Abstract
Background
Limited data is available from low-middle and upper-middle income countries of the factors associated with hospitalization or admission to pediatric intensive care unit (PICU) for children with COVID-19.
Objective
To describe the factors associated with hospitalization or PICU admission of children with COVID-19 in Latin America.
Method
Multicenter, analytical, retrospective study of children reported from 10 different Latin American countries to the Latin-American Society of Pediatric Infectious Diseases (SLIPE-COVID) research network from June 1, 2020, and February 28, 2021. Outpatient or hospitalized children <18 years of age with COVID-19 confirmed by polymerase chain reaction or antigen detection from the nasopharynx were included. Children with multisystem inflammatory syndrome in children (MIS-C) were excluded. Associations were assessed using univariate and multivariable logistic regression models.
Results
A total of 1063 children with COVID-19 were included; 500 (47%) hospitalized, with 419 (84%) to the pediatric wards and 81 (16%) to the ICU. In multivariable analyses, age <1 year (Odds Ratio [OR] 1.78; 95% CI 1.08–2.94), native race (OR 5.40; 95% CI 2.13–13.69) and having a co-morbid condition (OR 5.3; 95% CI 3.10–9.15), were associated with hospitalization. Children with metabolic or endocrine disorders (OR 4.22; 95% CI 1.76–10.11), immune deficiency (1.91; 95% CI 1.05–3.49), preterm birth (OR 2.52; 95% CI 1.41–4.49), anemia at presentation (OR 2.34; 95% CI 1.28–4.27), radiological peribronchial wall thickening (OR 2.59; 95% CI 1.15–5.84) and hypoxia, altered mental status, seizures, or shock were more likely to require PICU admission. The presence of pharyngitis (OR 0.34; 95% CI 0.25–0.48); myalgia (OR 0.47; 95% CI 0.28–0.79) or diarrhea (OR 0.38; 95% CI 0.21–0.67) were inversely associated with hospital admission.
Conclusions
In this data analysis reported to the SLIPE research network in Latin America, infants, social inequalities, comorbidities, anemia, bronchial wall thickening and specific clinical findings on presentation were associated with higher rates of hospitalization or PICU admission. This evidence provides data for prioritization prevention and treatment strategies for children suffering from COVID-19.
Keywords: COVID-19, SARS-CoV-2, children, critical care, hospitalization
Introduction
The emergence and rapid global spread of SARS-CoV-2 in early 2020 posed a major threat to populations from low and middle income countries (LMIC), where data on clinical presentations and risk factors for serious COVID-19 infections in children were scarce (1, 2). There are now ~64 million cases of COVID-19 in Latin America (3), with 8–16% of these cases reported among those between 0 and 19 years of age in different countries of the region (4–8). Although COVID-19 presents as mild or asymptomatic in most children, severe disease and hospitalization can occur in ~1% of children and is likely to increase as children represent most of the unvaccinated in these countries (9).
Early identification of factors associated with severe disease in children allows clinicians and public health officials to triage those in need of advanced level of care and is critical as vaccination campaigns are rolled out, allowing the prioritization of those who will benefit the most from early protection. Although several observational studies have provided information on risk factors for hospitalization or critical care admission in children in high-income countries (10–16), detailed demographic, laboratory, radiological and clinical data obtained from a large sample in LMIC countries are scarce.
Pediatric societies around the world have established data registries of pediatric COVID-19 cases to understand the epidemiology, clinical presentation and morbidity and mortality rates in children (9, 17). The Sociedad Latinoamericana de Infectologia Pediatrica (SLIPE) started a multinational collaboration to create a data registry on children being affected with COVID-19 in Latin American countries. The purpose of the present study was to describe the factors associated with hospitalization or pediatric intensive care unit (PICU) admission in children with COVID-19 in Latin America.
Materials and Methods
Independent pediatricians collected and reported cases of COVID-19 using an online registry created by the SLIPE-COVID network that utilized a standardized data collection form developed in Research Electronic Data Capture (REDCap) hosted by the University of Colorado, Aurora, USA. Investigators from 17 sites in 14 cities from 10 different Latin American countries prospectively identified patients between June 1, 2020, and February 28, 2021 and retrospectively inputted their data. All study sites were level 3 referral hospitals. The registry and study protocol were reviewed and approved by the local institutional review board of each participating center. Since data was collected for routine clinical practice, research was deemed of minimal risk and the requirement for informed consent was waived.
Children <18 years of age who presented to outpatient clinics or hospital emergency departments from participating sites with COVID-19-related symptoms and polymerase chain reaction (PCR) or antigen-confirmed SARS-CoV-2 infection were included. Children with MIS-C were excluded from this analysis.
Case ascertainment varied across sites. Seven cities used an active case surveillance system that allowed to identify children with confirmed COVID-19 from a list of all ambulatory or hospitalized encounters provided by the hospital's epidemiology or microbiology department, while a passive case ascertainment method was used in the remaining seven cities where children were identified after a pediatric infectious disease service consultation was obtained.
Patient characteristics including demographics, clinical, laboratory and radiological data were collected from medical records as documented by the site clinician who evaluated the patient. For children with one or more COVID-19-related consultations during the study period, only data from the first encounter was collected and for children with multiple laboratory reports during their care, only first values were recorded. Nutritional status was classified based on body mass index (BMI) standard deviations (SD) according to the World Health Organization (WHO) into underweight (below −2 SD), normal nutritional status (between −2 and +1 SD), overweight (between +1 and +2 SD) and obesity (above +2 SD) (18). Radiologic findings were categorized according to the density and extent of parenchymal changes. Anemia was defined as hemoglobin below 11 grams/deciliter (19) and thrombocytopenia as platelets < 150,000 × 103 cells/μL (20). C-reactive protein and neutrophil to lymphocyte ratio were dichotomized as previous reports (21–23). For symptoms that were not described in medical records, investigators recorded “non-applicable” when children were too young or too ill to describe their symptoms.
Outcome endpoints for this analysis were divided into (a) outpatient care, (b) hospitalization in a general pediatric ward, or (c) intensive care unit (ICU) admission. Final disposition was at the discretion of primary care team. Data were compared in children requiring admission in the general ward vs. ICU, in those requiring hospitalization (general ward or ICU) vs. outpatient care and in those who did or did not require supplementary oxygen. In the latter two groups laboratory or imaging data were not compared, as children receiving outpatient care seldom had blood drawn or chest X-rays obtained.
Data Analysis
Summary statistics were used for description of variables. Medians and interquartile ranges (IQR) were used for continuous variables; categorical variables were reported as absolute numbers and proportions. Chi2 tests and Fisher's exact tests were used for categorical variables as appropriate, and the Mann-Whitney U test was used for comparing median values of non-normally distributed variables. Associations of baseline characteristics and clinical findings with hospitalization, PICU and/or supplementary oxygen requirement were assessed using univariate and multivariable logistic regression models. Covariates that were significant at the 0.20 level in univariate analysis were included in multivariable models. A backward elimination algorithm was performed to select covariates that were independently associated with hospitalization, PICU and/or supplementary oxygen use, setting a p value of <0.05 as significant. Backward variable selection was performed using Rubin's Rules (24). Two multivariate models were created to explore associations with the outcomes of interest. One explored sociodemographic characteristics and comorbidities, and another explored signs/symptoms, laboratory values and radiologic findings. A sensitivity analysis including only children from the seven cities that used an active surveillance system was conducted. In addition, all co-morbidities were integrated in a model that explored the effect of any co-morbidity in the risk of hospital admission or PICU.
