Summary
This position paper summarises the current knowledge on the epidemiology, diagnosis and management of women of childbearing age with rheumatic heart disease (RHD) in Africa, as well as the available data on their use of reproductive health services. The aim is to provide guidance to health professionals on aspects of sexual and reproductive health in women with RHD. It reviews the diagnosis, management and counselling of women with RHD throughout their reproductive life. Additionally, this publication discusses potential ways of integrating obstetric and cardiovascular care at peripheral levels of the health systems, as a way of improving outcomes and reducing maternal mortality rates related to cardiovascular disease in Africa. Finally, the article proposes responses to fulfill the actual needs for better reproductive health services and improvement in care for women with RHD.
Keywords: sexual and reproductive health, cardiovascular disease, rheumatic heart disease
The social cluster of the African Union Commission hosted a consultation with rheumatic heart disease (RHD) experts, convened by the Pan-African Society of Cardiology (PASCAR) in Addis Ababa, Ethiopia from 21 to 22 February 2015.1 The objective of this meeting was to develop a ‘roadmap’ of key actions that need to be taken by governments to eliminate acute rheumatic fever (ARF) and eradicate RHD in Africa, following a call for action the previous year.2 Seven priority areas for intervention were adopted: (1) create prospective disease registers at sentinel sites in affected countries to measure disease burden and track progress towards a reduction in mortality rate by 25% by the year 2025; (2) ensure an adequate supply of high-quality benzathine penicillin for the primary and secondary prevention of ARF/RHD; (3) improve access to reproductive health services for women with RHD and other non-communicable diseases (NCD); (4) decentralise technical expertise and technology for diagnosing and managing ARF and RHD (including cardiac ultrasound); (5) establish national and regional centres of excellence for essential cardiac surgery for the treatment of affected patients and training of cardiovascular practitioners of the future; (6) initiate national multi-sectoral RHD programmes within NCD control programmes of affected countries; and (7) foster international partnerships with multi-national organisations for resource mobilisation, monitoring and evaluation of the programme to end RHD in Africa. Since then, African Union heads of state have endorsed this Addis Ababa communique.
Following the Mosi-o-Tunya Declaration in 2014.2 PASCAR created seven taskforces as part of the plan to implement a roadmap to end ARF and RHD in Africa. These PASCAR technical groups aim to move forward each of these key areas of intervention. We present the work of the taskforce on ‘Access to reproductive health services for women with RHD and other non-communicable diseases in Africa’ here, which emanated out of face-to-face and virtual meetings.
This position paper reviews the current knowledge on reproductive care for women with RHD, particularly during their reproductive years. Its objective is to provide guidelines for (1) the use of contraceptive methods by girls and women of reproductive age with RHD; (2) antenatal care and counselling; (3) diagnosis and management of pregnant women with RHD; (4) post-delivery counselling and follow up for women with RHD. Acknowledging that sexual and reproductive health services do not function in isolation, but instead are nested within the broader health system, consideration is given to health system factors, by reflecting on what needs to be in place to adequately provide equitable care to women with RHD.
Rationale
The Millennium Development Goal 5 of achieving a 75% reduction in maternal mortality rate between 2000 and 2015 has failed. During these years, the global maternal mortality rate was reduced by only 45%.3 Around 80% of all maternal deaths occur in areas of high birthrate and low healthcare access and there is an alarming geographical disparity. Women from sub-Saharan Africa (SSA) have a 100-fold greater risk of having a pregnancyrelated death than those living in North America.4
Indirect maternal mortality is responsible for one-quarter of the total maternal mortality rate.5 Pre-existing cardiovascular disease, pregnancy-related cardiomyopathy and aggravated hypertension are among the conditions that contribute significantly to high indirect maternal mortality in low- and middle-income countries (LMIC). RHD, a disease linked to poverty, which has almost disappeared in developed countries, remains an important determinant of morbidity and mortality in LMIC, particularly in SSA where a high prevalence of RHD coincides with high maternal mortality rates. RHD contributes to almost 30% of the cardiovascular disease seen in pregnancy,6 is associated with a maternal mortality rate of 34% and is responsible for substantial foetal loss.7
The Global Rheumatic Heart Disease Registry (the REMEDY study)8 has shown a prevalence ratio of women to men of 2:1. This multi-national, hospital-based prospective registry enrolled 3 343 patients presenting at 25 hospitals, mostly from 12 African countries. Young females were in the majority (median age 28 years, females 66.2%) and had a higher prevalence of major cardiovascular complications. There was sub-optimal use of contraception, even in high-risk women. Only 5% of women with prosthetic heart valves and 2% of those with severe mitral stenosis were on contraception.
When the participating countries in REMEDY were grouped into the income categories of low-income (LIC), low- and middle-income (LMIC), and upper-middle-income (UMIC) countries, according to 2011 World Bank definitions, there was no difference in the predominance of females with regard to males in the three groups: 728/1 110 (65.8%), 867/1 370 (63%) and 616/863 (71.3%). However, a statistically significant difference was found in the proportion of women in their childbearing years between the three groups of countries, with a higher number in low- and low-middle-income countries: 86.5% in LIC, 90.3% in LMIC and 66.9% in UMIC (p < 0.01).
Among the 1 825 women of childbearing age (12–51 years) recruited into REMEDY, only 65 (3.6%) were on contraception.8 This poor provision of family planning for women with heart disease occurs in many regions of the world.9,10 Among the most important reasons for this are difficult access to health facilities and/or providers with capacity to ensure comprehensive family planning in several parts of the continent, as well as low education levels and awareness of health personnel, inadequate cardiovascular diagnosis, inadequate referral pattern and poor overall management. Long distances to health facilities and lack of funding to cover the travel fare are also important aspects of late presentation to healthcare. Lack of women empowerment, social pressure on women to conceive, and cultural aspects related to use of contraception also play a role in determining high parity.11 Health concerns related to contraception and opposition to family planning are equally leading reasons hampering women from using modern contraception.
