Abstract
The cardiovascular disease myocarditis is characterized by inflammation and necrosis of cardiac muscle. This disease has been associated with various viral etiologies, including cytomegalovirus (CMV). Murine CMV (MCMV) infection of adult BALB/c mice produces a disease with acute and chronic phases similar to that found in humans. In our murine model, we have investigated the therapeutic efficacy of antiviral drug administration on myocarditis. Two drugs commonly used for CMV treatment, ganciclovir and cidofovir, were subjected to trials, with both drugs showing potent antiviral activity against MCMV both in vitro and in vivo. The acute phase of myocarditis was significantly reduced when antiviral therapy commenced 24 h postinfection. Such treatment also reduced the severity of the chronic phase of myocarditis. In contrast, antiviral treatment commencing after the acute phase had no effect on chronic myocarditis. Reinfection of mice with MCMV caused exacerbation of myocardial inflammation. Such an increase in severity of myocarditis could be prevented with either ganciclovir or cidofovir treatment, but the preexisting inflammation and necrosis of the myocardium persisted. These data highlight possible therapeutic uses of antiviral drugs in viral myocarditis as well as further elucidating the pathogenic nature of the disease.
The cardiovascular disease myocarditis is characterized by inflammation and necrosis of cardiac muscle. The disease ranges from transient inflammation to a fulminant syndrome with manifestations that may include heart failure, arrhythmias, and sudden death. Acute and chronic myocarditis can be induced by a number of agents, including viruses (31). Although enterovirus infections are recognized as a leading cause of myocarditis (27), evidence is accumulating that cytomegalovirus (CMV) is also of importance in inducing myocarditis in humans (8, 11, 21).
The strict host specificity of CMV has not allowed the development of a direct animal model for infection with human CMV. However, murine CMV (MCMV) infection of mice provides an excellent experimental model for investigation of the development of disease. Previous work has shown that MCMV infection of BALB/c mice induces myocarditis (17). A mixed cellular infiltrate composed of polymorphonuclear neutrophils, macrophages, and mononuclear lymphocytes is observed at 3 to 5 days, reaching a peak at 7 to 11 days after MCMV infection. The chronic, autoimmune phase of myocarditis is characterized by a predominantly mononuclear cellular infiltrate, which gradually increases in severity and is maintained for at least 100 days postinfection.
A number of pathogenic mechanisms have been suggested to account for the inflammation and necrosis found in this disease. Early insult to cardiac tissue may involve direct viral infection of the heart. Alternatively, the production of an antiviral immune response during viral infection may cause necrosis of infected myocytes through the release of inflammatory mediators (23, 25, 30, 32). Furthermore, an antiviral immune response that cross-reacts with self antigens, such as myosin, may lead to myocyte necrosis and autoimmune disease (9, 11, 12, 15, 18, 34). However, despite considerable advances in medicine and a greater understanding of myocarditis, treatment for this disease remains primarily supportive. The therapy regime is focused on the myocardial dysfunction and includes such measures as oxygenation and the administration of inotropic agents to increase cardiac output.
The role of virus as a trigger for myocarditis is very clear; therefore, the therapeutic effects of antiviral drugs on the subsequent course of myocarditis has been the focus of recent research. Studies of the coxsackievirus B3 (CB3) myocarditis mouse model have demonstrated a biphasal pattern of disease (26) and have shown some promise for antiviral drugs, with ribavirin being subjected to trials as a possible therapeutic agent (13). Early administration of ribavirin resulted in inhibition of viral replication, a reduction in myocardial damage during the acute phase of disease, and increased survival. However, when treatment commenced on day 4 of the infection, myocardial damage was reduced but there was no survival benefit. Our previous research has shown that MCMV infection of susceptible mice clearly causes the onset of myocarditis (17). We have therefore examined the therapeutic efficacies of several antiviral agents in our MCMV myocarditis model.
