Abstract
A literature survey revealed no suitable “reversed phase-high performance thin layer chromatography (RP-HPTLC)” method for the analysis of rivaroxaban in nanoparticle (NP) formulations. Therefore, a novel rapid, simple, economical and environment friendly RP-HPTLC method has been established for the quantification of rivaroxaban in NP formulations and commercial tablets. RP-HPTLC analysis of rivaroxaban was performed using “RP-18 silica gel 60 F254S HPTLC plates”. The binary mixture of green solvents ethanol : water (7 : 3 v/v) was utilized as the mobile phase. The quantification of rivaroxaban was carried out in densitometric mode at λmax = 253 nm. Rivaroxaban peaks from NP formulations was confirmed by comparing its single spot at Rf = 0.71 ± 0.02 with that of the standard. The proposed RP-HPTLC method was found to be linear in the range of 50–600 ng per band with R2 = 0.9994. The equation for linear regression analysis was obtained as Y = 13.28x + 1189.4. The proposed RP-HPTLC technique was validated for “precision, accuracy, robustness and sensitivity”. The accuracy of the method was obtained as 97.97–99.67%. The % RSD in repeatability and intermediate precision was recorded as 0.46–0.64 and 0.48–0.86%, respectively. The LOD and LOQ for rivaroxaban were obtained as 18.45 and 55.35 ng per spot, respectively. The % content of rivaroxaban in marketed tablets and NPs was recorded as 99.20 and 98.08%, respectively. The proposed RP-HPTLC technique could be successfully applied for the pharmaceutical assay of rivaroxaban in NPs, marketed tablets and related formulations.
A literature survey revealed no suitable “reversed phase-high performance thin layer chromatography (RP-HPTLC)” method for the analysis of rivaroxaban in nanoparticle (NP) formulations.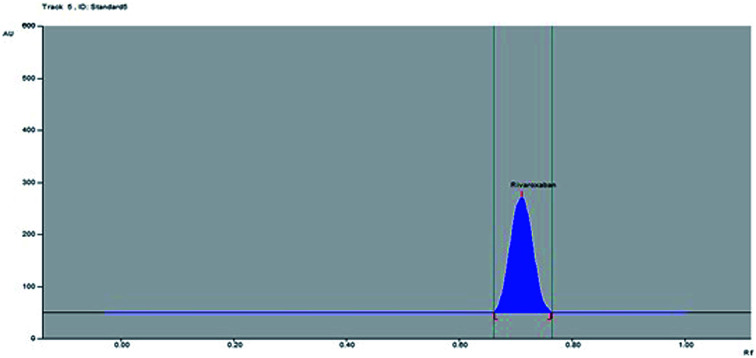
Introduction
Rivaroxaban, an oral anticoagulant, acts at the crucial points to stop the formation of blood clots.1 It has been reported as a potent, selective and direct inhibitor of factor Xa which prevents thromboembolism during surgical operations.1,2 It has been proposed as practically insoluble in water and freely soluble in organic solvents.3 It is rapidly absorbed from the gut and produces maximum inhibition of factor Xa within 4 h of oral administration.4,5 It does not exert direct effects on platelets.5 In the literature, many “high-performance liquid chromatography (HPLC)” methods are available for the quantification of rivaroxaban in its dosage forms especially tablet dosage forms.3,6–19 Some HPLC methods are also available for the analysis of rivaroxaban in human or rat plasma.6,20 Some “high-performance thin layer chromatography (HPTLC)”3,11,21 and spectrophotometric methods11,22,23 have also been proposed for the analysis of rivaroxaban in its tablet formulations. A “liquid chromatography-mass spectrometry (LC-MS)” method has also been proposed for pharmacokinetic assessment of rivaroxaban in humans.20 A TLC-densitometry, HPLC and LC-MS method had also been applied to study the degradation products of rivaroxaban.3 Square-wave voltammetry method has also been used for the electrochemical determination of rivaroxaban in pharmaceutical dosage forms.24 Most of the routine analytical methods mentioned above for the analysis of rivaroxaban used toxic solvents as the mobile phase. Because not a single method using green solvents as the mobile has been reported for the analysis of rivaroxaban in literature, we have considered our method as the eco-friendly one. Recently, there is an issue in the sustainability of the pharmaceutical industries due to the huge use of toxic solvents in pharmaceutical and biomedical analysis. Most of the chemicals/solvents or their combinations used for the analysis of drugs and pharmaceutics are highly toxic to the environment. Moreover, a little attention had been paid towards the environmental effects of analytical methods.25 In recent years, the research reports available in the literature related to “clean analytical chemistry or environmentally-benign analytical methods” have been increased substantially.26–28
Despite various favorable characteristics like “non-toxic, non-volatility, non-inflammable, non-aggressiveness, high biodegradability and cost effectiveness”, the complete potential of environmentally safe solvents either alone or in combinations as an eluent for HPTLC analysis of drugs and pharmaceuticals had not been fully utilized.29–31
Green HPTLC technique offers several advantages over routine HPTLC in the analysis of drugs or pharmaceutical compounds.32–34 Interestingly, no eco-friendly analytical methodology had been applied yet for the analysis of rivaroxaban in pharmaceutical formulations or biological fluids. Moreover, no reports on the determination of rivaroxaban biomarker in nanoparticles (NPs) formulation utilizing the RP-HPLC technique are available in the literature so far. In the view of these facts, the objective of the present work was to develop and validate an ecofriendly, simple, precise, accurate, robust and sensitive RP-HPTLC technique for the analysis of rivaroxaban in NPs formulation utilizing RP18 silica gel plates. The proposed green analytical methodology was also validated as per “International Conference on Harmonization (ICH)” guidelines.35
Experimental section
Materials
Rivaroxaban and chloroform were obtained from “Sigma Aldrich (St. Louis, MO, USA)”. Chromatography grade ethanol was procured from “E-Merck (Darmstadt, Germany)”. Chromatography grade water was obtained from “Milli-Q unit”. Marketed tablets of rivaroxaban were purchased from local market in “Riyadh, Saudi Arabia”. NPs formulation of rivaroxaban was prepared and characterized in Pharmaceutics Laboratory in Prince Sattam bin Abdulaziz University. All the solvents or reagents used were of analytical/pharmaceutical grades.
