Abstract
The antimicrobial activities of chloroquine (CQ) and several 4-aminoquinoline drugs were tested against Penicillium marneffei, an opportunistic fungus that invades and grows inside macrophages and causes disseminated infection in AIDS patients. Human THP1 and mouse J774 macrophages were infected in vitro with P. marneffei conidia and treated with different doses of drugs for 24 to 48 h followed by cell lysis and the counting of P. marneffei CFU. CQ and amodiaquine exerted a dose-dependent inhibition of fungal growth, whereas quinine and artemisinin were fungistatic and not fungicidal. The antifungal activity of CQ was not due to an impairment of fungal iron acquisition in that it was not reversed by the addition of iron nitrilotriacetate, FeCl3, or iron ammonium citrate. Perl's staining indicated that CQ did not alter the ability of J774 cells to acquire iron from the medium. Most likely, CQ's antifungal activity is due to an increase in the intravacuolar pH and a disruption of pH-dependent metabolic processes. Indeed, we demonstrate that (i) bafilomycin A1 and ammonium chloride, two agents known to alkalinize intracellular vesicles by different mechanisms, were inhibitory as well and (ii) a newly synthesized 4-amino-7-chloroquinoline molecule (compound 9), lacking the terminal amino side chain of CQ that assists in drug accumulation, did not inhibit P. marneffei growth. These results suggest that CQ has a potential for use in prophylaxis of P. marneffei infections in human immunodeficiency virus-infected patients in countries where P. marneffei is endemic.
Chloroquine (CQ) is a widely available and inexpensive drug belonging to the family of 7-chloro-4-aminoquinoline compounds (22). It is currently employed for malaria prophylaxis and therapy; its hydroxy derivative is also used as an anti-inflammatory agent in the treatment of rheumatoid arthritis and systemic lupus erythematosus. Depending on the chemical characteristics and the length of the side chain in position 4, the aminoquinolines are monoprotic or diprotic weak bases: they diffuse freely in the membranes in the unprotonated form, but once they reach an intracellular acidic environment, they rapidly become protonated and accumulate, raising the intravacuolar pH (9, 16). Hence, these molecules can alter the activity of the acidic vesicle system that in mammalian cells includes the endosomes, the lysosomes, the Golgi complex, and, in the case of several types of intracellular pathogens, the phagosomes and phagolysosomes. The activity of CQ against intraerythrocytic Plasmodium spp. is accomplished by its accumulation via pH trapping in the acidic food vacuole of the parasites; binding to a specific substrate, heme, derived from the proteolysis of host hemoglobin; and finally, inhibition of heme detoxification (4, 10, 16, 20). Alkalization of the endosome and/or phagolysosome seems to explain the antimicrobial activity of CQ against several intracellular microorganisms, such as Histoplasma capsulatum (21), Cryptococcus neoformans (18, 19), Legionella pneumophila (6), and Francisella tularensis (12).
These antimicrobial properties prompted us to investigate the potential inhibitory effects of CQ and several quinoline derivatives on Penicillium marneffei, an opportunistic pathogen endemic in Southeast Asia that causes disseminated infection in AIDS patients (8, 26, 27). P. marneffei is dimorphic: it grows at 25°C as a mold and at 37°C as a yeast. In vivo, it invades the cells of the mononuclear phagocyte system where it divides by fission as a yeast (7, 8). An in vitro macrophage model that allows evaluation of the extent of intracellular P. marneffei growth was developed. It was demonstrated that the activation of J774 murine macrophages or human THP1 cells by treatment with lipopolysaccharide (LPS) plus gamma interferon (IFN-γ) reduces the recovery of live fungi via nitric oxide-dependent and -independent mechanisms (28). This system was used in the present study to assess the potential activity of CQ against P. marneffei and to study its mechanism of action.
MATERIALS AND METHODS
Mice.
Pathogen-free female CD1 mice, 6 to 8 weeks old, were obtained from Charles River Italia (Calco, Como, Italy) and were housed, fed, and handled in compliance with prescriptions for the care and use of laboratory animals.
Reagents.
