical properties of injectable CaP based bone cements could be improved by the addition of BG as a second solid phase [62,63]. Also, the incorporation of BG into CPC can lead to a compact cement microstructure [118,124]. Meanwhile, incorporation of a polymeric liquid phase could strongly help the cohesion of the composite while leading to a homogenous distribution of both CaP and BG particles in the cement matrix [[115], [116], [117],123,126]. The characterization of setting and handling properties of the injectable composites is necessary to understand the hardening mechanism of the prepared cement [127]. For an injectable material both an adequate working time and short setting time are required so that the defect filling would be done in an optimal time window and material leakage out of the defect site would be prevented [5,128]. O'Neill et al. [5] have indicated in their publication, the final setting time should be less than 15 min for clinical applications. Summarizing the data from all available publications, where BG was use as a modifier for CPC, it is confirmed that the BG incorporation into CPC affected significantly the setting time of the final cement (see Fig. 7).
Fig. 7.
How BG incorporation affects the injectable CaP cements. Data (final setting time of the composites) were taken from the references indicated in Table 2. The red data points indicate the setting time of BG-free CaP cement. Red dotted line at 15 min represents the clinically approved setting time. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The variations in the setting time can be explained with the differences in the liquid phase composition, liquid/powder phase ratio as well as powder or composite composition [5,7]. Since the formulations were composed not only of different BG and CaP powder ratios but also diverged in BG and CaP chemistry, setting mechanisms described are unique for each individual BG and CaP combination. According to Fig. 7, the majority of the studies did not match the clinical requirements of the cement setting time. The increase of the final setting time was the general trend after incorporation of BG into the CPC. Also, the injectability was enhanced if BG was added to the CPC [108,110,112,113,115,116]. Accordingly, the addition of 30 wt% BG (23.75 Na2O, 23.75 CaO, 48.5 SiO2–4P2O5 (wt.%)) increased the final setting time of CPC from 7 min to 14 min in the study of Renno et al. [116]. Obtained results by Yu et al. [117] revealed that the setting time could be increased from 21 min to 25 min, when the weight percentage of 45S5 BG in CPC varied from 10 wt% to 20 wt% at a P/L of 2.0 g/ml. Although the setting time of the BG incorporated CPC exceeded the clinical requirements, authors pointed out that the prolonged setting time (about 10 min) could provide increased time window for surgeons to prepare the cement with a good injectability [117]. Dadkhah et al. [121] described the cements with setting time increase from 37 min to 57 min by the addition of 10 wt% BG (composition of SiO2/CaO: 80/20 mol%) particles. They compared their findings with commercially available cement with the final setting time of 51 min [121]. This setting time also was recognized as clinically suitable, as it can improve the injectability and handling of the cement during surgery [121,124,125,131]. It should be also noted that the evaluation of setting time at 37 °C could possibly extend the hardening time of the cement phase [132].
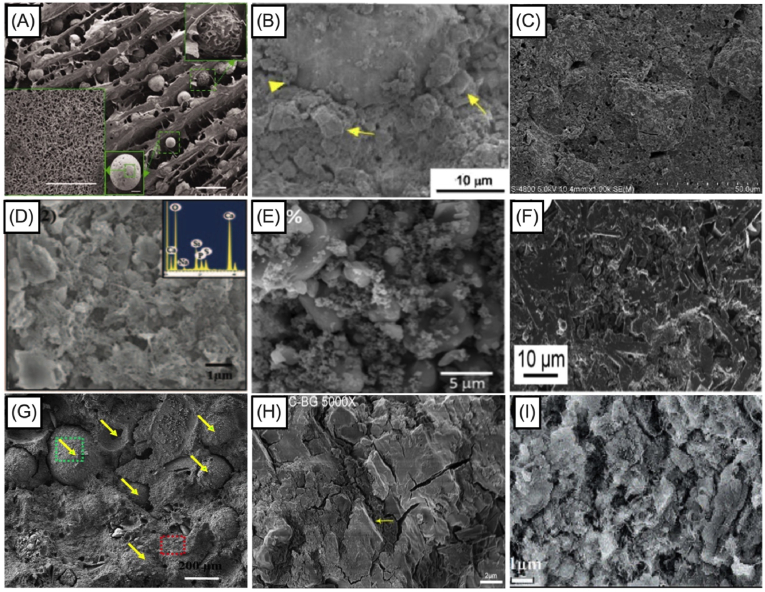
Despite the positive effect that polymer addition into the liquid phase can bring to the cohesion and rheology of the composite [118], such additives as polyethylene glycol and alginate could restrain the setting reaction by coating the cement powders with a polymer layer [113]. In some of the studies, the addition of BG into the CPC showed a contrary effect and decreased the setting time [118,119,123]. While the setting time of BG-free CaP cement composites was recorded as 43 min, the setting time decreased to 36 min with 10 wt% BG addition, to 28 min with 20 wt% BG addition, and to 24 min with 30 wt% BG addition with the composition of 60% SiO2, 36% CaO and 4% P2O5 by mol as Sadiasa et al. reported [118]. Additionally, these results were accredited to the presence of citric acid in the liquid component, which acted as a water reducing agent resulting in chelation reaction between Ca2+ and citrate ions. During the chelation reaction, the powder component absorbed the citric acid molecules leading to hydration and dissolution of the cement phase [118]. Incorporation of BG increased the dissolved concentration of Ca2+ ions for chelation reaction in which the powder component absorbed the citric acid molecules, thus reducing the setting time. However, some researchers have reported completely different effects of citrate ions, showing an increased setting time of BG incorporated CPC and CPC composites by the addition of citric acid into the liquid phase [123,133,134]. For example Hasan et al. [123] have described an opposite result regarding the effect of citrate ions in the liquid phase. They attributed this outcome as well as the reverse relationship between setting time and compressive strength to the reaction properties of BG microsphere production in which size and sphericity of the BG microspheres were retained [123]. Sadiasa et al. [118] defined the particles of produced BG as fine particles; however, the particle size was not given in their study. Nevertheless, they concluded that the particles detected in their scanning electron microscopy (SEM) images (Fig. 8(B), [118]) appeared as nanosized BG powder. In the study of Hasan et al. [123], the particle size of the BG microspheres ranged between 150 and 600 μm, as shown in Fig. 8 [123]. Consequently, the different influence of citrate ions on the setting time could be explained by particle size differences of the fabricated BGs.
Fig. 8.
SEM images of fabricated bioactive glasses in the study of (A) Renno et al. [116], (B) Sadiasa e t al. [118], (C) and optical microscopy images of (D) Dadkhah et al. [121] and Hasan et al. [123] (Reproduced from the references with permission).
Addition of small amount of mesoporous bioactive glass (mBG) nano-component has also been shown to accelerate the setting time of CaP cements by absorbing free (extra) water molecules from the liquid phase [119]. In addition, authors agreed that mesoporous BG nanoparticles could adsorb water of the liquid phase, which increased the initial P/L ratio. Then the increment of the P/L ratio caused the decrease in setting time of BG incorporated CaP cements.
Considering such input parameters as initial powder type of both BG and CaP, liquid-to-powder ratio, or other additives used in the composite synthesis such as polymers or liquefiers, it was observed that they all have contributed to differences in the final cement setting time. Additionally, the particle size of the powder phase and the composite synthesis method can not only optimize the injectability but also the setting mechanism of the final product.
3.3. BG impact on CPC mechanical properties
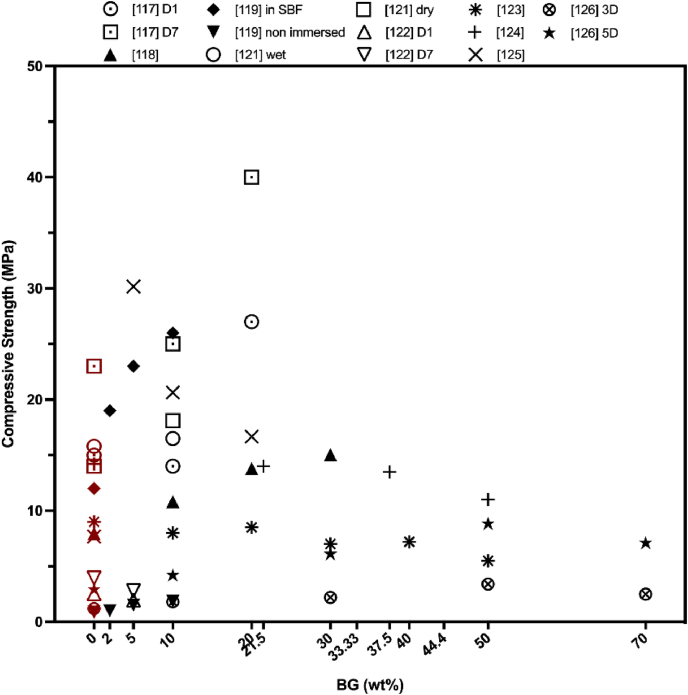
When evaluating the relationship between the CPC setting time and compressive strength, a reverse correlation between these two parameters can be directly observed as prolonged setting time leads to the decrease of the compressive modulus [135,136]. However, by addition of BG the compressive strength of the CPC could be enhanced, despite the prolonged setting times (see Fig. 9) [108,112,116].
Fig. 9.
