Abstract
The heterogeneous nature of children with symptoms of autism spectrum disorder (ASD) makes it difficult to identify risk factors and effective treatment options. We sought to identify behavioral and developmental features that best define the heterogeneity and homogeneity in 2–5-year-old children classified with ASD and subthreshold ASD characteristics. Children were enrolled in a multisite case–control study of ASD. Detailed behavioral and developmental data were gathered by maternal telephone interview, parent-administered questionnaires, child cognitive evaluation, and ASD diagnostic measures. Participants with a positive ASD screen score or prior ASD diagnosis were referred for comprehensive evaluation. Children in the ASD group met study criteria based on this evaluation; children who did not meet study criteria were categorized as having subthreshold ASD characteristics. There were 1480 children classified as ASD (81.6% boys) and 594 children classified as having subthreshold ASD characteristics (70.2% boys) in the sample. Factors associated with dysregulation (e.g., aggression, anxiety/depression, sleep problems) followed by developmental abilities (e.g., expressive and receptive language skills) most contributed to heterogeneity in both groups of children. Atypical sensory response contributed to homogeneity in children classified as ASD but not those with subthreshold characteristics. These findings suggest that dysregulation and developmental abilities are clinical features that can impact functioning in children with ASD and other DD, and that documenting these features in pediatric records may help meet the needs of the individual child. Sensory dysfunction could be considered a core feature of ASD and thus used to inform more targeted screening, evaluation, treatment, and research efforts.
Keywords: autism, characteristics, heterogeneity, homogeneity, phenotypes
Lay summary
The diverse nature of autism spectrum disorder (ASD) makes it difficult to find risk factors and treatment options. We identified the most dissimilar and most similar symptom(s) in children classified as ASD and as having subthreshold ASD characteristics. Factors associated with dysregulation and developmental abilities contributed to diversity in both groups of children. Sensory dysfunction was the most common symptom in children with ASD but not those with subthreshold characteristics. Findings can inform clinical practice and research.
INTRODUCTION
Autism spectrum disorder (ASD) is a developmental disability characterized by persistent deficits in social interaction and communication and the presence of restricted interests and repetitive behaviors (RRB). The Centers for Disease Control and Prevention report that ASD is recognized in about 1 in 44 children in the United States (Maenner et al., 2020), although prevalence estimates vary according to methodology and geographic locale. ASD often co-occurs with other behavioral, developmental, medical, and psychiatric conditions (Hyman et al., 2020; Levy et al., 2010). The developmental trajectory of ASD can vary widely with some individuals improving in one or both diagnostic domains while others remain stable or worsen over time (Fountain et al., 2012). The complex nature of ASD suggests that it is likely associated with multiple biological and environmental risk factors that interact to influence presentation and course (Chaste & Leboyer, 2012). This type of phenotypic heterogeneity complicates the search for risk factors and effective treatment options.
Some researchers have attempted to simplify ASD phenotypes to guide screening, diagnosis, and treatment efforts and inform etiologic research. Most of these analyses have utilized data reducing and/or data portioning techniques (e.g., cluster analysis, latent class analysis, factor analysis) at a time when ASD symptoms are first recognized and/or diagnosed (Frazier et al., 2008, 2012; Georgiades et al., 2013; Hu & Steinberg, 2009; Kim et al., 2016; Munson et al., 2008; Sacco et al., 2012; Stevens et al., 2000; Zheng et al., 2020). These techniques assume that ASD represents discrete underlying conditions that emerge based on patterns of observed characteristics. The consensus from available studies is that there are between two and four subgroups of preschool-age children with ASD defined by a severity gradient (i.e., subgroups are ordered according to scaled aggregate performance across different measures). Components of this severity gradient depend on which variables were included in the study, but often represent measures of developmental functioning (e.g., cognitive abilities) and dysregulation (aggression, anxiety/depression, sleep problems) (Hu & Steinberg, 2009; Sacco et al., 2012; Stevens et al., 2000). Conversely, some studies report that subgroups do not differ on measures of sensory dysfunction (Sacco et al., 2012; Zheng et al., 2020).
Yet more than 20 years of subtyping analyses have yielded little evidence of their clinical utility or predictive validity. Most have not been replicated and the number of distinct ASD subgroups is variable across analyses. It is unclear whether subgroups are stable over time and how they relate to etiological underpinnings. Other limitations of previous subgroup analyses are the wide ranges of participant ages (Frazier et al., 2008, 2012; Hu & Steinberg, 2009), restricted regional variability (Kim et al., 2016; Sacco et al., 2012), and inclusion of data from only one parent interview (Frazier et al., 2008; Georgiades et al., 2013; Hu & Steinberg, 2009). Consequently, more fruitful research endeavors could define the clinical value of identifying specific features that contribute to heterogeneity in diverse samples of children with ASD, and how features that contribute to homogeneity can help prioritize screening, evaluation, treatment, and risk factor research.
An alternate approach to identifying constellations of ASD symptoms is to quantify the most distinguishing and common features of preschool children with ASD versus those with subthreshold characteristics, or how specific features explain variance from the most common ASD profile. Quantifying the most discriminating features would highlight associated clinical symptoms that can impact functioning and therefore be documented in pediatric records to best meet the unique needs of the individual child. Quantifying the most common features—especially those most common in children with only well-defined ASD—could promote more informed and targeted screening, evaluation, treatment, and research efforts that focus on those common features. An enumeration approach may also facilitate data replication, and encourage future longitudinal analyses on the stability and predicative validity of identified features over time.
The Study to Explore Early Development (SEED) is a multisite, case–control study of preschool children designed to investigate the behavioral manifestation and risk factors of ASD. At the time of this study, the SEED sample was comprised of almost 1500 children classified as ASD and almost 600 children classified as having subthreshold characteristics after a comprehensive child evaluation. A previous latent class analysis on a sample of those children revealed four subgroups that diverged on measures of dysregulation and developmental functioning and converged on measures of sensory dysfunction (Wiggins et al., 2017). However, like other subgroup attempts, the number of classes was not replicated even though the pattern of data was similar in multiple analyses (unpublished data). The same data has not yet been used to quantify the most distinguishing and common features of preschool children with ASD versus those with subthreshold characteristics and determine if these findings can be replicated.
The objectives of our study were to use SEED data to: (1) define behavioral and developmental features that explain the most variance in preschool-age children classified as ASD versus those with subthreshold ASD characteristics, (2) define behavioral and developmental features most common among these two groups of children, and (3) explore whether results can be replicated across independent samples of children. Based on previous research, we hypothesized that heterogeneity would be defined by developmental abilities and dysregulation in both study groups, and homogeneity would be defined by sensory dysfunction only within the ASD group.
