Abstract
Purpose
Serum calcium and phosphorus abnormalities are associated with cardiovascular disorders in general population, but evidence among patients with established coronary heart disease (CHD) is limited and controversial. This study aimed to investigate the associations of baseline serum calcium and phosphorus levels with long-term mortality risk among patients with CHD.
Methods
We conducted a prospective cohort study among 3187 patients with CHD from October 2008 and December 2011 in China. Cox proportional hazards model was used to assess the associations of serum calcium and phosphorus at baseline with the risk of death.
Results
During follow-up (mean, 4.9 years), 295 patients died, 193 of which resulted from cardiovascular causes. Multivariable-adjusted hazard ratios (HR) for each 1 mmol/L increase in serum calcium at baseline were 0.27 (95% confidence interval (CI) 0.14–0.51) for all-cause mortality and 0.26 (95% CI 0.12–0.54) for cardiovascular mortality. Patients in the highest compared to the lowest quartile of serum calcium were at lower risk of all-cause mortality (HR, 95% CI 0.57, 0.40–0.82) and cardiovascular mortality (0.50, 0.32–0.79) (both Ptrend < 0.001). This inverse association between serum calcium and the risk of mortality did not change when participants were stratified by sex, age groups, level of overweight, types of CHD, and history of diabetes. We also observed a graded positive association between baseline serum phosphorus and the risks of mortality.
Conclusions
The present study is the first to report that lower serum calcium at baseline is associated with an increased risk of all-cause and cardiovascular mortality in a Chinese coronary heart disease cohort. Further studies are required to investigate the causal relationship and actual mechanisms.
Keywords: Mineral metabolism, Cardiovascular disease, Mortality, Cohort study
Introduction
Serum calcium and phosphorus are essential for many physiological progresses which might be directly or indirectly related to cardiovascular disease (CVD), the leading cause of deaths worldwide [1]. While the prognosis of coronary heart disease (CHD) has improved substantially over the recent decades in the developed countries [2], CHD mortality still remains high in the developing countries, especially in China [3]. To improve the survival of CHD patients, further researches are needed to focus on the associations of not only conventional cardiac risk factors but also non-traditional risk factors, such as serum calcium and phosphorus, with the long-term outcomes.
Abnormalities in calcium and phosphorus homeostasis have been reported as risk factors in cardiovascular disorders [4]. Previous studies have reported that higher serum calcium and phosphorus levels, even within the normal range, were associated with abnormal vascular function, such as increased carotid intima–media thickness, arterial stiffness and the presence of calcified plaque [5–8]. Several studies have examined the associations of serum calcium and phosphorus with the major cardiovascular outcomes in subjects with chronic kidney disease (CKD) [9] or in the general population [10, 11]. Patients with established CVD might have renal insufficiency, impaired gastrointestinal function, or heightened neurohormonal activation, which could affect calcium and phosphorus homeostasis; whether the abnormalities of calcium and phosphorus would in turn affect the prognosis of CVD was unknown. Furthermore, recent cross-sectional studies have indicated that the decrease of serum calcium following the occurrence of CVD was associated with cardiovascular disorder in Chinese patients, which was inconsistent with previous Western studies [12]. The association of lower serum calcium levels with the long-term adverse outcomes in Chinese CHD patients has not been clarified. Thus, we aimed to investigate the associations of baseline serum calcium and phosphorus with the risks of all-cause and cardiovascular mortality among Chinese patients with CHD.
Methods
Study population
We evaluated the association of baseline serum calcium and phosphorus with the risk of all-cause and cardiovascular mortality among CHD patients, who participated in the Guangdong Coronary Artery Disease Cohort (GCADC) study. The sampling methods of the GCADC study setting have been described previously in detail [13, 14]. Using the same selection, criteria and ascertainment of CHD, we first recruited 1984 patients during 2008–2011 [13], and then further included 1615 patients via electronic medical records during 2013–2014. In total, we recruited 3599 consecutive inpatients who were admitted to the Cardiology Department of three superior specialty hospitals in Guangdong, China (Guangzhou Military General Hospital, Sun Yat-sen Memorial Hospital, and First Affiliated Hospital of Sun Yat-sen University) between October 2008 and December 2011 and were diagnosed as CHD [International Classification of Diseases (ICD)-10 codes I20–I25] according to the World Health Organization 1999/2000 guidelines [15, 16]. Patients with acute coronary syndrome were defined as the occurrence of any of unstable angina pectoris, ST-segment elevation myocardial infarction, and non-ST-segment elevation myocardial infarction within 3 months. The present study included 3187 CHD patients aged 40 years and over after excluding the participants lacking data on general information at baseline (n = 248) and serum calcium or phosphorus measures (n = 164). Informed consent was obtained from each of the first recruited patients from the GCADC study. We did not obtain informed consent from those participants recruited during 2013–2014 because we used anonymized data compiled from electronic medical records. The study plan for the first recruited patients and analysis plan for the whole patients were approved by the Sun Yat-sen University Ethics Committee, and all clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki.
Measurements
Every patient’s general information, including admission date, birth date, gender, address, contact information, marriage status, education level, leisure-time physical activity, smoking habits, alcohol consumption, history of diseases and use of medication before admission was obtained by a standardized questionnaire through a face-to-face interview and/or extracted from the computerized hospitalization records. Smoking habits and alcohol consumption were classified into three groups: never, past, or current. Current smoking was defined as regularly at least one cigarette per day and lasting for more than 6 months before the study, and current alcohol drinking was defined as drinking any type of alcoholic beverage at least once a week and lasting for half a year.
Clinical measurements of each participant were extracted from an electronic case record system. At admission, trained nurses measured height, weight and blood pressure using a standard protocol [17]. Body mass index (BMI) was defined as the weight in kilograms divided by the square of height in meters. All blood samples were drawn in the next morning after admission with at least 12 h fasting. Lipids, fasting blood glucose, serum creatinine, serum calcium or phosphorus were determined by standard methods using the Hitachi Automatic Analyzer 7600–020 (Hitachi, Tokyo, Japan). We estimated glomerular filtration rate (GFR) using the Modification of Diet in Renal Disease (MDRD) Study equation: [18] estimated GFR (eGFR) = 175 × (standardized serum creatinine in mg/dL)−1.154 × age−0.203 × 0.742 (if female).
Prospective follow-up
Since enrollment, all study participants have been followed for all-cause and cardiovascular deaths from hospital medical records of readmission, telephone contacts with patients or their immediate family members (only for the first recruited patients), and death registration at the Guangdong Provincial Center for Disease Control and Prevention annually. In the current study, follow-up duration was calculated from the date of enrollment to the date of death, the date of last contact, or September 30, 2015. There was no patient lost in the follow-up in the current study. We used the ICD codes to identify the cause of deaths, and the ICD codes I00–I99 were classified as cardiovascular deaths.