Calibration curve of rivaroxaban
Standard stock solution of rivaroxaban was prepared by dissolving 10 mg of drug in 10 mL of chloroform. About 1 mL of this solution was taken and diluted with mobile phase to obtain the final concentration of 100 μg mL−1 (stock solution of rivaroxaban). Various volumes of stock solution i.e., 0.5, 1, 2, 3, 4, 5 and 6 mL (50, 100, 200, 300, 400, 500, and 600 ng, respectively) were applied on TLC. The peak area of rivaroxaban at each concentration was recorded and calibration curve was plotted in the range of 50–600 ng per spot.
Sample preparation for the estimation of rivaroxaban in marketed tablets
Ten marketed tablets (each containing 10 mg of rivaroxaban) were weighed and the average weight was calculated. The tablets were then crushed using pestle and mortar and finely powdered. A weight of the powder equivalent to 10 mg of rivaroxaban was dispersed in chloroform and diluted with mobile phase to get 100 mL stock solution. The obtained solution was filtered to remove undissolved materials and sonicated for 10 min. About 1.0 mL of this stock was taken and diluted suitably with mobile phase. The diluted solution was analyzed for the content of rivaroxaban in marketed tablets by the proposed RP-HPTLC method.
Preparation of rivaroxaban loaded PLGA NPs
“Emulsion solvent evaporation method” was used for the preparation rivaroxaban loaded PLGA NPs.36 Briefly, an accurately weighed amount of rivaroxaban (20 mg) was dissolved in 4 mL of PLGA polymeric solution in dichloromethane (DCM). Separately, aqueous phases were prepared by dissolving the required amount of polyvinyl alcohol (PVA) (500 mg) in distilled water (8 mL). Furthermore, the prepared organic phase was emulsified with the aqueous phase dropwise under probe sonication for 3 min at 60% voltage efficiency using “Probe Sonicator (Fisher Scientific, USA)” with 10 s on–off pulse. The dispersion was subsequently placed over a “Magnetic Stirrer (Labtec, Korea)” for 24 h at 700 rpm to evaporate the organic phase. After complete evaporation, the dispersion was centrifuged “(Hermle Labort Echnik, Germany)” at 14 000 × g for 15 min. To remove the adsorbed drug as well as the excessive PVA, the sediment was washed thrice with Milli-Q water; both the supernatant and filtrate were preserved for drug content estimations. Prepared NPs were characterized well for physicochemical properties. The concentrated NPs were lyophilized and preserved for further analysis.
Sample preparation for analysis of rivaroxaban in PLGA NPs
Accurately weighed 1.0 g of NPs (containing 10 mg of rivaroxaban) was dispersed in chloroform and diluted with mobile phase to get 100 mL stock solution. The obtained solution was filtered to remove undissolved materials and sonicated for 10 min. About 1.0 mL of this stock was taken and diluted suitably with mobile phase. The diluted solution was analyzed for the content of rivaroxaban in NPs by the proposed RP-HPTLC method.
Chromatographic conditions
Densitometric RP-HPTLC quantification of rivaroxaban was carried out on 10 × 20 cm glass-backed plates coated with 0.2 mm layers of “RP-18 silica gel 60 F254S (E-Merck, Germany)”. Various concentrations of rivaroxaban were applied on the TLC plates as 6 mm bands with the help of a “CAMAG Automatic TLC Sampler 4 (ATS4) sample applicator (Geneva, Switzerland)” fitted with a “CAMAG microliter syringe”. The sample application rate was kept constant at 150 nL s−1. The TLC plates were developed at linear ascending mode at a distance of 80 mm with ethanol : water 7 : 3 (%, v/v) as a mobile phase in a “CAMAG Automatic Developing Chamber 2 (ADC2)”. The chamber was previously saturated with mobile phase vapor for 30 min at 22 °C.