Ferric ammonium citrate, FeCl3 · 6H2O, iron nitriloacetate (FeNTA), CQ, amodiaquine (AM), and quinine were from Sigma Italia (Milan, Italy). Artemisinin was a kind gift from R. Carter, Knoll, Switzerland. The 4-aminoquinoline derivatives used in this study (compounds 9 and 15) (Fig. 1) were a kind gift of Timothy J. Egan, Department of Chemistry, University of Cape Town, Cape Town, South Africa. They were synthesized from 4-chloroquinoline as previously described (10).
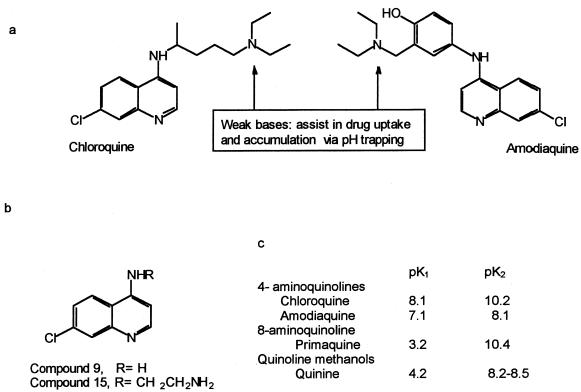
FIG. 1.
Structure and numbering of CQ and AM (a) and the 4-aminoquinoline compounds used in this study (b) and their pK values (c) (10, 15; T. J. Egan, personal communication).
Strain.
The strain of P. marneffei used in this study, IUM 885346, was kindly provided by M. A. Viviani. It was isolated from the blood of a human immunodeficiency virus (HIV)-positive Italian male intravenous drug user who had been infected in Thailand (7). The fungus was cultured on yeast morphology agar (YMA) (Biolife, Milan, Italy) at 25°C for 10 to 14 days. Conidia were then collected by flooding the culture surface with saline, centrifuged at 4,000 × g for 20 min, and filtered to remove mixed mycelial debris, as previously described (28).
Macrophage cell lines.
The murine macrophage-like cell line J774 was maintained in minimal essential medium (MEM) (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum (Euroclone, Ltd.), 1% glutamine, 2% HEPES buffer, 1% penicillin-streptomycin (Biological Ind., Kibbutz, Israel), and 1% nonessential amino acids (complete medium) (GIBCO BRL) in 5% CO2 at 37°C in petri dishes (Cellstar; Greiner, Ltd., Kremsmuster, Austria). J774 macrophages were mechanically collected with a cell lifter (Costar Italia, Milan, Italy) and transferred to fresh medium every 3 to 4 days. Human THP1 cells were grown in suspension in complete RPMI 1640 medium (GIBCO BRL). They were differentiated by treatment with 0.32 μM phorbol myristate acetate (PMA) for 72 h (28).
Assay of antifungal activity of macrophages.
PMA-differentiated THP1 cells or J774 macrophages were seeded in triplicate in 24-well Costar plates at 105 cells/well in complete medium and incubated at 37°C in 5% CO2 for 24 h; confluent monolayers were then treated with P. marneffei conidia (2 × 105/well) for 2 h of phagocytosis in the absence of opsonins. Since the microscopic count of conidia cannot distinguish viable from dead fungi, the number of CFU at 72 h did not always match the number of conidia of the initial inoculum but varied depending on conidium viability. We did not include experiments in which the viability of conidia was below 60%. After phagocytosis, cell monolayers were washed with warm phosphate-buffered saline to remove nonphagocytized conidia, and either lysed, treated with different concentrations of drugs, or stimulated with 100 U of recombinant IFN-γ (Genzyme)/ml and 1 μg of LPS (from E. coli O111:B4; Sigma)/ml in complete medium, as previously described (28). The number of CFU recovered from the lysis of J774 or THP1 cells after 2 h of phagocytosis was considered the initial inoculum and indicated as the “baseline” value in all figures and the table. At the end of 24 or 48 h of incubation, macrophages were lysed with 0.1% Tween 20 in saline and the phagocytized yeasts were recovered, centrifuged, and plated in serial dilution in triplicate in YMA for 72 h at 25°C. The results are expressed as mean CFU ± standard errors of the mean (SEM) from three or four different experiments.