In the present study, we have examined the therapeutic effects of two antiviral drugs, ganciclovir (GCV) and cidofovir (CDV). GCV (9-[1,3-dihydroxy-2-propoxymethyl]guanine) has been used extensively in the treatment of CMV retinitis in immunocompromised patients, especially those with AIDS (4). CMV possesses a unique viral kinase capable of phosphorylating GCV to GCV monophosphate in infected cells (20). GCV monophosphate is readily converted by cellular enzymes to GCV triphosphate (33), which is the active form of the drug and inhibits viral DNA polymerase (7). CDV, 1-[(S)-3-hydroxy-2-(phosphonomethoxy)propyl] cytosine dihydrate, is a monophosphate nucleotide analogue and undergoes cellular phosphorylation to an active diphosphate form (10). CDV competitively inhibits the incorporation of deoxycytosine-5′-triphosphate by viral DNA polymerase into viral DNA (36). Once incorporated into viral DNA, it slows further DNA synthesis and causes DNA destabilization. While the antiviral potency of these drugs is unquestionable, their therapeutic value for CMV-induced myocarditis is unknown. In this study, we have used our well-established MCMV myocarditis mouse model to examine the efficacies of GCV and CDV. We have examined various treatment regimes, focusing on the effect that these drugs have on the acute and chronic phases of myocarditis. Our results provide valuable insights into the efficacy of antiviral treatments for virus-induced myocarditis as well as further elucidating the pathogenesis of the disease.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free inbred male BALB/c mice (6 to 8 weeks old; seronegative for MCMV) were obtained from the Animal Resources Centre (Perth, Western Australia) and maintained under minimal disease conditions.
Virus.
Virulent MCMV (K181 strain [Perth]) was prepared by salivary gland passage in mice and stored in the gas phase of liquid nitrogen (16). The virus was originally obtained from D. Lang (Duke University, Durham, N.C.) and after serial passages was designated the K181 MCMV (Perth) strain. The virulent wild isolate of MCMV designated G4 was originally isolated by T. Scalzo (University of Western Australia) from wild mice trapped in Geraldton, Western Australia, and was similarly prepared as a salivary gland virus stock. Quantification of infectious MCMV was determined by plaque assay using mouse embryo fibroblast (MEF) monolayers, as previously described (16). Virus titers are expressed as the mean PFU per gram of tissue ± standard deviation (SD), and the limit of detection was 100 PFU/g.
Drugs.
GCV (Roche Products, Sydney, Australia) and CDV (Pharmacia & UpJohn, Sydney, Australia) were used in our MCMV model. These antiviral drugs dissolved in pyrogen-free saline were given to mice via the intraperitoneal (i.p.) route.
Plaque reduction assay.
The sensitivities of the K181 laboratory strain and the G4 wild isolate of MCMV to antiviral drugs were assessed by plaque reduction assay using MEF monolayers (19). The infected cells were overlaid with a medium containing 1% methylcellulose and various concentrations of an antiviral drug and were further incubated for 3 days. The 50% inhibitory concentration corresponds to the drug dose achieving 50% plaque reduction.
Primary MCMV infection.
Mice were infected by the i.p. route with 104 PFU of MCMV diluted in pyrogen-free saline. In studies examining the acute phase of myocarditis, mice were infected with MCMV on day 0. Drug treatments of various doses commenced on day 1 postinfection (p.i.) and continued daily. Saline was similarly administered as a placebo. Hearts, livers, spleens, and salivary glands were removed from groups of five mice on day 7 p.i. The hearts were transected along the midline and processed with Bouin's fixative for histological examination. The spleens, livers, and salivary glands were homogenized, and infectious virus was quantified by plaque assay. In experiments examining the chronic phase of myocarditis, mice were infected on day 0 and treatment commenced on day 1 p.i. The mice received either 40 mg of GCV per kg of body weight per day or 5 mg of CDV/kg/day (effective doses were determined from earlier experiments with acute virus infection). Treatment continued until day 7 p.i. The hearts were removed on day 35 p.i. and processed for histology. In a separate experiment, mice were infected on day 0 and treatment, with either 40 mg of GCV/kg/day or 5 mg of CDV/kg/day, commenced on day 14 p.i. and continued until day 35 p.i., when the hearts were removed for histology. Control mice were either uninfected and drug treated or MCMV infected without drug treatment.
Secondary MCMV infection.