How BG incorporation affects the injectable CaP composites. Data were taken from the references in Table 2. The red data points indicate the compressive strength of BG-free CPC. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These results can be attributed to the fact that the prolonged setting time is still in the clinically acceptable limit and cohesion of the composites is adequate enough to not wash-out during the hardening reaction. Additionally, BG incorporation into CPC forms dense and homogeneous cement microstructure as seen in Fig. 10, which in fact improves the mechanical properties of the BG added CPC composites. Pu et al. [125] confirmed that after 5 wt% 75S25C BG (with the mole composition of 75% SiO2 and 25% CaO) addition, compressive strength of CPC increased from 21.52 ± 2 MPa to 30.17 ± 1 MPa. Nevertheless, further increase of BG amount inside the CaP phase led to the reduced strength of the composite [125,126]. Later on, authors examined bioactivity by the morphological observation of BG incorporated CPC composites with the aim to identify the compressive strength fall. Their results indicated that increased BG content could be destructive for the bonds among the CPC crystals and polypeptide poly (γ-glutamic acid) which was presented in their liquid phase, since the crystal entanglement was not detected for 20 wt% BG incorporated samples [125]. In the study of Dadkhah et al. [121], compressive strength of BG (composition of SiO2/CaO: 80/20 mol%) incorporated CPC was lower, if compared to unmodified CPC samples, but higher than that of commercial Cerament®. After setting for seven days, the compressive strength was elevated from 14.01 ± 0.7 MPa to 18.1 ± 0.8 MPa because of the continuous accumulation and hydration process of the cement phase. The reason behind it could also be explained by the existence of nano-sized glass-ceramic particles inside the composites which endures the architecture of the composites after the hardening process. El-Fiqi et al. [119] also introduced mesoporous BG (composition of 85% SiO2 and 15% CaO in mol) particles into the CPC, which elevated the compressive strength of the final BG added CPC composite from 12 MPa (in case of CPC) to 26 MPa (10 wt% of BG incorporated in CPC) after incubation in SBF for 7 days at 37 °C. Microstructure analysis (see Fig. 10) supported these findings by showing that mesoporous BG nanoparticles surrounded the CaP microparticles in such a way that space between the larger particles of the CPC was being filled by the addition of mesoporous BG nanoparticles [118]. This also increased the crystal growth which further supported the enhancement of mechanical properties.
Fig. 10.
Morphological images of BG incorporated CaP cement after setting. (A) Yang et al. [115] showed how BG nanoparticles and porous CaP microspheres placed inside hydrogel complex. (B) Renno et al. [116] showed the composite micrograph, yellow arrow represents the BG and arrow head represents the PLGA-microparticles. (C) Yu et al. [117] showed the micrograph of 20 wt% BG incorporated CaP cement composite. (D) Sadiasa et al. [118] showed the micrograph of 30 wt% BG incorporated CaP cement composite. (E) El-Fiqi et al. [119] showed the micrograph of 10 wt% BG incorporated CaP cement composite. (F) Dadkhah et al. [121] showed the homogenous distribution of cement calcium sulfate hemihydrate, mesoporous particles and glass-ceramic particles. (G) Hasan et al. [123] showed greyscale 3D reconstructed view of composite sample from micro-CT scans showing distribution of microspheres with in the brushite cement, yellow arrows indicate microspheres position inside the CaP cement. (H) Li et al. [124] showed the morphology of BG particles which were embedded and dispersed in the CaP paste as the yellow arrows indicated. (I) Pu et al. [125] showed the micrograph of 5 wt% BG incorporated CaP composite (Reproduced from the references with permission). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Comparison of the available research findings on the mechanical properties of injectable BG incorporated CPC composites indicated that the compressive strength could mimic the strength of trabecular bone. Therefore, injectable BG containing CPC composites have an acceptable performance to further assess their clinical translation capability. The interfacial bonding between the powder and liquid phase could be improved to eliminate possible failure mechanisms while setting and to increase the initial mechanical strength. Both the particle size of CaP powder and the rounded and smaller size of BG particles may also lead to an enhancement of interfacial bond between the composite and the surrounding bone tissue after implantation.
3.4. BG impact on CPC in vitro performance
The bone-like apatite formation and deposition on the material surface provide insightful information related to bone bonding ability of the CPC. Thus, bioactivity evaluation of injectable BG incorporated CPC is needed to verify the bone-like apatite accumulation on the surface of the composite. The apatite layer precipitation on the surface of both CPC and BG incorporated CPC composites was analyzed by several researchers using SEM and X-ray diffractometry (XRD) [[108], [109], [110],112,114,116]. Characteristic XRD reflections of apatite (2θ peaks approximately at 26°, 29° and 32°) appeared with greater intensity for BG added CPC composites, indicating that the bioactivity is improved with BG modification. The morphological examination proved excess amount of formed apatite particles on the surface of the BG incorporated CPC composites compared to pure CPC. These differences can be attributed to the apatite formation mechanism exhibited by BG. The presence of BG accumulates the formation of SiO2− groups on the cement surface in the form of a film layer, when composites come into the contact with the aqueous environment. This layer leads to precipitation of CaO–P2O5 groups, which then provides the nucleation sites for apatite crystal formation. In the case of pure CPC, apatite formation takes place when Ca2+ and PO43− ions are supersaturated in surrounding environment, which requires more time to form an apatite layer on the surface of the composites [117]. El-Fiqi et al. [119] pointed out that the higher surface area of mesoporous BG particles, which also contributes to an increased overall surface area of the BG incorporated CPC, leads to the formation of microspherical apatite islands on the surface of BG incorporated CPC composites, that facilitates protein adsorption (Cytochrome C (Cyto.c) was used as model protein), if compared to pure CPC [119]. Mendes et al. [122] incorporated mesoporous particles into brushitic cement and a needle-like crystal morphology after 7 days of incubation in SBF solution at 37 °C was observed. After 14 days of incubation, the presence of the particles was no longer detected by using energy-dispersive spectroscopy (EDS) analysis. This finding was attributed to the secondary nucleation of apatite crystals, which could be notably observed in SEM images as morphological changes of the needle-like crystals to a globular structure formation. Likewise, Hasan et al. [123] added 45S5 BG derived microspheres, sizes ranging from 150 to 600 μm, into brushite cement. After 7 days of incubation in SBF, XRD results revealed that corresponding reflections of β-TCP converted to DCPD [123].
In order to predict the cement biological response, during the sample incubation in medium (PBS, SBF or other) both the monitoring of medium pH changes as well as the ion release kinetics is required. This has been done by several authors and the results have revealed that CPC composites released Ca2+ and PO34− whereas BG incorporated samples additionally released silicate species [115,116,119,121,124,125]. In the reported studies, additional released Ca2+ ions from the BG containing composites has led to rapid mineralization and accelerated re-precipitation effect. The release profile of silicate ions as given in Table 3 remained at a higher and sustained level from the indicated first time measurement until the end of the incubation period, which pointed out that Si ions might not only contribute to the precipitation process but also could be involved in the osteogenic differentiation and bone regeneration processes [115,119,121,137]. Additionally, these ions released from the BG incorporated CPC samples regulate the pH of the incubation medium by increasing it to neutral pH. This effect can be attributed to Ca2+ ion release and the formation of apatite. Neutralization time of the pH value varied among the studies because of the differences between the CaP powder phase and the liquid phase, different dissociation rates, ion composition in BG as well as the hydration product differences [124]. Besides the close relationship between the ion release and pH changes, also the degradation of the composites is strongly affected by the pH variations and these two parameters have a reciprocal relationship as well. For example, the degradation of β-TCP from the mesoporous silica incorporated CPC composites of Mendes et al. [122] reduced the pH of the surrounding medium from pH 7.4 to pH 6.0, and then the pH was stabilized when the DCPD crystals and the remaining β-TCP equilibrated. BG incorporated CaP/PLGA composites reported by Renno et al. [116] revealed a pH decrease during the 9 weeks of incubation in PBS at 37 °C. This finding broadly supported the work of other researchers in the area of linking pH decrease with PLGA presence which is due to the release of lactic and glycolic acid monomers [40,116]. In the end, incorporation of BG within CaP/PLGA composites modulated the pH at physiological level during the composite degradation process. Summarizing the findings above, it should be considered that for BG incorporated CPC composites higher degradation rates will be observed, if compared with pure CPC, mainly due to the relatively high BG solubility [116,117,[121], [122], [123], [124], [125],129,130]. It can be also inferred that the degradation rate can be effectively controlled by the weight fraction and composition of BG incorporated in the CPC. Therefore, the examination of the apatite formation, ion release, pH change, and degradation rate is pivotal to predict the in vivo response in terms of bone bonding ability through the in-situ formation of an apatite layer. The high surface area and reactivity of BG as a secondary powder phase significantly elevated HAp deposition on the composites after immersion in SBF solution. HAp deposition on the scaffolds also increased the mechanical strength of the composites.
Table 3.
Data on released Si4+ ions from BG incorporated injectable CaP cement composites, according to literature.
| BG content (wt.%) | Incubation solution | Incubation time | Released Si4+ ionsa | Ref |
|---|---|---|---|---|
| 0.8 | DMEM, pH 7.4 | 12 h | 42 ppm | [115] |
| 96 h | 47 ppm | |||
| 10 | Tris Buffer, pH 7.4 | Day 1 | 1.5 mM | [119] |
| Day 14 | 2 mM | |||
| 5 | SBF, pH 7.4 | Day 1 | 20 mg/L | [122] |
| Day 14 | 170 mg/L | |||
| 25 | SBF, pH 7.4 | Day 1 | 0.3 mg/L | [124] |
| Day 7 | 0.6 mg/L | |||
| 5 | PBS, pH 7.4 | Day 1 | 50 ppm | [125] |
| Day 21 | 70 ppm | |||
| 10 | Day 1 | 58 ppm | ||
| Day 21 | 75 ppm | |||
| 20 | Day 1 | 60 ppm | ||
| Day 21 | 80 ppm |
Indicates that data was taken from the graphical representation of the publications. Therefore, values are representing the approximate amounts of released Si4+ ions. BG – bioactive glass, DMEM – Dulbecco's Modified Eagle's Medium, SBF – Simulated body fluid, PBS – Phosphate buffer saline, h – hour, Ref – references.
