Abstract
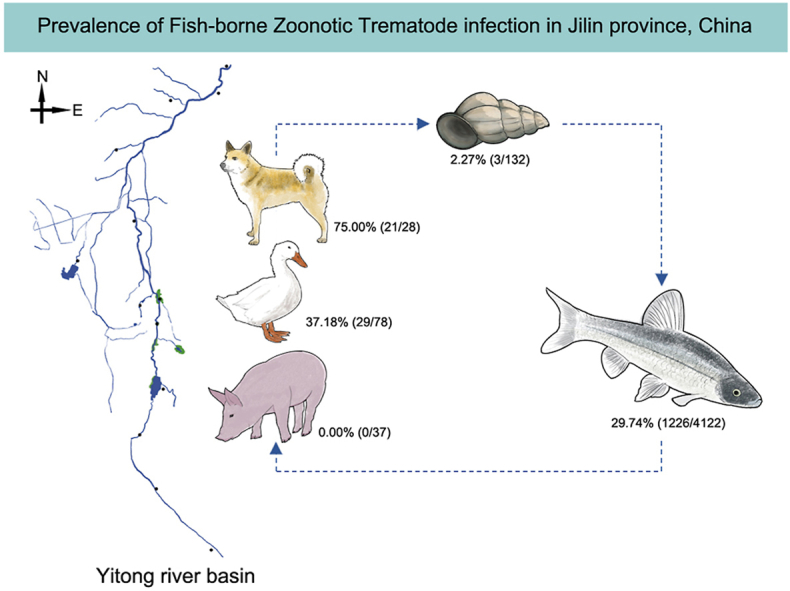
Fish-borne zoonotic trematodes (FZTs) are the most serious food-borne parasites in Asia and have become a burden to public health and a new challenge in food safety. In Jilin Province, China, the prevalence of FZTs in intermediate and definitive hosts has not been extensively explored. In the present study, we investigated the prevalence of FZTs in Jilin Province, China. From July to November 2020, a total of 132 freshwater snails (the first intermediate host of FZTs), 4122 wild freshwater fishes (the second intermediate host of FZTs) and 143 fecal samples from canines, ducks and swine (the definitive host of FZTs) were collected from the Yitong River basin of Jilin Province. FZT species were identified by morphological observation combined with internal transcribed spacer (ITS) sequence analysis. The prevalence of FZTs was then calculated. The results showed that the prevailing species of FZTs in Jilin Province, China, were Clonorchis sinensis, Metorchis orientalis and Echinochasmus japonicus. The total prevalence of FZTs was 29.74% (1226/4122) in fish, the total infection rates were 2.27% (3/132) in snails, 75.00% (21/28) in canines and 37.18% (29/78) in ducks. The coinfection rates of the two trematodes were 13.39% (552/4122) in fish, 35.71% (10/28) in canines and 7.69% (6/78) in ducks. The coinfection rate of the three flukes was 2.60% (107/4122) in fish. Nine of the 12 fish species examined were infected with FZT metacercariae.
Keywords: Fish-borne zoonotic trematodes, Prevalence, Clonorchis sinensis, Metorchis orientalis, Echinochasmus japonicus
Graphical abstract
Highlights
-
• C.
sinensis, M. orientalis and E. japonicus were reported in Jilin province of China.
-
•
The prevalence of FZTs in freshwater fish were 29.74% (1226/4122).
-
•
Four fish species were identified as new second intermediate hosts of E. japonicus.
1. Introduction
Fish-borne zoonotic trematodes (FZTs) are an important group of zoonotic parasites transmitted by fish and fish products (Hop et al., 2007). A total of 59 species of FZTs have been found to parasitize humans. FZTs’ life cycle involve a first intermediate host (freshwater snails), a second intermediate host (freshwater fish) and a definitive host (human or other animals). These trematodes can be grouped into two main categories, the liver fluke group (Opisthorchiidae: 12 species) and the intestinal fluke group (Heterophyidae: 36 species, Echinostomatidae: 10 species and Nanophyetidae: 1 species) (Qiu et al., 2017). They are primarily related to liver and biliary disorders and intestinal diseases, causing severe disease and economic burden (Tang et al., 2016; Yu and Mott, 1994).
FZTs are primarily distributed in Asia, including China, South Korea, Japan, Laos, Vietnam, Thailand and the Far East of Russia (Qian et al., 2016; Stefan et al., 2013; WHO, 1995). More than 26 million people have been infected with liver flukes, including 15 million infected with C. sinensis (Qian et al., 2016; Sripa et al., 2007). In cases of intestinal FZTs, an estimated 40 to 50 million people are infected with one or several species (Hung et al., 2015; Keiser and Utzinger, 2009). The estimates of at-risk populations for FZTs are more than 1.1 billion (Santos and Howgate, 2011; Sripa et al., 2007, 2010). The World Health Organization recently added FZT infections to its list of emerging infectious diseases (Clausen et al., 2012). The prevalence of C. sinensis in freshwater fish is 67.5% in Korea and 12.5% in Vietnam (Sohn et al., 2018). In China, the prevalence of C. sinensis metacercariae in fish is 35.1% (in 19 provinces/municipalities) (Zhang et al., 2020).
FZT species that have been reported in China include C. sinensis, E. japonicus, M. orientalis, Isthmiophora hortensis, Procerovum varium, Metagonimus yokogawai, Haplorchis pumilio, Stellantchasmus falcatus, Haplorchis taichui, and Centrocestus formosanus (Choi et al., 2006; Qiu et al., 2017). Jilin Province, located in the northeast region of China, has a high infection rate of C. sinensis in humans (Li et al., 2011) because the local people have a habit of eating raw and undercooked fish (Lun et al., 2005). The prevalence of C. sinensis in the Songhua River, Fuyu County, Western Jilin Province, is 31.96% (Zhang et al., 2014, 2020). However, data on the FZT species and prevalence in Jilin Province have not been explored extensively, which is very detrimental to the prevention and control of FZTs in Jilin Province. Therefore, this study conducted an epidemiological investigation on FZTs in Jilin Province from July to November 2020.