Validation of RP-HPTLC method
RP-HPTLC densitometric method was validated for “linearity, precision, accuracy, robustness, sensitivity and specificity” using the guidelines of ICH.35 The linearity of RP-HPTLC method for rivaroxaban was studied by plotting the concentration against measured peak area of rivaroxaban. The linearity was evaluated in the range of 50–600 ng per band.
The accuracy (as percent of recovery) was estimated using standard addition technique. In this technique, previously analyzed sample of rivaroxaban (200 ng per spot) was spiked with extra 0, 50, 100 and 150% contents of rivaroxaban. These mixtures were analyzed again by the proposed RP-HPTLC technique. The % recovery for rivaroxaban at each concentration level was estimated.
Precision of the proposed RP-HPTLC technique was obtained in terms of “repeatability and intermediate precision”. Repeatability (intra-day precision) was obtained by the analysis of samples on the same day at three different concentrations i.e., 200, 300 and 400 ng per spot in 6 replicates. Intermediate precision (inter-day precision) was obtained by the analysis of samples on three different days at 200, 300 and 400 ng per spot in 6 replicates.
Robustness of the proposed RP-HPTLC method was obtained by changing in the mobile phase composition during the estimation of rivaroxaban.
Detection (LOD) and quantification (LOQ) limits of the RP-HPTLC technique were obtained using standard deviation (SD) method. LOD and LOQ were calculated by applying eqn (1) and (2):
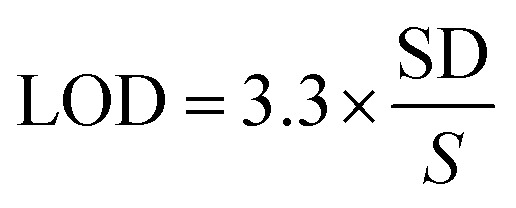 |
1 |
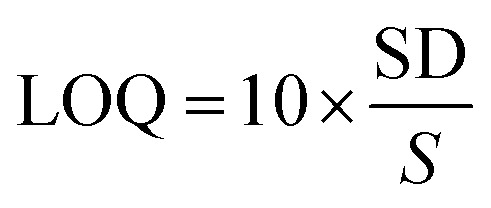 |
2 |
In which, S is the slope of the calibration curve.
The specificity of the proposed RP-HPTLC technique was evaluated by comparing the Rf values and ultra-violet (UV) spectra of the rivaroxaban in the samples with that of the standard.
Estimation of rivaroxaban in marketed tablets and NPs formulation
The samples of marketed tablets and NPs were applied and chromatograms were recorded under the same conditions as for analysis of standard rivaroxaban. The peak area of rivaroxaban in commercial tablets and prepared NPs was recorded. The amount of rivaroxaban present in both formulations was obtained from the calibration curve of standard rivaroxaban.
Results and discussion
Preparation and characterization of rivaroxaban loaded PLGA NPs
Rivaroxaban loaded PLGA NPs formulation was prepared by solvent evaporation technique.36 The formulation composition and characterization parameters of prepared NPs are summarized in Table 1. The purpose of preparation of PLGA NPs was to apply the proposed analytical methodology for the determination of rivaroxaban in NPs formulation. Only tablets of rivaroxaban are available in the market. Therefore, NPs formulation was prepared additionally in order to increase the application of proposed analytical methodology. DCM was used as the solvent for the preparation of rivaroxaban PLGA NPs because both polymer (PLGA) as well as rivaroxaban are soluble in DCM. PLGA NPs were prepared by solvent evaporation method and hence the used of organic solvent in which both drug as well as polymer are soluble is recommended.36 Moreover, the solvent DCM was evaporated in order to get solid PLGA NPs. Hence in final formulation, DCM was not present. In this way, prepared PLGA NPs are safe and non-toxic to human beings. The particle size and polydispersity index of PLGA NPs were obtained as 496 nm and 0.47, respectively. The zeta potential and entrapment efficiency of formulation were recorded as −18.41 mV and 87.91%, respectively. The physicochemical parameters recorded in this study suggested the formation of polymeric NPs of rivaroxaban.
Formulation composition and physicochemical characterization of PLGA NPs of rivaroxaban (n = 3).
| Formulation composition | Characterization parameter | ||
|---|---|---|---|
| Rivaroxaban (mg) | 20 | Particle size ± SD (nm) | 496.00 ± 8.50 |
| PLGA (mg) | 125 | Polydispersity index | 0.47 |
| PVA (mg) | 500 | Zeta potential ± SD (mV) | −18.41 ± 3.14 |
| Entrapment efficiency ± SD (%) | 87.91 ± 8.60 | ||
Method development
According to literature survey, there is no report on the determination of rivaroxaban in NPs using RP-HPTLC method. Therefore, attempts were made to develop and validate a cost effective, economical and environment friendly method for the determination of rivaroxaban. In RP-HPTLC technique, the mobile phase was prepared by the simple mixture of water and ethanol (green solvent) as compared to normal phase HPTLC technique. The adaptation of reverse phase methodology over normal phase helped in avoiding the non-polar fractions from the sample in the TLC, which gives a very clear elution pattern. Moreover, it helped in avoiding the interference due to impurities in the chromatograms, formation of compact spot and detection clarity.37 Eco-friendly RP-HPTLC method for the analysis of rivaroxaban will reduce the environmental toxicity caused by routine toxic solvents used in routine pharmaceutical analysis.33,34 Our research work gave a very good analytical technique for the estimation of the rivaroxaban in NPs formulation.
