Abstract
Indwelling medical devices are associated with infectious complications. Incorporating antimicrobials into indwelling materials may reduce bacterial colonization. Bismuth thiols are antibiofilm agents with up to 1,000-fold-greater antibacterial activity than other bismuth salts. Staphylococci are particularly sensitive, as determined by agar diffusion and broth dilution susceptibility testing. Bismuth-ethanedithiol inhibited 10 methicillin-resistant Staphylococcus epidermidis strains at 0.9 to 1.8, Staphylococcus aureus ATCC 25923 at 2.4, and S. epidermidis ATCC 12228 at 0.1 μM Bi3+. Antiseptic-resistant S. aureus was sensitive to bismuth-2-3-dimercaptopropanol (BisBAL) at ≤7 μM Bi3+. Hydrogel-coated polyurethane rods soaked in BisBAL inhibited S. epidermidis for 39 days (inhibitory zone diameter in agar, ≥30 mm for >25 days). Slime from 16 slime-producing S. epidermidis strains was inhibited significantly by bismuth-3,4-dimercaptotoluene (BisTOL), but not by AgNO3, at subinhibitory concentrations. In conclusion, bismuth-thiols are bacteriostatic and bactericidal against staphylococci, including resistant organisms, but are also inhibitors of slime at subinhibitory concentrations. At subinhibitory concentrations, BisTOL may be useful in preventing the colonization and infection of indwelling intravascular lines, since staphylococci are important pathogens in this setting.
Staphylococci are ubiquitous skin microflora and important nosocomial and community-acquired pathogens. Coagulase-negative staphylococci are the most common cause of foreign-body device infection and have become increasingly prevalent in nosocomial bacteremia and infections in the immunocompromised host (10, 11, 15). Staphylococcus aureus is frequently implicated in wound infections, osteomyelitis, endocarditis, and sepsis (20, 24). Multiresistant strains (methicillin-resistant S. aureus [MRSA] and methicillin-resistant Staphylococcus epidermidis [MRSE]) commonly colonize the skin and nares of hospitalized patients and personnel, serving as a reservoir for nosocomial infection and antibiotic resistance genes transferable to staphylococci and other bacteria (3, 12, 21). Staphylococci can adhere to a variety of foreign materials. Once established, such infections are difficult to resolve. The emergence of resistant staphylococci has raised concern that effective antimicrobial regimens may not be available in the near future (23).
Bismuth thiols (BTs) are a group of novel biocides with potent, broad-spectrum activity (8). BTs inhibit bacteria at up to 1,000-fold-lower concentrations than other bismuth salts. At subinhibitory concentrations, BTs suppress bacterial exopolysaccharide expression in Klebsiella and Pseudomonas spp., which prevents biofilm formation and renders the bacteria susceptible to host defenses (9). At concentrations from 0.6 to 1 ppm (3 to 5 μM Bi3+), bismuth-2-3-dimercaptopropanol (BisBAL) suppressed capsule and slime production by 70 to 90%, with only marginal effects on growth (9). Exopolysaccharides are also important virulence factors for staphylococci (18, 22).
One approach to preventing infection due to indwelling devices is the bonding of antimicrobial agents to catheter material. Silver-impregnated devices have proven efficacy, as have other antiseptics (14, 17). BTs may prove superior in this regard. The possible advantages of BTs over other metal-based antiseptics and antifouling agents (e.g., silver, copper, and organotins) include safety, since bismuth is relatively nontoxic compared to other heavy metals. BTs also inhibit slime expression and are particularly effective at inhibiting and killing staphylococci (17, 24). This report documents the potent antibacterial and antibiofilm activities of BTs against various staphylococci, including resistant strains.
MATERIALS AND METHODS
Bacteria and culture conditions.