3.5. BG impact on CPC cellular response
The formed hydroxyapatite (HAp)-like layer following the (partial) dissolution of BG incorporated CPC has a leading role in creating a strong bond to bone, stimulating osteoblast cell proliferation and differentiation, inducing angiogenesis and presenting antibacterial effects [123,137,138]. Indirect cell culture techniques in which the cells are exposed to the extracted medium of the incubated samples revealed that the dissolution products of BG incorporated injectable CaP cement composites are responsible for changes in cellular activity [121,122,125]. A cytotoxic effect of high concentration extracts (200 mg/ml) of both CaP cements and BG incorporated CaP cements was determined in the study of Mendes et al. [122] by using Chinese hamster ovary cells (CHO–K1). A possible explanation for this effect might be the rapid pH drop in the incubation medium at day 2 due to the ion release. The authors explained this as an effect of surplus Ca2+ ion release, which can induce the cell apoptosis by endoplasmic reticulum stress [122]. On the contrary, significant stimulative effect on cell proliferation was recorded on bone marrow mesenchymal stem cells (BMMSC) for both BG incorporated and BG-free composite extracts [125]. Li et al. [124] incorporated borosilicate BG composition of 23.6 g Na2CO3, 40.96 g K2CO3, 24.92 g MgCO3, 81.64 g CaCO3, 40.08 g SiO2, 165.64 g H3BO3 and 23.16 g NaH2PO4·2H2O into the magnesium phosphate bone cement (MPC). The percentage of the cellular viability decreased for both MPC and BG incorporated MPC (BG/MPC) composites when the concentration of the extracted solution increased. Besides, extracts of MPC (for the extract concentrations ranging from 9.375 to 150 mg/ml) showed 20–50% higher cellular viability, if compared to the extracts of BG/MPC (when extract concentration was below 37.5 mg/ml) composites. This notable difference was attributed to the more dominant effect of magnesium ions on the proliferation of BMMSC, than that obtained when Si4+ or boron (B) ions were present in the extracted medium [124]. Additionally, B and Si ions at higher concentrations had limited effect on the proliferation of BMSCs, while lower concentrations exhibited stimulative effects [124]. These results were attributed to the BG ability to regulate the pH of the surrounding medium. Also, the results of Fan et al. [126] on Mg and Sr ion incorporated BG/CPC composites revealed that the medium extracts taken on day 1, 3 and 5 showed good cytocompatibility with an increased number of MC3T3-E1 cells over the incubation period.
Although the indirectly measured cell response gives a comprehensive assessment about the effect that material composition has on cells, direct cell culture techniques provide more detailed insights in the cell-material interactions [63]. Osteoblast cells (obtained from calvariae of 1-to 2-day-old Sprague-Dawley rats) [117], MG63 cells [118], MC3T3-E1 cells [120,123]) were directly seeded on CPC and BG incorporated CPC composites, and results revealed that cell growth, attachment and proliferation were significantly promoted on BG added CPC composites due to the Ca2+ and Si4+ ion enriched environment. Yu et al. [117] found better cellular attachment on the surface of 20 wt% 45S5 BG incorporated CPC composites than on surfaces of BG-free composites after 8 h of cell seeding, indicating the positive effect of BG incorporation on the cellular attachment. This finding has important implications for early-enhanced cellular attachment, which is mostly governed by the adsorption of serum proteins such as fibronectin onto the BG [117,139]. Authors also pointed out that this strong irreversible adsorption of serum proteins was encouraged through the high negative-surface-charge density of the surface silanols (Si–OH) of BG [116,117,140]. Sadiasa et al. [118] further verified that the osteoblast adhesion, proliferation as well as the cell-material interaction were promoted by increased BG concentration in CPC composites with longer incubation time. The expression level of osteogenic gene markers, namely collagen-I, bone sialoprotein, osteonectin and osteopontin was found to be higher in 30 wt% BG (composition of 60% SiO2, 36% CaO and 4% P2O5 with balanced Na2O) incorporated CPC composites compared to BG-free composites, suggesting that BG incorporation can initiate an active mineralization in bone tissue [118]. Axrap et al. [120] studied BMP-2 and TGF-β expression levels, and found that they were significantly higher for BG incorporated CPC samples. As in vitro gene expression is regulated by the autocrine response to the ion release, higher levels of the growth factors and gene expression can be directly attributed to increased ion release from the BG component. Similarly, Hasan et al. [123] conducted immunocytochemical analysis by examining the expression of alkaline phosphatase (ALP) and osteopontin. Both expression results of bone formation markers displayed higher level of fluorescence, indicating that the BG incorporation directly influenced osteogenic differentiation. Finally, both indirect and direct cell studies showed that the high bioactivity of BG facilitates better cell behavior [141].
Recent studies have demonstrated that the incorporation of such ions as boron (B), zinc (Zn), magnesium (Mg) or strontium (Sr) into the BG or CPC simulates the osteogenic differentiation and mineralization both in vitro and in vivo [108,115]. Besides, addition of silver and B ions into the BG silicate network has been explored as a tool to develop antibacterial and antimicrobial materials [108]. Yang et al. [115], Li et al. [124], and Fan et al. [126] incorporated Zn and Sr, B, Mg and Sr ions, respectively. Their in vitro cellular results were in agreement with literature results in which cell proliferative effects were shown. Despite the positive effects of these ions on the cellular response, such important parameter as cytotoxic level of each individual ion needs to be considered very carefully. For example, Zn concentrations in the range of 2–8 ppm might be harmful for osteoblasts due to oxidative stresses [142], while Sr-containing BGs were found to be non-toxic [143]. Cu ions showed a negative effect on MG-63 cells when the concentration was higher than 1000 μg/ml [144]. Up to 10 wt% addition of boron ion into BG showed no cytotoxicity [145]. The effects of incorporation of less-common ions such as Ba2+, Bi3+, Cl−, Cr6+, Dy3+, Eu3+, Gd3+, Yb3+, Th3+, Ge2+, Au3+, Ho3+, I−, La3+, Mn2+, Mo6+, Ni2+, Nb5+, N3−, Pd2+, Rb+, Sm3+, Se4+, Ta5+, Te4+, Tb3+, Er3+, Sn2+, W6+, V5+, Y3+, and Zr4+ on the biological properties of BGs have been comprehensively reviewed, showing the potential beneficial effect of these rare ions on biological response when applied in certain concentrations [146]. Obtained results revealed that in most cases the ionic dissolution products from BGs augment osteogenesis by regulating osteoblast proliferation, differentiation, and gene expression. Therefore, it is possible to improve the biological properties of BG incorporated CPC composites by doping the added BG component with different ions within their non-toxic range.
Although both indirect and direct cell studies on injectable BG added CPC composites have led to positive results, a deeper cell biology analysis is needed to further understand the biological responses of these composites. For example, direct cell culture techniques possess some limitations. Usually the material to be tested requires pre-treatment before cell seeding, while static 3D in vitro cell culture is insufficient to keep the cell numbers stable. Moreover the high degree of dissolution of BG-CPC composites is detrimental to cell in vitro growth because of pH changes (increase) of the incubated medium [62]. Therefore, it is essential to develop standardized methods and procedures to evaluate the biological responses to the materials such as cell attachment, adhesion and spreading at the initial stage of cell/material interactions and the subsequent cell proliferation, differentiation and gene expression [105]. Since bone tissue is not composed of a single cell type, versatile co-culture models such as endothelial cell/osteoblast, osteoclast/osteoblast, and chondrocytes/osteoblast should be used to mimic in-vitro in a more realistic manner the potential interaction of BG incorporated CPC composites with bone tissue [147]. In general, the biological responses of composites can be evaluated both qualitatively (by showing the organization of cytoskeletal proteins) and quantitatively (by performing cytotoxicity measurements and by characterizing the growth, proliferation, differentiation, phenotype, and gene expression of cells).
3.6. BG impact on CPC in vivo response
All researches directed to CPC modifications aim to improve the in vivo response of the final material composition by designing an injectable bone cement with enhanced biocompatibility, osteoconductivity and osteoinductivity, leading to adequate bone healing and new bone formation [[148], [149], [150]]. When the materials are implanted into the bone defect area, two scenarios are faced. In the first scenario, an intermediate fibrous tissue could be formed and the material fails both mechanically and biologically. In the second case, direct bone-material bonding occurs, which leads to the successful osteointegration [151]. After implantation and injection of BG incorporated CPC composites at the defect site of animal models, a biological apatite later is formed on the implant surface, which can act as a matrix for cell migration, adhesion and growth [115,[117], [118], [119],123,124]. El-Fiqi et al. [119] have shown that both CPC and BG (composition; 85% SiO2 and 15% CaO in mol) added CPC composites were biocompatible, if implanted subcutaneously in rat model. Higher bone volume was detected in the implanted area of BG incorporated CPC samples, indicating better bone ingrowth, if compared with BG-free composites. Obtained data suggested that bone formation was achieved due to the composites’ osteoconductivity, which further stimulated the osteogenic cell proliferation. Histological staining further proved that new bone formation around the composite has been detected after 6 weeks. The main difference between BG added composites and BG-free CPC composites was detected in their in vivo degradation profile, since the degradation of BG-free CPC composites was not notable [119]. Yang et al. [115] attributed similar findings to the homogeneous dispersion of CaP and BG particles in the porous hydrogel network and also to the critical amounts of silicon, strontium and zinc ions, which could induce the relevant genes of osteogenic cells. The interaction between the bone and BG incorporated CPC composite was clearly observed along the longitudinal depth of the composite verified by the collagen deposits and presence of osteoblasts. Meanwhile, μ-CT images of the extracted samples revealed that the BG added composites degraded more rapidly than BG-free CaP composites, supporting the previous findings of higher BG degradation and resorbability rates, while inducing the bone growth [115,117,118,123,124]. Due to the higher degradation rate of BG incorporated composites, the pore size of these composites increased as a function of time, which led to the new bone ingrowth into the material after 12 weeks of implantation [117,118,123]. According to these data, we can infer that biodegradation and bone ingrowth were in balance as dissolved ions positively influenced the microenvironment of cells, leading to cell-mediated degradation.