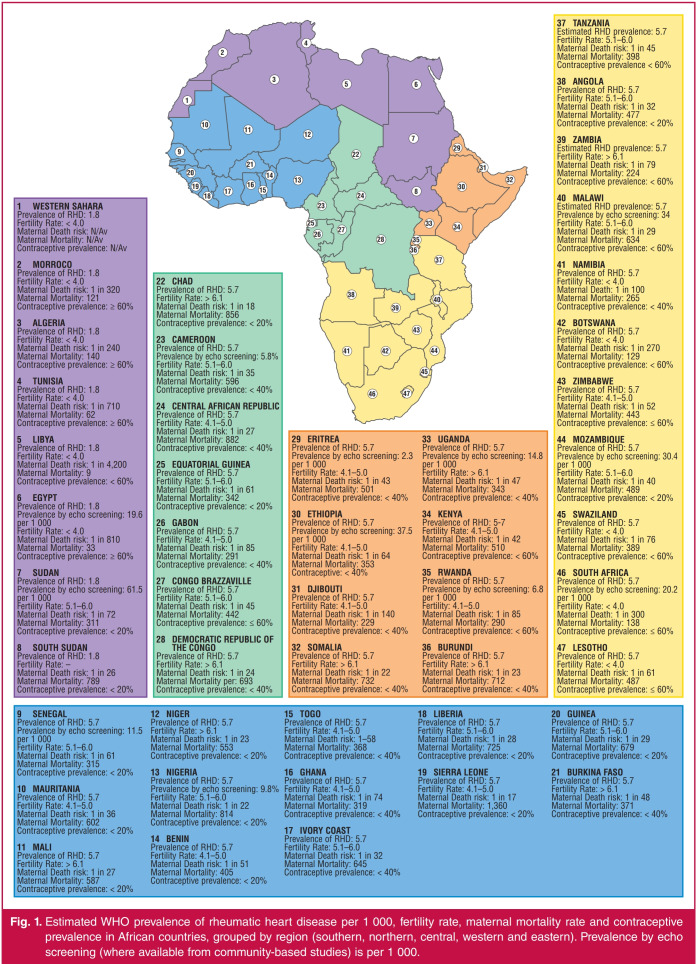
The low usage of contraception contributes to high fertility rates in Africa and explains why pregnancy in the context of RHD is very frequent in this region. In the REMEDY study, 72 women (3.7%) were pregnant at the time of enrolment, the youngest being 14 years and the oldest 51 years.8 The low use of contraceptive methods by young women and the predominance of high-risk women in the most under-resourced countries highlight the urgent need to address the reproductive health requirements of women with RHD in SSA. Fig. 1 shows the prevalence of RHD, contraceptive usage, and fertility and maternal mortality rates in the different regions of the African continent.12-23
Fig. 1.
Estimated WHO prevalence of rheumatic heart disease per 1 000, fertility rate, maternal mortality rate and contraceptive prevalence in African countries, grouped by region (southern, northern, central, western and eastern). Prevalence by echo screening (where available from community-based studies) is per 1 000.
Determinants of the low usage of reproductive health services by women with RHD in Africa are probably related to factors on the side of both the providers and the users. On the providers’ side, training of health professionals to better diagnose and manage women with RHD throughout their reproductive life is an important aspect to address. This position paper is expected to provide guidance and promote structural changes in the health services to accommodate the particular needs of women with RHD.
Methods
We performed a non-systematic literature review of published studies on RHD worldwide. Briefly, we searched PubMed for studies that had been published in the period 2000 to June 2017. Our strategy included search terms corresponding to RHD diagnosis, management or prevention, combined with the words ‘contraception’, ‘maternal mortality’, ‘pregnancy’ and ‘post-partum’. Additional searches were done in Google Scholar for categories that yielded very few results in PubMed. Studies were selected as relevant if they met any of the following criteria: contained data on RHD in women, and pregnancyrelated maternal and foetal outcomes. Preference was given to articles reporting data from LMIC worldwide, and from Africa in particular. Sentinel articles outside the time period were included.
In this position paper we especially aimed at reviewing scientific evidence available to explore and answer the following questions:
What specific issues need to be addressed in managing women with RHD in Africa?
Does any contraceptive method work better or increase the risk of complications?
Does pregnancy affect RHD? Does RHD worsen pregnancy outcomes?
Are there specific interventions that should be considered for pregnant women?
What service-provision models can be used to manage women with RHD?
What are the health system considerations for developing and supporting those services?
Diagnosis and management of RHD
RHD commonly affects the mitral, aortic and tricuspid valves, in isolation or in combination.24,25 It is one of the drivers of maternal mortality beyond six weeks’ postpartum in SSA, because highrisk women with RHD are often diagnosed during pregnancy.
During pregnancy, assessment of the valves affected, the severity of lesions and risk prediction are complex, requiring considerable experience. Despite the scarce information published on the subject, it is well known that valve dysfunction, especially stenotic lesions, results in significant physiological effects during pregnancy, increasing maternal mortality rate and foetal loss.8 This is mainly due to the increase in cardiac output associated with advance in gestational stage, which increases the transvalvular gradient.25
In addition, the fall in peripheral vascular resistance will provoke fluid retention and volume expansion, which may be more marked in women with a stenotic lesion because they are less able to increase cardiac output in response to pressure drop.26 Furthermore, the increased heart rate may be poorly tolerated, as left ventricular filling depends on an adequate diastolic filling time. Women may therefore complain of shortness of breath, heart failure and arrhythmia. Left-sided regurgitant valve lesions are better tolerated in pregnancy because of the reduction in the regurgitant volume caused by a fall in systemic vascular resistance, except if the regurgitation is acute and or occurs in the context of poor ventricular function.
Imaging diagnosis
Cardiovascular imaging may be required in pregnant women with known or suspected RHD. The diagnostic imaging modalities that may be used in pregnancy include chest radiography, fluoroscopy, echocardiography, invasive angiography, cardiovascular computed tomography, computed tomographic pulmonary angiography, cardiovascular magnetic resonance (CMR) imaging and nuclear techniques. Echocardiography and CMR appear to be safe in pregnancy and are not associated with any adverse foetal effects. Despite concerns related to imaging modalities that involve ionising radiation, namely teratogenesis, mutagenesis and childhood malignancy, evidence shows that no single imaging study approaches the cautionary dose of 5 rad (50 mSv or 50 mGy).27 Currently, it seems that a single cardiovascular radiological study during pregnancy is safe and should be undertaken when clinically justified.27
Echocardiography is mandatory for the diagnosis of rheumatic heart valve disease, but in pregnancy transmitral and transaortic gradients need to be evaluated with caution. They tend to be over-estimated due to the physiologically increased heart rate, but may be underestimated in the presence of impaired systolic function.25,28 Furthermore, the increased heart rate per se affects the peak and mean systolic gradients, as calculated from the Bernoulli transformation; the calculation of the valve orifice using the continuity equation should also be used.
Management
Healthcare systems and the socio-economic conditions of most families in Africa do not allow easy provision of the level of care required by RHD patients to prevent complications, and therefore many patients reach hospitals with established heart failure, atrial fibrillation and pulmonary hypertension.8 When managing women of childbearing age, key issues related to medical and surgical management must be taken into account, including the desire for a future pregnancy, the need for intervention prior to pregnancy, and the type of valve surgery, if this procedure is needed. Discussion on contraceptive options, risks of pregnancy, as well as maternal and foetal risks related to pregnancy and delivery, are also mandatory.