The bacteria employed include clinical isolates of S. aureus, MRSA and MRSE, the reference strains S. aureus ATCC 25923 and S. epidermidis ATCC 12228, and the antiseptic-resistant S. aureus strains FDA209P, L20A, N20, MEK23, and MEK24. Strains L20A and N20 show resistance to ethidium bromide, quaternary ammonium compounds, and glutaraldehyde (19). S. epidermidis RP62A (ATCC 35984), a slime-producing strain, was employed in coated-rod agar diffusion studies, as well as in slime production studies. Other bacteria employed include Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, and clinical isolates of vancomycin-resistant enterococci, E. coli, Legionella pneumophila, Burkholderia cepacia, and Pseudomonas aeruginosa. Slime assay organisms included S. epidermidis RP62A and 15 slime-producing S. epidermidis clinical isolates grown in Trypticase soy broth (TSB) with 1% glucose (4). The bacteria were subcultured weekly on Mueller-Hinton agar supplemented with 1% Fildes enrichment (Difco Laboratories, Detroit, Mich.), also used for agar diffusion studies. Broth dilution was performed in Mueller-Hinton II broth. The broth was supplemented with 1% Fildes enrichment and 20 mM NaCl.
BT preparation.
The agents employed were bismuth-1,2-ethanedithiol (BisEDT), BisBAL, bismuth-2-mercaptoethanol (BisβME), bismuth-3,4-dimercaptotoluene (BisTOL), and bismuth-pyrithione (BisPYR) (6). Liquid and powder forms were prepared at various molar ratios. The proposed structures of some of these compounds have been published (1). BT solutions were prepared in propylene glycol using bismuth nitrate and commercially prepared thiols (Sigma-Aldrich Corporation, St. Louis, Mo.). Five micromolar BTs is approximately equal to 1 μg of bismuth/ml.
Susceptibility studies.
Staphylococci were tested for susceptibility in agar and broth media. In agar diffusion studies, plates were streaked with 106 bacteria. Sterile paper disks (6-mm diameter) were placed on the surface and impregnated with 5 μl of agent in propylene glycol. Inhibitory-zone diameters were measured at 24 h. Broth dilution studies were performed in accordance with NCCLS standards. The bacteria were tested for susceptibility to several BT agents at a wide range of concentrations (0.1 to 100 μM bismuth; 5 μM = 1 μg/ml). The MIC is expressed as the concentration of BT inhibiting visual growth for 24 ± 2 h. Minimum bactericidal concentrations (MBC) are expressed as the BT concentration that reduced viability by 99.9%, as determined by plating the bacteria on agar medium.
Catheter studies.
Uncoated polyurethane catheter material (3-mm diameter) was treated with SurModics PhotoLink technology, a polyvinylpyrrolidone-based hydrogel. Catheters were soaked overnight at 25°C in 50/75 mM BisBAL (pH 12.5), rinsed in sterile distilled H2O for 5 s to remove excess BisBAL, and embedded in Mueller-Hinton agar with a lawn of S. epidermidis RP62A or E. coli ATCC 25922 at 106 CFU/ml. The plates were incubated overnight at 37°C, and the zone diameter perpendicular to the rod in which no growth was apparent was measured. The rods were transferred daily to fresh lawns on Mueller-Hinton agar. The diameter of the zone of inhibition (in millimeters) is expressed as the mean from three samples. The diameter of the rod was 3 mm, which was equivalent to no zone.
Slime assay.
A quantitative adherence assay was employed to test for slime production (4). Briefly, 1:100 dilutions of overnight cultures in TSB were used to inoculate wells in a microtiter polystyrene plate. After growth for 24 or 48 h at 37°C, the plates were gently washed three times with phosphate-buffered saline, and the adherent bacteria were fixed at 60°C for 1 h and then stained with Hucker's crystal violet; excess stain was washed off with tap water. The optical density (OD) of the biofilm was measured at 570 nm in a spectrophotometer (Novapath Microplate Reader; BioRad Laboratories Inc.). To examine the effect of BT compounds or silver nitrate (2) on slime production, BTs were prepared as described above and diluted directly into culture medium. S. epidermidis growth was followed by determining the OD of cultures at 600 nm (OD600) and by counting CFU on blood agar media. The results were analyzed to evaluate the possible effect of growth rate and cell density on slime formation. Accordingly, the slime index was defined as an estimate of the density of the biofilm generated by a culture with an OD600 of 0.5 [slime index = mean OD of the biofilm × (0.5/mean OD growth)] (7).
Statistics.
Where possible, data are presented as the mean of at least three independent trials along with the standard deviation. Differences in slime production were analyzed by the Wilcoxon test for related rankable scores.
RESULTS
The susceptibility of MRSA to BTs was examined by agar diffusion. Forty-seven MRSA strains were tested with BisBAL and compared to other pathogenic bacteria (Table 1). BisBAL produced an average inhibition zone diameter of 18.7 mm against MRSA, compared to the relative absence of inhibition produced by the individual components that make up BisBAL. Isolates of other bacteria, though sensitive to one degree or another, were less sensitive than S. aureus.
