Abstract
Vaginal rings address a critical need for an independently initiated, long-acting HIV prevention method, but their design must be acceptable to promote uptake and adherence. Human-centered design (HCD) may help address design preference questions. In two Phase I studies of vaginal rings for HIV prevention conducted in the United States, we used qualitative interviews to assess participants' perceptions and opinions of the physical characteristics of the ring they used and of a ring's physical characteristics after comparing four ring designs presented via a visual tool. Users were found to prefer ring designs that appear easy to use, are physically comfortable, that function well, and are aesthetically pleasing. The parameters for these features varied widely. Product developers and marketers should consider marketing messages in which the target users feel this product is made to meet their needs and desires. Product developers are encouraged to design using HCD early in ring development (Clinical Trial Registration number: NCT03234400 and NCT03670355).
Keywords: HIV prevention, qualitative research, human-centered design, vaginal rings, visual tools
Introduction
An estimated 1.7 million people worldwide became infected with HIV in 2019, 48% of whom were women and girls.1 Effective, acceptable biomedical interventions to prevent HIV acquisition are critically needed. Oral pre-exposure prophylaxis offers effective HIV prevention when taken daily. However, adhering to a daily regimen is a significant barrier to effectiveness and acceptability.2
Vaginal rings are polymeric controlled-release drug delivery platforms that can be used for several indications, including HIV prevention. As long-acting (1–3 months) drug delivery devices, rings may address challenges with adherence to daily HIV prevention methods.2,3 Furthermore, to optimize real-world effectiveness, rings must be acceptable to users. Previous research on rings indicated that overall, women find them acceptable and easy to use; however, some users experience discomfort, specifically unacceptable impacts on sex.4 Rings' visual and physical characteristics may play a role in those drawbacks.5–9
Human-centered design (HCD), a methodology with growing application in global and public health fields,10 can help design solutions that meet users' needs by incorporating their feedback throughout the design process. HCD in health research can be beneficial to implement at the developmental or prototyping stage for new products to ensure that products and services are tailored to end-users' needs and context and can seamlessly fit into their lives. HCD may also be useful to develop marketing strategies that connect with the target end user to facilitate successful rollout.11 HCD uses participatory activities to engage potential end users in reflective discussions on the potential delivery, acceptability, and usability of products or services.
Visual and interactive tools may be used to facilitate these discussions, enabling communication in multiple ways.12,13 A growing body of research has been dedicated to incorporating user evaluations into the design of rings and other vaginal drug delivery platforms, utilizing tools of this type.7,8,14,15 These preclinical studies exploring users' sensory perceptions and experiences with vaginal delivery prototypes have a strong relationship with their acceptability.7,16–18 They call for further research across geographical regions and cultures,7 among potential future prevention product users,8 and across varied stages of product development.19
In the context of two Phase I trials of extended-release vaginal rings for HIV prevention conducted in the United States,20,21 we sought to explore preferences for attributes of vaginal rings among a random set of participants using a user-centered approach and in-depth interviews (IDIs) at trial completion of each study. Unlike previous studies that explore various socioecological influencing factors of acceptability,22 this analysis narrows in on products' design, exploring physical characteristics and potentially modifiable elements of rings.
Two rings, the dapivirine (DPV) ring (International Partnership for Microbicides, Silver Spring, Maryland) in the Microbicide Trials Network (MTN)-036/IPM 047 and the tenofovir (TFV) ring (CONRAD, Eastern Virginia Medical School, Arlington, Virginia) in MTN-038, were studied, yet both trials used the same measures for product preference and acceptability assessments and IDI guides, with a goal of comparing attributes in combined analyses. Our joint qualitative analysis on the preferred characteristics of these vaginal rings is presented in this article, which focuses on appearance and use.
Materials and Methods
Study design
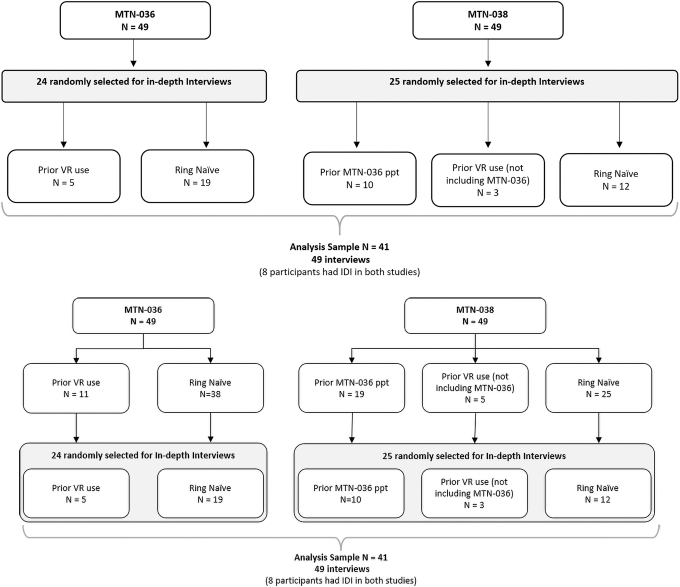
MTN-036/IPM 047 was a 3-month Phase I randomized pharmacokinetic and safety study of extended-duration DPV vaginal rings, conducted between November 2017 and February 2019 at two clinical research sites in Birmingham, Alabama, and San Francisco, California, among 49 participants, 24 of whom were interviewed for this analysis.
Participants were randomly assigned to active platinum-cured silicone rings with three DPV dosages, but all rings were visually identical with an outer diameter of 56 mm and a cross-sectional diameter of 7.7 mm. MTN-038 was a Phase I randomized pharmacokinetic and safety study of a 90-day intravaginal ring containing TFV conducted between January and August 2019 at three clinical research sites in Birmingham, Alabama; San Francisco, California; and Pittsburgh, Pennsylvania, among 49 participants, 25 of whom were interviewed for this analysis (Fig. 1).
FIG. 1.
Flowchart for MTN-036 and MTN-038 joint qualitative analysis.
Participants were randomized to receive an active or placebo hydrophilic polyether urethane ring (2:1 ratio); both rings were visually identical with an outer diameter of 55 mm and a cross-sectional diameter of 5.5 mm.23 Eligibility criteria were identical for the two studies, including being assigned female at birth* and being 18–45 years old, healthy, and HIV uninfected. Participants from MTN-036 could enroll into MTN-038, and 19 women eventually participated in both trials (Fig. 1).
Study products
The study products in MTN-036 were three silicone matrix polymer rings containing 25 mg of DPV worn monthly or 100 or 200 mg of DPV worn for ∼91 days. In MTN-038, participants received a polyurethane tubing ring containing 1.4 g of TFV or a matched placebo ring24; both rings were worn for ∼91 days.
Procedures
Procedures and findings of the MTN-036 and MTN-038 studies have previously been described.25–27 For behavioral evaluation, all participants in both studies completed identical computer-assisted self-interviews (CASIs), including assessment of prior vaginal ring use. Clinic staff collected demographic data by CASI and in person.