For missing data, a multiple imputation chained equation with 90 imputed datasets was used. This value was set using the rule of thumb (25). All variables, including the binary outcomes, were included in the imputation model and further sensitivity analysis was conducted using complete-case data. To control for variability in case ascertainment and non-measured social or cultural confounders, clustered standard errors were estimated to adjust for the correlation between children from the same city in each model. Hospitals from the same city had the same case ascertainment method. Variables that could not be assessed in all children due to age (i.e. anosmia or dysgeusia) were not imputed nor included in multivariable analysis. All statistical analyses were carried out in Stata version 16.0 (StataCorp, College, Station, TX) and p values of <0.05 (two-sided) were considered statistically significant. Imputation was not attempted for variables that had more than 50% of their data missing in the complete dataset except for the C-reactive protein values; this variable was imputed given its relevance in predicting severe outcomes in other studies (23, 26).
Results
Study Participants
A total of 1063 children were included (median age, 3 years [IQR 1–9]; age range 22 days to 17.8 years, 46.4% female, 63% with normal weight and 29% with comorbidity). Of all, 563 (53%) received ambulatory care, while 500 (47%) were hospitalized (419 [84%] in the general ward and 81 [16%] in the ICU) (Table 1). Most children were included from 2 hospitals in Bogotá, Colombia (n = 387); 1 hospital in Quilmes, Argentina (n = 264); 2 hospitals in Cali, Colombia (n = 160) and 2 hospitals in Panamá (n = 109). The remaining cases (n = 143) were included from Asunción, Paraguay; David, Panamá; Guatemala City, Guatemala; La Paz, Bolivia; Lara, Venezuela; Monterrey, México; Rosario, Argentina; San Lorenzo, Paraguay; San Salvador, El Salvador and Uberlandia, Brazil (Figure 1).
Table 1.
Demographics and clinical characteristics of pediatric patients with COVID-19.
| Characteristic | All patients | Outpatient care | Hospitalized | |
|---|---|---|---|---|
| General ward | PICU | |||
| (n = 1,063) | (n = 563) | (n = 419) | (n = 81) | |
| Age, median (IQR), yr | 3 (1–9) | 4 (1–10) | 3 (1–9) | 3 (1–9) |
| Age groups, yr No. (%) | ||||
| <1 | 241 (22.7) | 109 (19.4) | 113 (27.0) | 19 (23.5) |
| 1–5 | 386 (36.3) | 214 (38.0) | 141 (33.6) | 31 (38.3) |
| 6–9 | 176 (16.6) | 95 (16.9) | 67 (16.0) | 14 (17.3) |
| ≥10 | 260 (24.5) | 145 (25.7) | 98 (23.4) | 17 (21.0) |
| Sex, No. (%) | ||||
| Male | 570 (53.6) | 291 (51.7) | 237 (56.6) | 42 (51.9) |
| Female | 493 (46.4) | 272 (48.3) | 182 (43.4) | 39 (48.1) |
| Race or ethnic group a , No. (%) | ||||
| No.* | 987 | 517 | 396 | 74 |
| Caucasian | 459 (46.5) | 285 (55.1) | 149 (37.6) | 25 (33.8) |
| Native | 16 (1.6) | 3 (0.6) | 11 (2.8) | 2 (2.7) |
| Black or African American | 9 (0.9) | 6 (1.2) | 3 (0.8) | 0 (0.0) |
| Mixed race | 503 (51.0) | 223 (43.1) | 233 (58.8) | 47 (63.5) |
| Population, No. (%) | ||||
| No.* | 880 | 415 | 385 | 80 |
| Urban | 807 (91.7) | 393 (94.7) | 347 (90.1) | 67 (83.8) |
| Rural | 73 (8.3) | 22 (5.3) | 38 (9.9) | 13 (16.2) |
| Level of Education of caregiver, No. (%) | ||||
| No.* | 248 | 115 | 110 | 23 |
| No education | 87 (35.1) | 25 (21.7) | 53 (48.2) | 9 (39.1) |
| Primary education | 40 (16.1) | 17 (14.8) | 18 (16.4) | 5 (21.7) |
| High school | 79 (31.8) | 40 (34.8) | 32 (29.1) | 7 (30.4) |
| University degree | 42 (16.9) | 33 (28.7) | 7 (6.4) | 2 (8.7) |
| Full immunization coverage, No. (%) | ||||
| No.* | 859 | 477 | 310 | 72 |
| Yes | 770 (89.6) | 435 (91.2) | 274 (88.4) | 61 (84.7) |
| Nutritional Status b , No. (%) | ||||
| No.* | 562 | 259 | 242 | 61 |
| Underweight | 65 (11.6) | 17 (6.6) | 37 (15.3) | 11 (18.0) |
| Normal Weight | 356 (63.3) | 173 (66.8) | 149 (61.6) | 34 (55.7) |
| Overweight | 83 (14.8) | 47 (18.1) | 31 (12.8) | 5 (8.2) |
| Obese | 58 (10.3) | 22 (8.4) | 25 (10.3) | 11 (18) |
| Co-morbidities- No. (%) | ||||
| Chronic lung disease | 123 (11.6) | 54 (9.6) | 58 (13.8) | 11 (13.6) |
| Congenital heart disease | 18 (1.7) | 6 (1.1) | 9 (2.1) | 3 (3.7) |
| Cirrhosis/Biliary atresia | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0.0) |
| Chronic gastrointestinal disease | 9 (0.8) | 2 (0.4) | 6 (1.4) | 1 (1.2) |
| Renal insufficiency | 8 (0.7) | 0 (0.0) | 8 (1.9) | 0 (0.0) |