TABLE 1.
Susceptibilities of bacteria to BisBAL in agar diffusiona
| Bacterium (no. of isolates) | Diam of zone of inhibition (mm)
|
||
|---|---|---|---|
| Bi(NO3)3 (157 μg) | BAL (186 μg) | BisBAL (157/31 μg) | |
| MRSA (47) | 6.8 ± 2.2 | ≤6.0 | 18.7 ± 3.1 |
| VRE (18) | 6.7 ± 1.2 | ≤6.0 | 10.9 ± 1.6 |
| E. coli (6) | 7.3 ± 0.6 | ≤6.0 | 12.5 ± 1.3 |
| L. pneumophila (4) | ≤6.0 | ≤6.0 | 15.6 ± 1.0 |
| P. aeruginosa (3) | ≤6.0 | ≤6.0 | 16.0 ± 1.1 |
Suspensions of bacteria (106CFU/ml) were spread on Mueller-Hinton agar plates. Bismuth nitrate and BAL were used to impregnate paper disks on the agar surface. The plates were incubated overnight at 37°C, and the zones of inhibition were measured the following day. The data are expressed as the mean for all isolates within a group, along with the standard deviation.
The susceptibilities of S. aureus to various BTs in broth dilution studies are summarized in Table 2. BTs were prepared in propylene glycol at 5 and 2.5 mM. BisTOL was superior to the other compounds with regard to MBC. BisEDT was the most inhibitory but was not an effective bactericidal agent, as was BisTOL, BisPYR, or BisBAL. Propylene glycol alone at 1% in culture medium had no inhibitory effect.
TABLE 2.
BT bacteriostatic and bactericidal concentrations against S. aureusa
| BT | MIC (μM Bi3+) | MBC (μM Bi3+) |
|---|---|---|
| BisTOL | 4.4 ± 0.1 | 8.8 ± 0.3 |
| BisPYR | 6.7 ± 0.2 | 14.2 ± 0.8 |
| BisBAL | 8.3 ± 0.4 | 18.0 ± 0.5 |
| BisEDT | 2.4 ± 0.0 | 73.3 ± 2.3 |
As determined for S. aureus ATCC 25923 by broth dilution. The broth consisted of Mueller-Hinton broth with 1% Fildes enrichment and 20 mM NaCl. BTs, prepared in propylene glycol at a 2:1 molar ratio, were added to medium containing bacteria in accordance with NCCLS standards. The data represent the means resulting from three or more independent trials. Five micromolar BT contains approximately 1 μg of bismuth/ml.
Several BTs, formulated as either liquids or powders at various molar ratios, were tested against a variety of staphylococci (Table 3). BisBAL 3:1 powder inhibited 87 S. aureus strains on average below 15 μM compared to BisPYR 2:1 powder, which inhibited an MRSA strain at 2.5 μM. Among the liquid preparations, BisEDT was most active, inhibiting 10 MRSE strains at an average of 1.1 μM Bi3+, S. epidermidis ATCC 12228 at 0.09 μM Bi3+, and S. aureus ATCC 25923 at 2.4 μM Bi3+. BisβME at 1:1.4 inhibited 65 S. aureus clinical isolates at an average of 23.7 μM Bi3+.
TABLE 3.
Susceptibilities of staphylococci to BTsa
| Agent | Bacteria | MIC (μM) |
|---|---|---|
| BisBAL 3:1 | 87 S. aureus isolates | 5.5 ± 0.9 |
| BisPYR 2:1 | 1 MRSA clinical strain | 2.5 |
| BisEDT 2:1 | 10 MRSE clinical strains | 1.1 ± 0.4 |
| BisEDT 2:1 | S. aureus ATCC 25923 | 2.4 |
| BisEDT 2:1 | S. epidermidis ATCC 12228 | 0.09 |
| BisßME 1:1.4 | 65 S. aureus clinical strains | 23.7 ± 4.7 |
Dilutions (1:100) of a 0.5 MacFarland standard suspension of staphylococci in Mueller-Hinton broth containing 1% Fildes enrichment and 20 mM NaCl were incubated at 34°C with rocking in an Abbott Avantage. Five micromolar bismuth-thiol contains approximately 1 μg of bismuth/ml.
