Abstract
Background
Cardiovascular disease and cancer remain the most prevalent conditions worldwide. The relationship between the two is becoming increasingly recognized, with both sharing similar risk factors. Currently, there are no guidelines or substantial data for the management of this subset of patients. This case presents the management of a patient with advanced malignancy and ischaemic cardiomyopathy, exploring the difficult decision making process for revascularization, ensuring an individualized approach is used for each patient.
Case summary
A 68-year-old man with Stage II lung cancer and overall poor prognosis with multiple comorbidities limiting his functional status presented with a non-ST elevation myocardial infarction and ischaemic cardiomyopathy due to extrinsic tumour compression and left internal mammary artery graft-obtuse marginal fibrosis due to chest wall radiation. He had a prolonged admission with the heart team discussions regarding optimal management. He subsequently underwent percutaneous coronary intervention to his native left circumflex chronic total occlusion. He was discharged home on dual antiplatelet and heart failure therapy.
Discussion
This case reflects that the management of patients with cancer and coronary artery disease is complex. Many factors including patient comorbidities adversely impact on prognosis, and treatment goals need to be clearly defined. The evidence in the area is lacking but continues to grow; however, the care for such patients needs to be individualized.
Keywords: PCI, LIMA, Tumor, Ischaemic cardiomyopathy, Case report
Learning points.
Cancer survivors have a three-fold increased risk of myocardial infarction.
Occurrence of acute coronary syndromes in cancer patients (whether in remission or not) has a large impact on quality of life, hospital admissions, and accelerated adverse outcomes (such as death).
No major evidence base to guide revascularization in cancer patients as they are largely excluded from clinical trials and only limited registry data are available.
Percutaneous coronary intervention is a preferred revascularization modality especially in palliative patients, but this decision should be individualized.
Primary specialties involved
Cardiology, cardiothoracic surgery, critical care, medical oncology.
Introduction
The relationship between ischaemic heart disease and cancer is becoming increasingly recognized with both sharing similar risk factors and pathogenesis of inflammation and oxidative stress.1 Ongoing development in cancer diagnosis and treatment has improved mortality rates, also increasing the number of patients with cancer that develop coronary artery disease. Cancer, in itself, is a cardiovascular risk factor, with survivors having a three-fold increase in 6-month cumulative incidence of myocardial infarction.2 Currently, there are no guidelines or substantial data for the management of this subset of patients as they have largely been excluded from clinical trials and national registries. However, as the number of cancer survivors or those undergoing active treatment increases, so does the frequency of percutaneous coronary intervention (PCI) performed on these patients.
Timeline
| Date | Significant event |
|---|---|
| May 23, 2021 | Presentation to the Emergency Department with Type 2 respiratory failure and chest pain. Admitted to ICU and commenced on milrinone, dual antiplatelet therapy, intravenous heparin, and antibiotics |
| May 24, 2021 | TTE showed severe left ventricular dysfunction with akinesis of the anterior wall |
| June 1, 2021 | CT chest showed tumour encasing the LIMA-OM graft |
| June 4, 2021 | Diagnostic coronary angiogram showed diffuse segment of disease in the LIMA-OM graft with a mid-segment 99% lesion |
| June 11, 2021 | PCI to the native LCx CTO |
| June 15, 2021 | Discharged home after optimization of heart failure therapy |
Case presentation
A 68-year-old man presented with Type 2 respiratory failure after several days of increasing dyspnoea and pleuritic chest pain. He had left upper lobe Stage II squamous cell lung carcinoma diagnosed 1 year ago, for which he had completed 20 fractions of 55 Gy radiotherapy 6 months before. He was considered unsuitable for chemotherapy with cisplatin/paclitaxel due to his poor Eastern Cooperative Oncology Group performance status, and history of coronary bypass grafting [left internal mammary artery (LIMA)-obtuse marginal (OM), saphenous vein graft (SVG)-OM, SVG-right posterior descending artery (RPDA)] 7 years prior, poorly controlled diabetes, endovascular aneurysm repair for abdominal aortic aneurysm, hearing loss, peripheral neuropathy, and voice hoarseness due to tumour involvement of the recurrent laryngeal nerve. Transthoracic echocardiogram 1-year prior had shown normal left ventricular (LV) systolic function with abnormal septal motion.
