Abstract
The Microbicide Trials Network-017 study was undertaken to characterize the safety, acceptability, pharmacokinetic (PK), and pharmacodynamic profile of the reduced-glycerin (RG) 1% tenofovir (RG-TFV) gel compared to oral emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). The study was a Phase 2, three-period, randomized sequence, open-label, expanded safety and acceptability crossover study. In each 8-week study period, HIV-1-uninfected participants were randomized to RG-TFV rectal gel daily or RG-TFV rectal gel before and after receptive anal intercourse (RAI) (or at least twice weekly in the event of no RAI), or daily oral FTC/TDF. A mucosal substudy was conducted at sites in the United States and Thailand. Samples were collected to evaluate PK and ex vivo biopsy challenge with HIV-1. A total of 195 men who have sex with men and transgender women were enrolled in the parent study and 37 in the mucosal substudy. As previously reported, both products were found to be safe and acceptable. Systemic TFV concentrations were significantly higher following oral exposure and daily rectal administration compared to RAI-associated product use (p < .001). All three routes of pre-exposure prophylaxis (PrEP) administration resulted in the inhibition of explant infection (p < .05), and there was a significant inverse correlation between explant HIV-1 p24 and tissue concentrations of TFV and FTC (p < .0001). Despite significant differences in systemic and mucosal drug concentrations, all three PrEP regimens were able to protect rectal explants from ex vivo HIV infection. These data suggest that there is a rationale for co-development of oral and topical antiretroviral PrEP for HIV prevention.
Clinical Trial Registration number: NCT01687218.
Keywords: rectal, microbicide, HIV, prevention, tenofovir, emtricitabine
Introduction
Randomized, placebo-controlled clinical studies of the oral antiretroviral combination emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) tablet taken daily or around the time of sexual intercourse have demonstrated efficacy in reducing incidence of HIV infection in men who have sex with men (MSM).1–3 Oral FTC/TDF and FTC/tenofovir alafenamide fumarate are now available by prescription for prevention of HIV infection in at-risk individuals in high- and low-income countries.
While the increased availability of FTC/TDF or FTC/TAF pre-exposure prophylaxis (PrEP) is an important step in HIV prevention, it remains to be seen if oral PrEP will be accessible and acceptable for long-term use by at-risk groups, including young women, black MSM, and transgender men.4–6
While oral PrEP will fulfill HIV prevention needs for some individuals, options such as a rectal gel, suppository, or enema may be more desirable to others at risk of HIV from receptive anal intercourse (RAI).7,8 Lubricating gel is frequently used to facilitate anal intercourse9 and topical HIV prevention candidates formulated as lubricants are likely to be acceptable and easily incorporated into the sexual practices of populations having RAI. Topical PrEP in the form of a rectal microbicide has been in development for over 15 years. Recently, much of this effort has focused on the development of 1% formulation of tenofovir (TFV) gel, initially using the vaginal formulation and later the reduced-glycerin (RG) gel with a lower osmolality.10 The Microbicide Trials Network (MTN) 007 Phase 1 study demonstrated that this modified formulation was both safe and acceptable to men and women following daily rectal application for up to seven consecutive days.11 Two subsequent Phase I studies of this RG formulation, CHARM-01 and CHARM-02, confirmed gel safety as well as demonstrating a favorable mucosal pharmacokinetic (PK) profile associated with simulated RAI.11,12 It is this RG product that was taken into the Phase 2 MTN-017 study.
The objectives of the MTN-017 study were to compare the safety profiles of daily oral FTC/TDF tablet, daily rectal TFV RG 1% gel, and RAI-associated rectal TFV RG 1% gel, and to evaluate and compare their acceptability as potential HIV prevention methods. The overall safety and acceptability findings from the MTN-017 study have previously been published.8,13 This article describes the PK and pharmacodynamic (PD) data generated in the MTN-017 mucosal substudy.
Materials and Methods
Study design
MTN-017 was a Phase 2 randomized sequence open-label expanded safety and acceptability crossover study of the oral FTC/TDF tablet and rectally applied RG-TFV 1% gel. Participants were randomized to one of six sequences consisting of three 8-week periods with different product use regimens: daily oral FTC (200 mg)/TDF (300 mg), daily rectal RG-TFV 1% gel, or rectal RG-TFV 1% gel used before and after RAI. not exceeding two doses within 24 h, consistent with the method used for vaginal application of 1% TFV gel in the CAPRISA 004 study in South African women14 (Table 1). If participants did not engage in RAI, they were instructed to use two doses of the RG-TFV 1% gel at least once weekly. Product use was assessed by mixed methods, including unused product return count, text messaging reports, and qualitative plasma TFV PK results.15 Participants were evaluated at weeks 0, 4 (mid-period), and 8 (end period). There was a 1-week washout between periods.