Medical therapy
Due to limited access to interventional cardiology and cardiothoracic surgery in Africa,8 the majority of RHD patients are managed medically. Medical therapy is indicated for all symptomatic patients and is used as a bridge to surgery or interventional catheterisation, as well as for those with contraindications to surgery.
Diuretics are used in most symptomatic patients to treat congestive heart failure. Diuretics are usually combined with ACE inhibitors where there is valve regurgitation and with betablockers in patients with mitral stenosis. Despite the indication for using corticosteroids for the treatment of episodes of active carditis, in some instances, aspirin at high doses is preferred due to fear of reactivation of tuberculosis during pregnancy.29 Patients may need anticoagulants for the prevention of valve thrombosis and/or thrombo-embolism in severe mitral stenosis or atrial fibrillation. Finally, medicines may also be needed for the prevention or treatment of infective endocarditis.
Drugs are an important component of a patient’s management after surgery or balloon valvotomy. An important aspect of medical management of RHD is secondary prophylaxis for the prevention of new attacks of acute rheumatic fever and progression of valve lesions. Benzathine penicillin G has been the gold standard for secondary prophylaxis, including for pregnant women.29,30
Percutaneous mitral dilatation (PMD)
Since its introduction in the early 1980s, PMD has had a significant impact on the treatment of mitral stenosis.31 Randomised trials have demonstrated successful results obtained with this technique, and explain why it has largely replaced surgical commissurotomy.32-34 PMD is particularly important in the management of women with RHD in Africa, where haemodynamically severe mitral stenosis presents earlier in life, and young patients have thickened valve leaflets presenting with or without concurrent regurgitation.35,36
When planning for PMD measurement for valve area, this should be done using planimetry with two-dimensional echocardiography. Only if planimetry is not feasible should the Doppler pressure half-time method be used. Continuous-wave Doppler must be used to assess the mean mitral gradient, and the structural abnormalities should be described using the Wilkins score.37 Presence (and quantification) of mitral and/or aortic regurgitation should be assessed, and pulmonary artery pressure measured in all cases.
Only experienced operators should perform PMD, using the Inoue or a double-balloon technique. The procedure is stopped when there is complete opening of at least one commissure, with a valve area ≥ 1.5 cm2 (≥ 1.0 cm2/m2 of BSA). The appearance of regurgitation or its increase by more than one-quarter also determines the end of the procedure.34
Marijon and colleagues have shown that despite candidates for PMD from non-Western countries being younger, with more severe valve stenosis and pulmonary hypertension, this procedure was an effective treatment for mitral stenosis in these populations.36 In this study of 350 patients (mean age: 41 years, 81% women) with mitral valve area of less than 1.5 cm2, the results of PMD were similar in patients from non-Western and Western countries; 6% of women submitted to PMD in non-Western countries were pregnant, compared to only 2% in developed countries.
Surgery
There is a growing array of repair procedures aimed at preserving the patient’s own heart valves.25,38,39 They can be used during pregnancy and as an emergency intervention.25,40-44 The different risk and benefit profiles of bioprostheses and mechanical valves with regard to valve haemodynamics, durability, incidence of thrombotic events, need for anticoagulation, and impact on foetal outcome must be considered in women of childbearing age.45-47
The type of operation for the treatment of mitral valve disease and the decision to preserve the native valve have been determined mainly by the surgeon’s skill to perform mitral repair or commissurotomy, which are the interventions of choice in young women. PMD and repair need pliable valves that are not heavily calcified. Limited data are available on pregnancy outcomes in women with aortic homographs and aortic valve repair (David’s operation), as well on those who have had the Ross procedure.
The Ross procedure is a complex operation that involves removal of the patient’s own pulmonary valve and pulmonary artery, which is then used to replace the diseased aortic valve, with re-implantation of the coronary arteries into the graft, as well as the insertion of a human homograft into the pulmonary artery. This procedure can provide an excellent haemodynamic result, with the added benefit that the valves are not thrombogenic. However, the procedure is difficult and seldom performed. The reported pregnancies had an overall good maternal and foetal outcome.48,49
In general, there is very limited access for young females in LMICs to procedures such as mitral and aortic valve repair, aortic homograft insertion and the Ross procedure. This is due to the complexity of the surgery, limited resources and considerably fewer surgeons available in most endemic areas.
Contraception
Health professionals in Africa should adopt a pro-active attitude to holistically address the reproductive and cardiovascular health needs of women with RHD. Owing to the high risk associated with pregnancy, these women should be prioritised for appropriate contraceptive advice.39,50 Patients with moderate mitral stenosis and a dilated left atrium have an increased risk of stroke during pregnancy, and this should be openly discussed with them prior to conception.50 Women of childbearing age with severe mitral stenosis should be prioritised for family planning advice, since they have an extremely high risk of morbidity and mortality. When pregnancy is strongly desired, PMD and/or surgery should be considered.51
Contraceptive counselling should consider factors such as the known risk of pregnancy for the women, the risks of a given contraceptive method (failure rates and availability), the individual’s preferences, protection against infections, and costs. Since there are no studies performed in women with RHD to investigate the relative risks and benefits of different contraceptive methods,25,52 and no studies on contraceptive devices have been performed in these women, the relative risks and benefits of different contraceptive methods are based on consensus only. Input may be necessary from all specialists involved in care to select the best method.
Fig. 2 summarises the most commonly recommended contraceptives. As women with mitral stenosis, mechanical valve prostheses and/or left ventricular dysfunction are at a substantial risk of thrombo-embolic events, hormonal contraceptives with a pro-thrombotic effect should be avoided. The risk of venous thrombosis is significantly increased (up to seven-fold) by the oestrogen component in oral contraceptives, irrespective of the type of progestin used.53 Oestrogen-containing oral contraceptives also increase the risk of arterial thrombosis and hypertension.54 The most effective types of contraceptives are the long-acting reversible forms, including intra-uterine contraceptive devices or progesterone cutaneous implants. The progestogen (etonogestrel) implant has no cardiac effects, is effective and has fewer side effects compared to other implants.25 The new progesterone-releasing intra-uterine systems for longacting contraception are now preferred to the older copper intra-uterine device.55
Fig. 2.