IDIs were conducted with a randomly selected subset of participants across sites (Table 1) who had engaged in penile-vaginal intercourse in the past year to gather qualitative data on acceptability of ring use during sex and among a population engaging in sexual practices known to be associated with HIV acquisition risk.
Table 1.
Demographic Data for Study and In-Depth Interview Subset Samples
| Demographic variables | MTN-036 study sample | MTN-038 study sample | MTN-036 IDI subset | MTN-038 IDI subset | MTN-036 and MTN-038 IDI combined totala |
|---|---|---|---|---|---|
| N | 49 | 49 | 24 | 25 | 41 |
| Age [median (IQR)] | 29 (26–34) | 29 (24–35) | 29 (27–34) | 30 (26–35) | 30 (26–34) |
| Race/ethnicity | |||||
| Non-Hispanic white | 18 (37%) | 25 (51%) | 8 (33%) | 14 (56%) | 21 (51%) |
| African American | 20 (41%) | 14 (29%) | 7 (29%) | 5 (20%) | 11 (27%) |
| Hispanic or Latino | 5 (10%) | 3 (6%) | 3 (13%) | 1 (4%) | 2 (5%) |
| Asian | 4 (8%) | 5 (10%) | 4 (17%) | 3 (12%) | 5 (12%) |
| Biracialb | 2 (4%) | 2 (4%) | 2 (8%) | 2 (8%) | 2 (5%) |
| Gender identity (self-report) | |||||
| Cisgender femalec | 46 (94%) | 46 (94%) | 23 (96%) | 24 (96%) | 40 (98%) |
| Study site | |||||
| San Francisco, CA | 24 (49%) | 15 (31%) | 13 (54%) | 9 (36%) | 17 (41%) |
| Birmingham, AL | 25 (51%) | 13 (27%) | 11 (46%) | 7 (28%) | 15 (37%) |
| Pittsburgh, PA | N/A | 21 (43%) | N/A | 9 (36%) | 9 (22%) |
| Any penile-vaginal sex in the past 4 weeks | 27 (55%) | 45 (92%) | 19 (79%) | 25 (100%) | 39 (95%) |
| Ring naive | 38 (78%) | 25 (51%) | 19 (79%) | 12 (48%) | 23 (56%) |
| Participation in both trials | N/A | 19 (39%) | N/A | 10 (40%) | 13 (32%) |
| Interviewed with experience in both trials | N/A | N/A | N/A | N/A | 10 (24%) |
Demographic data were collected in person by interviewers, responses to sexual activity and ring use questions were collected by CASI, participants' enrollment in one versus both trials was collected from study coordinators.
Participants with IDIs in both studies (n = 8) are represented once using responses from MTN-038.
Includes Asian and Caucasian, and American Indian and Caucasian.
Other participants were “transgender male” or “gender nonconforming/gender variant” based on self-definition.
CASI, computer-assisted self-interview; IDI, in-depth interview; IQR, interquartile range; MTN, Microbicide Trials Network.
IDIs were ∼1 h long each, conducted in English privately via video call during participants' last study visit (day 91) or shortly thereafter at the research clinics by one of three female interviewers trained in qualitative methods at a postgraduate level (including the lead author) and working as analysts for RTI International who never interacted with participants before the interview. Interviewers used the same pretested semistructured guide in both studies, with topics including acceptability of the assigned ring, disclosure of ring use, types of sexual activity during study participation, experience using the ring during sex and/or menses, and preferences for alternative ring designs and durations (e.g., monthly vs. extended 3-month duration).28,29
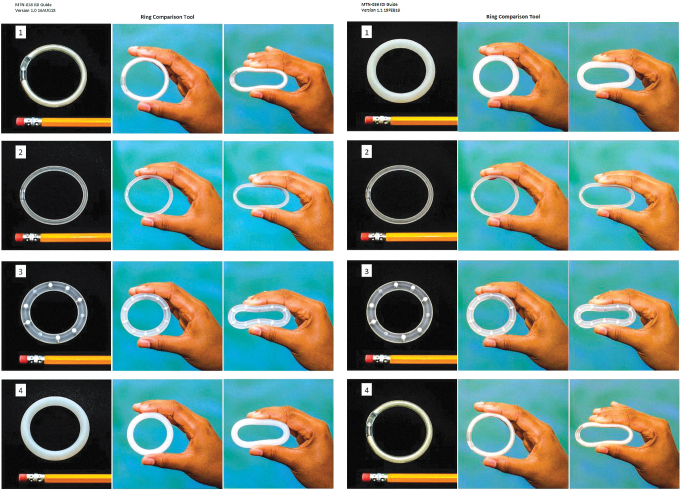
Next, interviewers asked participants to remove a visual tool from a large envelope (Fig. 2). The tool used in each interview showed photographs of four different rings side by side: the MTN-036 and MTN-038 study rings, the NuvaRing (Merck & Co), and the pod ring (Oak Crest).30 Presenting photographs or blueprints of product designs offers a means of structuring data collection around product preferences and allows researchers to summarize, clarify, confirm, and probe on preferred elements of the designs.31 With IDIs, this tool allowed researchers to obtain nuanced descriptions of the contextual desires and needs surrounding users' product preferences.
FIG. 2.
Visual comparison tool used in the MTN-036 and MTN-038 IDIs, respectively. IDIs, in-depth interviews; MTN, Microbicide Trials Network. Color images are available online.
Interviewers explained that the purpose of the visual tool was to compare attributes of the ring participants used (shown first on the visual tool as Number 1 in each trial) with perceived attributes of other rings on the market or in development. Interviewers distinguished the purpose of having three photographs of each ring: life-size images of the rings in the first column, rings held in an unflexed position in the second column, and pinched rings with roughly the same amount of pressure applied to depict relative flexibility in the third column (although this was not standardized). They pointed out the variation in size, flexibility, and material of each ring (Table 2).32 Participants were asked to think aloud as they compared the rings on the tool.
Table 2.
Rings Shown on the Visual Ring Comparison Tool
| Ring | Developer | Type | Material | Size (outer diameter, cross-sectional diameter) | Hardness shore A | Color | Notes |
|---|---|---|---|---|---|---|---|
| DPV ring | International Partnership for Microbicides | Matrix | Platinum-cured silicone | 56 mm, 7.7 mm | 67–6932 | Opaque off-white | MTN-036 study ring. Referred to as Number 1 on the MTN-036 visual tool and Number 4 on the MTN-038 visual tool. |
| NuvaRing | Merck & Co. | Reservoir | Ethylene vinyl acetate copolymers and magnesium stearate | 54 mm, 4.0 mm | 8332 | Transparent | Referred to as Number 2 on the MTN-036 and MTN-038 visual tools. |
| Pod ring | Oak crest | Pod reservoir | Silicone | 56 mm, 8.0 mm | 42–47a,b | Transparent visible drug pods | Referred to as Number 3 on the MTN-036 and MTN-038 visual tools. |
| TFV ring | CONRAD | Reservoir | Hydrophilic polyether urethane | 55 mm, 5.5 mm | 100a,c | Transparent visible drug paste | MTN-038 study ring. Referred to as Number 1 on the MTN-038 visual tool and Number 4 on the MTN-036 visual tool. |
Hardness Shore A measurement of vaginal ring polymer. Shore hardness of the hydrophilic reservoir designs varies when taking in water and swelling during use.