METHOD
Participants were children classified as having ASD or subthreshold characteristics in SEED. This analysis includes children enrolled between 2007 and 2011 (SEED1) or 2012–2016 (SEED2) in communities in California, Colorado, Georgia, Maryland, North Carolina, and Pennsylvania. To be eligible for the study, a child had to be 2–5 years of age at enrollment, born and residing in one of the study catchment areas, and living with a caregiver who was competent to communicate in English or, in California and Colorado, English, or Spanish. The SEED protocol was approved by Institutional Review Boards at each study site.
Children with a diagnosis of ASD or non-ASD developmental delay or disorder (DD) were identified from multiple educational and health providers or family or physician referral. Children with a DD diagnosis other than ASD were recruited to serve as a comparison group and to detect children with ASD symptoms who were not yet identified as having ASD. Schendel et al. (2012) provided a detailed description of eligibility criteria, ascertainment methods, enrollment methods, and data collection procedures in SEED.
Data collection included a maternal telephone interview and self-administered forms described below. Children were screened for ASD symptoms to determine procedures for an in-person assessment. All children included in this analysis received a cognitive evaluation and a standardized ASD observation, and their mothers completed a standardized ASD diagnostic interview.
Variable selection
For this study, we chose behavioral and developmental variables previously used in a latent class analysis conducted on some children with ASD in the current sample (Wiggins et al., 2017). Each of the variables used in analyses represented a construct that emerged as a significant predictor of ASD subgroup membership in previous studies (Table 1), suggesting their potential to explain variance from the most common ASD phenotype. Once a construct was identified (e.g., expressive language abilities) authors searched the SEED dataset to identify measurement sources. Continuous variables used in the SEED1 latent class analysis were dichotomized for this study to plot each datapoint among multiple axes. Cut points used to dichotomize variables were based on expert opinion (e.g., late walking), norm-based data (e.g., delays found on a standardized assessment), parent endorsement of a condition or problem (e.g., presence of sensory dysfunction), or ASD symptoms beyond the median of the ASD sample (i.e., social communication problems noted on an ASD screen). Dependent variables and cut-off scores are provided in Table 1.
TABLE 1.
Variables used in multiple correspondence analysis among preschool-aged children with autism spectrum disorder and those with subthreshold characteristics and associated references to support their inclusion in the analysis
| Multiple correspondence variables | Categorical scores used in analysis | |
|---|---|---|
| Autism Diagnostic Observation Schedule (ADOS) |
0 = Low to moderate symptoms 1 = High level of symptoms |
|
| Autism Diagnostic Interview—Revised (ADI-R) |
0 = 15 months or younger 1 = 16 months or older |
|
| 0 = Not reported 1 = Reported |
||
| Maternal interview | 0 = Not reported 1 = Reported |
|
| Child Behavior Checklist (CBCL) |
0 = t scores of 64 or lower 1 = t scores of 65 or higher |
|
| Mullen Scales of Early Learning (MSEL) |
0 = t scores of 40 or higher 1 = t scores of 39 or lower |
|
| Social Communication Questionnaire (SCQ) |
0 = Scores lower than 17 1 = Scores of 17 or higher |
Note: In general, scores of 1 represent a delay or deficit in the child.
Associated references represented as superscript numbers.
Measurement sources
Social Communication Questionnaire
The Social Communication Questionnaire (SCQ) (Rutter et al., 2003) is a 40-item standardized screen for ASD. In SEED, children with a cut-off score of ≥11 received a comprehensive developmental evaluation since this cut-off score maximizes sensitivity and specificity in preschool-age children (Allen et al., 2007). A SCQ score of less than 17 was used to determine social communication deficits relative to other children in the ASD sample (i.e., the mean and median SCQ scores among children with ASD were 17.4 and 17.0, respectively).
The SEED maternal interview
Responses on a comprehensive interview determined maternal age, education, and race/ethnicity. Mothers comprised 99% of respondents. Mothers were also asked if their child was ever diagnosed by a healthcare professional with epilepsy or seizure disorder. Positive endorsement determined the presence of seizure disorder in the child.
Child Behavior Checklist/1½–5 years
Mothers completed the Child Behavior Checklist (CBCL) (Achenbach, 2013) to assess child behavior problems. The CBCL is a widely used standardized instrument that measures symptoms of aggression, anxiety/depression, attention problems, emotional reactivity, somatic complaints, and sleep problems in the child. CBCL t scores of 65 or higher indicate borderline to clinically significant problems and were used to define the presence of behavior problems in this study.
Mullen Scales of Early Learning
The Mullen Scales of Early Learning (MSEL) (Mullen, 1995) is a standardized in-person evaluation of the early learning abilities of young children in four areas of functioning: expressive language, receptive language, fine motor, and visual reception skills. The MSEL yields domain t scores that have a mean of 50 and SD of 10. Children with a MSEL domain t score of 40 or below were classified as having below average functioning in that domain. Children with an overall standard score (mean 100 and SD 15) below 70 were classified as having intellectual disability (ID).
Autism Diagnostic Interview—Revised
The Autism Diagnostic Interview (ADI-R) (Lord et al., 1994) is a comprehensive semi-structured interview used to classify children as ASD or non-ASD. Mothers reported age at verbal language development (i.e., first meaningful word[s]) and age the child “first walked independently without holding on.” Mothers also reported the presence or absence of each of the following: history of regression, insistence on sameness (i.e., compulsions/rituals, difficulties with minor changes in routines, and resistance to trivial changes in the environment), repetitive behavior with objects, repetitive motor mannerisms (i.e., finger mannerisms and other complex mannerisms), restricted interests (i.e., unusual preoccupations, circumscribed interests, and unusual attachment to objects), self-injurious behavior, and unusual sensory response (i.e., unusual sensory interests, undue sensitivity to noise, and negative response to specific sensory stimuli).
Autism Diagnostic Observation Schedule
The Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1999) is a standardized diagnostic observation used to classify children as ASD or non-ASD. During an ADOS administration, the clinician tries to elicit communication and social interaction using structured play activities. ADOS classification is determined by the total score, which is converted into a calibrated severity score to compare children of different ages and developmental stages. The ADOS calibrated severity score ranges from 1 to 10 with scores of 1–7 indicating low to moderate symptoms and 8–10 indicating a high level of ASD symptoms.
Study classification
SEED ASD case status was based on the results of the ADOS and ADI-R, scored without consideration of previous clinical diagnoses. Briefly, children classified as having ASD were those who met ASD criteria on both the ADI-R and ADOS, or who met ASD criteria on the ADOS and one of three alternate criteria on the ADI-R (i.e., met criteria on the social domain and was within two points on the communication domain, met criteria on the communication domain and was within two points on the social domain, or met criteria on the social domain and had two or more points noted on the behavioral domain). Using standardized diagnostic instruments to classify children with ASD in SEED offered a uniform method of characterizing ASD symptoms that can be replicated in other studies (Wiggins, Levy, et al., 2015).