The degree of iron loading was evaluated histochemically. J774 macrophages were seeded into chamber slides (Nunc) at 5 × 105 cells/well in complete medium and treated with 250 μM FeCl3 plus 10 μM CQ or FeCl3 alone for 48 h. At the end of the incubation, slides were rinsed with saline, fixed in 4% buffered formalin, and then stained with Prussian blue according to the method of Perl (23).
Cell viability.
The MTT [3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (M-2128; Sigma) assay was used to determine the viability of J774 macrophages after treatment with antimalarial drugs (29). Briefly, 105 J774 cells were seeded in quadruplicate in 96-well plates in complete medium and serial dilutions of the drugs. The plates were incubated for 48 h at 37°C in 5% CO2; then, 20 μl of a 5-mg/ml solution of MTT in phosphate-buffered saline was added, and incubation continued for an additional 3 h at 37°C. The plates were then centrifuged, the supernatants were discarded, and the dark blue formazan crystals were dissolved using 100 μl of lysing buffer consisting of a solution of 20% (wt/vol) sodium dodecyl sulfate (Sigma) and 40% N,N-dimethylformamide (Merck) in H2O at pH 4.7 adjusted with 80% acetic acid. The plates were then read on a microplate reader (Molecular Devices Co., Menlo Park, Calif.) using a test wavelength of 550 nm and a reference wavelength of 650 nm. The optical density at 650 nm (OD650) was subtracted from the OD550 to eliminate nonspecific background. A similar method was employed to measure the effects of antimalarial drugs on P. marneffei cultured in 96-well plates for 72 h, as described previously (17).
RESULTS
Effects of CQ and other antimalarial compounds on P. marneffei growth in human and mouse macrophages.
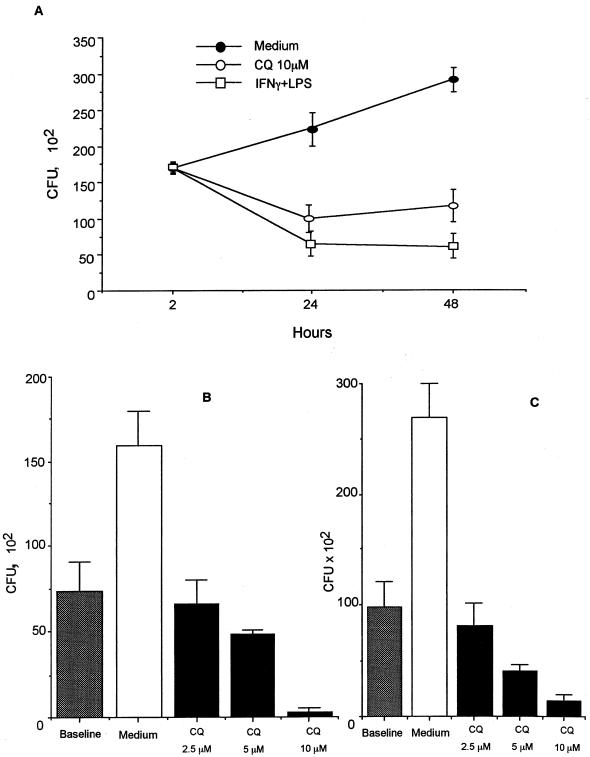
P. marneffei multiplies inside murine J774 cells, as evidenced by an approximately twofold increase in CFU after 48 h of culture (Fig. 2A). Treatment of J774 cells with CQ at 2 h postinfection induced a significant and dose-dependent inhibition of P. marneffei growth (Fig. 2A and B). CQ was fungistatic at 5 μM and fungicidal at 10 μM. The antifungal activity of CQ was comparable to that obtained by the activation of cells with IFN-γ plus LPS (Fig. 2A and reference 28). Similar results were obtained using the human monocyte/macrophage cell line THP1 (Fig. 2C) or fresh peritoneal macrophages from CD1 mice (data not shown).
FIG. 2.