2. Materials and methods
2.1. Description of sampling sites and sampling time
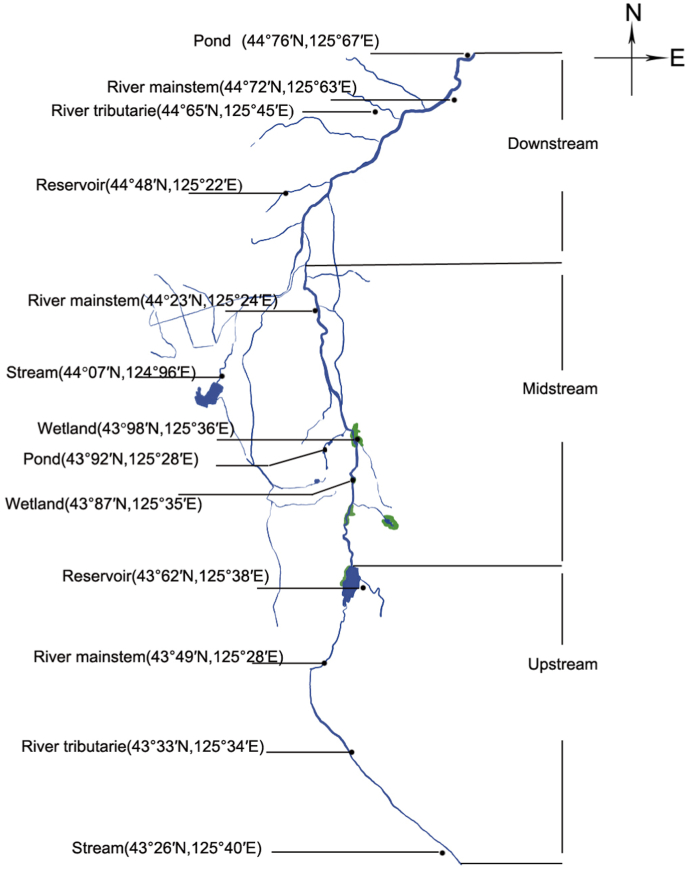
The Yitong River basin runs through the central area of Jilin Province, which is located in multiple important economic locations and is an important supply source for freshwater fish in Jilin Province. It is the second largest tributary of the Songhua River, with a basin area of 8440.00 square kilometers and a total length of 342.50 km. Freshwater fish and snail samples were collected in the upstream, midstream and downstream areas of the Yitong River basin, with 13 sampling points covering reservoirs, ponds, river mainstems, river tributaries, streams and wetlands. The fish samples were continuously harvested from July to November 2020. Fecal samples were collected from the definitive hosts living in villages or wildlife living around these 13 collection sites (Fig. 1). The definitive host feces and wild freshwater snails were harvested from July to November 2020.
Fig. 1.
Sampling sites of freshwater fish, freshwater snails and the definitive hosts' feces.
2.2. Sample collection
2.2.1. Freshwater fishes
A total of 4122 freshwater fishes from 12 species (Pseudorasbora parva, Hemiculter leucisculus, Perccottus glehnii, Rhodeus sinensis, Ctenogobius giurinus, Carassius auratus, Saurogobio dabryi, Misgurnus anguillicaudatus, Cyprinus carpio, Silurus asotus, Ophiocephalus argus Cantor and Hemisalanx prognathus Regan) were randomly collected from the Yitong River basin. We collected freshwater fish using fishing nets or purchased them from fishermen, and the collected fish are commonly and popularly consumed species in the area. The sampling information for the freshwater fish is provided in Additional File Table S1.
2.2.2. Freshwater snails
Freshwater snails were collected during the morning for 20 min per site by fully randomly handpicking or catching them with a net. In 9 of 13 sampling collection sites, a total 132 snails from 4 species (46 Parafossarulus striatulus, 11 Bithynia. sp., 68 Viviparidae gen. sp. and 7 Radix auricularia) were obtained for trematode detection.
2.2.3. Fecal samples of canines, swine and ducks
A total of 143 fecal samples from canines (28 samples), ducks (78 samples) and swine (37 samples) were randomly collected. The majority of animals were domestic and feral (12 ducks) and more than 12 months old (100% canines, 100% swine, 46.15% ducks). Feces from canines, ducks and swine were directly sampled immediately after defecation. The duck breeds were Partridge duck and Anas platyrhynchos. The dogs were primarily Chinese field dogs, and the swine were Landrace and Large Yorkshire. The sampling information from the definitive hosts is provided in Additional File Table S2.
2.3. Morphological observation of FZTs in the samples
2.3.1. FZT metacercariae observation in freshwater fishes
FZT metacercariae were detected using a combination of compression and artificial digestion methods as previously described (WHO, 1995). Briefly, a compression process was used for small-sized fish (≤10 g): each fish was compressed using two glass slides and observed under a microscope. The fish were repeatedly compressed and observed three times. Then, FZT-positive fish were used to isolate metacercariae. Artificial digestion was used for large-sized fish or to isolate metacercariae. Each whole fish was cut into small pieces and ground using a homogenizer, which was placed in a beaker with digestion solution (10 g pepsin, 6 g NaCl and 10 mL concentrated HCl in 1 L distilled water) at a ratio of 1 g tissue per 10 mL solution. The mixture was thoroughly stirred at 37 °C for 3 h. After digestion, the large particles were removed by filtering through a 250 μm/aperture copper sieve. The filtered solution was transferred to a triangle measuring cup, diluted in tap water and kept still for 2 h. The supernatant was discarded, and the sediment was retained. This procedure was repeated until the supernatant became clear. The sediment was transferred to a cell culture dish containing 5 mL sterile PBS, and the metacercariae were morphologically identified and classified under a microscope and counted.
The prevalence, metacercariae abundance and calculated intensity/100 g fish of FZTs were calculated as shown (Bush et al., 1997).
Metacercariae abundance: The number of metacercariae in each fish.
2.3.2. FZT examination in snails
Small snails were crushed using two glass slides, and FZT larvae were observed under a microscope. The large hard-shelled snails were opened, and visceral tissue and hemolymph were transferred onto glass slides for microscopic observation (Hung et al., 2015). The infection rate of FZTs was calculated (Bush et al., 1997).
2.3.3. FZT egg detection in fecal samples
Fecal samples were examined for FZT eggs using direct smears and microscopic observation. The smear was repeated three times for each sample to improve the detection rate. The infection rate of FZTs was calculated in definitive hosts (Bush et al., 1997).
2.4. ITS sequence amplification and analysis for FZTs
2.4.1. Genomic DNA extraction
All of the fecal samples and freshwater snails were stored at 4 °C until DNA extraction. Different kinds of metacercariae species from three independent experiments were stored in Alsever's solution at 4 °C prior to DNA extraction. Genomic DNA from different metacercariae species and freshwater snail samples was extracted using a genomic DNA Kit (TIANGEN BIOTECH, Beijing, China). DNA from the definitive host fecal samples was performed strictly according to the instructions of the Fecal Genomic DNA Kit (TIANGEN BIOTECH, Beijing, China). All DNA precipitates were stored at −80 °C.
2.4.2. Detection of ITS sequences for FZTs
The internal transcribed spacer (ITS) sequences of FZTs from all the extracted DNA were amplified by PCR using ITS sequence primers (Additional file Table S3) as previously described (Qiu et al., 2019; Zhou et al., 2010). After amplification, 10 μL of PCR product was examined by agarose gel (1.5%) electrophoresis and ethidium bromide substitution (Applygen Technologies Inc. Beijing, China) staining. All positive products were sequenced by Life Technology Company (Comate Bioscience Co., Ltd., Jilin, China).