Initial workup
Investigations on admission showed elevated serum creatinine of 151 µmol/L, eGFR of 46 mL/min/1.73 m2, and C-reactive protein of 35 mg/L. Serum high sensitivity troponin T measured 4 h apart was 134 ng/L (normal <14 ng/L) and 210 ng/L, peaking at 334 ng/L; consistent with non-ST elevated myocardial infarction (NSTEMI). An electrocardiogram showed sinus rhythm and pre-existing left bundle branch block. Chest X-ray showed left lower zone consolidation and pulmonary oedema. Transthoracic echocardiogram showed normal LV size with moderate–severe systolic dysfunction. Notably, akinesis of the whole anterior and anterolateral wall was seen. He was admitted to the intensive care unit for non-invasive ventilation, inotropic support with milrinone, intravenous diuretics, and intravenous antibiotic therapy for community-acquired pneumonia. He was also commenced on dual antiplatelet therapy and an intravenous heparin infusion for management of an NSTEMI. Chest computerized tomography (CT) showed an infiltrating mass of 10 cm × 6.5 cm in size invading the left hilum (Figure 1). There was a severe narrowing of the left upper lobe bronchus with complete occlusion of the small airways supplying the anterior portion of the lobe. Coronary angiography via the left radial artery showed a right dominant circulation, a discretely diseased left main coronary artery (LMCA) with 70% stenosis, minor diffuse disease of the left anterior descending artery (LAD), diffusely diseased non-dominant left circumflex artery (LCx) with 100% proximal chronic total occlusion (CTO) which had a multicentre CTO Registry of Japan score (J-CTO) score of 3 (calcification, length >20 mm and ambiguous cap), proximal dominant right coronary artery occlusion with patent SVG to the RPDA (Figure 2). The LIMA graft to the OM artery had a long segment of diffuse disease with a 99% lesion in the mid-portion (Figure 3). Fractional flow reserve (FFR) to the LMCA and proximal LAD was 0.80 and LMCA minimal luminal area (MLA) was 5.5 mm2 and was unlikely to be ischaemic.
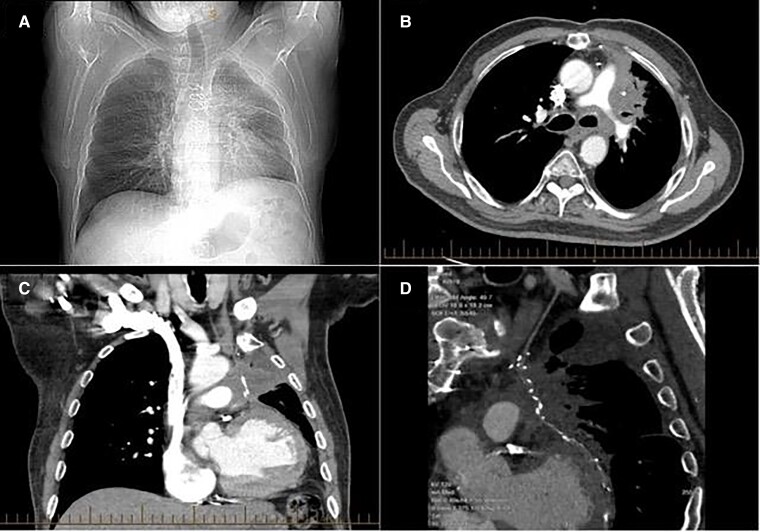
Figure 1.
Chest X-ray and computerized tomography chest at admission demonstrating infiltrating mass in the left lung. The chest X-ray in (A) demonstrates hyperinflation of the lungs with increased peribronchial markings in the left lung. The computerized tomography demonstrates in panel (B), an infiltrative irregular and speculated mass in the left hilum measuring ∼10 cm × 6.5 cm. There is peribronchovascular thickening and compression of the left upper lobe bronchus with complete occlusion of the small airways. There is involvement of the left pleura (B) and (C). The mass is encasing the left internal mammary artery graft, with resultant extrinsic compression (C) and (D).
