Abstract
Dapivirine (DPV), formulated as vaginal ring, demonstrated HIV risk reduction. MTN-026 explored DPV, formulated as rectal gel, for safety, pharmacokinetics (PK), and acceptability. HIV-uninfected men and women aged 18–45 years were enrolled at United States and Thailand sites and randomized 2:1 to receive DPV 0.05% or placebo gel via rectal applicator. A single-dose phase was followed by seven observed daily doses. Plasma and fluid and tissue from both rectum and cervix were collected at baseline and after the final dose over 72 h for PK, ex-vivo HIV-1 biopsy challenge, histology, and flow cytometry. Twenty-eight participants were randomized; 2 terminated early; 9 were female and 19 male; 12 were white, 11 Asian, 4 black, and 1 other race/ethnicity. Mean age was 28.5 and 34.2 years in the DPV and placebo arms, respectively. Thirty adverse events occurred (all Grade 1 or 2, except one unrelated Grade 3) without study arm differences. DPV rectal tissue concentrations [median (interquartile range)] 0.5–1 and 2 h after a single dose were 256 ng/g [below the lower limit of quantification (BLQ)–666] and BLQ (BLQ–600), respectively, then BLQ (BLQ–BLQ) from 24 to 72 h; concentrations following multiple doses were similar. The largest median DPV plasma concentrations were 0.33 ng/mL (0.15–0.48) after one dose and 0.40 (0.33–0.49) after seven doses. The DPV rectal gel was acceptable and without safety concerns. While DPV plasma concentrations were similar to the vaginal ring, rectal tissue concentrations were well below vaginal ring tissue concentrations, suggesting need for reformulation.
Clinical trial number: NCT03239483.
Keywords: HIV, dapivirine, rectal, HIV prevention, microbicides
Introduction
The biomedical HIV prevention landscape has in recent years added oral methods, including tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC)1,2 and tenofovir alafenamide/FTC.3 Similar to the field of female contraception, one type of HIV prevention may not meet the needs of all at-risk demographics, and there is currently a robust portfolio of HIV prevention research investigating alternative methods of administration, formulations, and drugs. These include long-acting antiretroviral injectables/implants, broadly neutralizing antibodies, vaccines, and topical products. Both oral pre-exposure prophylaxis (PrEP) and one study of vaginal gel have shown efficacy when used on demand.2,4 Rectal microbicides, therefore, have appeal for some due to their potential on-demand use if protective tissue concentrations could be achieved rapidly after dosing and their reduced toxicity due to greatly reduced systemic drug exposure as seen with vaginal microbicides.5–7 To date, no rectal microbicide has been studied in an efficacy trial.
Dapivirine (DPV), a non-nucleoside reverse transcriptase inhibitor is a substituted di-amino-pyrimidine derivative with potent antiviral activity against HIV-1 and was initially developed as an oral antiretroviral treatment. A vaginal ring formulation of DPV was evaluated for HIV-1 prevention in two Phase 3 studies that demonstrated a risk reduction of between 31% [hazard ratio, 0.69; 95% confidence interval (CI), 0.49–0.99; p = .04] and 27% (95% CI, 1–46; p = .046) for HIV among women in four African countries.8,9 This ring received a positive scientific opinion from the European Medicines Agency (EMA) under the Article 58 procedure for use by cisgender women aged 18 years and older in developing countries to reduce their risk of HIV-1 infection and a recommendation from the World Health Organization (WHO). These positive opinions will facilitate national regulatory reviews in Africa where women face the highest risk for HIV and the ring is intended for initial rollout, pending approvals.10
In the context of an efficacious DPV vaginal ring, there is an imperative to initiate investigation of a rectal microbicide DPV gel with the potential to protect this epithelium from HIV infection from receptive anal intercourse (RAI). The current study is the first to evaluate the safety, Pharmacokinetic (PK) and acceptability of 0.05% DPV gel administered rectally.
Materials and Methods
This was a Phase 1, randomized, double-blind, three-site, placebo-controlled trial with planned enrollment of 27 participants at the University of Pittsburgh, Pennsylvania, the University of Alabama at Birmingham, Alabama, and the Silom Community Clinic, Bangkok, Thailand clinical research sites. The participants were randomized (2:1) to receive DPV gel (0.05%) as used in previous studies or placebo gel under direct observation. This trial was registered in the ClinicalTrials.gov database.
Study objectives
The primary objectives of the study were to assess the safety and PK of DPV gel (0.05%), secondary and exploratory objectives assessed the acceptability and various aspects of mucosal safety, including preliminary (ex vivo) efficacy of the gel after rectal application.
Participants and regulatory
Healthy HIV-negative men and women between the ages of 18 and 45 years were recruited from a variety of sources across sites, including outpatient clinics, universities, and community-based locations, using approved recruitment materials. Ethics approval was obtained from the Universities of Pittsburgh and Alabama, Birmingham, the Thai Ministry of Public Health, the Faculty of Tropical Medicine, Mahidol University, the Bamrasnaradura Infectious Diseases Institute, and the U.S. Centers for Disease Control and Prevention before the study commencing. All participants signed informed consent.
Participant instructions and schedule
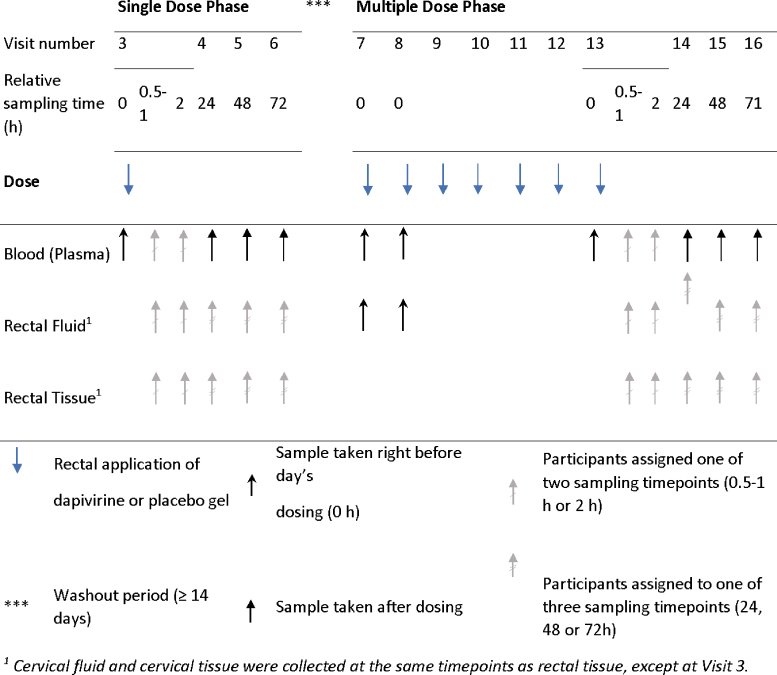
Twenty-seven participants were randomized (2:1) to receive rectally administered DPV gel (0.05%) or placebo gel. Participants initially received a single dose of the gel administered by the study staff. Following a minimum 2-week washout period, participants were instructed to present to the clinic daily for the 7-day directly observed dosing phase where the product was administered either by the participant (under observation by study staff) or by study staff, depending upon site and/or participant preference. Study staff who administered the gel were not the same staff who assessed the participant's safety (Fig. 1).