| Neurologic disease | 59 (5.5) | 7 (1.2) | 41 (9.8) | 11 (13.6) |
| Metabolic or endocrine disorder | 8 (0.7) | 1 (0.2) | 4 (0.9) | 3 (3.7) |
| Immune deficiency | 88 (8.3) | 13 (2.3) | 57 (13.6) | 18 (22.2) |
| Preterm birth | 33 (3.1) | 9 (1.6) | 17 (4.1) | 7 (8.6) |
| Any co-morbidity- No. (%) | 305 (28.7) | 84 (14.9) | 175 (41.8) | 46 (56.8) |
| Source of infection- No. (%) | ||||
| Hospital | 27 (2.5) | 1 (0.2) | 19 (4.5) | 7 (8.6) |
| Community | 79 (7.4) | 26 (4.6) | 42 (10.0) | 11 (13.6) |
| Traveling | 5 (0.5) | 2 (0.4) | 3 (0.7) | 0 (0.0) |
| Home | 376 (35.4) | 248 (44.0) | 113 (27.0) | 248 (44.0) |
| Other | 6 (0.6) | 4 (0.7) | 1 (0.2) | 1 (1.2) |
| Unknown | 585 (55.0) | 290 (51.5) | 247 (58.9) | 48 (59.3) |
| Symptoms or Signs described or present on admission- No. (%) | ||||
| Anosmia No.* |
25 (3.4) 745, NA = 318 |
19 (4.7) 403, NA = 160 |
3 (1.1) 283, NA = 136 |
3 (5.1) 59, NA = 22 |
| Dysgeusia No.* |
27 (3.6) 756, NA = 307 |
19 (4.7) 407, NA = 156 |
6 (2.1) 289, NA = 130 |
2 (3.3) 60, NA = 21 |
| Skin rash No.* |
34 (3.2) 1,059 |
11 (2.0) 562 |
21 (5.0) 416 |
2 (2.5) 81 |
| Conjunctivitis No.* |
15 (1.4) 1,059 |
8 (1.4) 562 |
6 (1.4) 416 |
1 (1.2) 81 |
| Abdominal pain No.* |
127 (12.6) 1,011, NA = 51 |
62 (11.4) 543, NA = 20 |
54 (13.7) 394, NA = 24 |
11 (14.9) 74, N = 7 |
| Fever No.* |
745 (70.1) 1,062 |
424 (75.3) 563 |
263 (62.9) 418 |
58 (71.6) 81 |
| Cough No.* |
580 (54.7) 1,061 |
324 (57.5) 563 |
209 (50.1) 417 |
47 (58.0) 81 |
| Pharyngitis No.* |
248 (23.4) 1,059 |
177 (31.4) 563 |
59 (14.2) 415 |
12 (14.8) 81 |
| Rhinitis No.* |
314 (29.6) 1,060 |
181 (32.1) 563 |
111 (26.7) 416 |
22 (27.2) 81 |
| Headache No.* |
162 (15.3) 1,058 |
114 (20.2) 563 |
39 (9.4) 414 |
9 (11.1) 81 |
| Myalgia No.* |
90 (8.5) 1,055 |
60 (10.7) 561 |
22 (5.3) 413 |
8 (9.9) 81 |
| Malaise No.* |
341 (32.2) 1,058 |
182 (32.4) 562 |
123 (29.6) 415 |
36 (44.4) 81 |
| Diarrhea No.* |
219 (20.7) 1,059 |
139 (24.7) 563 |
61 (14.7) 415 |
19 (23.5) 81 |
| Vomit No.* |
199 (18.8) 1,059 |
107 (19.0) 563 |
77 (18.6) 415 |
15 (18.5) 81 |
| Dyspnea No.* |
231 (21.8) 1,060 |
41 (7.3) 561 |
137 (32.8) 418 |
53 (65.4) 81 |
| Hypoxia No.* |
175 (16.5) 1,060 |
8 (1.4) 561 |
115 (27.5) 418 |
52 (64.2) 81 |
| Hemoptisis No.* |
5 (0.5) 1,058 |
1 (0.2) 561 |
3 (0.7) 416 |
1 (1.2) 81 |
| Altered mental status No.* |
43 (4.1) 1,058 |
4 (0.7) 561 |
13 (3.1) 416 |
26 (32.1) 81 |
| Seizures No.* |
46 (4.3) 1,058 |
8 (1.4) 561 |
21 (5.0) 417 |
17 (21.2) 80 |
| Dehydration No.* |
67 (6.3) 1,055 |
7 (1.2) 560 |
41 (9.9) 415 |
19 (23.7) 80 |
| Shock No.* |
28 (2.6) 1,057 |
1 (0.2) 559 |
5 (1.2) 417 |
22 (27.2) 81 |
| Onset of symptoms to, median (IQR) | 2 (1–3) | 1 (0–3) | 1 (0–3) | 1 (1–3) |
IQR, Interquartile range; PICU, pediatric intensive care unit.
Race/ethnic group was collected by study personnel based on auto reporting by the study participants. “Mixed race” refers to an individual of mixed European/Native heritage.
Nutritional status was classified based on standard deviations for body mass index according to the World Health Organization into thinness (below −2 standard deviations [SD]), normal nutritional status (between −2 and +1 SD), overweight (between +1 and +2 SD) and obesity (above +2 SD). Body mass index was calculated as weight in kilograms divided by height in meters squared.
Number of patients with available data.
Figure 1.
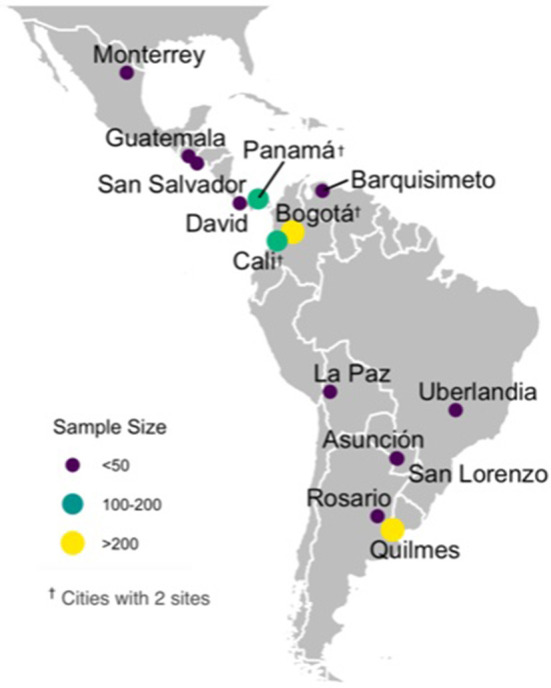
SLIPE-COVID research network-city locations and sites.
Most children had fever (70%) and cough (55%). Fewer than one third had signs or symptoms of upper respiratory tract infection (Table 1). Ninety-seven children (9%) had diarrhea without respiratory symptoms while 104 children (9.8%) did not have either fever or respiratory symptoms at admission. The frequency of these clinical presentations varied by age groups (Supplementary Table 1). Frequency of missing data is presented in Supplementary Table 2.
Eighteen children died (median age 6.2 years [IQR 1.8–12.2], 11 [61%] males), 11 (61%) had comorbidities (mainly immune deficiency [n = 6, 33%] or neurologic [n = 3, 17%]), and 22% came from a rural area. The majority presented with fever (n = 15, 83%), hypoxia (n = 14, 78%), dyspnea (n = 13, 72%) and cough (n = 11, 61%). Characteristics of hospital admission among 500 hospitalized children are described in Supplementary Table 3.