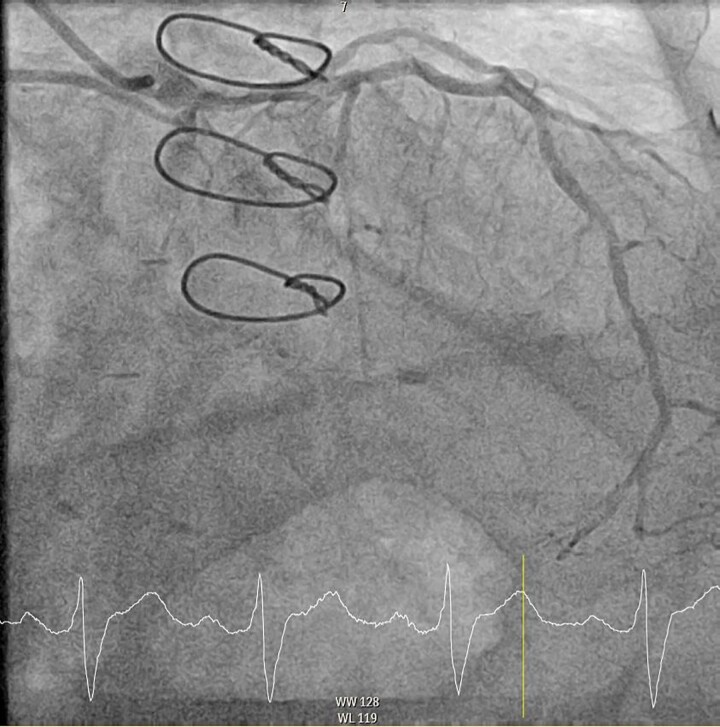
Figure 2.
Diagnostic coronary angiogram demonstrating the left circumflex artery chronic total occlusion. These RAO −27.0° and Caudal −35.4° coronary angiography images show the sternotomy wires from prior coronary artery bypass surgery, 70% distal stenosis of the left main coronary artery, minor diffuse disease of the left anterior descending artery, and an occluded proximal chronic total occlusion of the left circumflex artery.
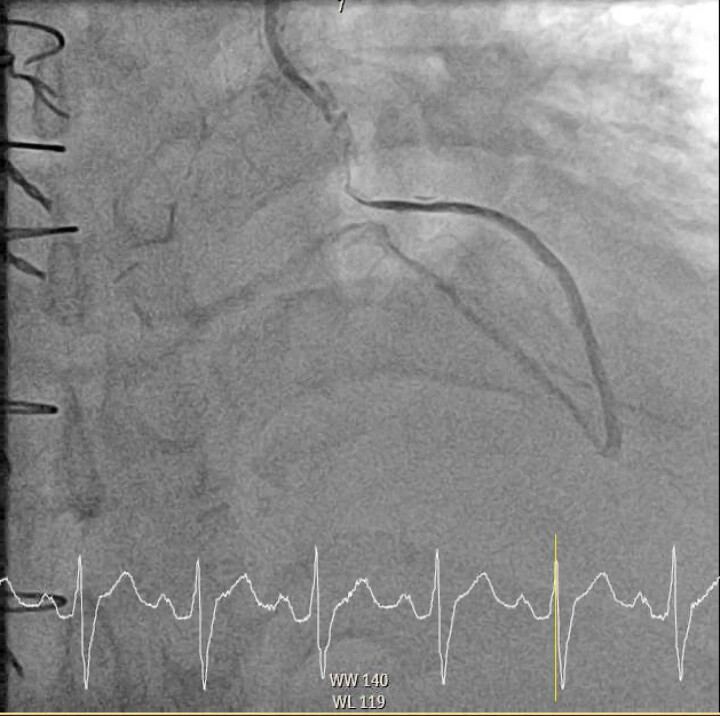
Figure 3.
Diagnostic coronary angiogram demonstrating compression of the left internal mammary artery to obtuse marginal graft. This is a LAO 2.8° and cranial 27.2° view with the left internal mammary artery graft selectively engaged. There is a diffuse segment of flow-limiting disease in the mid-section of the left internal mammary artery to obtuse marginal graft with flow distal to the lesion, secondary to extrinsic compression of the tumour. There is no atherosclerotic disease in the rest of the graft.
