Abstract
We have attempted to validate in Aspergillus flavus the main in vitro methodologies that have been used to detect resistance in Aspergillus fumigatus. We developed a murine model with two A. flavus isolates, one that was apparently resistant in vitro to amphotericin B (AFL5) and another that was resistant to itraconazole (AFL8). No correlation was found for amphotericin B in AFL5, since the in vivo response was compatible with a susceptible isolate. Modification of the in vitro susceptibility test methodology for amphotericin B was unsuccessful. Although AFL8 was apparently resistant to itraconazole in vitro, it was found to be susceptible in vivo. Additional in vitro work has detected weaknesses in the in vitro susceptibility methodology validated for A. fumigatus when applied to A. flavus. The principal problems are that changes in the inoculum have a large effect on the MICs of itraconazole for some A. flavus strains and that a trailing end point and spore sediment often appear when an inoculum with a higher colony count is used. We propose a modified method using a final inoculum of 2.5 × 104 CFU per ml of RPMI 1640 medium with 2% glucose buffered to pH 7.0 in a microtiter format, incubated for 48 h with no growth end point. Validation of this methodology requires one or more itraconazole-resistant A. flavus isolates, which have yet to be identified.
Aspergillus spp. are among the most ubiquitous of the airborne saprophytic fungi. Aspergillus has low pathogenicity for humans and rarely invades an immunologically competent host (26). During the past two decades, however, the incidence of invasive aspergillosis has risen inexorably (14). This increase is almost certainly the consequence of more widespread use of aggressive cancer chemotherapy regimens, expansion of organ transplant programs, and the advent of the AIDS epidemic (2, 12). Invasive aspergillosis is now the most common invasive mold infection worldwide (3). Aspergillus flavus is one of the most common agents of Aspergillus infections (3) and is of economic importance in the food industry due to its production of aflatoxins (15).
Itraconazole (ITZ) and amphotericin B (AMB) are the only two agents licensed for the treatment of Aspergillus infections. Clinical failure is frequent with AMB therapy in patients with invasive aspergillosis. In vivo resistance to AMB has been reported for Aspergillus fumigatus (29), A. flavus (22), and Aspergillus terreus (11; P. Warn, personal communication). MICs to AMB are in a narrow range with most testing systems, typically ranging from 0.5 to 4 μg/ml. A reliable in vitro method to separate genuinely resistant from AMB-susceptible isolates in Aspergillus unfortunately has not been identified despite many attempts (11). The frequency of such AMB “resistance” is unclear. The mechanism(s) of resistance is also unclear. In the last decade, azole resistance has been increasingly observed in other fungi such as Candida spp. (4, 28). Resistance to ITZ (confirmed in an animal model) has only recently been described in A. fumigatus (6).
A. flavus was therefore chosen to study ITZ and AMB resistance. At the same time, we attempted to validate in vitro susceptibility testing for both drugs using animal models. Until now, this validation has been achieved in only one species of the genus, A. fumigatus, and only for ITZ and SCH 56592 (7, 21). A. flavus drug susceptibility to some antifungal agents has been studied in animal models (1, 8, 16, 23, 25, 27), but only one study has examined susceptibility to ITZ and/or AMB (22). That model was part of a larger study testing other filamentous fungi, and only one A. flavus strain was tested. The isolate was susceptible to both AMB and ITZ in vitro. Therefore, validation of an in vitro susceptibility testing method was not possible.
The two A. flavus strains we studied in vivo were selected based on in vitro susceptibility data available from Hope Hospital, Manchester, United Kingdom, using a methodology that was validated for A. fumigatus and ITZ in the same animal model system (7, 20). The AFL5 strain was apparently resistant to AMB (MIC, 32 μg/ml), and susceptible to ITZ (MIC, 1 μg/ml), whereas the AFL8 strain was resistant to ITZ (MIC, 32 μg/ml), and susceptible to AMB (MIC, 2 μg/ml) (19). A total of eight A. flavus strains were tested with this method, and the MIC50 for ITZ was 0.25 μg/ml and for AMB was 0.5 μg/ml. Thus, the in vivo outcome in our treatment model would be capable of definitively validating or invalidating this in vitro methodology.
MATERIALS AND METHODS
Strains.
Sixteen selected clinical isolates of A. flavus were studied in vitro. Two of the isolates, AFL5 and AFL8, were studied in vivo as well. Isolates of A. flavus were obtained from many locations including the United Kingdom, the United States, Oslo, Norway (OSL-60A), Madrid, Spain (CM-900), and Nijmejen, the Netherlands (NIJ-766), and one was an American Type Culture Collection (ATCC) isolate. Isolate AFL5 was obtained from the urine of a drug addict with renal aspergillosis in San Francisco General Hospital, San Francisco, Calif., in 1988. For this isolate, the MIC for ITZ was 1 μg/ml and for AMB was 32 μg/ml (19). Isolate AFL8 was obtained from a sinus aspirate of a leukemic patient with invasive sinus aspergillosis, also in 1988. For this isolate, the MIC for ITZ was 32 μg/ml and for AMB was 2 μg/ml (19). The isolates were maintained in slopes of nutrient broth containing 10% glycerol in liquid nitrogen for subculturing.
In vivo studies. (i) Animals.