Inhibition of intracellular P. marneffei growth by CQ. (A) Antifungal activity of J774 macrophages treated with 10 μM CQ or stimulated with IFN-γ plus LPS. The data are expressed as mean CFU ± SEM recovered from triplicate cultures of a representative of three experiments. (B) Dose-dependent effect of CQ against P. marneffei conidia phagocytized by J774 macrophages for 2 h. The data are expressed as mean CFU ± SEM recovered from three different experiments in triplicate. (C) Dose-dependent effect of CQ against P. marneffei conidia phagocytized by PMA-differentiated human THP1 cells for 2 h. The data are expressed as mean CFU ± SEM recovered from three different experiments in triplicate.
In addition to CQ, several other antimalarials were assayed for anti-P. marneffei activity in J774 cells. Table 1 shows that CQ and AM, both 4-aminoquinoline drugs, but not primaquine, an 8-aminoquinoline, all tested at 10 μM, were active in reducing P. marneffei survival. The effect was significant at 24 h and even more marked at 48 h. Between 24 and 48 h, the intracellular multiplication of P. marneffei was completely inhibited in the CQ- and AM-treated cells, whereas a 2.4-fold increase in CFU was seen in control groups. In contrast, quinine and artemisinin exerted fungistatic effects that were mostly prominent after 48 h. As measured by the MTT assay, none of the drugs employed was toxic for J774 macrophages (Table 1, last column).
TABLE 1.
Effects of several antimalarials on the intracellular growth of P. marneffei yeasts in J774 macrophages
| Treatmenta | CFU (102) ± SD (% of control) atb:
|
MTT, assay result (OD550 ± SD) | ||
|---|---|---|---|---|
| 2 h | 24 h | 48 h | ||
| Baseline | 105.7 ± 12 | |||
| Medium | 134.6 ± 3.5 (100) | 300 ± 41 (100) | 0.452 ± 0.03 | |
| CQ (10 μM) | 60.2 ± 14 (44.6)∗ | 71 ± 14 (24)∗∗ | 0.451 ± 0.05 | |
| AM (10 μM) | 34.3 ± 6.6 (25.5)∗ | 27 ± 4 (9)∗∗ | 0.476 ± 0.04 | |
| Quinine (10 μM) | 130.2 ± 12.1 (96.6) | 137 ± 8 (46)∗ | 0.356 ± 0.01 | |
| Primaquine (10 μM) | 145.3 ± 17.6 (100) | 272 ± 76 (91) | 0.486 ± 0.05 | |
| Artemisinin (10 μM) | 149.3 ± 9.5 (100) | 156 ± 17 (52)∗ | ND | |
P. marneffei conidia were phagocytized by J774 cells at a 2:1 ratio in complete MEM for 2 h. Cell monolayers were then washed to remove nonphagocytized conidia and incubated with the different drugs at 37°C.
After 2 h (baseline) and at the end of 24 or 48 h of incubation, J774 cells were lysed and intracellular yeasts were plated in YMA in triplicate for 72 h at room temperature. The experiments were repeated six times for CQ and three times for the other drugs. ∗, P < 0.05 by analysis of variance; ∗∗, P < 0.01 by analysis of variance; ND, not done.
Effect of exogenous iron supplementation.
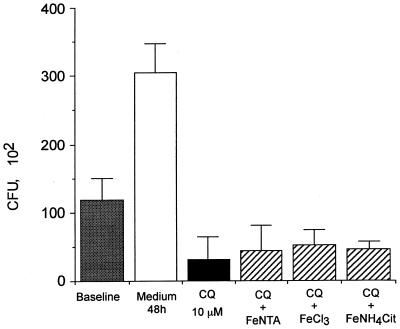
Since some of the antimicrobial effects of CQ have been attributed to its ability to restrict iron availability to intracellular pathogens (6, 12, 21), the activity of CQ was examined in the presence of exogenous iron supplementation. The addition of FeNTA, ferric ammonium citrate, or ferric chloride did not modify the antifungal activity of CQ at 10 μM against P. marneffei (Fig. 3). To ascertain whether CQ could impair cellular accumulation of exogenous iron by interfering with the endocytic pathway, J774 cells were examined by Perl's staining after incubation with 250 μM ferric chloride. The positive stain of CQ-treated and control J774 cells demonstrates that cellular iron loading was not affected by treatment with 10 μM CQ. It is therefore unlikely that CQ exerts its anti-P. marneffei activity by impairing the acquisition of iron by the fungus.