Diagnosis and management
Left internal mammary artery grafts are generally resistant to atherosclerosis and the appearance of the mid-LIMA lesion raised the suspicion of extrinsic tumour compression. Computerized tomography chest confirmed recurrent lung neoplasm encasing and compressing the mid-LIMA graft. It is unclear as to how much tissue fibrosis secondary to previous irradiation was contributing to the occlusive lesion. There was extensive discussion about the optimal revascularization management approach post-NSTEMI with new severe ischaemic cardiomyopathy due to extrinsic tumour compression of the LIMA graft. He was at high risk for redoing CABG, and PCI was preferred. The anterior akinesis was likely due to progression of LMCA disease, but PCI was not required given negative FFR and adequate MLA. A differential cause for LV dysfunction due to the direct effects of radiotherapy was considered. Stenting of the mid-LIMA graft lesion was thought to have a low success, given the ability for rapid tumour regrowth leading to re-occlusion. An alternate approach with a covered stent would be prone to thrombotic occlusion. Not only was he was thought to be at higher thrombotic risk from cancer but wiring and balloon dilatation of the LIMA lesion would have concomitant risks of bleeding and perforation as it is within a friable tumour with increased vascularity. The consensus opinion was that LIMA lesion stenting was a suboptimal option as rapid re-occlusion is likely due to tumour progression, based on previous clinical experience at our centre. Furthermore, the pathological mechanisms responsible for in-stent restenosis via neointimal hyperplasia are well described. However, there is little known about the mechanical factors that might contribute to stent failure.3 Anatomical factors that increase the likelihood of longitudinal stent deformation include lesion calcification, length, and vessel tortuosity.4 With an expected median survival of 18 months, PCI to the native LCx CTO lesion vs. medical therapy alone was discussed with the patient and his family who agreed to proceed with percutaneous revascularization. Chronic total occlusion -PCI was also high risk, given the J-CTO score but likely to be a more durable result if successful. He underwent intravascular ultrasound (IVUS)-guided PCI to the LCx CTO lesion 4 days later using bilateral radial artery access. Antegrade wire escalation strategy was successful with 1.7 Fx mizuki microcatheter, and IVUS showed extensive calcification and intravascular lithotripsy (C2 3.0 × 12 mm—proximal 30 pulses and mid-40 pulses) and placement of two drug-eluting stents (Orsiro 3.0 × 22 mm and 2.5 × 26 mm) (Figure 4). There were no procedural complications, and he was discharged home on aspirin and clopidogrel and heart failure therapy including beta-blocker, angiotensin receptor–neprilysin inhibitor, and mineralocorticoid receptor antagonist.
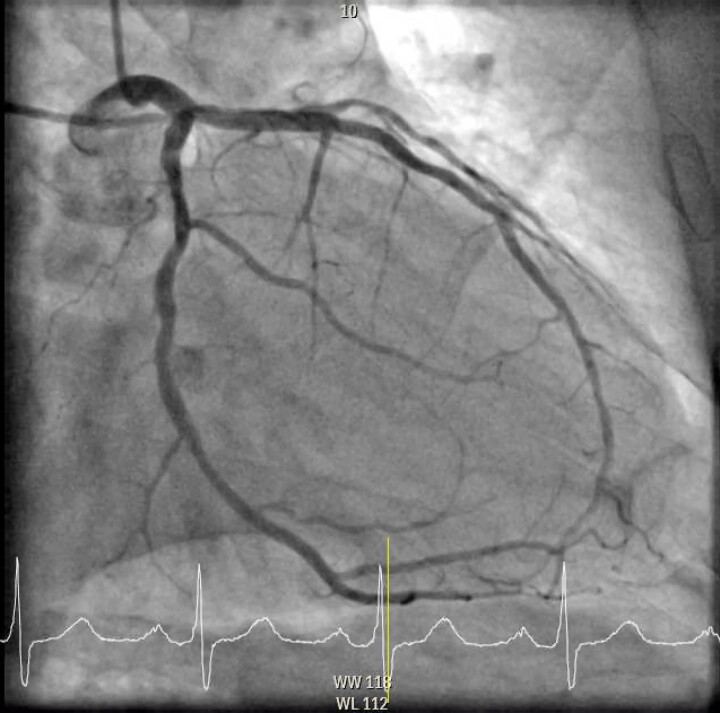
Figure 4.
Final image of left circumflex artery after the intravascular ultrasound-guided percutaneous coronary intervention of the chronic total occlusion. In the LAO 49.7° and caudal −15.0° view with engagement of the left main coronary artery showing flow restored to a revascularized proximal left circumflex artery after the intravascular ultrasound-guided percutaneous coronary intervention with intravascular lithotripsy and placement of two drug-eluting stents (Osiro 3.0 × 22 mm + 2.5 × 26 mm).