Children classified as having subthreshold characteristics were those who received a comprehensive developmental evaluation due to a previous ASD diagnosis or SCQ score of ≥11 points but did not meet SEED ASD criteria. The most common diagnoses given by a healthcare provider for children with subthreshold characteristics were language delay, sensory integration disorder, motor delay, and attention deficit hyperactivity disorder (ADHD) (Wiggins, Levy, et al., 2015). They differed from children with ASD in that they had fewer ASD symptoms and behavior problems and more advanced cognitive abilities (Wiggins, Levy, et al., 2015). Children in this group had significantly more diagnoses of ADHD and significantly fewer diagnoses of sensory integration disorder and vision problems than children with ASD (Wiggins, Reynolds, et al., 2015).
SEED clinicians rated their degree of certainty the child had ASD after the in-person developmental evaluation. Ratings of 1–3 were categorized as “not certain” and ratings of 4–5 were categorized as “certain.” This measure was used as a proxy for clinical judgment. Clinicians were certain of ASD in 88.9% of children classified as ASD compared to 14.6% of those classified as having subthreshold characteristics (χ2 p < 0.01). The correlation between ASD case status and clinician certainty was 0.71 (r p < 0.01). Details on the SEED final classification algorithm can be found in Wiggins, Reynolds, et al. (2015).
Data analyses
We used multiple correspondence analysis (MCA) to address the research objectives. MCA is a statistical technique that summarizes response profiles among numerous categorical variables to reveal patterns in complex datasets (Abdi & Valentin, 2007; Beh, 2004; Clausen, 1998; Greenacre, 1994, 2007; Higgs, 1991; Sourial et al., 2010). Each data point is plotted in multi-dimensional space to determine its proximity to an origin defined by chi square distance measures. Datapoints farthest from the origin support the hypothesis of dependence (i.e., considerable variation from the most common profile). Datapoints closest to the origin support the hypothesis of independence (i.e., little variation from the most common pattern).
MCA is different from other techniques in that dimensions that produce the most variance in a sample are quantified according to their relative importance in explaining heterogeneity. Specific characteristics are also quantified according to their relative importance in explaining variance within a dimension. Numeric values of the characteristics that define heterogeneity are then used to calculate values to define homogeneity. SEED1 and SEED2 samples were analyzed separately for purposes of cross-validation of results. Five steps of MCA were followed in this study and are outlined below.
First, we identified the number of dimensions that represent the most important deviations among 23 variables (46 categories) chosen for the study (Abdi & Valentin, 2007; Beh, 2004; Clausen, 1998; Greenacre, 1994, 2007). The first dimension identifies variables that contribute to the greatest amount of variance within the data set; subsequent dimensions identify variables that explain the greatest proportion of remaining variance. The percent and cumulative percent of the inertia decomposition represent how much variance is explained by each dimension, and the total amount of variance explained considering all previous dimensions. We used a threshold of 70% of the cumulative inertia decomposition to determine the number of dimensions to retain in the analysis (Higgs, 1991).
Second, we examined coordinates that revealed the farthest distance from the origin along the first dimension. We considered the top 10% of variables (n = 5) with the largest absolute values in SEED1 that were replicated in SEED2 to define factors that most contributed to heterogeneity in the first dimension.
Third, we examined coordinates that revealed the farthest distance from the origin along any remaining dimension until 70% of the cumulative inertia decomposition or variance was explained. We considered the top 10% of variables (n = 5) with the largest absolute values in SEED1 that were replicated in SEED2 to define secondary factors that contributed to heterogeneity in subsequent dimensions.
Fourth, we named dimensions according to similarities among variables that contributed to heterogeneity in each of the retained dimensions.
Finally, we summed the absolute values of column coordinates in each dimension to determine the least amount of distance along the retained dimensions. We considered the top 10% of variables (n = 5) with the smallest absolute values along each dimension to define homogeneity, with interest in those variables that met this criterion in both the SEED1 and SEED2 datasets.
All analyses were conducted using SAS 9.4.
RESULTS
There were 707 children classified with ASD in SEED1 and 773 children classified with ASD in SEED2. There were 306 children classified with subthreshold ASD characteristics in SEED1 and 288 children classified with subthreshold ASD characteristics in SEED2. There were more boys in the SEED1 and SEED2 ASD samples compared to those with subthreshold characteristics (χ2 p < 0.01). The presence of ID was also more prevalent among children classified with ASD than those with subthreshold characteristics (χ2 p < 0.01). These and other characteristics of the study samples are provided in Table 2.
TABLE 2.
Characteristics of preschool-aged children with autism spectrum disorder and subthreshold characteristics in the Study to Explore Early Development
| Autism spectrum disorder | p value | Subthreshold characteristics | p value | |||
|---|---|---|---|---|---|---|
| SEED1 N = 707 | SEED2 N = 773 | SEED1 N = 306 | SEED2 N = 288 | |||
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Child intellectual disability | ||||||
| No | 255 (36.64) | 275 (35.99) | 0.80 | 198 (65.78) | 194 (67.36) | 0.68 |
| Yes | 441 (63.36) | 489 (64.01) | 103 (34.22) | 94 (32.64) | ||
| Child sex | ||||||
| Boys | 580 (82.04) | 628 (81.24) | 0.73 | 230 (75.16) | 187 (64.93) | <0.01 |
| Girls | 127 (17.96) | 145 (18.76) | 76 (24.84) | 101 (35.07) | ||
| Maternal age | ||||||
| 40 years or older | 190 (26.87) | 168 (21.73) | 0.14 | 67 (21.90) | 59 (20.49) | 0.87 |
| 35–39 years | 231 (32.67) | 274 (35.45) | 80 (26.14) | 74 (25.69) | ||
| 30–34 years | 188 (26.59) | 214 (27.68) | 83 (27.12) | 75 (26.04) | ||
| 29 years or younger | 98 (13.88) | 117 (15.14) | 76 (24.84) | 80 (27.78) | ||
| Maternal education | ||||||
| College/advanced degree | 361 (52.02) | 423 (54.79) | 0.31 | 104 (34.10) | 111 (38.81) | 0.46 |
| Associates degree | 219 (31.56) | 248 (32.12) | 106 (34.75) | 98 (34.27) | ||
| High school diploma | 79 (11.38) | 73 (9.46) | 53 (17.38) | 48 (16.78) | ||
| No high school diploma | 35 (5.04) | 28 (3.63) | 42 (13.77) | 29 (10.14) | ||
| Maternal race/ethnicity | ||||||
| Non-Hispanic white | 386 (55.70) | 354 (46.52) | <0.01 | 130 (42.62) | 119 (41.90) | 0.98 |
| Non-Hispanic black | 137 (19.76) | 198 (26.02) | 95 (31.15) | 92 (32.39) | ||
| Non-Hispanic other | 85 (12.27) | 88 (11.56) | 27 (8.85) | 26 (9.15) | ||
| Hispanic | 85 (12.27) | 121 (15.90) | 53 (17.38) | 47 (16.55) | ||
Note: 8 children from SEED1 and 9 children from SEED2 were missing data on intellectual disability; 13 mothers from SEED1 and 1 mother from SEED2 were missing data on education; 14 mothers from SEED1 and 12 mothers from SEED2 were missing data on race/ethnicity.
