Abstract
The prevention, early discovery and effective treatment of patients with hepatocellular carcinoma (HCC) remain a global medical challenge. At present, HCC is still mainly treated by surgery, supplemented by vascular embolization, radio frequency, radiotherapy, chemotherapy and biotherapy. The application of multikinase inhibitor sorafenib, chimeric antigen receptor T cells, or PD-1/PD-L1 inhibitors can prolong the median survival of HCC patients. However, the treatment efficacy is still unsatisfactory due to HCC metastasis and postoperative recurrence. During the process of hepatocyte malignant transformation, HCC tissues can express and secrete many types of specific biomarkers, or oncogenic antigen molecules into blood, for example, alpha-fetoprotein, glypican-3, Wnt3a (one of the key signaling molecules in the Wnt/β-catenin pathway), insulin-like growth factor (IGF)-II or IGF-I receptor, vascular endothelial growth factor, secretory clusterin and so on. In addition, combining immunotherapy with non-coding RNAs might improve anti-cancer efficacy. These biomarkers not only contribute to HCC diagnosis or prognosis, but may also become molecular targets for HCC therapy under developing or clinical trials. This article reviews the progress in emerging biomarkers in basic research or clinical trials for HCC immunotherapy.
Keywords: Hepatocellular carcinoma, Immunotherapy, Carcinoembryonic proteins, Specific biomarkers, Wnt/β-catenin pathway, Signal molecules
Core Tip: Tissues in hepatocellular carcinoma (HCC) or hepatocyte malignant transformation can express and secrete a variety of molecules such as specific biomarkers or oncogenic antigens into blood. These biomarkers not only contribute to the diagnosis or prognosis of HCC, but may also become molecular targets for HCC therapy under developing or clinical trials. This article reviews the recent novel progress of some emerging biomarkers in basic studies or clinical trials for HCC immunotherapy.
INTRODUCTION
The prevention, early monitoring or diagnosis and accurate or effective treatment of hepatocellular carcinoma (HCC) are still urgent medical problems[1,2]. The occurrence of HCC is mainly associated with chronic persistent infection of hepatitis B virus (HBV) or hepatitis C virus (HCV), intake of chemical carcinogens, and nonalcoholic fatty liver disease (NAFLD)[3]. In the past decade, NAFLD has become a leading cause of chronic hepatitis and liver cirrhosis, as well as an important risk factor for HCC[4]. Innate and adaptive immunity play a pivotal role in determining tumor control vs progression. Genomic instability and abnormal signaling in the setting of chronic liver inflammation that promotes fibrogenesis and angiogenesis lead to tumorigenesis, and it is necessary to determine how they may be exploited in the development of novel therapeutics[5]. The activation of oncogenes or HCC-related genes, inactivation of anti-oncogenes or activation of some oncogenes during the embryonic period can induce malignant transformation of hepatocytes[5], many types of specific markers can be expressed, and then secreted into blood during the process of initiation, promotion and evolution[1]. Notably, HCC oncoimmunology depends on diverse genetic and environmental factors that together shape cancer-promoting inflammation and immune dysfunction-critical processes that control HCC malignant progression and response to therapy[6,7].
Currently, HCC is still treated mainly by surgery, with auxiliary vascular embolization, radio frequency, radiotherapy, chemotherapy, and biological therapy[8,9]. Application of the multikinase inhibitor sorafenib can prolong the median survival of HCC patients. However, its efficacy in HCC treatment remains unsatisfactory due to tumor metastasis or postoperative occurrence[10,11]. Undoubtedly, the integration of data obtained from both preclinical models and human studies can help to accelerate the identification of robust predictive biomarkers of response to targeted or immune- therapy[12,13]. HCC tissues express specific antigens such as the key molecules of HCC-related signal pathways, growth factors and receptors, vascular endothelial growth factor (VEGF), and the products of oncogenes that mediated tumor progression and could be potential molecular targets for anti-cancer therapy with high specificity and application prospects[14,15]. This review presents new advances in a few promising carcinoembryonic biomarkers for HCC immunotherapy from basic studies or clinical trials.
ALPHA-FETOPROTEIN
A glycoprotein of alpha-fetoprotein (AFP) synthesized from fetal liver or HCC tissues[16], consisting of 609 single-chain amino acid polypeptides and containing 24 leading signal points (9 ~ 10 amino acid) residues located in three N-terminal domains, the major histocompatibility complex (MHC) class I or II molecules recognize these precursor signals and present them to CD4+ T cells and CD8+ T cells, and the activated T cells recognize the body’s immunodominant or sub-immunodominant epitopes[17]. Amino acid peptide sequences and immunogenicity of human AFP epitopes are shown in Table 1. These immunogenic or sub-immunogenic AFP peptide chains could play an immunomodulatory role in humans, as they have the function and ability of a polypeptide vaccine, and could induce or stimulate anti-AFP specific immune responses.
Table 1.
Amino acid peptide sequences and immunogenicity of alpha-fetoprotein epitopes
|
No.
|
Starting
|
Numbers
|
Fragment
|
Immunogenicity
|
| 1 | 7 | 9 | IFLIFLLNF | Sub-immunodominant Ag |
| 2 | 137 | 9 | PLFQVPEPV | Immunodominant Ag |
| 3 | 150 | 9 | AYEEDRETF | Sub-immunodominant Ag |
| 4 | 158 | 9 | FMNKFIYEI | Immunodominant Ag |
| 5 | 218 | 9 | LLNQHACAV | Sub-immunodominant Ag |
| 6 | 235 | 9 | FQAITVTKL | Sub-immunodominant Ag |
| 7 | 249 | 10 | KVNFTEIQKL | Immunodominant Ag |
| 8 | 307 | 9 | TTLERGQCII | Sub-immunodominant Ag |
| 9 | 321 | 9 | KPEGLSPNL | Immunodominant Ag |
| 10 | 325 | 10 | GLSPNLNRFL | Immunodominant Ag |
| 11 | 357 | 9 | EYSRRHPQL | Immunodominant Ag |
| 12 | 364 | 10 | QLAVSVILRV | Immunodominant Ag |
| 13 | 403 | 9 | KYIQESQAL | Immunodominant Ag |
| 14 | 414 | 9 | RSCGLFQKL | Immunodominant Ag |
| 15 | 424 | 9 | EYYLQNAFL | Immunodominant Ag |
| 16 | 434 | 9 | AYTKKAPQL | Immunodominant Ag |
| 17 | 485 | 10 | CIRHEMTPV | Sub-immunodominant Ag |
| 18 | 492 | 9 | PVNPGVGQC | Sub-immunodominant Ag |
| 19 | 503 | 9 | SYANRRPCF | Sub-immunodominant Ag |
| 20 | 507 | 10 | NRRPCFSSLV | Sub-immunodominant Ag |
| 21 | 542 | 9 | GVALQTMKQ | Immunodominant Ag |
| 22 | 547 | 10 | TMKQEFLINL | Sub-immunodominant Ag |
| 23 | 555 | 9 | NLVKQKPQI | Sub-immunodominant Ag |
| 24 | 591 | 9 | CFAEEGQKL | Sub-immunodominant Ag |
Ag: Antigen; Fragment: Fragment of alpha-fetoprotein (AFP) peptide chain; Numbers: Amino acid numbers of AFP peptide chain; Starting: Starting point of AFP peptide chain.