Table 1.
Study Regimen
| Sequence | Period 1 (8 weeks) | Washout (1 week) | Period 2 (8 weeks) | Washout (1 week) | Period 3 (8 weeks) |
|---|---|---|---|---|---|
| 1 | Daily oral | Daily rectal | RAI rectal | ||
| 2 | RAI rectal | Daily oral | Daily rectal | ||
| 3 | Daily rectal | RAI rectal | Daily oral | ||
| 4 | Daily rectal | Daily oral | RAI rectal | ||
| 5 | Daily oral | RAI rectal | Daily rectal | ||
| 6 | RAI rectal | Daily rectal | Daily oral |
RAI, receptive anal intercourse.
The primary study objectives were to assess both safety and acceptability of daily oral FTC/TDF, daily rectal RG-TFV 1% gel, and RAI-associated rectal RG-TFV 1% gel. Secondary objectives were to compare systemic and local PK, and to evaluate and compare adherence between the three product use regimens. The MTN-017 study protocol is available at www.mtnstopshiv.org/studies/4495.
Study sites
There were eight study sites: four in the United States (Boston, Pittsburgh, San Francisco, and San Juan), two in Thailand (Bangkok and Chiang Mai), and one each in Peru (Lima) and South Africa (Cape Town). The mucosal substudy described in this article was conducted at the Pittsburgh and Bangkok sites.
Ethical considerations
Before implementation, the study protocol was reviewed and approved by the institutional review boards/ethics committees at each participating site. In addition, the protocol was approved by the Prevention Sciences Review Committee of the National Institute of Allergy and Infectious Diseases of the U.S. National Institutes of Health. All participants provided written informed consent. The trial was registered with ClinicalTrials.gov.
Participants
Healthy HIV-uninfected MSM and TGW ≥18 years of age with a history of RAI (protected or unprotected) at least once in the previous 3 months were recruited through social and traditional media, online advertising, flyers, community engagement, and word of mouth. Individuals with abnormalities of the colorectal mucosa, significant gastrointestinal symptoms, rectal Chlamydia trachomatis or Neisseria gonorrhoeae infection or any sexually transmitted infection requiring treatment, chronic hepatitis B infection, hepatitis C exposure, a requirement to use drugs that were likely to increase the risk of bleeding following mucosal biopsy, or symptoms suggestive of HIV seroconversion were excluded from the study.
Study products
CONRAD (Arlington, VA) supplied the RG-TFV 1% gel, which was provided in prefilled applicators (HTI Plastics, Lincoln, NE) containing 4 mL gel. RG-TFV 1% gel (weight/weight) is a transparent gel formulation of tenofovir (PMPA, 9-[(R)-2-(phosphonomethoxy)propyl]adenine monohydrate), formulated in purified water with edetate disodium, citric acid, glycerin, methylparaben, propylparaben, and hydroxyethylcellulose, and pH adjusted to 4–5. The RG-TFV 1% gel has lower glycerin content than the TFV 1% gel (original vaginal formulation) and a significantly reduced osmolality (∼800 vs. ∼3,000 mmol/kg, respectively). Oral FTC/TDF was supplied by Gilead Sciences (Foster City, CA). Participants were asked to take either one oral FTC/TDF tablet with water daily or to deliver intrarectally the content of an applicator filled with TFV RG 1% gel using a sachet of lubricant to facilitate insertion (Good Clean Love, Inc., Eugene, OR) either daily or before and after RAI. Study product adherence was monitored through the use of real-time plasma PK and behavioral interviews.15
PK analysis
Rectal biopsies for PK and PD (explant infection) studies were collected at the same time. TFV, FTC, and tenofovir diphosphate (TFV-DP) concentrations in plasma, rectal fluid, tissue, and peripheral blood mononuclear cells (PBMCs) were conducted through previously described liquid chromatographic-mass spectrometric methods by the Johns Hopkins University School of Medicine Clinical Pharmacology Analytical Laboratory.16,17 Assays were validated in accordance with FDA: Guidance for Industry, Bioanalytical Method Validation, recommendations.18 Briefly, assay lower limits of quantification were as follows: plasma TFV and FTC: 0.31 ng/mL; rectal fluid TFV: 1.25 ng/sponge; rectal fluid FTC: 5 ng/sponge; tissue TFV: 0.05 ng/sample; tissue FTC: 0.25 ng/sample; PBMC; and tissue TFV-DP: 50 fmol/sample. Rectal fluid concentrations were normalized to the weight of rectal fluid collected on the Merocel sponge (Beaver-Visitec International, Inc., Waltham, MA) and reported as ng/mg. Intracellular metabolite concentrations were normalized to cell counts and tissue weights and reported as fmol/106 cells (PBMC) and fmol/mg (tissue), respectively.