Risk Factors for Hospital Admission
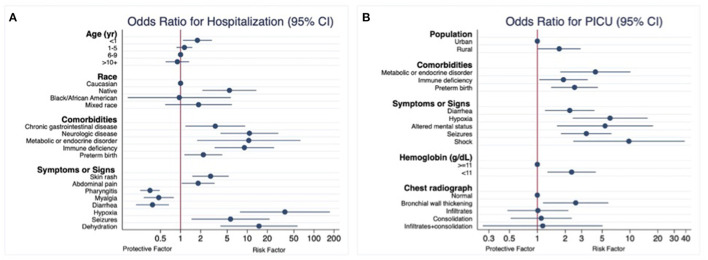
Five hundred hospitalized children were compared with 563 children who received outpatient care. In the multivariable model using multiple imputation, younger age (<1 year), native race, and the presence of certain underlying medical conditions were associated with an increased risk for hospital admission (Table 2 and Figure 2). In a model that considered all underlying medical conditions together, the odds for hospital admission were 5.3 times higher (95% CI 3.10 to 9.15) in children with any underlying medical conditions. Both sensitivity analysis (using the complete dataset that included missing data and only children who were enrolled from the seven cities that used an active surveillance system) showed similar results (Supplementary Table 4).
Table 2.
Demographic and underlying medical conditions associated with hospitalization (general ward and intensive care), among pediatric patients with COVID-19 using multiple imputation chained equations for missing data.
| Characteristic | Outpatient care | Hospitalization | Univariate analysis | Multivariable analysis |
|---|---|---|---|---|
| (n = 563) | (n = 500) | OR (95% CI) | OR (95% CI) | |
| Age groups, yr. No. (%) | ||||
| <1 | 109 (19.4) | 132 (26.4) | 1.42 (0.86–2.34) | 1.78 (1.08–2.94) |
| 1–5 | 214 (38.0) | 172 (344.4) | 0.94 (0.75–1.18) | 1.14 (0.87–1.50) |
| 6–9 | 95 (16.9) | 81 (16.2) | Ref. | Ref. |
| ≥10 | 145 (25.7) | 115 (23.0) | 0.93 (0.66–1.31) | 0.89 (0.60–1.34) |
| Sex, No. (%) | ||||
| Male | 291 (51.7) | 279 (55.8) | Ref. | - |
| Female | 272 (48.3) | 221 (44.2) | 0.85 (0.66–1.06) | - |
| Race or ethnic group a , No. (%) | ||||
| Caucasian | 285 (55.1) | 174 (37.0) | Ref. | Ref. |
| Native | 3 (0.6) | 13 (2.8) | 6.03 (1.99–18.22) | 5.40 (2.13–13.69) |
| Black or African American | 6 (1.2) | 3 (0.6) | 0.90 (0.11–7.07) | 0.96 (0.16–5.66) |
| Mixed race | 223 (43.1) | 280 (59.6) | 2.02 (0.55–7.14) | 1.86 (0.59–5.88) |
| Population, No. (%) | ||||
| Urban | 393 (94.7) | 414 (89.0) | Ref. | - |
| Rural | 22 (5.3) | 51 (11.0) | 1.95 (1.14–3.35) | - |
| Full immunization coverage, No. (%) | ||||
| Yes | 435 (91.2) | 335 (87.7) | 0.63 (0.30–1.35) | - |
| Nutritional Status b , No. (%) | ||||
| Underweight | 17 (6.6) | 48 (15.8) | 1.57 (0.85–2.90) | - |
| Normal Weight | 173 (66.8) | 183 (60.4) | Ref. | - |
| Overweight | 47 (18.1) | 36 (11.9) | 0.83 (0.54–1.28) | - |
| Obese | 22 (8.5) | 36 (11.9) | 1.13 (0.67–1.93) | - |
| Co-morbidities- No. (%) | ||||
| Chronic lung disease | 54 (9.6) | 69 (13.8) | 1.51 (0.75–3.04) | - |
| Congenital heart disease | 6 (1.1) | 12 (2.40) | 2.28 (0.55–9.51) | - |
| Chronic gastrointestinal disease | 2 (0.4) | 7 (1.40) | 3.98 (1.40–11.31) | 3.31 (1.18–9.29) |
| Neurologic disease | 7 (1.2) | 52 (10.4) | 9.22 (3.80–22.34) | 10.77 (3.97–29.81) |
| Metabolic or endocrine disorder | 1 (0.2) | 7 (1.40) | 7.98 (0.99–64.09) | 10.53 (1.77–62.79) |
| Immune deficiency | 13 (2.3) | 75 (15.0) | 7.47 (2.78–20.07) | 9.05 (3.24–25.27) |
| Preterm birth | 9 (1.6) | 24 (4.8) | 3.10 (1.58–6.10) | 2.19 (1.14–4.22) |
OR, odds ratio; IQR, interquartile range.
Race/ethnic group was collected by study personnel based on auto reporting by the study participants. “Mixed race” refers to an individual of mixed European/Native heritage.
Nutritional status was classified based on standard deviations for body mass index according to the World Health Organization into thinness (below −2 standard deviations [SD]), normal nutritional status (between −2 and +1 SD), overweight (between +1 and +2 SD) and obesity (above +2 SD). Body mass index was calculated as weight in kilograms divided by height in meters squared.
Figure 2.
Risk factors among pediatric patients with COVID-19. Multiple imputation chained equations for missing data. (A) Hospital admission (general pediatric ward or pediatric intensive care unit). (B) Pediatric intensive care admission among hospitalized patients.
Children presenting with skin rash, abdominal pain, dehydration, hypoxia, or seizures were at increased risk for hospital admission, while pharyngitis, myalgia and diarrhea reduced the odds of hospital admission (Table 3). Similar results were obtained from both sensitivity analysis (Supplementary Table 5).
Table 3.
Signs or symptoms described or present on initial evaluation associated with hospitalization (general ward and intensive care), among pediatric patients with COVID-19 using multiple imputation chained equations for missing data.
| Symptoms or Signsa - | Outpatient care | Hospitalization | Univariate analysis | Multivariable analysis |
|---|---|---|---|---|
| No. (%) | (n = 563) | (n = 500) | OR (95% CI) | OR (95% CI) |
| Anosmia | 19 (4.7) | 6 (1.8) | - | - |
| Dysgeusia | 19 (4.7) | 8 (2.3) | - | - |
| Skin rash | 11 (2.0) | 23 (4.6) | 2.42 (1.40–4.19) | 2.81 (1.51–5.27) |
| Conjunctivitis | 8 (1.4) | 7 (1.4) | 1.00 (0.47–2.12) | - |
| Abdominal pain | 62 (11.4) | 65 (13.9) | 1.22 (0.90–1.64) | 1.83 (1.04–3.24) |
| Fever | 424 (75.3) | 321 (64.3) | 0.59 (0.29–1.21) | - |
| Cough | 324 (57.5) | 256 (51.4) | 0.78 (0.46–1.32) | - |
| Pharyngitis | 177 (31.4) | 71 (14.3) | 0.36 (0.29–0.45) | 0.34 (0.25–0.48) |
| Rhinitis | 181 (32.1) | 133 (26.8) | 0.77 (0.31–1.90) | - |
| Headache | 114 (20.2) | 48 (9.7) | 0.42 (0.23–0.77) | - |
| Myalgia | 60 (10.7) | 30 (6.1) | 0.53 (0.27–1.06) | 0.47 (0.28–0.79) |
| Malaise | 182 (32.4) | 159 (32.1) | 0.98 (0.47–2.08) | - |
| Diarrhea | 139 (24.7) | 80 (16.1) | 0.58 (0.33–1.03) | 0.38 (0.21–0.67) |
| Vomit | 107 (19.0) | 92 (18.5) | 0.97 (0.55–1.69) | - |
| Dyspnea | 41 (7.3) | 190 (38.1) | 7.79 (4.61–13.18) | - |
| Hypoxia | 8 (1.4) | 167 (33.5) | 34.64 (7.58–158.36) | 36.69 (7.76–173.39) |
| Altered mental status | 4 (0.7) | 39 (7.8) | 11.72 (3.57–38.53) | - |
| Seizures | 8 (1.4) | 38 (7.6) | 5.72 (1.74–18.76) | 5.61 (1.46–21.58) |
| Dehydration | 7 (1.2) | 60 (12.1) | 10.90 (3.85–30.87) | 14.99 (3.96–58.84) |
| Shock | 0 | 27 (5.4) | - | - |
| Onset of symptoms to, median (IQR) | 2 (1–3) | 1 (0–3) | 1.00 (0.96–1.05) | - |
Laboratory or radiologic data not included due to high frequency of missing data.