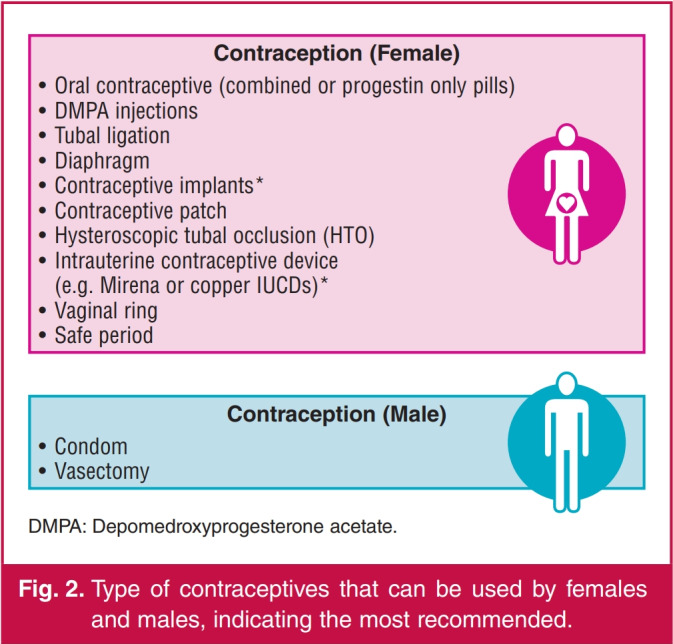
Type of contraceptives that can be used by females and males, indicating the most recommended.
Pre-conception evaluation
Ideally, pre-conception evaluation and advice on risk prediction should be given to all women with RHD when pregnancy is planned.52 Pre-conception evaluation usually includes a careful history, detailed physical examination, electrocardiogram and cardiac ultrasound,50 but an exercise test may be considered for objective assessment of functional classification (Table 1). The type of lesion, presence of impaired left ventricular function, and need for anticoagulation are among the issues that need to be addressed when anticipating pregnancy.56
Table 1. Pre-conception evaluation in women with rheumatic heart valve disease planning a pregnancy or assessment in early pregnancy.
|
Particular attention must be given to a woman with prosthetic heart valves wanting to fall pregnant. The choices of anticoagulation therapy (e.g. heparin, warfarin or enoxaparin) during pregnancy must be discussed, with a clear plan to prevent complications and mortality. Severe symptomatic valve disease should be corrected prior to pregnancy, because cardiac surgery during pregnancy carries high risks for the foetus.52
Pre-natal care
For optimal cardiac and obstetric care, high-risk pregnant patients with RHD should preferably be cared for in centres with expertise and availability of diagnostic and therapeutic options.25 Pregnant women with known or suspected RHD often require laboratory evaluation and use of cardiovascular imaging modalities that involve ionising radiation, which has been related to teratogenesis, mutagenesis and childhood malignancy.57 As stated before, concerns related to safety of imaging tests must always be balanced against the importance of accurate diagnosis and thorough assessment of the pathological condition.27 The indications for and limitations of the different diagnostic procedures must be discussed, as well as their potentially harmful effects during pregnancy, but if needed, chest radiography, fluoroscopy, echocardiography and invasive angiography may all be used. Echocardiography appears to be completely safe for both the mother and foetus.58
Counselling for pregnant women should be given according to the CARPREG (CARdiac disease in PREGnancy) risk score47 or the modified World Health Organisation (WHO) classification.59 The fact that most women present to antenatal clinics after 20 weeks of gestation has implications for their functional assessment, and limits the option for termination of pregnancy. In any case, care should be given to discussing maternal and offspring risks, namely choices of anticoagulant therapy, as well as risks of miscarriage, early delivery and smallfor- gestational-age babies. Complications such as heart failure and valve thrombosis must also be discussed as they may occur beyond the immediate delivery period. Finally, side effects of common drugs need to be openly discussed.
Algorithms for the identification and management of pregnant patients with cardiovascular disease can help by improving care, guiding screening of heart disease in all pregnant women, and detecting those with RHD (Fig. 3). They should distinguish between women with known or recently detected cardiovascular disease who are controlled and should undergo risk assessment, from those in heart failure needing immediate ambulance transfer to a tertiary centre.25 More importantly, the algorithms must identify women at high risk who may need careful monitoring beyond the usual peripartum period.
Fig. 3.
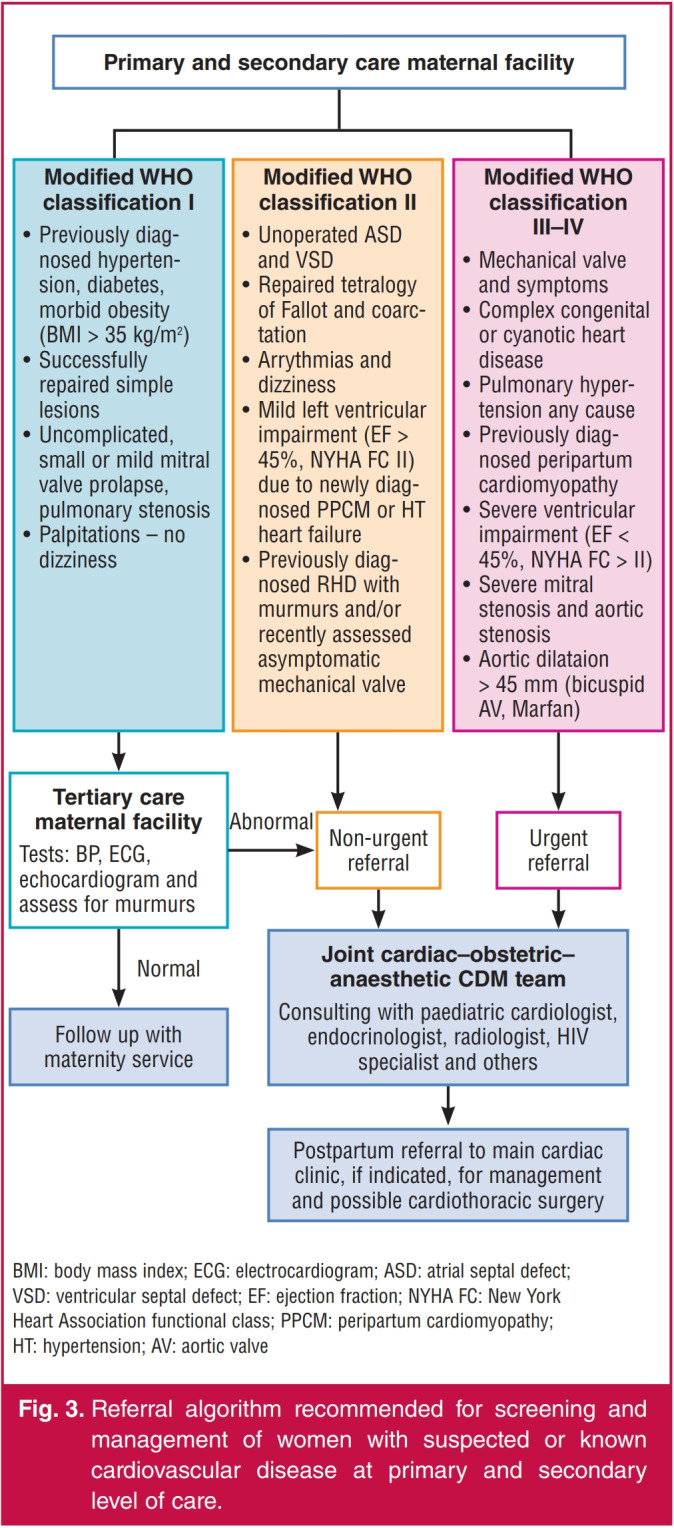
Referral algorithm recommended for screening and management of women with suspected or known cardiovascular disease at primary and secondary level of care.