In experiments examining the treatment of myocarditis upon virus reinfection, mice were infected with K181 MCMV on day 0 and then reinfected with either K181 or G4 MCMV on day 56 p.i. Treatment with either 40 mg of GCV/kg/day or 5 mg of CDV/kg/day commenced on day 57 post-initial infection. Hearts, livers, spleens, and salivary glands were removed from groups of five mice on day 63 post-initial infection (i.e., day 7 postreinfection). The hearts were processed for histology, and the spleens, livers, and salivary glands were homogenized for determination of virus titer by plaque assay. Control mouse groups were either K181 MCMV infected with no reinfection, K181 MCMV infected with K181 MCMV reinfection, or K181 MCMV infected with G4 MCMV reinfection.
Heart histopathology.
Myocarditis was scored from hematoxylin- and eosin-stained heart sections as the average number of foci of cellular infiltration ± SD from five mice per group, as previously described (6).
Statistical analysis.
The Student t test was used to assess differences between groups of data. P values of 0.05 or less were considered to be statistically significant.
RESULTS
In vitro antiviral activities of GCV and CDV for MCMV.
The antiviral activities of GCV and CDV were determined by plaque reduction assays. The K181 laboratory strain and the G4 wild isolate of MCMV were inhibited with GCV concentrations of 8.9 and 5.6 μM, respectively, for 50% reduction in plaques formed in virus-infected MEF monolayers (data not shown). The 50% inhibitory concentrations for CDV were 0.17 and 0.23 μM, respectively, against K181 and G4 viruses. These values are similar to previously reported values for GCV and CDV (24) and confirm that these antiviral drugs are effective against our laboratory strain and the wild isolate of MCMV.
Antiviral treatment of acute MCMV infection and myocarditis in mice.
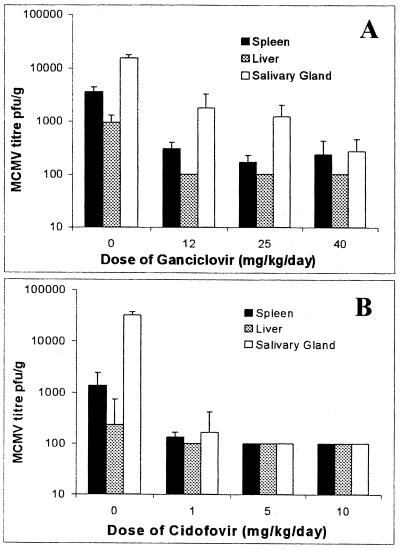
The antiviral effect of GCV and CDV on virus replication in the major target organs, liver, spleen, and salivary gland, and the therapeutic effect of such antiviral agents on the acute phase of MCMV-induced myocarditis were investigated. Adult BALB/c mice were infected with K181 MCMV and then treated with various doses of GCV or CDV commencing day 1 p.i. MCMV-infected mice treated with GCV showed significant (P < 0.05) reductions in virus titers in the spleen and salivary gland with all doses of this drug compared to untreated MCMV-infected mice (Fig. 1A). MCMV-infected mice treated with CDV showed a significant (P < 0.05) reduction in virus titers in the salivary gland with all doses compared to the untreated MCMV-infected mice (Fig. 1B). While the reductions in the spleen and liver were not statistically significant, doses of 5 and 10 mg of CDV/kg/day reduced viral titers in these organs to the limit of detection of the plaque assay (Fig. 1B).
FIG. 1.
Antiviral therapy reduces MCMV titers in major target organs. BALB/c mice were inoculated with 104 PFU of MCMV i.p. at day 0 and treated with GCV (A) or CDV (B) daily from day 1 to 7 p.i. or treated with saline as a placebo. The average numbers (+SD) of PFU per gram of liver, spleen, and salivary gland at day 7 p.i. from groups of five mice per dose are shown.