AFP peptide chains have several fragments showing immunodominant or sub- immunodominant epitopes, which can be recognized by the MHC-I molecules, and specifically induce T cells to activate or recognize AFP antigen. AFP positive peripheral blood mononuclear cells (PBMC) containing five human leukocyte antigen (HLA)-A*24:02 restricted T cell epitopes, AFP-derived peptide induces cytotoxic T lymphocytes (CTL) to produce interferon-γ (INF-γ), which can kill AFP-positive cancer cells. Although it has been shown in clinical trials, the function of dendritic cells (DC), specific CTL, and CD8+ T cell response, targeting therapy for AFP positive cancer cells remains to be studied. The T cell receptor (TCR) has been prepared by induction and screening in vitro, which can specifically recognize and bind AFP/HLA-A*02 antigen that is restricted to AFP158-166 peptide (FMNKFIYEI) to lay the foundation for HCC immuno-therapy[18]. A novel HLA-A*24:02 antigen was found to be more common than the HLA-A*02:01 among Asian HCC patients. Its restrictive peptide (KWVESIFLIF, AFP2-11 signal) was found to be soluble in healthy human monocyte AFP 2-11-HLA-A*24:02-specific TCR (KWV3.1). T cells could be activated specifically and kill AFP-positive T2-A24 HCC cells that contained AFP 2-11 and HLA-A*24:02+ antigen, indicating that AFP+HLA-A*24:02+ antigen might be a new immunotherapeutic target for HCC[19].
The combination of anti-CTL-A-4 therapy (tremelimumab) together with ablation in advanced HCC cases has shown that killing tumors by direct methods can result in the immune system being activated or switched on. There are new drugs available known as immune checkpoint inhibitors (ICIs) which can enhance the anti-HCC effect. In patients treated with tremelimumab, blood CD4+-HLA-DR+, CD4+PD-1+, CD8+HLA-DR+, CD8+PD-1+, CD4+ICOS+, and CD8+ICOS+ T cells increased, the patients with higher CD+PD-1+ cells responded well to treatment, with increasing specific CD8+PD-1 T cells for AFP and survivin, and higher CD3+T cells for tumor infiltration, suggesting that tremelimumab with ablation is a novel potential method for increasing CD8+ T cells and decreasing circulating HCV, and an effective therapy for advanced HCC patients[20].
ANGIOGENIC FACTORS
Most patients with HCC are diagnosed at an advanced stage of disease. Until recently, systemic treatment options that showed survival benefits in HCC have been limited to tyrosine kinase inhibitors, antibodies targeting oncogenic signaling pathways or VEGF receptors[21]. Angiogenesis plays an important role in HCC progression, and VEGF and angiopoietin (Ang) are key drivers of tumor angiogenesis. A better understanding of the relation between VEGF and angiogenesis or progression may reveal their potential as biomarkers for liver cancer diagnosis and therapy. VEGF-targeting strategies already represent an important component of today's systemic treatment for HCC, whereas targeting the Ang/Tie2 signaling pathway may harbor future potential in this context due to reported beneficial anticancer effects when targeting this pathway[22,23]. Following a decade of negative Phase III trials since the approval of sorafenib, more recently several drugs have proven efficacy both in first line vs sorafenib (lenvatinib) or in second line vs placebo (regorafenib, cabozantinib, ramucirumab/ Cyramza®). A fully human anti-VEGFR-2 recombinant IgG1 monoclonal antibody (ramucirumab) has been approved as monotherapy for HCC patients with AFP levels over 400 ng/mL who have been treated with sorafenib, with significantly prolonged overall survival (OS) and progression-free survival. Its safety profile was consistent with that expected for agents targeting the VEGF/VEGFR axis. The potential clinical development of systemic treatments for HCC, focuses on combination therapies with immunotherapy and treatment sequences as a way to maximize survival benefit[24,25].
The HCC microenvironment is characterized by dysfunction of the immune system through multiple mechanisms, including accumulation of various immunosuppressive factors, recruitment of regulatory T cells and myeloid-derived suppressor cells, and induction of T cell exhaustion accompanied by the interaction between immune checkpoint ligands and receptors. ICIs interfere in this interaction and have altered the therapeutic landscape of multiple cancer types including HCC. HCC patients with different levels of liver function, tumor size, and number of lesions may all have intermediate-stage disease according to the BCLC staging system. Their treatment includes conventional or drug-eluting bead transarterial chemoembolization, yttrium-90 radioembolization, thermal ablation, bland embolization, and combination therapy with VEGF inhibitors or ICIs. Clinical evidence supports the available locoregional treatment options for intermediate-stage HCC[26]. Although optimal sequencing is an area of ongoing investigation, multiple targeted therapies have improved OS in intermediate or advanced HCC[27]. Several targeted agents including multi-tyrosine kinase inhibitors and immunotherapy agents have been approved for use beyond the frontline setting in advanced HCC patients, and combining therapeutic strategies is an evolving approach showing early promise[23,28]. The success of PD-1 monotherapy, combining regimens with PD-1/PD-L1 inhibitors plus VEGF targeted agents has shown positive results in various malignancies including HCC. These innovative approaches enhance the intensity of cancer-directed immune responses and will potentially impact the outcome of this aggressive disease[29].
GLYPICAN-3
With regard to HCC, a promising antigen appears to be glypican-3 (GPC3) which is over-expressed in HCC tissues and has been associated with worse disease-free survival and OS. GPC3 is involved in many signaling cascades that promote cell growth and invasion, including the Wnt pathway that is well-known for its role in embryogenesis. GPC3 as an oncofetal proteoglycan anchored to the cell membrane of HCC, and is normally detected in the fetal liver but not in the healthy adult liver[30,31]. However, abnormal GPC3 in tissues or sera of HCC patients is expressed as GPC3 mRNA gene transcription or protein levels, and predicts a poor prognosis of HCC. Mechanistic studies have revealed that GPC3 functions by binding to molecules such as the Wnt/β-catenin signaling or growth factors during HCC formation and progression. Moreover, specific serum GPC3 has been used as a diagnostic or prognostic serological marker, and a molecular target for molecular imaging or therapeutic intervention in HCC[32-34]. GPC3 as a molecular target for HCC immunotherapy is shown in Table 2. To date, GPC3-targeted magnetic resonance imaging, positron emission tomography, and near-infrared imaging have investigated the early stage of HCC, and immunotherapeutic protocols targeting GPC3 have been developed, including the use of humanized anti-GPC3 cytotoxic antibodies, peptide/DNA vaccines, immuno-toxin therapies, and genetic therapies.
Table 2.
Glypican-3 as molecule-target for hepatocellular carcinoma immunotherapy
|
Group
|
Name
|
Species
|
Epitopes
|
Verifying/applying
|
| Antibody | M18D04/19B11 | Mouse | N-terminal (aa: 25-358) | Basic studies |
| A1836A | Mouse | N- terminal | Basic studies | |
| GPC3-C02 | Mouse | C- terminal | Basic studies | |
| GC33 | Mouse | C-terminal (aa: 524-563) | Preclinical trial studies | |
| hGC33 | Human | C- terminal (aa: 524-563) | Clinical trial-II | |
| HS20 | Human | Heparan sulfate chain | Preclinical trial | |
| sGPC3 | Human | — | Preclinical trial | |
| Vaccines | GPC3298-306 | Mouse | 298-306 peptide | Clinical trial-II |
| GPC3144-152 | Mouse | 144-152 peptide | Clinical trial-II | |
| miRNA | miR-219-5p | Human | — | In vitro or in vivo studies |
| miR-520c-3p | Human | — | In vitro studies | |
| miR-1271 | Human | — | In vitro studies | |
| shRNA | GPC3 shRNA | Human | — | In vitro or in vivo studies |
| siRNA | GPC3 siRNA | Human | — | In vitro or in vivo studies |
GPC-3: Glypican-3; aa: Amino acid; miR: MicroRNA; shRNA: Small hairpin RNA; siRNA: Small interfering RNA.