2.4.3. Phylogenetic analysis of ITS sequences
To study the phylogenetic relationships of the isolated and other FZTs, ITS sequences of liver flukes (M. orientalis, M. ussuriensis, C. sinensis, Opisthorchis sudarikovi, Opisthorchis felineus, Erschoviorchis anuiensis and Euryhelmis costaricensis) and intestinal flukes (Echinostoma trivolvis, Echinoparyphium mordvilkowi, Stephanoprora uruguayense, Stephanoprora chasanensis, Stephanoprora pseudoechinata, E. japonicus, Echinochasmus milvi, Echinochasmus coaxatus) from GenBank of the National Center of Biotechnology Information (NCBI) were used for phylogenetic analysis using Aspidogaster chongqingens as the outgroup. The phylogenetic tree for ITS genes of FZTs was reconstructed using MEGA X software. The methods of maximum likelihood (ML), maximum parsimony (MP) and neighbor joining (NJ) were used for phylogenetic construction, and the initial trees were automatically obtained by selecting the topology with a superior log likelihood value (Wang et al., 2020).
2.5. Data analysis
The overall prevalence of FZT metacercariae in freshwater fish and their respective prevalence with respect to different factors (fish species, sampling location, sampling water source, sampling time) were statistically analyzed using SPSS24.0 (IBM, Armonk, NY, USA), and the analysis shows the 95% confidence intervals (95% CI). Differences in data between groups and statistical graph plotting were performed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). A p value < 0.05 was considered to indicate a significant difference.
3. Results
3.1. The prevalence of FZTs in wild freshwater fish
3.1.1. The prevalence of FZTs in wild freshwater fish in Jilin Province
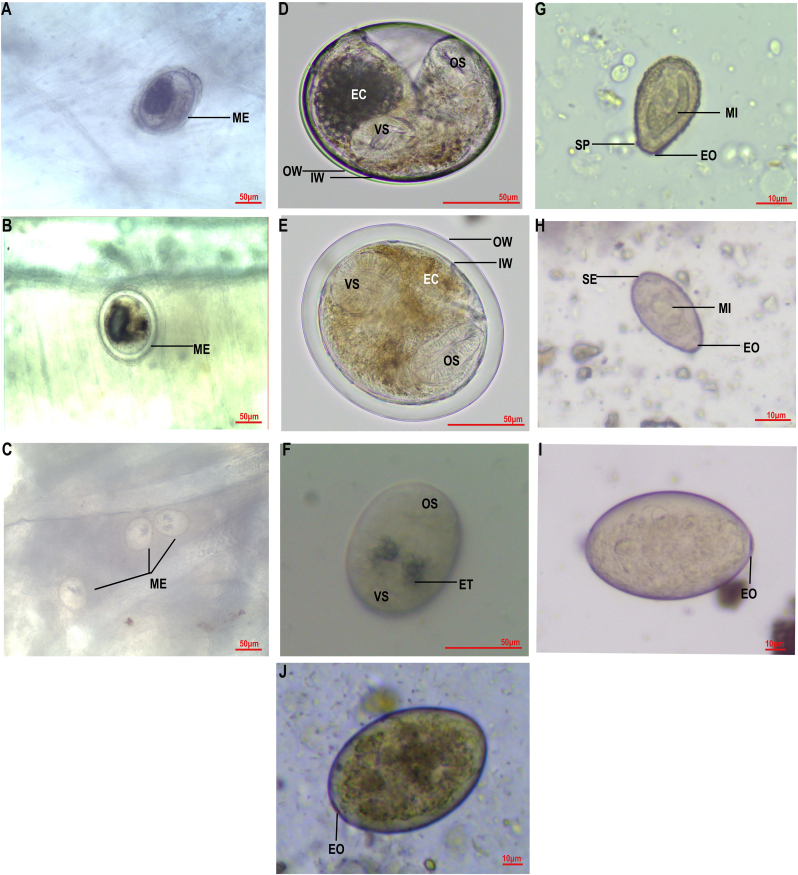
Morphological observation of metacercariae combined with ITS sequence analysis revealed that the prevailing FZTs in Jilin Province were C. sinensis, M. orientalis and E. japonicus (Fig. 2, Fig. 3). The metacercariae of C. sinensis were oblate or elliptical, 135.65 ± 27.65 μm × 93.23 ± 12.76 μm in size and contained an active larva with two layers of thin cyst walls. The excretory sac of the larva was dark brown, and the oral sucker and ventral sucker were similar in size (Fig. 2 A and D). The metacercariae of M. orientalis were oval or round, 113.37 ± 12.52 μm × 101.89 ± 13.87 μm in size, with two layers of thick cyst walls (10.05 ± 2.50 μm thick). The metacercariae contained a movable larva with a slower activity frequency than that of C. sinensis, with a brownish yellow excretory sac with a similar size between the oral and ventral suckers (Fig. 2 B and E). The metacercariae of E. japonicus were elliptical and very small, approximately 78.63 ± 12.18 μm × 43.66 ± 8.95 μm in size. The metacercariae were translucent, with various round particles distributed in the excretion tube (Fig. 2 C and F).
Fig. 2.
The metacercariae and eggs of C. sinensis, M. orientalis and E. japonicus.C. sinensis metacercariae encysted on the flesh of P. parva, scale bar = 50 μm (A). M. orientalis metacercariae encysted on the flesh of P. parva, scale bar = 50 μm (B). E. japonicus metacercariae encysted on the gills of R. sinensis, scale bar = 50 μm (C). C. sinensis metacercariae isolated from P. parva, scale bar = 50 μm (D). M. orientalis metacercariae isolated from P. parva, scale bar = 50 μm (E); E. japonicus metacercariae isolated from R. sinensis, scale bar = 50 μm (F). C. sinensis egg in feces of canine, scale bar = 10 μm (G). M. orientalis egg in feces of duck, scale bar = 10 μm (H). E. japonicus eggs in feces of canine and duck, scale bar = 10 μm (I, J). EC, excretion cyst; ET, excretion tube; EO, egg operculum; IW, inner cyst wall; OW, outer cyst wall; ME, metacercariae; MI, miracidium; SP, shoulder peaks; SE, small spine at the rear end; OS, oral sucker; VS, ventral sucker.
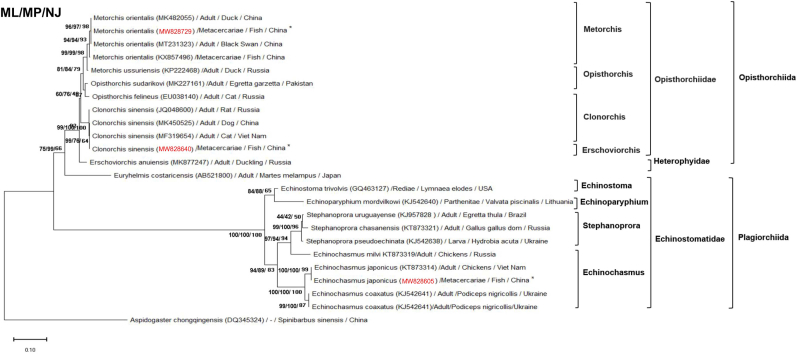
Fig. 3.