BTs were also tested against antiseptic-resistant S. aureus. The antiseptic-resistant strains L20A and N20 were actually more sensitive to BisBAL (MICs, ≤1 μM Bi3+) than the antiseptic-sensitive strain FDA209P (MIC, 6 μM Bi3+) Moreover, the triclosan- and mupirocin-resistant strains MEK23 (MIC, 7 μM Bi3+) and MEK24 (MIC, 6 μM Bi3+) were as sensitive to BisBAL as was the antiseptic-sensitive strain. All strains were susceptible to BisBAL at or below 7 μM (1.4 μg of Bi3+/ml).
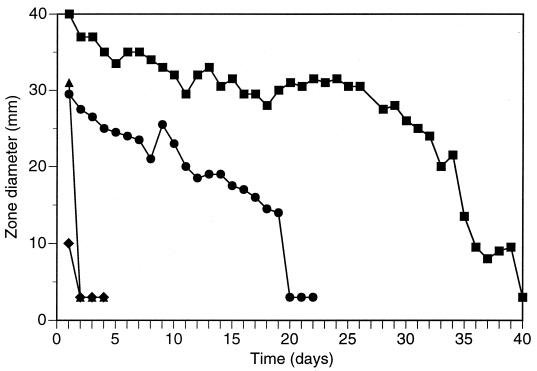
BTs were also tested for long-term slow release from catheter-related materials. Hydrogel-coated polyurethane rods saturated with BisBAL were tested daily in agar diffusion studies. The diameters of zones BT inhibition around hydrogel-coated and uncoated rods for a slime-forming S. epidermidis strain were measured and are summarized in Fig. 1. Hydrogel-coated rods exhibited strong antibacterial activity for over 1 month. Against E. coli, zones were produced for 19 days (Fig. 1). Rods without hydrogel coating showed no inhibition of bacteria after day 1.
FIG. 1.
Inhibitory action of BisBAL-coated polyurethane rods. Hydrogel-coated rods were soaked in 50 or 75 mM BisBAL overnight and embedded int agar plates seeded with S. epidermidis (■) or E. coli (●). Rods without BisBAL were also tested against S. epidermidis (▴) and E. coli (⧫). The plates were incubated overnight at 37°C, and the zones of inhibition were measured. The rods were transferred daily to fresh plates. Zone diameters are expressed as the means of three samples. The diameter of the rod was 3 mm, which was equivalent to no zone.
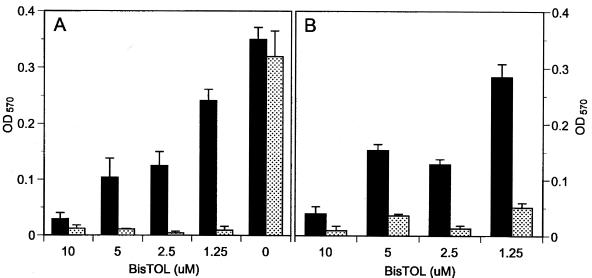
To analyze glycocalyx formation, S. epidermidis strain RP62A was treated with several BT compounds at subinhibitory concentrations. Slime production was affected at sub-MICs of BisTOL, BisBAL, BisPYR, and BisEDT (data not shown). However, only sub-MIC BisTOL strongly limited slime production under these conditions. Figure 2 shows 24- and 48-h slime inhibition at 1.25 μM BisTOL, though culture growth was apparent (OD600 = 0.30; 5 × 108 CFU/ml). Higher BisTOL levels were more growth inhibitory. The slime-inhibiting effect of BisTOL was verified by examining its effect at 24 h on 15 additional slime-producing S. epidermidis clinical isolates (Table 4). Biofilm ODs were significantly lower (P < 0.002) after growth in 1.25 μM BisTOL, with an average reduction in slime of 86.4% ± 0.07% and a range from 68.1 to 96.0%. All BisTOL-treated bacteria exhibited growth at 24 h.
FIG. 2.
Effect of BisTOL on growth (solid bars) and on biofilm formation (stippled bars) by S. epidermidis RP62A after 24 (A) and 48 (B) h. Bacteria adhering to microtiter plates were stained and measured for absorbence as described in Materials and Methods. The values are means of triplicate determinations (± standard deviations).