Among children classified with ASD, the 70% threshold of cumulative inertia was achieved with two dimensions. Similarly, among children classified with subthreshold characteristics, the 70% threshold of cumulative inertia was achieved with two dimensions. These results indicate that two dimensions explained at least 70% of the variance in symptoms among children classified with ASD and in those with subthreshold characteristics, and the addition of other dimensions did not significantly contribute to explaining heterogeneity in ASD phenotypes. We therefore retained two dimensions for subsequent analyses (Table 3).
TABLE 3.
Adjusted inertia and percentage of inertia explained in multiple correspondence analysis among preschool-aged children with autism spectrum disorder and those with subthreshold characteristics in the Study to Explore Early Development
| Autism spectrum disorder | |||
|---|---|---|---|
| SEED1 sample | |||
| Adjusted inertia | Percent of inertia | Cumulative percent of inertia | |
| Dimension 1 | 0.01 | 52.41 | 52.41 |
| Dimension 2 | 0.01 | 27.50 | 79.91 |
| Dimension 3 | <0.01 | 2.73 | 82.64 |
| SEED2 sample | |||
| Adjusted inertia | Percent of inertia | Cumulative percent of inertia | |
| Dimension 1 | 0.01 | 49.24 | 49.24 |
| Dimension 2 | 0.01 | 30.62 | 79.86 |
| Dimension 3 | <0.01 | 3.02 | 82.88 |
| Subthreshold characteristics | |||
| SEED1 sample | |||
| Adjusted inertia | Percent of inertia | Cumulative percent of inertia | |
| Dimension 1 | 0.01 | 50.61 | 50.61 |
| Dimension 2 | 0.01 | 25.14 | 75.75 |
| Dimension 3 | <0.01 | 6.08 | 81.85 |
| SEED2 sample | |||
| Adjusted inertia | Percent of inertia | Cumulative percent of inertia | |
| Dimension 1 | 0.01 | 65.65 | 65.65 |
| Dimension 2 | 0.01 | 15.16 | 80.80 |
| Dimension 3 | <0.01 | 2.90 | 83.70 |
Note: The cumulative inertia decomposition represents the total percent of phenotypic variance explained by chosen dimensions. Bold indicates the number of dimensions retained, which were those that defined a threshold of 70% of the cumulative inertia decomposition (Higgs, 1991).
Dimension 1 explained between 49% and 65% of the variance in ASD symptoms (Table 3). Factors that most contributed to phenotypic heterogeneity in Dimension 1 for both SEED phases and for both study groups were anxiety/depression, aggression, and sleep problems. For children classified with ASD, the absence of sensory dysfunction also contributed to heterogeneity in Dimension 1 (Tables 4 and S1 and Figure 1). For children classified with subthreshold characteristics, emotional reactivity also contributed to heterogeneity in Dimension 1 (Tables 4 and S1). We therefore refer to Dimension 1 as “dysregulation.”
TABLE 4.
Variables that most contributed to the heterogeneity and homogeneity among preschool-aged children with autism spectrum disorder and those with subthreshold characteristics in the Study to Explore Early Development Phase1 and Phase2
| Autism spectrum disorder | Subthreshold characteristics | ||||
|---|---|---|---|---|---|
| SEED1 | SEED2 | SEED1 | SEED2 | ||
| Heterogeneity Dimension 1: Dysregulation | Anxiety/depression | + | + | + | + |
| Sleep problems | + | + | + | + | |
| Aggression | + | + | + | + | |
| No sensory dysfunction | + | + | |||
| Emotional reactivity | + | + | |||
| Heterogeneity Dimension 2: Developmental abilities | Expressive language skills | + | + | + | + |
| Receptive language skills | + | + | + | + | |
| Fine motor skills | + | + | |||
| Visual reception skills | + | + | |||
| Visual reception delays | + | + | |||
| Homogeneity | Sensory dysfunction | + | + | ||
| Age of walking 15 months or younger | + | + | |||
| Absence of epilepsy/seizure disorder | + | + | + | + | |
| Absence of developmental regression | + | + | |||
| Absence of moderate–severe ASD symptoms | + | + | |||
Note: Developmental skills in Dimension 2 in the average range unless otherwise noted.
FIGURE 1.