PD analysis
At the baseline visit and end of dosing period (week 8), rectal biopsies were collected in 20 mL RPMI [with 1.125 μg/mL of Amphotericin Band 0.5 mg/mL of Zosyn (piperacillin and tazobactam)] and transported to the laboratory for ex vivo infection within 1–2 h using a common viral stock of HIV-1BaL (104 TCID50), as previously described.19 Supernatants for p24 quantification were collected on days 4, 7, 10, and 14 post-HIV challenge, stored, and later assayed for p24 antigen (p24 HIV antigen ELISA; NCI, Bethesda, MD) where the assay's lower limit of quantification (LLOQ) was 10 pg/mL. Nondetectable cumulative p24 measures were converted to 1/2 the LLOQ (5 ng/mL). The sum of all four supernatant p24 values (cumulative p24) was divided by biopsy weight. The median (of up to four biopsies) cumulative p24 with and without biopsy weight-adjusted p24 was the unit of analysis. For PK/PD analysis, drug concentrations below the LLOQ were imputed as LLOQ/2. For analysis and plotting purposes, predose (no drug) concentration values were imputed as LLOQ/20.
Statistical analyses
TFV, TFV-DP, and FTC for all sampled biological matrices were summarized by study visit using median [interquartile range (IQR)] and box plots. Paired comparisons of PK and PD values among and between regimens were made using the nonparametric Friedman test and Wilcoxon signed rank test, respectively. Pairwise correlations between PK and/or PD variables used the Spearman rank order correlation test.
Generalized estimating equation models with a gamma log link, exchangeable correlation structure, and robust errors were used to evaluate the relationship between cumulative p24 antigen (dependent variable) and drug concentration and regimen as independent variables. Explant p24 was evaluated with and without biopsy weight adjustment. All PK variables were explored.
Antiretroviral drug concentration-p24 response modeling explored (1), 2-, 3-, and 4-parameter Imax models (E0 baseline p24 without drug, Imax maximum p24 change on drug, IC50 molar drug concentration at half-maximal effect, and slope term [Hill coefficient]), (2) weighting schemes for heteroscedasticity, (3) ± biopsy weight adjustment, and (4) ± imputation of baseline and/or below the limit of quantification values. Drug concentration explored all matrices assayed. Goodness-of-fit was assessed using the correlation matrix, coefficient of variation, and Schwartz and Akaike information criterion (Phoenix WinNonlin v.8, Certara, Cary, NC).
Results
A total of 195 MSM and transgender women (TW) were enrolled in the parent study and 37 in the mucosal substudy conducted in Pittsburgh, PA, and Bangkok, Thailand. Nineteen participants were enrolled in Pittsburgh and 18 in Bangkok.
PK analysis
TFV and FTC PK data from all biological matrices and across six observation times are summarized in Table 2. Comparing end of treatment period (end period) concentration data, plasma TFV concentrations were highest during the oral administration period (p < .001) and tissue and RF TFV were highest in the daily rectal administration period (p < .001). TFV concentrations in blood and tissue were lowest during the RAI-associated dosing period (Table 2). Tissue TFV-DP was highest during the daily rectal dosing period and similar between oral and RAI rectal dosing periods. Comparing plasma concentrations of TFV (oral and rectal administration) and FTC (oral only) did not demonstrate any significant difference for either analyte at the end of the dosing period (week 8) or the mid-period (week 4) (Wilcoxon test, all p > .57). Comparing the TFV and FTC plasma concentrations during oral dosing periods against established oral adherence benchmarks (HPTN 06616) indicates that 82.8% to 90.8% of participants took 7 doses in the week before sampling (Table 3). This agrees with PBMC TFV-DP data, indicating 87% of participants took seven doses in the prior week, based on HPTN 066 benchmarks. Furthermore, so-called white coat adherence (e.g., dosing only the day before study visits and not on earlier occasions) was rare with only 2% of daily adherent participants (based on plasma TFV) having concomitant PBMC TFV-DP, indicating less than daily dosing
Table 2.