J. Moss, personal communication.
M. Clark, personal communication.
DPV, dapivirine; TFV, tenofovir.
Interviewers probed on preferences related to size, flexibility, color, translucency, and visible construction elements such as the pod reservoir or tube connections.
Interviewers completed debrief reports after each IDI to summarize the main and novel findings. Audio recordings from the interviews were transcribed verbatim and reviewed for quality assurance before coding and analysis.
Analysis
Qualitative textual data were analyzed thematically. A codebook was developed iteratively through an inductive and deductive process using the debrief reports from MTN-036 to initially identify codes. A team of four analysts (including the lead author and one other interviewer) used Dedoose software v7.0.23 to code MTN-036 transcripts. Two analysts (including the lead author) applied the same codebook to MTN-038 data. In both studies, the coding team maintained ∼80% intercoder reliability across nine key codes representing main topics of interest. Weekly analytical meetings were held during the coding processes of both studies to reach consensus on intercoder discrepancies when 80% intercoder reliability was not achieved.
Here, text excerpts coded with “PHYSICAL CHARACTERISTICS” and “VISUAL COMPARISON” were extracted (Table 3). Data were stratified by study and participants' experience using a vaginal ring before enrollment (ring-naive vs. prior ring experience). The MTN-038 data also included a stratum of participants who previously participated in MTN-036 to examine how their experience influenced their perception of ring attributes. The lead author wrote memoranda for each excerpt included in the stratified coded data sets, summarizing findings related to physical characteristics of the vaginal rings and the visual comparison tool. Then a second analyst developed an inductive codebook (Table 4) in Dedoose to code the excerpt memoranda, refining major themes and parsing out interesting or divergent opinions in a final summary report.
Table 3.
Codes and Child Codes Extracted for Analysis
| Code | Child code | Description |
|---|---|---|
| Physical characteristics | Apply to physical properties or characteristics of the ring(s) not covered in the grandchild codes. Double code with ATTITUDES as necessary. Double code with EFFICACY for form-function attribution comments related to how well the ring might protect against HIV. | |
| Flexibility | Use for discussions or comments about the ring(s) actual or perceived flexibility. | |
| Looks | Use for discussions or comments about the ring(s) “look” or external design, including texture, pods, closures, and color. | |
| Size | Use for discussions or comments about the ring(s) size including diameter and thickness. | |
| Visual comparison | Apply for discussions about or during the visual comparison tool exercise. For specific references to each ring pictured in the tool, apply child code. Double code with other codes regarding product attributes, as necessary. | |
| IPM (DPV)a | (Number 1) Use for discussions or comments about the DPV ring developed by the International Partnership for Microbicides. Participants may refer to the ring as Number 1 (MTN-036 study product). | |
| NUVA | (Number 2) Use for discussions or comments about the NuvaRing. Participants may refer to the ring as Number 2. | |
| POD | (Number 3) Use for discussions or comments about the pod ring. Participants may refer to the ring as Number 3. | |
| CONRAD (TFV)a | (Number 4) Use for discussions or comments about the TFV ring developed by CONRAD. Participants may refer to the ring as Number 4 (MTN-038 study product). |
These definitions were the only code definitions modified in the MTN-038 codebook: the visual ring comparison tool showed the MTN-036 study ring as Number 1 in MTN-036 and Number 4 in MTN-038, and the MTN-038 study ring was shown as Number 4 in MTN-036 and Number 1 in MTN-038.
Table 4.
Inductive Codebook for Refining Major Themes in Analysis
| Code | Child code | Description |
|---|---|---|
| Attributes | Parent code was used as a title for child codes and not applied to any excerpts. | |
| Hardness/flexibility | Excerpts describing the flexibility, hardness, or stiffness of the rings, including comments on the participant's experience bending the ring for insertion and removal. | |
| Construction | Excerpts related to the way that the ring is contrasted or fabricated, including whether it was made in a mold or as a tube with conjoined ends/connector. | |
| Drug visibility | Excerpts related to the visibility of the drug within the ring, including comments about the appearance of the drug itself, or ability to monitor drug absorption. | |
| Duration | Excerpts related to the duration of the ring, including how long participants anticipate the ring to last based on its physical features. | |
| Color | Excerpts related to the color or transparency of the ring, including preferences and recommendations for the color and transparency of rings. | |
| Shape | Excerpts related to the shape of the rings, including recommendations for different shapes such as ovular rings. | |
| Size/thickness | Excerpts related to the size, diameter, or thickness of the rings, including comments on the participants' first impressions of the ring size/thickness and experiences using the ring related to its size/thickness | |
| Texture | Excerpts related to the texture of the ring, either experienced or anticipated from the visual tool, including comments about the material's smoothness. | |
| Influence | Parent code was used as a title for child codes and not applied to any excerpts. | |
| Ease of use | Excerpts describing participants' ability to use the ring easily or with challenges, including their experiences inserting, wearing, and removing the ring, as well as facilitators or barriers to easily using the ring. | |
| Awareness | Excerpts describing participants' mental awareness of the ring, such as cognitive burdens during daily use, forgetting that they have a ring, emotions related to having the ring on their mind, and other related content of their consciousness of the ring. | |
| Comfort | Excerpts related to how comfortable or uncomfortable the ring was to use, including comments from participants about pain or lack of pain. | |
| Discretion | Excerpts related to how discreet the ring may be, including their ability to use the product with or without others knowing, and their liking or disliking of that quality. | |
| Ancillary effects | Excerpts related to participants' perception of added benefits or drawbacks of using the ring, beyond its primary purpose of HIV prevention, including perceived side effects of ring use. | |
| Familiarity | Excerpts about how familiar the ring may be to the participant, including prior experiences using rings or other vaginal products such as menstrual cups. | |
| Functionality | Excerpts related to the way that the ring works or functions as a HIV prevention device, including how or how well the drug is dispensed. Also includes perceived effectiveness of the product to prevent HIV. | |
| Visual impression | Excerpts related to participants' impression of how the rings appear aesthetically unrelated to attributes' effect on ring use. | |
| Change in opinion | Excerpts describing the participants changing their opinion of the ring or ring characteristics over time, including first impressions compared with impression after use. | |
Regular meetings were held with the lead author and analyst during the memorandum and coding processes to discuss themes, as well as similarities and differences between strata.