Risk Factors for ICU Admission
The 81 children who required ICU admission were compared with the remaining 419 children who were hospitalized in the general pediatric ward. In the multiple imputation model, residing in a rural area was the only sociodemographic factor associated with increased odds of ICU care, while metabolic or endocrine disorder, immune deficiency and preterm birth were the underlying medical conditions significantly associated with ICU admission (Table 4). Sensitivity analysis showed similar results (Supplementary Table 6).
Table 4.
Demographic and underlying medical conditions associated with intensive care, among pediatric patients hospitalized due to COVID-19 using multiple imputation chained equations for missing data.
| Characteristics | Place of hospitalization | Multiple Imputation | ||
|---|---|---|---|---|
| General ward | Pediatric intensive care unit | Univariable analysis | Multivariable analysis | |
| (n = 419) | (n = 81) | OR (95% CI) | OR (95% CI) | |
| Age groups, yr. No. (%) | ||||
| <1 | 113 (27.0) | 19 (23.5) | 0.80 (0.53–1.22) | - |
| 1–5 | 141 (33.6) | 31 (38.3) | 1.05 (0.66–1.66) | - |
| 6–9 | 67 (16.0) | 14 (17.3) | Ref. | - |
| ≥10 | 98 (23.4) | 17 (21.0) | 0.83 (0.47–1.46) | - |
| Sex, No. (%) | ||||
| Male | 237 (56.6) | 42 (51.9) | Ref. | - |
| Female | 182 (43.4) | 39 (48.1) | 1.21 (0.73–2.01) | - |
| Race or ethnic group a , No. (%) | ||||
| Caucasian | 149 (37.6) | 25 (33.8) | Ref. | - |
| Native | 11 (2.8) | 2 (2.7) | 1.06 (0.26–4.24) | - |
| Black or African American | 3 (0.8) | 0 (0.0) | - | - |
| Mixed race | 233 (58.8) | 47 (63.5) | 1.22 (0.38–3.88) | - |
| Population, No. (%) | ||||
| Urban | 347 (90.1) | 67 (83.8) | Ref. | Ref. |
| Rural | 38 (9.9) | 13 (16.2) | 1.76 (1.03–2.99) | 1.72 (1.02–2.92) |
| Full immunization coverage, No. (%) | ||||
| Yes | 274 (88.4) | 61 (84.7) | 0.70 (0.38–1.28) | - |
| Nutritional Status b , No. (%) | ||||
| Underweight | 37 (15.3) | 11 (18.0) | 1.28 (0.77–2.14) | - |
| Normal Weight | 149 (61.6) | 34 (55.8) | Ref. | - |
| Overweight | 31 (12.8) | 5 (8.2) | 0.69 (0.25–1.89) | - |
| Obese | 25 (10.33) | 11 (18.0) | 1.36 (0.60–3.10) | - |
| Co-morbidities- No. (%) | ||||
| Chronic lung disease | 58 (13.8) | 11 (13.6) | 0.98 (0.24–3.91) | - |
| Congenital heart disease | 9 (2.1) | 3 (3.7) | 1.75 (0.54–5.69) | - |
| Chronic gastrointestinal disease | 6 (1.4) | 1 (1.2) | 0.86 (0.07–10.18) | - |
| Neurologic disease | 41 (9.8) | 11 (13.6) | 1.45 (0.74–2.83) | - |
| Metabolic or endocrine disorder | 4 (0.9) | 3 (3.7) | 3.99 (1.35–11.76) | 4.22 (1.76–10.11) |
| Immune deficiency | 57 (13.6) | 18 (22.2) | 1.81 (0.96–3.43) | 1.91 (1.05–3.49) |
| Preterm birth | 17 (4.1) | 7 (8.6) | 2.24 (1.26–3.95) | 2.52 (1.41–4.49) |
OR, odds ratio; IQR, interquartile range.
Race/ethnic group was collected by study personnel based on auto reporting by the study participants. “Mixed race” refers to an individual of mixed European/Native heritage.
Nutritional status was classified based on standard deviations for body mass index according to the World Health Organization into thinness (below −2 standard deviations [SD]), normal nutritional status (between −2 and +1 SD), overweight (between +1 and +2 SD) and obesity (above +2 SD). Body mass index was calculated as weight in kilograms divided by height in meters squared.
Children presenting with diarrhea, hypoxia, altered mental status, seizures or shock were at higher risk for ICU admission. Anemia and bronchial wall thickening in chest radiograph were also associated with increased risk (Table 5). Sensitivity analysis showed similar results (Supplementary Table 7).
Table 5.
Signs or symptoms, laboratory values and chest radiograph findings described or present on initial evaluation associated with intensive care, among pediatric patients hospitalized due to COVID-19 using multiple imputation chained equations for missing data.