Drug Concentration by Analyte and Regimen
| Regimen |
Daily oral |
Daily rectal |
RAI rectal |
|||
|---|---|---|---|---|---|---|
| Matrix analyte | Mid-period | End period | Mid-period | End period | Mid-period | End period |
| Plasma TFV (ng/mL) | 82.2 (53.7, 133.0); 185 | 80.5 (46.0, 145.0); 186 | 2.9 (0.8, 7.9); 184 | 2.3 (0.5, 8.1);177a | 0.5 (0.0, 2.7); 185 | 0.5 (0.0, 3.4); 182b,c |
| Plasma FTC (ng/mL) | 240.0 (92.7, 947.0); 185 | 251.0 (72.1, 1,060.0); 186 | all BLQ; 184 | all BLQ; 177a | all BLQ; 185 | all BLQ; 182b |
| PBMC TFV-DP (fmol/106 cells) | 58.8 (32.2, 101.8); 185 | |||||
| Rectal tissue TFV (fmol/mg) | 1.5 (0.7, 4.7); 37 | 9.2 (4.8, 14.6); 36a | 1.0 (0.3, 4.9);36c | |||
| Rectal tissue FTC (ng/mg) | 0.4 (0.3, 0.7);37 | all BLQ; 36a | all BLQ; 36b | |||
| Rectal tissue TFV-DP (fmol/106 cells) | 32.9 (22.2, 74.8);37 | 100.0 (68.1, 172.8); 36a | 36.0 (9.1, 117.2); 36 | |||
| Rectal fluid TFV (ng/mg) | 10.1 (1.2, 39.3); 185 | 10.0 (0.8, 42.9); 186 | 17.3 (1.0, 160.1); 182 | 13.0 (1.6, 89.3); 173a | 0.9 (0.1, 12.1); 186 | 1.0 (0.1, 17.4); 182b,c |
| Rectal fluid FTC (ng/mg) | 2.2 (0.5, 15.2); 185 | 2.1 (0.4, 10.7); 186 | all BLQ; 182 | all BLQ; 173a | all BLQ; 186 | all BLQ; 182b |
Data are median (lower quartile and upper quartile); sample number.
p < .02, aDaily oral vs. Daily rectal, bDaily oral vs. RAI rectal, cDaily rectal vs. RAI rectal.
BLQ, below the limit of quantification; FTC, emtricitabine; PBMC, peripheral blood mononuclear cell; TFV, tenofovir; TFV-DP, tenofovir diphosphate.
Table 3.
Percent of Study Participants Within Specified Adherence Ranges in the Oral Emtricitabine/Tenofovir Disoproxil Fumarate Period Based on Pharmacologic Benchmarks
| Drug-period | 7 doses per week | 4 doses per week | <4 doses per week |
|---|---|---|---|
| TFV mid | 88.6% | 7.6% | 3.8% |
| TFV end | 82.8% | 9.7% | 7.5% |
| FTC mid | 90.8% | 5.4% | 3.8% |
| FTC end | 82.8% | 10.7% | 6.5% |
Pharmacodynamics
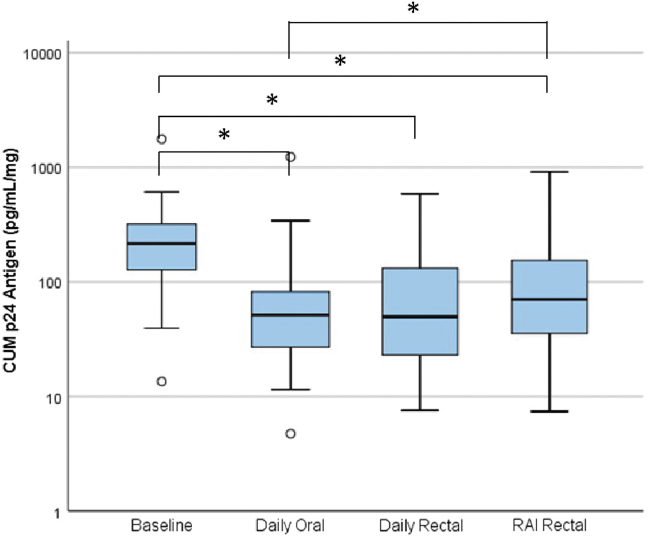
Ex vivo explant HIV challenge cumulative p24 antigen results with and without biopsy weight adjustment were highly correlated (Spearman, r = 0.975, p < .001). There was a statistically significant decrease in explant infection compared to predrug baseline in all three regimens with median (IQR) log10 declines as follows: daily oral 0.55 (0.21, 1.00), p < .001; daily rectal 0.68 (0.02, 0.97), p < .001; and RAI rectal 0.34 (−0.19, 0.78), p = .025 (Fig. 1). There was no significant difference in the degree of explant infection between oral and daily rectal dosing or daily rectal dosing and RAI-associated dosing, but inhibition of explant infection was significantly lower in RAI-associated rectal dosing compared to oral dosing (p = .026).
FIG. 1.