FIG. 1.
Diagram of dosing schedule and sampling of blood, rectal fluid and rectal tissue for PK endpoint. Specimen sampling done at dosing visits was targeted at 0.5–1 or 2 h after the dosing. Sampling of rectal fluid and rectal tissue specimens was targeted within 30 min of the blood sample. The seven daily doses were spaced by ∼24 h. PK, pharmacokinetic. Color images are available online.
Participants received one prefilled applicator of DPV gel (0.05%) or placebo gel at Visit 7 in case they could not attend one of their seven daily dosing clinic visits and were informed of the ideal storage conditions and correct disposal of the used applicator. If a participant was not able to attend a clinic visit, the participant was instructed to administer the dose at home at approximately the same time of day as all other daily doses, unless the next dose was due within the next 6 h. If the next dose was due within 6 h, the dose was skipped, and the next dose was administered as originally scheduled. Participants who missed more than one application of the product were instructed to contact the site for further direction.
Products
DPV gel (0.05%) was formulated as an aqueous semisolid gel with a pH of 4.7 and osmolality of 767 mOsm/kg. The excipients in the gel were pharmacopoeia grade components with a history of use in currently approved vaginal products, including purified water, sodium hydroxide, polycarbophil, hydroxyethyl cellulose (HEC), propylene glycol, methylparaben, and propylparaben. Each prefilled applicator contained ∼2.5 g (2.5 mL) of DPV gel (0.05%) that was stored at room temperature 25°C (77°F) before use with excursions permitted between 15°C and 30°C (59°F to 86°F).
The Universal Placebo gel contained HEC as the gel thickener, purified water, sodium chloride, sorbic acid, and sodium hydroxide. The gel was isotonic and formulated at a pH of 4.4. Each prefilled applicator contained ∼2.5 g (2.5 mL) and was stored at room temperature 25°C before use with excursions permitted between 15°C and 30°C.
MTN-026 used a prefilled applicator previously used in other rectal microbicide studies, including RMP02/MTN-006, MTN-007, Project GEL, CHARM 01/03, and MTN-017. The applicator was manufactured by HTI Plastics (Lincoln, NE) in accordance with HTI's quality assurance procedures and the Good Manufacturing Practices as established by the U.S. Food and Drug Administration.
Safety
Routine safety laboratory evaluations included testing for renal and liver function, hematology, and HIV. PK (plasma, rectal/cervical fluid and tissue), and pharmacodynamic (PD; rectal/cervical tissue) testing was undertaken as previously described.11–13
Adverse events (AEs) were graded using the NIH Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.1, July 2017 and Addenda 1, 2, and 3 [Female Genital (Dated November 2007), Male Genital (Dated November 2007), and Rectal (Clarification dated May 2012) Grading Tables for Use in Microbicide Studies]; (https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables). In cases where a genital or anorectal AE was covered in multiple tables, the Rectal Grading Table for Use in Microbicide Studies took priority.
All AEs were classified using the MedDRA organ system class/preferred term. The proportion of participants with at least one Grade 2 or higher AE was estimated for each arm, with exact 95% CIs (using the Clopper-Pearson method) provided, and these were compared using Fisher's exact test.
The primary safety endpoints were evaluated on all enrolled participants who received at least one dose of the study product (either DPV or placebo gel).
Behavioral assessments
Participants responded to brief Computer-Assisted Self-Interview (CASI) at the Enrollment visit, after the single dose and at a follow-up assessment that occurred after the seven daily doses were applied. Evaluation of acceptability was focused on two main questions from the computer-assisted self-interview CASI taken at the final visit (“Overall how easy was it to use the gel?,” “Overall how did it feel to have the gel inside you?”). The endpoints were operationalized as the proportion of participants who gave a positive response (“Easy” or “Very easy” and “Very comfortable” or “Comfortable,” respectively), for which 95% CIs are provided.
Specimen collection for PK, PD, flow cytometry, and histological assessment
At the enrollment visit, participants had samples collected by cervicovaginal lavage, rectal tissue biopsies, and rectal sponges for rectal fluid for baseline safety (histology and flow cytometry) evaluation. Participants were randomly assigned to have rectal fluid and rectal biopsies collected by flexible sigmoidoscopy after both the single and seven daily applications at (1) either 30–60 or 120 min and (2) at either 24, 48, or 72 h, after the application. Plasma was collected at either 30–60 or 120 min on the dosing days and at 24, 48 and 72 h postdosing. Cleansing enemas (either saline or Fleet), consistent within participant and according to site, were used after collection of rectal fluid and before flexible sigmoidoscopy for rectal tissue biopsies. Female participants were also randomized to provide samples of cervicovaginal fluid (Dacron swab) and cervical biopsies (Tischler forceps) at the above-described times only after the seventh daily dose.
PK assessment
DPV was quantified via validated liquid-chromatographic–tandem mass spectrometric methods in rectal and cervical tissue,13 cervical fluid collected on the Dacron swab,14 and plasma.15 DPV was also quantified in rectal fluid collected on the Dacron swab using the same methodology as cervical fluid, with the exception that DPV was extracted from rectal fluid using 3 mL 1:1 methanol:water solution. Assays were validated in accordance with FDA Bioanalytical Guidelines and testing was performed by the Clinical Pharmacology Analytical Laboratory at the Johns Hopkins University School of Medicine. Assay lower limits of quantification (LLOQs) were as follows: plasma DPV: 20 pg/mL; rectal fluid (collected on Dacron swabs) DPV: 0.250 ng/swab; cervical fluid (collected on Dacron swabs) DPV: 0.250 ng/swab; and cervical and rectal tissue biopsies: 0.05 ng/sample. Results for rectal and cervical fluid/tissue samples were normalized to net swab or tissue weights and reported as drug concentration per mg of swab or tissue, respectively. Median weight-normalized LLOQs are listed in Table 1.