| Symptoms or signs - | General ward | Pediatric intensive care unit | Univariable analysis | Multivariable analysis |
|---|---|---|---|---|
| No. (%) | (n = 419) | (n = 81) | OR (95% CI) | OR (95% CI) |
| Anosmia | 3 (1.1) | 3 (5.1) | - | - |
| Dysgeusia | 6 (2.1) | 2 (3.3) | - | - |
| Skin rash | 21 (5.0) | 2 (2.5) | 0.48 (0.15–1.52) | - |
| Conjunctivitis | 6 (1.4) | 1 (1.2) | 0.84 (0.07–10.47) | - |
| Abdominal pain | 54 (13.7) | 11 (14.9) | 1.04 (0.36–2.97) | - |
| Fever | 263 (62.9) | 58 (71.6) | 1.48 (1.06–2.08) | - |
| Cough | 209 (50.1) | 47 (58.0) | 1.38 (0.74–2.54) | - |
| Pharyngitis | 59 (14.2) | 12 (14.8) | 1.05 (0.37–3.00) | - |
| Rhinitis | 111 (26.7) | 22 (27.2) | 1.02 (0.44–2.39) | - |
| Headache | 39 (9.4) | 9 (11.1) | 1.21 (0.64–2.30) | - |
| Myalgia | 22 (5.3) | 8 (9.9) | 1.96 (0.52–7.40) | - |
| Malaise | 123 (29.6) | 36 (44.4) | 1.89 (0.76–4.71) | - |
| Diarrhea | 61 (14.7) | 19 (23.5) | 1.78 (1.13–2.82) | 2.23 (1.21–4.12) |
| Vomit | 77 (18.6) | 15 (18.5) | 1.00 (0.59–1.71) | - |
| Dyspnea | 137 (32.8) | 53 (65.4) | 3.89 (1.84–8.25) | - |
| Hypoxia | 115 (27.5) | 52 (64.2) | 4.73 (2.08–10.80) | 6.10 (2.40–15.50) |
| Altered mental status | 13 (3.1) | 26 (32.1) | 14.50 (5.02–41.92) | 5.38 (1.63–17.75) |
| Seizures | 21 (5.0) | 17 (21.2) | 5.08 (3.30–7.82) | 3.37 (1.80–6.32) |
| Dehydration | 41 (9.9) | 19 (23.7) | 2.90 (1.12–7.50) | - |
| Shock | 5 (1.2) | 22 (27.2) | 28.69 (11.92–69.04) | 9.74 (2.44–38.89) |
| Onset of symptoms to, median (IQR) | 1 (0–3) | 1 (0–3) | 0.99 (0.94–1.04) | - |
| Laboratory values | ||||
| Hemoglobin (g/dL) | ||||
| No.* | 372 | 77 | ||
| Median (IQR) | 12.3 (10.9–13.5) | 11.4 (9.5–13.3) | 0.90 (0.81–0.99) | |
| Anemiaa No. (%) | 106 (28.5) | 34 (44.2) | 1.98 (1.25–3.16) | 2.34 (1.28–4.27) |
| Platelets (103 cells/uL) | ||||
| No.* | 391 | 77 | 0.998 (0.997–0.999) | |
| Median (IQR) | 299.0 (218–386) | 276.0 (157.5–374.5) | 2.46 (1.51–4.01) | |
| Thrombocytopeniab No. (%) | 42 (10.7) | 18 (23.4) | – | |
| WBC (103 cells/uL) | ||||
| No.* | 397 | 78 | ||
| Median (IQR) | 10.0 (6.8–15.0) | 12.2 (6.5–14.9) | 1.00 (0.97–1.04) | |
| Neutrophils (103 cells/uL) | ||||
| No.* | 397 | 76 | ||
| Median (IQR) | 5.2 (2.5–9.3) | 6.0 (3.0–9.3) | 0.99 (0.94–1.05) | |
| Lymphocytes (103 cells/uL) | ||||
| No.* | 394 | 76 | ||
| Median (IQR) | 2.8 (1.6–4.6) | 3.0 (0.9–5.2) | 1.03 (0.96–1.09) | – |
| Neutrophil to lympho ratio | ||||
| No.* | 393 | 76 | ||
| Median (IQR) | 1.8 (0.7–4.3) | 1.8 (0.8–4.8) | 1.01 (0.95–1.07) | |
| Values > 5 | 86 (21.9) | 18 (23.7) | 1.10 (0.62–1.96) | – |
| C-reactive protein (mg/dL) | ||||
| No.* | 332 | 70 | ||
| Median (IQR) | 12.9 (4.2–39.5) | 18.9 (7.0–102.7) | 1.004 (1.002–1.006) | |
| Values > 50 mg/dL. No. (%) | 70 (21.1) | 23 (32.9) | 1.88 (1.16–3.06) | – |
| Chest radiograph performed, No. (%) | ||||
| Yes | 313 (74.7) | 71 (87.65) | ||
| Normal | 194 (62.0) | 29 (40.8) | Ref. | Ref. |
| Bronchial wall thickening | 28 (8.9) | 10 (14.1) | 2.27 (0.97–5.28) | 2.59 (1.15–5.84) |
| Interstitial infiltrates | 55 (17.6) | 16 (22.5) | 2.08 (1.19–3.63) | 1.01 (0.48–2.16) |
| Consolidation | 23 (7.3) | 8 (11.3) | 2.53 (1.28–4.98) | 1.10 (0.51–2.35) |
| Interstitial infiltrates + consolidation | 13 (4.1) | 8 (11.3) | 4.32 (1.17–15.93) | 1.14 (0.26–5.04) |
WBC, white blood cell count.
Values < 11 g/dL.
Platelets < 150,000 × 103 cells/μ L.
Number of patients with available data.
Risk Factors Associated With Supplementary Oxygen Use
Risk factors associated with supplementary oxygen requirement were similar to those associated with hospital admission. In addition, history of chronic lung disease increased the risk for supplementary oxygen requirement (OR 4.45, 95% CI 2.35–8.41) (Supplementary Table 8).
Discussion
In this multi-country case registry of children with COVID-19 (excluding MIS-C), we identified several factors independently associated with ICU admission, hospitalization and need for supplementary oxygen. Similar to other reports, age under 1-year, comorbidities such as metabolic/endocrine disorders, preterm birth or immune deficiency, certain presenting signs/symptoms and social determinants of health were associated with increased COVID-19-related morbidity (10, 15, 27–30). In addition, low hemoglobin concentrations and bronchial wall thickening on chest radiographs were independently associated with the need for PICU admission.
Social determinants of health have long been recognized to be important predictors as to how epidemics are experienced in terms of infection rates and morbidity (31), particularly in more socially unequal regions, such as Latin America. A multicenter cohort study reported that rates of pediatric PICU admission and deaths due to COVID-19 were higher in Latin American than European children (32). Disparities in health determinants like economic instability, insurance status and housing conditions of patients and their families have consistently placed social, racial, and ethnic minorities at greater risk for severe illness by COVID-19 (31). This is especially true for children and their social determinants influencing life opportunities, disease characteristics and health outcomes. We described greater risks in children with demographic characteristics indicative of lower socio-economic status in Latin America such as native ethnic group or living in rural areas. Social inequalities and low socioeconomic status have also been described as risk factors for death (28), so it is possible that unmeasured socioeconomic or cultural disparities that increase the risk of a more severe or late presentation in children living in rural areas may have been present in this study. For example, although level of education of the caregiver was not imputed or included in multivariable analysis due to the high frequency of missing data, there was a higher proportion of uneducated caregivers among those children who required hospitalization.