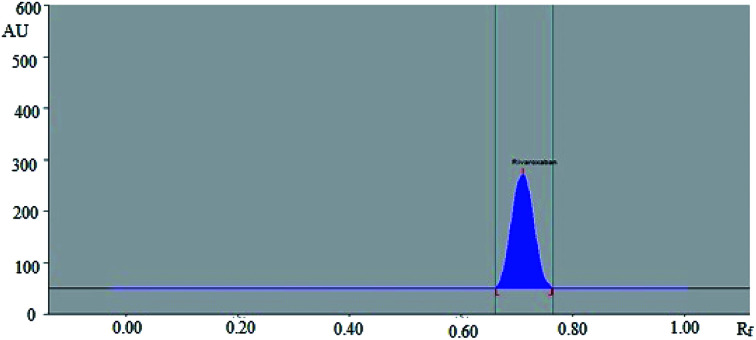
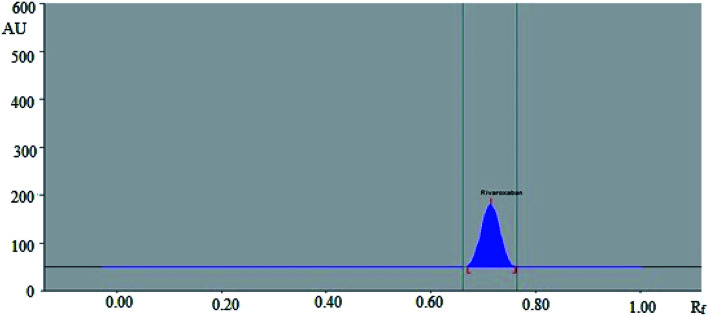
In this study, the composition of mobile phase was optimized to develop a suitable band for densitometric RP-HPTLC technique. The chromatogram was obtained for standard rivaroxaban under chamber saturation conditions using ecofriendly mobile phase ethanol : water 7 : 3 (%, v/v) as the green solvent system (Fig. 1). The densitometric estimation of rivaroxaban was carried out at 253 nm in the reflectance/absorbance mode. The mobile phase composed of ethanol : water 7 : 3 (%, v/v) presented a compact, symmetrical and well resolved peak at Rf value of 0.71 ± 0.02 (Fig. 2). The spectra of the bands were measured and maximum HPTLC response under reflectance/absorbance mode was obtained at the wavelength of 253 nm.
Fig. 1. Picture of developed TLC plate.
Fig. 2. HPTLC densitogram of standard rivaroxaban.
Method validation
The data for linear regression analysis of calibration curve of rivaroxaban is tabulated in Table 2. The calibration curve of rivaroxaban was found to linear in the range 50–600 ng per spot. Linear regression analysis showed good linear relationship (Table 2). The coefficient of determination (R2) was recorded as 0.9994 and found to be significant (P < 0.05). The equation for regression line was obtained as Y = 13.28x + 1189.4, in which Y represents the peak area and x is the concentration of rivaroxaban. The values of slope and intercept were obtained as 13.28 ± 0.75 and 1189.40 ± 52.21, respectively. The values for 95% confidence interval of slope and intercept were recorded as 11.98–14.57 and 1097.38–1281.41, respectively. The values of slope and intercept depend on x-axis and y-axis values, respectively. The values in x-axis are the concentrations of rivaroxaban which were ranged as 50–600 ng per spot. However, the values in y-axis are the measured HPTLC area of rivaroxaban which were obtained in the range of 1791–9025. It is obvious that the values of HPTLC response are much higher than concentration and hence intercept value is much higher than slope of the calibration curve.
Linear regression data for the calibration curve of rivaroxaban (n = 6).
| Linearity range (ng per spot) | 50–600 |
| Regression equation | Y = 13.28x + 1189.4 |
| R 2 | 0.9994 |
| Slope ± SD | 13.28 ± 0.75 |
| Intercept ± SD | 1189.40 ± 52.21 |
| Standard error of slope | 0.30 |
| Standard error of intercept | 21.39 |
| 95% confidence interval of slope | 11.98–14.57 |
| 95% confidence interval of intercept | 1097.38–1281.41 |
| LOD ± SD (ng per spot) | 18.45 ± 1.87 |
| LOQ ± SD (ng per spot) | 55.35 ± 5.61 |
Accuracy of the proposed RP-HPTLC method is expressed as the % recovery. The % recovery of the rivaroxaban for the proposed analytical methodology was recorded as 97.97–99.67% (Table 3). The % RSD for the recovery studies was obtained as 0.55–0.93%. The higher values of % recovery and lower % RSD values suggested the accuracy of the proposed analytical methodology.