Different synergisms have been postulated based on the potential interplay between anti-angiogenic drugs and immunotherapy, with several clinical trials currently ongoing. As the most extensively tested combination regimens for advanced HCC comprise anti-PD-1/anti-PD-L1 agents plus anti-angiogenic agents, oncogenic GPC3 is an ideal promising candidate for HCC immunotherapy as it is highly expressed in cancerous tissues but limited in normal livers. Recently, the adoptive transfer of hGPC3-specific chimeric antigen receptor T (CAR-T) cells for HCC treatment has been conducted in clinical trials. Due to rigid construction, conventional CAR-T cells have some intrinsic limitations, such as uncontrollable overactivation and inducing severe cytokine release syndrome. By using co-culturing assays and a xenograft mouse model, the in vitro and in vivo cytotoxicity and cytokine release of the split anti-hGPC3 CAR-T cells were evaluated against various HCC cell lines and compared with conventional CAR-T cells. In vitro data demonstrated that split anti-hGPC3 CAR-T cells could recognize and lyse hGPC3-positive HepG2 or Huh7 cells in a dose-dependent manner. Impressively, the split anti-hGPC3 CAR-T cells produced and released a significantly lower amount of pro-inflammatory cytokines, including IFN-γ, TNF-α, IL-6, and GM-CSF, than conventional CAR-T cells. When injected into immune-deficient mice inoculated subcutaneously with HepG2 cells, the split anti-hGPC3 CAR-T cells could suppress HCC growth, but released significantly lower levels of cytokines than conventional CAR-T cells. The split anti-hGPC3 CAR-T cells reduced the level of cytokine release, and represent a more versatile and safer alternative to conventional CAR-T cells for HCC treatment[35,36]. The most recent data indicate novel combination strategies and targets, and a future role for molecular therapies in the treatment of advanced HCC. Current barriers in CAR-T therapy include its high production cost and the need to identify validated extracellular HCC-specific antigens[33,37].
WNT3a
Several signaling pathways involved in HCC have been studied, including STAT3- NFκB, JAK-STAT, RAS MAPK, PI3K-AKT-mTOR and Wnt-β-catenin. Of these, cascades involving mitogen-activated protein kinase (MAPK) emerge as key regulators of HCC. Both HBV and HCV infection can induce activation of the Wnt/β-catenin signal pathway and participate in HCC progression[38,39]. Oncogenic HBx of HBV can activate Src kinase to inhibit GSK3 activity and induce intracellular β-catenin accumulation, promote DNA methyl-transferase I expression and Wnt3a to bind and silence secreted frizzled related protein 1 and 5[40]. HBx can reduce the inhibitory role of deacetylase 1 to β-catenin, and activation of the Wnt pathway promotes HCC development[41]. Also, the core protein of HCV can promote Wnt3a expression, induce TCF dependent transcription, inhibit GSK3, increase and stabilize intracellular β-catenin to nucleus transport, and up-regulate the expression of cyclinDl, c-myc, WISP2, Wnt3a, Wnt1 and CTGF to promote HCC growth, and DNA synthesis for HCC progression[42]. Wnt3a is a critical signal molecule among the 19 mammalian Wnt proteins. A higher level of Wnt3a expression was only found in the sera or tissues of HCC patients from a cohort of cases with chronic liver diseases[43,44], and it is the first report of Wnt3a as a novel specific marker for HCC diagnosis and prognosis[45,46].
Abnormal Wnt3a expression is involved in the development and metastasis of HCC[47], and may be a novel strategy for HBV or HCV-related HCC therapy. High hepatic LINC00662 correlated with poor survival of HCC patients[48,49], and might up-regulate Wnt3a expression by competitively binding miR-15a, miR-16 and miR-107, with tumor-associated macrophages as a major component of the HCC microenvironment, and they have been revealed to have associations with Wnt3a signaling and cancer initiation, tumor growth, metastasis, dormancy, immunity and tumor stem cell maintenance[40]. Wnt3a is one of HCC-related Wnt signals exhibiting numerous genetic abnormalities as well as epigenetic alterations including modulation of DNA methylation. Targeted Wnt3a gene transcription might be an effective molecule-targeted therapy. The novel Crispr/Cas9-gsRNA lentiviral vector system with the advantages of higher targeting accuracy has been successfully used to inhibit Wnt3a in liver cancer cell lines at the mRNA level in vitro and confirmed at the protein level in vivo in transplanted tumor studies[44,50].
The inhibitory effect of Wnt3a on the proliferation of HCC cells or HCC xenograft growth has been demonstrated and interfering with Wnt3a could significantly inhibit the expression of down-stream β-catenin and related-signal molecules[51]. The xenograft model of knockout Wnt3a in HepG2 cells resulted in slower growth, and a significant reduction in tumor size or loss of weight. The molecular mechanism of the Wnt3a cascade reaction involving multiple targets, can block upstream GPC-3 signals and downstream β-catenin to nucleus transport[52,53], and inhibiting or delaying HCC progression can be carried out using specific antibodies (OMP-54F28, OTSA101)[54] and small size peptide SAH-BCL-9[55]. The abnormal liver or circulating Wnt3a in HCC has provided initial evidence, and the tumor volume after intervening in Wnt3a mRNA transcription with specific shRNA was 355.0 ± 99.9 mm3 in the intervention group which was significantly lower than that (869.4 ± 222.5 mm3) in the negative group, and the time to tumor formation in the intervention group was longer than that in the negative group; the tumor weight (0.35 ± 0.11 g) in the intervention group was markedly lower than that (0.88 ± 0.20 g) in the negative group. Immunohistochemistry confirmed that Wnt3a was strongly inhibited in the intervention group[56], and indicated that targeted-Wnt3a signaling could result in effective inhibition of HCC growth.
CLUSTERIN
Secretory clusterin (sCLU) is a stress-induced heterodimer sulfated glycoprotein, located on chromosome 8q21-q12, which is highly conserved between species and has a cytoprotective effect. Its biological function as a small molecule partner is almost similar to that of heat shock protein[57]. Basic and clinical studies have shown that sCLU expression was low in normal liver tissues and its activation during the malignant transformation of hepatocytes was progressively over-expressed[58,59], which was closely associated with HCC progression by contributing to angiogenesis, chemo-resistance, cell survival, and metastasis[60]. The positive rate of hepatic sCLU expression was up to 73.3% in stage I HCC by immunohistochemical analysis. Its expression at the mRNA or protein level was increased with clinical staging of HCC, which indicated that sCLU could be a biomarker for differentiating benign from malignant liver diseases[61].
Recurrence and metastasis after hepatectomy are the main causes of poor prognosis of HCC[62]. Hepatic sCLU plays an important role in the proliferation, multidrug resistance, invasion and metastasis of HCC cells[63,64]. sCLU mediated the expression of MMP-2, p-AKT and E-cadherin in HCC BEL-7402 or SMMC-7721 cell lines, and down-regulating sCLU expression can significantly reduce the invasive ability of HCC cells by the selective COX-2 inhibitor meloxicam plus specific sCLU-shRNA plasmids[65,66]. These data indicated that sCLU is a new effective target for the occurrence, invasion and metastasis of HCC, and should have a bright future in HCC immunotherapy.