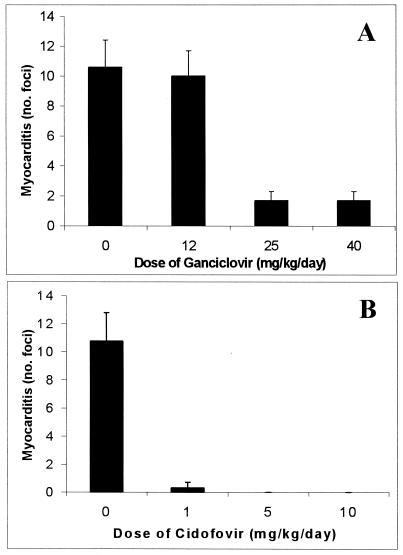
Myocarditis was significantly reduced (6.2-fold reduction; P < 0.05) on day 7 in MCMV-infected mice treated with either 25 or 40 mg of GCV/kg/day (Fig. 2A). However, 12 mg/kg/day had no observed effect on the severity of myocarditis compared to that in the untreated MCMV-infected mice. CDV-treated mice showed significant reduction (36-fold reduction; P < 0.05) in myocarditis on day 7 for all doses of CDV, with no detectable inflammation observed in mice given doses of 5 and 10 mg/kg/day (Fig. 2B). As expected, myocarditis was undetectable in control uninfected BALB/c mice treated with the above-mentioned defined doses of either GCV or CDV (data not shown).
FIG. 2.
Early antiviral treatment reduces the severity of acute MCMV-induced myocarditis. BALB/c mice were inoculated with 104 PFU of MCMV i.p. at day 0 and treated with either GCV (A) or CDV (B) daily from day 1 to 7 p.i. or treated with saline as a placebo. The average numbers (+SD) of inflammatory foci per heart section at day 7 p.i. from groups of five mice per dose are shown.
Antiviral treatment of chronic MCMV-induced myocarditis.
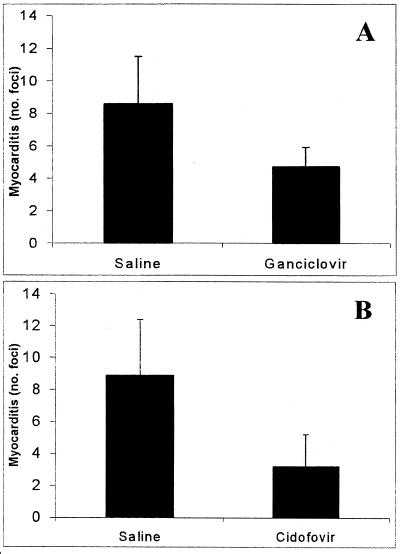
The efficacy of antiviral treatment on the chronic phase of MCMV-induced myocarditis was next examined. A significant reduction in chronic myocarditis was observed under both the GCV and CDV treatment regimes (1.8- and 2.8-fold reduction, respectively; P < 0.05) (Fig. 3); however, the disease was not completely abolished.
FIG. 3.
Early antiviral therapy reduces the severity of chronic MCMV-induced myocarditis. BALB/c mice were inoculated with 104 PFU of MCMV i.p. on day 0 and treated with either GCV at 40 mg/kg/day (A) or CDV at 5 mg/kg/day (B) from day 1 to 7 p.i. or treated with saline as a placebo. The average numbers (+SD) of inflammatory foci per heart section on day 35 p.i. from groups of five mice are shown.
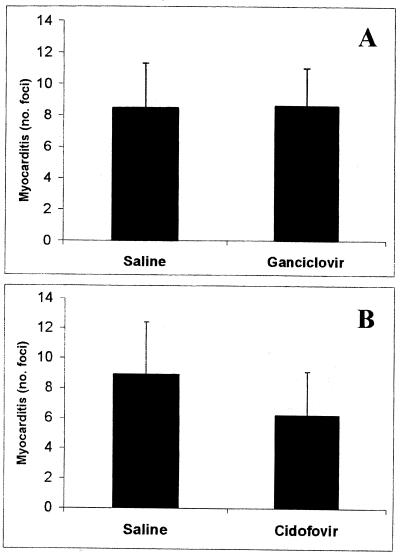
In a separate experiment, antiviral treatment commenced after myocarditis was established, with no decrease in myocarditis observed on day 35 with either the GCV or CDV treatment regime (Fig. 4).
FIG. 4.
Late antiviral therapy has no therapeutic effect on chronic MCMV-induced myocarditis. BALB/c mice were inoculated with 104 PFU of MCMV i.p. on day 0 and treated with either GCV at 40 mg/kg/day (A) or CDV at 5 mg/kg/day (B) from day 14 to 35 p.i. or treated with saline as a placebo. The average numbers (+SD) of inflammatory foci per heart section on day 35 p.i. from groups of five mice are shown.