Side-by-side box plots of cumulative HIV-1 p24 antigen (pg/mL/mg biopsy tissue shown in log scale) for all study conditions. Box indicates IQR, horizontal bar indicates median, whiskers indicate 1.5 times the IQR, and circles indicate outliers beyond the whiskers and less than three times the IQR.*p < .05. CUM, cumulative; IQR, interquartile range; RAI, receptive anal intercourse.
PK/PD relationship
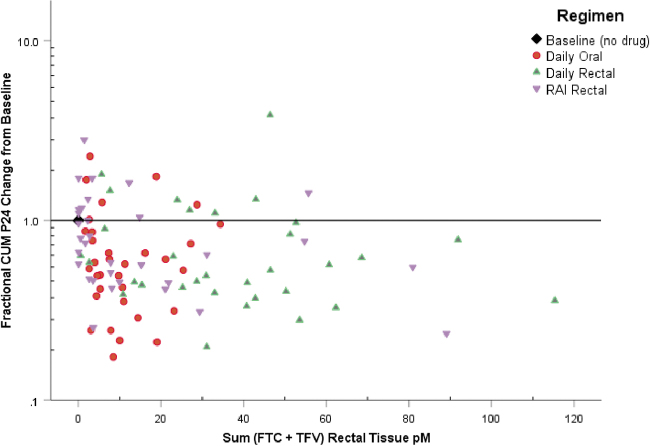
Ex vivo infection results in RT were negatively correlated with paired PK drug concentrations in the respective tissue and fluids or plasma across all visits (correlation coefficient ranged from −0.38 to −0.427, all p < .001). Figure 2 shows the relative decrease from baseline cumulative p24 antigen with increasing total molar drug concentration.
FIG. 2.
Fractional change from baseline in median cumulative p24 antigen expressed as ratio of regimen to baseline ratio (y-axis). Molar concentration sum (FTC plus TFV) on x-axis. Reference line (1.0) indicates no change from baseline. FTC, emtricitabine; TFV, tenofovir.
Generalized estimating equations were used to quantify the impact of drug concentration increase and drug regimen on the cumulative p24 antigen response in the explant assay (Supplementary Table S1). The statistically significant negative slope of the rectal tissue molar sum indicates a 13 ng/mg reduction in p24 antigen for every additional 1 ng/mL increase in colon tissue TFV or FTC concentration. In addition, the daily oral dosing period was independently associated with reduced HIV infectivity, although with a minor p24 effect compared to changes in tissue drug concentration.
The data were also fit to an inhibitory PD concentration-response model (data not shown), which indicated a statistically significant parameter estimate for a 2-parameter inhibitory Imax model (E0 and IC50). More complex models (adding Imax and gamma steepness exponent [Hill coefficient] terms) failed to identify statistically significant parameter values for all parameters. Even though we estimated statistically significant PD parameter estimates, we rejected the 2-parameter model on grounds of biologic implausibility because (1) the model underestimated E0 (for which we had empiric baseline [no drug] estimates for comparison), (2) very few observations above the fitted IC50 estimate, and (3) the inhibitory p24 asymptote at infinite drug concentration did not approach the minimum cumulative p24 value (LLOQ), which we have observed in other studies using the same explant methods and drugs.
Discussion
In this study, we have demonstrated that both oral and rectal administration of antiretroviral PrEP can prevent ex vivo HIV challenge infection of colorectal explants. This provides a rationale for the continued development of topical antiretroviral PrEP for the prevention of RAI-associated HIV infection.