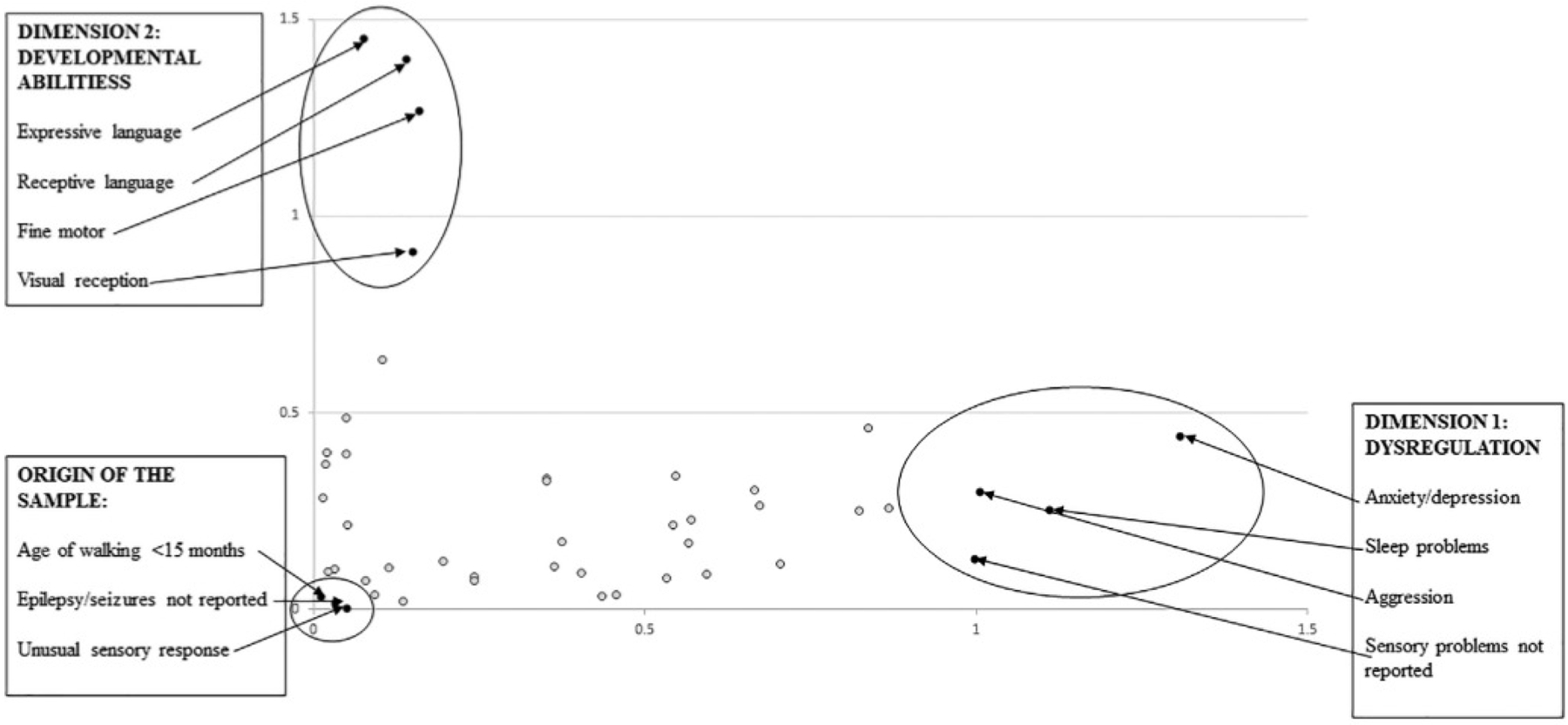
Variables that most contributed to the heterogeneity (i.e., those furthest from the origin) and homogeneity (i.e., those closest to the origin) of preschool-aged children with autism spectrum disorder in the Study to Explore Early Development combined sample
Dimension 2 explained between 15% and 30% of the variance in ASD symptoms (Table 3). Factors that most contributed to phenotypic heterogeneity in Dimension 2 for both SEED phases and for both study groups were expressive and receptive language skills. For children classified with ASD, the fine motor skills and visual reception skills also contributed to heterogeneity in Dimension 2 (Tables 4 and S2 and Figure 1). For children classified with subthreshold ASD characteristics, visual reception delays also contributed to heterogeneity in Dimension 2 (Tables 4 and S2). We therefore refer to Dimension 2 as “developmental abilities.”
Homogeneity was defined by identifying coordinates closest to the origin of all data points when plotted together (i.e., those with the smallest absolute values along each dimension). Interestingly, the absence of certain features was most prevalent in both groups of children: absence of walking later than 15 months and absence of epilepsy/seizure disorder for children classified with ASD and absence of developmental regression, epilepsy/seizure disorder, and moderate–severe ASD symptoms for children classified with subthreshold characteristics. The presence of sensory dysfunction was the only symptom that contributed to homogeneity in children classified with ASD—but not those with subthreshold characteristics—in both SEED phases (Tables 4 and S3 and Figure 1).
DISCUSSION
Ours is the first study to identify and replicate specific behavioral and developmental features that best define the heterogeneity and homogeneity among a large regionally diverse sample of preschool children classified with ASD and with subthreshold characteristics. Factors associated with dysregulation (i.e., anxiety/depression, aggression, and sleep problems) were responsible for 49%–65% of the variance and factors associated with developmental abilities (i.e., expressive and receptive language skills) were responsible for an additional 15%–30% of the variance in both groups of children. The presence of sensory dysfunction was the only symptom that defined homogeneity among children classified with ASD in both SEED phases; this finding did not apply to children with subthreshold characteristics. These results support previous analyses and suggest that dysregulation and developmental abilities are associated clinical features that can impact functioning among children with ASD and other DD and sensory dysfunction could be considered a core feature of ASD (Bitsika et al., 2018).
Importantly, factors associated with dysregulation and factors associated with developmental abilities clustered within dimensions in this analysis and previous subgroup analyses (Frazier et al., 2012; Kim et al., 2016; Munson et al., 2008; Stevens et al., 2000). Our findings enhance previous research in several ways. First, we were able to demonstrate independence of these dimensions by quantifying their relative importance in dissecting heterogeneity in early childhood phenotypes. Second, we were able to quantify specific factors within each dimension that best define this heterogeneity. Third, we were able to confirm that similar factors contributed to heterogeneity among children who meet stringent ASD criteria and those with subthreshold characteristics. These findings are valuable when promoting systematic documentation of characteristics that highlight the unique needs of preschool children with atypical development.
Signs of dysregulation are particularly important to evaluate and document in clinical records given these symptoms contribute to at least 49% of the variance in early childhood phenotypes. Anxiety/depression, aggression, and sleep problems are much more prevalent in children with ASD symptoms than other children and can emerge early in life (Reynolds et al., 2019; Wiggins, Levy, et al., 2015). However, they may not be systematically recorded without consensus recommendations and diagnostic specifiers. Adding specifiers related to dysregulation to diagnostic criteria for childhood disorders—like those for intellectual impairment; language impairment; catatonia; and cooccurring, medical, mental, or behavioral disorders for ASD—could encourage clinical monitoring and tailor treatments that warrant specialized care. Distinguishing sleep problems from other medical conditions and basing specifiers on symptoms rather than diagnosed disorders are especially relevant to preschool-age children. This approach would retain “lumping” diagnostic categories similar to current taxonomies but “splitting” diagnostic specifiers and measures of levels of support needed by the child (Abu-Akel et al., 2019).
Unusual sensory response was the most common deficit among preschool children classified with ASD but not children classified with subthreshold characteristics in SEED. This finding is reinforced in several other studies and self-reports of individuals with ASD and supports the addition of sensory dysfunction to ASD diagnostic criteria. More importantly, this finding highlights the need to screen, evaluate, and treat sensory concerns early in life to avert potential developmental debilities that may be caused by sensory deficits. The Sensory Processing Measure (SPM) (Parham et al., 2007), Sensory Processing Measure—Preschool (SPM-P (Parham & Ecker, 2010), and Sensory Profile—2nd edition (SP-2) (Dunn, 2010) are brief parent-report screens appropriate for children 2–12 years (SPM and SPM-P) and birth through 14 years (SP-2). An occupational therapist trained in sensory evaluation and treatment can provide a thorough assessment and develop an individualized treatment plan for the child (Schaaf et al., 2014). Therapies designed to address sensory dysfunction can improve individualized treatment goal attainment for young children with ASD (Pfeiffer et al., 2011; Schaaf et al., 2014).