Antiviral treatment of MCMV reinfection.
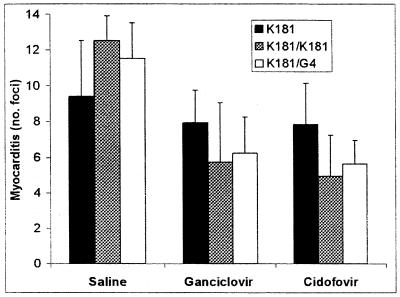
The effect of GCV and CDV was examined in mice reinfected with either the same or a different strain of MCMV during the chronic phase of myocarditis. On day 7 postreinfection, livers, spleens, and salivary glands were removed for virus quantitation, and hearts were examined histologically for myocarditis. Reinfection of mice with K181 significantly exacerbated myocarditis (1.3-fold increase). An increase in cardiac inflammation was also observed after reinfection of mice with G4 MCMV (1.2-fold increase); however, this was not statistically significant (Fig. 5). Treatment with GCV or CDV resulted in a slight decrease in myocarditis in unreinfected mice, but both drugs caused a significant decrease in disease in both the K181 and G4 MCMV-reinfected animals (2.2- and 2.6-fold reduction, respectively; P < 0.05) (Fig. 5). As expected, no virus was detected by plaque assay in any of the target organs taken from previously infected mice challenged with either MCMV strain (data not shown).
FIG. 5.
Exacerbation of myocarditis by virus reinfection is prevented with antiviral therapy. BALB/c mice were inoculated with 104 PFU of MCMV (K181 strain) i.p. on day 0. The mice were then reinfected with 104 PFU of either K181 (K181/K181) or G4 (K181/G4) MCMV i.p. on day 56 p.i. and then treated with either GCV at 40 mg/kg/day or CDV at 5 mg/kg/day from day 57 to day 63 p.i. or treated with saline as a placebo. The average numbers (+ SD) of inflammatory foci per heart section at day 63 p.i. from groups of five mice are shown.
DISCUSSION
This study examined the potential use of the antiviral drugs GCV and CDV as therapeutic agents in a CMV-induced myocarditis model using adult BALB/c mice. Antiviral therapy was tried, as the role of MCMV in the induction of cardiac inflammation is unquestionable. Our results clearly support this viral role, but additional mechanisms also contribute to the development of myocarditis. We have shown that early treatment (day 1 p.i.) with either GCV or CDV significantly reduces myocarditis in the acute phase of disease (day 7 p.i.). This finding supports the hypothesis that early myocardial inflammation is mediated either directly or indirectly by virus infection. Data from the CB3 model of myocarditis shows that the virus alone can cause direct myocyte death without involving any immune mechanism of host defense (9, 22). During the early stage of CB3 disease, virus can be readily obtained from the heart tissue (12), and in situ hybridization studies using CB3-infected mice demonstrated that viral infection leading directly to myocyte necrosis and inflammatory foci were consistently associated with virus-infected cells (9, 12, 14). In our MCMV model of myocarditis, virus titers in the heart are extremely low—indeed, 100-fold lower than CB3 titers in the heart (6, 28, 30). Although treatment with 12 mg of GCV/kg/day significantly reduced MCMV titers in the spleen, liver, and salivary gland, this dose of GCV had no effect on myocarditis severity. However, higher doses of the drug further reduced viral titers and caused significant decreases in myocardial inflammation. This indicates that MCMV-induced myocarditis cannot be treated in a dose-dependent manner with antiviral drugs; rather, viral titers must be reduced to a relatively low level before any therapeutic effect can be observed in the acute phase. Acute viral burdens in the liver have also been shown to be decreased with GCV treatment of weanling BALB/c mice infected i.p. with MCMV (2).