Table 1.
Concentration of Dapivirine in Plasma, Rectal Fluid, Rectal Tissue, Cervical Fluid, and Cervical Tissue from Samples Collected Before and After the Single- and Multiple-Dose Phases
| Evaluable participants in DPV arm (n = 18) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma LLOQ: 20.0 pg/mL |
Rectal fluid LLOQa: 0.01 (0.01–0.02) ng/mg |
Rectal tissue LLOQa: 0.003 (0.003–0.004) ng/mg |
||||||||||
| Nb | Not validc | BLQ (%)d | Median (IQR) | Nb | Not validc | BLQ (%)d | Median (IQR) | Nb | Not validc | BLQ (%)d | Median (IQR) | |
| Single-dose phase | ||||||||||||
| Predose | 18 | 0 | 18 (100) | BLQ (BLQ–BLQ) | — | — | — | — | — | — | — | — |
| 0.5–1 h | 8 | 0 | 3 (38) | 31.5 (BLQ–54.6) | 8 | 0 | 0 (0) | 30.10 (16.10–43.90) | 8 | 0 | 4 (50) | 0.256 (BLQ–0.666) |
| 2 h | 10 | 0 | 0 (0) | 326.0 (153.0–481.0) | 10 | 2 | 0 (0) | 12.77 (6.06–141.50) | 10 | 0 | 6 (60) | BLQ (BLQ–0.600) |
| 24 h | 18 | 0 | 9 (50) | 20.1 (BLQ–56.6) | 8 | 0 | 6 (75) | BLQ (BLQ–0.02) | 8 | 0 | 8 (100) | BLQ (BLQ–BLQ) |
| 48 h | 18 | 0 | 11 (61) | BLQ (BLQ–27.3) | 4 | 0 | 2 (50) | 0.01 (BLQ–0.04) | 4 | 0 | 4 (100) | BLQ (BLQ–BLQ) |
| 72 h | 18 | 0 | 14 (78) | BLQ (BLQ–BLQ) | 6 | 0 | 5 (83) | BQL (BLQ–BLQ) | 6 | 0 | 6 (100) | BLQ (BLQ–BLQ) |
| Multiple-dose phase | ||||||||||||
| Day 1, predose | 18 | 0 | 18 (100) | BLQ (BLQ–BLQ) | 18 | 1 | 17 (94) | BLQ (BLQ–BLQ) | — | — | — | — |
| Day 2, predose | 18 | 0 | 2 (11) | 67.9 (43.1–111.0) | 18 | 0 | 6 (33) | 0.18 (BLQ–1.91) | — | — | — | — |
| Day 7, predose | 17 | 0 | 0 (0) | 167.0 (136.0–219.0) | — | — | — | — | — | — | — | — |
| 0.5–1 h | 8 | 0 | 0 (0) | 290.5 (147.0–506.5) | 8 | 1 | 0 (0) | 89.70 (44.90–258.0) | 8 | 1 | 6 (75) | BLQ (BLQ–BLQ) |
| 2 h | 9 | 0 | 0 (0) | 403.0 (330.0–493.0) | 9 | 1 | 0 (0) | 26.65 (4.95–278.50) | 8e | 0 | 6 (75) | BLQ (BLQ–0.151) |
| 24 h | 17 | 0 | 0 (0) | 119.0 (86.4–172.0) | 7 | 0 | 3 (43) | 0.01 (BLQ–0.10) | 7 | 0 | 7 (100) | BLQ (BLQ–BLQ) |
| 48 h | 17 | 0 | 0 (0) | 113.0 (69.0–132.0) | 4 | 0 | 3 (75) | BLQ (BLQ–0.04) | 3e | 0 | 3 (100) | BLQ (BLQ–BLQ) |
| 72 h | 17 | 0 | 1 (6) | 80.3 (47.3–129.0) | 6 | 0 | 4 (67) | BLQ (BLQ–0.01) | 6 | 0 | 6 (100) | BLQ (BLQ–BLQ) |
| Evaluable female participants in DPV gel arm (n = 3) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cervical fluid LLOQf: 0.009 (0.004–0.022) ng/mg |
Cervical tissue LLOQf: 0.003 (0.002–0.006) ng/mg |
|
|
|
|
||||||
| Nb | Not validc | BLQ (%)d | Median (IQR) | Nb | Not validc | BLQ (%)d | Median (IQR) | |||||
| Single-dose phase, all times |
7 |
0 |
7 (100) |
BLQ (BLQ–BLQ) |
7 |
0 |
7 (100) |
BLQ (BLQ–BLQ) |
|
|
|
|
| Multiple-dose Phase, all times | 7 | 0 | 7 (100) | BLQ (BLQ–BLQ) | 7 | 0 | 7 (100) | BLQ (BLQ–BLQ) | ||||
Median (IQR) of the weight-normalized LLOQ.
Number of participants providing samples at the corresponding timepoint.
Number of samples for which a valid concentration value could not be obtained, excluded from the calculation of descriptive statistics.
Number of samples with concentration below the LLOQ (percent relative to the total of samples collected at the corresponding timepoint).
One participant, assigned to the 2 and 48 h timepoints, declined to provide samples of rectal tissue at the multiple-dose phase.
Median (range) of the weight-normalized LLOQ.
BLQ, below the lower limit of quantification; DPV, dapivirine; IQR, interquartile range; LLOQ, lower limit of quantification.
DPV concentration versus time plots and descriptive statistics were used to summarize PK data. Samples with concentrations below the LLOQ were imputed a value equivalent to 0 and resulting summaries below the LLOQ are reported as BLQ. Samples were too sparse to estimate peak concentration or area under the curve. To calculate each individual's elimination constant rate (β) of plasma DPV, we used linear regression on the log-transformed DPV concentrations from the 24, 48, and 72 h timepoints, both after single and multiple doses (BLQ observations were excluded). Terminal elimination half-life (t1/2) was estimated as log(2)/β. Due to the large number of BLQs after the single dose, the estimation of β and t1/2 relied only on concentrations after the multiple dosing for most of the participants.