Our findings also emphasize the need to better characterize sensory problems in young children with ASD and risk factors for their development. Previous studies have found a range of pre-, peri-, and neo-natal factors associated with sensory dysfunction: assisted delivery, breech presentation, low birthweight, infections during pregnancy, lead exposure, maternal stress during pregnancy, prenatal alcohol exposure, placental abruptions, and premature birth (Cai et al., 2019; Crepeau-Hobson, 2009; Crozier et al., 2016; Fieldsted & Xue, 2019; Jirikowic et al., 2020; May-Benson et al., 2009; Ryckman et al., 2017; Szczepara-Fabian et al., 2018; Wickremasinghe et al., 2013). There is a dose–response relationship in terms of the number of exposures and the presence of sensory dysfunction in the child. Prenatal alcohol exposure and maternal stress during pregnancy have also been shown to increase sensory processing deficits in non-human primates (Schneider et al., 2008). Future research is needed to determine risk factors for sensory dysfunction most relevant to preschool children and how to prevent or diminish their developmental consequences.
Epilepsy/seizure disorder is more common among individuals with ASD (Lukmanji et al., 2019). Our analyses found that absence of epilepsy/seizure disorder contributed to homogeneity in the ASD sample. Yet most individuals with ASD who develop epilepsy start having seizures after 10 years of age (Bolton et al., 2011), and our sample was restricted to preschool age children. Age of walking at 15 months or younger also contributed to homogeneity in the ASD sample. This finding could suggest that evaluation of gross motor abilities, albeit important in general pediatric examination, is less useful than evaluation of other developmental abilities in detecting and characterizing ASD in preschool-age samples.
MCA is a unique analytic approach that utilizes categorical data. There are benefits of using MCA over other analytic techniques to explore the research questions posed in this study. Examining relationships between categories of variables can help identify monotonic or nonmonotonic relationships. Other techniques such as factor analysis or principal components analysis are appreciative of only uniform relationships (monotonic positive or negative relationship) across continuous variables. Individual categories of variables on extracted MCA dimensions can be displayed in a two-dimensional plot, which facilitates interpretation of independent relationships between dimensions and weighs schemes of categories along those dimensions. Consequently, MCA quantifies factors that contribute to variance in ASD phenotypes based on chi square distance measures from the most common ASD profile. However, we recognize individual variation within each of the factors dichotomized for this study. We also recognize that variables used in this analysis represent broad constructs that lack developmental specificity. Our approach to dissecting ASD phenotypes can therefore be considered a first step in explaining heterogeneity and homogeneity in ASD and identifying specific characteristics that warrant for further study.
It is important to remember that any study of ASD phenotypes relies on the measures used to operationalize features of the disorder, as well as the statistical application. The measures used in this study are common in ASD research and clinical practice but are not without limitations. Most of the variables used in our MCA analysis were from the ADI-R, CBCL, and MSEL. ADI-R items were used to define features of restricted interests and repetitive behaviors; however, internal consistency of the ADI-R behavioral domain is weaker than the social or communication domains (de Bildt et al., 2013). One study found more measurement invariance in the CBCL among samples of children with ASD and ID than ASD alone (Dovgan et al., 2019). Another study noted that most young children with ASD show general developmental delays on the MSEL and therefore questioned its clinical sensitivity (Burns et al., 2013). Improvements in measurement sources are needed to better define the developmental constructs measured in diverse ASD samples.
There are several limitations of our study. Data were collected at a specific point in time and cannot be generalized to other stages of development. Many of the variables included in analyses were based on parent-report and not direct examination of the child. Some variables were taken from instruments used to select the ASD sample, although children varied on individual characteristics despite ASD versus subthreshold status. Most children with ASD in our sample (63.4%) were classified with ID, although measures of cognitive performance become more reliable after 6 years of age. Results may therefore be less applicable to children without cognitive delays measured in preschool and identified with ASD later in life. Finally, our study focused on ASD phenotypes based on behavioral and developmental data without consideration of their biological foundations. Future studies could examine whether the disparate dimensions and features within those dimensions found in this study are related to different biological mechanisms and how those mechanisms are predictive of ASD variability over time and at the individual level (Wolfers et al., 2019).
Regardless of these limitations, our findings are the first to identify specific factors that best define heterogeneity and homogeneity in children with ASD and those with subthreshold characteristics that can be replicated across independent samples of children. These findings can be used to inform clinical practice on features relevant to both children with ASD and other DD that impact functioning (i.e., dysregulation and developmental abilities) and guide research on features most shared among children with ASD (i.e., sensory dysfunction). We used data from a large multisite case–control study that classified children with ASD according to standardized diagnostic instruments and collected detailed phenotypic information on each child. These strengths support the robustness of our results and their implications for clinical practice and future research.