TABLE 4.
Effect of BisTOL on growth and slime production of S. epidermidisa
| Bacterial strain | Growth (OD600) | Slime production in TSB (OD570) | Slime production in TSB-BisTOL (OD570) | Slime index |
|---|---|---|---|---|
| SE 35D | 0.270 ± 0.020 | 0.265 ± 0.030 | 0.052 ± 0.015 | 0.096 |
| SE 39 | 0.298 ± 0.030 | 0.350 ± 0.027 | 0.064 ± 0.022 | 0.107 |
| SE A73 | 0.394 ± 0.033 | 0.378 ± 0.040 | 0.040 ± 0.003 | 0.05 |
| SE R2 | 0.450 ± 0.072 | 3.000 ± 0.110 | 0.120 ± 0.023 | 0.133 |
| SE R22 | 0.352 ± 0.031 | 0.430 ± 0.020 | 0.076 ± 0.004 | 0.107 |
| SE R25 | 0.348 ± 0.023 | 0.540 ± 0.050 | 0.073 ± 0.031 | 0.104 |
| SE R27 | 0.352 ± 0.024 | 0.420 ± 0.022 | 0.053 ± 0.019 | 0.07 |
| SE R33 | 0.390 ± 0.025 | 1.980 ± 0.221 | 0.082 ± 0.002 | 0.105 |
| SE R35 | 0.567 ± 0.025 | 0.307 ± 0.024 | 0.098 ± 0.017 | 0.086 |
| SE R55 | 0.298 ± 0.031 | 0.562 ± 0.031 | 0.053 ± 0.019 | 0.088 |
| SE R57 | 0.301 ± 0.025 | 0.305 ± 0.015 | 0.026 ± 0.001 | 0.043 |
| SE R66 | 0.254 ± 0.033 | 0.327 ± 0.032 | 0.039 ± 0.015 | 0.076 |
| SE 5/P | 0.298 ± 0.027 | 0.346 ± 0.021 | 0.041 ± 0.018 | 0.068 |
| SE 21/P | 0.305 ± 0.042 | 0.427 ± 0.038 | 0.062 ± 0.004 | 0.101 |
| SE 49/P | 0.402 ± 0.019 | 0.310 ± 0.019 | 0.051 ± 0.022 | 0.063 |
BisTOL tested at 1.25 μM (0.25 μg of Bi3+/ml) in TSB, and biofilm OD quantitated at 24 h, using a microtiter assay.
Silver nitrate inhibited the growth rate and slime production of S. epidermidis (MIC, 94 μg/ml), but no effect on slime expression was seen at subinhibitory concentrations (Table 5). Thus, for AgNO3, the slime index from RP62A samples showing growth ranged from 1.29 to 2.20 (Table 5), while that for sub-MIC BisTOL ranged from 0.05 to 0.133 among the 15 strains tested (Table 4). The slime index for the untreated control was 1.22.
TABLE 5.
Effect of AgNO3 on growth and slime production of S. epidermidis
| Silver concn (μg/ml) | Growth (OD600) | Slime production (OD570) | CFU/ml |
|---|---|---|---|
| 3,020 | 0.083 ± 0.007 | 0.020 ± 0.009 | <100 |
| 1,510 | 0.071 ± 0.009 | 0.016 ± 0.001 | <100 |
| 755 | 0.081 ± 0.024 | 0.014 ± 0.005 | <100 |
| 378 | 0.110 ± 0.015 | 0.019 ± 0.006 | <100 |
| 189 | 0.080 ± 0.010 | 0.015 ± 0.010 | <100 |
| 94 | 0.063 ± 0.017 | 0.010 ± 0.004 | <100 |
| 47 | 0.256 ± 0.114 | 0.661 ± 0.396 | 2.7 × 107 |
| 24 | 0.339 ± 0.036 | 1.490 ± 0.122 | 2.3 × 108 |
| 12 | 0.411 ± 0.016 | 1.712 ± 0.010 | 4.5 × 108 |
| 6 | 0.480 ± 0.023 | 1.686 ± 0.163 | 9 × 108 |
| 0 | 0.640 ± 0.038 | 1.560 ± 0.120 | 3.2 × 109 |
DISCUSSION
Infection due to multiple antibiotic-resistant staphylococci is a growing problem. Emerging resistance to several agents, including macrolides, quinolones, ethidium bromide, antiseptics, disinfectants, and heavy metals (3, 5, 19), is of major concern. Moreover, the production of slime by S. epidermidis is associated with the clinical features of infection and enables bacteria to colonize catheters (16). Slime (biofilm) provides an ecological niche that attracts nutrients and protects against antimicrobial agents and cellular immunity (6). As a result, successful therapy of many staphylococcal infections has proven difficult to attain.