Obesity and diabetes mellitus are comorbidities that have been identified as risk factors for disease severity in other studies (12, 15, 33–35). In this study, obesity was not significantly associated with higher risk of hospitalization or ICU admission. However, obesity was more frequent in children requiring ICU admission than in children requiring outpatient care (18 vs. 8.4%, respectively), although no multivariable model comparison was made for these two categories. In addition, obese children were at higher odds of hospitalization (overall and in the ICU), although this was of limited statistical significance. Yet, in studies from high-income countries, obesity has been consistently demonstrated as a risk factor for disease severity in children, especially in adolescents (15, 36). In animal studies, angiotensin-converting enzyme 2 (ACE2) protects against SARS-CoV-2 associated acute respiratory distress syndrome (ARDS) (37). ACE2 expression is decreased in children with diabetes mellitus likely due to glycosylation (38), which may explain their higher risk for hospital or ICU admission in this and other series (33). Although asthma has been suggested as a risk factor for severe illness in children with COVID-19, our study, as well as a registry-based (39) and a cohort study (16) did not confirm this association. Overall, the underlying medical conditions associated with hospital or ICU admission in this Latin American pediatric registry are similar to those described for the same outcomes in US children (39).
As with children with COVID-19 from European and North American countries (11, 40), fever and cough were the predominant clinical features at presentation (70 and 55%, respectively) and approximately a third of children presented with gastrointestinal symptoms. Some clinical manifestations are related to disease progression and complications and are predictive of a higher level of care. As reported in adults (41), children presenting with hypoxia, altered mental status, seizures, shock, dyspnea, or dehydration were more likely to require hospital admission or intensive care. Pharyngitis, myalgia, and diarrhea were identified in this series as inversely associated with hospital admission; and in the complete dataset anosmia and dysgeusia were also found to be protective although of limited statistical significance. A UK study also found that children presenting with upper respiratory signs (rhinorrhea) were less likely to require admission to critical care (35). Preferential distribution of ACE-2 receptors in the upper respiratory or intestinal epithelium (42) may explain the lower frequency of hospitalization in children with these clinical features. Between 9 and 10% of children in this series presented without fever or respiratory symptoms, or only developed diarrhea, findings that need to be considered when developing diagnostic algorithms, especially in settings with actively circulating virus.
Several laboratory findings have been associated with the severity of COVID-19 in adult patients (43). However, data on laboratory values as risk factors for the need for ICU admission in children are scarce. Of routinely collected data in children, only the C-reactive protein has been shown to be a predictor in one study (12), but not in others (44). Similar to our findings, leukocyte indices do not appear to be reliable indicators of disease severity in the pediatric population (45). We found that anemia was the only laboratory predictor for the risk of ICU admission. Whereas this finding has not been described in children, it has been documented as a significant risk factor in adults (46) and should be looked at in detail when assessing children with COVID-19. Like adults (47), bronchial wall thickening was associated with increased risk of ICU admission. Other more severe radiologic abnormalities often described in severe cases (48) were not independently associated with risk of ICU admission in this series, likely due to low number of cases.
Several studies have described risk factors for severe COVID-19 in high-income countries, but data is still scarce from LMIC. Our findings are similar to those from the UK cohort of children for admission to critical care (35). In both studies, children who required PICU were more likely to be of younger age, and had associated comorbidities. Studies from high-income countries have shown that adolescents and patients with elevated C-reactive protein have an increased risk for more severe outcomes (12, 16, 40, 49). These associations were not evident in our data, although different exposure categories and outcome measures may explain these discrepancies.
This study has several limitations. First, seven of the 14 reporting cities used a passive case-ascertainment method which probably introduced a selection bias. Thus, our study population does not represent the full spectrum of COVID-19, but rather children with more severe disease. This is unlikely to impact our results or conclusions given that our goal was to identify factors associated with hospital or ICU admission, and the multilevel regression model clustered by region. To further explore for selection bias, a sensitivity analysis including only children from the seven cities that used an active surveillance system was compared with the analysis that included all children. Factors associated with severity were similar in both analyses suggesting that the effect on our data was minimal.
Second, some relevant variables had missing data, which could reduce the statistical power of the study and produce biased estimates. We tried to overcome this limitation by using multiple imputation, allowing appropriate estimation of the underlying distribution of the data. Results from analyses using the complete dataset (including missing data) and the multiple imputation dataset were similar. Third, we excluded children with MIS-C, who represent one of the most severe clinical forms of pediatric post-infectious COVID-19. A separate full analysis of MIS-C children in Latin American children is ongoing by investigators from the REKAMLATINA network in a cohort of children admitted at several hospitals in the region (50). Finally, due to the multisite nature of the study, full standardization of criteria utilized by all managing clinicians for hospitalization or ICU care was not possible. Therefore, children's outcome disposition may differ by hospitals' capacity and census demands, notwithstanding the severity of the COVID-19 presentation. However, the need for ICU admission or oxygen is an objective measure for a more serious condition.
In conclusion, this evolving collaborative network allowed the collection of detailed data in one of the largest studies from a LMIC region to provide a first description of associated factors for hospital or ICU admission in developing countries. The demographic, clinical and laboratory parameters that were identified could help care providers in different settings (outpatient clinic, emergency room or general pediatric wards) to identify children at higher risk for a complicated disease course, and direct policy makers to prioritize pediatric subgroups for prevention and treatment strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and the registry and study protocol were reviewed and approved by the local Institutional Review Board of each participant center. The research was deemed of minimal risk because data was collected for routine clinical practice and the requirement for informed consent was waived. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
EL-M and EA: study conception and design. EL-M, GC-M, MB, DD, JT, RU-G, PL, RD, PP, JP, XN, CM, ML, GE, CD, KL, PQ, MR, JR-A, AE-V, MC, OM, EB, JC, AM, AJ-Z, LD, MM, and NG: data collection. EL-M, EC, and EA: analysis and interpretation of results. E-LM and EA: draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Funding
The Research Electronic Data Capture (REDCap) database used for this study was supported by the Grant Number UL1 TR002535, from NIH/NCATS Colorado CTSA.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the contributions of the physicians, nurses and the administrative team at SLIPE for the work in caring for these patients and to the contributing institutions for participating in the SLIPE-COVID network.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.868297/full#supplementary-material
References
- 1.Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID-19 in Children in the United States: Intensive Care Admissions, Estimated Total Infected, and Projected Numbers of Severe Pediatric Cases in 2020. J Public Health Manag Pract. (2020) 26:325–33. 10.1097/PHH.0000000000001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta NS, Mytton OT, Mullins EWS, Fowler TA, Falconer CL, Murphy OB, et al. SARS-CoV-2 (COVID-19): What Do We Know About Children? A Systematic Review. Clin Infect Dis. (2020) 71:2469–79. 10.1093/cid/ciaa556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panamerican Health Organization . PAHO. Geo-Hub COVID-19 - Information System for the Region of the Americas. (2022). Available online at: https://paho-covid19-response-who.hub.arcgis.com (accessed February 24, 2022).
- 4.Gobierno de Chile. Plan de Acción Coronavirus (2022). Available online at: https://www.gob.cl/coronavirus/cifrasoficiales/#datos (accessed February 24, 2022).
- 5.Dirección general de Epidemiología . Gobierno de Mexico. COVID-19 Mexico Tablero general CONACYT. Available online at: https://datos.covid-19.conacyt.mx (accessed February 24, 2022).
- 6.Ministerio de Salud Argentina . Vigilancia Epidemiologica. Nuevo Coronavirus. Vigilancia, diagnóstico y manejo institucional de casos en pediatría. (2021). Available online at: https://www.argentina.gob.ar/salud/coronavirus/casos-pediatria (accessed February 24, 2022).