Male CD-1 strain mice, 4 to 5 weeks old and weighing between 18 and 20 g each, were purchased from Charles River United Kingdom, Ltd. (Margate, Kent, United Kingdom). The mice were virus-free and were allowed free access to food and water. Mice were randomized into groups of 10 animals. Each cage was inspected twice daily, and any infected animals unable to reach the drinker were culled.
(ii) Immunosuppression.
Cyclophosphamide (Sigma-Aldrich Poole, Dorset, United Kingdom) was administered intravenously via the lateral tail vein to all animals at a dose of 200 mg/kg. A state of profound neutropenia was achieved 3 days after administration and lasted for 4 days (5).
(iii) Inoculum.
The isolates were grown in a vented tissue culture flask containing Sabouraud dextrose agar (Oxoid; Unipath Limited, Basingstoke, United Kingdom) plus chloramphenicol (SAB+C) (Sigma) for 10 days. The conidia were harvested in 25 ml of sterile phosphate-buffered saline (PBS) with 0.05% Tween 80 (PBS-Tween), the flask was shaken gently and stored at 4°C. The day of infection the stock solution was adjusted to an inoculum that would give a 90% lethal dose (LD90) (2.6 × 105 and 2.2 × 105 CFU/ml for AFL5 and AFL8, respectively) according to preliminary studies based on viability counts, using the following inocula: AFL8, 3 × 107, 8 × 106, 1.7 × 106, 7.6 × 105, 4.3 × 104, 1.3 × 104, and 5 × 103; AFL5, 3.5 × 107, 8 × 106, 4.8 × 106, 1.7 × 106, 7 × 105, 2.7 × 105, 8 × 104, and 2 × 103. Three days after immunosuppression, all animals were infected with 0.15 ml of the LD90 suspension via the tail vein (day 0). The number of CFU per milliliter of the inoculum was rechecked from the remaining conidial suspension after the animals were infected.
(iv) Antifungal therapy.
Deoxycholate AMB (Fungizone; E. R. Squibb, Hounslow, Middlesex, United Kingdom) was dissolved in 5% dextrose (Baxter Healthcare, Norfolk, United Kingdom) to a stock concentration of 5 mg/ml. Three concentrations of AMB were used in the course of the experiment: 5, 2, and 0.5 mg/kg per dose. The stock solution of AMB was diluted accordingly in 5% dextrose. All doses of AMB (and of 5% dextrose in a control group of mice) were administered via intraperitoneal injection (0.15 ml) once daily at 24, 48, and 96 h postinfection, and at 7 days postinfection.
ITZ (Janssen Research Foundation, Beerse, Belgium) was dissolved to a stock concentration of 25 mg/ml in a mixture of 1,2-propanediol (Fluka, Poole, Dorset, United Kingdom) and concentrated HCl in a water bath and then mixed with (2-hydroxypropyl)-β-cyclodextrin (HPBC) (Fluka) solubilized in water, and the pH was adjusted to 2.0 with NaOH. Three concentrations of ITZ were used: 75, 25, and 15 mg/kg per dose. The stock solution was diluted accordingly, in water for the higher dose and in an aqueous solution of HPBC for the two lower doses, because ITZ precipitated in a more diluted HPBC solution. All doses of ITZ (and an aqueous solution of HPBC given to a control group of mice) were administered by gavage (0.15 ml) three times daily on days 1 and 2 and twice a day on days 3 to 7.
(v) ITZ pharmacokinetics.
A separate group of four uninfected cyclophosphamide mice was treated with the 75- and 15-mg/kg ITZ doses (two mice for each dose). Blood samples were collected on day 4 by cardiac puncture 3 h after administration of the morning dose to determine the pharmacokinetics of ITZ during treatment, in duplicate. Samples were collected into plain tubes and allowed to clot at room temperature. Serum was then removed and stored at −20°C until analyzed. Samples were thawed and analyzed as a batch in bioassays using 10% yeast nitrogen base plus glucose plus trisodium citrate agar and Candida kefyr San Antonio strain.
(vi) Cultures.
On day 11 of the experiment, all surviving mice were culled. The lungs, liver, and kidneys (and brain for mice given AFL5 due to observation of middle-ear or cerebellar infection) were removed and transferred into 2 ml of PBS. The organs were homogenized in a tissue grinder (Polytron; Kinematica AG, Luzern, Switzerland) for approximately 15 to 30 s and then diluted to 10−1 and 10−2 of the original concentration. One hundred microliters of the undiluted and diluted suspensions was then transferred to SAB+C plates, and the liquid was spread over the surface of the plates. The plates were incubated at 37°C in a moist atmosphere and examined daily for 5 days. Colony counts were recorded from all plates that showed growth.
(vii) Statistical analysis.
Mortality and culture data were analyzed using the Mann-Whitney U test or the Kruskall-Wallis test if the Mann-Whitney test was not possible (i.e., if all values were identical in one group). Two-sided P values are given. Mice that died before day 10 were assumed to have organ counts at least as high as the highest counts in surviving mice in the calculation of culture result statistics. All data analysis was performed using the computer package Arcus Quik Stat (Addison Wesley Longman, Ltd.)
In vitro susceptibility testing procedures.