Ethical statement
The MTN-036 and MTN-038 study protocols were approved by the Institutional Review Boards at each study site and were overseen by the regulatory infrastructure of the Division of AIDS (DAIDS) and MTN. All participants provided written informed consent before study participation and the IDI participants provided further verbal consent before being interviewed.
Results
This analysis included 49 interviews from a total of 41 participants; 24 interviews conducted in MTN-036 and 25 conducted in MTN-038. Ten participants interviewed in MTN-038 took part in both studies, 8 of whom had been interviewed previously in MTN-036. Participants in this combined IDI sample averaged 30 years of age.
Approximately half were non-Hispanic white, 27% were African American, and 12% were Asian, 5% were Hispanic or Latino, and 5% were biracial (Table 1); 98% were cisgender women and 95% engaged in penile-vaginal sex within 4 weeks before their enrollment visit. Over half of the participants had never used a vaginal ring before the study (i.e., “ring naive”; Supplementary Tables S1 and S2). The 10 interviews with participants who had experience using the rings in MTN-036 and MTN-038 at the time of the interview provide unique perspectives on ring characteristics from their actual experience using different rings.
Discussions using the visual ring comparison tool in MTN-036 and MTN-038 yielded several major themes that reached saturation, which focused on functionality and use attributes, categorized by the underlying preference and broken down by specific attributes associated with each preference. Attribute preferences related to the aesthetic appearance of the ring emerged as a minor theme, cited as less important characteristics than use attributes. Emic terms and quotes are italicized throughout the results to express participants' voices, and Table 5 shows additional longer exemplary quotes for each theme. Attributes that were discussed more by participants in one versus the other study by virtue of the ring they had used in that study are noted where applicable.
Table 5.
Exemplary Quotes
| Theme, subtheme | ID | Quote—study, participant ID, analysis stratum, age |
|---|---|---|
| Attributes related to ease of use | ||
| Size/thickness | 1A | “To be real, I've only tried like once or twice and like I pinched it and I tried to push it up but like I just really couldn't. Like I don't even know how to describe it, I don't even think I got anywhere in, but like I'm a person that uses tampons, like I'm, I'm quite comfortable like with myself so, I don't know, just the fact that it was hard for me kind of said something about it.”—MTN-036, 764-10478-2, no prior use, age 19 |
| 1B | “I like the thickness of the NuvaRing and I, you know, I probably wouldn't be that concerned about the thickness had I not tried the NuvaRing first. But it's really easy to get out just because it's so, it's thin so you can slip your finger just around it super quick, whereas like these, this one [IPM ring] you kind of really had to like fish in there to get it out.”—MTN-038, 764-50051-6, 036 PPT, age 30 | |
| Pliability | 2A | “It would just make it a little easier to like grip and insert properly. I remember with, with number four [IPM ring] I had to kind of fight with it a little bit. Because you're kind of, when you're putting it in you're kind of at a weird angle anyway, so the more flexible it is the easier it is to get in there.”—MTN-038, 764-50051-6, 036 PPT, age 30 |
| 2B | “Well, I'm curious about how things, like problems that people have with things falling out, because I think having something that's a little more rigid and a little larger will actually, might, I mean, this is total hypothesis but would actually maybe stay in better than something that's really small and like super flexible.”—MTN-038, 764-90486-6, no prior use, age 22 | |
| 2C | “I like that number four [IPM ring] is, and number three [pod ring], they're, they're both kind of similar in this way, there's no seam so you don't have that one spot where it's a little harder to bend.”—MTN-038, 764-50051-6, 036 PPT, age 30 | |
| Attributes related to physical comfort | ||
| Size/thickness | 3A | “I know from experience that the width doesn't make a difference in how it felt, but I feel like maybe for some people, if they knew have the option for a smaller, thinner one, would probably want a smaller, thinner one”—MTN-038, 764-42065-2, 036 PPT, age 29 |
| Softness | 4A | “I mean, again with the caveat that I did not partake in sexual intercourse while wearing the ring. My answer might change if it turned out that I could feel it significantly, or my partner could feel it significantly, then maybe I would want something like a little more flexible, or. But based on just like my day-to-day experience of having the ring inside of me and not having an issue with that, like I'm going to assume that one is a design that works for me.”—MTN-036, 764-38270-2, no prior use, age 34 |
| 4B | “What I didn't expect was the way that it was removed because like there's no really way to pinch it and then pull it out, like it expands and they just pull it out, and like it's like an unsettling feeling. Like it's not that it's very painful but like the fact that like, I can't describe it, I don't, [laughter] I don't know what words I can use to describe like a jade bangle being pulled out of my vagina. [laughter]”—MTN-036, 764-10478-2, no prior use, age 19 | |
| Attributes related to perception of effectiveness | ||
| Size/thickness | 5A | “Okay. It's, I hope this doesn't sound funny but the first one I like, I almost liked that it was hefty and that it was thick because I felt protected by it. I was like, ‘Oh, this definitely looks like something that will be protecting me from evil HIV coming my way.’ [laughter] And looking at the second one, perhaps this is just because I had the first one, but it's almost like, ‘This flimsy thing is supposed to do that? How is, how is that supposed to happen?’”—MTN-036, 764-27737-3, no prior use, age 27 |
| Drug visibility | 6A | “I would personally like to see the way the drug looks, what is, what is, is my body absorbing. I think that will make the patient a whole lot more comfortable in using it instead of just being able to put just like a really opaque colored ring and inserting it in and you're, and you're taking a chance. I think it'll make someone a bit more comfortable for them to see what exactly, you know, the consistency and the color of the medication that is being absorbed in their body.”—MTN-038, 821-19430-0, no prior use, age 38 |
| 6B | “I like, and just because I'm a curious person by nature, I like to see, ‘Ooh, let me see,’ I'm always asking my sisters, whenever something happens to them, ‘Let me see, let me see, let me see.’ I'm a let-me-see type of person. So I would like a ring to where maybe there was some kind of indicator on it that indicates that the ring is full, now it's empty, some kind of indicator that would indicate that the medication is no longer in the ring.”—MTN-036, 821-97322-9, prior ring use, age 41 | |
| Construction | 7A | “Oh, I wondered about that because you can see where they're connected in, in one [CONRAD ring], well, yeah, I guess you can in two [NuvaRing], too. But today when I saw it when they took it out I, you could see the, where it connects. And I did worry about that, I was like, ‘Is this going to be a point of like, like will it come loose there?’ So that worried me. That's interesting, that almost makes me feel more confident in three [pod ring] and four [IPM ring] as, because of them being molds. But also like integrity and like, you know, the integrity of the ring, like I don't want something that's going to pop open or break or, you know, exposed to the environment of whatever drug that I'm using, yeah.”—MTN-038, 702-29388-5, no prior use, age 28 |
| 7B | “I guess the tube thing, I'm sure it feels [inaudible] it's been tested many times, but I would be worried about like the connected, like the connected tube, like two and four, like coming undone inside of me. […] I don't know, whereas like a mold just feels more secure.”—MTN-036, 764-38270-2, no prior use, age 34 | |
| 7C | “I would be concerned about bacteria if all those holes weren't sealed. […]As long as those little holes were filled I would be fine with it, but if those, if there were any holes that were left open, that would concern me as far as infection goes.”—MTN-036, 821-70544-0, no prior use, age 34 | |
Attributes that make rings easier to use
The ease of using rings drove discussions about different characteristics of the rings. Ease of use was determined by perceived challenges related to inserting and removing the ring and by the ring staying in place or involuntarily slipping out of place. Ease of use was a central component of how a ring would be designed or considerations for design modification. In both studies, the rings' size, thickness, and flexibility were perceived to affect ease of use.