In this regard, BTs may be of benefit. Slow release of BisBAL from hydrogel-coated polyurethane rods retarded the growth of slime-forming S. epidermidis for 39 days, producing an average zone of inhibition diameter of 27.8 mm. The 1:1.5 molar ratio of bismuth to BAL was significant, since other ratios and concentrations were not as active (data not shown), nor was a catheter which was not coated with hydrogel. This molar ratio produced a neutral BT compound, thereby increasing the hydrophobicity and facilitating a linkage to the plastic surface. Medical devices that use polyurethane include central venous and hemodialysis catheters, pacemaker leads, guidewires, synthetic vascular grafts, heart valves, cardiac assist devices, artificial organs, breast implants, and wound dressings, to name a few. The duration of BT release from polyurethane devices in a liquid medium would probably have been more reflective of the in vivo situation than the agar medium used, which constitutes a potential drawback to these catheter studies.
Equally promising is the effect of BTs on staphylococcal glycocalyx. BTs suppress staphylococcal exopolysaccharides at subinhibitory concentrations. BisTOL was superior to the other BTs tested, preventing slime formation on polystyrene microtiter plates at 1.25 μM (0.25 μg/ml) after 24- and 48-h incubations. It is not known why BisTOL was more active than other BTs. BisTOL is the only aromatic BT tested, which may conceivably boost activity against gram-positive bacteria. The antibiofilm properties of BisTOL also compare favorably to those of silver (Table 6). BisTOL inhibited biofilms at 0.25 μg/ml versus 94 μg of silver/ml. The effect of silver also appeared to be on growth rather than on biofilm formation, whereas BisTOL suppressed biofilm even in the presence of growth (Table 4).
For comparison, Klebsiella pneumoniae capsule expression was inhibited by BisBAL or BisEDT at ≤1 μg/ml (9). Glycocalyx inhibition by BTs thus appears to be broad spectrum in that it affects both gram-positive and gram-negative bacteria. Glycocalyx inhibition is achievable in defined or complex media, though some medium effects have been documented. The selection of BT may be important, depending on the bacteria involved.
At higher concentrations, BTs inhibited all staphylococci tested, including resistant strains. Staphylococci resistant to various antiseptics and antibiotics were tested for sensitivity to BTs. None were resistant to BTs, including methicillin-resistant strains. Bacteria resistant to mupirocin, triclosan, and quaternary ammonium compounds were sensitive to BTs. Thus no cross-resistance was noted. BTs readily gain entry into bacteria, due to their cationic detergentlike structure, and interfere with redox enzymes (8, 13). Exopolysaccharide expression is energy intensive and is inhibited early on, due to a rapid drop in intracellular ATP levels (unpublished data).
Several BTs were strongly bactericidal. The MBC against S. aureus was, on average, twofold greater than the MIC. BisEDT was not appreciably bactericidal, though it was quite effective at inhibiting growth. Generally speaking, BTs were more active against the staphylococci than against streptococci or gram-negative bacteria (Table 1) (8).
The unique bacteriostatic, bactericidal, and antibiofilm properties of BTs may be useful to prevent or treat staphylococcal infections. Against staphylococci, BTs are potent agents, show no cross-resistance, are released slowly from implants for long-term antisepsis, and retard slime expression at safe concentrations. Their broad-spectrum effects may render BTs useful as anti-infectives and preservatives against bacteria in a variety of settings.