The phylogenetic tree of FZTs obtained in this study with other trematodes based on ITS sequences.
ITS sequences of C. sinensis, M. orientalis and E. japonicus from fish were obtained and compared. They had the same similarity and were deposited into NCBI (No. MW828640, MW828729 and MW828605). The phylogenetic relationship between the FZTs obtained in this study and other trematodes based on ITS sequences was analyzed via MP, NJ and ML using A. chongqingens as the outgroup. The scale bar indicates an evolutionary distance of 0.10 substitutions per site in the sequence. The ITS sequences of C. sinensis, M. orientalis and E. japonicus obtained in this study (marked with *) was 100% consistent with the sequences of C. sinensis (MF319654), M. orientalis (MK482055) and E. japonicus (KT873314) deposited in NCBI GenBank.
The ITS sequences of C. sinensis, M. orientalis and E. japonicus metacercariae from different fish species were analyzed by molecular biology and deposited in NCBI GenBank (Nos. MW828640, MW828729 and MW828605), which were 100% homologous to the reported ITS sequences of the trematodes deposited in NCBI GenBank (Nos. MF319654, MK482055 and KT873314) (Fig. 3) (Besprozvannykh et al., 2017; Na et al., 2016; Tatonova et al., 2017). The ITS sequence phylogenetic tree indicated that the sequences were classified as C. sinensis, M. orientalis and E. japonicus (marked with * in Fig. 3).
The prevalence of FZTs in freshwater fish in Jilin Province was 29.74% (1226/4122) (Table 1). The infection rates of C. sinensis, M. orientalis and E. japonicus were 22.59% (931/4122), 9.92% (409/4122) and 18.10% (746/4122), respectively (Table 1). The total coinfection rate of FZTs was 13.39% (552/4122). The coinfection rate of C. sinensis and M. orientalis was 4.95% (204/4122), C. sinensis and E. japonicus was 7.23% (298/4122), M. orientalis and E. japonicus was 3.81% (157/4122), and the coinfection rate of the three species FZTs was 2.60% (107/4122) (Table 1).
Table 1.
The prevalence of FZTs in freshwater fish in Jilin Province of China.
| FZT species | No. of fish examined | No. of fish infected | Prevalence (%) | 95% CI |
|---|---|---|---|---|
| C. sinensis | 4122 | 931 | 22.59 | 21.30–23.86 |
| M. orientalis | 4122 | 409 | 9.92 | 9.01–10.83 |
| E. japonicus | 4122 | 746 | 18.10 | 16.92–19.28 |
| Total | 4122 | 1226 | 29.74 | 28.34–31.14 |
| C. sinensis and E. japonicus | 4122 | 298 | 7.23 | 6.44–8.02 |
| C. sinensis and M. orientalis | 4122 | 204 | 4.95 | 4.29–5.61 |
| M. orientalis and E. japonicus | 4122 | 157 | 3.81 | 3.23–4.39 |
| Total | 4122 | 552 | 13.39 | 5.83–20.95 |
| C. sinensis, M. orientalis and E. japonicus | 4122 | 107 | 2.60 | 2.11–3.09 |
3.1.2. Difference in FZT prevalence in different wild freshwater fishes
FZT infection was observed in 9 out of 12 fish species, including P. parva, H. leuciclus, P. glenii, R. sinensis, C. giurinus, C. auratus, S. dabryi, M. anguillicaudatus, and C. carpio, while S. asotus, O. argus Cantor and H. prognathus Regan were not infected with FZTs. C. sinensis metacercariae were found in P. parva, H. leuciclus, P. glenii, R. sinensis, C. giurinus, C. auratus, C. carpio and S. dabryi, and the prevalence of C. sinensis among infected fishes ranged from 3.10% (C. auratus, 24/773) to 60.76% (P. parva, 607/999). The calculated intensity/100 g fish of C. sinensis varied from 0.06 to 6528.54, with the highest prevalence and the maximum metacercariae abundance observed in P. parva (Table 2). P. parva, H. leuciclus, P. glenii, R. sinensis and S. dabryi were infected with M. orientalis metacercariae. The prevalence of M. orientalis among infected fishes ranged from 2.00% (P. glenii, 5/250) to 28.73% (P. parva, 287/999), and the calculated intensity/100 g fish ranged from 0.30 to 366,78, with the highest prevalence and the maximum metacercariae abundance observed in P. parva (Table 2). E. japonicus metacercariae were detected in P. parva, H. leuciclus, R. sinensis, C. giurinus, M. anguillicaudatus and S. dabryi, and the prevalence of E. japonicus among infected fishes ranged from 0.81% (C. giurinus, 5/619) to 40.20% (R. sinensis, 318/791). The calculated intensity/100 g fish ranged from 6.76 to 700.89 (Table 2). R. sinensis was the freshwater fish with the highest prevalence and maximum metacercariae abundance of E. japonicus (Table 2).
Table 2.
The prevalence of FZTs in different freshwater fishes in Jilin Province of China.