Generally, thinner rings were thought to be easier to pinch into a figure 8 (as instructed by clinic staff), making them easier to insert. In MTN-036, some participants explained that the DPV ring was difficult to pinch together because it was too thick to grasp easily (Table 5, 1A). Participants imagined that the thinner NuvaRing or TFV ring would be easier to pinch together. Participants who were ring naive or had experience using a thin ring such as the NuvaRing appeared more concerned about using a thicker ring (Table 5, 1B). Participants noted that insertion was a hard-to-overcome challenge and that thinner rings appeared easier to insert, making the procedure less “intimidating.”
In MTN-038, participants agreed that thicker rings (compared with the TFV ring) would be more challenging to use because they would be harder to position correctly during insertion. Participants in both trials reflected on the ease of twisting the TFV ring to insert it due to its thinness compared with the DPV ring. On the contrary, thicker rings were perceived to be easier to use once inserted. Participants typically assumed that thicker rings would be less likely to move or slip out of place in the vagina. Those who used the DPV ring in MTN-036 tended to think that thick rings stay in place better, noting their experience using a thicker ring with few or no issues of the ring slipping or expelling. A participant in both studies reported that the TFV ring “sagged” more than the DPV ring.
Similarly, some participants saw a larger external diameter as a positive characteristic because it was associated with “pushing against the walls” of the vagina. Users thought this would help the ring “stay out of the way” and may relieve them of the potential burden of the ring slipping. A 28-year-old participant in MTN-038 who was ring naive explained:
“I don't know why but it makes me feel more confident that it's [TFV ring] like a wider diameter, that makes me feel like it'll stay better. Because if it was small I'd really worry that it would come out.”
The ring's pliability in relation to how easily it would bend was another dominant theme. Overall, participants indicated that more flexible rings would be easier to insert. Some MTN-036 participants recalled difficulty inserting the DPV ring, explaining that they had to “fight with it.” They imagined that a more pliable ring would be more compliant and therefore easier to use (Table 5, 2A). Yet, others thought that the ring's “rigidity” may have helped keep it in place (Table 5, 2B). A related finding from MTN-038 involved the lack of flexibility at the TFV ring's welding of the reservoir tube that forms the ring. Participants thought the welded section felt stiffer than the rest of the ring, making it more challenging to bend to insert (Table 5, 2C).
Participants also lacked consensus on whether more or less flexible rings would be easier to remove. On one hand, stiff rings were seen as challenging to pull out of the vaginal opening. However, a few participants felt there was a threshold at which more flexibility would be useful, after which the ring would become harder to use. If the ring was too flexible, they thought it may not stay in place well or that the ring shape might “collapse” from the pressure of the vaginal walls.
Attributes that make the ring physically comfortable
The perceived comfort of wearing each ring was a key theme relating to being unable to feel or notice the ring once in place. Conversely, being aware of the ring during everyday use was undesirable. Many participants recognized that before they had ever used a vaginal ring, they worried about it being uncomfortable, emphasizing that the physical discomfort may cause some mental distress or burden. In contrast, a handful of participants thought a completely unnoticeable ring could cause worry that the ring had involuntarily fallen out.
Size and thickness were, again, key characteristics affecting the rings' perceived comfort. Smaller thinner rings were thought to be more comfortable, less noticeable, or less “invasive” during use because they would apply less pressure to the user's vaginal walls. Thicker rings appeared “intimidating” or “scary” to some participants uncertain of how comfortable they would feel when inserted and reinforced their initial concerns of using a vaginal ring at the beginning of the study. A 29-year-old participant in MTN-036 who had previous experience using rings recalled:
“I saw it [study ring] and I was like, ‘Holy cow, this thing is huge.’ [laughter] And it doesn't bend very well so it takes like a lot of maneuvering to get it in the first time. […] It just, it looked like it was going to be uncomfortable the first time I saw it because it was so thick, but then I didn't even notice it once it was in.”
Interestingly, several participants in MTN-036 stated that they thought the thinner rings on the visual tool would be more comfortable to use even though they had no discomfort with their DPV ring (Table 5, 3A). Some participants thought the ring's comfort would depend on its size relative to the size of the user's vagina. A few suggested considering different sizes for the ring or even being fitted by a professional for the size of ring, feeling that “it may not be a ‘one-size-fits-all’ thing.”
The rings' material was also discussed in terms of the rings' comfort or noticeability. Participants thought a “softer” or “squishier” material was likely to be comfortable or unnoticeable. This factor was particularly important to participants who were concerned about their sex partners feeling the ring during penile-vaginal sex. Participants worried a firmer “rigid” ring might hurt their sex partners (Table 5, 4A). Drawing on experience removing their rings, participants thought softer rings would be more comfortable to remove (Table 5, 4B). They viewed rings with a softer looking material, such as the pod ring, as more desirable than a hard plastic material.
A participant in both MTN-038 and MTN-036 expressed preference for a more flexible ring that can “move with the changing [position] of the vagina” throughout the day “rather than having something like big and clumpy back there.” Despite these expectations about how firm rings may feel during everyday use, in both studies, the rings were rarely felt after insertion. Much like their experiences with the study rings' size, participants stated a preference for something more comfortable even if their ring was rarely noticeable in situ. Unlike the concern about rings being too flexible to use easily, no one thought a ring could be too flexible to be comfortable.
Attributes related to perceptions of effectiveness
Participants described several physical characteristics of the rings that they perceived to be related to how well a ring would function as an HIV prevention device. These physical characteristics were cited as those that affected the perception that the ring “works” properly, that it is efficacious, or that it is safe. The most “functional” attributes instilled more confidence in the participants, increased fit into their everyday life, created less disruption, contributed to a feeling of mental comfort while using the ring, and in turn may also affect participants' willingness to use the ring in the future.
Although thinner rings were viewed as more “approachable” overall based on their perceived ease of use and comfort, several participants noted that the thicker rings looked “sturdier” and more reliable than thin rings, which appeared “flimsy” and thus less efficacious (Table 5, 5A). Participants stated that the “heftier” rings appeared more “potent” or more likely to protect them from HIV compared with smaller “daintier” rings. Nevertheless, a thicker ring was seen as disadvantageous even if it were easy to use, comfortable, and longer lasting, because its appearance would be a barrier to initiating use. Participants cautioned that the “daunting” size may turn potential users away from trying the ring. A 30-year-old participant in MTN-038 who also participated in MTN-036 explained:
“I don't know, I really don't know why, I don't know. I mean, I guess like the size, like looking at the big size, you know, just to like alleviate any like visual displeasure from looking at it in the beginning, like just having like a smaller size maybe.”