FIG. 3.
Effect of exogenous iron supplementation on the activity of CQ against intracellular growth of P. marneffei. P. marneffei conidia were incubated with J774 cells for 2 h at a 2:1 ratio in triplicate. After removal of nonphagocytized conidia, cultures were treated with CQ in the presence of FeNTA (50 μg/ml), FeCl3 (250 μM), or ferric NH4 citrate (100 μg/ml) and incubated for a further 48 h. The data are expressed as mean CFU ± SEM recovered from triplicate cultures of a representative of three experiments.
Effects of vacuolar pH-neutralizing agents on P. marneffei survival.
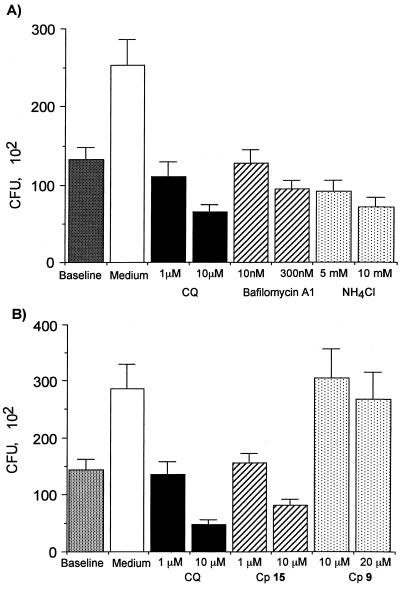
To investigate whether the activity of CQ was related to its ability to increase vesicular pH, both a structurally unrelated weak base (ammonium chloride) and an inhibitor of vacuolar H+-ATPase (bafilomycin A1) (5, 13) were used. Both compounds increased the antifungal activity of J774 (Fig. 4A) and THP1 (data not shown) macrophages in a concentration-dependent manner, suggesting that a pH increase within the P. marneffei-containing phagosome may explain the antifungal activity of CQ. Testing of compounds 9 and 15, two newly synthesized 4-aminoquinolines with different side chains and base properties (10), further supported this hypothesis. The structures of compound 9, a 4-amino-7-chloroquinoline, and compound 15, an N-(7-chloro-4 quinolinyl)-1,2-ethanediamine, are shown in Fig. 1 together with those of CQ and AM, as well as the pK values of the studied aminoquinolines. Compound 9 lacks the terminal amino side chain of CQ that assists in drug accumulation in the acidic compartments of macrophages via pH trapping, whereas compound 15 is more similar to CQ. However, compounds 9 and 15 are both less lipophilic than CQ. When assayed on J774 macrophages, compound 9, even at 20 μM, was completely inactive against P. marneffei (Fig. 4B), whereas compound 15 at 10 μM inhibited P. marneffei growth by 50%.
FIG. 4.
Effect of vacuolar pH-neutralizing agents on P. marneffei growth inside J774 macrophages. (A) Inhibition of P. marneffei growth by treatment with bafilomycin A1 and ammonium chloride. The data are expressed as mean CFU ± SEM recovered from triplicate cultures of a representative of three experiments. (B) Inhibition of P. marneffei growth by treatment with CQ, compound 15 (Cp 15), or compound 9 (Cp 9). The data are expressed as mean CFU ± SEM recovered from triplicate cultures of a representative of three experiments.
Effects of antimalarial compounds on P. marneffei growth in axenic medium.
The aminoquinoline compounds were also tested against P. marneffei conidia in a cell-free system using MEM and the MTT assay to measure fungal viability (17, 28). None of the drugs showed any direct activity against P. marneffei at the concentrations used in the J774 assay (10 μM). Higher concentrations (50 μM) of CQ and AM were required to inhibit the fungus by 75 and 86%, respectively. Treatment with 50 μM concentrations of compounds 9 and 15 resulted in a similar degree of inhibition of P. marneffei growth (69 and 66%, respectively).