In vitro susceptibility testing was performed by comparing three different methodologies with eight A. flavus strains (including the two studied in an animal model). Method A is derived from published work from our laboratory that validated in vivo a method of detecting ITZ resistance in A. fumigatus but failed to do so for AMB (7, 11). Method A2 differs from A only in the reading criterion. Method B is the NCCLS M38-P proposed standard (18).
(i) Method A.
RPMI-1640 medium (with l-glutamine, without bicarbonate) (Sigma) supplemented with 2% glucose buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) was used for method A. Deoxycholate AMB was dissolved in distilled water to a stock concentration of 1,600 mg/liter and stored in the dark at −20°C. ITZ was dissolved at a concentration of 3,200 mg/liter in acetone and hydrochloric acid in a water bath at 60°C and then stored in aliquots at −20°C. A broth microdilution method was employed. Doubling dilutions of the drug were prepared in 100-μl volumes of the medium in the wells of a flat-bottom microtiter plate, giving final drug concentrations that ranged from 16 to 0.015 μg/ml. The last column of the wells was the drug-free positive control.
To prepare the inoculum, colonies were grown for 3 days on SAB+C at 37°C. Conidia were harvested by scraping a swap wetted with sterile PBS-Tween (Sigma) along the surface of the colonies and then suspended in sterile PBS-Tween. The suspension was then vortexed for 10 s to break up clumps of conidia. The conidial suspension was used on the same day. The inoculum suspension densities were counted with an improved Neubauer hemocytometer without having been diluted, and densities were then adjusted in the medium.
The final inoculum in the wells contained 5 × 105 CFU/ml. Viability counts were performed retrospectively as a check on the inoculum. Conidial suspensions before inoculation of the wells (106 CFU/ml) were diluted 1,000-fold, and 100 μl of inoculum was plated onto SAB+C plates and incubated at 37°C for 24 to 48 h. A variation up to 50% in the colony count in the inoculum was considered acceptable. Microtiter trays were incubated at 37°C for 46 to 50 h. The MICs were read visually and were defined as the lowest drug concentration with no visible growth.
(ii) Method A2.
The same plates prepared by method A were read visually, but trailing end points (reduced but persistent homogeneous growth in wells with high concentrations of the antifungal agent) were ignored in reading the end point. Thus, we considered the MIC to be the lowest ITZ concentration found in any well with a major reduction in homogenous growth.
(iii) Method B.
Method B is the NCCLS M-38P proposed standard (18). It differs from method A and A2 as follows. The same medium was used but not supplemented with glucose. Drug dilutions were prepared as batch dilutions instead of as doubling dilutions. AMB and ITZ stocks were diluted in dimethyl sulfoxide. Colonies were grown on potato dextrose agar at 35°C for 7 days. Spores were harvested by covering the colonies with 1 ml of sterile 0.85% saline, adding one drop of Tween, and probing the colonies with the tip of a Pasteur pipette. Heavy particles were allowed to settle for 5 min, and the upper homogeneous suspension was collected and vortexed for 15 s. Conidial suspensions were adjusted by a spectrophotometric procedure, read at 530 nm, and adjusted to an optical density of 0.09 to 0.11 and then diluted to 1:50 on the medium. This gave a final inoculum of 0.4 × 104 to 5 × 104 CFU/ml. Plates were incubated for 46 to 50 h at 35°C. MICs were read visually—AMB at 100% inhibition (no growth) and ITZ at 50 to 75% inhibition. This latter end point was inexact, so we considered the MIC to be the ITZ concentration of the well with the sharpest reduction in fungal growth compared to an adjacent well with a lower ITZ concentration.
(iv) Inoculum dependence MIC studies.
A modification of method A was used for inoculum dependence MIC studies, differing in inoculum preparation and the use of three different inoculum sizes. The inoculum was prepared as for method A except that the conidial suspension was stored in PBS-Tween 80 at 4°C and used for up to 1 month. The final inocula used were 1 × 106, 5 × 105, and 2.5 × 104 CFU/ml. When 106 CFU/ml was used, a sediment of spores formed that gave the impression that the organism had grown in all wells. For this inoculum, therefore, the MIC was read microscopically and defined as the well with the lowest ITZ concentration in which at least 75% of the spores were ungerminated when observed under an inverted microscope. Fourteen A. flavus isolates were tested.
Each experiment was repeated twice on separate occasions. For statistical and descriptive purposes, an MIC of >16 μg/ml is considered as an MIC of 32 μg/ml.
National Culture Collection accession numbers.
Both AFL5 and AFL8 have been deposited with the National Collection of Pathogenic Fungi (PHLS Mycology Reference Laboratory, Bristol, United Kingdom) as NCPF 7508 and 7509, respectively.
RESULTS
In vivo and in vitro studies.
Our prior expectation, based on preliminary in vitro data, was that infection caused by AFL5 would be refractory to AMB and responsive to ITZ and that infection caused by AFL8 would be refractory to ITZ and responsive to AMB. However, the survival rates were similarly high for both drugs in models using both Aspergillus strains.