Despite advances in the diagnosis and treatment of HIV infection, new cases are still occurring at an unacceptable level. The 2019 UNAIDS report estimated that ∼1.7 million new HIV infections occurred in 2018. Consequently, increased efforts are being made to develop and implement safe and effective HIV prevention strategies. Antiretroviral PrEP is the most advanced HIV prevention intervention and oral PrEP with FTC/TDF is now licensed in several countries. In addition, FTC/TAF (Descovy®) was approved for PrEP use in 2019, although the indication excluded individuals at risk of HIV infection through vaginal sex.20 Ensuring that the most at-risk populations have access to oral PrEP and support to maximize PrEP adherence during periods of risk for HIV infection remains a challenge.21 In this setting, other antiretroviral PrEP modalities are being developed, including topical products (microbicides), vaginal rings, and long-acting injectable PrEP.22 We and others have advocated for the development of topical rectal microbicides for the prevention of HIV infection associated with condomless RAI.23
A critical question in the development of topical PrEP is whether a sufficient amount of antiretroviral drug can be delivered to the mucosal tissue at risk of HIV infection. Rectal 1% TFV gel has been shown to protect non-human primates from SIV infection,24 and has been evaluated in two Phase 1 studies where it was able to significantly inhibit HIV infection in colorectal explants.11,12 The MTN-017 mucosal PK/PD study was unique as it provided an opportunity to compare the safety, acceptability, and PK/PD profile of both oral and rectal PrEP in a sexually active population of MSM.13
The plasma PK concentration of TFV was greater after daily oral FTC/TDF compared to rectal administration and rectal tissue TFV concentrations were greater after daily rectal use of TFV gel in comparison to oral administration of FTC/TDF. These differences in oral dosing and topical dosing were similar to those seen between oral FTC/TDF and vaginal TFV dosing in MTN-001, a clinical trial using similar size, crossover design, and the same drug analytical methods as in MTN-017.25
In the HPTN 066 directly observed PK study, participants received oral FTC/TDF in a range of dosing regimens (once weekly to once daily) for 5 weeks.16 When comparing the HPTN 066 daily FTC/TDF regimen PK data to the MTN-017 oral FTC/TDF data, there were a number of observations: (1) the number of MTN-017 participants with daily adherence was consistently high (>82%); (2) blood concentrations of both FTC and TFV were higher in MTN-017 participants, which may have been due to collection of samples less than 24 h postdosing (the sampling time in HPTN 066); and (3) tissue concentrations of FTC, TFV, and TFV-diphosphate in MTN-017 were largely overlapping those reported in HPTN 066. The data presented herein add substantially to the published rectal tissue data for both oral FTC/TDF and rectal TFV dosing. The duration of dosing in the MTN-017 study (8 weeks) was greater than that seen in the HPTN 066 study (5 weeks) and the MTN-017 participants did not have their product administration directly observed, but they did receive motivational counseling linked to real-time PK data, which may have improved overall product adherence.15,26
Ex vivo/in vitro explant models have been used to screen candidate PrEP agents including rectal, vaginal, oral, and injectable forms of PrEP.11,19,27–30 Because the daily (83%–91%) and four weekly (5%–11%) adherence frequencies were so high during our oral regimen periods, and given the demonstrated efficacy of oral FTC/TDF dose observed with daily, four weekly, and four dose on demand regimens, the rectal tissue concentrations achieved in MTN-017 are associated with a high level of protection.1,3,31 Consistent with that observation, the magnitude of reduced HIV infectivity as assessed by the ex vivo explant HIV challenge may be associated with a high level of clinical HIV protection in this study population of MSM and TW.
It remains uncertain if the mucosal tissue antiretroviral concentration alone—and by extension, the rectal tissue ex vivo HIV challenge alone as a biomarker —is sufficient for HIV protection or if a combination of systemic and local mucosal drug concentration is necessary. There has never been a clinical PrEP efficacy trial of a rectal microbicide (low systemic, high rectal drug concentrations) to compare to oral and injectable PrEP trials (high systemic, lower rectal drug concentration), which would be needed to answer this. Topical vaginal dosing of TFV or dapivirine demonstrate reduced HIV infectivity in the ex vivo HIV explant challenge model32–34; these drugs have also proven effective in randomized clinical trials, although with less magnitude of protection—even with post hoc adherence corrections—than oral FTC/TDF combinations and injectable cabotegravir dosing.35–39
The MTN-017 explant studies were conducted at two separate clinical sites (Pittsburgh and Bangkok), although the quantification of supernatant HIV-1 p24 antigen from both trial sites was undertaken in Pittsburgh. Two articles have explored the variability of explant infection in studies conducted across multiple sites. In the first study, sites were provided with a common source of virus and experimental compound and were able to demonstrate that multiple investigators could identify when a drug was efficacious when providing standardized reagents and analytical techniques were used.40 The second study was able to show that using four tissue explants (as used in the MTN-017 study) per experimental time point was adequate for comparative analyses.41
Despite differences in plasma and tissue PK exposure related to oral or rectal product administration, significant inhibition of colorectal explant HIV infection was seen with all three dosing regimens. These data are encouraging in terms of generating a rationale for the use of rectal PrEP regimens for HIV prevention. Understanding the PK/PD profile of candidate topical products used on an intermittent basis is important as participants in the MTN-017 study expressed a preference for RAI-associated dosing compared to daily rectal use.15 While RAI-associated dosing had the lowest PK exposure in plasma and rectal tissue and the smallest reduction in HIV infectivity, this finding is consistent with less frequent dosing, associated reduction in drug accumulation, and more distant sample timing with RAI-associated dosing when compared to daily rectal dosing. Whether intermittent dosing achieves similar drug concentrations at the time of HIV exposure could be estimated using PK models, but the duration for which such HIV suppressive concentrations need to be sustained after an HIV exposure has not been established.