These results prove that BG incorporation also supports osteogenesis and has potential for neovascularization. Some publications in literature have reported the in vivo effects of BG and CaP pastes (see Table 4). These publications are helpful to understand the synergetic effect of combining these two phases together. In these studies, CPCs showed slower bone healing process compared to BG cements [[152], [153], [154]]. These findings could be attributed to the high resorption rates of BG material. Also, a slower bone healing process was explained by the foreign body immune reaction processes. Since BG cements are able to form an apatite layer when they are in contact with body fluids, this layer could decrease fibrous tissue formation. Ding et al. [155] and Cui et al. [156] described in vivo drug release profiles from both borate-BG and CaP cement. In vivo drug release kinetics was also correlated with the cement biodegradation pattern, which was found to be higher for the borate-BG cement [156]. A study of Araújo et al. [153] compared in vivo properties of the fabricated low temperature CaP (LTCaP) with other commercially available bone substitutes including BG (Bio-Gran®). Although the higher bone volume was detected for BG and the lowest bone volume was noticed for the fabricated LTCaP, LTCaP displayed complete bone remodeling after 16 weeks compared to the commercially available alternatives (resorption of an organic bovine bone (AB), bioactive glass (BG), and demineralized bone matrix (DBM) was 8.6%, 32.4% and 37.5% in volume from week 3–16, respectively). Authors attributed these results to such physical properties of LTCaP as increased surface area and microporosity [153]. In another study, newly formed blood vessels were detected along with newly bone formation after 2 weeks of injectable mesoporous BG nanoparticle implantation (the ratio of Ca:Si was varied; either at 5:95, 15:85, or 25:75 by mole, however authors did not indicate which composition was implanted) [154]. The main differences between the mesoporous BG nanoparticles and conventional CaP cement were explained by their effect on the bone formation process [154]. While mesoporous BG nanoparticles presented osteoinductive process, conventional CaP cement revealed osteoconductive properties. Trace amount of silicon, strontium and zinc ions incorporated into the octacalcium phosphate (OCP)-BG composite could synergistically influence osteogenic gene expression which would further improve bone ingrowth capability as Yang et al. stated [115]. Moreover, it has been pointed out that the addition of ions such as strontium, zinc, boron and zirconium has a co-stimulatory effect on angiogenesis together with the dissolution products of BG [117,123,124,157,158].
Table 4.
In vivo experimentation overview performed by an injectable BG incorporated CaP cement composite.
| Species | Defect Details | Implantation Time | Analysis Techniques | Examination | Ref |
|---|---|---|---|---|---|
| Sprague-Dawley rats | Femoral bone marrow cavity, 1.2-mm diameter holes | 1, 2, 4 and 8 weeks | DXA | BMD | [115] |
| Radiographic and μCT | Bone maturation corresponding to the appearance of injected implant | ||||
| Histological analysis | Bone-material interaction | ||||
| New bone ingrowth | |||||
| Biodegradation | |||||
| New Zealand white rabbits | Femoral condyle 6 mm diameter and 10 mm height) | 4 and 12 weeks | Macroscopic evaluation | Degree of specimen incorporation and tissue reactions adjacent to the specimens | [117] |
| μCT | In vivo resorption of the implant | ||||
| RMVF | |||||
| Histological analysis | BVF | ||||
| Bone formation | |||||
| Biodegradation | |||||
| Sprague-Dawley rats | subcutaneous tissues | 2 weeks | Histological analysis | Biocompatibility | [119] |
| Bone formation | |||||
| calvarial bone defects with 6-mm diameter | 6 weeks | Tissue-material interaction | |||
| Biodegradation | |||||
| New Zealand rabbits | Femur heads 6 mm diameter and 5 mm thickness | 4 and 12 weeks | μCT | Quantitative new bone formation | [123] |
| Tissue-material interaction | |||||
| Histological analysis | Bone formation | ||||
| Biodegradation | |||||
| New Zealand white rabbit | Femoral condyle 5 mm diameter and 5–10 mm depth | 4 and 8 weeks | X-ray and μCT | Biodegradation | [124] |
| Bone regeneration | |||||
| Histological analysis | Bone formation | ||||
| Tissue-material interaction | |||||
| New Zealand white rabbit | Femoral bone 4 mm diameter | 3 months | μCT | Tissue-material interaction | [118] |
| Quantitative bone formation | |||||
| Histological analysis and microscopic evaluation | Bone formation | ||||
| Biodegradation |
DXA– dual energy x-ray analysis, BMD – bone marrow density, μCT– micro computerized tomography, RMVF – the residual material volume fraction, BMF – bone volume fraction.
Properties of unset BG incorporated CPC composites such as setting time, cohesion, injectability and ease of application to the surgical site and properties of set BG added CPC composites such as mechanical properties, porosity, biodegradation and biocompatibility showed that all mentioned features could contribute synergistically to build injectable BG/CPC composites which are attractive for clinical translation. The reviewed studies have contributed to our understanding that a bone-like apatite layer on the surface of the materials should be induced to build a strong bond to bone tissue already at the early stage of the implantation. BG incorporation into CaP paste revealed that a sustained apatite formation can be more likely achieved, if they have integrated; because the CaP phase degrades more slowly than the glassy phase. In addition, pH and ion release can be controlled by tuning the physical properties of the added BG particles. By doing this, sustained release of Ca2+, Si4+ ions and other incorporated biologically active ions might be obtained in order to improve osteogenesis and angiogenesis.
4. Conclusion and future outlook
CPCs are highly desirable for bone tissue substitution because of their injectability, bioactivity and biocompatibility. They are either utilized as pre-set scaffolds or injectable pastes. Their injectable property is promising for minimally invasive application, so that they can fill irregular bone defects. Although most CPC formulations provide the properties of an ‘ideal’ injectable bone cement, CPCs still possess two main drawbacks. One of them is their mechanical weakness which limits their application in load bearing bone sites. Secondly, CPCs have inadequate biodegradation which fails to achieve degradation rate suitable for the bone formation process. Many attempts have been done during the last decades to overcome these drawbacks and several respective reviews on CPCs describe how their mechanical properties, processing approach, drug delivery ability and handling properties can be enhanced. One of the more recent attempts is to combine BGs with CPC, since BGs are well-known for their direct bone bonding ability as well as their high bioactivity. Although meritorious reviews have been carried out on combination of BGs and CaP, no systemic review exists which summarizes the currently available published scientific literature data on BG incorporated injectable CaP bone cements analysing BG incorporated CPC composite properties based on the criteria that make an ideal injectable bone cement.
Since all these parameters of injectable bone cements, namely degree of injectability, rheology, setting time, anti-washout properties and cohesiveness, are dependent each on other, results should be discussed accordingly. Thus, prospective studies should include all these parameters to build up a comparable experimental design in order to interpret obtained outputs effectively and then further improve in vitro and in vivo performance of the designed materials. However, none of the published papers on BG incorporated injectable CPC has presented all the desired properties relevant to injectable bone cements as one data set. For example, rheological properties of BG incorporated CPC composites had only been characterized by Yang et al. [115]. Considerably more rheological characterization would be beneficial to provide insightful information about the setting and hardening mechanisms of BG incorporated CPC composites. Also, it should be noted that all these experiments would benefit if performed by using ISO or ATCC standards, thereby building uniform study designs, where outcomes can be compared directly with each other.
Application of different types of CPC, BG compositions and types (melt-derived, sol-gel-derived, microsized, nanosized etc.) as well as liquid phases to form injectable BG added CPC composites has resulted in wide differences between setting time and compressive strength results. Despite these variances, handling properties of injectable BG incorporated CPC composites were found to be adequate, based on their injectability and anti-wash-out characterization. Although the mechanical properties of BG added CPC composites in general mimicked those of trabecular bone, it must be pointed out that the mechanical properties of injectable CPCs cannot be further improved to make them appropriate for load bearing bone tissue applications. Therefore, efforts should be channeled to improve the degradation rate of CPCs, which should be well-balanced to the dynamics of bone formation. In this regard, BG inclusion represents a significant element to achieve controlled degradation.
Summarizing available research data on injectable BG incorporated CPC composites, it can also be concluded that induction of a bone-like apatite layer on the surface of the developed material is key to build a bond to bone tissue at the early stage of implantation. Studies on BG incorporation into CaP paste revealed that sustained apatite formation can be more likely achieved in such systems compared to unmodified CPC composites. In addition, pH and ion release can be controlled by tunning the physicochemical properties and composition of BG particles. By this approach, sustained release of Ca2+ and Si4+ ions might be obtained in order to improve the osteogenic and angiogenetic properties of the injectable BG incorporated CPC. Taking all together, individual properties of BG and CaP cements can be boosted by integrating them for better physicochemical properties, biodegradation, osteoconductivity, osteoinductivity, and angiogenecity. Especially angiogenic effects could be enhanced by incorporation of biologically active ions in BGs.
Further experiments, using unified characterization techniques to evaluate injectable bone cements, could shed more light on the effect of BG addition on hardening mechanism and physicochemical properties of CPCs. Toughness and fatigue resistance of BG incorporated CPC composites should be also evaluated to achieve a better mechanical performance, considering that highly bonded materials are stiffer than natural bone which could cause implant failure. Therefore, future research should be also targeted to examine and improve the interfacial strength rather than changing the properties of powder or liquid phases. Despite the promising in vitro and in vivo performance of injectable BG incorporated CPCs, further research should be undertaken to explore cell-mediated degradation mechanisms of injectable BG incorporated CaP, CaP cements and BG cement composites. Meanwhile, the released ions from BGs, namely Si4+ ions or incorporated ions such as boron, zinc and strontium, should be monitored, while performing in vivo studies in order to confirm their non-nephrotoxicity or their elimination from the body. The developed BG incorporated CPC composites can be also utilized for the controlled and targeted delivery of bioactive agents such as anti-cancer drugs, anti-inflammatory drugs or growth factors. Due to the presence of the BG component, it is also possible to tailor these composites to serve as dual drug/ion delivery vehicles and in this way to increase their biological functionality.
Declaration of competing interest
Authors do not have any conflicts of interest to declare.
Acknowledgement
The authors acknowledge financial support from the European Union's Horizon 2020 research and innovation programme under the grant agreement No 857287 (BBCE).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Aldo R. Boccaccini, Email: aldo.boccaccini@fau.de.
Dagnija Loca, Email: Dagnija.Loca@rtu.lv.