Mortality in each experiment is shown in Fig. 1 and 2. Both Aspergillus strains caused a lethal infection in mice in the inoculum-finding studies. The LD90 inocula were 2.6 × 105 and 2.2 × 105 CFU/ml for AFL5 and AFL8, respectively. The survival rates in the control groups were low (0 to 20%). The infection was disseminated in that lung, brain, liver, and kidney tissues were all infected. As with other species of Aspergillus in this model, pulmonary infection is less than that in the liver and kidneys, but overall the infection is still rapidly fatal. The concentration of ITZ in sera at the 15-mg/kg dose in these models ranged from 2.6 to 4.7 μg/ml, similar to that obtained by our group in previous similar models with similar ITZ doses (6, 21). The concentration at 75 mg/kg could not be determined due to technical problems. Despite the torticollis and staggering noticed in some mice infected with the AFL5 isolate, very low numbers of CFU were retrieved from the brains of the surviving mice and most of the brains were sterile.
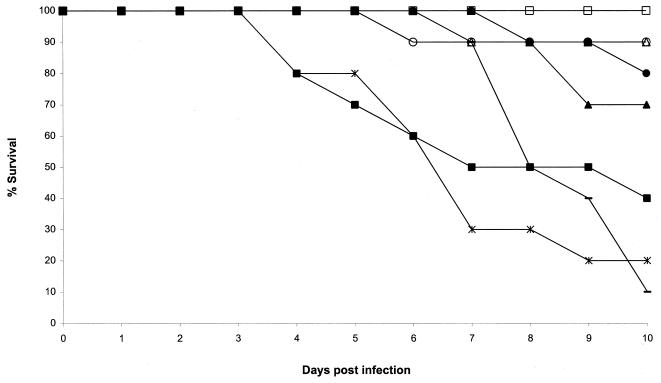
FIG. 1.
Plot of cumulative mortality against time for a murine model against A. flavus AFL5. □, ITZ, 75 mg/kg; ○, ITZ, 25 mg/kg; ▵, ITZ, 15 mg/kg, ■, AMB, 5 mg/kg; ●, AMB, 2 mg/kg; ▴, AMB, 0.5 mg/kg; —, 5% glucose; ×|, cyclodextrin.
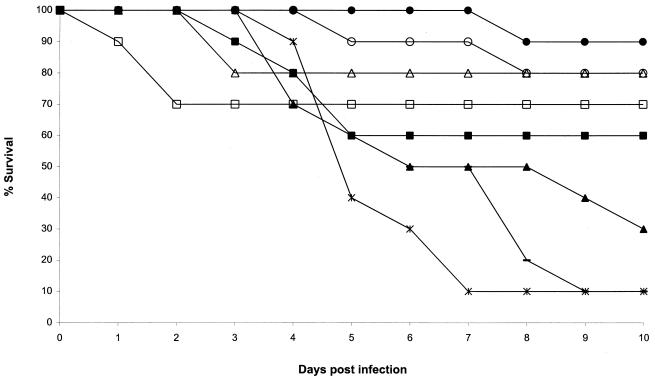
FIG. 2.
Plot of cumulative mortality against time for a murine model against A. flavus AFL8. □, ITZ, 75 mg/kg; ○, ITZ, 25 mg/kg; ▵, ITZ, 15 mg/kg; ■, AMB, 5 mg/kg; ●, AMB, 2 mg/kg; ▴, AMB, 0.5 mg/kg; —, 5% glucose; ×|, cyclodextrin.
AMB.
In our murine model, both AFL5 and AFL8 appeared to be susceptible to AMB. With AFL5 the control mice succumbed (Fig. 1), whereas the survival was high with AMB at 2 and 0.5 mg/kg (80 and 70% survival, respectively). The higher dose (5 mg/kg) was less effective (40% survival). These differences were not statistically significant for any of the three doses. AFL8 was responsive to AMB as well (Fig. 2). The 2-mg/kg dose was again the best AMB treatment (90% survival; P = 0.001). The lower AMB dose (0.5 mg/kg) was almost ineffective (30% survival; P = not significant [ns]) compared to the control group (10% survival). The higher AMB dose was again less effective than the 2-mg/kg dose (60% survival; P = ns).
Colony counts were calculated from the culture results, and a detailed summary is shown in Table 1. For AFL5, AMB organ burdens in mice that received the 2- and 0.5-mg/kg doses were significantly lower than those in the controls for all organs (P = 0.011 and P = 0.018, respectively), whereas counts were significantly lower only for liver tissue at the 5-mg/kg dose level (P = 0.04). For AFL8, colony counts in mice that received 5 and 2 mg/kg per dose were significantly lower than the control for all organs (P = 0.02 and P = 0.004 respectively) (brain counts not available). Comparison of the culture results showed higher organ burdens with AFL5 than AFL8 (about 30-fold at 2 mg/kg of AMB compared with two- to threefold higher burdens in controls), but the number of animals sampled was small.
TABLE 1.