In the present study, we report that chronic MCMV-induced myocarditis cannot be prevented by early antiviral treatment with either GCV or CDV given during the acute stage of infection. While the severity of chronic myocarditis was reduced, inflammation and necrosis were still observed. During the chronic phase of disease, infectious MCMV cannot be detected in the heart, spleen, liver, or salivary gland of susceptible mice, providing supporting evidence that this phase is not mediated by direct viral damage. We have also found that treatment of mice with foscarnet (500 mg/kg/day), using a regime similar to that for GCV and CDV, reduced virus titers in the spleen and liver but was not effective at reducing acute myocarditis (data not shown). However, this observation may reflect the inadequacy of systemic delivery of foscarnet in our animal model, since this drug is often administered to patients as an infusion. Furthermore, antiviral treatment with either GCV or CDV after the acute phase of disease and extending into the chronic phase had no significant therapeutic effect. This finding supports an important role for other governing factors besides virus in the development of myocarditis following virus infection.
We also found that susceptible mice reinfected with the same or a different strain of MCMV during the chronic phase of disease showed a significant increase in myocarditis. While a previous CMV infection allowed rapid clearance of virus upon reinfection, with no infectious virus detected on day 7 postreinfection, it did not protect against the development of myocarditis. Antiviral treatment with GCV or CDV reduced myocarditis severity in reinfected mice; however, inflammation and necrosis indicative of the chronic phase were still evident. Clearly, further myocardial damage is being caused by subsequent viral infections, with such reinfections able to be treated with antiviral drugs. However, such treatment is not effective for the existing chronic inflammation and necrosis, which is possibly autoimmune in nature, induced by the primary CMV infection.
Clinical trials of CDV for CMV retinitis in AIDS patients have demonstrated efficacy in slowing the progression of this disease and provided insights into toxicity, an adverse side effect of this antiviral drug (28). However, emergence of drug-resistant CMV mutants has been observed for CDV- and GCV-treated AIDS patients (5). The molecular basis of such de novo resistance is often described as mutations in the UL54 DNA polymerase gene (3, 29, 35). Combinatorial therapy using such antiviral agents, not necessarily administered simultaneously, may have desirable synergistic effects in vivo without potential problems associated with the development of drug resistance. Furthermore, incorporation of new analogues of such antiviral compounds in therapeutic regimes may enhance the potency of these agents at affecting early virus replication (1).
Our results show that antiviral therapy is an effective treatment for MCMV-induced myocarditis when initiated very early in infection. Such early treatment provides protection against severe acute myocarditis and reduces the level of chronic myocarditis. However, our studies of treatment during the chronic phase of disease showed no therapeutic effect in the murine model, providing further evidence that an immunopathogenic mechanism perpetuates the late phase of disease. Nonetheless, antiviral therapy is a viable option to prevent exacerbation of disease when reinfection is recognized as a high risk factor, especially in transplant patient groups. Our study has highlighted possible clinical applications of antiviral drugs as therapeutic agents in CMV-induced myocarditis as well as providing further insights into the pathogenesis of this disease.
ACKNOWLEDGMENTS
This work was supported by the Australian National Health and Medical Research Council (project grant 96-1312) and SmithKline Beecham International.
REFERENCES