Ex vivo explant HIV challenge PD assessment
Four rectal biopsies were obtained from each participant at each assigned visit and subjected to ex vivo HIV challenge as previously described.16 HIV-1 p24 antigen assays were performed on the biopsies' explant cultures at days 3, 7, 10, and 14. The cumulative p24 antigen value was the sum of the four supernatant p24 antigen concentrations. Cumulative p24 was biopsy weight-adjusted by dividing the reported cumulative p24 concentration (pg/mL) by the weight of each biopsy (mg), yielding final pg/mL/mg units.17 Values below the LLOQ, 10 pg/mL (also weight adjusted) were imputed as either LLOQ or LLOQ/2.
A multilevel hierarchical random effects model was fitted, including random effects (intercept) for participants and for sample time within the participants, with adjustment for enema type, to estimate the difference in mean log10 cumulative p24 relative to DPV arm baseline and placebo. p-Values are adjusted for multiple comparisons using the Benjamini-Hochberg procedure.
PK-PD assessment
Concentration-response PK-PD relationship was assessed in several ways. First, log10 cumulative p24 from participants in the DPV Gel arm with detectable (>LLOQ) rectal tissue DPV concentrations were compared to those with no detectable levels and placebo gel recipients. Differences in mean (95% CI) log10 cumulative p24 were estimated using generalized estimating equations, adjusting by type of enema (fleet vs. saline). Second, we used linear regression to estimate the mean change in the log10 cumulative p24 per unit change in the log10 DPV concentration in rectal tissue. A multilevel hierarchical random effects model was fitted, including random effects (intercept) for participants and for collection times within the participants. Adjustment for type of enema was included. The primary analysis imputed values below the LLOQ as LLOQ/2 for participants in the DPV Gel arm and predose baseline and placebo arm values as LLOQ/20 to enable assessment of concentration-response. A sensitivity analysis using a variety of imputed of DPV placebo values (exclusion or LLOQ/20), DPV arm <LLOQ values (LLOQ, LLOQ/2, and LLOQ/20), and p24 (LLOQ and LLOQ/2) were also explored.
Histology and mucosal T cell phenotype assessment
For the exploratory mucosal safety endpoints, histology used standard hematoxylin and eosin staining and evaluated microscopically using a qualitative injury and inflammation score as previously described.18 Additional rectal biopsies collected at designated biopsy visits underwent enzymatic digestion and flow cytometric characterization of mucosal T cell phenotype as previously described.12
Fisher's exact test was used to compare the scores resulting from the histological evaluation of rectal mucosal tissue samples. The distribution of biomarkers of T cell phenotyping obtained by flow cytometry were compared, between arms, using a Wilcoxon rank sum test, while differences between samples collected at baseline and after dosing were tested using a Wilcoxon signed rank test. To account for multiple testing, a false discovery rate adjustment was performed using the Benjamini-Hochberg procedure.19
Results
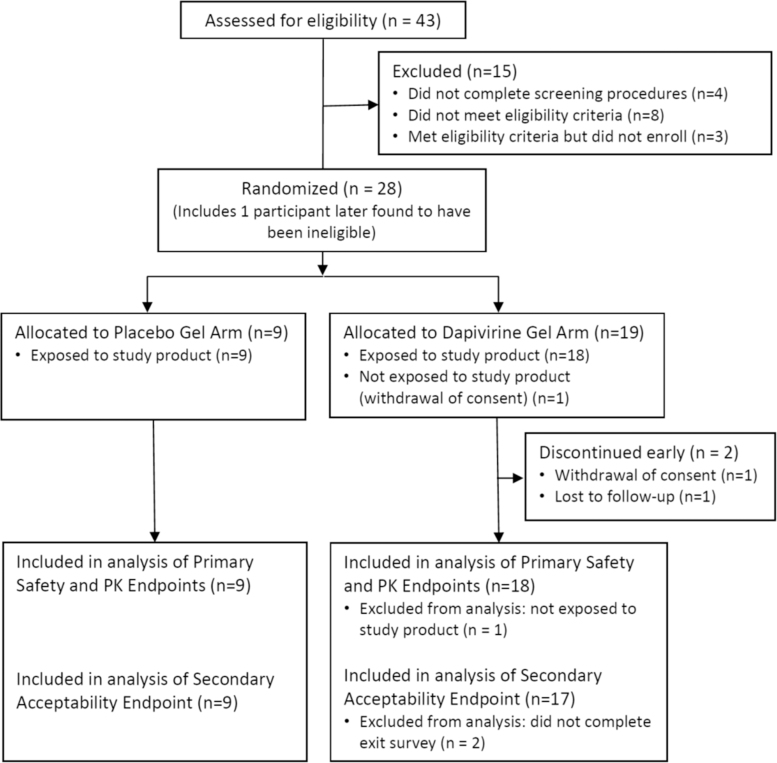
Of the 43 participants who were screened, 27 were eligible and were enrolled, 10 at Birmingham, AL, and 9 each at Pittsburgh, PA, and Bangkok, Thailand. One additional participant, who was found to have been ineligible only after completing all visits was enrolled at the Bangkok site (enrolled in another HIV prevention study) and is included in the analysis of primary and secondary endpoints, making a total of 28 enrolled participants. Fifteen individuals were screened but not enrolled as follows: 4 (27%) did not complete all screening procedures, 3 (20%) were eligible but did not enroll, and the remaining 8 (53%) were not eligible. From the 28 enrolled participants, 26 (93%) completed the study at their scheduled exit/end of study visit. One participant discontinued the study early due to withdrawal of consent and one other participant was lost to follow-up. Both participants were from the Birmingham site and both had been randomized to the DPV gel arm (Fig. 2).
FIG. 2.
CONSORT diagram for the disposition of participants in MTN-026.
Demographics
The mean age of participants was 28.5 years in the DPV gel arm and 34.2 years in the placebo gel arm. Nine (32%) of the 28 participants reported their sex at birth to be female, representing a lower proportion of participants in the DPV gel arm (4 out of 19 participants, 21%) than in the placebo gel arm (5 out of 9 participants, 56%). Except for one participant (sex at birth male) who reported “other” identified gender (specified “gay”), all other participants reported identifying their gender to be the same as their sex at birth. From the U.S. sites, 12 (63%) of the participants were white, 4 (21%) were black or African American, 2 (11%) were Asian, and one (5%) reported “Other” race (specifying “Hispanic/Latino”). From the Bangkok site, all 9 enrolled participants were Thai. Two (7%) of the participants were married and 6 (21%) reported currently living with their partner. Twenty-six participants (93%) attended college or university, one participant (4%) completed secondary school and one (4%) had incomplete secondary school.