INSULIN-LIKE GROWTH FACTOR AXIS
The hepatic insulin-like growth factor (IGF) axis contains ligands, receptors, substrates, and ligand binding proteins. Accumulating data have demonstrated that aberrant IGF signaling might lead to malignant transformation of hepatocytes or HCC progression, in particular, IGF-II or IGF-I receptor (IGF-IR) are key molecules in hepatocarcinogenesis[67] or rat xenograft models[68], and affect the molecular pathogenesis of HCC, thus providing the rationale for targeting the IGF axis in HCC[69]. The biological activities of IGF-II or IGF-IR not only promote HCC cell proliferation or xenograft growth, but also confer resistance to standard treatments[70]. Several strategies targeting this system including monoclonal antibodies against IGF-1R or small molecule inhibitors of the tyrosine kinase function of IGF-1R are under active investigation. For example, DX-2647, a recombinant human antibody, potently neutralizes the action of IGF-II, which is overexpressed in HCC[71] and impairs xenograft growth of the Hep3B but not HepG2 cell line with high p-STAT3 levels, suggesting that STAT3 activation is one pathway that mediates resistance to IGF-II-targeted therapy in HCC[72].
The over-expression of hepatic IGF-IR in human HCC promotes HCC cell proliferation, and attaching importance to IGF-IR might improve the prognostic or therapy of HCC[73]. Enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) is a regulator of promoted IGF-IR induced sorafenib resistance of HCC in vitro by directly transcriptionally repressing a set of microRNAs including miR-101, miR-122, miR-125b, and miR-139[74-76]. A model of an EZH2-miRNAs-IGF-IR regulatory axis might provide insights into how to reverse sorafenib resistance in HCC. Silencing the IGF-IR gene by a specific shRNA to induce inhibition of cell proliferation in vitro or rat xenograft growth in vivo may be a novel molecular-targeted therapy for HCC. Several strategies targeting this system including monoclonal antibodies against IGF-IR and inhibitors of the tyrosine kinase function of IGF-IR are under active investigation. Gene-specific shRNA against IGF-signaling molecules as well as IGF-IR selective receptor tyrosine kinase (RTK)-inhibitors (tyrphostins) may therefore offer new therapeutic options[77,78]. However, as a specific shRNA is currently not applicable in HCC therapy, selective RTK-inhibitors represent the most promising approach for future therapeutic strategies.
SYNERGY OF NON-CODING RNAS
While immunotherapy holds great promise for combating cancer, its limited efficacy due to an immunosuppressive tumor microenvironment and systemic toxicity hinder the broader application of immunotherapy[79,80]. Combinatorial immunotherapy approaches that use a highly efficient and tumor-selective gene carrier can improve anticancer efficacy and circumvent the systemic toxicity. HCC is one of the multi-genetic diseases, and multiple studies have highlighted the key roles of noncoding RNAs (ncRNAs) in the chemo-resistance of HCC such as biomarkers and functional modulation of the cellular response to sorafenib[81-83]. Targeted chemotherapeutic agent, sorafenib, is known to show a statistically significant but limited OS advantage in advanced HCC, linked with the modulation of several intracellular signaling pathways through diverse operating biomolecules including ncRNAs[84-86]. Accumulated evidence has demonstrated that ncRNAs (miRNAs, long ncRNAs or lncRNAs, and circular RNA or circRNA) could serve as biomarkers in the diagnosis, prognosis, and treatment of HCC[87,88] and have been well-documented to participate in HCC progression with promoting or inhibiting roles[89,90].
Interestingly, varied responses to miRNAs have been linked with the modulation of several intracellular signaling pathways[91]. An abnormality of miR-218 expression was investigated in human HCC tissues or HCC cell lines to evaluate its function and the underlying mechanisms of HCC. Gain-of-function and loss-of-function assays indicated forced expression of miR-218 by inhibited HCC cell migration/invasion and reversed epithelial-mesenchymal transition to mesenchymal-epithelial transition. Serpine mRNA binding protein 1 (SERBP1) is a target gene of miR-218, and targeting the miR-218/SERBP1 signal pathway that inhibits malignant phenotype formation might be a potential novel strategy for HCC therapeutics, as miR-218 functions as a HCC suppressor and is involved in many biological processes such as tumor initiation, development, and metastasis[92]. Nanotechnology-enabled dual delivery of siRNA and plasmid DNA that selectively targets and reprograms the immune-suppressive tumor microenvironment has been shown to improve HCC immunotherapy[93-95].
HCC-associated circRNAs are abundant, and their over/low expression might promote/inhibit HCC cell proliferation or tumor growth[96-98]. An abnormality of circ-homer1 in HCC cells or tissues was related to tumor size, lymph node metastasis, high clinical staging and poor prognosis. The mechanism of circ-homer1 over-expression promoted HCC growth or invasiveness via the mir-1322/cxc16 axis[99]; conversely, interfering with circ-homer1 activation inhibited the proliferation, migration and invasion of liver cancer cells via apoptosis. The circ-0051443 from circulating exosomes or HCC tissues regulated BAK1 expression by combining with mir-331-3p to promote cell apoptosis or cell cycle arrest in HCC, and inhibit the biological behavior of HCC cells in vivo or nude mice HCC xenografts[100]. Another interesting study also showed that has_circ_0008450 expression in HCC tissues or cells might inhibit HCC progression by regulating the mir-214-3p/ezh2 axis[101,102]. These data suggested that specific ncRNAs were useful molecular targets for HCC therapy.
CONCLUSION
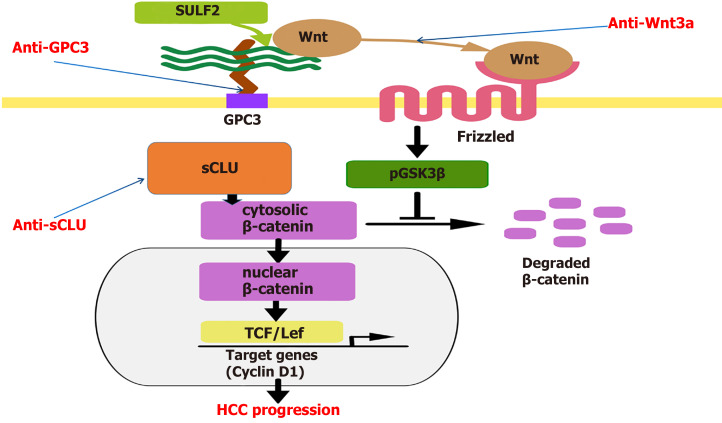
In conclusion, HCC is a multi-gene variant malignant tumor with DNA methylation, microRNA, lncRNA expression and immune response[103]. Immunotherapy for HCC has begun to produce better results, and HCC-specific molecules may be combined with comprehensive interventions such as surgery, interventional therapy, radiotherapy, and chemotherapy to improve the efficacy and prolong the survival time of HCC patients[104]. Despite the rapid development of genomics and proteomics, advances in molecular pathology, pharmacology and genetic engineering, DNA splicing, gene silencing, transcription interference, and monoclonal antibodies for more specific and less side effects immune therapy techniques[105] that can directly block the signaling molecules involved in HCC growth related signaling pathways (Figure 1) or serve as molecular targets such as radionuclide, drug carriers, and immunotherapy play a unique role in the specific or comprehensive treatment of HCC.
Figure 1.