Statistical analyses
We used the general linear model after adjustment for age or Chi-square analysis to test differences in continuous or categorical risk factors between different categories of serum calcium or phosphorus. The associations of different levels of serum calcium or phosphorus at baseline with the risks of all-cause and cardiovascular mortality were tested using Cox proportional hazards model. Both serum calcium and phosphorus were considered as continuous variables and in quartiles (phosphorus: ≤1.01 (reference group), 1.02–1.15, 1.16–1.29, >1.29 mmol/L; calcium: <2.18 (reference group), 2.18–2.27, 2.28–2.36, and ≥2.37 mmol/L). All analyses were adjusted for age and sex, and further for education, marital status, smoking, alcohol drinking, leisure-time physical activity, BMI, eGFR, systolic blood pressure (SBP), triglyceride, high-density lipoprotein cholesterol (HDL-C), types of CHD, history of diabetes, use of antihypertensive medications, use of glucose-lowering medications, use of lipid-lowering medications, and use of antiplatelet medications. Serum phosphorus was included in the final models when evaluating serum calcium with mortality; and serum calcium was included in the final models when evaluating serum phosphorus with mortality. The proportional hazard assumption in the Cox models was assessed with graphic methods and also by adding to the model an interaction term between a function of time and the variable of interests. In general, the graphical methods showed that the curves of every model were roughly parallel; and there was no violation of all proportionality assumptions using models including time-by-covariate interactions (all P for interaction term >0.05). The different categories of serum calcium or serum phosphorus were included in the models as dummy variables, and the significance of the trend over different categories was tested in the same models with the median of each category as a continuous variable. Since the interaction between sex and serum calcium and death risk was not statistically significant, data for men and women were combined in the analyses to maximize the statistical power.
In addition, we used restricted cubic splines in Cox models to test whether there was a dose–response or non-linear association of serum phosphorus or calcium as a continuous variable with the risks of all-cause and cardiovascular mortality, respectively.
Statistical significance was considered to be P < 0.05 and all probability values were two sides. Analyses were performed using PASW for Windows, version 20.0 (IBM SPSS Inc., Chicago, IL, USA) and SAS for Windows, version 9.4 (SAS Institute, Cary, NC, USA).
Results
General characteristics of the study population according to baseline quartiles of serum calcium or phosphorus are presented in Tables 1 and 2, respectively. Of the 3187 patients, the mean age was 64.2 years and 62.9% were men.
Table 1.
Baseline characteristics according to different levels of serum calcium among patients with coronary heart disease
| Characteristics | Serum calcium (mmol/L) | P value | |||
|---|---|---|---|---|---|
| <2.18 | 2.18–2.27 | 2.28–2.36 | ≥2.37 | ||
| Male (%) | 70.3 | 63.4 | 62.6 | 55.4 | <0.001 |
| Age (years) | 65.8 (0.39) | 64.7 (0.38) | 63.2 (0.40) | 62.9 (0.39) | <0.001 |
| Serum phosphorus (mmol/L) | 1.13 (0.01) | 1.14 (0.01) | 1.17 (0.01) | 1.21 (0.01) | <0.001 |
| Body mass index (kg/m2) | 23.6 (0.12) | 23.8 (0.12) | 24.1 (0.12) | 24.5 (0.12) | <0.001 |
| Systolic blood pressure (mmHg) | 131 (0.77) | 134 (0.76) | 138 (0.79) | 139 (0.77) | <0.001 |
| Diastolic blood pressure (mmHg) | 75.3 (0.45) | 77.8 (0.44) | 79.0 (0.45) | 80.4 (0.44) | <0.001 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.88 (0.04) | 3.00 (0.03) | 3.04 (0.04) | 3.04 (0.03) | 0.002 |
| High-density lipoprotein cholesterol (mmol/L) | 1.06 (0.01) | 1.13 (0.01) | 1.17 (0.01) | 1.21 (0.01) | <0.001 |
| Triglycerides (mmol/L) | 1.64 (0.05) | 1.85 (0.05) | 1.92 (0.05) | 1.98 (0.05) | <0.001 |
| Total cholesterol (mmol/L) | 4.54 (0.04) | 4.78 (0.04) | 4.89 (0.04) | 4.97 (0.04) | <0.001 |
| Fasting glucose (mmol/L) | 6.71 (0.10) | 6.41 (0.10) | 6.60 (0.10) | 6.71 (0.10) | 0.12 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2, %) | <0.001 | ||||
| ≥90 | 17.8 | 22.9 | 23.7 | 20.3 | |
| 60–89 | 48.9 | 46.2 | 52.2 | 53.8 | |
| 30–59 | 28.6 | 29.3 | 23.1 | 24.6 | |
| <30 | 4.8 | 1.6 | 1.1 | 1.2 | |
| Types of coronary heart disease (%) | <0.001 | ||||
| Acute coronary syndrome | 65.9 | 57.7 | 51.5 | 51.7 | |
| Stable coronary heart disease | 34.1 | 42.3 | 48.5 | 48.3 | |
| Married (%) | 70.7 | 75.8 | 79.4 | 85.8 | <0.001 |
| Years of education (%) | 0.61 | ||||
| ≤9 | 68.3 | 67.1 | 66.3 | 67.6 | |
| 10–12 | 17.5 | 15.0 | 15.5 | 15.7 | |
| ≥13 | 14.3 | 17.9 | 18.2 | 16.7 | |
| Smoking (%) | 0.006 | ||||
| Never | 58.4 | 60.3 | 61.6 | 66.1 | |
| Past | 8.7 | 9.8 | 11.0 | 10.0 | |
| Current | 32.8 | 29.9 | 27.4 | 23.9 | |
| Alcohol drinking (%) | 0.001 | ||||
| Never | 80.3 | 82.7 | 84.7 | 88.1 | |
| Past | 5.1 | 5.8 | 4.1 | 2.6 | |
| Current | 14.6 | 11.5 | 11.2 | 9.3 | |
| Leisure-time physical activity (%) | <0.001 | ||||
| None | 22.8 | 18.3 | 13.4 | 10.1 | |
| <30 min/day | 11.6 | 11.7 | 11.1 | 7.6 | |
| ≥30 min/day | 29.0 | 24.5 | 21.1 | 14.7 | |
| No data | 36.5 | 45.5 | 54.3 | 67.6 | |
| History of diseases (%) | |||||
| Hypertension | 81.0 | 76.5 | 75.9 | 72.9 | 0.002 |
| Diabetes | 23.8 | 22.4 | 21.7 | 25.4 | 0.33 |
| Uses of medications before admission (%) | |||||
| Antihypertensive medication | 45.3 | 49.3 | 54.0 | 54.1 | 0.001 |
| Glucose-lowering medication | 14.9 | 16.3 | 16.5 | 19.7 | 0.07 |
| Lipid-lowering medication | 7.3 | 11.3 | 13.0 | 12.0 | 0.003 |
| Antiplatelet medication | 13.6 | 17.7 | 16.2 | 15.1 | 0.17 |
The values are mean (SE) or percentage. Continuous variables are adjusted for age, except for age
Table 2.