Preferences varied widely between opaque and translucent rings. Those who preferred a translucent ring, such as the TFV ring used in MTN-038, noted that they liked being able to see and “monitor” how much drug remained in the ring (Table 5, 6A). “Monitoring” by occasionally removing their ring to view how much drug had been absorbed or was left was appealing to participants because it provided a tangible sense of the ring's efficacy.
Participants viewed the amount of drug in the ring as indicative of how well the ring was working, which would provide ongoing “reassurance,” an important characteristic for any new vaginal ring user. Viewing the amount of drug in the ring would also give participants some agency in their daily ring use, which appealed to some participants who felt a temptation to “check on” their ring rather than “forget” about it (Table 5, 6B). Indeed, some participants stated a preference to periodically remove their ring for cleaning or simply to check that it is still there.
In comparison, disliking the drug's visibility in a translucent ring was less commonly cited. Some participants stated that seeing the drug was unsettling because it caused them to fear that if the ring were to break, it would release all the drug inside the user. Opaque rings seemed less likely to raise this concern about the ring's safety. One participant explained that being able to see the drug made the temptation to remove the ring too strong, causing poorer adherence and leading to less protection from HIV. This theme was discussed most among participants in MTN-038, who experienced seeing the drug in their study ring.
Another salient theme in both studies was the preference for rings fabricated in a mold, such as the DPV and pod rings, rather than as a tube with ends visibly welded, such as the NuvaRing or TFV ring. Participants likened the TFV ring's appearance to a “glo-stick bracelet” and imagined it constructed similarly, fearing that the place where it connects could “pop open” or break during use (Table 5, 7A and 7B). This led to some emphatic concerns about the drug “spill out” into one's body or the ring disconnecting when pinched during insertion. The NuvaRing, which has a smaller, less visible connector, did not garner similar concerns.
The pod ring, which has visible drug “pods” embedded, raised some concerns about safety and hygiene. Some participants erroneously assumed the pods were hollow and uncovered. This raised concern that vaginal fluids might collect in the pods and need to be cleaned meticulously or lead to vaginal infections over time (Table 5, 7C). When interviewers explained to participants that the pods are backfilled with silicone and asked if that changed how participants felt, many were still skeptical. Others thought the pod ring's construction was “cool” and liked that the pods could distribute different drugs without coformulating them.
Attributes that are aesthetically pleasing
Aside from the elements of a ring that affected participants' experience using it, a less salient theme centered on preferences for rings' “aesthetic” appearance. Having an attractive ring mattered to participants; however, appearance mattered less than attributes related to rings' functionality. These characteristics differed among participants despite being regarded as items that “look good,” highlighting the subjective nature of aesthetic preference. Aesthetic attributes included the ring design's “simplicity,” the ring's color, and whether the ring had visible pod reservoirs.
Discussion
In this study, participants of two multisite HIV prevention trials in the United States viewed photographs of four vaginal rings at different stages of the product pipeline. All participants had experience using at least one of the four rings for ∼3 months. Our findings provide new insights into these participants' preferences and considerations for the continued development and marketing of rings. First, although participants shared preferences for rings that are easy to use, are physically comfortable, that function well, and are aesthetically pleasing, they cited diverse characteristic specifications or parameters within those categories.
We found that different users had varied preferences in ring designs to fulfill those characteristics. Furthermore, even if their experience using a study ring was satisfactory, participants still preferred design features different from those of the ring they used. Some of the design features of these rings are fixed, whereas others may be changeable. Messaging about the rings can be tailored to attend to these attribute preferences. Participants also cited some preferences that enable further engagement from the user, such as monitoring their drug release or periodically cleaning their ring, which may conflict with some assumptions about the “set it and forget it” heuristic for long-acting prevention products. Thus, using a user-centered approach to vaginal rings, our study invites a renewed discussion on ring designs and marketing considerations.
We found many shared opinions about the size and flexibility of rings to simplify use and increase comfort, but there was no consensus about the specific parameters to change. Diversity in preferred parameters emphasizes that multiple vaginal ring designs may be welcomed to suit a range of tastes in future users. These differences in opinion suggest a demand for multiple options even for a single product such as the HIV prevention ring and to allow for “trialability,” an important stage in the successful adoption of a technology innovation.33
This analysis also sheds light on preferences reported in other HIV prevention ring studies. For instance, in studies conducted in sub-Saharan Africa, women mentioned size and softness as attributes that affect comfort.5,6 In this domestic sample, ring softness was linked to the comfort of the users and their sexual partners, but was also associated with less stability. Many participants also preferred a smaller thinner ring for ease of use, although a thicker or larger ring was perceived to stay in place better and made the user feel protected.
In this study and for future research, a user-centered or HCD approach can help product developers strike a balance between users' preferences and structural requirements of the product34; for instance, to compromise between a soft ring that is more comfortable and firm enough to stay in place. Concerns about product attributes highlighted in this research can also help messaging to educate users on product misconceptions or instructions for use; for instance, to explain how the ring can be pinched or twisted for easy insertion and removal, or to educate users of a ring with visible drug that if the welding “popped open” the tube itself would still be sealed and nothing would spill out.
Importantly, familiarity with rings factored into users' preferences for ring designs. Evidently, those with prior experience using vaginal rings had less apprehension about the rings' complexity, similar to previous findings.35 This is also similar to user evaluations of the pod ring by Guthrie et al., in which participants were more mentally comfortable and less psychologically aware of the ring with each subsequent ring they used.19 Furthermore, this highlights the importance of experience using the product under evaluation to assess the acceptability of its attributes. Whereas some preclinical studies have enrolled participants with experience using a similar type of product before presenting alternative designs visually,8,14 this study allowed participants to consider their experience of using at least one of the visually presented rings to form their opinions.
As Rosen et al. point out, meaning-making is based on experience, presumptions, and desired effects for prevention products.8 However, participants' preferences were not always aligned with the attributes they were familiar with. For instance, although some participants expressed a desire for a thinner ring that is easy to use such as the NuvaRing they once used, others with NuvaRing experience noted that a thicker ring is just as comfortable. Furthermore, participants often cited preferences for attributes regardless of their experience; for instance, preferring a thinner ring for comfort despite finding a thick ring comfortable throughout the study.
Although rings are advantageous from a user burden perspective because they do not require any maintenance once in place,36,37 some participants in this study also wanted to exercise agency in their HIV prevention product choice and use. They liked a translucent ring that would allow them to monitor drug release, opportunities to remove the ring occasionally for cleaning, and the idea of trying several rings before determining which one to use.