Labour and postpartum care
Induction, management of labour, delivery and postpartum care require specific expertise and joint management by the obstetrician, cardiologist and anesthaesiologist, preferably in an experienced tertiary care centre. This is true for women with native valve pathology but is more important in those with prosthetic valves.
The treating specialist should prepare a detailed management plan, considering access to care outside of normal working hours. Management needs to be individualised due to the complexity of cases but also due to lack of prospective data.25 Based on consensus, the preferred mode of delivery is vaginal. A delivery plan should be prepared until week 34, including information on the timing of delivery (spontaneous/induced), method of induction, use of general or regional anaesthesia, level of monitoring, needs and details for post-partum monitoring, and sub-acute bacterial endocarditis prophylaxis. In addition, specific instructions for anticoagulation should be documented.
As recently summarised by Sliwa et al.,25 delivery in anticoagulated women with prosthetic valves needs to follow a certain algorithm of care. At 36 weeks, most patients are converted to either low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH). Delivery is usually planned to allow a UFH infusion to be started 36 hours prior to induction/ Caesarean section, and to be discontinued six hours before planned delivery. If there are no bleeding complications during the delivery, then, according to common practice, the UFH infusion is usually restarted four to six hours after delivery. However, this is not evidence-based practice and needs more research. In the case of significant vaginal tears, haematoma or postpartum haemorrhage (PPH), a later start of heparin could be considered, depending on the clinical situation and the risk of valve thrombosis (higher risk for mitral position).
Caesarean delivery could be considered in a number of case scenarios, for example for patients with valvular lesions presenting in pre-term labour on oral anticoagulants, in patients with symptomatic severe stenotic lesions (aortic or mitral stenosis), or in the presence of an ascending aorta > 45 mm, severe pulmonary hypertension or acute heart failure.25 If an emergency delivery must be carried out while the patient is taking warfarin, then a Caesarean section should be performed under general anaesthetic, with fresh frozen plasma cover and prothrombin complex concentrate added if necessary, to reverse anticoagulation.
Peripartum and postpartum obstetric complications are more common in patients with valvular heart disease and can include PPH, defined as blood loss > 500 ml (vaginal delivery) or > 1 000 ml (Caesarian section), which require transfusion or are accompanied by a drop in haemoglobin > 2.0 g/l. Ergometrine is relatively contra-indicated due to its effects on blood pressure and the potential to cause coronary artery spasm. Oxytocin can also have adverse effects, inducing vasodilatation in the subcutaneous vessels, and vasoconstriction in the splanchnic bed and coronary arteries. The direct effect on cardiac receptors increases heart rate, with the overall effect of hypotension, tachycardia and myocardial ischaemia.60,61 If a PPH does occur, oxytocin should be given and prostaglandins are generally well tolerated, but early intervention is key to keeping control of the situation. One should use mechanical approaches, including an intra-uterine balloon and uterine compression sutures.
Infective endocarditis is rare in pregnancy in patients with valvular lesions, and has been reported with an incidence of 0.5%.47 Endocarditis prophylaxis is recommended for highrisk patients (prosthetic valve) with high-risk procedures, such as dental procedures. During delivery, the indication is controversial and at present antibiotic prophylaxis is not routinely recommended for vaginal or Caesarian delivery.62 However, in our practice, we use prophylaxis with any mode of delivery other than an uncomplicated vaginal delivery, especially in patients with a mechanical valve.
The need for integrated and responsive health systems
Understanding the clinical interventions and requirements is crucial, but due consideration must be given as to how these interventions can and should be supported in health system contexts of resource-poor settings, as is the case in many of the LMICs. A very important consideration is how cardiovascular requirements for women with RHD are integrated with sexual and reproductive health (SRH) and general cardiovascular and other relevant services.
In general, the current high adolescent fertility rates mean that almost one-fifth of women in Africa have an unmet need for family planning.63 This points to a general challenge with access to SRH services, including young women at risk of or with established RHD. In a recent study in school-going children in South Africa and Ethiopia, the odds of having asymptomatic RHD increased with worsening socio-economic circumstances and the condition affected predominantly girls. Therefore young women, particularly those living in poverty, may present with undetected disease to reproductive health services for contraception and obstetric care.64 This confirms the need for an integrated and responsive health system, so as to enable the identification, treatment and follow-up support of women with RHD, in particular young women of reproductive age.
Global efforts to develop health systems that are resilient, responsive and able to meet the multiple needs of health service users include the development of sound maternal and reproductive health services, particularly considering the unfinished millennium development goals agenda, which strongly emphasised maternal health and the reduction of maternal mortality rates. This unfinished agenda is now incorporated into the sustainable development goals, in particular goals 3, 4 and 5, which speak to equitable development to promote healthy lives for women and girls.28
Women with RHD require a continuum of care, from prevention of the disease, which involves addressing the social determinants of health; to interventions at primary care level following a Streptococcus group A sore throat, including the necessary long-term follow up; through to first diagnosis of rheumatic fever in childhood and subsequent follow up and support in their schooling, adolescence and young adulthood; through their reproductive years and into later adulthood.
In their reproductive years, the school setting and primary level health facilities (with reproductive and obstetric health services) become crucial sites for care and support. Therefore SRH services must be equipped to diagnose women who have undetected RHD, provide secondary prophylaxis after rheumatic fever, treat symptomatic RHD, and provide sexual healthcare, contraceptive care, and antenatal, obstetric and postnatal care. Since health systems across Africa are subject to significant stressors, the development of a pro-active service response to this demand poses an ongoing challenge. However, with appropriate protocols and interventions to enable good antenatal, obstetric and postnatal care, some settings have reduced bad outcomes to a minimum.65 The major gaps in access to SRH described for women in SSA apply to those with RHD and call for innovative approaches to improve the current situation in this high-risk group of people.