Baseline characteristics according to different levels of serum phosphorus among patients with coronary heart disease
| Characteristics | Serum phosphorus (mmol/L) | P value | |||
|---|---|---|---|---|---|
| ≤1.01 | 1.02–1.15 | 1.16–1.29 | >1.29 | ||
| Male (%) | 78.8 | 67.3 | 56.6 | 48.6 | <0.001 |
| Age (years) | 64.8 (0.39) | 65.2 (0.39) | 64.5 (0.39) | 62.2 (0.39) | <0.001 |
| Serum calcium (mmol/L) | 2.24 (0.01) | 2.27 (0.01) | 2.29 (0.01) | 2.29 (0.01) | <0.001 |
| Body mass index (kg/m2) | 23.8 (0.12) | 24.0 (0.12) | 24.1 (0.12) | 24.1 (0.12) | 0.19 |
| Systolic blood pressure (mmHg) | 134 (0.77) | 136 (0.77) | 136 (0.78) | 136 (0.78) | 0.26 |
| Diastolic blood pressure (mmHg) | 77.6 (0.45) | 78.0 (0.45) | 78.3 (0.45) | 78.5 (0.45) | 0.46 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.93 (0.03) | 2.97 (0.03) | 3.04 (0.04) | 3.01 (0.04) | 0.12 |
| High-density lipoprotein cholesterol (mmol/L) | 1.11 (0.01) | 1.16 (0.01) | 1.18 (0.01) | 1.13 (0.01) | <0.001 |
| Triglycerides (mmol/L) | 1.71 (0.05) | 1.71 (0.05) | 1.85 (0.05) | 2.12 (0.05) | <0.001 |
| Total cholesterol (mmol/L) | 4.66 (0.04) | 4.75 (0.04) | 4.90 (0.04) | 4.88 (0.04) | <0.001 |
| Fasting glucose (mmol/L) | 6.82 (0.10) | 6.55 (0.10) | 6.49 (0.10) | 6.57 (0.10) | 0.10 |
| Estimated glomerular filtration rate (mL/min per 1.73 m2, %) | <0.001 | ||||
| ≥90 | 22.9 | 21.1 | 18.0 | 22.6 | |
| 60–89 | 50.6 | 52.2 | 54.0 | 44.2 | |
| 30–59 | 25.4 | 24.8 | 27.4 | 28.3 | |
| <30 | 1.1 | 2.0 | 0.6 | 4.9 | |
| Types of coronary heart disease (%) | <0.001 | ||||
| Acute coronary syndrome | 64.3 | 54.0 | 52.2 | 56.4 | |
| Stable coronary heart disease | 35.7 | 46.0 | 47.8 | 43.6 | |
| Married (%) | 74.5 | 79.8 | 78.9 | 78.5 | 0.051 |
| Years of education (%) | 0.89 | ||||
| ≤9 | 65.7 | 66.5 | 69.4 | 67.8 | |
| 10–12 | 17.2 | 16.0 | 14.7 | 15.9 | |
| ≥13 | 17.2 | 17.5 | 15.9 | 16.3 | |
| Smoking (%) | <0.001 | ||||
| Never | 54.4 | 60.8 | 64.2 | 67.2 | |
| Past | 11.2 | 11.3 | 9.9 | 7.0 | |
| Current | 34.4 | 27.9 | 25.9 | 28.5 | |
| Alcohol drinking (%) | 0.001 | ||||
| Never | 78.9 | 84.4 | 86.0 | 86.9 | |
| Past | 6.1 | 4.8 | 3.7 | 2.8 | |
| Current | 15.1 | 10.8 | 10.2 | 10.3 | |
| Leisure-time physical activity (%) | 0.002 | ||||
| None | 19.0 | 17.3 | 13.5 | 13.8 | |
| <30 min/day | 11.8 | 8.7 | 10.8 | 10.6 | |
| ≥30 min/day | 24.8 | 20.6 | 22.3 | 20.6 | |
| No data | 44.4 | 53.4 | 53.3 | 55.1 | |
| History of diseases (%) | |||||
| Hypertension | 78.7 | 76.0 | 77.3 | 74.2 | 0.18 |
| Diabetes | 23.8 | 21.7 | 23.4 | 24.5 | 0.59 |
| Uses of medications before admission (%) | |||||
| Antihypertensive medication | 47.7 | 51.9 | 53.5 | 49.7 | 0.12 |
| Glucose-lowering medication | 15.9 | 15.1 | 17.6 | 18.8 | 0.22 |
| Lipid-lowering medication | 9.9 | 10.7 | 10.2 | 12.6 | 0.39 |
| Antiplatelet medication | 16.7 | 14.4 | 16.4 | 15.0 | 0.56 |
The values are mean (SE) or percentage. Continuous variables are adjusted for age, except for age
During a mean follow-up period of 4.9 years, 295 deaths were identified, 193 of which were due to CVD. Multivariable-adjusted hazard ratios (age, gender, marital status, education, smoking, alcohol consumption, leisure-time physical activity, types of CHD, eGFR, HDL-C, TG, history of diabetes, SBP, BMI, use of antihypertensive drugs, use of anti-diabetic drugs, use of lowering-lipid drugs, and use of anti-platelet drugs) associated with different levels of baseline serum calcium (<2.18 (reference group), 2.18–2.27, 2.28–2.36, and ≥2.37 mmol/L) were 1.00, 0.72, 0.46, and 0.57 (Ptrend < 0.001) for all-cause mortality, and 1.00, 0.75, 0.32, and 0.50 (Ptrend < 0.001) for cardiovascular mortality, respectively (Table 3). Compared with CHD patients with the lowest serum phosphorus levels (≤1.01 mmol/L) at baseline, those with the highest baseline serum phosphorus (>1.29 mmol/L) had 1.86 times (95% confidence interval (CI) 1.32–2.63) and 1.62 times (95% CI 1.06–2.48) risks of all-cause and cardiovascular mortality, respectively.
Table 3.