Discontinuations
Two participants, both randomized to the DPV gel arm, and both from the Birmingham site, discontinued treatment early. The reasons for early discontinuation of treatment were recorded as “Unable/unwilling to comply with study procedures” (for the participant who terminated the study at Visit 3 due to withdrawal of consent) and “Other” (for the participant who was lost to follow-up after Visit 8).
Adherence to study product schedule
For the 26 participants who completed the study, all their gel applications were observed in the clinic except for one participant in the placebo arm for whom the application corresponding to Visit 11 was reported as done at home.
Primary endpoint: safety
From the 28 enrolled participants, 27 were exposed to the study product and thus considered evaluable for the primary safety endpoint. The proportion of participants with at least one Grade 2 or higher AE was lower in the DPV gel arm (3 out of 18, 17%, 95% CI: 4%–41%) than in the placebo gel arm (5 out of 9, 56%, 95% CI: 21%–86%, p value = .072).
Overall, there were 30 total AEs reported from 13 participants. From the 18 participants receiving DPV gel, 6 (33.3%) reported at least one AE, for a total of 11 AEs, with 7 AEs (63.3%) of Grade 1 (Mild severity) and 4 AEs (36.4%) of Grade 2 (Moderate severity). From these 11 AEs observed among participants receiving DPV Gel, 3 AEs [27.3%, two Grade 1 (diarrhea, and anal itching), and one Grade 2 (diarrhea)] were considered related to the study product. From the 9 participants receiving the placebo gel, 7 reported at least one AE for a total of 19 AEs, with 10 AEs (52.6%) of Grade 1 (Mild severity), 8 AEs (42.1%) of Grade 2 (Moderate severity), and 1 (5.3%) of Grade 3 (Severe). From the 19 AEs observed among participants receiving the placebo gel, 2 AEs [10.5%, both Grade 1 (diarrhea, dyschezia)] were considered related to the study product.
Primary endpoint: PK
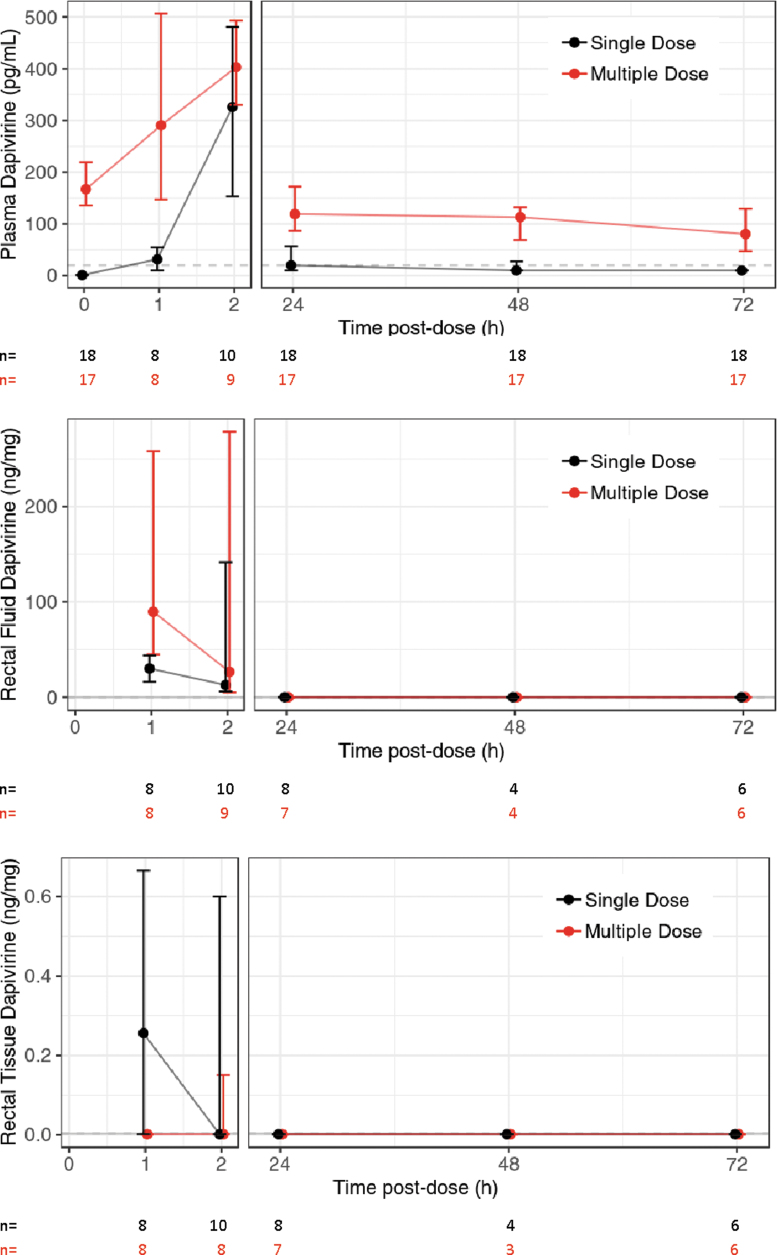
From the 19 participants randomized to the DPV gel arm, 18 are included in this endpoint analysis, as one participant was never administered study product. Also, one of the 18 evaluable participants dropped out during the daily dose phase, after receiving two doses. For participants in the DPV gel arm, the mean number of days between these visits was 24 days (range: 21–43 days). Aggregate plasma, rectal fluid, rectal tissue, cervical fluid, and cervical tissue concentration results are summarized in Table 1 and concentration versus time plots are provided for plasma, rectal fluid, and rectal tissue DPV (Fig. 3 and Supplementary Fig. S1). In both the single- and multiple-dose phases, DPV concentrations in plasma rose rapidly through the first 120 min postdose after which they fell from 24 through 72 h postdose. Median single-dose plasma DPV fell below the LLOQ by 24 h postdose. In the multiple-dose phase, plasma DPV had accumulated median [interquartile range (IQR)]: 167.0 (136.0–219.0) pg/mL predose and remained above the LLOQ throughout the 72-h observation period. The median (IQR) plasma terminal elimination half-life was 52.7 h (35.3–86.2) among 16 participants with valid estimates (two participants had a missing or negative estimate of β). For 9 of these 16 evaluable participants, the paucity of plasma values above the LLOQ left only the multiple-dose phase data for half-life estimation.