| FZT species | Fish species | No. of fish examined | No. of fish infected | Prevalence (%) | 95% CI | Metacercariae abundance | Calculate intensity/100 g fish |
|---|---|---|---|---|---|---|---|
| C. sinensis | P. parva | 999 | 607 | 60.76 | 57.73–63.79 | 0–1044 | 6528.54 |
| H. leuciclus | 365 | 103 | 28.22 | 23.60–32.84 | 0–65 | 119.96 | |
| P. glenii | 250 | 34 | 13.60 | 9.35–17.85 | 0–37 | 4.10 | |
| R. sinensis | 791 | 86 | 10.87 | 8.70–13.04 | 0–18 | 55.91 | |
| C. giurinus | 619 | 31 | 5.00 | 3.28–6.72 | 0–11 | 10.13 | |
| C. auratus | 773 | 24 | 3.10 | 1.88–4.32 | 0–9 | 0.31 | |
| S. dabryi | 153 | 44 | 28.76 | 21.59–35.93 | 0–68 | 317.57 | |
| M. anguillicaudatus | 60 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| C. carpio | 44 | 2 | 4.55 | 1.61–10.71 | 0–13 | 0.06 | |
| S. asotus | 18 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| O. argus Cantor | 2 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| H. prognathus Regan | 48 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| M. orientalis | P. parva | 999 | 287 | 28.73 | 25.91–31.53 | 0–127 | 366.78 |
| H. leuciclus | 365 | 44 | 12.05 | 8.71–15.39 | 0–36 | 6.58 | |
| P. glenii | 250 | 5 | 2.00 | 0.26–3.73 | 0–8 | 0.30 | |
| R. sinensis | 791 | 53 | 6.70 | 4.96–8.44 | 0–16 | 18.94 | |
| C. giurinus | 619 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| C. auratus | 773 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| S. dabryi | 153 | 20 | 13.07 | 7.73–18.41 | 0–54 | 144.72 | |
| M. anguillicaudatus | 60 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| C. carpio | 44 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| S. asotus | 18 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| O. argus Cantor | 2 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| H. prognathus Regan | 48 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| E. japonicus | P. parva | 999 | 327 | 32.73 | 29.82–35.64 | 0–46 | 396.38 |
| H. leuciclus | 365 | 75 | 20.55 | 16.40–26.70 | 0–25 | 30.68 | |
| P. glenii | 250 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| R. sinensis | 791 | 318 | 40.20 | 36.78–43.62 | 0–76 | 700.89 | |
| C. giurinus | 619 | 5 | 0.81 | 0.10–1.52 | 0–8 | 6.76 | |
| C. auratus | 773 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| S. dabryi | 153 | 15 | 9.80 | 5.09–14.51 | 0–14 | 60.22 | |
| M. anguillicaudatus | 60 | 6 | 10.00 | 2.40–17.59 | 0–28 | 11.63 | |
| C. carpio | 44 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| S. asotus | 18 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| O. argus Cantor | 2 | 0 | 0.00 | 0.00–0.00 | 0 | 0 | |
| H. prognathus Regan | 48 | 0 | 0.00 | 0.00–0.00 | 0 | 0 |
3.1.3. Association of the size of fish with FZT prevalence
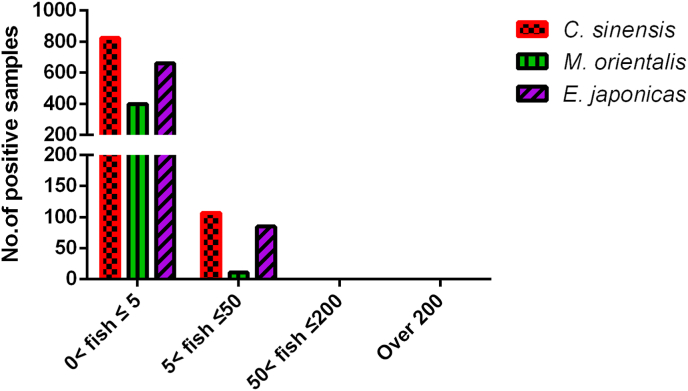
To understand the relationship between fish size and FZT metacercariae infection, we counted FZT metacercariae in fish of different weights. The results showed that small-sized fish (0<fish≦5 g) were more vulnerable to three FZTs than middle-sized fish (5<fish≦50 g), and no FZT metacercariae infection was observed in large-sized fish (>50 g) (Fig. 4).
Fig. 4.
The number of FZTs infecting wild freshwater fish of different sizes.
3.1.4. The FZT prevalence in different reaches and water sources
Wild freshwater fish from upstream, midstream and downstream were examined. The results showed that C. sinensis, M. orientalis and E. japonicus were present in all three reaches of the Yitong River basin. The prevalence rates of C. sinensis, M. orientalis and E. japonicus were 19.38% (304/1569), 17.46% (274/1569) and 15.55% (244/1569) upstream; 20.35% (292/1435), 7.11% (102/1435) and 17.70% (254/1435) midstream; and 29.96% (335/1118), 2.95% (33/1118) and 22.18% (248/1118) downstream. The highest prevalence of M. orientalis was upstream (17.46%, 274/1569). The highest prevalence of C. sinensis and E. japonicus was downstream, with prevalence rates of 29.96% (335/1118) and 22.18% (248/1118), respectively (Table 3).
Table 3.
The prevalence of FZTs in wild freshwater fish from different reaches of Yitong River basin.
| FZT species | Drainage reaches | No. of fish examined | No. of fish infected | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|
| C. sinensis | Upstream | 1569 | 304 | 19.38 | 11.64–20.48 |
| Midstream | 1435 | 292 | 20.35 | 18.37–27.77 | |
| Downstream | 1118 | 335 | 29.96 | 24.61–34.46 | |
| M. orientalis | Upstream | 1569 | 274 | 17.46 | 12.41–20.41 |
| Midstream | 1435 | 102 | 7.11 | 4.47–10.07 | |
| Downstream | 1118 | 31 | 2.95 | 0.94–4.43 | |
| E. japonicus | Upstream | 1569 | 244 | 15.55 | 9.98–18.22 |
| Midstream | 1435 | 254 | 17.70 | 14.79–23.27 | |
| Downstream | 1118 | 248 | 22.18 | 17.72–26.68 |
The sampling sites were classified according to different water sources (reservoirs, ponds, river mainstems, river tributaries, streams and wetlands). The prevalence among different water sources ranged from 8.24% (22/267 in streams) to 34.44% (186/540 in ponds) for C. sinensis, from 4.12% (11/267 in streams) to 12.56% (57/454, in reservoirs) for M. orientalis and from 10.49% (28/267 in streams) to 25.19% (237/941 in wetlands) for E. japonicus (Table 4).
Table 4.
The prevalence of FZTs in wild freshwater fish from different water sources in Jilin Province of China.
| FZT species | Water sources | No. of fish examined | No. of fish infected | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|