References
- 1.Bauer T.W., Muschler G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000:10–27. [PubMed] [Google Scholar]
- 2.Laurencin Cato E.-A.F.S., Khan Yusuf. Bone graft substitutes. Princ. Pract. Wrist Surg. 2009:277–287. [Google Scholar]
- 3.Precheur H.V. Bone graft materials, dent. Clin. North Am. 2007;51:729–746. doi: 10.1016/j.cden.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Şahin E. Cem. Based Mater. IntechOpen; 2018. Calcium phosphate bone cements; pp. 206–230. [DOI] [Google Scholar]
- 5.O'Neill R., McCarthy H.O., Montufar E.B., Ginebra M.P., Wilson D.I., Lennon A., Dunne N. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 2017;50:1–19. doi: 10.1016/j.actbio.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Rangabhatla A.S.L., Tantishaiyakul V., Oungbho K., Boonrat O. Fabrication of pluronic and methylcellulose for etidronate delivery and their application for osteogenesis. Int. J. Pharm. 2016;499:110–118. doi: 10.1016/j.ijpharm.2015.12.070. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Zhang X., Kang L., Xu F., Wang Z., Cui F.-Z., Guo Z. Recent progress in injectable bone repair materials research. Front. Mater. Sci. 2015;9:332–345. doi: 10.1007/s11706-015-0310-z. [DOI] [Google Scholar]
- 8.Perez R.A., Shin S.-H., Han C.-M., Kim H.-W. Bioactive injectables based on calcium phosphates for hard tissues: a recent update. Tissue Eng. Regen. Med. 2015;12:143–153. doi: 10.1007/s13770-015-0096-1. [DOI] [Google Scholar]
- 9.Loca D., Sokolova M., Locs J., Smirnova A., Irbe Z. Calcium phosphate bone cements for local vancomycin delivery. Mater. Sci. Eng. C. 2015;49:106–113. doi: 10.1016/J.MSEC.2014.12.075. [DOI] [PubMed] [Google Scholar]
- 10.Irbe Z., Loca D., Vempere D., Berzina-Cimdina L. Controlled release of local anesthetic from calcium phosphate bone cements. Mater. Sci. Eng. C. 2012;32:1690–1694. doi: 10.1016/J.MSEC.2012.04.069. [DOI] [PubMed] [Google Scholar]
- 11.V Dorozhkin S. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015;6 doi: 10.3390/jfb6030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang J.H., Shin S., Kim H.J., Jeong J., Jin H.E., Desai M.S., Lee S.W., Kim S.Y. Improvement of physical properties of calcium phosphate cement by elastin-like polypeptide supplementation. Sci. Rep. 2018;8:1–11. doi: 10.1038/s41598-018-23577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow L.C. Calcium phosphate cements: chemistry, properties, and applications. MRS Proc. 1999;599:27. doi: 10.1557/PROC-599-27. [DOI] [Google Scholar]
- 14.Liao H., Walboomers X.F., Habraken W.J.E.M., Zhang Z., Li Y., Grijpma D.W., Mikos A.G., Wolke J.G.C., Jansen J.A. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater. 2011;7:1752–1759. doi: 10.1016/j.actbio.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Yang H.L., Zhu X.S., Chen L., Chen C.M., Mangham D.C., Coulton L.A., Aiken S.S. Bone healing response to a synthetic calcium sulfate/β-tricalcium phosphate graft material in a sheep vertebral body defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100 B:1911–1921. doi: 10.1002/jbm.b.32758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verron E., Pissonnier M.L., Lesoeur J., Schnitzler V., Fellah B.H., Pascal-Moussellard H., Pilet P., Gauthier O., Bouler J.M. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater. 2014;10:4887–4895. doi: 10.1016/j.actbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 17.O'Neill R., McCarthy H.O., Montufar E.B., Ginebra M.-P., Wilson D.I., Lennon A., Dunne N. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 2017;50:1–19. doi: 10.1016/J.ACTBIO.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Ginebra M., Canal C., Espanol M., Pastorino D., Montufar E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Ginebra M.P., Traykova T., Planell J.A. Calcium phosphate cements as bone drug delivery systems: a review. J. Contr. Release. 2006;113:102–110. doi: 10.1016/j.jconrel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Brown W.E., Chow L.C. A new calcium-phosphate setting cement. J. Dent. Res. 1983;62:672. [Google Scholar]
- 21.Brown W.E., Chow L.C. A new calcium phosphate, water-setting cement. Cem. Res. Prog. 1986:351–379. http://ci.nii.ac.jp/naid/10004554570/en/ [Google Scholar]
- 22.Thai V.V., Lee B.T. Fabrication of calcium phosphate – calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010;21:1867–1874. doi: 10.1007/s10856-010-4058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez R.A., Kim H.W., Ginebra M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012;3:1–20. doi: 10.1177/2041731412439555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajzer I., Castaño O., Engel E., Planell J.A. Injectable and fast resorbable calcium phosphate cement for body-setting bone grafts. J. Mater. Sci. Mater. Med. 2010;21:2049–2056. doi: 10.1007/s10856-010-4078-5. [DOI] [PubMed] [Google Scholar]
- 25.Sarda S., Fernández E., Nilsson M., Balcells M., Planell J.A. Kinetic study of citric acid influence on calcium phosphate bone cements as water-reducing agent. J. Biomed. Mater. Res. 2002;61:653–659. doi: 10.1002/jbm.10264. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama A., Yamamoto S., Kawasaki T., Kohgo T., Nakasu M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials. 2002;23:1091–1101. doi: 10.1016/S0142-9612(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.J., Kim B., Padalhin A.R., Lee B.T. Incorporation of chitosan-alginate complex into injectable calcium phosphate cement system as a bone graft material. Mater. Sci. Eng. C. 2019;94:385–392. doi: 10.1016/j.msec.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 28.Ginebra M.P., Rilliard A., Fernández E., Elvira C., San Román J., Planell J.A. Mechanical and rheological improvement of a calcium phosphate cement by the addition of a polymeric drug. J. Biomed. Mater. Res. 2001;57:113–118. doi: 10.1002/1097-4636(200110)57:1<113::AID-JBM1149>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Song H.Y., Rahman A.H.M.E., Lee B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using chitosan and citric acid. J. Mater. Sci. Mater. Med. 2009;20:935–941. doi: 10.1007/s10856-008-3642-8. [DOI] [PubMed] [Google Scholar]
- 30.Shi H., Zhang W., Liu X., Zeng S., Yu T., Zhou C. Synergistic effects of citric acid - sodium alginate on physicochemical properties of α-tricalcium phosphate bone cement. Ceram. Int. 2019;45:2146–2152. doi: 10.1016/j.ceramint.2018.10.124. [DOI] [Google Scholar]
- 31.Zhong W., Sun L., Yu T., Zhou C. Preparation and characterization of calcium phosphate cement with enhanced tissue adhesion for bone defect repair. Ceram. Int. 2021;47:1712–1720. doi: 10.1016/j.ceramint.2020.08.288. [DOI] [Google Scholar]
- 32.Li D.X., Fan H.S., Zhu X.D., Tan Y.F., Xiao W.Q., Lu J., Xiao Y.M., Chen J.Y., Zhang X.D. Controllable release of salmon-calcitonin in injectable calcium phosphate cement modified by chitosan oligosaccharide and collagen polypeptide. J. Mater. Sci. Mater. Med. 2007;18:2225–2231. doi: 10.1007/s10856-007-3084-8. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez Caballero S.S., Ferri-Angulo D., Debret R., Granier F., Marie S., Lefèvre F.X., Bouler J.M., Despas C., Sohier J., Bujoli B. Combination of biocompatible hydrogel precursors to apatitic calcium phosphate cements (CPCs): influence of the in situ hydrogel reticulation on the CPC properties. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109:102–116. doi: 10.1002/jbm.b.34685. [DOI] [PubMed] [Google Scholar]
- 34.Nezafati N., Farokhi M., Heydari M., Hesaraki S., Nasab N.A. In vitro bioactivity and cytocompatibility of an injectable calcium phosphate cement/silanated gelatin microsphere composite bone cement. Compos. B Eng. 2019;175 doi: 10.1016/j.compositesb.2019.107146. [DOI] [Google Scholar]
- 35.Oğuz Ö.D., Ege D. Preparation of graphene oxide-reinforced calcium phosphate/calcium sulfate/methylcellulose-based injectable bone substitutes. MRS Commun. 2019;9:1174–1180. doi: 10.1557/mrc.2019.125. [DOI] [Google Scholar]
- 36.Babo P.S., Santo V.E., Gomes M.E., Reis R.L. Development of an injectable calcium phosphate/hyaluronic acid microparticles system for platelet lysate sustained delivery aiming bone regeneration. Macromol. Biosci. 2016;16:1662–1677. doi: 10.1002/mabi.201600141. [DOI] [PubMed] [Google Scholar]
- 37.Kucko N.W., Li W., García Martinez M.A., ur Rehman I., Ulset A.S.T., Christensen B.E., Leeuwenburgh S.C.G., Herber R.P. Sterilization effects on the handling and degradation properties of calcium phosphate cements containing poly (D,L-lactic-co-glycolic acid) porogens and carboxymethyl cellulose. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:2216–2228. doi: 10.1002/jbm.b.34306. [DOI] [PubMed] [Google Scholar]
- 38.Thai V.V., Lee B.T. Fabrication of calcium phosphate-calcium sulfate injectable bone substitute using hydroxy-propyl-methyl-cellulose and citric acid. J. Mater. Sci. Mater. Med. 2010;21:1867–1874. doi: 10.1007/s10856-010-4058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demir Ö., Oğuz, Ege D. Rheological and mechanical properties of thermoresponsive methylcellulose/calcium phosphate-based injectable bone substitutes. Materials. 2018;11 doi: 10.3390/ma11040604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Link D.P., van den Dolder J., Jurgens W.J.F.M., Wolke J.G.C., Jansen J.A. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006;27:4941–4947. doi: 10.1016/j.biomaterials.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Low K.L., Tan S.H., Zein S.H.S., Roether J.A., Mouriño V., Boccaccini A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94B:273–286. doi: 10.1002/jbm.b.31619. [DOI] [PubMed] [Google Scholar]
- 42.Sarda S., Fernández E., Nilsson M., Balcells M., Planell J.A. Kinetic study of citric acid influence on calcium phosphate bone cements as water-reducing agent. J. Biomed. Mater. Res. 2002;61:653–659. doi: 10.1002/jbm.10264. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama A., Yamamoto S., Kawasaki T., Kohgo T., Nakasu M. Development of calcium phosphate cement using chitosan and citric acid bone substitute materials. Biomaterials. 2002;23:1091–1101. doi: 10.1016/S0142-9612(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 44.Leroux L., Hatim Z., Frèche M., Lacout J.L. Effects of various adjuvants (lactic acid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone. 1999;25:31S–34S. doi: 10.1016/S8756-3282(99)00130-1. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Ye J., Wang Y. Influence of a novel radiopacifier on the properties of an injectable calcium phosphate cement. Acta Biomater. 2007;3:757–763. doi: 10.1016/j.actbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Chen F., Liu C., Wei J., Chen X., Zhao Z., Gao Y. Preparation and characterization of injectable calcium phosphate cement paste modified by polyethylene glycol-6000. Mater. Chem. Phys. 2011;125:818–824. doi: 10.1016/j.matchemphys.2010.09.050. [DOI] [Google Scholar]
- 47.Zhang J., Liu W., Gauthier O., Sourice S., Pilet P., Rethore G., Khairoun K., Bouler J.-M., Tancret F., Weiss P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2015;31 doi: 10.1016/j.actbio.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 48.Xiupeng W., Ye J., Wang Y., Chen L. Self-setting properties of a b-dicalcium silicate-reinforced calcium phosphate cement. J. Biomed. Mater. Res. B Appl. Biomater. 2007;82:93–99. doi: 10.1002/jbm.b.30709. [DOI] [PubMed] [Google Scholar]
- 49.Hench L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 50.Wu Z., Lin Z., Yao A., Ye S., Pan H., Cui X., Wang D. Influence of particle size distribution on the rheological properties and mathematical model fitting of injectable borosilicate bioactive glass bone cement. Ceram. Int. 2020;46:24395–24406. doi: 10.1016/j.ceramint.2020.06.222. [DOI] [Google Scholar]
- 51.T.A. van Vugt, J.A.P. Geurts, J.J. Arts, N.C. Lindfors, 3 - Biomaterials in treatment of orthopedic infections, in: J.J.C. Arts, J.B.T.-M. of P.J.I. (PJIs) Geurts (Eds.), Woodhead Publishing, 2017: pp. 41-68. doi:https://doi.org/10.1016/B978-0-08-100205-6.00003-3.