REFERENCES
- 1.Agocs L, Briand G G, Burford N, Cameron T S, Kwiatkowski W, Robertson K N. The structurally flexible bicyclic bis(2-hydroxyethanethiolato)bismuth(III) complex: a model for asymmetric monoanionic chelation of bismuth(III) Inorg Chem. 1997;36:2855–2860. doi: 10.1021/ic961262y. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Yamasaki O, Kanzaki H, Tada J, Arata J. Effects of sucrose and silver on Staphylococcus aureus biofilms. J Antimicrob Chemother. 1998;42:629–634. doi: 10.1093/jac/42.5.629. [DOI] [PubMed] [Google Scholar]
- 3.Archer G L, Climo M W. Antimicrobial susceptibility of coagulase-negative staphylococci. Antimicrob Agents Chemother. 1994;38:2231–2237. doi: 10.1128/aac.38.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldassarri L, Simpson W A, Donelli G, Christensen G D. Variable fixation of staphylococcal slime by different histochemical fixatives. Eur J Clin Microbiol Infect Dis. 1993;12:866–868. doi: 10.1007/BF02000411. [DOI] [PubMed] [Google Scholar]
- 5.Boyce J M. Methicillin-resistant Staphylococcus aureus: detection, epidemiology, and control measures. Infect Dis Clin N Am. 1989;3:901–913. [PubMed] [Google Scholar]
- 6.Costerton J W, Cheng K J, Geesey G G, Ladd T I, Nickel J C, Dasgupta M. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- 7.Deighton M, Borland R. Regulation of slime production in Staphylococcus epidermidis by iron limitation. Infect Immun. 1993;61:4473–4479. doi: 10.1128/iai.61.10.4473-4479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domenico P, Salo R J, Novick S G, Schoch P E, Van Horn K, Cunha B A. Enhancement of bismuth antibacterial activity with lipophilic thiol chelators. Antimicrob Agents Chemother. 1997;41:1697–1703. doi: 10.1128/aac.41.8.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domenico P, Tomas J M, Merino S, Rubires X, Cunha B A. Surface antigen exposure by bismuth-thiol suppression of Klebsiella pneumoniae capsular polysaccharide. Infect Immun. 1999;67:664–669. doi: 10.1128/iai.67.2.664-669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eykyn S J. Infection and intravenous catheters. J Antimicrob Chemother. 1984;14:23–25. doi: 10.1093/jac/14.3.203. [DOI] [PubMed] [Google Scholar]
- 11.Haslett T M, Isenberg H D, Hilton E, Tucci V, Kay B G, Vellozzi E M. Microbiology of indwelling central intravascular catheters. J Clin Microbiol. 1988;26:696–701. doi: 10.1128/jcm.26.4.696-701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kluytmans J, Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahony D E, Lim-Morrison S, Bryden L. Antimicrobial activities of synthetic bismuth compounds against Clostridium difficile. Antimicrob Agents Chemother. 1999;43:582–588. doi: 10.1128/aac.43.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maki D G, Stolz S M, Wheeler S, Mermel L A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: a randomized, controlled trial. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Needham C A, Stempsey W. Incidence, adherence and antibiotic resistance of coagulase-negative staphylococcus species causing human disease. Diagn Microbiol Infect Dis. 1984;2:293–299. doi: 10.1016/0732-8893(84)90060-9. [DOI] [PubMed] [Google Scholar]
- 16.Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- 17.Raad I, Darouiche R, Hachem R, Mansouri M, Bodey G P. The broad spectrum activity and efficacy of catheters coated with minocycline and rifampicin. J Infect Dis. 1996;73:418–424. doi: 10.1093/infdis/173.2.418. [DOI] [PubMed] [Google Scholar]
- 18.Rupp M E, Ulphani J S, Fey P D, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutination of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model. Infect Immun. 1999;67:2627–2632. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasatsu M, Shibata Y, Noguchi N, Kono M. High-level resistance to ethidium bromide and antiseptics in Staphylococcus aureus. FEMS Microbiol Lett. 1992;93:109–114. doi: 10.1016/0378-1097(92)90514-o. [DOI] [PubMed] [Google Scholar]
- 20.Sheagren J N. Staphylococcus aureus: the persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. , 1437–1442. [DOI] [PubMed] [Google Scholar]
- 21.Shuter J, Hatcher V B, Lowy F D. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thakker M, Park J-S, Carey V, Lee J C. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–5189. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomasz A. Multiple-antibiotic resistant pathogenic bacteria: a report on the Rockefeller University Workshop. N Engl J Med. 1994;330:1247–1251. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 24.Waldvogel F A. Staphylococcus aureus. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practices of infectious diseases. 4th ed. New York, N.Y: Churchill Livingstone; 1995. pp. 1754–1777. [Google Scholar]