School-based reproductive health services
The school setting is the place where children and adolescents can be: alerted to the importance of seeking care for a strep sore throat; sensitised to the symptoms and signs of rheumatic heart disease; exposed to counselling and support if they are on penicillin prophylaxis; and targeted for appropriate sexual health education and contraceptive provisioning. School health services must be linked to the appropriate primary level services, including SRH services, to provide a comprehensive continuum of care.
However, in the majority of African countries, school health services either do not exist or function sub-optimally. Where they do function, the main focus is on screening and prevention in younger children.22,66 This represents a huge missed opportunity to support and educate young women as they enter their sexually active and reproductive health period. Where school health services exist, these should extend their activities to include health promotion and SRH to support young women in general, incorporating modules for endemic cardiovascular diseases, such as RHD, peripartum cardiomyopathy and hypertension in pregnancy. Where these do not exit, other mechanisms via existing primary healthcare services should be initiated to accommodate the SRH support to young women with RHD.
Maternal health services
A recent systematic review, conducted on the state of maternal health services for women with disabilities in SSA,67 found that current maternal health services for women who live and are dependent on public sector service provision in SSA are wholly inadequate. This state of affairs extends to all women and not just those with disabilities, and would include women with RHD. A key factor is the significant lack of human resources, both in numbers and technical skills, across all countries, including obstetricians and gynecologists, midwives, and support staff such as laboratory technicians. This needs to be considered when planning for screening, diagnosis, management and follow up of women with RHD in these services.
Linked to the availability of human resources are issues such as financial resources to support diagnostics, drug supply chain, and support schemes for women who fall pregnant and face the possibility of cardiac complications. Finally, we must take into consideration that the selective development of any service response, as demonstrated by several vertical diseaselinked programmes, poses many drawbacks to the current global efforts to support strengthening of the overall health system, and therefore the recommendation of this review for an integrated service response to RHD.
The service response to address the needs of women with RHD will therefore have to be considered in the context of strengthening reproductive and maternal health services in general. Within such a system, we must ensure that health workers are sufficiently aware of the importance of RHD and that the system requirements are in place to identify, diagnose and treat women with this condition.
The Lancet series on sexual and reproductive health services68 emphasises the importance of matching sexual and reproductive health rights with accessible and good-quality services. Recognising the rights of more vulnerable groups, such as women with RHD who generally live in conditions of poverty, challenges the current status quo where SRH services are generally designed to address the needs of women without additional medical complications.
Fathalla et al.68,69 call for action to prioritise SRH services for all, and they strongly question the decision of leaving out SRH as an explicit and stand-alone sustainable development goal as they posit that this has the potential to influence progress in access to SRH services in SSA. For instance, a significant number of countries in Africa have no or restricted termination of pregnancy services, and in at least nine countries, the unmet needs for family planning services are more than 30% for married women. The extent of the unmet needs is unknown for unmarried women, many of whom would be in their adolescence and most likely to present with RHD in pregnancy.
Research needs and the way forward
Based on this review, the PASCAR taskforce identified several areas that need research. These include increase in awareness about RHD and its complications, improvement in management, and adequate counselling for women of reproductive age. The use of protocols that incorporate equipment such as hand-held echocardiography and point-of-care laboratory testing (for early detection of rheumatic fever and follow up of anticoagulation) should be explored. Access to reproductive health services, availability and affordability of efficient contraceptive methods, and acceptability of pregnancy termination options are among the issues that need to be understood in the different African contexts.
Shortage of human resources with adequate technical abilities and supplies to appropriately diagnose, treat and follow up women with RHD requires careful consideration. Education of health professionals and testing of models for integration of services may avoid loss of opportunities for the diagnosis of RHD and promote adequate management when patients make contact with the health system. Innovative financing and logistical mechanisms are required to support diagnosis, ensure consistent drug supply, and increase availability of interventions, allowing a continuum of care. Community-based financing mechanisms that ensure community engagement and sustainability must also be explored.
Health promotion and educational campaigns directed at young people, in particular girls, should be prioritised. The vehicles to deliver information need to be adapted to the young population in both rural and urban communities. The education and research areas identified stress and the need for a comprehensive approach to prevention and control of this condition of poverty, involving sectors such as health, education, social services, communications and finance.
Acknowledgments
We acknowledge the work of Janette Lombard in scheduling and organising the working meetings. We also express our appreciation to Sylvia Dennis for her help in editing and formatting the manuscript for submission.
Contributor Information
Olga Mocumbi Ana, Email: amocumbi@gmail.com, Division of Non-Communicable Diseases, Instituto Nacional de Saúde; and Faculty of Medicine, Eduardo Mondlane University, Maputo, Mozambique.
Keila KF Jamal, Mozambique Institute of Health Education and Research, Maputo, Mozambique.
Amam Mbakwem, Departments of Internal Medicine and Cardiology, University of Lagos, Lagos, Nigeria.
Maylene Shung-King, Health Policy and Systems Division, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
Karen Sliwa, Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Faculty of Health Sciences, University of Cape Town; Soweto Cardiovascular Research Group, University of the Witwatersrand, Johannesburg, South Africa; and Mary McKillop Institute, ACU, Melbourne, Australia.