Some signals in the Wnt/β-catenin pathway by anti-signaling antibodies or intervening in their gene transcription to inhibit hepatocellular carcinoma growth. Using anti-signaling molecule antibodies or intervening in their gene transcription to inhibit Wnt/β-catenin pathway activation could suppress proliferation of hepatocellular carcinoma (HCC) cells or HCC growth. GPC-3: Glypican-3; HCC: Hepatocellular carcinoma; sCLU: Secretory clusterin; TCF: T-cell factor; SULF2: Sulfatase 2; pGSK3β: Phosphorylated glycogen synthase kinase 3β.
Footnotes
Conflict-of-interest statement: The authors report no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 19, 2021
First decision: May 1, 2021
Article in press: May 25, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li Y S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Yu HG
Contributor Information
Min Yao, Research Center of Clinical Medicine & Department of Immunology, Medical School of Nantong University, Nantong 226001, Jiangsu Province, China.
Jun-Ling Yang, Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China.
De-Feng Wang, Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China.
Li Wang, Department of Medical Informatics, Medical School of Nantong University, Nantong 226001, Jiangsu Province, China.
Ying Chen, Department of Oncology, Affiliated Second Hospital of Nantong University, Nantong 226001, Jiangsu Province, China.
Deng-Fu Yao, Research Center of Clinical Medicine, Affiliated Hospital of Nantong University, Nantong 226001, Jiangsu Province, China. yaodf@ahnmc.com.
References
- 1.Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17:139–152. doi: 10.1038/s41575-019-0229-4. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen JG, Zhu J, Zhang YH, Zhang YX, Yao DF, Chen YS, Lu JH, Ding LL, Chen HZ, Zhu CY, Yang LP, Zhu YR, Qiang FL. Cancer survival in Qidong between 1972 and 2011: A population-based analysis. Mol Clin Oncol. 2017;6:944–954. doi: 10.3892/mco.2017.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negro F. Natural history of NASH and HCC. Liver Int. 2020;40 Suppl 1:72–76. doi: 10.1111/liv.14362. [DOI] [PubMed] [Google Scholar]
- 5.Wang SZ, Lee SD, Sarkar D, Lee HM, Khan A, Bhati C, Sharma A, Kumaran V, Bruno D, Cotterell A, Levy MF. Immunological characterization of hepatocellular carcinoma. Hepatoma Res. 2021;7:6. [Google Scholar]
- 6.Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: Basic concepts and therapeutic implications. J Hepatol. 2020;72:167–182. doi: 10.1016/j.jhep.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215–229. doi: 10.1016/j.jhep.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Zhu AX, Farah W, Almasri J, Zaiem F, Prokop LJ, Murad MH, Mohammed K. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology. 2018;67:422–435. doi: 10.1002/hep.29486. [DOI] [PubMed] [Google Scholar]
- 9.Doycheva I, Thuluvath PJ. Systemic Therapy for Advanced Hepatocellular Carcinoma: An Update of a Rapidly Evolving Field. J Clin Exp Hepatol. 2019;9:588–596. doi: 10.1016/j.jceh.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Agnano I, Berardi AC. Extracellular Vesicles, A Possible Theranostic Platform Strategy for Hepatocellular Carcinoma-An Overview. Cancers (Basel) 2020;12 doi: 10.3390/cancers12020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia S, Pan Y, Liang Y, Xu J, Cai X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2020;51:102610. doi: 10.1016/j.ebiom.2019.102610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reig M, da Fonseca LG, Faivre S. New trials and results in systemic treatment of HCC. J Hepatol. 2018;69:525–533. doi: 10.1016/j.jhep.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Casadei-Gardini A, Orsi G, Caputo F, Ercolani G. Developments in predictive biomarkers for hepatocellular carcinoma therapy. Expert Rev Anticancer Ther. 2020;20:63–74. doi: 10.1080/14737140.2020.1712198. [DOI] [PubMed] [Google Scholar]
- 14.Luo P, Wu S, Yu Y, Ming X, Li S, Zuo X, Tu J. Current Status and Perspective Biomarkers in AFP Negative HCC: Towards Screening for and Diagnosing Hepatocellular Carcinoma at an Earlier Stage. Pathol Oncol Res. 2020;26:599–603. doi: 10.1007/s12253-019-00585-5. [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307–319. doi: 10.1016/j.jhep.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Li XJ, Shao DH, He ML, Liang GW. Association of Common Variants in HNF1A Gene with Serum AFP Level in Healthy Chinese Individuals and HCC Patients. Dis Markers. 2019;2019:6273497. doi: 10.1155/2019/6273497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Docta RY, Ferronha T, Sanderson JP, Weissensteiner T, Pope GR, Bennett AD, Pumphrey NJ, Ferjentsik Z, Quinn LL, Wiedermann GE, Anderson VE, Saini M, Maroto M, Norry E, Gerry AB. Tuning T-Cell Receptor Affinity to Optimize Clinical Risk-Benefit When Targeting Alpha-Fetoprotein-Positive Liver Cancer. Hepatology. 2019;69:2061–2075. doi: 10.1002/hep.30477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Gong H, Liu Q, Wu W, Cheng J, Mei Y, Chen Y, Zheng H, Yu X, Zhong S, Li Y. Identification of an HLA-A*24:02-restricted α-fetoprotein signal peptide-derived antigen and its specific T-cell receptor for T-cell immunotherapy. Immunology. 2020;159:384–392. doi: 10.1111/imm.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agdashian D, ElGindi M, Xie C, Sandhu M, Pratt D, Kleiner DE, Figg WD, Rytlewski JA, Sanders C, Yusko EC, Wood B, Venzon D, Brar G, Duffy AG, Greten TF, Korangy F. The effect of anti-CTLA4 treatment on peripheral and intra-tumoral T cells in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:599–608. doi: 10.1007/s00262-019-02299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HW, Cho KJ, Park JY. Current Status and Future Direction of Immunotherapy in Hepatocellular Carcinoma: What Do the Data Suggest? Immune Netw. 2020;20:e11. doi: 10.4110/in.2020.20.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderborght B, Lefere S, Vlierberghe HV, Devisscher L. The Angiopoietin/Tie2 Pathway in Hepatocellular Carcinoma. Cells. 2020;9 doi: 10.3390/cells9112382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed A, Hildebrand H, Park R, Al-Jumayli M, Abbasi S, Melancon T, Saeed A, Al-Rajabi R, Kasi A, Baranda J, Williamson S, Sun W. Immune Checkpoint Inhibitors versus VEGF Targeted Therapy as Second Line Regimen in Advanced Hepatocellular Carcinoma (HCC): A Retrospective Study. J Clin Med. 2020;9 doi: 10.3390/jcm9092682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syed YY. Ramucirumab: A Review in Hepatocellular Carcinoma. Drugs. 2020;80:315–322. doi: 10.1007/s40265-020-01263-6. [DOI] [PubMed] [Google Scholar]