DISCUSSION
P. marneffei is assumed to enter the human body by the respiratory route in the conidial form. It is then internalized by macrophages, where it transforms and takes a yeast-like morphology (8). It was previously reported that the same conidium-to-yeast transition could be elicited in vitro following phagocytosis of P. marneffei conidia by J774 macrophages and incubation at 37°C (7). Intracellular fungus multiplication is also documented, as measured by the two- to threefold increase in CFU found after 48 h of culture compared to the amount of phagocytized conidia (reference 28 and this paper). The murine J774 cell line as well as its human promonocytic counterpart THP1 were utilized in the present study to evaluate the effects of CQ on the survival of P. marneffei within its macrophage habitat.
CQ inhibited intracellular P. marneffei growth in a dose-dependent manner in both the murine and the human cell lines, as well as in primary murine macrophages. This drug has previously been shown to exert in vitro and in vivo inhibitory effects on the growth of two other yeasts: H. capsulatum (21) and C. neoformans (18, 19). The beneficial effects of CQ have been attributed to different mechanisms. In the case of H. capsulatum, CQ impedes the pH-dependent acquisition of iron either from the transferrin-transferrin receptor complex in the endosome or from ferritin in the lysosome (21). By contrast, CQ inhibits C. neoformans growth by a mechanism that is independent of iron acquisition but is related to other consequences of the pH increase in the subcellular acidic compartments (18, 19). Concerning P. marneffei, it is unlikely that iron acquisition fully explains the inhibitory effects of CQ, as the addition of either FeNTA, ferric chloride, or ferric ammonium citrate did not abrogate the CQ-induced inhibition.
On the other hand, the following three arguments lend support to the concept that CQ, which is well recognized to increase vacuolar pH, exerts its anti-P. marneffei activity by some other pH-dependent mechanisms. First, it has previously been shown that the fungus, when cultured axenically, grows better at an acidic pH than at a neutral or basic pH (7). Second, CQ's effects can be mimicked by the use of compounds that increase vacuolar pH by different means, such as ammonium chloride, a totally unrelated molecule that also acts as a weak base, or bafilomycin A1, which inhibits the vacuolar proton-ATPase and hence vacuolar acidification (5, 13). Third, comparing the anti-P. marneffei activity of several quinoline derivatives yields interesting results. Indeed, the 4-aminoquinolines used, CQ and AM, share a similar inhibitory effect toward P. marneffei, whereas quinine and primaquine are less active. This may be explained by different pKa values of the corresponding drugs, resulting in a much higher accumulation rate for CQ and AM than for quinine and primaquine within the cells' acidic compartments (11, 22). AM, even if it has slightly lower pKa values than CQ (Fig. 1), accumulates more in vesicles for reasons unrelated to ion trapping (11, 22) which may explain its high anti-P. marneffei activity compared to CQ. The pK1 value of primaquine is 3.2 (15), resulting in a much lower vesicular accumulation, in agreement with its very limited anti-P. marneffei activity. Similarly, the low pKa1 value of quinine may explain its modest effect. Furthermore, we tested compound 9, which differs from CQ only by the absence of the terminal amino side chain and is therefore a much weaker base than CQ (Fig. 1) (reference 10, no precise pKa values reported; T. J. Egan personal communication). Accordingly, compound 9 has lost the anti-P. marneffei activity of its parent, CQ. In contrast, compound 15, in which the amino side chain is maintained, yielded anti-P. marneffei activity. These results provide strong support for the idea that CQ's inhibitory activity toward P. marneffei is related to the high rate of accumulation of the diprotic drug within the P. marneffei-containing phagolysosome and possibly in the acidic vacuole of P. marneffei itself. Very high concentrations of CQ at this specific site may result in a direct anti-P. marneffei activity. Indeed, when studied in a cell-free medium, CQ kills P. marneffei at concentrations 4 to 5 times higher than those needed inside macrophages. Similarly, CQ seems to directly affect the growth of C. neoformans (14).