- 7.Instituto Nacional de Salud . Ministerio de Salud y Protección Social Colombia. COVID-19 en Colombia. (2022). Available online at: https://www.ins.gov.co/Noticias/Paginas/coronavirus-casos.aspx (accessed February 24, 2022).
- 8.Ministerio de Salud Publica y Bienestar Social . Gobierno de Paraguay. Coronavirus/ COVID 19 en Paraguay. (2022). Available online at: https://www.mspbs.gov.py/reporte-covid19.html (accessed February 25, 2022).
- 9.American Academy of Pediatrics . Children and COVID-19: State-Level Data Report. Available online at: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/ (accessed September 14, 2021).
- 10.Bellino S, Punzo O, Rota MC, Del Manso M, Urdiales AM, Andrianou X, et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics. (2020) 146:e2020009399. 10.1542/peds.2020-009399 [DOI] [PubMed] [Google Scholar]
- 11.Gotzinger F, Santiago-Garcia B, Noguera-Julian A, Lanaspa M, Lancella L, Calo Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. (2020) 4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graff K, Smith C, Silveira L, Jung S, Curran-Hays S, Jarjour J, et al. Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J. (2021) 40:e137–e45. 10.1097/INF.0000000000003043 [DOI] [PubMed] [Google Scholar]
- 13.Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk Factors for Severity in Children with Coronavirus Disease 2019: A Comprehensive Literature Review. Pediatr Clin North Am. (2021) 68:321–38. 10.1016/j.pcl.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. (2021) 180:1659–63. 10.1007/s00431-021-03955-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, et al. Risk factors for severe COVID-19 in children. Pediatrics. (2021) e2021053418. [Epub ahead of print]. 10.1542/peds.2021-053418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk AL, Florin TA, Kuppermann N, Tancredi DJ, Xie J, Kim K, et al. Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study. JAMA Netw Open. (2022) 5:e2142322. 10.1001/jamanetworkopen.2021.42322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pudjiadi AH, Putri ND, Sjakti HA, Yanuarso PB, Gunardi H, Roeslani RD, et al. Pediatric COVID-19: Report From Indonesian Pediatric Society Data Registry. Front Pediatr. (2021) 9:716898. 10.3389/fped.2021.716898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization .WHO. Obesity and Overweight. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed September 14, 2021).
- 19.World Health Organization .WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online at: https://apps.who.int/iris/handle/10665/85839 (accessed September 14, 2021).
- 20.Linkins LA, Dans AL, Moores LK, Bona R, Davidson BL, Schulman S, et al. Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141(2 Suppl):e495S–e530S. 10.1378/chest.11-2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int J Clin Pract. (2021) 75:e13698. 10.1111/ijcp.13698 [DOI] [PubMed] [Google Scholar]
- 22.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. (2021) 325:1074–87. 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. (2020) 4:100055. 10.1016/j.lanwpc.2020.100055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood AM, White IR, Royston P. How should variable selection be performed with multiply imputed data? Stat Med. (2008) 27:3227–46. 10.1002/sim.3177 [DOI] [PubMed] [Google Scholar]
- 25.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. (2011) 30:377–99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Yan L, Fei Y, Zhang C. Laboratory abnormalities and risk factors associated with in-hospital death in patients with severe COVID-19. J Clin Lab Anal. (2020) 34:e23467. 10.1002/jcla.23467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Team CC-R. Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:422–6. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira EA, Colosimo EA, Simoes ESAC, Mak RH, Martelli DB, Silva LR, et al. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: an analysis of a nationwide database. Lancet Child Adolesc Health. (2021) 5:559–68. 10.1016/S2352-4642(21)00134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Dambrauskas S, Camporesi A, Cantillano EM, Dallefeld S, Dominguez-Rojas J, Gurbanov A, et al. Pediatric critical COVID-19 and mortality in a multinational cohort. Available online at: https://www.medrxiv.org/content/10.1101/2021.08.20.21262122v1.full.pdf (accessed January 15, 2022). [DOI] [PMC free article] [PubMed]
- 30.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. (2020) 174:868–73. 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bambra C, Riordan R, Ford J, Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. (2020) 74:964–8. 10.1136/jech-2020-214401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Saráchaga M, Salcedo-Lozada P, et al. COVID-19 and Multisystem Inflammatory Syndrome in Latin American Children: a multinational study. Pediatr Infect Dis J. (2021) 40:e1–6. 10.1097/INF.0000000000002949 [DOI] [PubMed] [Google Scholar]
- 33.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. (2020) 370:m3249. 10.1136/bmj.m3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin B, DeWitt PE, Russell S, Anand A, Bradwell KR, Bremer C, et al. Characteristics, Outcomes, and Severity Risk Factors Associated With SARS-CoV-2 Infection Among Children in the US National COVID Cohort Collaborative. JAMA Netw Open. (2022) 5:e2143151. 10.1001/jamanetworkopen.2021.43151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. (2005) 436:112–6. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Res Clin Pract. (2020) 162:108132. 10.1016/j.diabres.2020.108132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw Open. (2021) 4:e2111182. 10.1001/jamanetworkopen.2021.11182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drouin O, Hepburn CM, Farrar DS, Baerg K, Chan K, Cyr C, et al. Characteristics of children admitted to hospital with acute SARS-CoV-2 infection in Canada in 2020. CMAJ. (2021) 193:E1483–E93. 10.1503/cmaj.210053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. (2020) archdischild-2020-320338. [Epub ahead of print]. 10.1136/archdischild-2020-320338 [DOI] [PubMed] [Google Scholar]
- 43.Zeng Z, Yu H, Chen H, Qi W, Chen L, Chen G, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care. (2020) 24:525. 10.1186/s13054-020-03255-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry BM, Benoit SW, de Oliveira MHS, Hsieh WC, Benoit J, Ballout RA, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): A pooled analysis and review. Clin Biochem. (2020) 81:1–8. 10.1016/j.clinbiochem.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. (2020) 58:1135–8. 10.1515/cclm-2020-0272 [DOI] [PubMed] [Google Scholar]
- 46.Tao Z, Xu J, Chen W, Yang Z, Xu X, Liu L, et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J Med Virol. (2021) 93:1478–88. 10.1002/jmv.26444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. (2020) 55:327–31. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ong SWX, Hui TCH, Lee YS, Haja Mohideen SM, Young BE, Tan CH, et al. High-risk chest radiographic features associated with COVID-19 disease severity. PLoS ONE. (2021) 16:e0245518. 10.1371/journal.pone.0245518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisler G, Izard SM, Shah S, Lewis D, Kainth MK, Hagmann SHF, et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. (2020) 10:171. 10.1186/s13613-020-00790-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulloa-Gutierrez R, Ivankovich-Escoto G, Yock-Corrales A, Tremoulet AH. Multisystem Inflammatory Syndrome Surveillance and COVID-19 in Latin America. Pediatr Infect Dis J. (2020) 39:e473–e4. 10.1097/INF.0000000000002901 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.