- 1.Bedard J, May S, L'Heureux L, Stamminger T, Copsey A, Drach J, Huffman J, Chan L, Jin H, Rando R F. Antiviral properties of a series of 1,6-naphthyridine and 7,8-dihydroisoquinoline derivatives exhibiting potent activity against human cytomegalovirus. Antimicrob Agents Chemother. 2000;44:929–937. doi: 10.1128/aac.44.4.929-937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolger G, Lapeyre N, Rheaume M, Kibler P, Bousquet C, Garneau M, Cordingley M. Acute murine cytomegalovirus infection: a model for determining antiviral activity against CMV induced hepatitis. Antivir Res. 1999;44:155–165. doi: 10.1016/s0166-3542(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Bowen E F, Cherrington J M, Lamy P D, Griffiths P D, Johnson M A, Emery V C. Quantitative changes in cytomegalovirus DNAemia and genetic analysis of the UL97 and UL54 genes in AIDS patients receiving cidofovir following ganciclovir therapy. J Med Virol. 1999;58:402–407. [PubMed] [Google Scholar]
- 4.DeArmond B. Safety considerations in the use of ganciclovir in immunocompromised patients. Transplant Proc. 1991;23(Suppl. 1):26–29. [PubMed] [Google Scholar]
- 5.Emery V C. Cytomegalovirus drug resistance. Antivir Ther. 1998;3:239–242. [PubMed] [Google Scholar]
- 6.Fairweather D, Lawson C M, Chapman A J, Brown C M S, Booth T W M, Papadimitriou J M, Shellam G R. Wild isolates of murine cytomegalovirus induce myocarditis and antibodies that cross-react with virus and cardiac myosin. Immunology. 1998;94:263–270. doi: 10.1046/j.1365-2567.1998.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freitas V R, Smee D F, Chernow M, Boehme R, Matthews T R. Activity of 9-(1,3-dihydroxy-2-propoxymethyl) guanine compared with that of acyclovir against human, monkey, and rodent cytomegaloviruses. Antimicrob Agents Chemother. 1985;28:240–245. doi: 10.1128/aac.28.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friman G, Wesslen L, Fohlman J, Karjalainen J, Rolf C. The epidemiology of infectious myocarditis, lymphocytic myocarditis and dilated cardiomyopathy. Eur Heart J. 1995;16(Suppl. 0):36–41. doi: 10.1093/eurheartj/16.suppl_o.36. [DOI] [PubMed] [Google Scholar]
- 9.Herzum M, Ruppert V, Kuytz B, Jomaa H, Nakamura I, Maisch B. Coxsackievirus B3 infection leads to cell death of cardiac myocytes. J Mol Cell Cardiol. 1994;26:907–913. doi: 10.1006/jmcc.1994.1108. [DOI] [PubMed] [Google Scholar]
- 10.Ho H T, Woods K L, Bronson J J, DeBoeck H, Martin J C, Hitchcock M J. Intracellular metabolism of the antiherpes agent (s)-1-[3-hydroxy-2-(phosphonymethoxy) propyl] cytosine. Mol Pharmacol. 1992;41:197–202. [PubMed] [Google Scholar]
- 11.Huber S A. Autoimmunity in myocarditis: relevance of animal models. Clin Immunol Immunopathol. 1997;83:93–102. doi: 10.1006/clin.1997.4342. [DOI] [PubMed] [Google Scholar]
- 12.Kandolf R, Klingel K, Zell R, Canu A, Fortmuller U, Hohenadl C, Albrecht M, Reimann B Y, Franz W M, Heim A, Raab U, McPhee F. Molecular mechanisms in the pathogenesis of enteroviral heart disease: acute, and persistent infections. Clin Immunol Immunopathol. 1993;68:153–158. doi: 10.1006/clin.1993.1112. [DOI] [PubMed] [Google Scholar]
- 13.Kishimoto C, Crumpacker C S, Abelmann W H. Ribavirin treatment of murine coxsackievirus B3 myocarditis with analysis of lymphocyte subsets. J Am Coll Cardiol. 1988;12:1334–1341. doi: 10.1016/0735-1097(88)92618-6. [DOI] [PubMed] [Google Scholar]
- 14.Klingel K, Kandolf R. The role of enterovirus replication in the development of acute and chronic muscle disease in different immunocompetent mouse strains. Scand J Infect Dis Suppl. 1993;88:79–85. [PubMed] [Google Scholar]
- 15.Lawson C M. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell Mol Life Sci. 2000;57:552–560. doi: 10.1007/PL00000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson C M, Grundy J E, Shellam G R. Antibody responses to murine cytomegalovirus in genetically resistant and susceptible strains of mice. J Gen Virol. 1988;69:1987. doi: 10.1099/0022-1317-69-8-1987. [DOI] [PubMed] [Google Scholar]