- 25.De Luca E, Marino D, Di Maio M. Ramucirumab, A Second-Line Option For Patients With Hepatocellular Carcinoma: A Review Of The Evidence. Cancer Manag Res. 2020;12:3721–3729. doi: 10.2147/CMAR.S216220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chai NX, Chapiro J. Therapy of Intermediate-Stage Hepatocellular Carcinoma: Current Evidence and Clinical Practice. Semin Intervent Radiol. 2020;37:456–465. doi: 10.1055/s-0040-1719186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim H, Ramjeesingh R, Liu D, Tam VC, Knox JJ, Card PB, Meyers BM. Optimizing Survival and the Changing Landscape of Targeted Therapy for Intermediate and Advanced Hepatocellular Carcinoma: A Systematic Review. J Natl Cancer Inst. 2021;113:123–136. doi: 10.1093/jnci/djaa119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao CS, Yin H, Ning HB, Peng Z, Li K, Ding GQ. Levels of HBx, VEGF, and CEACAM1 in HBV-related hepatocellular carcinoma and their correlation with cancer prognosis. Eur Rev Med Pharmacol Sci. 2017;21:3827–3833. [PubMed] [Google Scholar]
- 29.Park R, Eshrat F, Al-Jumayli M, Saeed A. Immuno-Oncotherapeutic Approaches in Advanced Hepatocellular Carcinoma. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao M, Wang L, Fang M, Zheng W, Dong Z, Yao D. Advances in the study of oncofetal antigen glypican-3 expression in HBV-related hepatocellular carcinoma. Biosci Trends. 2016;10:337–343. doi: 10.5582/bst.2016.01176. [DOI] [PubMed] [Google Scholar]
- 31.Zhou F, Shang W, Yu X, Tian J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38:741–767. doi: 10.1002/med.21455. [DOI] [PubMed] [Google Scholar]
- 32.Cao W, Sharma M, Imam R, Yu J. Study on Diagnostic Values of Astrocyte Elevated Gene 1 (AEG-1) and Glypican 3 (GPC-3) in Hepatocellular Carcinoma. Am J Clin Pathol. 2019;152:647–655. doi: 10.1093/ajcp/aqz086. [DOI] [PubMed] [Google Scholar]
- 33.. Erratum for the Research Article: "PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma" by X. Tan, P. Banerjee, E. A. Pham, F. U. N. Rutaganira, K. Basu, N. Bota-Rabassedas, H.-F. Guo, C. L. Grzeskowiak, X. Liu, J. Yu, L. Shi, D. H. Peng, B. L. Rodriguez, J. Zhang, V. Zheng, D. Y. Duose, L. M. Solis, B. Mino, M. G. Raso, C. Behrens, I. I. Wistuba, K. L. Scott, M. Smith, K. Nguyen, G. Lam, I. Choong, A. Mazumdar, J. L. Hill, D. L. Gibbons, P. H. Brown, W. K. Russell, K. Shokat, C. J. Creighton, J. S. Glenn, J. M. Kurie. Sci Transl Med. 2020;12 [Google Scholar]
- 34.Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers (Basel) 2019;11 doi: 10.3390/cancers11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Wen J, Yi H, Hou X, Yin Y, Ye G, Wu X, Jiang X. Split chimeric antigen receptor-modified T cells targeting glypican-3 suppress hepatocellular carcinoma growth with reduced cytokine release. Ther Adv Med Oncol. 2020;12:1758835920910347. doi: 10.1177/1758835920910347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faivre S, Rimassa L, Finn RS. Molecular therapies for HCC: Looking outside the box. J Hepatol. 2020;72:342–352. doi: 10.1016/j.jhep.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 2018;53:164–181. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Piconese S, Cammarata I, Barnaba V. Viral hepatitis, inflammation, and cancer: A lesson for autoimmunity. J Autoimmun. 2018;95:58–68. doi: 10.1016/j.jaut.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Jiang XH, Xie YT, Cai YP, Ren J, Ma T. Effects of hepatitis C virus core protein and nonstructural protein 4B on the Wnt/β-catenin pathway. BMC Microbiol. 2017;17:124. doi: 10.1186/s12866-017-1032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA, Yan KS, Marecic O, Siepe D, Li X, Moody JD, Williams BO, Clevers H, Piehler J, Baker D, Kuo CJ, Garcia KC. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature. 2017;545:234–237. doi: 10.1038/nature22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timperi E, Barnaba V. Viral Hepatitides, Inflammation and Tumour Microenvironment. Adv Exp Med Biol. 2020;1263:25–43. doi: 10.1007/978-3-030-44518-8_3. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Yao M, Fang M, Zheng WJ, Dong ZZ, Pan LH, Zhang HJ, Yao DF. Expression of hepatic Wnt5a and its clinicopathological features in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2018;17:227–232. doi: 10.1016/j.hbpd.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Sai WL, Yao M, Zheng WJ, Wu MN, Sun JY, Pan LH, Dong ZZ, Yao DF. [Abnormal expression of Wnt3a and inhibiting role of its molecular-targeted intervening in hepatocellular carcinoma] Zhonghua Gan Zang Bing Za Zhi . 2019;27:866–871. doi: 10.3760/cma.j.issn.1007-3418.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, Yao M, Fang M, Pan L, Wang L, Yang J, Dong Z, Yao D. Oncogenic Wnt3a: A Candidate Specific Marker and Novel Molecular Target for Hepatocellular Carcinoma. J Cancer. 2019;10:5862–5873. doi: 10.7150/jca.31599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan L, Yao M, Zheng W, Gu J, Yang X, Qiu L, Cai Y, Wu W, Yao D. Abnormality of Wnt3a expression as novel specific biomarker for diagnosis and differentiation of hepatocellular carcinoma. Tumour Biol. 2016;37:5561–5568. doi: 10.1007/s13277-015-4413-z. [DOI] [PubMed] [Google Scholar]
- 46.Jingjing H, Hongna H, Wenfu Z, Jianlin L, Guochu H, Yuanjia L, Songlin C, Yueqiang H. Bie Jia Jian Pill Combined with Bone Mesenchymal Stem Cells Regulates microRNA-140 to Suppress Hepatocellular Carcinoma Stem Cells. Int J Stem Cells. 2021 doi: 10.15283/ijsc20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu C, He Y, Duan J, Yang Y, Zhong C, Zhang J, Liao W, Huang X, Zhu R, Li M. Expression of Wnt3a in hepatocellular carcinoma and its effects on cell cycle and metastasis. Int J Oncol. 2017;51:1135–1145. doi: 10.3892/ijo.2017.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian X, Wu Y, Yang Y, Wang J, Niu M, Gao S, Qin T, Bao D. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol Oncol. 2020;14:462–483. doi: 10.1002/1878-0261.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You Y, Que K, Zhou Y, Zhang Z, Zhao X, Gong J, Liu Z. MicroRNA-766-3p Inhibits Tumour Progression by Targeting Wnt3a in Hepatocellular Carcinoma. Mol Cells. 2018;41:830–841. doi: 10.14348/molcells.2018.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abd Elhameed AG, Helal MG, Said E, Salem HA. Saxagliptin Defers Thioacetamide-Induced Hepatocarcinogenesis in Rats; A Novel Suppressive Impact on Wnt/Hedgehog /Notch1 Signaling. Environ Toxicol Pharmacol. 2021:103668. doi: 10.1016/j.etap.2021.103668. [DOI] [PubMed] [Google Scholar]