Hazard ratios of all-cause and cardiovascular mortality according to different levels of serum calcium and phosphorus among patients with coronary heart disease
| Variable | No. of death | Person-years | Hazard ratios (95% confidence intervals) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Cardiovascular mortality | ||||||||
| Total | CVD | Model 1a | Model 2b | Model 3c | Model 1a | Model 2b | Model 3c | ||
| Calcium by quartile | |||||||||
| 1 (<2.18 mmol/L) | 125 | 88 | 3850 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 (2.18–2.27 mmol/L) | 82 | 58 | 4075 | 0.69 (0.52–0.91) | 0.72 (0.54–0.96) | 0.72 (0.54–0.96) | 0.70 (0.50–0.97) | 0.75 (0.53–1.05) | 0.75 (0.53–1.06) |
| 3 (2.28–2.36 mmol/L) | 39 | 18 | 3801 | 0.39 (0.27–0.56) | 0.46 (0.32–0.67) | 0.47 (0.32–0.68) | 0.26 (0.16–0.44) | 0.32 (0.19–0.54) | 0.32 (0.19–0.54) |
| 4 (≥2.37 mmol/L) | 49 | 29 | 3919 | 0.51 (0.36–0.71) | 0.57 (0.40–0.82) | 0.57 (0.40–0.81) | 0.44 (0.29–0.67) | 0.50 (0.32–0.79) | 0.50 (0.32–0.79) |
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| 1 mmol/L increase in calcium (continuous variable) | 295 | 193 | 15,645 | 0.22 (0.13–0.37) | 0.27 (0.14–0.51) | 0.33 (0.18–0.61) | 0.19 (0.10–0.36) | 0.26 (0.12–0.54) | 0.30 (0.14–0.64) |
| Phosphorus by quartile | |||||||||
| 1 (≤1.01 mmol/L) | 71 | 52 | 3951 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 (1.02–1.15 mmol/L) | 74 | 46 | 3903 | 1.10 (0.79–1.52) | 1.28 (0.92–1.78) | 1.29 (0.93–1.80) | 0.95 (0.64–1.41) | 1.07 (0.71–1.61) | 1.08 (0.72–1.62) |
| 3 (1.16–1.29 mmol/L) | 72 | 48 | 3874 | 1.22 (0.87–1.70) | 1.41 (1.00–1.98) | 1.49 (1.06–2.10) | 1.15 (0.77–1.72) | 1.35 (0.90–2.03) | 1.45 (0.96–2.19) |
| 4 (>1.29 mmol/L) | 78 | 47 | 3916 | 1.59 (1.14–2.22) | 1.86 (1.32–2.63) | 1.86 (1.32–2.63) | 1.40 (0.93–2.10) | 1.62 (1.06–2.48) | 1.63 (1.07–2.48) |
| Ptrend | 0.006 | <0.001 | <0.001 | 0.091 | 0.018 | 0.013 | |||
| 1 mmol/L increase in phosphorus (continuous variable) | 295 | 193 | 15,645 | 2.65 (1.75–4.00) | 2.56 (1.75–3.74) | 2.29 (1.58–3.33) | 2.82 (1.70–4.68) | 2.60 (1.65–4.08) | 2.32 (1.49–3.62) |
Adjusted for age and sex
Adjusted for age, sex, marriage, education, smoking, alcohol consumption, leisure-time physical activity, types of coronary heart disease, estimated glomerular filtration rate, high-density lipoprotein cholesterol, triglycerides, history of diabetes, systolic blood pressure, body mass index, use of antihypertensive drugs, use of anti-diabetic drugs, use of lowering-lipid drugs, and use of anti-platelet drugs
Adjusted for variables in model 2 and also for serum calcium in the analyses of serum phosphorus or serum phosphorus in the analyses of calcium
When baseline serum phosphorus and calcium were considered as a continuous variable using restricted cubic splines in Cox models, we observed that the nadirs of the association of phosphorus and calcium with the risk of mortality were at 0.8–1.00 mmol/L of serum phosphorus and 2.3–2.5 mmol/L of serum calcium, respectively (Fig. 1). Phosphorus levels appeared to have a positive association with the risk of all-cause mortality and cardiovascular mortality (both P for non-linear >0.05). When serum calcium was <2.3 mmol/L, lower serum calcium levels were associated with a higher risk of mortality; the spline slightly increased when the calcium levels were higher than 2.5 mmol/L (both P for non-linear <0.05) (Fig. 1). Multivariable-adjusted hazard ratios for each 1 mmol/L increase in serum phosphorus were 2.56 (95% CI 1.75–3.74) for all-cause mortality and 2.60 (95% CI 1.65–4.08) for cardiovascular mortality; each 1 mmol/L increase in serum calcium contributed to about 70% decreased risks of all-cause mortality (HR 0.27, 95% CI 0.14–0.51) and cardiovascular mortality (HR 0.26, 95% CI 0.12–0.54) (Table 3).
Fig. 1.
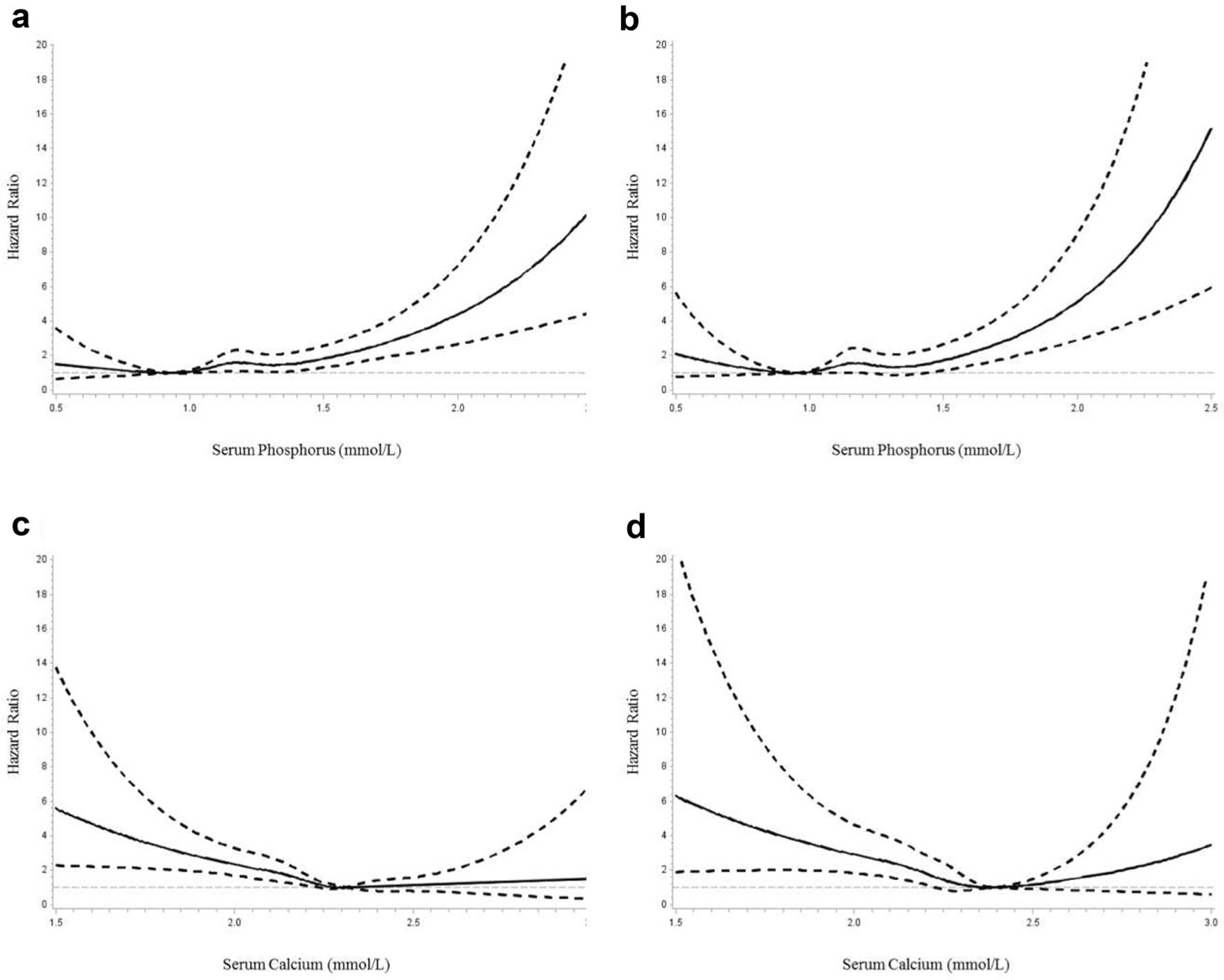
Spline plots displaying the risk of all-cause and cardiovascular mortality over the range of serum phosphorus and calcium. a HR for all-cause mortality by baseline serum phosphorus. b HR for cardiovascular mortality by baseline serum phosphorus. c HR for all-cause mortality by baseline serum calcium. d HR for cardiovascular mortality by baseline serum calcium. Adjusted for age, gender, body mass index, systolic blood pressure, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, history of diabetes, education, marriage, leisure-time physical activity, smoking, alcohol drinking, type of coronary heart disease, use of antihypertensive drugs, use of anti-diabetic drugs, use of lowering-lipid drugs and use of anti-platelet drugs. Dashed lines represented 95% CI