- 17.Lawson C M, O'Donoghue H, Bartholomaeus W N, Reed W D. Genetic control of mouse cytomegalovirus-induced myocarditis. Immunology. 1990;69:20–26. [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson C M, O'Donoghue H L, Reed W D. Mouse cytomegalovirus infection induces antibodies which cross react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992;75:513–519. [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Minagawa H, Tanaka S, Ryoichi M. Suppression of infectious virus spread to the liver by foscarnet following lethal infection of acyclovir-resistant herpes simplex virus type 2 in mice. Antiviral Res. 1995;27:111–121. doi: 10.1016/0166-3542(94)00087-o. [DOI] [PubMed] [Google Scholar]
- 20.Littler E, Stuart A D, Chee M S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the anti-viral nucleoside analogue ganciclovir. Nature. 1992;358:160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- 21.Maisch B, Schonian U, Crombach M, Wedl I, Bethge C, Herzum M, Klein H H. Cytomegalovirus associated inflammatory heart muscle disease. Scand J Infect Dis Suppl. 1993;88:135–148. [PubMed] [Google Scholar]
- 22.McManus B M, Chow L H, Wilson J E, Anderson D R, Gulizia J M, Gauntt C J, Klingel K E, Beisel K W, Kandolf R. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993;68:159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- 23.Neumann D A, Lane J R, Allen G S, Herskowitz A, Rose N R. Viral myocarditis leading to cardiomyopathy: do cytokines contribute to pathogenesis? Clin Immunol Immunopathol. 1993;68:181–190. doi: 10.1006/clin.1993.1116. [DOI] [PubMed] [Google Scholar]
- 24.Okleberry K M, Warren R P, Smee D F. Metabolism of ganciclovir and cidofovir in cells infected with drug-resistant and wild-type strains of murine cytomegalovirus. Antivir Res. 1997;35:83–90. doi: 10.1016/s0166-3542(97)00013-2. [DOI] [PubMed] [Google Scholar]
- 25.Rose N R. Myocarditis—from infection to autoimmunity. Immunologist. 1996;4:67–75. [Google Scholar]
- 26.Rose N R, Hill S L. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996;80:592–599. doi: 10.1006/clin.1996.0146. [DOI] [PubMed] [Google Scholar]
- 27.Rose N R, Neumann D A, Herskowitz A. Coxsackievirus myocarditis. In: Stollerman G H, editor. Advances in internal medicine. St. Louis, Mo: Mosby-Year Book Inc.; 1992. pp. 411–429. [PubMed] [Google Scholar]
- 28.Rose N R, Wolfgram L J, Herskowitz A, Beisel K W. Post infectious autoimmunity: two distinct phases of Coxsackievirus B3-induced myocarditis. Ann N Y Acad Sci. 1986;475:146–156. doi: 10.1111/j.1749-6632.1986.tb20864.x. [DOI] [PubMed] [Google Scholar]
- 29.Safrin S, Cherrington J, Jaffe H S. Clinical uses of cidofovir Rev. Med Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Sato S, Tsutsumi R, Burke A, Carlson G, Porro V, Seko Y, Okumura K, Kawana R, Virmani R. Persistence of replicating coxsackievirus B3 in the athymic murine heart is associated with development of myocarditic lesions. J Gen Virol. 1994;75:2911–2924. doi: 10.1099/0022-1317-75-11-2911. [DOI] [PubMed] [Google Scholar]
- 31.See D M, Tilles J E. Viral myocarditis. Rev Infect Dis. 1991;13:951–956. doi: 10.1093/clinids/13.5.951. [DOI] [PubMed] [Google Scholar]
- 32.Sherry B, Li X-Y, Tyler K L, Cullen J M, Virgin H W., IV Lymphocytes protect against and are not required for reovirus-induced myocarditis. J Virol. 1993;67:6119–6124. doi: 10.1128/jvi.67.10.6119-6124.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smee D F, Boehme R, Chernow M, Binko B P, Matthews T R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl) guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985;34:1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- 34.Von Herrath M G, Oldstone M B A. Role of viruses in the loss of tolerance to self-antigens and in autoimmune diseases. Trends Microbiol. 1995;3:424–430. doi: 10.1016/s0966-842x(00)88995-7. [DOI] [PubMed] [Google Scholar]
- 35.Wolfe D G, Smith I L, Lee D J, Freeman W R, Flores-Aguilar R, Spector S A. Mutations in human cytomegalovirus UL97 gene confer clinical resistance to ganciclovir and can be detected directly in patient plasma. J Clin Investig. 1995;95:257–263. doi: 10.1172/JCI117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong X, Smith J L, Kim C, Huang E S, Chen M S. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem Pharmacol. 1996;51:1563–1567. doi: 10.1016/0006-2952(96)00100-1. [DOI] [PubMed] [Google Scholar]