References
- 1.Watkins D, Zuhlke L, Engel M, Daniels R, Francis V, Francis G. et al. Seven key actions to eradicate rheumatic heart disease in Africa: the Addis Ababa communique. Cardiovasc J Afr. 2016;27:184–187. doi: 10.5830/CVJA-2015-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayosi BM, Gamra H, Dangou J-M, Kasonde J. Rheumatic heart disease in Africa: the Mosi-o-Tunya call to action. Lancet Global Health. 2014;2:e438–e439. doi: 10.1016/S2214-109X(14)70234-7. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh J. Beyond the millenium development goals: a southern perspective on a global new deal. J Int Develop. 2015;27:320–329. [Google Scholar]
- 4.GBD Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990-2015: a systemic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1775–1812. doi: 10.1016/S0140-6736(16)31470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. 2014;2:e323–333. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 6.Sliwa K, Libhaber E, Elliott C, Momberg Z, Osman A, Zuhlke L. et al. Spectrum of cardiac disease in maternity in a low-resource cohort in South Africa. Heart. 2014;100:1967–1974. doi: 10.1136/heartjnl-2014-306199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM. et al. Pregnancy in women with heart disease in sub-Saharan Africa. Arch Cardiovasc Dis. 2011;104:370–374. doi: 10.1016/j.acvd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Zuhlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B. et al. Characteristics, complications, and gaps in evidencebased interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J. 2015;36:1115–1122. doi: 10.1093/eurheartj/ehu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker KG, Human DG, De Moor MM, Sprenger KJ. The problem of compliance in rheumatic fever. S Afr Med J. 1987;72:781–783. [PubMed] [Google Scholar]
- 10.WHO Technical Report Series 923. Rheumatic fever and rheumatic heart disease. Geneva, 2005 29 October – 1 November 2001. [Google Scholar]
- 11.Mocumbi AO, Sliwa K. Women’s cardiovascular health in Africa. Heart. 2012;98:450–455. doi: 10.1136/heartjnl-2011-301025. [DOI] [PubMed] [Google Scholar]
- 12.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 13.Guengant J, May JF. African demography. Glob J Emerg Market Econ. 2013;12:215–267. [Google Scholar]
- 14.AFRI-DEV Info. Maternal dealth risk. http://wwwafri-devinfo/ 2016; Accessed 8 November 2017 [Google Scholar]
- 15.AFRI-DEV Info. Multisectoral information, data, research and evidence – for health, population, human and social development http:// wwwafri-devinfo/ 2016; Accessed 8 November 2017. [Google Scholar]
- 16.United Nations. Contraceptive prevalence: Unicef analysis based on United Nations, Department of Economic and Social Affairs, Population Division, model-based estimates and projections of family planning indicators. http://wwwtakepartcom/article/2014/11/07/africacontraception- child-population 2014. [Google Scholar]
- 17.Mucumbitsi J, Bulwer B, Mutesa L, Ndahindwa V, Semakula M, Rusingiza E. et al. Prevalence of rheumatic valvular heart disease in Rwandan school children: echocardiographic evaluation using the World Heart Federation criteria. Cardiovasc J Afr. 2017;28:1–8. doi: 10.5830/CVJA-2017-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D. et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. New Engl J Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 19.Gemechu T, Mahmoud H, Parry EH, Phillips DI, Yacoub MH. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. Eur J Prevent Cardiol. 2017;24:717–723. doi: 10.1177/2047487316687104. [DOI] [PubMed] [Google Scholar]
- 20.Yadeta D, Hailu A, Haileamlak A, Gedlu E, Guteta S, Tefera E. et al. Prevalence of rheumatic heart disease among school children in Ethiopia: A multisite echocardiography-based screening. Int J Cardiol. 2016;221:260–263. doi: 10.1016/j.ijcard.2016.06.232. [DOI] [PubMed] [Google Scholar]
- 21.Zuhlke LJ, Engel ME, Watkins D, Mayosi BM. Incidence, prevalence and outcome of rheumatic heart disease in South Africa: a systematic review of contemporary studies. Int J Cardiol. 2015;199:375–383. doi: 10.1016/j.ijcard.2015.06.145. [DOI] [PubMed] [Google Scholar]
- 22.Rossi E, Felici AR, Banteyrga L. Subclinical rheumatic heart disease in an Eritrean high-school population, detected by echocardiography. J Heart Valve Dis. 2014;23:235–239. [PubMed] [Google Scholar]
- 23.Sadoh WE, Omuemu VO, Israel-aina YT. Prevalence of rheumatic heart disease among primary school pupils in mid-western Nigeria. East Afr Med J. 2013;90:28–32. [PubMed] [Google Scholar]
- 24.Russell EA, Tran L, Baker RA, Bennetts JS, Brown A, Reid CM. et al. A review of outcome following valve surgery for rheumatic heart disease in Australia. BMC Cardiovasc Disord. 2015;15:103–103. doi: 10.1186/s12872-015-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sliwa K, Johnson MR, Zilla P, Roos-Hesselink JW. Management of valvular disease in pregnancy: a global perspective. Eur Heart J. 2015;36:1078–1089. doi: 10.1093/eurheartj/ehv050. [DOI] [PubMed] [Google Scholar]
- 26.Dennis A. Valvular heart disease in pregnancy. Int J Obstet Anesth. 2016;25:4–8. doi: 10.1016/j.ijoa.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Ntusi NA, Samuels P, Moosa S, Mocumbi AO. Diagnosing cardiac disease during pregnancy: imaging modalities. Cardiovasc J Afr. 2016;27:95–103. doi: 10.5830/CVJA-2016-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaelson-Cohen R, Elstein D, Ioscovich A, Armon S, Schimmel MS, Butnaru A. et al. Severe heart disease complicating pregnancy does not preclude a favourable pregnancy outcome: 15 years’ experience in a single centre. J Obstet Gynaecol. 2011;31:597–602. doi: 10.3109/01443615.2011.603064. [DOI] [PubMed] [Google Scholar]
- 29.Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, Taubert KA. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119:1541–1551. doi: 10.1161/CIRCULATIONAHA.109.191959. [DOI] [PubMed] [Google Scholar]
- 30.Manyemba J, Mayosi BM. Penicillin for secondary prevention of rheumatic fever. Cochrane Syst Rev 2002: CD002227. doi: 10.1002/14651858.CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. 1984;87:394–402. [PubMed] [Google Scholar]
- 32.Ben Farhat M, Gamra H, Betbout F, Maatouk F, Jarrar M, Addad F. et al. Percutaneous balloon mitral commissurotomy during pregnancy. Heart. 1997;77:564–567. doi: 10.1136/hrt.77.6.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyes VP, Raju BS, Wynne J, Stephenson LW, Raju R, Fromm BS. et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. New Engl J Med. 1994;331:961–967. doi: 10.1056/NEJM199410133311501. [DOI] [PubMed] [Google Scholar]
- 34.Vahanian A, Lung B, Pierard LA, Dion R, Pepper J. Valvular vascular diseases. In: Camm A, Luscher TF, Serruys P, eds. The ESC Textbook of Cardiovascular Medicine. Oxford: Blackwell; 2006: 625–670. [Google Scholar]