- 51.Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y, Feng M, Lee B, Gao W, Ho M. A Frizzled-Like Cysteine-Rich Domain in Glypican-3 Mediates Wnt Binding and Regulates Hepatocellular Carcinoma Tumor Growth in Mice. Hepatology. 2019;70:1231–1245. doi: 10.1002/hep.30646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Rajasekaran M, Xia H, Zhang X, Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL, Hong W, Hui KM. The microtubule-associated protein PRC1 promotes early recurrence of hepatocellular carcinoma in association with the Wnt/β-catenin signalling pathway. Gut. 2016;65:1522–1534. doi: 10.1136/gutjnl-2015-310625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Ye YC, Chen Y, Zhao JL, Gao CC, Han H, Liu WC, Qin HY. Crosstalk between hepatic tumor cells and macrophages via Wnt/β-catenin signaling promotes M2-like macrophage polarization and reinforces tumor malignant behaviors. Cell Death Dis. 2018;9:793. doi: 10.1038/s41419-018-0818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebio A, Kahn M, Lenz HJ. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther Targets. 2014;18:611–615. doi: 10.1517/14728222.2014.906580. [DOI] [PubMed] [Google Scholar]
- 55.Takada K, Zhu D, Bird GH, Sukhdeo K, Zhao JJ, Mani M, Lemieux M, Carrasco DE, Ryan J, Horst D, Fulciniti M, Munshi NC, Xu W, Kung AL, Shivdasani RA, Walensky LD, Carrasco DR. Targeted disruption of the BCL9/β-catenin complex inhibits oncogenic Wnt signaling. Sci Transl Med. 2012;4:148ra117. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chikhaliwala P, Rai R, Chandra S. Simultaneous voltammetric immunodetection of alpha-fetoprotein and glypican-3 using a glassy carbon electrode modified with magnetite-conjugated dendrimers. Mikrochim Acta. 2019;186:255. doi: 10.1007/s00604-019-3354-4. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Liu F, Zhou W, Zhang S, Chu P, Lin F, Wang HL. Diagnostic value of clusterin immunostaining in hepatocellular carcinoma. Diagn Pathol. 2020;15:127. doi: 10.1186/s13000-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng W, Yao M, Qian Q, Sai W, Qiu L, Yang J, Wu W, Dong Z, Yao D. Oncogenic secretory clusterin in hepatocellular carcinoma: Expression at early staging and emerging molecular target. Oncotarget. 2017;8:52321–52332. doi: 10.18632/oncotarget.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Udomsinprasert W, Poovorawan Y, Chongsrisawat V, Vejchapipat P, Honsawek S. Decreased circulating clusterin reflects severe liver complications after hepatoportoenterostomy of biliary atresia. Sci Rep. 2020;10:19736. doi: 10.1038/s41598-020-76875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Liu Y, Qin Q, Zheng T. Clusterin role in hepatocellular carcinoma patients treated with oxaliplatin. Biosci Rep. 2020;40 doi: 10.1042/BSR20200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park JS, Lee WK, Kim HS, Seo JA, Kim DH, Han HC, Min BH. Clusterin overexpression protects against western diet-induced obesity and NAFLD. Sci Rep. 2020;10:17484. doi: 10.1038/s41598-020-73927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao M, Sai W, Zheng W, Wang L, Dong Z, Yao D. Secretory Clusterin as a Novel Molecular-targeted Therapy for Inhibiting Hepatocellular Carcinoma Growth. Curr Med Chem. 2020;27:3290–3301. doi: 10.2174/0929867326666190624161158. [DOI] [PubMed] [Google Scholar]
- 63.Fu N, Du H, Li D, Lu Y, Li W, Wang Y, Kong L, Du J, Zhao S, Ren W, Han F, Wang R, Zhang Y, Nan Y. Clusterin contributes to hepatitis C virus-related hepatocellular carcinoma by regulating autophagy. Life Sci. 2020;256:117911. doi: 10.1016/j.lfs.2020.117911. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Zou F, Zhong J, Yue L, Wang F, Wei H, Yang G, Jin T, Dong X, Li J, Xiu P. Secretory Clusterin Mediates Oxaliplatin Resistance via the Gadd45a/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. J Cancer. 2018;9:1403–1413. doi: 10.7150/jca.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu B, Shang H, Liang X, Sun Y, Jing H, Han X, Cheng W. Preparation of novel targeting nanobubbles conjugated with small interfering RNA for concurrent molecular imaging and gene therapy in vivo. FASEB J. 2019;33:14129–14136. doi: 10.1096/fj.201900716RR. [DOI] [PubMed] [Google Scholar]
- 66.Kuo PC, Chau IY, Li AF, Chau YP, Hsia CY, Chau GY. Clusterin expression in nontumor tissue in patients with resectable hepatocellular carcinoma related with postresectional survival. J Chin Med Assoc. 2019;82:929–934. doi: 10.1097/JCMA.0000000000000195. [DOI] [PubMed] [Google Scholar]
- 67.Tai BJ, Yao M, Zheng WJ, Shen YC, Wang L, Sun JY, Wu MN, Dong ZZ, Yao DF. Alteration of oncogenic IGF-II gene methylation status associates with hepatocyte malignant transformation. Hepatobiliary Pancreat Dis Int. 2019;18:158–163. doi: 10.1016/j.hbpd.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Ngo MT, Jeng HY, Kuo YC, Diony Nanda J, Brahmadhi A, Ling TY, Chang TS, Huang YH. The Role of IGF/IGF-1R Signaling in Hepatocellular Carcinomas: Stemness-Related Properties and Drug Resistance. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, Yao M, Zheng W, Fang M, Wu M, Sun J, Dong Z, Yao D. Insulin-like Growth Factor I Receptor: A Novel Target for Hepatocellular Carcinoma Gene Therapy. Mini Rev Med Chem. 2019;19:272–280. doi: 10.2174/1389557518666181025151608. [DOI] [PubMed] [Google Scholar]
- 70.Hua H, Kong Q, Yin J, Zhang J, Jiang Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: a challenge for cancer therapy. J Hematol Oncol. 2020;13:64. doi: 10.1186/s13045-020-00904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799–806. doi: 10.1309/AJCPTFDSE2V3LCZP. [DOI] [PubMed] [Google Scholar]
- 72.Greenall SA, Donoghue J, Johns TG, Adams TE. Differential Sensitivity of Human Hepatocellular Carcinoma Xenografts to an IGF-II Neutralizing Antibody May Involve Activated STAT3. Transl Oncol. 2018;11:971–978. doi: 10.1016/j.tranon.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bie C, Chen Y, Tang H, Li Q, Zhong L, Peng X, Shi Y, Lin J, Lai J, Wu S, Tang S. Insulin-Like Growth Factor 1 Receptor Drives Hepatocellular Carcinoma Growth and Invasion by Activating Stat3-Midkine-Stat3 Loop. Dig Dis Sci. 2021 doi: 10.1007/s10620-021-06862-1. [DOI] [PubMed] [Google Scholar]
- 74.Cheng Z, Wei-Qi J, Jin D. New insights on sorafenib resistance in liver cancer with correlation of individualized therapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188382. doi: 10.1016/j.bbcan.2020.188382. [DOI] [PubMed] [Google Scholar]
- 75.Ghosh MK, Patra F, Ghosh S, Hossain CM, Mukherjee B. Antisense oligonucleotides directed against insulin-like growth factor-II messenger ribonucleic acids delay the progress of rat hepatocarcinogenesis. J Carcinog. 2014;13:2. doi: 10.4103/1477-3163.126761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei L, Wang X, Lv L, Liu J, Xing H, Song Y, Xie M, Lei T, Zhang N, Yang M. The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma. Mol Cancer. 2019;18:147. doi: 10.1186/s12943-019-1086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yao N, Yao D, Wang L, Dong Z, Wu W, Qiu L, Yan X, Yu D, Chen J, Sai W, Zhang H, Yang J. Inhibition of autocrine IGF-II on effect of human HepG2 cell proliferation and angiogenesis factor expression. Tumour Biol. 2012;33:1767–1776. doi: 10.1007/s13277-012-0436-x. [DOI] [PubMed] [Google Scholar]