Organ culture results for A. flavus AFL5 and AFL8
| Strain, treatment, and dose (mg/kg) | No. of survivors (%) | No. sterilized | Geometric mean CFUs
|
|||
|---|---|---|---|---|---|---|
| Braina | Kidneys | Liver | Lungs | |||
| AFL5 | ||||||
| ITZ, 75 | 10/10 (100) | 1 | 0 | 0 | 39 | 0 |
| ITZ, 25 | 9/10 (90) | 0 | 0 | 5 | 105 | 0 |
| ITZ, 15 | 9/10 (90) | 0 | 0 | 27 | 141 | 0 |
| AMB, 5 | 4/10 (40) | 0 | 3 | 281 | 87 | 5 |
| AMB, 2 | 8/10 (80) | 0 | 0 | 242 | 36 | 0 |
| AMB, 0.5 | 7/10 (70) | 0 | 1 | 115 | 143 | 1 |
| Glucoseb | 2/10 (20) | 0 | 30 | 1,500 | 300 | 30 |
| Cyclodextrinc | 0/10 (0) | 0 | 10 | 1,065 | 113 | 15 |
| AFL8 | ||||||
| ITZ, 75 | 7/10 (70) | 3 | N/A | 1 | 5 | 0 |
| ITZ, 25 | 8/10 (80) | 1 | N/A | 1 | 15 | 0 |
| ITZ, 15 | 8/10 (80) | 3 | N/A | 2 | 2 | 0 |
| AMB, 5 | 6/10 (60) | 2 | N/A | 13 | 4 | 1 |
| AMB, 2 | 9/10 (90) | 3 | N/A | 8 | 1 | 1 |
| AMB, 0.5 | 3/10 (30) | 0 | N/A | 240 | 34 | 10 |
| Glucoseb | 1/10 (10) | 0 | N/A | 675 | 79 | 19 |
| Cyclodextrinc | 1/10 (10) | 0 | N/A | 559 | 50 | 19 |
N/A, not available.
Control for AMB.
Control for ITZ.
The three in vitro methods of testing yielded elevated MICs for AMB against AFL5: 32 μg/ml with methods A and A2 and 4 to 8 μg/ml with method B. MICs of AMB against AFL8 were lower, varying from 2 to 4 μg/ml with methods A and A2 and from 1 to 2 μg/ml with method B. MICs of AMB against other isolates were also lower than those of AFL5, varying from 0.5 to 8 μg/ml with methods A and A2 and from 0.25 to 2 μg/ml with method B.
ITZ.
In our murine model both AFL5 and AFL8 were responsive to ITZ. This drug was very effective at all doses: 80 to 100% survival for AFL5 (P = 0.008, 0.048, and 0.031 for the 75-, 25-, and 15-mg/kg doses, respectively) and 70 to 80% for AFL8 (P = ns, 0.001, and 0.035, respectively), compared with the controls (10 to 20% survival). With AFL5 the three ITZ doses were numerically superior to any of the AMB treatments. Two animals died in the AFL8 75-mg/kg-per-dose group in the first 2 days of the experiment. These deaths were probably due to trauma during treatment.
For both Aspergillus strains, counts for animals treated with ITZ were significantly lower than those in the controls under all regimens and in all organs (except liver tissue in mice receiving the 15-mg/kg dose in the AFL5 model and the 25-mg/kg dose in the AFL8 model). Culture results show higher organ burdens with AFL5 than with AFL8.
MICs to ITZ for AFL5 were low with all methods. The MIC varied from 0.12 and 0.25 μg/ml with methods A and A2, respectively, to 0.12 μg/ml with method B. MICs to ITZ were variable for AFL8, depending on the method used: 32 and 1 μg/ml with method A, 0.5 and 1 μg/ml with method A2, and 0.12 μg/ml with method B.
Comparison of in vitro methodologies.
Some important differences in the ITZ susceptibility testing results were observed when the three methodologies were compared (Table 2). With method A, MICs of 32 μg/ml were obtained at least once in some of the isolates studied, whereas the maximum MIC with methods A2 and B was only 1 μg/ml. The high MIC values using method A were due to the presence of trailing end points. When trailing end points were ignored (method A2), much lower MICs were obtained (Table 2). Those wells showing a trailing end point were observed with an inverted microscope and revealed the presence of slightly germinated spores with abnormal hyphae, which were short and much thinner than those observed in the wells with lower ITZ concentrations and in plates without a trailing end point.
TABLE 2.
MICs for ITZ
| Aspergillus strain | MIC with the following methoda:
|
|||
|---|---|---|---|---|
| A | A2 | A2 (2.5 × 104 CFU/ml) | B | |
| AFL5 | 0.12–0.25 | 0.12–0.25 | 0.06–0.12 | 0.12 |
| AFL8 | 1–32 | 0.5–1 | 0.12 | 0.12 |
| NIJ-766 | 32 | 0.25–0.5 | 0.12–0.25 | 0.25 |
| OSL-60A | 0.25–32 | 0.25–0.5 | 0.06–0.12 | 0.06–0.12 |
| ATCC 15547 | 0.25–32 | 0.25 | 0.06–0.12 | 0.06–0.12 |
| AFL12 | 0.5–1 | 0.5–1 | 0.12 | 0.12–0.25 |
| AFL20 | 0.25 | 0.25 | 0.06–0.12 | 0.12 |
| CM-900 | 0.25 | 0.25 | 0.06 | 0.06 |
When the MICs were different in the two assays, the two different results are presented separated with a dash.
Apart from a trailing end point, differences in the MIC results between methods A and B seem to be related to the inoculum size differences. When method A was employed using a final inoculum within the range of method B (2.5 × 104 CFU/ml), MIC results were very similar to those observed for method B (Table 2).