The increase in pH within the phagocytic vacuole may have the following consequences. First, the increase in intravacuolar pH may directly reduce fungus growth (7). Second, it may inhibit pH-dependent yeast virulence factors. Both P. marneffei mycelia and yeast from different strains have recently been reported to express acid phosphatase activity (30), considered one of the virulence factors for intracellular pathogens such as F. tularensis (24) or Coxiella burnetii (1). Acid phosphatases, whose optimal pH is 5, seem to improve the survival of intracellular microorganisms by inhibiting the respiratory burst of phagocytic cells (24).
What may be the clinical relevance of the observed in vitro inhibitory effect of CQ toward P. marneffei? This fungus is endemic in Southeast Asia, where HIV type 1 (HIV-1) and malaria are also highly prevalent. In view of the fact that P. marneffei results in clinical infection when cellular immunity is devastated to a great extent, it is not astonishing that P. marneffei is the third most common AIDS-related opportunistic infection in Thailand (8, 27). It is unlikely that CQ will be of use in the treatment of established P. marneffei infections, as itraconazole, with a MIC of 0.009 μg/ml, is clearly effective (26, 27). However, CQ may possibly be of value in the primary prophylaxis of P. marneffei infection in HIV-1-infected individuals living in countries where it is endemic. Its low cost, established anti-HIV-1 effects (3, 25), and large spectrum of activity, which encompasses several other AIDS-opportunistic pathogens (2), are important characteristics of the drug in this setting.
ACKNOWLEDGMENTS
We thank T. J. Egan and C. H. Kaschula, from the Department of Chemistry, University of Cape Town, Cape Town, South Africa, and D. Monti and E. Pasini, from the Department of Organic and Industrial Chemistry, University of Milan, Milan, Italy, for synthesizing compounds 9 and 15 and for helpful discussion throughout this work.
This work was supported by the I.S.S., National Program for Research on AIDS, contract no. 50B.037 and 50C.030, Rome, Italy.
REFERENCES
- 1.Baca O G, Roman M J, Glew R H, Christner R F, Buhler J E, Aragon A S. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun. 1993;61:4232–4239. doi: 10.1128/iai.61.10.4232-4239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boelaert J R, Appelberg R, Gomes M S, Blasi E, Mazzolla R, Grosset J, Lounis N, Soteriadou K, Thiakaki M, Taramelli D, Tognazioli C. Experimental results on chloroquine and AIDS-opportunists. J Acquir Immune Defic Syndr. 2001;26:300–301. doi: 10.1097/00042560-200103010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Boelaert J R, Sperber K, Piette J. Chloroquine exerts an additive in vitro effect when associated with didanosine and hydroxyurea. AIDS Res Hum Retrovir. 1999;15:1241–1247. doi: 10.1089/088922299310133. [DOI] [PubMed] [Google Scholar]
- 4.Bray P, Janneth O, Raynes K, Mungthin M, Ginsburg H, Ward S. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J Cell Biol. 1999;145:363–376. doi: 10.1083/jcb.145.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchmüller-Rouiller Y, Corradin S B, Smith J, Mauël J. Effect of increasing intravesicular pH on nitrite production and leishmanicidal activity of activated macrophages. Biochem J. 1994;301:243–247. doi: 10.1042/bj3010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd T F, Horwitz M A. Chloroquine inhibits the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. A potential new mechanism for the therapeutic effect of chloroquine against intracellular pathogens. J Clin Investig. 1991;88:351–357. doi: 10.1172/JCI115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cogliati M, Roverselli A, Boelaert J R, Taramelli D, Lombardi L, Viviani M A. Development of an in vitro macrophage system to assess Penicillium marneffei growth and susceptibility to nitric oxide. Infect Immun. 1997;65:279–284. doi: 10.1128/iai.65.1.279-284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper C R., Jr From bamboo rats to humans: the odyssey of Penicillium marneffei. ASM News. 1998;64:390–397. [Google Scholar]