In conclusion, the phenotypic diversity in preschool children with ASD symptoms extends beyond diagnostic boundaries and is best defined by factors associated with dysregulation followed by developmental abilities. Atypical sensory response seems to be more relevant to those who meet stringent ASD criteria versus those with subthreshold characteristics and may therefore represent a core feature of ASD. Our findings support systematic documentation of factors that contribute to diversity of early childhood phenotypes and highlight the need to promote early evaluation and treatment of sensory concerns. These findings could also guide future research on risk factors that lead to sensory dysfunction.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank SEED study staff and the children and families who participated in the study. We would also like to thank the SEED Data Coordinating Center team at the Clinical and Translational Sciences Institute of Michigan State University for their support throughout this study. This publication was supported by cooperative agreements from the Centers for Disease Control and Prevention (CDC): U10DD000180–U10DD000184, U01000746–U01000750, and U10DD000901. The authors do not have any conflicts of interest to disclose. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding information
Centers for Disease Control and Prevention, Grant/Award Numbers: U10DD000180, U10DD000181, U10DD000182, U10DD000183, U10DD000184, U01000746, U01000747, U01000748, U01000749, U01000750, U10DD000901
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- Abdi H, & Valentin D (2007). Multiple correspondence analysis. In Salkind NJ (Ed.), Encyclopedia of measurement and statistics. Sage. [Google Scholar]
- Abu-Akel A, Allison C, Baron-Cohen S, & Heinke D (2019). The distribution of autistic traits across the autism spectrum: Evidence for discontinuous and dimensions subpopulations underlying the autism continuum. Molecular Autism, 10(24), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach T (2013). Child Behavior Checklist. Achenbach System of Empirically Based Assessment. [Google Scholar]
- Allen C, Silove N, Williams K, & Hutchins P (2007). Validity of the Social Communication Questionnaire in assessing risk of autism in preschool children with developmental problems. Journal of Autism and Developmental Disorders, 37(7), 1272–1278. [DOI] [PubMed] [Google Scholar]
- Baghdadli A, Assouline B, Sonié S, Pernon E, Darrou C, Michelon C, Picot MC, Aussilloux C, & Pry R (2012). Developmental trajectories of adaptive behaviors from early childhood to adolescence in a cohort of 152 children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42, 1314–1325. [DOI] [PubMed] [Google Scholar]
- Beh EJ (2004). Simple correspondence analysis: A bibliographic review. International Statistics Review, 72(2), 257–284. [Google Scholar]
- Bitsika V, Arnold WA, & Sharpley CF (2018). Cluster analysis of autism spectrum disorder symptomology: Qualitative distinct sub-types or quantitative degrees of severity of a single disorder? Research in Developmental Disabilities, 76, 65–75. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, & Rutter M (2011). Epilepsy in autism: Features and correlates. British Journal of Psychiatry, 198(4), 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns T, King T, & Spencer K (2013). Mullen scales of early learning: The utility in assessing children diagnosed with autism spectrum disorder, cerebral palsy, and epilepsy. Applied Neuropsychology: Child, 2, 33–42. [DOI] [PubMed] [Google Scholar]
- Cai H, Xu X, Zhang Y, Cong X, Lu X, & Huo X (2019). Elevated levels from e-waste exposure are linked to sensory integration difficulties in preschool children. Neurotoxicology, 71, 50–158. [DOI] [PubMed] [Google Scholar]
- Chaste P, & Leboyer M (2012). Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues in Clinical Neuroscience, 14, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen SE (1998). Applied correspondence analysis. Sage. [Google Scholar]
- Cohen IL, Liu X, Hudson M, Gillis J, Cavalari RN, Romanczyk RG, Karmel BZ, & Gardner JM (2016). Using the PDD behavior inventory as a level 2 screener: A classification and regression trees analysis. Journal of Autism and Developmental Disorders, 46, 3006–3022. [DOI] [PubMed] [Google Scholar]
- Crepeau-Hobson MF (2009). The relationship between perinatal risk factors and sensory processing difficulties in preschool children. Journal of Developmental and Physical Disability, 21, 315–328. [Google Scholar]
- Crozier SC, Goodson JZ, Mackay ML, Synnes AR, Grunau RE, Miller SP, & Zwicker JG (2016). Sensory processing patterns in children born very preterm. American Journal of Occupational Therapy, 70(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Cuccaro ML, Tuchman RF, Hamilton KL, Wright HH, Abramson RK, Haines JL, Gilbert JR, & Pericak-Vance M (2012). Exploring the relationship between autism spectrum disorder and epilepsy using latent class cluster analysis. Journal of Autism and Developmental Disorders, 42, 1630–1641. [DOI] [PubMed] [Google Scholar]
- de Bildt A, Oosterling I, van Lang N, Kuijper S, Dekker V, Sytema S, Oerlemans AM, van Steijn DJ, Visser JC, Rommelse NN, Minderaa RB, van Engeland H, van der Gaag RJ, Buitelaar JK, & de Jonge MV (2013). How to use the ADI-R for classifying autism spectrum disorders? Psychometric properties of criteria from the literature in 1,204 Dutch children. Journal of Autism and Developmental Disorders, 43, 2280–2294. [DOI] [PubMed] [Google Scholar]
- Dovgan K, Mazurek M, & Hansen J (2019). Measurement invariance of the child behavior checklist in children with autism spectrum disorder with and without intellectual disability: Follow-up study. Research in Autism Spectrum Disorders, 58, 19–29. [Google Scholar]
- Dunn W (2010). Sensory profile (2nd ed.). Pearson. [Google Scholar]
- Fieldsted B, & Xue L (2019). Sensory processing in young children with fetal alcohol spectrum disorder. Physical and Occupational Therapy in Pediatrics, 39(5), 553–565. [DOI] [PubMed] [Google Scholar]
- Fountain C, Winters A, & Bearman P (2012). Six developmental trajectories characterize children with autism. Pediatrics, 129, 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Kuba CS, Sinclair L, & Rezai A (2008). Exploratory and confirmatory factor analysis of the Autism Diagnostic Interview—Revised. Journal of Autism and Developmental Disorders, 38, 474–480. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Speer L, Embacher R, Law P, Constantino J, Findling RL, Hardan AY, & Eng C (2012). Validation of proposed DSM-5 criteria for autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 51(1), 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, Boyle M, Hanna S, Duku E, Zwaigenbaum L, Bryson S, Fombonne E, Volden J, Mirenda P, Smith I, Roberts W, Vaillancourt T, Waddell C, Bennett T, Thompson A, & Pathways in ASD Study Team. (2013). Investigating phenotypic heterogeneity in children with autism spectrum disorder: A factor mixture modeling approach. Journal of Child Psychology and Psychiatry, 54, 206–215. [DOI] [PubMed] [Google Scholar]
- Greenacre MJ (1994). Multiple and joint correspondence analysis. In Greenacre MJ & Blasius J (Eds.), Correspondence analysis in the social sciences. Academic Press. [Google Scholar]
- Greenacre MJ (2007). Correspondence analysis in practice. Chapman & Hall. [Google Scholar]
- Higgs NT (1991). Practical and innovative uses of correspondence analysis. The Statistician, 40(2), 183–194. [Google Scholar]
- Hu VW, & Steinberg ME (2009). Novel clustering of items from the Autism Diagnostic Interview—Revised to define phenotypes within autism spectrum disorders. Autism Research, 2, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Levy SE, Myers SM, & AAP Council on Children with Disabilities, Section on Developmental and Behavioral Pediatrics. (2020). Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics, 145(1), e20193447. [DOI] [PubMed] [Google Scholar]