When we stratified samples by sex, age, BMI, eGFR, type of CHD, history of hypertension, and history of diabetes, the inverse association of baseline serum calcium with risk of mortality was still significant in almost all subgroups. There was no effect modification to the association between lower calcium and an increased death risk according to different sexes, BMI, eGFR, type of CHD and history of diabetes. There were significant interactions of different ages and serum calcium with the risk of all-cause death, as well as history of hypertension and serum calcium with the risks of all-cause and cardiovascular mortality (Table 4). In addition, the significant interactions of sex, age, BMI, type of CHD, history of hypertension, history of diabetes and serum phosphorous with the risks of all-cause and cardiovascular mortality were found. The significant positive association between serum phosphorous and the risks of all-cause and cardiovascular mortality were present only in men, in CHD patients older than 65 years, in normal weight patients, in acute CHD patients only, and in patients without the history of either hypertension or diabetes (Table 4).
Table 4.
Hazard ratios of all-cause and cardiovascular mortality according to different levels of serum phosphorus and calcium by various subgroups
| Characteristics | Hazard ratios (95% confidence intervals) for phosphorus* | Hazard ratios (95% confidence intervals) for calcium* | ||||||
|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Cardiovascular mortality | All-cause mortality | Cardiovascular mortality | |||||
| Q4 vs. Q1 | P trend | Q4 vs. Q1 | P trend | Q4 vs. Q1 | P trend | Q4 vs. Q1 | P trend | |
| Gendera,b | ||||||||
| Male | 2.36 (1.55–3.60) | <0.001 | 2.00 (1.21–3.31) | 0.006 | 0.59 (0.37–0.93) | 0.002 | 0.60 (0.35–1.04) | 0.005 |
| Female | 1.14 (0.62–2.09) | 0.74 | 0.98 (0.43–2.22) | 0.96 | 0.58 (0.32–1.02) | 0.032 | 0.38 (0.17–0.86) | 0.006 |
| Age (years)a,c | ||||||||
| ≥65 | 1.62 (1.09–2.41) | 0.006 | 1.47 (0.90–2.40) | 0.046 | 0.57 (0.39–0.84) | 0.001 | 0.47 (0.29–0.77) | <0.001 |
| <65 | 2.36 (1.10–5.03) | 0.062 | 1.59 (0.67–3.76) | 0.58 | 0.34 (0.14–0.80) | 0.002 | 0.34 (0.11–1.02) | 0.017 |
| BMI (kg/m2)a,b | ||||||||
| ≥25 | 0.87 (0.45–1.68) | 0.61 | 0.66 (0.28–1.52) | 0.26 | 0.39 (0.19–0.80) | 0.006 | 0.35 (0.15–0.84) | 0.003 |
| <25 | 2.21 (1.46–3.36) | <0.001 | 1.99 (1.20–3.31) | 0.003 | 0.73 (0.49–1.11) | 0.029 | 0.62 (0.36–1.05) | 0.017 |
| eGFR (mL/min per 1.73 m2) | ||||||||
| ≥60 | 2.04 (1.26–3.31) | 0.013 | 1.62 (0.90–2.93) | 0.19 | 0.74 (0.47–1.16) | 0.030 | 0.62 (0.35–1.08) | 0.007 |
| <60 | 1.60 (0.96–2.67) | 0.052 | 1.60 (0.85–3.04) | 0.11 | 0.49 (0.27–0.88) | 0.015 | 0.47 (0.22–1.02) | 0.038 |
| Type of CHDb | ||||||||
| Acute | 1.73 (1.13–2.67) | 0.023 | 1.96 (1.17–3.29) | 0.011 | 0.56 (0.35–0.90) | 0.003 | 0.44 (0.24–0.81) | 0.001 |
| Stable | 1.44 (0.77–2.69) | 0.15 | 0.48 (0.20–1.15) | 0.24 | 0.73 (0.42–1.26) | 0.15 | 0.71 (0.35–1.43) | 0.16 |
| History of HBPa,b,c,d | ||||||||
| No | 4.13 (1.66–10.3) | 0.005 | 4.17 (1.34–13.0) | 0.019 | 2.04 (0.77–5.40) | 0.18 | 1.57 (0.42–5.86) | 0.67 |
| Yes | 1.58 (1.08–2.31) | 0.012 | 1.35 (0.84–2.17) | 0.11 | 0.48 (0.33–0.71) | <0.001 | 0.45 (0.27–0.73) | <0.001 |
| History of DMb | ||||||||
| No | 2.18 (1.40–3.39) | <0.001 | 1.96 (1.12–3.42) | 0.008 | 0.56 (0.35–0.88) | 0.002 | 0.43 (0.23–0.81) | 0.001 |
| Yes | 1.42 (0.79–2.57) | 0.28 | 1.18 (0.58–2.38) | 0.71 | 0.62 (0.35–1.12) | 0.053 | 0.61 (0.30–1.23) | 0.048 |
BMI body mass index, eGFR estimated glomerular filtration rate, CHD coronary heart disease, HBP hypertension, DM diabetes
Adjusted for age, gender, marriage, education, smoking, alcohol consumption, leisure-time physical activity, types of coronary heart disease, estimated glomerular filtration rate, high-density lipoprotein cholesterol, triglycerides, history of diabetes, systolic blood pressure, body mass index, use of antihypertensive drugs, use of anti-diabetic drugs, use of lowering-lipid drugs, and use of anti-platelet drugs, other than the variable in the analysis
P value for the interactions of variables and serum phosphorus with the risk of all-cause mortality <0.05;
P value for interactions of variables and serum phosphorus with the risk of cardiovascular mortality <0.05.