- 35.Karthikeyan G, Zuhlke L, Engel M, Rangarajan S, Yusuf S, Teo K, Mayosi BM. Rationale and design of a Global Rheumatic Heart Disease Registry: the REMEDY study. Am Heart J. 2012;163:535–540. doi: 10.1016/j.ahj.2012.01.003. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijon E, Iung B, Mocumbi AO, Kamblock J, Thanh CV, Gamra H. et al. What are the differences in presentation of candidates for percutaneous mitral commissurotomy across the world and do they influence the results of the procedure? Arch Cardiovasc Dis. 2008;101:611–617. doi: 10.1016/j.acvd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60:299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar AS, Rao PN, Dharmapuram AK, Chander H, Trehan H. Pulmonary autograft aortic valve replacement. Early experience with the Ross procedure. Texas Heart Inst J. 1995;22:177–179. [PMC free article] [PubMed] [Google Scholar]
- 39.Herrey A, Nelson-Piercy C. Cardiovascular disease in pregnancy. Medicine. 2010;10:555–560. [Google Scholar]
- Birincioglu CL, Unal EU, Celik IH, Ozen A, Tak S, Aksoyek A. et al. Surgery for rheumatic valve disease in pregnancy: what about the newborn? Heart Lung Circ. 2014:63–67. doi: 10.1016/j.hlc.2013.05.639. [DOI] [PubMed] [Google Scholar]
- 41.Demirbag R, Sade LE, Aydin M, Bozkurt A, Acarturk E. The Turkish registry of heart valve disease. Turk Kardiyol Dernegi Arsivi. 2013;41:1–10. doi: 10.5543/tkda.2013.71430. [DOI] [PubMed] [Google Scholar]
- 42.Martins LC, Freire CM, Capurucu CA, Nunes Mdo C, Rezende CA. Risk prediction of cardiovascular complications in pregnant women with heart disease. Arquivos Brasil Cardiol. 2016;106:289–296. doi: 10.5935/abc.20160028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nqayana T, Moodley J, Naidoo DP. Cardiac disease in pregnancy. Cardiovasc J Afr. 2008;19:145–151. [PMC free article] [PubMed] [Google Scholar]
- 44.Subbaiah M, Sharma V, Kumar S, Rajeshwari S, Kothari SS, Roy KK. et al. Heart disease in pregnancy: cardiac and obstetric outcomes. Arch Gynecol Obstet. 2013;288:23–27. doi: 10.1007/s00404-013-2730-2. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg ZL, Dominguez-Islas CP, Otto CM, Stout KK, Krieger EV. Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol. 2017;69:2681–2691. doi: 10.1016/j.jacc.2017.03.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Hagen IM, Roos-Hesselink JW, Ruys TP, Merz WM, Goland S, Gabriel H. et al. ERPT Investigators. Pregnancy in women with a mechanical heart valve: Data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation. 2015;132:132–142. doi: 10.1161/CIRCULATIONAHA.115.015242. [DOI] [PubMed] [Google Scholar]
- 47.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM. et al. Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). ESC guidelines on the management of cardiovascular diseases during pregnancy. Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 48.Heuvelman HJ, Arabkhani B, Cornette JM, Pieper PG, Bogers AJ, Takkenberg JJ, Roos-Hesselink JW. Pregnancy outcomes in women with aortic valve substitutes. Am J Cardiol. 2013;111:382–387. doi: 10.1016/j.amjcard.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 49.Arabkhani B, Heuvelman HJ, Bogers AJ, Mokhles MM, Roos-Hesselink JW, Takkenberg JJ. Does pregnancy influence the durability of human aortic valve substitutes? J Am Coll Cardiol. 2012;60:1991–1992. doi: 10.1016/j.jacc.2012.06.055. [DOI] [PubMed] [Google Scholar]
- 50.Zuhlke L, Acquah L. Pre-conception counselling for key cardiovascular conditions in Africa: optimising pregnancy outcomes. Cardiovasc J Afr. 2016;27:79–83. doi: 10.5830/CVJA-2016-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moghbeli N, Herrmann H. Mitral stenosis. Contemporary cardiology: Valvular Heart Dis. 2009;4:207–219. [Google Scholar]
- 52.Roos-Hesselink JW, Cornette J, Sliwa K, Pieper PG, Veldtman GR, Johnson MR. Contraception and cardiovascular disease. Eur Heart J. 2015;36:1728–1734. doi: 10.1093/eurheartj/ehv141. 34a–34b. [DOI] [PubMed] [Google Scholar]
- 53.Hugon-Rodin J, Gompel A, Plu-Bureau G. Epidemiology of hormonal contraceptives-related venous thromboembolism. Eur J Endocrinol. 2014;171:R221–230. doi: 10.1530/EJE-14-0527. [DOI] [PubMed] [Google Scholar]
- 54.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New Engl J Med. 2012;366:2257–226. doi: 10.1056/NEJMoa1111840. [DOI] [PubMed] [Google Scholar]
- 55.Costescu DJ. Levonorgestrel-releasing intrauterine systems for longacting contraception: current perspectives, safety, and patient counseling. Int J Women’s Health. 2016;8:589–598. doi: 10.2147/IJWH.S99705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghandi M, Martin S. Cardiac disease in pregnancy. S Austr Perinatal Pract Guidelines. 2014;42:1–28. [Google Scholar]
- 57.Westhoff-Bleck M, Podewski E, Hilfiker A, Hilfiker-Kleiner D. Cardiovascular disorders in pregnancy: diagnosis and management. Best Pract Res Clin Obstet Gynaecol. 2013;27:821–834. doi: 10.1016/j.bpobgyn.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC. et al. Cardiac disease in pregnancy I. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 59.Mocumbi AO, Sliwa K, Soma-Pillay P. Medical disease as a cause for maternal mortality: the pre-imminence of cardiovascular pathology. Cardiovasc J Afr. 2016;27(2):84–88. doi: 10.5830/CVJA-2016-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinder AJ, Dresner M, Calow C, Shorten GD, O’Riordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. Int J Obstet Anesth. 2002;11:156–159. doi: 10.1054/ijoa.2002.0970. [DOI] [PubMed] [Google Scholar]
- 61.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth. 2007;98:116–119. doi: 10.1093/bja/ael302. [DOI] [PubMed] [Google Scholar]
- 62.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I. et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 63.You D, Hug L, Anthony D. UNICEF report. Generation 2030. Africa calls upon investing in and empowering girls and young women. Reprod Health. 2015;12 doi: 10.1186/s12978-015-0007-x. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.United Nations. Transforming our world: the 2030 Agenda for Sustainable Development. Geneva. 2015 [Google Scholar]
- 65.Bundy D. Rethinking school health: A key component of education for all. The International Bank for Reconstruction and Development. The World Bank Group. 2011. https://doi.org/10.1596/978-0-8213-7907-3 . [Google Scholar]
- 66.Mheta D. Health system factors that impact on access to maternal services for women with disabilities in Sub-Saharan Africa: A systematic review. Unpublished thesis. Univesity of Cape Town, 2015. [Google Scholar]
- 67.Shaw D. Sexual and reproductive health: rights and responsibilities. Lancet. 2006;368:1941–1943. doi: 10.1016/S0140-6736(06)69487-7. [DOI] [PubMed] [Google Scholar]
- 68.Fathalla MF, Sinding SW, Rosenfield A, Fathalla MM. Sexual and reproductive health for all: a call for action. Lancet. 2006;368:2095–2100. doi: 10.1016/S0140-6736(06)69483-X. [DOI] [PubMed] [Google Scholar]
- 69.Cleland J, Bernstein S, Ezeh A, Faundes A, Glasier A, Innis J. Family planning: the unfinished agenda. Lancet. 2006;368:1810–1827. doi: 10.1016/S0140-6736(06)69480-4. [DOI] [PubMed] [Google Scholar]