- 9.De Duve C, De Barsy T, Poole T, Trouet A, Tulkens P, Van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 10.Egan T J, Hunter R, Kaschula C H, Marques H M, Misplon A, Walden J. Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, and antiplasmodial activity. J Med Chem. 1999;43:283–291. doi: 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- 11.Foley M, Tilley L. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther. 1998;79:55–87. doi: 10.1016/s0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 12.Fortier A H, Leiby D A, Naranayan R B, Asafoadjei E, Crawford R M, Nacy C A, Meltzer M S. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect Immun. 1995;63:1478–1483. doi: 10.1128/iai.63.4.1478-1483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagliardi S, Rees M, Farina C. Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase. Curr Med Chem. 1999;6:1197–1212. [PubMed] [Google Scholar]
- 14.Harrison T S, Griffin G E, Levitz S M. Conditional lethality of the diprotic weak bases chloroquine and quinacrine against Cryptococcus neoformans. J Infect Dis. 2000;182:283–289. doi: 10.1086/315649. [DOI] [PubMed] [Google Scholar]
- 15.Hufford C D, McChesney J D. Assignments of dissociation constants of primaquine by 13C-NMR spectroscopy. J Heterocycl Chem. 1983;20:273–275. [Google Scholar]
- 16.Krogstad G J, Schlesinger P H. Acid-vesicle function, intracellular pathogens, and the action of chloroquine against Plasmodium falciparum. N Engl J Med. 1987;317:542–549. doi: 10.1056/NEJM198708273170905. [DOI] [PubMed] [Google Scholar]
- 17.Levitz S M, Diamond R D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985;152:938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- 18.Levitz S M, Harrison T S, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Investig. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzolla R, Barluzzi R, Brozzetti A, Boelaert J R, Luna T, Saleppico S, Bistoni F, Blasi E. Enhanced resistance to Cryptococcus neoformans infection induced by chloroquine in a murine model of meningoencephalitis. Antimicrob Agents Chemother. 1997;41:802–807. doi: 10.1128/aac.41.4.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mungthin M, Bray P G, Ridley R G, Ward S A. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob Agents Chemother. 1998;42:2973–2977. doi: 10.1128/aac.42.11.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman S L, Gootee L, Brunner G, Deepe G S. Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J Clin Investig. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Neill P M, Bray P G, Hawley S R, Ward S A, Park B K. 4-Aminoquinolines—past, present, and future: a chemical perspective. Pharmacol Ther. 1998;77:29–58. doi: 10.1016/s0163-7258(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 23.Pearse A G E. Histochemistry. 2nd ed. Boston, Mass: Little, Brown; 1961. pp. 998–1002. [Google Scholar]
- 24.Reilly T J, Baron G S, Nano F E, Kuhlenschmidt M S. Characterization and sequencing of a respiratory burst-inhibiting acid phosphatase from Francisella tularensis. J Biol Chem. 1995;271:10973–10983. doi: 10.1074/jbc.271.18.10973. [DOI] [PubMed] [Google Scholar]
- 25.Sperber K, Kalb T H, Stecher V J, Banerjee R, Mayer L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res Hum Retrovir. 1993;9:91–98. doi: 10.1089/aid.1993.9.91. [DOI] [PubMed] [Google Scholar]
- 26.Supparatpinyo K, Nelson K E, Merz W G, Breslin B J, Cooper C R, Jr, Kamwan C, Sirisanthana T. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37:2407–2411. doi: 10.1128/aac.37.11.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supparatpinyo K, Khamwan C, Baosoung V, Nelson K E, Sirisanthana T. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344:110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 28.Taramelli D, Brambilla S, Sala G, Bruccoleri A, Tognazioli C, Riviera-Uzielli L, Boelaert J R. Effects of iron on extracellular and intracellular growth of Penicillium marneffei. Infect Immun. 2000;68:1724–1726. doi: 10.1128/iai.68.3.1724-1726.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twentyman P M, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:2279–2285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youngchim S, Vanittanakom N, Hamilton A J. Analysis of the enzymatic activity of mycelial and yeast phases of Penicillium marneffei. Med Mycol. 1999;37:445–450. doi: 10.1046/j.1365-280x.1999.00235.x. [DOI] [PubMed] [Google Scholar]