| C. sinensis | Reservoirs | 454 | 63 | 13.88 | 10.70–17.06 |
| Ponds | 540 | 186 | 34.44 | 30.43–38.45 | |
| River mainstems | 600 | 202 | 33.67 | 29.89–37.45 | |
| River tributaries | 1320 | 199 | 15.08 | 13.15–17.01 | |
| Streams | 267 | 22 | 8.24 | 4.94–11.54 | |
| Wetlands | 941 | 259 | 27.52 | 24.67–30.37 | |
| M. orientalis | Reservoirs | 454 | 57 | 12.56 | 9.51–15.61 |
| Ponds | 540 | 26 | 4.81 | 3.01–6.61 | |
| River mainstems | 600 | 62 | 10.33 | 7.89–12.77 | |
| River tributaries | 1320 | 157 | 11.89 | 10.14–13.66 | |
| Streams | 267 | 11 | 4.12 | 1.74–6.50 | |
| Wetlands | 941 | 96 | 10.20 | 8.27–12.13 | |
| E. japonicus | Reservoirs | 454 | 54 | 11.89 | 8.91–14.86 |
| Ponds | 540 | 135 | 25.00 | 21.53–28.85 | |
| River mainstems | 600 | 133 | 22.17 | 18.85–25.49 | |
| River tributaries | 1320 | 159 | 12.05 | 10.29–13.81 | |
| Streams | 267 | 28 | 10.49 | 6.81–14.17 | |
| Wetlands | 941 | 237 | 25.19 | 22.31–27.85 |
3.1.5 The effect of different months on FZT prevalence in wild freshwater fish.
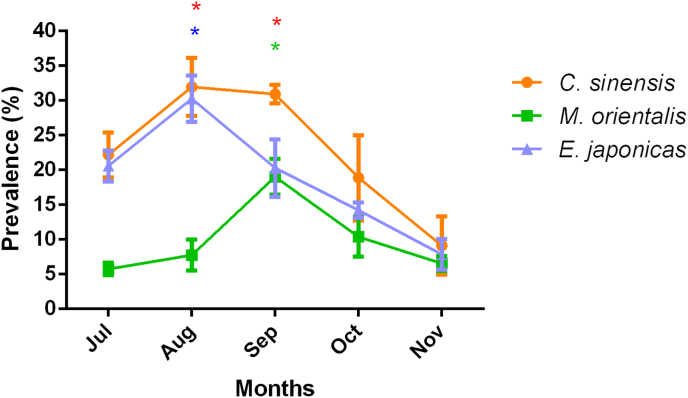
We continuously examined FZT prevalence in freshwater fish from July to November. The prevalence of C. sinensis, M. orientalis and E. japonicus in wild freshwater fish exhibited similar trends, increasing from July to August or September. The highest prevalence of C. sinensis (31.96%) and E. japonicus (30.25%) was observed in August, while that of M. orientalis (19.03%) was observed in September (Fig. 5).
Fig. 5.
The prevalence of FZTs in different months in wild freshwater fish in Jilin Province, China.
The prevalence of FZTs in freshwater fish gradually increased and then decreased, with the highest prevalence of C. sinensis and E. japonicus in August and the highest prevalence of M. orientalis in September. *p < 0.05 was considered to be a significant difference, and the prevalence in November was used as a control. The significances of C. sinensis, M. orientalis and E. japonicus in different months are marked in red *, green * and blue *, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Infection rate of FZTs in wild freshwater snails
We determined FZT infection rates in freshwater snails using direct microscopic observation combined with ITS sequence analysis. No FZT larvae were detected by direct observation in snails. ITS sequence analysis showed that C. sinensis ITS sequences were amplified in two P. striatulus samples (4.35%, 2/46), the M. orientalis ITS sequence was amplified in one P. striatulus sample (2.17%, 1/46), and no E. japonicus ITS sequences were amplified in snail samples. The overall infection rate of FZTs in snails was 2.27% (3/132), and no coinfection was observed.
3.3. FZT infection in the definitive hosts
Morphological identification and ITS sequence analysis were combined to assess FZT infection in fecal samples from canines, swine and ducks. The infection rates of C. sinensis and E. japonicus in canines were 46.43% (13/28) and 64.29% (18/28), respectively, and the infection rates of M. orientalis and E. japonicus in ducks were 19.23% (15/78) and 25.64% (20/78), respectively. The coinfection rate of C. sinensis and E. japonicus in canines was 35.71% (10/28), and the coinfection rate of M. orientalis and E. japonicus in ducks was 7.69% (6/78). No FZT infection was detected in swine.
The eggs of C. sinensis were bulb-shaped and yellow colored, approximately 24 ± 2.58 μm × 15.6 ± 2.33 μm in size, with egg operculum and shoulder peaks, containing a well-developed miracidium and a small spine at the bottom (Fig. 2 G). M. orientalis eggs were oval and yellow–brown colored, 25.87 ± 3.21 μm × 16.32 ± 1.65 μm in size, with egg operculum and without shoulder peaks, containing a well-developed miracidium and a small spine at the bottom (Fig. 2 H). The eggs of E. japonicus were oval and large, golden yellow colored, 84.25 ± 3.17 μm × 62.5 ± 2.55 μm in size, with a small egg operculum and containing yellow follicles (Fig. 2 I and J).
4. Discussion
In the present study, infection by FZTs in the first intermediate hosts (snails), the second intermediate hosts (freshwater fish) and the definitive hosts (canines, swine and ducks) in Jilin Province of China was investigated. Three trematodes (C. sinensis, M. orientalis and E. japonicus) were detected to be prevalent in this region. Nine species of fish could serve as the second intermediate hosts of FZTs, with P. striatulus serving as the first intermediate host and canines and ducks serving as the definitive hosts.
The different prevalences in different regions might be attributed to geographical conditions and local ecological status (climate, temperature, water quality, environment, etc.). The prevalence of C. sinensis was 37.09% (2160/5824) in Guangdong (Chen et al., 2010), 16.97% (52/307) in Guangxi (Sohn et al., 2009,b) and 19.96% (643/3221) in Heilongjiang (Zhang et al., 2014). The prevalence of M. orientalis was 10.54% (718/6815) in Heilongjiang (Qiu et al., 2017) and 6.33% (19/300) in Anhui (Li et al., 2017). Although E. japonicus metacercariae was found in freshwater fish (Sohn, 2009), the prevalence data for this trematode in freshwater fish are still lacking. In this study, the prevalence rates of C. sinensis, M. orientalis, and E. japonicus in freshwater fish in Jilin Province were 22.59%, 9.92% and 18.10%, respectively. Among 4122 samples, 13.39% of fish were infected with two species of trematodes, and 2.60% of fish were coinfected with three species of trematodes. This is the first report of the coinfection rate of FZTs in freshwater fish in Jilin Province. The suggests that ingestion of small amounts of raw fish might also cause FZT coinfection in definitive hosts.
A total of nine species of fish were found to be infected with FZTs in our survey, of which eight species were infected with C. sinensis, five species with M. orientalis and six species with E. japonicus. Unlike the survey in Heilongjiang Province (Qiu et al., 2017; Zhang et al., 2014), we did not find C. sinensis and M. orientalis in M. anguillicaudatu and C. auratus. In Guangdong Province, C. sinensis was prevalent in C. idellus, H. nobilis, C. molitorella, T. mossambica, C. auratus, H. molitrix, P. pekinensis and P. parva (Chen et al., 2010). The prevalence of FZTs in different fishes in Jilin Province was not consistent with that in other regions, which might be influenced by factors such as sampling time, sampling location, sampling water sources and sample size. Of note, P. parva was the most vulnerable fish to C. sinensis and M. orientalis in Jilin Province, which is consistent with observations in other provinces (Chen et al., 2010; Qiu et al., 2017; Zhang et al., 2014). Locally, the most commonly eaten fish are P. parva, H. leuciclus, R. sinensis, C. auratus, S. dabryi, and C. carpio. P. parva, H. leuciclus, R. sinensis and S. dabryi, which are susceptible to FZTs, are often eaten by deep-frying or stewing. However, insufficient cooking can result in incomplete extinction of the metacercariae in the muscle of fish and cause infection. Additional advice should be given to strengthen local disease publicity to avoid raw and accidental ingestion of susceptible fish. This is the first study to report the prevalence of E. japonicus in R. sinensis, H. leuciclus, C. giurinus, and S. dabryi, which enriches data on the second intermediate host of E. japonicus.