P value for interactions of variables and serum calcium with the risk of all-cause mortality <0.05;
P value for the interactions of variables and serum calcium with the risk of cardiovascular mortality <0.05
The join effects of baseline serum phosphorus and calcium with the risks of all-cause and cardiovascular mortality are shown in Fig. 2. We used two categories of baseline serum phosphorus (low risk ≤1.29 and high risk >1.29 mmol/L) and serum calcium (high risk <2.18 and low risk ≥2.18 mmol/L). Compared with CHD patients with serum phosphorus at ≤1.29 mmol/L and calcium at ≥2.18 mmol/L, patients at higher level of phosphorus (>1.29 mmol/L) and lower level of calcium (<2.18 mmol/L) had a 2.46-fold risk of all-cause mortality (95% CI 1.63–3.70) and a 2.36-fold risk of cardiovascular mortality (95% CI 1.40–4.00).
Fig. 2.
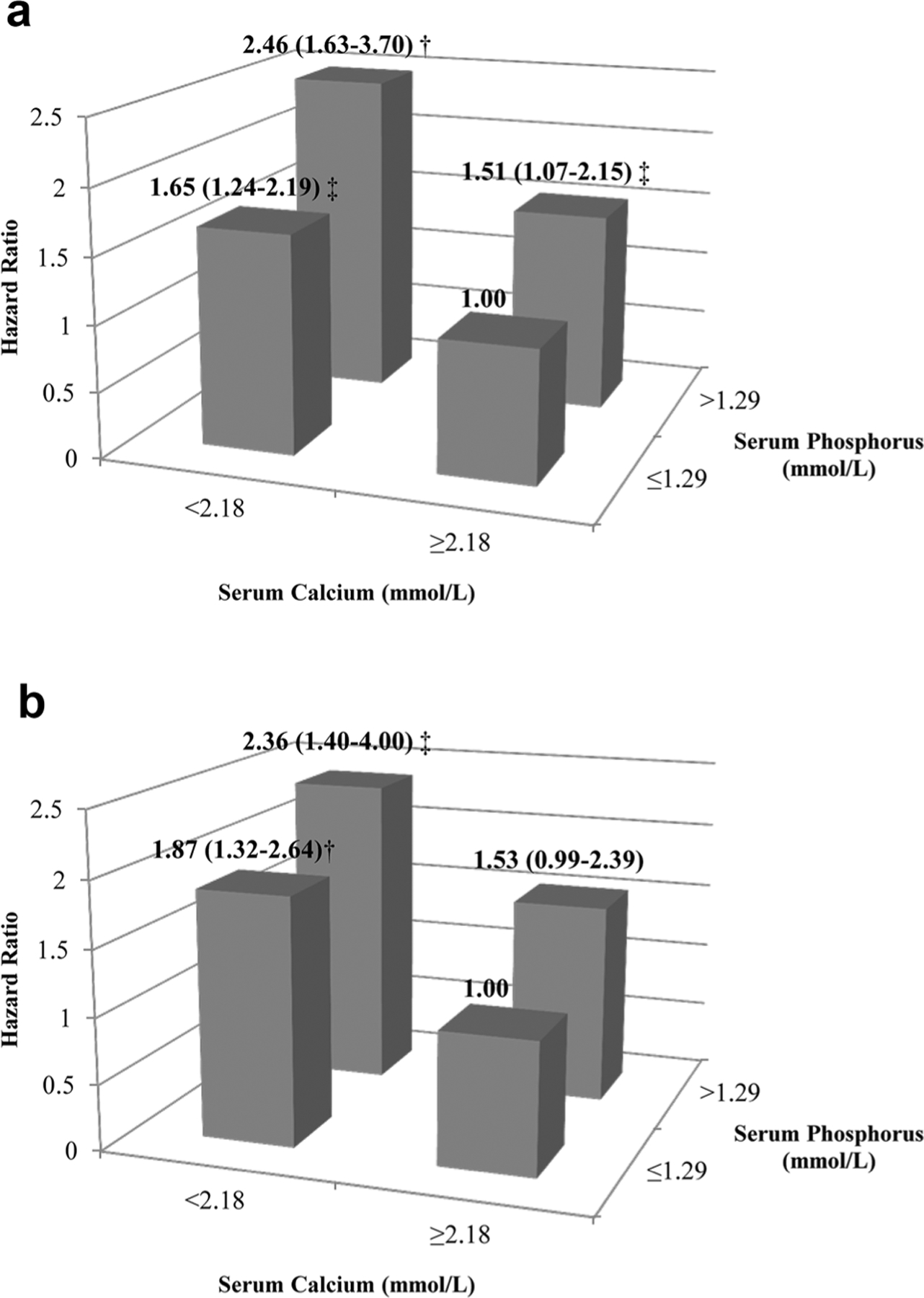
Hazard ratios of all-cause (a) and cardiovascular (b) mortality according to different levels of serum phosphorus and calcium. Adjusted for age, gender, body mass index, systolic blood pressure, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, history of diabetes, education, marriage, leisure-time physical activity, smoking, alcohol drinking, type of coronary heart disease, use of antihypertensive drugs, use of anti-diabetic drugs, use of lowering-lipid drugs and use of anti-platelet drugs. †P < 0.001; ‡P < 0.05
Discussion
To our knowledge, this is the first study to document that decreased serum calcium at baseline was associated with a long-term increased risk of all-cause and cardiovascular mortality among patients with CHD. In current study, we also found a graded positive association between baseline serum phosphorus levels and the risks of all-cause and cardiovascular mortality, especially among men with CHD.