Accuracy of the proposed method (n = 6).
| Excess drug added to analyte (%) | Theoretical content (ng) | Conc. found (ng) ± SD | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 0 | 200 | 199.33 ± 1.51 | 99.67 | 0.76 |
| 50 | 300 | 294.50 ± 2.74 | 98.17 | 0.93 |
| 100 | 400 | 395.00 ± 2.19 | 98.75 | 0.55 |
| 150 | 500 | 489.83 ± 4.40 | 97.97 | 0.90 |
Precision of the proposed RP-HPTLC method is expressed as % RSD of the measured concentrations for rivaroxaban. The precision was determined as repeatability and intermediate precision and results are tabulated in Table 4. The % RSD for repeatability was obtained as 0.46–0.64%. However, the % RSD for intermediate precision was recorded as 0.48–0.86%. The values of standard error for repeatability and intermediate precision were obtained as 7.63–13.72 and 9.33–18.72, respectively. The lower RSD values indicated the precision of the proposed analytical methodology.
Precision of the proposed method (n = 6).
| Conc. (ng per spot) | Repeatability (intraday precision) | Intermediate precision (interday) | ||||
|---|---|---|---|---|---|---|
| Area ± SD (n = 6) | Standard error | % RSD | Area ± SD (n = 6) | Standard error | % RSD | |
| 200 | 3891.20 ± 18.63 | 7.63 | 0.48 | 3895.20 ± 22.78 | 9.33 | 0.58 |
| 300 | 5273.60 ± 33.49 | 13.72 | 0.64 | 5291.60 ± 45.69 | 18.72 | 0.86 |
| 400 | 6546.40 ± 29.80 | 12.21 | 0.46 | 6544.40 ± 31.51 | 12.91 | 0.48 |
Results of robustness for the proposed RP-HPTLC method are tabulated in Table 5. The values of % RSD after introducing small changes in mobile phase composition were obtained as 0.66–0.82%. The Rf value for rivaroxaban after this small deliberate change was obtained in the range of 0.68–0.72. These observations suggested that small deliberate changes into the densitometric TLC procedure proved the robustness of the proposed HPTLC technique. The sensitivity of the HPTLC technique was estimated in terms of LOD and LOQ. The values of LOD and LOD are tabulated in Table 2. The LOD and LOQ of the RP-HPTLC technique were recorded as 18.45 and 55.35 ng per spot, respectively for rivaroxaban. This observation suggested that the proposed RP-HPTLC technique had good sensitivity which could be applied in wide range for detection and quantification of rivaroxaban effectively.
Robustness of the proposed HPTLC method (n = 6).
| Conc. (ng per spot) | Mobile phase composition (ethanol : water) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | Area ± SD (n = 6) | % RSD | R f | ||
| 7.2 : 2.8 | +0.2 | 3900.83 ± 25.59 | 0.66 | 0.72 | ||
| 300 | 7 : 3 | 7 : 3 | 0.0 | 3902.50 ± 28.71 | 0.74 | 0.71 |
| 6.8 : 3.2 | −0.2 | 3904.17 ± 32.05 | 0.82 | 0.68 | ||
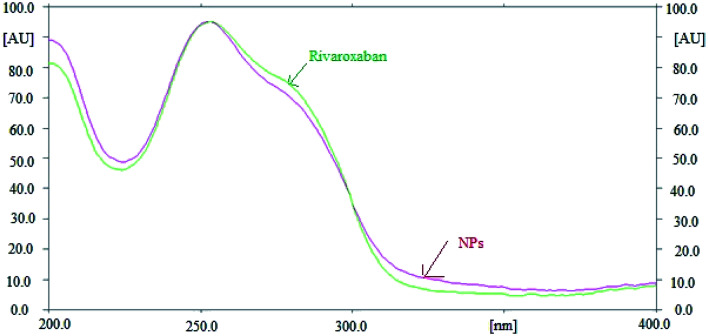
The specificity and peak purity of rivaroxaban was evaluated by comparing the overlaid spectra of rivaroxaban in sample and standard. The overlaid spectra of rivaroxaban in standard and prepared NPs formulation are presented in Fig. 3. The maximum HPTLC densitometric response of rivaroxaban in standard and NPs sample were obtained at the wavelength of 253 nm under reflectance/absorbance mode. The x-axis in Fig. 3 represents the wavelength (nm) and y-axis represents the HPTLC densitometric area instead of UV absorbance. In simple UV spectra, y-axis value is absorbance and x-axis value is the concentration. However, in case of HPTLC-densitometry analysis, the y-axis value represents the HPTLC area against the wavelength in x-axis. This HPTLC area is expressed in percent when we record UV absorption spectra using HPTLC-densitometry. Overall, the results presented in Fig. 3 suggested the specificity of the proposed RP-HPTLC technique.
Fig. 3. Overlay UV absorption spectra of standard rivaroxaban and their NPs formulation.