Follow-up
The patient was followed up 1 month following PCI and was limited by exertional dyspnoea primarily due to lung cancer and anaemia (Hb 78 g/L) but remained angina-free and did not require re-hospitalization.
Discussion
In general, patients with metastatic cancer have a poor prognosis post-PCI, with an increased risk of in-hospital mortality and bleeding complications despite optimal medical therapy.1,5 Overall, a conservative approach is preferred; but even in patients with a life expectancy of <1 year who present with an acute coronary syndrome, PCI should be considered, as these patients have lower mortality than those managed conservatively.6 Management of cancer patients requiring PCI mandate balancing bleeding and thrombotic risks as dual antiplatelet therapy is routine. Underlying primary or inherited thrombocytopenias and coagulopathies can restrict the use of dual antiplatelet therapy and increase the risk of bleeding post-PCI. This is balanced with the higher risk of stent thrombosis in a physiologically pro-coagulant state.7,8
The challenge for patient management is defining the goals of therapy and balancing the risks of treatment. In this case, percutaneous intervention was preferred to revascularize a terminally ill patient with recurrent lung cancer refractory to chest radiation therapy who has already had CABG. There was expected viability in the LCx territory and revascularization of the LIMA graft or native LCx lesion and their antecedent risks was complex. Left internal mammary artery graft intervention posed uncontrollable and unacceptable risks if bleeding occurred leading to haemo-mediastinum formation, which is extremely difficult to manage. Even if LIMA lesion stenting was simple, the likelihood of medium-term patency is poor. Left circumflex artery CTO-PCI with complex JCTO3 lesion was associated with higher complication risks compared with non-CTO-PCI.
Understandably, the HEART team, patient, and family discussions were extensive and prolonged. The expected benefits of PCI and revascularization are attenuated in the setting of advanced Stage II lung cancer which was the primary determinant of his prognosis, and other comorbidities limiting his function and quality of life even in the absence of angina. Hospital discharge with reduced re-admission and well-being were the primary objectives of treatment. To be a resident at home with controllable symptomatology and reduced hospitalization duration was a goal. The patient had an advanced care directive and was not for resuscitation in the event of a cardiac arrest or ventilation.
This case highlights that patients with cancer and coronary artery disease may have multiple complex issues all impacting on prognosis and clearly defining treatment goals should be a priority. Evidence in the area is lacking but continues to evolve but each patient’s care needs to be individualized.
Acknowledgements
The authors greatly appreciate Patrick Pender MBBS, James Leung MBBS, and Kishore Kaddapu MBBS, PhD for their input into the care of this patient.
Consent: The author confirms that verbal consent was obtained or submission and publication of this case report including images and associated text have been obtained from the patient and his family detailed in this case report.
Conflict of interest: All authors have no relevant disclosures.
Funding: None declared.
References
- 1. Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol 2012;35:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017;70:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenfield K, Schainfeld R, Pieczek A, Haley L, Isner JM. Restenosis of endovascular stents from stent compression. J Am Coll Cardiol 1997;29:328–338. [DOI] [PubMed] [Google Scholar]
- 4. Wiktor DM, Waldo SW, Armstrong EJ. Coronary stent failure: fracture, compression, recoil, and prolapse. Interv Cardiol Clin 2016;5:405–414. [DOI] [PubMed] [Google Scholar]
- 5. Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, De Belder MA, Kwok CS, Rashid M, Fischman DL, Mamas MA. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J 2019;40:1790–1800. [DOI] [PubMed] [Google Scholar]
- 6. Han XJ, Li JQ, Khannanova Z, Li Y. Optimal management of coronary artery disease in cancer patients. Chronic Dis Transl Med 2019;5:221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas KP, Leesar MA, Marmagkiolis K. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of India, and Sociedad Latino Americana de Cardiologıa intervencionista). Catheter Cardiovasc Interv 2016;87:E202–E223. [DOI] [PubMed] [Google Scholar]
- 8. Bottinor W. Expert analysis; PCI: risks and outcomes in patients with cancer. American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2019/05/23/22/24/pci-risks-and-outcomes-in-patients-with-cancer. [Google Scholar]