FIG. 3.
Median and IQR of the DPV concentration in plasma (pg/mL), rectal fluid (ng/mg), and rectal tissue (ng/mg) after single and multiple doses of gel applied rectally. Horizontal gray dashed lines indicate the DPV assay LLOQ for each matrix (plasma 20 pg/mL, rectal fluid 0.01 ng/mg, and rectal tissue homogenate 0.003 ng/mg). DPV, dapivirine; IQR, interquartile range; LLOQ, lower limit of quantification. Color images are available online.
The highest rectal fluid DPV concentrations were observed with the 30–60 min sample and had dropped below the LLOQ by 24 h postdose in both single and multiple-dose phases. Median rectal tissue concentrations never rose above the LLOQ at any time point postdose in single- or multiple-dose phase; only a few participants had transient concentrations above the LLOQ within the first 2 h postdose.
One of the four women randomized to the DPV gel arm did not receive the study product (early termination) and was considered as nonevaluable. For the remaining three participants, cervical fluid and tissue concentration were below the LLOQ at all times sampled.
Secondary endpoint: acceptability
The acceptability endpoint focuses on participants' ease of use and comfortability of the study product. From the 28 enrolled participants, 26 completed an Exit Survey by CASI at visit 14, of which 16 received the DPV Gel and 9 the Placebo Gel. Twenty-five out of the 26 evaluable participants reported that the gel was “Easy” or “Very Easy” to use (96% with 95% CI: 80%–100%). Twenty-two out of the 26 evaluable participants reported that the gel felt “Comfortable” or “Very comfortable” (85% with 95% CI: 65%–96%). There were no statistically significant differences in acceptability endpoints between the arms.
Pharmacodynamics
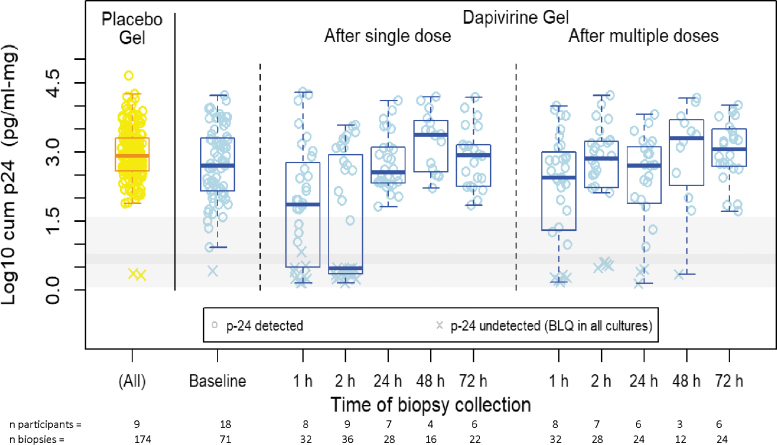
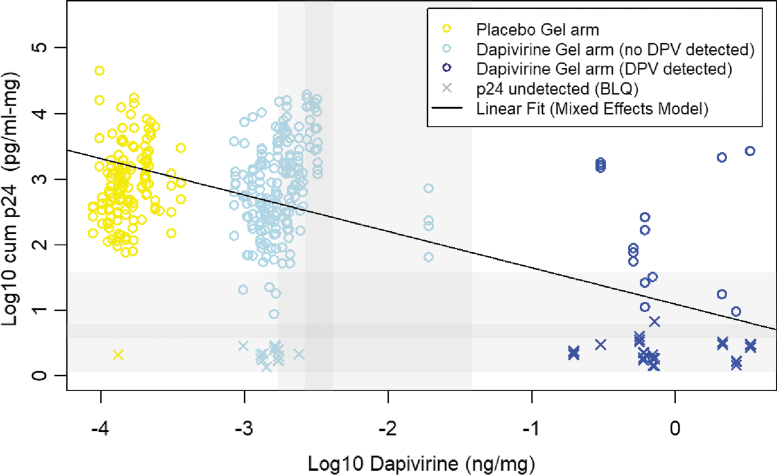
Following a single DPV gel dose, when compared to baseline values with no drug in the same participants, the mean (95% CI) log10 cumulative p24 antigen was −0.81 (−1.34 to −0.27) and −1.3 (−1.81 to −0.79) pg/mL/mg lower in the biopsies collected at 30–60 and 120 min, respectively (Table 2). No statistically significant differences, relative to baseline, were observed at later timepoints. For samples collected after seven daily doses, no difference in mean log10 cumulative 24 were observed relative to baseline (Fig. 4). Where DPV concentration was above the LLOQ, the mean log10 cumulative p24 was significantly lower when compared to biopsies of placebo participants and DPV arm participants with samples below the LLOQ, with a mean difference (95% CI) of −1.98 (−2.49 to −1.47) and −1.77 (−2.22 to −1.32) pg/mL/mg, respectively. A 10-fold change in the DPV concentration (ng/mg) was associated with a mean (95%CI) reduction of −0.56 (−0.69 to −0.40) pg/mL/mg (Fig. 5) and was not affected by enema type (saline or Fleet). While the magnitude of this concentration-response varied with imputed values for baseline and BLQ DPV concentrations, all models indicated statistically significant reductions in log10 cumulative p24 with increases in DPV tissue concentration.
Table 2.