Quantification of rivaroxaban in marketed tablets and NPs formulation
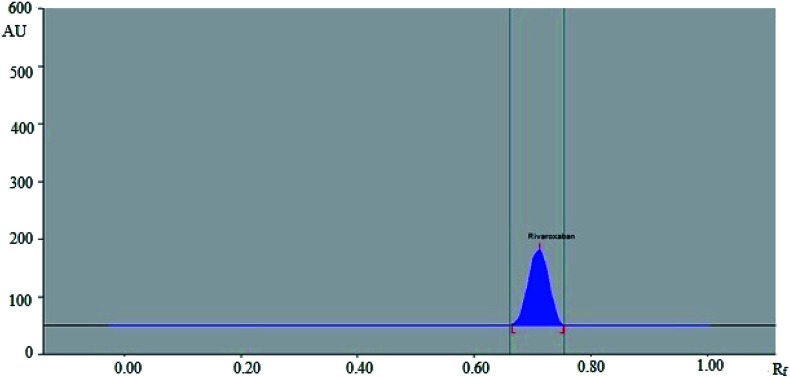
No green analytical methodology has been reported for the estimation of rivaroxaban in commercial formulations. However, green analytical technologies have already been applied for the estimation of drugs and pharmaceuticals.38,39 Low dimensional un-doped iron oxide NPs prepared by a solution method were applied as a green analytical technique in the analysis of antiemetic drug aprepitant.38 Hydrothermally prepared inorganic NPs were used as a green analytical technique for the electrochemical detection of olmesartan medoxomil in tablet dosage forms.39 Both methods were found sensitive for the determination of aprepitant and olmesartan medoxomil. Hence, green analytical methods could be the alternative approach for the determination of drugs and pharmaceuticals in commercial formulation. Rivaroxaban peaks from marketed tablet (Fig. 4) and NPs formulation (Fig. 5) were identified by comparing their single spot at Rf = 0.71 ± 0.02 with that of standard rivaroxaban under the same analytical conditions. The amount of rivaroxaban in marketed tablets and prepared NPs was calculated using the linear regression equation of the calibration curve of rivaroxaban. The results of the quantification of rivaroxaban in marketed tablets and prepared NPs are tabulated in Table 6. The amount of rivaroxaban in marketed tablets was found as 9.92 ± 0.86 mg out of 10 mg of theoretical drug content. However, the amount of rivaroxaban in prepared NPs was recorded as 9.80 ± 1.07 mg out of 10 mg of theoretical drug content. The % assay of rivaroxaban in marketed tablets and prepared NPs was obtained as 99.20 and 98.08%, respectively. These results showed that the proposed RP-HPTLC technique could be successfully used for the pharmaceutical assay of rivaroxaban in pharmaceutical formulations containing rivaroxaban as an active ingredient.
Fig. 4. HPTLC densitogram of standard rivaroxaban in marketed formulation.
Fig. 5. HPTLC densitogram of standard rivaroxaban in prepared NPs.
Quantification of rivaroxaban in marketed tablets and prepared NPs formulations (n = 3).
| Samples | Theoretical content (mg) | Content found (ng) ± SD | Content (%) |
|---|---|---|---|
| Marketed tablets | 10 | 9.92 ± 0.86 | 99.20 |
| NPs | 10 | 9.80 ± 1.07 | 98.08 |
Conclusion
The RP-HPTLC technique developed for the estimation of rivaroxaban was found to be simple, accurate, precise, robust, sensitive and specific. It is applicable in the quantification of rivaroxaban in a wide variety of pharmaceutical products containing rivaroxaban as one of the ingredient. The proposed RP-HPTLC methodology is the first validated technique for the analysis of rivaroxaban in NPs formulation using RP-18 silica gel. The results of this study prove that the method is selective for the estimation of rivaroxaban with added advantages of “short time, environmental friendly, minimal sample preparation in addition to the low cost”. The proposed RP-HPTLC technique could be successfully utilized for the assay of rivaroxaban in pharmaceutical products containing rivaroxaban as one of the ingredient.
Conflicts of interest
The authors report no conflict of interest associated with this manuscript.
Supplementary Material
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project no. RG-1435-072.