A further observation in our study was the problem of the insolubility of ITZ in the medium used in all the methods. When the plates were observed with an inverted microscope after 48 h, a crystal or amorphous precipitation of ITZ could be seen at the bottom of the wells with the higher ITZ concentrations, especially at 16 and 8 μg/ml but also at 4 μg/ml. The use of acetone-hydrochloric acid or dimethyl sulfoxide as a solvent made no difference. Fungal growth was sometimes noted in those wells (mainly at 16 and 8 μg/ml) near ITZ precipitation, even when the remainder of the well and wells with a lower ITZ concentration were clear of growth.
Inoculum dependence MIC studies.
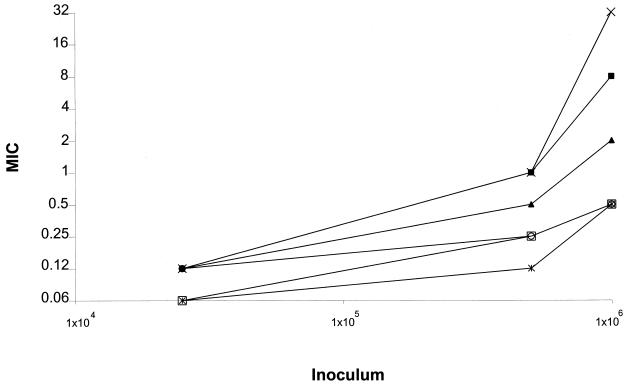
Changes in inoculum have a large effect on the MICs to ITZ of some strains of A. flavus (inoculum dependency) (Fig. 3). An increase in the size of the final inoculum from 2.5 × 104 to 1 × 106 CFU/ml raised the MIC 4- to 256-fold (two to eight wells) depending on the strain. Using a final inoculum size of 2.5 × 104 CFU/ml, within the range of method B (0.4 × 104 to 5 × 104 CFU/ml), all 14 of the A. flavus isolates studied had a MIC of 0.06 to 0.12 μg/ml. However, the MIC varied from 0.5 to 32 μg/ml when an inoculum of 106 CFU/ml was used.
FIG. 3.
MIC inoculum dependence in A. flavus. The results of only 14 of the more than 20 isolates tested are presented here. Isolates include those that gave the most and the least inoculum dependence. When two different MIC values were obtained with the same inoculum for one strain (always within a one-well variation) the higher value was plotted. ×, AFL12; ■, AFL8; ▴ F3934, NIJ-766, F1972, OSL-60A, and OSL-70A; ○, AFL18, ATCC 15547, and F1898; □, AFL19, AFL20, and CM-1264; ×|, AFL5.
DISCUSSION
A. flavus appears to cause a more virulent disseminated infection than other species within the genus. LD90 inocula were 2.6 × 105 and 2.2 × 105 CFU/ml for A. flavus AFL5 and AFL8 isolates, respectively. This is about 4- to 50-fold lower than that observed in similar mice models with A. fumigatus, in which LD90s varied from 1 × 106 to 1.2 × 107 CFU/ml (7), and 40- to 100-fold lower than with A. terreus, in which LD90s varied from 1 × 107 to 2 × 107 (11).
AMB.
We have consistently obtained high AMB MICs when testing AFL5 with all the three methods (4 to 32 μg/ml). Nevertheless, in our in vivo model the two isolates tested were responsive to AMB when mortality was taken into account. With the lowest AMB dose, the survival was lower in the AFL8 model despite the lower MICs obtained (1 to 4 μg/ml). It must be noted that kidney and liver burdens at 5 and 2 mg/kg per dose were higher for the AFL5 model.
A lack of correlation between in vitro results and in vivo outcome for AMB has been shown by Johnson et al. (11) in A. fumigatus. Odds et al. (22) studied the in vivo response of an in vitro-susceptible strain of A. flavus with a MIC to AMB of 0.5 to 4 μg/ml (other A. flavus strains tested did not differ significantly) (9). They found that strain of A. flavus to be highly resistant to AMB in vivo in intravenously infected mouse and guinea pig models. Thus, the most common currently used methods to detect AMB resistance seem to be meaningless with A. flavus as well as with A. fumigatus. Nevertheless, a correlation was obtained between in vitro susceptibility testing and clinical outcome in hematology patients with invasive aspergillosis (13). In this series, most of the isolates were A. flavus and A. terreus and an MIC of ≥2 μg/ml was predictive of death.
ITZ.
The two A. flavus strains we tested in an animal model were susceptible to ITZ, even with relatively low drug doses, despite the perception based on preliminary in vitro data from our laboratory and the high MIC obtained in this study using method A that AFL8 could be ITZ resistant.
Method A correlates with in vivo outcomes for A. fumigatus (7), but we have found difficulties when using it with A. flavus. The high MICs for ITZ we sometimes obtained with method A for some strains (Table 2) are due to the appearance of a trailing end point. This occurrence was variable within single strains in our studies and was never found by us with inocula containing fewer CFU per milliliter. We have not observed it when testing A. fumigatus. Inspection of wells showing a trailing end point were examined with an inverted microscope. Poor conidial germination was observed, and hyphae were thin. Trailing end points have arisen as a problem when testing Candida spp. with azoles (24). According to Marr et al. (17), this phenomenon is pH dependent in Candida spp.