Mean of the Log-10 Cumulative p24 (SD) and Estimated Difference of Means, Relative to the Placebo Arm and Relative to the Baseline Levels in the Dapivirine Gel Arm, with 95% Confidence Interval
| Arm/collection time | Mean (SD) | Mean difference (95% CI) |
|
|---|---|---|---|
| vs. Placebo | vs. DPV baseline | ||
| Placebo arm, all times | 2.94 (0.6) | ||
| DPV arm | |||
| Baseline | 2.72 (0.81) | −0.21 (−0.72 to 0.3) | |
| After single dose | |||
| 30–60 min | 1.84 (1.29) | −1.02 (−1.64 to −0.41)a | −0.81 (−1.34 to −0.27)a |
| 120 min | 1.48 (1.33) | −1.51 (−2.11 to −0.92)a | −1.3 (−1.81 to −0.79)a |
| 24 h | 2.76 (0.61) | −0.08 (−0.72 to 0.56) | 0.13 (−0.43 to 0.7) |
| 48 h | 3.22 (0.64) | −0.13 (−0.91 to 0.64) | 0.08 (−0.63 to 0.79) |
| 72 h | 2.87 (0.66) | 0.09 (−0.59 to 0.76) | 0.3 (−0.3 to 0.9) |
| After seven daily doses | |||
| 30–60 min | 2.22 (1.2) | −0.65 (−1.26 to −0.03) | −0.44 (−0.97 to 0.1) |
| 120 min | 2.58 (1.09) | −0.44 (−1.08 to 0.2) | −0.23 (−0.79 to 0.33) |
| 24 h | 2.36 (1.04) | −0.54 (−1.21 to 0.13) | −0.33 (−0.93 to 0.27) |
| 48 h | 2.95 (1.12) | −0.44 (−1.29 to 0.42) | −0.22 (−1.03 to 0.58) |
| 72 h | 3.02 (0.65) | 0.27 (−0.41 to 0.94) | 0.48 (−0.12 to 1.08) |
Statistically significant p-value under a 5% significance level after adjustment for multiple comparisons by the Benjamini-Hochberg procedure.
CI, confidence interval.
FIG. 4.
Vertical boxplots of log-10 weight-adjusted cumulative p24 (pg/mL-mg) from rectal tissue biopsies collected after a single or multiple dosing of study product administered rectally, by collection time and study product arm. Individual observations are indicated by yellow (placebo gel) and light blue (DPV gel) open circles. Horizontal bands show IQR (dark gray) and overall range of the weight-adjusted LLOQ for the cumulative p24. Color images are available online.
FIG. 5.
Log-10 cumulative p24 (pg/mL-mg) from four rectal tissue biopsies versus DPV concentration in rectal tissue biopsies collected at the same time, with a linear fit from a mixed effects model (samples with DPV concentration BLQ from participants in the DPV gel arm were imputed a value of LLOQ/2 while samples from participants in the Placebo gel arm were imputed a value of LLOQ/20). The light gray and dark gray regions indicate the range and interquartilc range, respectively, of the LLOQs for the cumulative p24 (horizontal gray bars) and the DPV concentration (vertical gray bars). BLQ, below the lower limit of quantification. Color images are available online.
Mucosal safety
Histology evaluation
Most participants had scores of 0 or 1 (0 = No abnormality, 1 = Mononuclear cell infiltrate), with only one score of 2 (Neutrophilic infiltrate-lamina propria, after single application of placebo gel) and one score of 3 (Neutrophilic infiltrate-epithelium, after multiple doses of DPV gel) observed. From the Fisher's exact test, we found no evidence of differences between the DPV gel arm and the placebo gel arm in the histology scores after rectal application of a single dose (Visit 3, p value = .516) or after multiple doses (Visit 13, p value = 1.000).
Flow cytometry
To evaluate immunological response to the study product, mucosal T cell phenotyping (CD45+, Viable CD3+, CD4+, CD8+, CCR5, and CXCR4) by flow cytometry was performed on rectal tissue biopsies collected at Visit 2 (Enrollment), Visit 3 (Single Dose visit), and Visit 13 (last of the Daily Dosing visits). None of the comparisons, either postdosing relative to placebo or between arms, remained statistically significant after adjusting for false discovery rate of 10% using the Benjamini-Hochberg procedure. Data not shown.
Discussion
Rectal application of DPV 0.05% gel was acceptable and without safety concerns in HIV-uninfected male and female adults with no alterations in sensitive investigational mucosal parameters, including histology and T cell subset phenotypes. PK analyses showed detectable median DPV levels in both plasma and rectal fluid after single administration out to 24 h, while after seven daily doses, the median rectal fluid detection was maintained for 24 h, and median plasma DPV was detected up to 72 h. In both dosing schedules, DPV was detected in rectal tissue of only a few participants and only at the 30–60- and 120-min timepoints. However, measurable DPV in rectal tissue corresponded to a graded decrease in tissue infectivity as measured using the in vitro HIV explant challenge. No DPV was detected in the female genital tract following single or multiple rectal DPV administrations.
The hypothesis underpinning the development of rectal microbicides is that the populations practicing RAI who have the highest risk of HIV are also the most familiar with the use of gel products (e.g., sexual lubricants) to facilitate intercourse. Thus, there would be only minimal behavioral adaptation required to use a gel product for HIV prevention just before intercourse. Per protocol, and to give context to rectal microbicide use, all participants in MTN-026 verified a history of consensual RAI within the last 12 months. Thus, participants likely had some degree of familiarity with anorectal product administration that validates the acceptability data showing that most found the gel administration both easy and comfortable. In the current study, we did not assess whether the DPV gel could act as an alternative to an anal lubricant before intercourse. The feasibility of using the DPV gel as a behaviorally congruent product resembling an anal lubricant (i.e., without needing a rectal applicator) will be addressed in the MTN-033 study.
The extensive PK analyses conducted in MTN-026 reveal significant challenges for the current formulation of this product. For efficacy, the antiretroviral product needs to be delivered in suppressive concentrations to the area most at risk of incident infection – in this case, the lower rectum. Following the single rectal gel dose, although no participants had a plasma dose BLQ, 60% of rectal tissue values were below the LLOQ at 120 min following dosing. Although it is unknown if efficacy predominantly depends upon local or systemic drug concentrations, the absence of detectable drug in tissue so near to the time of application is of concern for a product that could potentially be used on demand. Reasons for this may include rapid absorption into the systemic circulation that can be corroborated with detectable plasma values, sampling issues that may include sample dilution due to residual enema fluid, and the possibility that in rectal tissue, DPV may have dissociated rapidly ex vivo and was thus not measured. Although a protective signal was seen in the ex vivo HIV challenge models, it was seen in far fewer than after both oral daily FTC/TDF dosing or one-off rectal dosing of tenofovir.20–22 In addition, the tissue concentrations of DPV in MTN-026 were much lower than the in vitro IC50 as well as cervicovaginal tissue concentrations seen with the DPV vaginal ring that subsequently demonstrated HIV risk reduction in women in Phase 3 studies.13 However, rectal and vaginal explant methods differ significantly, requiring caution in such comparisons. Finally, there was no evidence of rectal to vaginal drug migration, not dissimilar to tenofovir for which there is a steep concentration gradient from rectal to cervicovaginal tissue concentration after rectal dosing; thus, indicating that rectal dosing could not be assumed to provide any female genital tract protection.6
Although routine daily dosing of a rectal gel product is unlikely to be either practical or acceptable in at-risk populations,11 a more sexually active and likely younger population may have cause to use the product daily – especially if these populations have access, availability, and adherence barriers to daily oral PrEP regimens. Nearly every one of our research participants reported that the gel was “Easy” or “Very Easy” to use as well as the gel feeling “Comfortable” or “Very comfortable,” which is not surprising given our inclusion criteria. However, whether frequent dosing of this DPV gel product is sufficient to prevent HIV infection remains unanswered.