- 78.Mohamed YI, Lee S, Xiao L, Hassan MM, Qayyum A, Hiatia R, Pestana RC, Haque A, George B, Rashid A, Duda DG, Elghazaly H, Wolff RA, Morris JS, Yao J, Amin HM, Kaseb AO. Insulin-like growth factor 1/Child-Turcotte-Pugh composite score as a predictor of treatment outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncotarget. 2021;12:756–766. doi: 10.18632/oncotarget.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanthaje S, Makol A, Chakraborti A. Sorafenib response in hepatocellular carcinoma: MicroRNAs as tuning forks. Hepatol Res. 2018;48:5–14. doi: 10.1111/hepr.12991. [DOI] [PubMed] [Google Scholar]
- 80.Huang KW, Hsu FF, Qiu JT, Chern GJ, Lee YA, Chang CC, Huang YT, Sung YC, Chiang CC, Huang RL, Lin CC, Dinh TK, Huang HC, Shih YC, Alson D, Lin CY, Lin YC, Chang PC, Lin SY, Chen Y. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci Adv. 2020;6:eaax5032. doi: 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Niu L, Liu L, Yang S, Ren J, Lai PBS, Chen GG. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer. 2017;1868:564–570. doi: 10.1016/j.bbcan.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Zhou B, Yang H, Yang C, Bao YL, Yang SM, Liu J, Xiao YF. Translation of noncoding RNAs and cancer. Cancer Lett. 2021;497:89–99. doi: 10.1016/j.canlet.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Gramantieri L, Pollutri D, Gagliardi M, Giovannini C, Quarta S, Ferracin M, Casadei-Gardini A, Callegari E, De Carolis S, Marinelli S, Benevento F, Vasuri F, Ravaioli M, Cescon M, Piscaglia F, Negrini M, Bolondi L, Fornari F. MiR-30e-3p Influences Tumor Phenotype through MDM2/TP53 Axis and Predicts Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2020;80:1720–1734. doi: 10.1158/0008-5472.CAN-19-0472. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, He X, Zhang X, Xu Y, Wu Y, Xu X. Immune-related microRNA signature for predicting prognosis and the immune microenvironment in hepatocellular carcinoma. Life Sci. 2021;265:118799. doi: 10.1016/j.lfs.2020.118799. [DOI] [PubMed] [Google Scholar]
- 85.Yugawa K, Yoshizumi T, Mano Y, Itoh S, Harada N, Ikegami T, Kohashi K, Oda Y, Mori M. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur J Surg Oncol. 2021;47:384–393. doi: 10.1016/j.ejso.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Lim LJ, Wong SYS, Huang F, Lim S, Chong SS, Ooi LL, Kon OL, Lee CG. Roles and Regulation of Long Noncoding RNAs in Hepatocellular Carcinoma. Cancer Res. 2019;79:5131–5139. doi: 10.1158/0008-5472.CAN-19-0255. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324. doi: 10.1186/s13046-018-0965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun C, Xiao T, Xiao Y, Li Y. Silencing of long noncoding RNA NEAT1 inhibits hepatocellular carcinoma progression by downregulating SMO by sponging microRNA503. Mol Med Rep. 2021;23 doi: 10.3892/mmr.2020.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sukowati CHC, Cabral LKD, Tiribelli C, Pascut D. Circulating Long and Circular Noncoding RNA as Non-Invasive Diagnostic Tools of Hepatocellular Carcinoma. Biomedicines. 2021;9 doi: 10.3390/biomedicines9010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salerno D, Chiodo L, Alfano V, Floriot O, Cottone G, Paturel A, Pallocca M, Plissonnier ML, Jeddari S, Belloni L, Zeisel M, Levrero M, Guerrieri F. Hepatitis B protein HBx binds the DLEU2 lncRNA to sustain cccDNA and host cancer-related gene transcription. Gut. 2020;69:2016–2024. doi: 10.1136/gutjnl-2019-319637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020;20:53–65. doi: 10.3727/105221620X15880179864121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang T, Xu L, Jia R, Wei J. MiR-218 suppresses the metastasis and EMT of HCC cells via targeting SERBP1. Acta Biochim Biophys Sin (Shanghai) 2017;49:383–391. doi: 10.1093/abbs/gmx017. [DOI] [PubMed] [Google Scholar]
- 93.Aspichueta P. Lipid-rich environment: a key role promoting carcinogenesis in obesity-related non-alcoholic fatty liver disease. Gut. 2018;67:1376–1377. doi: 10.1136/gutjnl-2018-316047. [DOI] [PubMed] [Google Scholar]
- 94.Zayac A, Almhanna K. Hepatobiliary cancers and immunotherapy: where are we now and where are we heading? Transl Gastroenterol Hepatol. 2020;5:8. doi: 10.21037/tgh.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gan L, Liu Z, Sun C. Obesity linking to hepatocellular carcinoma: A global view. Biochim Biophys Acta Rev Cancer. 2018;1869:97–102. doi: 10.1016/j.bbcan.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H, Deng T, Ge S, Liu Y, Bai M, Zhu K, Fan Q, Li J, Ning T, Tian F, Li H, Sun W, Ying G, Ba Y. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination-related USP7. Oncogene. 2019;38:2844–2859. doi: 10.1038/s41388-018-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Zhu C, Su Y, Liu L, Wang S, Liu Y, Wu J. Circular RNA hsa_circ_0004277 Stimulates Malignant Phenotype of Hepatocellular Carcinoma and Epithelial-Mesenchymal Transition of Peripheral Cells. Front Cell Dev Biol. 2020;8:585565. doi: 10.3389/fcell.2020.585565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S, Zhang K, Tan S, Xin J, Yuan Q, Xu H, Xu X, Liang Q, Christiani DC, Wang M, Liu L, Du M. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol Cancer. 2021;20:13. doi: 10.1186/s12943-020-01298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao M, Dong G, Meng Q, Lin S, Li X. Circ-HOMER1 enhances the inhibition of miR-1322 on CXCL6 to regulate the growth and aggressiveness of hepatocellular carcinoma cells. J Cell Biochem. 2020;121:4440–4449. doi: 10.1002/jcb.29672. [DOI] [PubMed] [Google Scholar]
- 100.Chen W, Quan Y, Fan S, Wang H, Liang J, Huang L, Chen L, Liu Q, He P, Ye Y. Exosome-transmitted circular RNA hsa_circ_0051443 suppresses hepatocellular carcinoma progression. Cancer Lett. 2020;475:119–128. doi: 10.1016/j.canlet.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 101.Du Q, Han J, Gao S, Zhang S, Pan Y. Hypoxia-induced circular RNA hsa_circ_0008450 accelerates hepatocellular cancer progression via the miR-431/AKAP1 axis. Oncol Lett. 2020;20:388. doi: 10.3892/ol.2020.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin T, Dai Y, Guo X, Chen W, Zhao J, Cao L, Wu Z. Silencing Of hsa_circ_0008450 Represses Hepatocellular Carcinoma Progression Through Regulation Of microRNA-214-3p/EZH2 Axis. Cancer Manag Res. 2019;11:9133–9143. doi: 10.2147/CMAR.S222716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen S, Peng H, Wang Y, Xu M, Lin M, Xie X, Peng B, Kuang M. Screening for immune-potentiating antigens from hepatocellular carcinoma patients after radiofrequency ablation by serum proteomic analysis. BMC Cancer. 2018;18:117. doi: 10.1186/s12885-018-4011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishida N, Kudo M. Immune Phenotype and Immune Checkpoint Inhibitors for the Treatment of Human Hepatocellular Carcinoma. Cancers (Basel) 2020;12 doi: 10.3390/cancers12051274. [DOI] [PMC free article] [PubMed] [Google Scholar]