While there were some attributes participants correctly assumed to influence the way in which a ring works, such as a greater width affecting its biomechanical compressibility,38 participants also erroneously interpreted some attributes to have implications for the rings' efficacy. For instance, thicker rings were associated with a more protective and reliable quality. Other research has found similar interpretations made about the sensory experiences of vaginal microbicides, such as porosity of a ring affecting perceived efficacy,7,8,39 suggesting that product developers should seek to incorporate properties that are not only efficacious but also perceived to be protective.15,39
The inclusion of HCD research is normally beneficial at an early stage of technology development to inform design attributes before features are locked in.40,41 Our results, however, point toward the benefit of a user-centered approach in advertising the compatibility of various vaginal rings with a diverse population of users. Those who liked the more “complex” rings were compelled by the idea that these rings were doing more for them. Those who liked the “simple” rings were attracted to the inert safe appearance of the molded ring. If HIV prevention rings are approved after clinical trials, their rollout and implementation must consider strategic marketing messages in which the target users feel this product is made to meet their needs and desires.
Furthermore, this study expands on previous studies of vaginal ring attribute preferences that used prototypes to inform preclinical product specifications,7,8,19 by offering visual representations of rings at various stages of the product pipeline. The use of a visual tool simulated a potential real-life consumer process of choosing an HIV prevention ring from multiple rings on the market in the future. Users' first choice for a ring may be informed by what looks best for them, without the opportunity to touch or try out more than one ring. Consistent with prior studies, participants conveyed the importance of rings that are comfortable, easy to insert and remove, appear efficacious, may be periodically removed, and allow the user to monitor drug usage.7–9,19
This analysis has several limitations. First, participants in MTN-036 and MTN-038 were healthy, HIV uninfected, and at low risk of HIV due to study-required condom use or abstinence from penile-vaginal sex. Consequently, our findings may not represent the same preferences of those at higher risk of acquiring HIV. Second, the tool used to facilitate discussions of ring design preferences was limited by representing each ring two dimensionally. There were some misconceptions about the rings' design, such as the perception that the pods of the pod ring were hollow or made of a metallic material, which led to unfounded concerns about hygiene and safety.19,30 Each ring's flexibility was also challenging to depict accurately with a photographic hand model.
Actual handling of placebo prototypes during IDIs, as done in other end-user studies,5,7,42,43 would have addressed this limitation; unfortunately, material transfer agreements were not obtained before study implementation. Because participants were asked to describe their first impressions of their study rings before appraising the other types of rings using the visual tool, there may have been saliency bias.
Other studies of rings have reported the saliency of first impressions of the rings, particularly by those new to rings.6 Last, self-reported past vaginal ring use in MTN-038 was not aligned with the list of participants who took part in MTN-036, indicating that some participants who took part in both studies did not report any past vaginal ring use, perhaps interpreting the question as vaginal ring use outside a clinical trial setting. Ascertaining more thoroughly all past vaginal ring use, including prior investigational ring use, may have prevented possible misclassification in understanding how experience with rings affected design preferences.
Conclusions
Stated preferences of design features from participants with varying levels of experience with vaginal rings provide important insights for messaging and design considerations of future vaginal rings. Product developers are encouraged to consider human-centered approaches early, while design considerations are still modifiable. Ring designs and the messaging accompanying their promotion may benefit from drawing on simple intentional attributes that signal safety, efficacy, comfort, and ease of use.
Marketing strategies for rings already commercially available may also benefit from positioning users' needs and comfort at the center of their campaign, instilling confidence in potential ring adopters. Finally, the market of rings for HIV prevention may benefit from the development and licensure of multiple rings that appeal to different types of users, to improve adoption and coverage.
Supplementary Material
Acknowledgments
The authors thank the MTN-036 and MTN-038 study team members who implemented the trials, and the manufacturers of the rings presented in the visual comparison tools. They also thank the participants of these studies, Ariana Katz and Mary Kate Shapely-Quinn for interviewing participants, Ellen Luecke and Jonah Leslie for analytical contributions, and Theresa Wagner, Faye Heard, and Carol Sprinkle for research coordination.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, CONRAD, IPM, or CONRAD and IPM's donors.
Authors' Contributions
I.H., M.S., R.S., T.M., J.P., B.A.C., C.H., A.Y.L., and A.V.S. assisted with the protocol development and design of this analysis. I.H., B.A.C., C.H., and A.Y.L. collected data for the studies. I.H. conducted the analysis and wrote the article. All authors reviewed, edited, and approved the final article.
Author Disclosure Statement
A.Y.L. has received investigator-sponsored research grants from Gilead Sciences and ViiV Health care and has led studies in which the study drug was donated by Gilead Sciences. B.A.C. receives research support from Medicines 360 and Sebela, managed by the Magee-Womens Research Institute.
Funding Information
The MTN-036 and MTN-038 studies were designed and implemented by the MTN funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, and UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the National Institutes of Health (NIH). The MTN-036/IPM 047 trial was sponsored by the International Partnership for Microbicides (IPM), which also developed and supplied the rings for the trial. The MTN-038 trial was sponsored by the DAIDS.
Supplementary Material
Not all participants were cisgender women. For gender neutrality, results reference participants by singular they/them/their pronouns.
References
- 1. UNAIDS: Global HIV & AIDS statistics 2020 fact sheet. Available at https://www.unaids.org/en/resources/fact-sheet (2019), accessed November 20, 2020.