Conclusions
The MTN-017 study demonstrated that both oral and topical antiretroviral PrEP are safe and acceptable to sexually active MSM at risk of HIV infection. This PK/PD substudy confirms that both routes of administration have the potential to inhibit explant infection. These data support further development of topical PrEP for HIV prevention. However, the MTN-017 participants expressed a preference for RAI-associated dosing,8 and it was this regimen that was associated with the lowest PK exposure in plasma and rectal tissue. It is therefore unclear whether a single dose of TFV gel administered before RAI would be enough to prevent HIV infection. For RAI-associated dosing to move forward, additional studies, such as those conducted for oral FTC/TFV, will be needed to better understand the relationships between drug exposure and mucosal protection from HIV infection. Higher concentrations of TFV, currently being explored as a rectal douche in the DREAM program, may provide a more viable solution for the development of a safe and effective RAI-associated topical PrEP strategy for HIV prevention.42
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the other institutions
Supplementary Material
Acknowledgments
We are grateful to the study participants for their participation and dedication. We thank the study team members at the research sites, the protocol management team, and the MTN leadership operation center for their contributions. We would also like to acknowledge the contributions of Dr. Charlene Dezzutti, who provided oversight of the laboratory assays performed in this study. We are grateful to Gilead Sciences who provided the FTC/TDF and CONRAD for providing RG-TFV under USAID PPRD #GPO-A-00-08-00005-00 using TFV drug substance, also graciously provided by Gilead. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Contributor Information
Collaborators: the MTN-017 Protocol Team
Authors' Contributions
I.M.M.: designed the study and generated the final draft of the article. R.P.K.N.A.: laboratory support for mucosal studies. M.A.M.: responsible for PK analysis. C.W.H.: study design and PK analysis. S.J.: operational support for the study. J.M.P.: NIH representative for regulatory and safety support. T.H.H.: CDC representative for regulatory and safety support. M.C.: laboratory oversight at the Bangkok site. A.C.: laboratory support at the Bangkok site. B.R.: laboratory support at the Bangkok site. G.D.: study design and provision of tenofovir gel. J.L.S.: safety support for tenofovir gel. J.F.R.: study design and provision of FTC/TDF. R.D.C.: principal investigator of the MTN-017 study.
Author Disclosure Statement
I.M.M.: is an employee and owns stock in Orion Biotechnology. M.A.M.: research funding (Gilead Sciences, ViiV/GSK, and Merck). C.W.H.: research funding (Gilead Sciences, ViiV/GSK, and Merck), Scientific Advisory Board (Gilead Sciences, ViiV/GSK, and Merck), Safety Monitoring Committee (Orion Biotechnology), and Founder/Officer of Prionde Biopharma. J.F.R.: employee and stockholder in Gilead Sciences. R.P.K.N.A., R.M.B., S.J., J.P., M.E.C., A.C., B.R., G.D., and R.D.C.: No competing financial interests exist.
Funding Information
This work was supported by National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health.
Supplementary Material
References
- 1. Grant RM, Lama JR, Anderson PL, et al. : Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormack S, Dunn DT, Desai M, et al. : Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Molina JM, Capitant C, Spire B, et al. : On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015;373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 4. Celum C, Hosek S, Tsholwana M, et al. : PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: Results from HPTN 082, a randomized controlled trial. PLoS Med 2021;18:e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schumacher CM, Tao X, Chandran A, et al. : Reaching those most at risk for HIV acquisition: Evaluating racial/ethnic disparities in the preexposure prophylaxis care continuum in Baltimore City, Maryland. J Acquir Immune Defic Syndr 2021;87:1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reisner SL, Moore CS, Asquith A, Pardee DJ, Mayer KH: The pre-exposure prophylaxis cascade in at-risk transgender men who have sex with men in the United States. LGBT Health 2021;8:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tingler RC, Connochie D, Bauermeister JA: Rectal douching and microbicide acceptability among young men who have sex with men. AIDS Behav 2020;24:1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carballo-Dieguez A, Giguere R, Dolezal C, et al. : Preference of oral tenofovir disoproxil fumarate/emtricitabine versus rectal tenofovir reduced-glycerin 1% gel regimens for HIV prevention among cisgender men and transgender women who engage in receptive anal intercourse with men. AIDS Behav 2017;21:3336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carballo-Diéguez A, Stein Z, Sáez H, et al. : Frequent use of lubricants for anal sex among men who have sex with men: The HIV prevention potential of a microbicidal gel. Am J Public Health 2000;90:1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGowan I, Hoesley C, Cranston RD, et al. : A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One 2013;8:e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGowan I, Cranston RD, Duffill K, et al. : A phase 1 randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% gel (the CHARM-01 study). PLoS One 2015;10:e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hiruy H, Fuchs EJ, Marzinke MA, et al. : A phase 1 randomized, blinded comparison of the pharmacokinetics and colonic distribution of three candidate rectal microbicide formulations of tenofovir 1% gel with simulated unprotected sex (CHARM-02). AIDS Res Hum Retroviruses 2015;31:1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cranston RD, Lama JR, Richardson BA, et al. : MTN-017: A rectal phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin Infect Dis 2017;64:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. : Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carballo-Dieguez A, Balan IC, Brown W, 3rd, et al.: High levels of adherence to a rectal microbicide gel and to oral Pre-Exposure Prophylaxis (PrEP) achieved in MTN-017 among men who have sex with men (MSM) and transgender women. PLoS One 2017;12:e0181607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hendrix CW, Andrade A, Bumpus NN, et al. : Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence venchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bushman LR, Kiser JJ, Rower JE, et al. : Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal 2011;56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US DHHS, FDA and CDER: Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine, 2001. Available at https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf/ accessed October 8, 2021.