Interestingly, our study found that the sampling water sources acted as constraints on the prevalence of FZTs, demonstrating that C. sinensis, E. japonicus and M. orientalis have selectivity for the environment, which has not been reported in previous investigations. A previous report demonstrated that the prevalence of C. sinensis varied in different water sources, with the prevalence of C. sinensis in rivers being much higher than that in ponds, reservoirs and streams (Zhang et al., 2020). In Heilongjiang Province, the prevalence of C. sinensis and M. orientalis was higher in the Songhua and Nenjiang Rivers than in lakes and ponds (Qiu et al., 2017; Zhang et al., 2014). In this study, the prevalence of C. sinensis was higher in ponds than in other water sources in Jilin Province. We found that the ponds had freshwater snails living in them with the necessary conditions for the transmission of C. sinensis. Additionally, the definitive hosts around the ponds may be responsible for the high prevalence of C. sinensis in the ponds. The prevalence of M. orientalis was higher in the reservoirs than in other water sources in our study due to the abundance of water, fish resources, and a large number of wild birds. M. orientalis is transmitted in a variety of wild birds (Wang et al., 2020), which may be responsible for the high prevalence of M. orientalis in these reservoirs in our study. The prevalence of E. japonicus was higher in the wetlands than in other water sources due to the rich biodiversity of the wetland park, which had all the hosts necessary for the prevalence of E. japonicus. Wetland waters are virtually immobile, making them more conducive to E. japonicus infection. We also found that M. orientalis was primarily prevalent upstream, while the prevalence of C. sinensis and E. japonicus was similar among the three reaches. It is recommended that surveys of FZTs should cover various water sources and basins as much as possible to avoid data bias or errors.
The differences in FZT prevalence in wild fish in Jilin Province among different months reflect the important influence of temperature and precipitation on FZT prevalence. Analyzing the prevalence of C. sinensis in freshwater fish in different seasons revealed that the prevalence of C. sinensis was higher in summer, but there were no significant differences among the four seasons (Zhang et al., 2020). However, that study did not mention sampling locations, and the influence of sampling time on the prevalence of FZTs may not be suitable for Jilin Province, China. It is known that precipitation and temperature variation affect the prevalence of C. sinensis metacercariae in fish (Zhang et al., 2020). The prevalence of FZTs in cultured freshwater fish in Jilin Province was highest in July (Yang et al., 2019), which was earlier than our finding (the prevalence of FZTs was highest in August and September), which may be caused by the lower temperature in 2020. The earlier increases in temperature and precipitation are more favorable for the development of FZTs in freshwater snails.
Transmission of FZTs is strictly restricted by the first and second intermediate hosts and the definitive hosts (Zhang et al., 2020). Our study identified FZT infection in the first intermediate hosts and in the definitive hosts in Jilin Province. These findings suggested that a complete life cycle of FZTs has formed in Jilin Province. The activities of definitive hosts may pose new challenges to local public safety to enact measures against FZTs, and the surveillance and treatment of FZTs in local livestock and poultry may be needed. The results of two FZT coinfections in canines and ducks further supported the inference that FZT coinfection in freshwater fish could ultimately result in FZT coinfection in definitive hosts.
This study identified three FZTs in intermediate and definitive hosts in Jilin Province. However, the low number of samples of the definitive hosts and the first intermediate hosts may affect the assessment of the prevalence of FZTs among the first intermediate hosts and definitive hosts in Jilin Province, and more work is required in future studies to confirm these findings.
5. Conclusion
This study is a detailed epidemiological investigation of FZTs in Jilin Province, including the first and second intermediate hosts, definitive hosts, species and prevalence of FZTs and coinfection rates.
Funding
This study was supported by National Key R&D Program of China (Nos. 2017YFD0501200, 2017YFD0501305), Science and Technology Development Program of Jilin Province (Nos. 20200402044NC). The experiments conducted in this study complied with the current laws of China.
Data availability statement
The newly generated sequences were submitted to the NCBI GenBank database under the accession No. MW828640, MW828729 and MW828605.
Authors’ contributions
Jianhua Li, Yuru Wang and Xin Li supervised the study and designed the experiments. Yuru Wang, Yanhui Yu and Yanyan Ren conducted these experiments and wrote the manuscript. Xiaocen Wang, Xichen Zhang, Jianhua Li and Zhiteng Zhao revised the manuscript. Nan Zhang, Pengtao Gong and Yeting Ma provided technical assistance.
Ethics statement
The project was approved by the animal welfare and research ethics committee of Jilin University. Prior to the start of the study, written informed consent was obtained from the owners of dogs, ducks and swine.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the animal owners for supporting diagnostic works. We thank Haoyang Zhang for the help in drawing of the sampling sites. We thank local fishermen for their help in catching freshwater fish. We thank Fucheng Ma for his help with fish collecting and animal fecal sampling.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2022.04.004.
Contributor Information
Yuru Wang, Email: yuruwang0109@126.com.
Xiaocen Wang, Email: wangxiaocen2016@163.com.
Pengtao Gong, Email: gongpt@jlu.edu.cn.
Yanhui Yu, Email: 1053408972@qq.com.
Nan Zhang, Email: n_zhang@jlu.edu.cn.
Yanyan Ren, Email: ry073088@163.com.
Yeting Ma, Email: 18535444856@163.co.
Zhiteng Zhao, Email: zhitengzhao@163.com.
Xichen Zhang, Email: xczhang@jlu.edu.cn.
Xin Li, Email: jlulixin0928@163.com.