Serum calcium and mortality
Though previous studies have shown that elevated serum calcium was a risk factor for mortality among CKD patients [19] and the general population [10]; the appearance of this positive association between serum calcium and mortality recently aroused controversy among patients with particular conditions. A meta-analysis, including 47 cohort studies (n = 327,644 patients), found no significant association between all-cause mortality and serum level of calcium among individuals with CKD [20], while several researchers have reported that reduced serum calcium levels following critical illness are associated with an increased mortality risk [21–23]. However, evidence among patients with CHD is scarce, especially in China. Two cross-sectional studies have already observed that total serum calcium levels had a negative association with the angiographic severity of CHD among stable patients [24], and low serum calcium was independently associated with left ventricular systolic dysfunction in CHD patients [12]. The present study first indicated that a low serum calcium level at baseline might be a predictor of long-term all-cause and cardiovascular mortality among CHD patients. Even after adjusting for traditional cardiometabolic risk factors and possible confounders, the inverse association was still present. In addition, in our study, high serum calcium levels (≥2.37 mmol/L) did not show an association with an increased risk of long-term mortality among patients with CHD.
Decreased serum calcium levels might imply impaired kidney function [9]. In the present study, the adjustment for eGFR, or stratifying for CHD patients as eGFR <60 or ≥60 mL/min per 1.72 m2 did not change the significant association between decreased serum calcium levels at baseline and increased risks of all-cause and cardiovascular mortality. Thus, our study suggested that a lower serum calcium level may be an independent risk factor for the prognosis of CHD rather than a surrogate marker of lower GFR. In fact, lower serum calcium could further promote the deterioration in renal function [25], which might play an adverse role in the progress of CHD, resulting in poor long-term outcomes.
Even though the mechanism of an increased risk of mortality associated with low levels of serum calcium is poorly understood, several putative mechanisms might be proposed. Decreased serum calcium levels could be detected in established CVD patients and be related to CVD severity [12, 26], which suggested that low serum calcium at baseline might make sense to have an impact on the prognosis of CHD patients. It has been assumed that a low calcium level might indicate an increased calcium consumption, partially reflect more plaques or thrombi formed and worsen coronary conditions, resulting in poor outcomes through platelet activation and/or intracellular calcium overload [25, 27, 28]. Several other studies indicated that a decreased serum calcium level was associated with secondary hyperparathyroidism, increased circulating fibroblast growth factor (FGF-23) levels, and the severity of vitamin D deficiency [25, 29], which were associated with an increased risk of mortality among patients with CHD [30, 31]. However, these clinical measurements were not available in the present study for additional analyses. Whether a decreased baseline serum calcium level is associated with an increased risk of long-term mortality and whether it could become a predictor of long-term mortality among CHD patients remain to be elucidated and still need further research.
Serum phosphorus and mortality
Most but not all studies have found an association between baseline higher serum phosphorus and an increased risk of mortality in subjects with impaired kidney function, end-stage rental disease, and in the general population with normal renal function [11, 20, 32, 33]. In agreement with these studies, this study provided additional supportive evidence that baseline serum phosphorus level was an independent predictor for the risk of mortality among patients with established CHD. The mechanisms of this positive association were complicated. A high level of phosphorus plays a critical role in multiple and diverse biological dysfunctions, such as rental deterioration [9], vascular stiffness [34] and endothelial dysfunction [35, 36], which might link to poor cardiovascular outcomes. Elevated levels of serum phosphorus would also promote coronary arteries calcification [37], which is positively correlated with atherosclerotic plaque burden and instability [4, 38, 39], and might be one of the major explanations for the relations. Previous studies indicated that higher levels of serum phosphorus might also be associated with hyperparathyroidism, which has been associated with poor survival. As described above, parathyroid hormone levels were not detected at admission, however, after adjusting for eGFR and serum calcium, which might be associated with hyperparathyroidism, an increased level of serum phosphorus was still associated with the risk of all-cause and cardiovascular mortality. Gender heterogeneity in serum phosphorus with the risk of mortality was observed in the present study. The positive association between serum phosphorus and the risks of all-cause and cardiovascular mortality was only seen in men but not in women, which was consistent with one previous study included 92,756 individuals without kidney disease [11]. Higher levels of serum phosphorus were found in men, which might be partially due to the sex hormone (mainly estradiol) production [40, 41].
There are several limitations in the present study. First, our study did not have data on some related risk factors which were not typically clinical measurements for CHD patients, such as FGF-23 and parathyroid hormone. Second, we only measured the serum calcium and phosphorus at baseline and did not measure them during the follow-up. Third, the causal link between baseline serum calcium and long-term mortality, and the related mechanism have not been illuminated in the present study. Based on the limitations above, our findings may need to be further confirmed by large RCTs or cohorts.
In conclusion, we found an inverse association between baseline serum calcium and death risk, and a positive association between baseline serum phosphorus and the risk of mortality among Chinese patients with CHD.
Acknowledgements
This study was supported by the State Key Program of National Natural Science Foundation of China (Grant 81130052 and Grant 81402671). Qian Chen is supported by International Program for Ph.D. Candidates, Sun Yat-sen University.