We also observed “false” trailing end points when plates were read visually after wells were inoculated with a higher inoculum than the one used in methods A and A2 (i.e., a final inoculum of >5 × 105 CFU/ml). This problem was due to the settling of the conidia on the bottom of the wells, causing sediment without growth. The problem was less apparent in other important pathogenic Aspergillus species such as A. fumigatus and A. terreus, since their conidia are smaller in size.
The in vivo outcome for the AFL5 and AFL8 strains in our model was in concordance with the in vitro results for both strains using methods A2 and B. It also showed that the appearance of trailing end points using method A does not accord with genuine resistance. We feel that trailing end points did not appear with method B because fewer CFU were used in the inoculum. The lower inoculum probably accounts for the lower MICs. When the inoculum recommended by the NCCLS (method B) was used with method A2, MICs with method B were within a one-well difference (Table 2). In our inoculum dependency studies, lower inocula gave rise to lower MICs (Fig. 3). Former work of Gehrt et al. (10) showed a less important inoculum effect on MICs, but they studied a small number of isolates of Aspergillus spp.
We believe that more CFU in the final inoculum employed in methods A and A2 is inappropriate for MIC testing of ITZ against A. flavus, due to the settling of conidia and the appearance of a trailing end point. A lower final inoculum containing 2.5 × 104 CFU/ml, within the range recommended by the NCCLS, seems a better option according to our results. With inocula in this range, other problematic phenomena in antifungal susceptibility testing using a microbroth method would be partially overcome. These problems include growth of only a small percentage of the conidia, growth of clumps of conidia at higher ITZ concentrations (we recommend that heavy particles be allowed to settle for 10 min and the supernatant suspension taken), and variable growth on the top of the wells. In addition, because MICs obtained with the low level of CFUs in the final inoculum we recommend are lower, a larger ITZ concentration-testing range would be available. A lower ITZ concentration range such as 4 to 0.004 μg/ml might be employed. Thus, false results in wells with ITZ precipitation would be avoided. We also argue that a narrow inoculum range should be employed due to the important inoculum dependence we have shown for some A. flavus strains. The use of an inoculum size range, as in the NCCLS M-38P document (0.4 × 104 to 5 × 104 CFU/ml), could give rise to very different MICs for strains with intermediate CFU in vitro.
We have also tested six in vitro ITZ-resistant A. fumigatus strains, two of them validated in vivo (7), following method A but using the lower level of inoculum we recommend here. All of those strains showed very high MICs compared to ITZ-susceptible strains (data not shown). Thus it seems that the use of an inoculum of 2.5 × 104 CFU/ml would still allow the detection of ITZ resistance in Aspergillus spp. A genuinely resistant strain is required to fully validate the NCCLS M-38P or any other methodology against ITZ for A. flavus, to elucidate the best inoculum to obtain reproducible meaningful results, and to detect more subtle phenomena such as intermediate resistance. No ITZ-resistant A. flavus strains have been reported in the literature, and we have not identified one in-house.
Regarding antifungal treatment, our results showed that AMB and ITZ appear to be good treatments for infections caused by A. flavus. However, with AMB effecacy was seen only within a limited dosing range. AMB was less effective at a low dose in our animal models, and there was more mortality with the higher dose, due perhaps to toxicity. Higher mortality with AMB doses of 5 mg/kg has not been observed in a similar model using A. fumigatus (7), and its relevance for patient management is unclear.
This work has shown that much still remains to be done regarding antifungal susceptibility testing of Aspergillus spp. The only method that, at this point, has been shown to correlate ITZ in vitro testing results with in vivo outcome for A. fumigatus does not work adequately with A. flavus. It has yet to be proven that the method detects A. fumigatus strains with intermediate resistance to ITZ. The lack of correlation of AMB susceptibility testing with the in vivo outcome, using all methods including the NCCLS M-38P method, parallels the findings in A. fumigatus. Our study also showed that AMB and ITZ are effective in the treatment of experimental A. flavus infections.
ACKNOWLEDGMENTS
This work was supported by the European Commission Training and Mobility of Researchers grant FMRX-CT970145, Eurofung, and the Fungal Research Trust.