Despite the challenges both investigators and funders face to design ethically robust HIV prevention studies that demonstrate efficacy in the presence of highly effective oral combination products, this must not limit the provision of expanded choice for individuals at risk of HIV infection. The use of a rectal gel product that may include both an antiretroviral with the capacity for lubrication may still be seen as giving added value in the HIV prevention landscape, especially for at-risk individuals for whom oral TDF or TAF with FTC is unacceptable or contraindicated. DPV has demonstrated efficacy in a vaginal ring formulation. Should a rectal gel product be pursued with DPV, this will require reformulation where consideration should be given to both a higher dose and longer lasting PK profile.
Supplementary Material
Acknowledgments
The study team gratefully acknowledges the study participants of MTN 026. The study was designed and implemented by the MTN with study product provided by the International Partnership for Microbicides (IPM) and the MTN. This study was first presented at the fourth HIV Research for Prevention conference in 2021. This article is dedicated to the memory of our friend and colleague Dr. Charlene S. Dezzutti.
Contributor Information
Collaborators: MTN-026 Protocol Team
Authors' Contributions
Protocol development (All), Laboratory analyses (C.S.D., R.P.K.N.A., R.M.B., L.W., M.A.M., and C.W.H.), data interpretation (All), article development, composition, and review and submission of the article (R.D.C., E.B., J.B, E.F.D., C.H., K.H., S.J., J.L., C.D.-I., H.G., M.P., C.E.J., L.K., D.S., R.P.K.N.A., L.W., M.A.M., J.P., B.D., J.N., I.McG., and C.W.H.).
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. National Institutes of Health, U.S. Centers for Disease Control and Prevention, or donors of the International Partnership for Microbicides.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was funded by the National Institute of Allergy and Infectious Diseases through individual grants (UM1AI068633, UM1AI068615, and UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. I.P.M. receives generous support from the Danish Ministry of Foreign Affairs, Flanders Department of Foreign Affairs, Irish Aid, the German Federal Ministry of Education and Research (BMBF) through the KfW Development Bank, the Ministry of Foreign Affairs of the Netherlands, United Kingdom aid from the UK Government's Foreign, Commonwealth & Development Office, the American people through the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) in partnership with the United States Agency for International Development (USAID), and the Bill & Melinda Gates Foundation.
Supplementary Material
References
- 1. McCormack S, Dunn DT, Desai M, et al. : Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): Effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016;387:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molina JM, Capitant C, Spire B, et al. : On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015;373:2237–2246. [DOI] [PubMed] [Google Scholar]
- 3. Mayer KH, Molina JM, Thompson MA, et al. : Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020;396:239–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. : Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nel A, Haazen W, Nuttall J, Romano J, Rosenberg Z, van Niekerk N: A safety and pharmacokinetic trial assessing delivery of dapivirine from a vaginal ring in healthy women. AIDS 2014;28:1479–1487. [DOI] [PubMed] [Google Scholar]
- 6. Justman JE, Nair GL, Hendrix CW, et al. : Pharmacokinetics and pharmacodynamics of tenofovir reduced-glycerin 1% gel in the rectal and vaginal compartments in women: A cross-compartmental study with directly observed dosing. J Acquir Immune Defic Syndr 2018;78:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW: Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. Hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr 2010;55:161–169. [DOI] [PubMed] [Google Scholar]
- 8. Nel A, van Niekerk N, Kapiga S, et al. : Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016;375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 9. Baeten JM, Palanee-Phillips T, Brown ER, et al. : Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dapivirine Vaginal Ring 25mg. European Medicines Agency, Amsterdam, The Netherlands, 2020. [Google Scholar]
- 11. Cranston RD, Lama JR, Richardson BA, et al. : MTN-017: A rectal phase 2 extended safety and acceptability study of tenofovir reduced-glycerin 1% gel. Clin Infect Dis 2017;64:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGowan I, Anton PA, Elliott J, et al. : Exploring the feasibility of multi-site flow cytometric processing of gut associated lymphoid tissue with centralized data analysis for multi-site clinical trials. PLoS One 2015;10:e0126454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen BA, Panther L, Marzinke MA, et al. : Phase 1 safety, pharmacokinetics, and pharmacodynamics of dapivirine and maraviroc vaginal rings: A double-blind randomized trial. J Acquir Immune Defic Syndr 2015;70:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parsons TL, Emory JF, Seserko LA, Aung WS, Marzinke MA: Dual quantification of dapivirine and maraviroc in cervicovaginal secretions from ophthalmic tear strips and polyester-based swabs via liquid chromatographic-tandem mass spectrometric (LC-MS/MS) analysis. J Pharm Biomed Anal 2014;98:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seserko LA, Emory JF, Hendrix CW, Marzinke MA: The development and validation of an UHPLC-MS/MS method for the rapid quantification of the antiretroviral agent dapivirine in human plasma. Bioanalysis 2013;5:2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGowan I, Wilkin T, Landovitz RJ, et al. : The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 2019;33:237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anton PA, Cranston RD, Kashuba A, et al. : RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012;28:1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGowan I, Hoesley C, Cranston RD, et al. : A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007). PLoS One 2013;8:e60147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I: Controlling the false discovery rate in behavior genetics research. Behav Brain Res 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 20. McGowan I, Cranston RD, Duffill K, et al. : A Phase 1 Randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% Gel (the CHARM-01 Study). PLoS One 2015;10:e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hendrix CW, Andrade A, Bumpus NN, et al. : Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses 2016;32:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoang T, Date AA, Ortiz JO, et al. : Development of rectal enema as microbicide (DREAM): Preclinical progressive selection of a tenofovir prodrug enema. Eur J Pharm Biopharm 2019;138:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.