References
- Perzborn E. Roehrig S. Straub A. Kubitza D. Mueck W. Laux V. Rivaroxaban: a new oral factor Xa inhibitor. Arterioscler., Thromb., Vasc. Biol. 2010;30:376–381. doi: 10.1161/ATVBAHA.110.202978. [DOI] [PubMed] [Google Scholar]
- Roehrig S. Straub A. Pohlmann J. Lampe T. Pernerstorfer J. Schlemmer K. H. Reinemer P. Perzborn E. Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene-2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor. J. Med. Chem. 2005;48:5900–5908. doi: 10.1021/jm050101d. [DOI] [PubMed] [Google Scholar]
- Abdallah M. A. Al-Ghobashy M. A. Lofty H. M. Investigation of the profile and kinetics of degradation of rivaroxaban using HPLC, TLC densitometry and LC/MS/MS: application to pre-formulation studies. Bull. Fac. Pharm. Cairo Univ. 2015;53:53–61. doi: 10.1016/j.bfopcu.2015.01.002. [DOI] [Google Scholar]
- Eerenberg E. S. Kamphuisen P. W. Sijpkens M. K. Meijers J. C. Buller M. Levi M. Reversal of rivaroxaban and dabigatran by pro-thrombin complex concentrate a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- Turpie A. G. G. New oral anticoagulants in atrial fibrillation. Eur. Heart J. 2008;29:155–165. doi: 10.1093/eurheartj/ehm575. [DOI] [PubMed] [Google Scholar]
- Hadagali M. D. Determination of rivaroxaban in pure, pharmaceutical formulations and human plasma samples by RP-HPLC. Int. J. Adv. Pharm. Anal. 2015;5:65–68. [Google Scholar]
- Celebier M. Recber T. Kocak E. Altnoz S. RP-HPLC method development and validation for estimation of rivaroxaban in pharmaceutical dosage forms. Braz. J. Pharm. Sci. 2013;49:359–366. doi: 10.1590/S1984-82502013000200018. [DOI] [Google Scholar]
- Sahoo S. Mekap S. K. Assay comparison of rivaroxaban by new HPLC method with an existing method in tablet dosage form. Pharm. Biol. Eval. 2017;4:180–182. doi: 10.26510/2394-0859.pbe.2017.27. [DOI] [Google Scholar]
- Girase Y. N. Srinivasrao V. Soni D. Development and validation of stability-indicating RP-HPLC method for rivaroxaban and its impurities. SOJ Biochem. 2018;4:1–6. doi: 10.15226/2376-4589/4/1/00127. [DOI] [Google Scholar]
- Sunny A. Sreedhar C. Rao T. S. Akkama H. G. Mahapatra A. Development of new analytical method for quantitative estimation of rivaroxaban in formulation and bulk drug. International Journal of Scientific Research And Education. 2017;5:6469–6478. [Google Scholar]
- Lories I. B. Mostafa A. A. Girges M. A. High performance liquid chromatography, TLC densitometry, first derivative and first derivative ratio spectrophotometry for determination of rivaroxaban and its alkaline degradates in bulk powder and its tablets. J. Chromatogr. Sep. Tech. 2013;4:E202. [Google Scholar]
- Seshamamba B. S. V. Satyanarayana P. V. V. Sekaran C. B. Application of stability indicating HPLC method with UV detector to the analysis of rivaroxaban in bulk and tablet dosage form. Chem. Sci. Trans. 2014;3:1546–1554. [Google Scholar]
- Kasad P. A. Photolytic-thermal degradation study and method development of rivaroxaban by RP-HPLC. Int. J. PharmTech Res. 2013;5:1254–1263. [Google Scholar]
- Kasad P. A. Muralikrishna K. S. Method development and acid degradation study of rivaroxaban by RP-HPLC in bulk. Asian J. Pharm. Anal. 2013;3:62–65. [Google Scholar]
- Kasad P. A. Muralikrishna K. S. Base degradation study and method development of rivaroxaban by RP-HPLC in bulk. Asian J. Pharm. Res. 2013;3:98–101. [Google Scholar]
- Chandra K. Satya P. Dhana A. Anupama C. Devanaboyina N. A new method for development and validation for analysis of rivaroxaban in formulation by RP-HPLC. Research Desk. 2012;1:24–33. [Google Scholar]
- Jebaliya H. Dabhi B. Patel M. Jadeja Y. Shah A. Stress study and estimation of a potent anticoagulant drug rivaroxaban by a validated HPLC method: technology transfer to UPLC. J. Chem. Pharm. Res. 2015;7:749–765. [Google Scholar]
- Mustafa C. Tuba R. Engin K. Sacide A. HPLC method development and validation for estimation of rivaroxaban in pharmaceutical dosage forms. Braz. J. Pharm. Sci. 2013;49:359–366. doi: 10.1590/S1984-82502013000200018. [DOI] [Google Scholar]
- Shivashankar V. Gandhimathi M. Ravi T. K. Development of validated RP-HPLC method for estimation of rivaroxaban in pharmaceutical formulation. Int. J. Pharm. Anal. Res. 2015;4:406–410. [Google Scholar]
- Rohde G. Determination of rivaroxaban-a novel, oral, direct Factor Xa inhibitor in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr., B. 2008;872:43–50. doi: 10.1016/j.jchromb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Vaghela D. Patel P. High performance thin layer chromatographic method with densitometry analysis for determination of rivaroxaban from its tablet dosage form. Int. J. Pharm. Pharm. Sci. 2014;6:383–386. [Google Scholar]
- Sekaran C. B. Bind V. H. Damayanthi M. R. Sireesha A. Development and validation of UV spectrophotometric method for the determination of rivaroxaban. Der Pharma Chem. 2013;5:1–5. [Google Scholar]