- 52.Xynos I.D., Edgar A.J., Buttery L.D., Hench L.L., Polak J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J. Biomed. Mater. Res. 2001;55:151–157. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 53.Ojansivu M., Mishra A., Vanhatupa S., Juntunen M., Larionova A., Massera J., Miettinen S. The effect of S53P4-based borosilicate glasses and glass dissolution products on the osteogenic commitment of human adipose stem cells. PLoS One. 2018;13 doi: 10.1371/journal.pone.0202740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gorustovich A.A., Roether J.A., Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng. B Rev. 2010;16:199–207. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 55.Zhang D., Leppäranta O., Munukka E., Ylänen H., Viljanen M.K., Eerola E., Hupa M., Hupa L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. A. 2010;93:475–483. doi: 10.1002/jbm.a.32564. [DOI] [PubMed] [Google Scholar]
- 56.Day R.M., Boccaccini A.R. Effect of particulate bioactive glasses on human macrophages and monocytes in vitro. J. Biomed. Mater. Res. A. 2005;73:73–79. doi: 10.1002/jbm.a.30262. [DOI] [PubMed] [Google Scholar]
- 57.V Thomas M., Puleo D.A., Al-Sabbagh M. Bioactive glass three decades on. J. Long Term Eff. Med. Implants. 2005;15:585–597. doi: 10.1615/jlongtermeffmedimplants.v15.i6.20. [DOI] [PubMed] [Google Scholar]
- 58.Rahaman M.N., Day D.E., Bal B.S., Fu Q., Jung S.B., Bonewald L.F., Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011;7:2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang W., Day D.E., Kittiratanapiboon K., Rahaman M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006;17:583–596. doi: 10.1007/s10856-006-9220-z. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen F.H. New essential trace elements for the life sciences. Biol. Trace Elem. Res. 1990;26–27:599–611. [PubMed] [Google Scholar]
- 61.Pemmer B., Roschger A., Wastl A., Hofstaetter J.G., Wobrauschek P., Simon R., Thaler H.W., Roschger P., Klaushofer K., Streli C. Spatial distribution of the trace elements zinc, strontium and lead in human bone tissue. Bone. 2013;57:184–193. doi: 10.1016/j.bone.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bellucci D., Sola A., Cannillo V. Hydroxyapatite and tricalcium phosphate composites with bioactive glass as second phase: state of the art and current applications. J. Biomed. Mater. Res. 2016;104:1030–1056. doi: 10.1002/jbm.a.35619. [DOI] [PubMed] [Google Scholar]
- 63.Karadjian M., Essers C., Tsitlakidis S., Reible B., Moghaddam A., Boccaccini A.R., Westhauser F. Biological properties of calcium phosphate bioactive glass composite bone substitutes: current experimental evidence. Int. J. Mol. Sci. 2019;20:1–22. doi: 10.3390/ijms20020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Au - Robinson T.E., Au - Hughes E.A.B., Au - Eisenstein N.M., Au - Grover L.M., Au - Cox S.C. The quantification of injectability by mechanical testing. JoVE. 2020 doi: 10.3791/61417. [DOI] [PubMed] [Google Scholar]
- 65.Liu C., He H. 2018. Developments and Applications of Calcium Phosphate Bone Cements. [DOI] [Google Scholar]
- 66.Bohner M., Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 67.Pina S., Olhero S.M., Gheduzzi S., Miles A.W., Ferreira J.M.F. Influence of setting liquid composition and liquid-to-powder ratio on properties of a Mg-substituted calcium phosphate cement. Acta Biomater. 2009;5:1233–1240. doi: 10.1016/j.actbio.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 68.Nehdi M., Al Martini S. Estimating time and temperature dependent yield stress of cement paste using oscillatory rheology and genetic algorithms. Cement Concr. Res. 2009;39:1007–1016. doi: 10.1016/j.cemconres.2009.07.011. [DOI] [Google Scholar]
- 69.Dapporto M., Gardini D., Tampieri A., Sprio S. Nanostructured strontium-doped calcium phosphate cements: a multifactorial design. Appl. Sci. 2021;11:2075. doi: 10.3390/app11052075. [DOI] [Google Scholar]
- 70.Sohrabi M., Hesaraki S., Kazemzadeh A., Alizadeh M. Development of injectable biocomposites from hyaluronic acid and bioactive glass nano-particles obtained from different sol-gel routes. Mater. Sci. Eng. C. 2013;33:3730–3744. doi: 10.1016/j.msec.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Sohrabi M., Hesaraki S., Kazemzadeh A. The influence of polymeric component of bioactive glass-based nanocomposite paste on its rheological behaviors and in vitro responses: hyaluronic acid versus sodium alginate. J. Biomed. Mater. Res. B Appl. Biomater. 2014;102:561–573. doi: 10.1002/jbm.b.33035. [DOI] [PubMed] [Google Scholar]
- 72.Şahin E., Kalyon D.M. The rheological behavior of a fast-setting calcium phosphate bone cement and its dependence on deformation conditions. J. Mech. Behav. Biomed. Mater. 2017;72:252–260. doi: 10.1016/j.jmbbm.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Wu Z., Lin Z., Yao A., Ye S., Pan H., Cui X., Wang D. Influence of particle size distribution on the rheological properties and mathematical model fitting of injectable borosilicate bioactive glass bone cement. Ceram. Int. 2020;46:24395–24406. doi: 10.1016/j.ceramint.2020.06.222. [DOI] [Google Scholar]
- 74.Zhang J., Liu W., Schnitzler V., Tancret F., Bouler J.M. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10:1035–1049. doi: 10.1016/j.actbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 75.Chen F., Song Z., Liu C. Fast setting and anti-washout injectable calcium-magnesium phosphate cement for minimally invasive treatment of bone defects. J. Mater. Chem. B. 2015;3:9173–9181. doi: 10.1039/c5tb01453k. [DOI] [PubMed] [Google Scholar]
- 76.Li X., He F., Ye J. Preparation, characterization and: in vitro cell performance of anti-washout calcium phosphate cement modified by sodium polyacrylate. RSC Adv. 2017;7:32842–32849. doi: 10.1039/c7ra03221h. [DOI] [Google Scholar]
- 77.Bohner M., Doebelin N., Baroud G. Theoretical and experimental approach to test the cohesion of calcium phosphate pastes. Eur. Cell. Mater. 2006;12:26–35. doi: 10.22203/eCM.v012a03. [DOI] [PubMed] [Google Scholar]
- 78.Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76:456–468. doi: 10.1002/jbm.b.30398. [DOI] [PubMed] [Google Scholar]
- 79.López A., Engqvist H. 2014. Compositions Comprising Injectable Biomaterial Cement and Radiopacity Improving Agent. WO 2014/016707 A2. [Google Scholar]
- 80.Zhao S., Pei Z., Zhang K., Li G., Liu P., Chang J., Zhang K., Mi L. Injectable calcium phosphate containing zirconia short fiber with enhanced radiopacity. Mater. Technol. 2020:1–6. doi: 10.1080/10667857.2020.1797327. [DOI] [Google Scholar]
- 81.Chen F., Liu C., Mao Y. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010;6:3199–3207. doi: 10.1016/j.actbio.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 82.Wu C.C., Yang K.C., Yang S.H., Lin M.H., Kuo T.F., Lin F.H. In vitro studies of composite bone filler based on poly(propylene fumarate) and biphasic α-tricalcium phosphate/hydroxyapatite ceramic powder. Artif. Organs. 2012;36:418–428. doi: 10.1111/j.1525-1594.2011.01372.x. [DOI] [PubMed] [Google Scholar]
- 83.Persson C., Berg S. Strategies towards injectable, load-bearing materials for the intervertebral disc: a review and outlook. J. Mater. Sci. Mater. Med. 2013;24:1–10. doi: 10.1007/s10856-012-4776-2. [DOI] [PubMed] [Google Scholar]
- 84.Liu H., Zhang Z., Gao C., Bai Y., Liu B., Wang W., Ma Y., Saijilafu, Yang H., Li Y., Chan A., Yang L. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C. 2020;116 doi: 10.1016/j.msec.2020.110904. [DOI] [PubMed] [Google Scholar]
- 85.Di Filippo M.F., Dolci L.S., Albertini B., Passerini N., Torricelli P., Parrilli A., Fini M., Bonvicini F., Gentilomi G.A., Panzavolta S., Bigi A. A radiopaque calcium phosphate bone cement with long-lasting antibacterial effect: from paste to injectable formulation. Ceram. Int. 2020;46:10048–10057. doi: 10.1016/j.ceramint.2019.12.272. [DOI] [Google Scholar]
- 86.Schröter L., Kaiser F., Stein S., Gbureck U., Ignatius A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020;117:1–20. doi: 10.1016/j.actbio.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 87.Charrière E., Terrazzoni S., Pittet C., Mordasini P., Dutoit M., Lemaitre J., Zysset P. Mechanical characterization of brushite and hydroxyapatite cements. Biomaterials. 2001;22:2937–2945. doi: 10.1016/S0142-9612(01)00041-2. [DOI] [PubMed] [Google Scholar]