- 2. Sidebottom D, Ekström AM, Strömdahl S: A systematic review of adherence to oral pre-exposure prophylaxis for HIV—How can we improve uptake and adherence? BMC Infect Dis 2018;18:581–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Straten A, Montgomery ET, Musara P, et al. : Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS (London, England) 2015;29:2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffin JB, Ridgeway K, Montgomery E, et al. : Vaginal ring acceptability and related preferences among women in low- and middle-income countries: A systematic review and narrative synthesis. PLoS One 2019;14:e0224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Musara P, Milford C, Shapley-Quinn MK, et al. : Preferences and acceptability of vaginal delivery forms for HIV prevention among women, male partners and key informants in South Africa and Zimbabwe: Qualitative findings. AIDS Behav 2021;25:124–138. [DOI] [PubMed] [Google Scholar]
- 6. van der Straten A, Browne EN, Shapley-Quinn MK, et al. : First impressions matter: How initial worries influence adherence to the dapivirine vaginal ring. J Acquir Immune Defic Syndr 2019;81:304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrow Guthrie K, Vargas S, Shaw JG, et al. : The promise of intravaginal rings for prevention: User perceptions of biomechanical properties and implications for prevention product development. PLoS One 2015;10:e0145642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosen RK, van den Berg JJ, Vargas SE, et al. : Meaning-making matters in product design: Users' sensory perceptions and experience evaluations of long-acting vaginal gels and intravaginal rings. Contraception 2015;92:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauermeister JA, Golinkoff JM, Carballo-Diéguez A, et al. : A mixed-methods study examining adherence to and acceptability of intravaginal rings for HIV prevention: Behavioral results of MTN-027. AIDS Behav 2020;24:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holeman I, Kane D: Human-centered design for global health equity. Inf Technol Dev 2020;26:477–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biroscak BJ, Schneider T, Martinez Tyson D, Aguado Loi CX, Bryant CA: Applying tools from human-centered design to social marketing planning. Soc Mar Q 2018;24:63–73. [Google Scholar]
- 12. Wang Y, Masoodian M: Designing visual tools to facilitate human-centered design. Proceedings of the International Conference on Advanced Visual Interfaces. Salerno, Italy: Association for Computing Machinery, 2020:Article 81. [Google Scholar]
- 13. Glegg SMN: Facilitating interviews in qualitative research with visual tools: A typology. Qual Health Res 2019;29:301–310. [DOI] [PubMed] [Google Scholar]
- 14. Guthrie KM, Rohan L, Rosen RK, et al. : Vaginal film for prevention of HIV: Using visual and tactile evaluations among potential users to inform product design. Pharm Dev Technol 2018;23:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morrow KM, Fava JL, Rosen RK, et al. : Willingness to use microbicides is affected by the importance of product characteristics, use parameters, and protective properties. J Acquir Immune Defic Syndr 2007;45:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tolley EE, Morrow KM, Owen DH: Designing a multipurpose technology for acceptability and adherence. Antiviral Res 2013;100 Suppl:S54–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morrow KM, Hendrix C: Clinical evaluation of microbicide formulations. Antiviral Res 2010;88 Suppl 1(Suppl 1):S40–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guthrie KM, Dunsiger S, Vargas SE, et al. : Perceptibility and the ‘Choice Experience’: User sensory perceptions and experiences inform vaginal prevention product design. AIDS Res Hum Retroviruses 2016;32:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guthrie KM, Rosen RK, Vargas SE, et al. : User evaluations offer promise for pod-intravaginal ring as a drug delivery platform: A mixed methods study of acceptability and use experiences. PLoS One 2018;13:e0197269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinicaltrials.gov. A phase 1, randomized pharmacokinetics and safety study of extended duration dapivirine vaginal rings. Available at https://clinicaltrials.gov/ct2/show/NCT03234400 (2019), accessed November 9, 2020.
- 21. Clinicaltrials.gov. Pharmacokinetic and safety study of a 90 day intravaginal ring containing tenofovir. https://clinicaltrials.gov/ct2/show/NCT03670355 (2019), accessed November 9, 2020.
- 22. Mensch BS, van der Straten A, Katzen LL: Acceptability in microbicide and PrEP trials: Current status and a reconceptualization. Curr Opin HIV AIDS 2012;7:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson TJ, Clark MR, Albright TH, et al. : A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob Agents Chemother 2012;56:6272–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thurman AR, Schwartz JL, Brache V, et al. : Randomized, placebo controlled phase I trial of safety, pharmacokinetics, pharmacodynamics and acceptability of tenofovir and tenofovir plus levonorgestrel vaginal rings in women. PLoS One 2018;13:e0199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts ST, Hawley I, Luecke EH, et al.: Acceptability and preference for 3-month versus 1-month vaginal rings for HIV-1 risk reduction among participants in a phase 1 trial. Paper presented at: AIDS 2020 Virtual: 23rd International AIDS Conference, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu AY, Dominguez Islas C, Gundacker H, et al. : Phase 1 pharmacokinetics and safety study of extended duration dapivirine vaginal rings in the United States. J Int AIDS Soc 2021;24:e25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stoner MCD BE, Gundacker HM, Hawley I, et al.: Acceptability of an extended duration vaginal ring for HIV prevention and interest in a multi-purpose ring. Plos One. Under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MTNstopshiv.org. MTN-036/IPM 047 Study Implementation Materials. Available at https://mtnstopshiv.org/research/studies/mtn-036ipm-047/mtn-036ipm-047-study-implementation-materials, accessed August 21, 2021.
- 29. MTNstopshiv.org. MTN-038 Study Implementation Materials. Available at https://mtnstopshiv.org/research/studies/mtn-038/mtn-038-study-implementation-materials, accessed August 21, 2021.
- 30. Vincent KL, Moss JA, Marzinke MA, et al. : Safety and pharmacokinetics of single, dual, and triple antiretroviral drug formulations delivered by pod-intravaginal rings designed for HIV-1 prevention: A Phase I trial. PLoS Med 2018;15:e1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bischof N, Comi A, Eppler MJ: Knowledge visualization in qualitative methods—Or How Can I See What I Say? Paper presented at: 2011. 15th International Conference on Information Visualisation, 2011. [Google Scholar]
- 32. McCoy CF, Millar BG, Murphy DJ, et al. : Mechanical testing methods for drug-releasing vaginal rings. Int J Pharm 2019;559:182–191. [DOI] [PubMed] [Google Scholar]
- 33. Rogers E: Diffusion of innovations, 5th ed. New York, NY: Simon and Schuster, 2003. [Google Scholar]
- 34. Steen M: Tensions in human-centred design. CoDesign 2011;7:45–60. [Google Scholar]
- 35. Montgomery ET, van der Straten A, Chitukuta M, et al. : Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS (London, England) 2017;31:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinrib R, Browne EN, Shapley-Quinn MK, et al. : Perspectives from young South African and Zimbabwean Women on attributes of four (placebo) vaginal microbicide delivery forms. AIDS Behav 2020;24:637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Agot K, Lutnick A, Shapley-Quinn M, et al. : I felt like a TRIO champion? End-user perspectives on their role as co-designers of multi-purpose technologies [version 1; peer review: Awaiting peer review]. Gates Open Res 2020;4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kiser PF, Johnson TJ, Clark JT: State of the art in intravaginal ring technology for topical prophylaxis of HIV infection. AIDS Rev 2012;14:62–77. [PubMed] [Google Scholar]
- 39. Morrow KM, Underhill K, van den Berg JJ, Vargas S, Rosen RK, Katz DF: User-identified gel characteristics: A qualitative exploration of perceived product efficacy of topical vaginal microbicides. Arch Sex Behav 2014;43:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krogstad EA, Atujuna M, Montgomery ET, et al. : Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc 2018;21:e25170–e25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Velsen L, Evers M, Bara C-D, Op den Akker H, Boerema S, Hermens H: Understanding the acceptance of an ehealth technology in the early stages of development: An end-user walkthrough approach and two case studies. JMIR Form Res 2018;2:e10474–e10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lutnick A, Shapley-Quinn MK, Manenzhe KN, et al. : Two birds with one stone: Health care providers' perspectives about prevention technologies in Kenya and South Africa. J Int Assoc Providers of AIDS Care 2019;18:2325958219841366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Straten A, Ryan JH, Reddy K, et al. : Influences on willingness to use vaginal or oral HIV PrEP during pregnancy and breastfeeding in Africa: The multisite MAMMA study. J Int AIDS Soc 2020;23:e25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.