- Jirikowic TL, Thorne JC, McLaughlin SA, Waddington T, Lee A, & Hemingway S (2020). Prevalence and patterns of sensory processing behaviors in a large clinical sample of children with prenatal alcohol exposure. Research in Developmental Disabilities, 100, 103617. [DOI] [PubMed] [Google Scholar]
- Kim SH, Macari S, Koller J, & Chawarska K (2016). Examining the phenotypic heterogeneity of early autism spectrum disorder: Subtypes and short-term outcomes. Journal of Child Psychology and Psychiatry, 57(1), 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SE, Giarelli E, Lee LC, Schieve L, Kirby R, Cunniff C, Nicholas J, Reaven J, & Rice CE (2010). Autism spectrum disorder and cooccurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. Journal of Developmental and Behavioral Pediatrics, 31(4), 267–275. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M,L, & Couteur AL (1994). Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24, 659–685. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, & Risi S (1999). Autism Diagnostic Observation Schedule. Western Psychological Services. [Google Scholar]
- Lukmanji S, Manji SA, Kadhim S, Sauro KM, Wirrell EC, Kwon C-S, & Jette N (2019). The co-occurrence of epilepsy and autism: A systematic review. Epilepsy and Behavior, 98(PtA), 238–248. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, Christensen DL, Wiggins LD, Pettygrove S, Andrews JG, Lopez M, Hudson A, Baroud T, Schwenk Y, White T, Rosenberg CR, Lee L-C, Harrington RA, Huston M, … Dietz PM (2020). Prevalence of autism spectrum disorder among children aged 8 years — Autism and developmental disabilities monitoring network, 11 Sites, United States, 2016. MMWR. Surveillance Summaries, 69(4), 1–12. 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Benson TA, Koomar JA, & Teasdale A (2009). Incidence of pre-, peri-, and post-natal birth and developmental problems of children with sensory processing disorder and children with autism spectrum disorder. Frontiers in Integrative Neuroscience, 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E (1995). Mullen scales of early learning. Pearson. [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Koehler E, Lord C, Rogers S, Sigman M, Estes A, & Abbott R (2008). Evidence for latent classes of IQ in young children with autism spectrum disorder. American Journal of Mental Retardation, 113, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham DL, & Ecker C (2010). Sensory processing measure—Preschool. Pearson. [Google Scholar]
- Parham DL, Ecker C, Kuhaneck HM, Henry DA, & Glennon TJ (2007). Sensory processing measure. Pearson. [Google Scholar]
- Pfeiffer BA, Koenig K, Kinnealey M, Sheppard M, & Henderson L (2011). Effectiveness of sensory integration interventions in children with autism spectrum disorders: A pilot study. American Journal of Occupational Therapy, 65(1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AM, Soke GN, Sabourin KR, Hepburn S, Katz T, Wiggins LD, Schieve LA, & Levy SE (2019). Sleep problems in 2- to 5-year-olds with autism spectrum disorder and other developmental delays. Pediatrics, 143(3), e20180492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter MA, Bailey A, & Lord C (2003). The Social Communication Questionnaire. Western Psychological Services. [Google Scholar]
- Ryckman J, Hilton C, Rogers C, & Pineda R (2017). Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior. Early Human Development, 113, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R, Lenti C, Saccani M, Curatolo P, Manzi B, Bravaccio C, & Persico AM (2012). Cluster analysis of autistic patients based on principal pathogenetic components. Autism Research, 5, 137–147. [DOI] [PubMed] [Google Scholar]
- Schaaf RC, Benevides T, Mailloux Z, Faller P, Hunt J, van Hooydonk E, Freeman R, Leiby B, Sendecki J, & Kelly D (2014). An intervention for sensory difficulties in children with autism: A randomized trial. Journal of Autism and Developmental Disorders, 44, 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel D, DiGuiseppi C, Croen L, Fallin D, Reed P, Schieve L, Wiggins LD, Daniels J, Grether J, Levy SE, Miller L, Newschaffer C, Pinto-Martin J, Robinson C, Windham GC, Alexander A, Aylsworth AS, Bernal P, Bonner JD, … Yeargin-Allsopp M (2012). The Study to Explore Early Development (SEED): A multi-site epidemiologic study of autism by the centers for autism and developmental disabilities research and epidemiology (CADDRE) network. Journal of Autism and Developmental Disorders, 42, 2121–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Gajewski LL, Larson JA, Roberts AD, Converse AK, & DeJesus OT (2008). Sensory processing disorder in a primate model: Evidence from a longitudinal study of prenatal stress effects. Child Development, 79(1), 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourial N, Wolfson C, Zhu B, Quail J, Fletcher J, Karunananthan S, Bandeen-Roche K, Béland F, & Bergman H (2010). Correspondence analysis is a useful tool to uncover the relationships of categorical variables. Journal of Clinical Epidemiology, 63(6), 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Fein DA, Dunn M, Allen D, Waterhouse LH, Feinstein C, & Rapin I (2000). Subgroups of children with autism by cluster analysis: A longitudinal examination. Journal of the American Academy of Child and Adolescent Psychiatry, 39(3), 346–352. [DOI] [PubMed] [Google Scholar]
- Szczepara-Fabian M, Emich-Widera E, Kazek B, Kaniewska A, & Paprocka J (2018). The prenatal and perinatal risk variables of sensory processing disorder. Clinics in Mother and Child Health, 15(1), 1–5. [Google Scholar]
- Visser JC, Rommelse NJ, Lappenschaar M, Servatius-Oosterling IJ, Greven CU, & Buitelaar JK (2018). Variation in the early trajectories of autism symptoms is related to the development of language, cognition, and behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 659–668. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe AC, Rogers EE, Johnson BC, Shen A, Barkovich AJ, & Marco EJ (2013). Children born prematurely have atypical sensory profiles. Journal of Perinatology, 33(8), 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Levy SE, Daniels J, Schieve L, Croen LA, DiGuiseppi C, Blaskey L, Giarelli E, Lee LC, Pinto-Martin J, Reynolds A, Rice C, Rosenberg CR, Thompson P, Yeargin-Allsopp M, Young L, & Schendel D (2015). Autism spectrum disorder symptoms among children enrolled in the Study to Explore Early Development (SEED). Journal of Autism and Developmental Disorders, 45(10), 3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Reynolds A, Rice C, Moody EJ, Bernal P, Blaskey L, Rosenberg SA, Lee LC, & Levy SE (2015). Using standardized diagnostic instruments to classify children with autism in the Study to Explore Early Development. Journal of Autism and Developmental Disorders, 45, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Tian L, Levy S, Rice C, Lee L-C, Schieve L, Pandey J, Daniels J, Blaskey L, Hepburn S, Landa R, Edmondson-Pretzel R, & Thompson W (2017). Homogeneous subgroups of children with autism improve phenotypic characteristics in the Study to Explore Early Development. Journal of Autism and Developmental Disorders, 47, 3634–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T, Floris DL, Dinga R, van Rooij D, Isakoglou C, Kia SM, Zabihi M, Llera A, Chowdanayaka R, Kumar VJ, Peng H, Laidi C, Batalle D, Dimitrova R, Charman T, Loth E, Lai M-C, Jones E, Baumeister S, … Beckmann CF (2019). From pattern classification to stratification: towards conceptualizing the heterogeneity of Autism Spectrum Disorder. Neuroscience & Biobehavioral Reviews, 104, 240–254. 10.1016/j.neubiorev.2019.07.010 [DOI] [PubMed] [Google Scholar]
- Zheng S, Hume KA, Able H, Bishop SL, & Boyd B (2020). Exploring developmental and behavioral heterogeneity among preschoolers with ASD: A cluster analysis on principal components. Autism Research, 13(5), 796–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