- 88.Espanol M., Perez R.A., Montufar E.B., Marichal C., Sacco A., Ginebra M.P. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009;5:2752–2762. doi: 10.1016/j.actbio.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 89.Ginebra M.P., Driessens F.C.M., Planell J.A. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials. 2004;25:3453–3462. doi: 10.1016/j.biomaterials.2003.10.049. [DOI] [PubMed] [Google Scholar]
- 90.Mariño F.T., Torres J., Hamdan M., Rodríguez C.R., Cabarcos E.L. Advantages of using glycolic acid as a retardant in a brushite forming cement. J. Biomed. Mater. Res. B Appl. Biomater. 2007;83B:571–579. doi: 10.1002/jbm.b.30830. [DOI] [PubMed] [Google Scholar]
- 91.Wang S., Xu C., Yu S., Wu X., Jie Z., Dai H. Citric acid enhances the physical properties, cytocompatibility and osteogenesis of magnesium calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 2019;94:42–50. doi: 10.1016/j.jmbbm.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 92.Low K.L., Tan S.H., Zein S.H.S., Roether J.A., Mouriño V., Boccaccini A.R. Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res. B Appl. Biomater. 2010;94:273–286. doi: 10.1002/jbm.b.31619. [DOI] [PubMed] [Google Scholar]
- 93.Krüger R., Groll J. Fiber reinforced calcium phosphate cements – on the way to degradable load bearing bone substitutes? Biomaterials. 2012;33:5887–5900. doi: 10.1016/j.biomaterials.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 94.V Dorozhkin S., Epple M. Biological and medical significance of calcium phosphates. Angew. Chem., Int. Ed. Engl. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 95.Bohner M. Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury. 2000;31:D37–D47. doi: 10.1016/S0020-1383(00)80022-4. [DOI] [PubMed] [Google Scholar]
- 96.Gisep A., Wieling R., Bohner M., Matter S., Schneider E., Rahn B. Resorption patterns of calcium-phosphate cements in bone. J. Biomed. Mater. Res. 2003;66:532–540. doi: 10.1002/jbm.a.10593. [DOI] [PubMed] [Google Scholar]
- 97.LeGeros R.Z., Lin S., Rohanizadeh R., Mijares D., LeGeros J.P. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003;14:201–209. doi: 10.1023/A:1022872421333. [DOI] [PubMed] [Google Scholar]
- 98.Basu S., Basu B. Doped biphasic calcium phosphate: synthesis and structure. J. Asian Ceram. Soc. 2019;7:265–283. doi: 10.1080/21870764.2019.1636928. [DOI] [Google Scholar]
- 99.LeGeros R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008;108:4742–4753. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- 100.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971;5:117–141. doi: 10.1002/jbm.820050611. [DOI] [Google Scholar]
- 101.Giocondi J.L., El-Dasher B.S., Nancollas G.H., Orme C.A. Molecular mechanisms of crystallization impacting calcium phosphate cements. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010;368:1937–1961. doi: 10.1098/rsta.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davies J.E. Bone bonding at natural and biomaterial surfaces. Biomaterials. 2007;28:5058–5067. doi: 10.1016/j.biomaterials.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 103.Rahaman M.N. In: 3 - Bioactive Ceramics and Glasses for Tissue Engineering. Boccaccini A.R., PXB T.-T.E.U.C., Second P., Ma E., editors. Woodhead Publishing; 2014. pp. 67–114. [DOI] [Google Scholar]
- 104.Hench L.L. Bioceramics, a clinical success. Am. Ceram. Soc. Bull. 1998;77:67–74. https://www.scopus.com/inward/record.uri?eid=2-s2.0-0032118515&partnerID=40&md5=2cb30f49d303543b5264f8c18d7c85c8 [Google Scholar]
- 105.Ingole V.H., Sathe B., Ghule A.V. Elsevier Ltd.; 2018. Bioactive Ceramic Composite Material Stability, Characterization, and Bonding to Bone. [DOI] [Google Scholar]
- 106.Profeta A.C., Prucher G.M. Bioactive-glass in periodontal surgery and implant dentistry. Dent. Mater. J. 2015;34:559–571. doi: 10.4012/dmj.2014-233. [DOI] [PubMed] [Google Scholar]
- 107.Campion C.R., Ball S.L., Clarke D.L., Hing K.A. Microstructure and chemistry affects apatite nucleation on calcium phosphate bone graft substitutes. J. Mater. Sci. Mater. Med. 2013;24:597–610. doi: 10.1007/s10856-012-4833-x. [DOI] [PubMed] [Google Scholar]
- 108.Gerhardt L.-C., Boccaccini A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Mater. 2010;3 doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crush J., Hussain A., Seah K.T.M., Khan W.S. Bioactive glass: methods for assessing angiogenesis and osteogenesis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.643781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li W., Boccaccini A.R. Bioactive glasses: traditional and prospective applications in healthcare. Hot Top. Biomater. 2014:56–68. doi: 10.4155/EBO.13.585. [DOI] [Google Scholar]
- 111.Zhang J., Liu W., Schnitzler V., Tancret F., Bouler J.-M. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10:1035–1049. doi: 10.1016/j.actbio.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Thompson I.D., Hench L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998;212:127–136. doi: 10.1243/0954411981533908. [DOI] [PubMed] [Google Scholar]
- 113.Hum J., Boccaccini A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: a review. J. Mater. Sci. Mater. Med. 2012;23:2317–2333. doi: 10.1007/s10856-012-4580-z. [DOI] [PubMed] [Google Scholar]
- 114.Ginebra M.-P., Traykova T., Planell J.A. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials. 2006;27:2171–2177. doi: 10.1016/j.biomaterials.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 115.Yang X., Gan Y., Gao X., Zhao L., Gao C., Zhang X., Feng Y., Ting K., Gou Z. Preparation and characterization of trace elements-multidoped injectable biomimetic materials for minimally invasive treatment of osteoporotic bone trauma. J. Biomed. Mater. Res. 2010;95:1170–1181. doi: 10.1002/jbm.a.32936. [DOI] [PubMed] [Google Scholar]
- 116.Renno A.C.M., Nejadnik M.R., Van De Watering F.C.J., Crovace M.C., Zanotto E.D., Hoefnagels J.P.M., Wolke J.G.C., Jansen J.A., Van Den Beucken J.J.J.P. Incorporation of bioactive glass in calcium phosphate cement: material characterization and in vitro degradation. J. Biomed. Mater. Res. 2013;101 A:2365–2373. doi: 10.1002/jbm.a.34531. [DOI] [PubMed] [Google Scholar]
- 117.Yu L., Li Y., Zhao K., Tang Y., Cheng Z., Chen J., Zang Y., Wu J., Kong L., Liu S., Lei W., Wu Z. A novel injectable calcium phosphate cement-bioactive glass composite for bone regeneration. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sadiasa A., Sarkar S.K., Franco R.A., Min Y.K., Lee B.T. Bioactive glass incorporation in calcium phosphate cement-based injectable bone substitute for improved in vitro biocompatibility and in vivo bone regeneration. J. Biomater. Appl. 2014;28:739–756. doi: 10.1177/0885328213478256. [DOI] [PubMed] [Google Scholar]
- 119.El-Fiqi A., Kim J.H., Perez R.A., Kim H.W. Novel bioactive nanocomposite cement formulations with potential properties: incorporation of the nanoparticle form of mesoporous bioactive glass into calcium phosphate cements. J. Mater. Chem. B. 2015;3:1321–1334. doi: 10.1039/c4tb01634c. [DOI] [PubMed] [Google Scholar]
- 120.Axrap A., Wang J., Liu Y., Wang M., Yusuf A. Study on adhesion, proliferation and differentiation of osteoblasts promoted by new absorbable bioactive glass injection in vitro. Eur. Rev. Med. Pharmacol. Sci. 2016;20:4677–4681. [PubMed] [Google Scholar]
- 121.Dadkhah M., Pontiroli L., Fiorilli S., Manca A., Tallia F., Tcacencu I., Vitale-Brovarone C. Preparation and characterisation of an innovative injectable calcium sulphate based bone cement for vertebroplasty application. J. Mater. Chem. B. 2017;5:102–115. doi: 10.1039/c6tb02139e. [DOI] [PubMed] [Google Scholar]
- 122.Mendes L.S., Saska S., Coelho F., Capote T.S.D.O., Scarel-Caminaga R.M., Marchetto R., Carrodeguas R.G., Gaspar A.M.M., Rodríguez M.A. Injectable β-TCP/MCPM cement associated with mesoporous silica for bone regeneration: characterization and toxicity evaluation. Biomed. Mater. 2018;13 doi: 10.1088/1748-605X/aa9085. 0–24. [DOI] [PubMed] [Google Scholar]
- 123.Hasan M.L., Kim B., Padalhin A.R., Faruq O., Sultana T., Lee B.T. In vitro and in vivo evaluation of bioglass microspheres incorporated brushite cement for bone regeneration. Mater. Sci. Eng. C. 2019;103 doi: 10.1016/j.msec.2019.109775. [DOI] [PubMed] [Google Scholar]
- 124.Li C., Hao W., Wu C., Li W., Tao J., Ai F., Xin H., Wang X. Injectable and bioactive bone cement with moderate setting time and temperature using borosilicate bio-glass-incorporated magnesium phosphate. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/ab633f. [DOI] [PubMed] [Google Scholar]