- Celebier M. Kaynak S. N. Altnoz S. Sahin S. UV spectrophotometric method for determination of the dissolution profile of rivaroxaban. Dissolution Technol. 2014;21:56–59. doi: 10.14227/DT210414P56. [DOI] [Google Scholar]
- Suslu I. Celebier M. Altnoz S. Electrochemical behaviour investigation and square-wave voltammetric determination of rivaroxaban in pharmaceutical dosage forms. Anal. Methods. 2014;6:9397–9403. doi: 10.1039/C4AY01871K. [DOI] [Google Scholar]
- Haq N. Shakeel F. Alanazi F. Alshora D. H. Ibrahim M. A. Development and validation of a green RP-HPLC method for the analysis of rosuvastatin: a step towards making liquid chromatography environmentally benign. Green Process. Synth. 2018;7:160–169. [Google Scholar]
- Haq N. Iqbal M. Alanazi F. K. Alsarra I. A. Shakeel F. Applying green analytical chemistry for rapid analysis of drugs: adding health to pharmaceutical industry. Arabian J. Chem. 2017;10:S777–S785. doi: 10.1016/j.arabjc.2012.12.004. [DOI] [Google Scholar]
- Ibrahim F. A. Elmansi H. Fathy M. E. Green RP-HPLC method for simultaneous determination of moxifloxacin combinations: investigation of the greenness for the proposed method. Microchem. J. 2019;148:151–161. doi: 10.1016/j.microc.2019.04.074. [DOI] [Google Scholar]
- Ostovan A. Ghaedi M. Arabi M. Yang Q. Li J. Chen L. Hydrophilic Multitemplate molecularly imprinted biopolymers based on a green synthesis strategy for determination of B family vitamins. ACS Appl. Mater. Interfaces. 2018;10:4140–4150. doi: 10.1021/acsami.7b17500. [DOI] [PubMed] [Google Scholar]
- Haq N. Siddiqui N. A. Alam P. Shakeel F. Alanazi F. K. Alsarra I. A. Estimation of sodium lauryl sulphate concentration in marketed formulations by stability-indicating ‘green’ planar chromatographic method. Chiang Mai J. Sci. 2018;45:1531–1542. [Google Scholar]
- Youssof A. M. E. Salem-Bekhit M. M. Shakeel F. Alanazi F. K. Haq N. Analysis of anti-neoplastic drug in bacterial ghost matrix, w/o/w double nanoemulsion and w/o nanoemulsion by a validated ‘green’ liquid chromatographic method. Talanta. 2016;154:292–298. doi: 10.1016/j.talanta.2016.03.086. [DOI] [PubMed] [Google Scholar]
- Alsuwyeh A. A. Alanazi F. Shakeel F. Salem-Bekhit M. M. Haq N. Estimation of anti-neoplastic drug doxorubicin in bacterial ghost matrix by new “environmentally benign” RP-HPLC method: a step towards sustainable development of pharmaceutical industry. Arabian J. Sci. Eng. 2018;43:181–190. doi: 10.1007/s13369-017-2664-2. [DOI] [Google Scholar]
- Yin D. Guan Y. Gu H. Jia Y. Zhang Q. Polymerized high internal phase emulsion monolithic material: a novel stationary phase of thin layer chromatography. RSC Adv. 2017;7:7303–7309. doi: 10.1039/C6RA27609A. [DOI] [Google Scholar]
- Al-Alamein A. M. A. Abd El-Rahman M. K. Abdel-Moety E. M. Fawaz E. M. Green HPTLC-densitometric approach for simultaneous determination and impurity-profiling of ebastine and phenylephrine hydrochloride. Microchem. J. 2019;147:1097–1102. doi: 10.1016/j.microc.2019.04.043. [DOI] [Google Scholar]
- Rezk M. R. Monir H. H. Marzouk H. M. Novel determination of a new antiviral combination; sofosbuvir and velpatasvir by high performance thin layer chromatographic method; application to real human samples. Microchem. J. 2019;146:828–834. doi: 10.1016/j.microc.2019.02.012. [DOI] [Google Scholar]
- International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human use, Harmonised Triplicate Guideline on Validation of Analytical Procedures: Methodology, Recommended for Adoption at Step 4 of the ICH process on November 1996 by the ICH Steering Committee, IFPMA, Geneva [Google Scholar]
- Anwer M. K. Mohammad M. Ezzeldin E. Fatima F. Alalaiwe A. Iqbal M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: in vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019;14:1587–1595. doi: 10.2147/IJN.S195048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. Al-Rehaily A. J. Alam P. Alqahtani A. S. Hidayatullah S. Rehman M. T. Mothana R. A. Abbas S. S. Khan M. U. Khalid J. M. Siddiqui N. A. Antidiabetic, antioxidant, molecular docking and HPTLC analysis of miquelianin isolated from Euphorbia schimperi C. Presl. Saudi Pharm. J. 2019;27:655–663. doi: 10.1016/j.jsps.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M. M. Khan S. B. Faisal M. Asiri A. M. Tariq M. A. Detection of aprepitant drug based on low-dimensional un-doped iron oxide nanoparticles prepared by a solution method. Electrochim. Acta. 2012;75:164–170. doi: 10.1016/j.electacta.2012.04.093. [DOI] [Google Scholar]
- Rahman M. M. Khan S. B. Faisal M. Rub M. A. Al-Youbi A. O. Asiri A. M. Electrochemical detection of olmesartan medoxomil using hydrothermally prepared nanoparticles composed SnO2–CO3O4 nanocubes in tablet dosage forms. Talanta. 2012;99:924–931. doi: 10.1016/j.talanta.2012.07.060. [DOI] [PubMed] [Google Scholar]