- 19. McGowan I, Dezzutti CS, Siegel A, et al. : Long-acting rilpivirine as potential pre-exposure prophylaxis for HIV-1 prevention (the MWRI-01 study): An open-label, phase 1, compartmental, pharmacokinetic and pharmacodynamic assessment. Lancet HIV 2016;3:e569–e578. [DOI] [PubMed] [Google Scholar]
- 20. Mayer KH, Molina JM, Thompson MA, et al. : Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020;396:239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venter WDF: Pre-exposure prophylaxis: The delivery challenge. Front Public Health 2018;6:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McGowan I: An overview of antiretroviral pre-exposure prophylaxis of HIV infection. Am J Reprod Immunol 2014;71:624–630. [DOI] [PubMed] [Google Scholar]
- 23. McGowan I, Dezzutti C: Rectal microbicide development. Curr Top Microbiol Immunol 2014;383:117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cranage M, Sharpe S, Herrera C, et al. : Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med 2008;5:e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendrix CW, Chen BA, Guddera V, et al. : MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One 2013;8:e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balan IC, Giguere R, Brown W, 3rd, et al.: Brief participant-centered convergence interviews integrate self-reports, product returns, and pharmacokinetic results to improve adherence measurement in MTN-017. AIDS Behav 2018;22:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anton PA, Cranston RD, Kashuba A, et al. : RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012;28:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen BA, Panther L, Marzinke MA, et al. : Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: A double-blind randomized trial. J Acquir Immune Defic Syndr 2015;70:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGowan I, Wilkin T, Landovitz RJ, et al. : The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 2019;33:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cranston RD, Dezzutti CS, Siegel A, et al. : A multiple dose phase 1 assessment of rilpivirine long acting in a model of preexposure prophylaxis against HIV. AIDS Res Hum Retroviruses 2019;35:794–804. [DOI] [PubMed] [Google Scholar]
- 31. Anderson PL, Glidden DV, Liu A, et al. : Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bunge KE, Dezzutti CS, Hendrix CW, et al. : FAME-04: A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of film and gel formulations of tenofovir. J Int AIDS Soc 2018;21:e25156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bunge KE, Dezzutti CS, Rohan LC, et al. : A phase 1 trial to assess the safety, acceptability, pharmacokinetics, and pharmacodynamics of a novel dapivirine vaginal film. J Acquir Immune Defic Syndr 2016;71:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robinson JA, Marzinke MA, Bakshi RP, et al. : Comparison of dapivirine vaginal gel and film formulation pharmacokinetics and pharmacodynamics (FAME 02B). AIDS Res Hum Retroviruses 2017;33:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marrazzo JM, Ramjee G, Richardson BA, et al. : Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dai JY, Hendrix CW, Richardson BA, et al. : Pharmacological measures of treatment adherence and risk of HIV infection in the VOICE study. J Infect Dis 2016;213:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baeten JM, Palanee-Phillips T, Brown ER, et al. : Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brown ER, Hendrix CW, van der Straten A, et al. : Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition: A secondary analysis from a randomized, placebo-controlled trial. J Int AIDS Soc 2020;23:e25634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nel A, van Niekerk N, Kapiga S, et al. : Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016;375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 40. Richardson-Harman N, Lackman-Smith C, Fletcher PS, et al. : Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol 2009;47:3530–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richardson-Harman N, Parody R, Anton P, et al. : Analytical advances in the ex vivo challenge efficacy assay. AIDS Res Hum Retroviruses 2017;33:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoang T, Date AA, Ortiz JO, et al. : Development of rectal enema as microbicide (DREAM): Preclinical progressive selection of a tenofovir prodrug enema. Eur J Pharm Biopharm 2019;138:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.