Footnotes
Conflict of interest The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.World Health Organization (2014) Global status report on non-communicable diseases 2014 [Google Scholar]
- 2.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S (2007) Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med 356(23):2388–2398. doi: 10.1056/NEJMsa053935 [DOI] [PubMed] [Google Scholar]
- 3.Critchley J, Liu J, Zhao D, Wei W, Capewell S (2004) Explaining the increase in coronary heart disease mortality in Beijing between 1984 and 1999. Circulation 110(10):1236–1244 [DOI] [PubMed] [Google Scholar]
- 4.Giachelli CM (2004) Vascular calcification mechanisms. J Am Soc Nephrol 15(12):2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4 [DOI] [PubMed] [Google Scholar]
- 5.Martin M, Valls J, Betriu A, Fernandez E, Valdivielso JM (2015) Association of serum phosphorus with subclinical atherosclerosis in chronic kidney disease. Sex makes a difference. Atherosclerosis 241(1):264–270. doi: 10.1016/j.atherosclerosis.2015.02.048 [DOI] [PubMed] [Google Scholar]
- 6.Ruan L, Chen W, Srinivasan SR, Xu J, Toprak A, Berenson GS (2010) Relation of serum phosphorus levels to carotid intima–media thickness in asymptomatic young adults (from the Bogalusa Heart Study). Am J Cardiol 106(6):793–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabanayagam C, Shankar A (2011) Serum calcium levels and hypertension among U.S. adults. J Clin Hypertens (Greenwich) 13(10):716–721. doi: 10.1111/j.1751-7176.2011.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR (2009) Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 4(3):609–615. doi: 10.2215/cjn.04100808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarz S, Trivedi BK, Kalantar-Zadeh K, Kovesdy CP (2006) Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin J Am Soc Nephrol 1(4):825–831. doi: 10.2215/CJN.02101205 [DOI] [PubMed] [Google Scholar]
- 10.Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, Sundstrom J (2010) Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol 30(2):333–339. doi: 10.1161/ATVBAHA.109.196675 [DOI] [PubMed] [Google Scholar]
- 11.Yoo KD, Kang S, Choi Y, Yang SH, Heo NJ, Chin HJ, Oh KH, Joo KW, Kim YS, Lee H (2016) Sex, age, and the association of serum phosphorus with all-cause mortality in adults with normal kidney function. Am J Kidney Dis 67(1):79–88. doi: 10.1053/j.ajkd.2015.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Ma H, Hao X, Yang J, Chen Q, Lu L, Zhang R (2016) Low serum calcium is associated with left ventricular systolic dysfunction in a Chinese population with coronary artery disease. Sci Rep 6:22283. doi: 10.1038/srep22283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding D, Qiu J, Li X, Li D, Xia M, Li Z, Su D, Wang Y, Zhang Y, Zhang J, Lv X, Xiao Y, Hu G, Ling W (2014) Hyperglycemia and mortality among patients with coronary artery disease. Diabetes Care 37(2):546–554. doi: 10.2337/dc13-1387 [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, Zhang Y, Ding D, Li D, Xia M, Li X, Yang Y, Li Q, Hu G, Ling W (2016) Metabolic syndrome and its individual components with mortality among patients with coronary heart disease. Int J Cardiol 224:8–14. doi: 10.1016/j.ijcard.2016.08.324 [DOI] [PubMed] [Google Scholar]
- 15.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O’Rourke RA, Schafer WP, Williams SV (1999) ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation 99(21):2829–2848 [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO, Smith SC Jr (2000) ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation 102(10):1193–1209 [DOI] [PubMed] [Google Scholar]
- 17.WHO Monica Project Principal Investigators (1988) The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol 41(2):105–114 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4):247–254 [DOI] [PubMed] [Google Scholar]
- 19.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK (2008) Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 52(3):519–530. doi: 10.1053/j.ajkd.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 20.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF (2011) Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305(11):1119–1127. doi: 10.1001/jama.2011.308 [DOI] [PubMed] [Google Scholar]
- 21.Cherry RA, Bradburn E, Carney DE, Shaffer ML, Gabbay RA, Cooney RN (2006) Do early ionized calcium levels really matter in trauma patients? J Trauma 61(4):774–779. doi: 10.1097/01.ta.0000239516.49799.63 [DOI] [PubMed] [Google Scholar]
- 22.Sauter TC, Lindner G, Ahmad SS, Leichtle AB, Fiedler GM, Exadaktylos AK, Haider DG (2015) Calcium disorders in the emergency department: independent risk factors for mortality. PLoS One 10(7):e0132788. doi: 10.1371/journal.pone.0132788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, Kalantar-Zadeh K (2010) Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol 32(5):403–413. doi: 10.1159/000319861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narang R, Ridout D, Nonis C, Kooner JS (1997) Serum calcium, phosphorus and albumin levels in relation to the angiographic severity of coronary artery disease. Int J Cardiol 60(1):73–79 [DOI] [PubMed] [Google Scholar]
- 25.Lim LM, Kuo HT, Kuo MC, Chiu YW, Lee JJ, Hwang SJ, Tsai JC, Hung CC, Chen HC (2014) Low serum calcium is associated with poor renal outcomes in chronic kidney disease stages 3–4 patients. BMC Nephrol 15:183. doi: 10.1186/1471-2369-15-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ising H, Gunther T, Bertschat F, Ibe K, Stoboy V, Heldman E (1987) Alterations of electrolytes in serum and erythrocytes after myocardial infarction. Magnesium 6(4):192–200 [PubMed] [Google Scholar]
- 27.Varga-Szabo D, Braun A, Nieswandt B (2009) Calcium signaling in platelets. J Thromb Haemost 7(7):1057–1066. doi: 10.1111/j.1538-7836.2009.03455.x [DOI] [PubMed] [Google Scholar]
- 28.Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA (2010) Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic RIsk Progression (TRIP) study. Platelets 21(5):360–367. doi: 10.3109/09537100903548903 [DOI] [PubMed] [Google Scholar]
- 29.Bilezikian JP, Khan A, Potts JT Jr, Brandi ML, Clarke BL, Sho-back D, Juppner H, D’Amour P, Fox J, Rejnmark L, Mosekilde L, Rubin MR, Dempster D, Gafni R, Collins MT, Sliney J, Sanders J (2011) Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res 26(10):2317–2337. doi: 10.1002/jbmr.483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH (2010) The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med 152(10):640–648. doi: 10.7326/0003-4819-152-10-201005180-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W (2008) Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med 168(12):1340–1349. doi: 10.1001/archinte.168.12.1340 [DOI] [PubMed] [Google Scholar]
- 32.Bellasi A, Mandreoli M, Baldrati L, Corradini M, Di Nicolo P, Malmusi G, Santoro A (2011) Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 6(4):883–891. doi: 10.2215/cjn.07810910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, Shafi T, Coresh J, Powe NR, Melamed ML (2015) Race, mineral homeostasis and mortality in patients with end-stage renal disease on dialysis. Am J Nephrol 42(1):25–34. doi: 10.1159/000438999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendrick J, Ix JH, Targher G, Smits G, Chonchol M (2010) Relation of serum phosphorus levels to ankle brachial pressure index (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 106(4):564–568. doi: 10.1016/j.amjcard.2010.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau WL, Pai A, Moe SM, Giachelli CM (2011) Direct effects of phosphate on vascular cell function. Adv Chronic Kidney Dis 18(2):105–112. doi: 10.1053/j.ackd.2010.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuto E, Taketani Y, Tanaka R, Harada N, Isshiki M, Sato M, Nashiki K, Amo K, Yamamoto H, Higashi Y, Nakaya Y, Takeda E (2009) Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 20(7):1504–1512. doi: 10.1681/asn.2008101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA (2009) Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20(2):397–404. doi: 10.1681/asn.2008020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke AP, Taylor A, Farb A, Malcom GT, Virmani R (2000) Coronary calcification: insights from sudden coronary death victims. Z Kardiol 89(Suppl 2):49–53 [DOI] [PubMed] [Google Scholar]
- 39.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS (1998) Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 31(1):126–133 [DOI] [PubMed] [Google Scholar]
- 40.Onufrak SJ, Bellasi A, Cardarelli F, Vaccarino V, Muntner P, Shaw LJ, Raggi P (2009) Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol 169(1):67–77. doi: 10.1093/aje/kwn285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cirillo M, Ciacci C, De Santo NG (2008) Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med 359(8):864–866. doi: 10.1056/NEJMc0800696 [DOI] [PubMed] [Google Scholar]