Jianhua Li, Email: jianhuali7207@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Besprozvannykh V.V., Rozhkovan K.V., Ermolenko A.V. Stephanoprora chasanensis n. sp. (Digenea: echinochasmidae): morphology, life cycle, and molecular data. Parasitol. Int. 2017;66:863–870. doi: 10.1016/j.parint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Bush A.O., Lafferty K.D., Lotz J.M., Shostak A.W. Parasitology meets ecology on its own terms: margolis et al. Revisited. J. Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Chen D., Chen J., Huang J., Chen X., Feng D., Liang B., Che Y., Liu X., Zhu C., Li X., Shen H. Epidemiological investigation of Clonorchis sinensis infection in freshwater fishes in the Pearl River Delta. Parasitol. Res. 2010;107:835–839. doi: 10.1007/s00436-010-1936-5. [DOI] [PubMed] [Google Scholar]
- Choi M.H., Kim S.H., Chung J.H., Jang H.J., Eom J.H., Chung B.S., Sohn W.M., Chai J.Y., Hong S.T. Morphological observations of Echinochasmus japonicus cercariae and the in vitro maintenance of its life cycle from cercariae to adults. J. Parasitol. 2006;92:236–241. doi: 10.1645/GE-354R1.1. [DOI] [PubMed] [Google Scholar]
- Clausen J.H., Madsen H., Murrell K.D., Van P.T., Thu H.N.T., Do D.T., Thi L.A.N., Manh H.N., Dalsgaard A. Prevention and control of fish-borne zoonotic trematodes in fish nurseries. Vietnam. Emerg. Infect. Dis. 2012;18:1438–1445. doi: 10.3201/eid1809.111076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hop N.T., De N. Van, Murrell D., Dalsgaard A. Occurrence and species distribution of fishborne zoonotic trematodes in wastewater-fed aquaculture in northern Vietnam. Trop. Med. Int. Health. 2007;12:66–72. doi: 10.1111/j.1365-3156.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- Hung N., Dung D., Lan Anh N., Van P., Thanh B., Van Ha N., Van Hien H., Canh L. Current status of fish-borne zoonotic trematode infections in Gia Vien district, Ninh Binh province, Vietnam. Parasites Vectors. 2015;8:21. doi: 10.1186/s13071-015-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Food-borne trematodiases. Clin. Microbiol. Rev. 2009;22:466–483. doi: 10.1128/CMR.00012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Wu H., Sun E., Zhu Y., Li C., Wu H., Sun E., Zhu Y. Investigation on the endemic characteristics of Metorchis orientalis in Huainan area. China. Nutr. Hosp. 2017;34:675. doi: 10.20960/nh.1333. [DOI] [PubMed] [Google Scholar]
- Li X., Chen Y., Ouyang Y., Zhang H., Lin R., Weil M. Overview of human clonorchiasis sinensis in China. Southeast Asian J. Trop. Med. Publ. Health. 2011;42:248–254. [PubMed] [Google Scholar]
- Lun Z.R., Gasser R.B., Lai D.H., Li A.X., Zhu X.Q., Yu X.B., Fang Y.Y. Clonorchis sinensis: a key foodborne zoonosis in China. Lancet Infect. Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- Na L., Gao J.F., Liu G.H., Fu X., Su X., Yue D.M., Gao Y., Zhang Y., Wang C.R. The complete mitochondrial genome of Metorchis orientalis (Trematoda: opisthorchiidae): comparison with other closely related species and phylogenetic implications. Infect. Genet. Evol. 2016;39:45–50. doi: 10.1016/j.meegid.2016.01.010. [DOI] [PubMed] [Google Scholar]
- Qian M.B., Utzinger J., Keiser J., Zhou X.N. Clonorchiasis. Lancet. 2016;387:800–810. doi: 10.1016/S0140-6736(15)60313-0. [DOI] [PubMed] [Google Scholar]
- Qiu J., Zhang Y., Zhang X., Gao Y., Li Q., Chang Q., Wang C. Metacercaria infection status of fishborne zoonotic trematodes, except for Clonorchis sinensis in Fish from the Heilongjiang province, China. Foodb. Pathog. Dis. 2017;14:440–446. doi: 10.1089/fpd.2016.2249. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Gao Y., Li Y., Lv Q. Comparative analyses of complete ribosomal DNA sequences of Clonorchis sinensis and Metorchis orientalis: IGS sequences may provide a novel genetic marker for intraspecific variation. Infect. Genet. Evol. 2019;78:104125. doi: 10.1016/j.meegid.2019.104125. [DOI] [PubMed] [Google Scholar]
- Santos C.A.M.L., Dos, Howgate P. Fishborne zoonotic parasites and aquaculture: a review. Aquaculture. 2011;318:253–261. [Google Scholar]
- Sohn W.M. a. Fish-borne zoonotic trematode metacercariae in the Republic of Korea. Kor. J. Parasitol. 2009;47:103–114. doi: 10.3347/kjp.2009.47.S.S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Eom K.S., Min D.Y., Rim H.J., Li X. b. Fishborne trematode metacercariae in freshwater fish from Guangxi Zhuang Autonomous Region, China. Kor. J. Parasitol. 2009;47:249–257. doi: 10.3347/kjp.2009.47.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W.M., Na B.K., Cho S.H., Ju J.W., Son D.C. Prevalence and intensity of Clonorchis sinensis metacercariae in freshwater fish from wicheon stream in Gunwi-Gun, Gyeongsangbuk-do, Korea. Kor. J. Parasitol. 2018;56:41–48. doi: 10.3347/kjp.2018.56.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B., Kaewkes S., Intapan P.M., Maleewong W., Brindley P.J. Food-borne trematodiases in Southeast Asia: epidemiology, pathology, clinical manifestation and control. Adv. Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Sripa B., Kaewkes S., Sithithaworn P., Mairiang E., Laha T., Smout M., Pairojkul C., Bhudhisawasdi V., Tesana S., Thinkamrop B., Bethony J.M., Loukas A., Brindley P.J. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:1148–1155. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan W., Hung N.M., Madsen H., Fried B. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitol. 2013;58:231–258. doi: 10.2478/s11686-013-0155-5. [DOI] [PubMed] [Google Scholar]
- Tang Z., Huang Y., Yu X.B. Current status and perspectives of Clonorchis sinensis and clonorchiasis: epidemiology, pathogenesis, omics, prevention and control. Infect. Dis. Poverty. 2016;5:1–12. doi: 10.1186/s40249-016-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatonova Y.V., Chelomina G.N., Nguyen H.M. Inter-individual and intragenomic variations in the ITS region of Clonorchis sinensis (trematoda: opisthorchiidae) from Russia and Vietnam. Infect. Genet. Evol. 2017;55:350–357. doi: 10.1016/j.meegid.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li X., Sun Q., Gong P., Zhang N., Zhang X., Wang X., Li G., Li J. First case report of Metorchis orientalis from black swan. Int. J. Parasitol. Parasites Wildl. 2020;13:7–12. doi: 10.1016/j.ijppaw.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Heal Organ; 1995. Control of Foodborne Trematode Infections; pp. 1–157. [PubMed] [Google Scholar]
- Yang S., Pei X., Yin S., Yu X., Mei L., Zhao W., Liang H., Wang Q., Yang D. Investigation and research of Clonorchis sinensis metacercariae and Metorchis orientalis metacercariae infection in freshwater fishes in China from 2015 to 2017. Food Control. 2019;104:115–121. [Google Scholar]
- Yu S., Mott K.E. Epidemiology and morbidity of food-borne intestinal trematode infections. World Heal Org. 1994;91:R125–R152. [Google Scholar]
- Zhang Y., Gong Q.L., Lv Q.B., Qiu Y.Y., Wang Y.C., Qiu H.Y., Guo X.R., Gao J.F., Chang Q.C., Wang C.R. Prevalence of Clonorchis sinensis infection in fish in South-East Asia: a systematic review and meta-analysis. J. Fish. Dis. 2020;43:1409–1418. doi: 10.1111/jfd.13245. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Na L., Wang W., Xu W., Gao D., Liu Z., Wang C., Zhu X. Prevalence of Clonorchis sinensis infection in freshwater fishes in northeastern China. Vet. Parasitol. 2014;204:209–213. doi: 10.1016/j.vetpar.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Zhou X., Disease F., Zhu X. Sequences of internal transcribed spacers and two mitochondrial genes: effective genetic markers for Metorchis orientalis. J. Anim. Vet. Adv. 2010;9:2371–2376. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The newly generated sequences were submitted to the NCBI GenBank database under the accession No. MW828640, MW828729 and MW828605.