- 125.Pu Z., Fan H., Zhang C., Gao C., Zhu P. Effect of bioglass on in vitro bioactivity and cytocompatibility of biphasic α-tricalcium phosphate/gypsum cements. Mater. Technol. 2020:1–12. doi: 10.1080/10667857.2020.1761654. 00. [DOI] [Google Scholar]
- 126.Fan H., Yang Q., Zheng D., Gao C., Zhu P. Synergistic effects of wheat gluten and poly (γ-glutamic acid) on physicochemical properties of bioglass modified beta-tricalcium phosphate bone cement. Mater. Technol. 2021:1–15. doi: 10.1080/10667857.2021.2016294. 00. [DOI] [Google Scholar]
- 127.Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76:456–468. doi: 10.1002/jbm.b.30398. [DOI] [PubMed] [Google Scholar]
- 128.Sarda S., Fernández E., Llorens J., Martínez S., Nilsson M., Planell J.A. Rheological properties of an apatitic bone cement during initial setting. J. Mater. Sci. Mater. Med. 2001;12:905–909. doi: 10.1023/A:1012832325957. [DOI] [PubMed] [Google Scholar]
- 129.Cao W., Peng Y., Zhang Y., Qiu F., Li M., Tang J., Wu Z. Novel bone wax based on tricalcium silicate cement and BGs mixtures. Biomed. Mater. 2018;13:65001. doi: 10.1088/1748-605x/aad73c. [DOI] [PubMed] [Google Scholar]
- 130.Cao W., Zong S., Zhang Y., Qiu F., Tang J., Wu Z., Luan J. Preparation of a novel bone wax with modified tricalcium silicate cement and BGs. Mater. Sci. Eng. C. 2019;99:979–985. doi: 10.1016/j.msec.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 131.Hatten H.P., Jr., Voor M.J. Bone healing using a bi-phasic ceramic bone substitute demonstrated in human vertebroplasty and with histology in a rabbit cancellous bone defect model. Intervent Neuroradiol. 2012;18:105–113. doi: 10.1177/159101991201800114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Driessens F.C.M., Boltong M.G., De Maeyer E.A.P., Verbeeck R.M.H., Wenz R. Effect of temperature and immersion on the setting of some calcium phosphate cements. J. Mater. Sci. Mater. Med. 2000;11:453–457. doi: 10.1023/A:1008944126664. [DOI] [PubMed] [Google Scholar]
- 133.Ma B., Xiao J., Tan H. Effect of citric acid on cement hydration. Adv. Mater. Res. 2012;393–395:49–53. doi: 10.4028/www.scientific.net/AMR.393-395.49. [DOI] [Google Scholar]
- 134.Zhang Y., Yang J., Cao X. Effects of several retarders on setting time and strength of building gypsum. Construct. Build. Mater. 2020;240 doi: 10.1016/j.conbuildmat.2019.117927. [DOI] [Google Scholar]
- 135.Oğuz Ö.D., Ege D. Effect of zoledronic acid and graphene oxide on the physical and in vitro properties of injectable bone substitutes. Mater. Sci. Eng. C. 2020;120 doi: 10.1016/j.msec.2020.111758. [DOI] [PubMed] [Google Scholar]
- 136.Shieh T.M., Hsu S.M., Chang K.C., Chen W.C., Lin D.J. Calcium phosphate cement with antimicrobial properties and radiopacity as an endodontic material. Materials. 2017;10 doi: 10.3390/ma10111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mandakhbayar N., El-Fiqi A., Lee J.H., Kim H.W. Evaluation of strontium-doped nanobioactive glass cement for dentin-pulp complex regeneration therapy. ACS Biomater. Sci. Eng. 2019;5:6117–6126. doi: 10.1021/acsbiomaterials.9b01018. [DOI] [PubMed] [Google Scholar]
- 138.Naruphontjirakul P., Tsigkou O., Li S., Porter A.E., Jones J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019;90:373–392. doi: 10.1016/j.actbio.2019.03.038. [DOI] [PubMed] [Google Scholar]
- 139.Kargozar S., Kermani F., Beidokhti S.M., Hamzehlou S., Verné E., Ferraris S., Baino F. Functionalization and surface modifications of bioactive glasses (BGs): tailoring of the biological response working on the outermost surface layer. Materials. 2019;12 doi: 10.3390/ma12223696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lobel K.D., Hench L.L. In-vitro protein interactions with a bioactive gel-glass. J. Sol. Gel Sci. Technol. 1996;7:69–76. doi: 10.1007/BF00401885. [DOI] [Google Scholar]
- 141.Ciraldo F.E., Boccardi E., Melli V., Westhauser F., Boccaccini A.R. Tackling bioactive glass excessive in vitro bioreactivity: preconditioning approaches for cell culture tests. Acta Biomater. 2018;75:3–10. doi: 10.1016/j.actbio.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 142.Aina V., Perardi A., Bergandi L., Malavasi G., Menabue L., Morterra C., Ghigo D. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem. Biol. Interact. 2007;167:207–218. doi: 10.1016/j.cbi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 143.O'Donnell M.D., Candarlioglu P.L., Miller C.A., Gentleman E., Stevens M.M. Materials characterisation and cytotoxic assessment of strontium- substituted bioactive glasses for bone regeneration. J. Mater. Chem. 2010;20:8934–8941. doi: 10.1039/c0jm01139h. [DOI] [Google Scholar]
- 144.Ben-Arfa B.A.E., Palamá I.E., Miranda Salvado I.M., Ferreira J.M.F., Pullar R.C. Cytotoxicity and bioactivity assessments for Cu2+ and La3+ doped high-silica sol-gel derived bioglasses: the complex interplay between additive ions revealed. J. Biomed. Mater. Res. 2019;107:2680–2693. doi: 10.1002/jbm.a.36772. [DOI] [PubMed] [Google Scholar]
- 145.Schuhladen K., Stich L., Schmidt J., Steinkasserer A., Boccaccini A.R., Zinser E. Cu, Zn doped borate bioactive glasses: antibacterial efficacy and dose-dependent: in vitro modulation of murine dendritic cells. Biomater. Sci. 2020;8:2143–2155. doi: 10.1039/c9bm01691k. [DOI] [PubMed] [Google Scholar]
- 146.Pantulap U., Arango-Ospina M., Boccaccini A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022;33 doi: 10.1007/s10856-021-06626-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jin G.Z., Han C.M., Kim H.W. In vitro co-culture strategies to prevascularization for bone regeneration: a brief update. Tissue Eng. Regen. Med. 2015;12:69–79. doi: 10.1007/s13770-014-0095-7. [DOI] [Google Scholar]
- 148.Larsson S., Hannink G. Injectable bone-graft substitutes: current products, their characteristics and indications, and new developments. Injury. 2011;42:S30–S34. doi: 10.1016/j.injury.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 149.No Y.J., Roohani-Esfahani S.I., Zreiqat H. Nanomaterials: the next step in injectable bone cements. Nanomedicine. 2014;9:1745–1764. doi: 10.2217/nnm.14.109. [DOI] [PubMed] [Google Scholar]
- 150.Kondiah P.J., Choonara Y.E., Kondiah P.P.D., Marimuthu T., Kumar P., Du Toit L.C., Pillay V. A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules. 2016;21 doi: 10.3390/molecules21111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nuss K.M.R., von Rechenberg B. Biocompatibility issues with modern implants in bone - a review for clinical orthopedics. Open Orthop. J. 2008;2:66–78. doi: 10.2174/1874325000802010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vertenten G., Vlaminck L., Gorski T., Schreurs E., Van Den Broeck W., Duchateau L., Schacht E., Gasthuys F. Evaluation of an injectable, photopolymerizable three-dimensional scaffold based on D,L-lactide and ε-caprolactone in a tibial goat model. J. Mater. Sci. Mater. Med. 2008;19:2761–2769. doi: 10.1007/s10856-008-3404-7. [DOI] [PubMed] [Google Scholar]
- 153.Araújo M.V.F., Mendes V.C., Chattopadhyay P., Davies J.E. Low-temperature particulate calcium phosphates for bone regeneration. Clin. Oral Implants Res. 2010;21:632–641. doi: 10.1111/j.1600-0501.2009.01864.x. [DOI] [PubMed] [Google Scholar]
- 154.Kang M.S., Lee N.H., Singh R.K., Mandakhbayar N., Perez R.A., Lee J.H., Kim H.W. Nanocements produced from mesoporous bioactive glass nanoparticles. Biomaterials. 2018;162:183–199. doi: 10.1016/j.biomaterials.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 155.Ding H., Zhao C.J., Cui X., Gu Y.F., Jia W.T., Rahaman M.N., Wang Y., Huang W.H., Zhang C.Q. A novel injectable borate bioactive glass cement as an antibiotic delivery vehicle for treating osteomyelitis. PLoS One. 2014;9:1–9. doi: 10.1371/journal.pone.0085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cui X., Zhao C., Gu Y., Li L., Wang H., Huang W., Zhou N., Wang D., Zhu Y., Xu J., Luo S., Zhang C., Rahaman M.N. A novel injectable borate bioactive glass cement for local delivery of vancomycin to cure osteomyelitis and regenerate bone. J. Mater. Sci. Mater. Med. 2014;25:733–745. doi: 10.1007/s10856-013-5122-z. [DOI] [PubMed] [Google Scholar]
- 157.Dadkhah M., Pontiroli L., Fiorilli S., Manca A., Tallia F., Tcacencu I., Vitale-Brovarone C. Preparation and characterisation of an innovative injectable calcium sulphate based bone cement for vertebroplasty application. J. Mater. Chem. B. 2017;5:102–115. doi: 10.1039/c6tb02139e. [DOI] [PubMed] [Google Scholar]
- 158.Hoppe A., Güldal N.S., Boccaccini A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]