REFERENCES
- 1.Cacciapuoti A, Loebenberg D, Parmegiani R, Antonacci B, Norris C, Moss E L, Jr, Menzel F, Jr, Yarosh-Tomaine T, Hare R S, Miller G H. Comparison of SCH 39304, fluconazole, and ketoconazole for treatment of systemic infections in mice. Antimicrob Agents Chemother. 1992;36:64–67. doi: 10.1128/aac.36.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning D W. Invasive aspergillosis in immunocompromised patients. Curr Opin Infect Dis. 1994;7:456–462. [Google Scholar]
- 3.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 4.Denning D W, Baily G G, Hood S V. Azole resistance in Candida. Eur J Clin Microbiol Infect Dis. 1997;16:1–20. doi: 10.1007/BF01695630. [DOI] [PubMed] [Google Scholar]
- 5.Denning D W, Hall L, Jackson M, Hollis S. Efficacy of D0870 compared with those of itraconazole and amphotericin B in two murine models of invasive aspergillosis. Antimicrob Agents Chemother. 1995;39:1809–1814. doi: 10.1128/aac.39.8.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning D W, Kenkateswarlu K, Oakley K L, Anderson M J, Manning N J, Stevens D A, Warnock D W, Kelly S L. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–1368. doi: 10.1128/aac.41.6.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J Antimicrobial Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 8.Eisenstein D J, Biddinger P W, Rhodes J C. Experimental murine invasive pulmonary aspergillosis. Am J Clin Pathol. 1990;93:510–515. doi: 10.1093/ajcp/93.4.510. [DOI] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gehrt A, Peter J, Pizzo P A, Walsh T J. Effect of increasing inoculum sizes of pathogenic filamentous fungi on MICs of antifungal agents by broth microdilution method. J Clin Microbiol. 1995;33:1302–1307. doi: 10.1128/jcm.33.5.1302-1307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson E M, Oakley K L, Radford S A, Moore C, Warn P, Warnock D W, Denning D W. Lack of correlation of in vitro amphotericin B susceptibility testing with outcome in a murine model of Aspergillus infection. J Antimicrob Chemother. 2000;45:85–93. doi: 10.1093/jac/45.1.85. [DOI] [PubMed] [Google Scholar]
- 12.Khoo S H, Denning D W. Invasive aspergillosis in patients with AIDS. Clin Infect Dis. 1994;19:541–548. doi: 10.1093/clinids/19.supplement_1.s41. [DOI] [PubMed] [Google Scholar]
- 13.Lass-Flörl C, Kofler G, Kropshofer G, Hermans J, Kreczy A, Dierich M P, Niederwieser D. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J Antimicrob Chemother. 1998;42:497–502. doi: 10.1093/jac/42.4.497. [DOI] [PubMed] [Google Scholar]
- 14.Latgé J. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liener I E. Toxin constituents of plant foodstuffs. New York, N.Y: Academic Press, Inc.; 1969. pp. 392–394. [Google Scholar]
- 16.Loebenberg D, Cacciapuoti A, Parmegiani R, Moss E L, Jr, Menzel F, Jr, Antonacci B, Norris C, Yarosh-Tomaine T, Hare R S, Miller G H. In vitro and in vivo activities of SCH 42427, the active enantiomer of the antifungal agent SCH 39304. Antimicrob Agents Chemother. 1992;36:498–501. doi: 10.1128/aac.36.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marr K A, Rustad T R, Rex J H, White T C. The trailing end point phenotype in antifungal susceptibility testing is pH dependent. Antimicrob Agents Chemother. 1999;43:1383–1386. doi: 10.1128/aac.43.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 19.Oakley K L, Moore C B, Denning D W. In-vitro activity of liposomal nystatin against Aspergillus species, abstr. P90. Proceedings of the 4th Congress European Confederation of Medical Mycology. 1998. Glasgow, Scotland. [Google Scholar]
- 20.Oakley K L, Moore C B, Denning D W. Comparison of in vitro activity of liposomal nystatin against Aspergillus species with those of nystatin, amphotericin B (AB) deoxycholate, AB colloidal dispersion, liposomal AB, AB lipid complex, and itraconazole. Antimicrob Agents Chemother. 1999;43:1264–1266. doi: 10.1128/aac.43.5.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and an itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odds F C, Gerven F V, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oji E O. Ketoconazole: a new imidazole antifungal agent has both prophylactic potential and therapeutic efficacy in keratomycosis of rabbits. Int Ophthalmol. 1982;5:163–167. doi: 10.1007/BF00149147. [DOI] [PubMed] [Google Scholar]
- 24.Revankar S G, Kirkpatrick W R, McAtee R K, Fothergill A W, Redding S W, Rinaldi M G, Patterson T F. Interpretation of trailing end points in antifungal susceptibility testing by the national committee for clinical laboratory standards method. J Antimicrob Chemother. 1998;36:153–156. doi: 10.1128/jcm.36.1.153-156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitt H J, Andrade J, Edwards F, Niki Y, Bernard E, Armstrong D. Inactivity of terbinafine in a rat model of pulmonary aspergillosis. Eur J Clin Microbiol Infect Dis. 1990;9:832–835. doi: 10.1007/BF01967386. [DOI] [PubMed] [Google Scholar]
- 26.Sharma O P, Chwogule R. Many faces of pulmonary aspergillosis. Eur Respir J. 1998;12:705–715. doi: 10.1183/09031936.98.12030705. [DOI] [PubMed] [Google Scholar]
- 27.Taylor J J, Burroughs E J. Experimental avian aspergillosis. Mycopathol Mycol Appl. 1973;51:131–141. doi: 10.1007/BF02141104. [DOI] [PubMed] [Google Scholar]
- 28.Vanden-Bossche H, Dromer F, Improvisi I, Lozano-Chiu M, Rex J H, Sanglard D. Antifungal drug resistance in pathogenic fungi. Med Mycol. 1998;36:119–128. [PubMed] [Google Scholar]
- 29.Verweij P E, Oakley K L, Morrissey J, Morrissey G, Denning D W. Efficacy of LY303366 against amphotericin B-susceptible and -resistant Aspergillus fumigatus in a murine model of invasive aspergillosis. Antimicrob Agents Chemother. 1998;42:873–878. doi: 10.1128/aac.42.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]