Abstract
Legumes have adaptive mechanisms that regulate nodulation in response to the amount of nitrogen in the soil. In Lotus japonicus, two NODULE INCEPTION (NIN)-LIKE PROTEIN (NLP) transcription factors, LjNLP4 and LjNLP1, play pivotal roles in the negative regulation of nodulation by controlling the expression of symbiotic genes in high nitrate conditions. Despite an improved understanding of the molecular basis for regulating nodulation, how nitrate plays a role in the signaling pathway to negatively regulate this process is largely unknown. Here, we show that nitrate transport via NITRATE TRANSPORTER 2.1 (LjNRT2.1) is a key step in the NLP signaling pathway to control nodulation. A mutation in the LjNRT2.1 gene attenuates the nitrate-induced control of nodulation. LjNLP1 is necessary and sufficient to induce LjNRT2.1 expression, thereby regulating nitrate uptake/transport. Our data suggest that LjNRT2.1-mediated nitrate uptake/transport is required for LjNLP4 nuclear localization and induction/repression of symbiotic genes. We further show that LjNIN, a positive regulator of nodulation, counteracts the LjNLP1-dependent induction of LjNRT2.1 expression, which is linked to a reduction in nitrate uptake. These findings suggest a plant strategy in which nitrogen acquisition switches from obtaining nitrogen from the soil to symbiotic nitrogen fixation.
LjNRT2.1 acts as a hub in the LjNLP1–LjNLP4 signaling pathway to control nodulation in response to nitrate.
IN A NUTSHELL.
Background: Through symbiosis with nitrogen-fixing bacteria, legumes can use nitrogen from the atmosphere as a nutrient. However, root nodule symbiosis requires energy and plants save energy when they can. For example, plants temporarily halt root nodule symbiosis when the soil contains an abundance of nitrogen-containing nutrients such as nitrate. In other words, plants have a mechanism to control root nodule symbiosis upon changes in the amount of nitrate. Using the model legume Lotus japonicus, we previously identified the NIN-LIKE PROTEINs LjNLP1 and LjNLP4 as key transcription factors that play pivotal roles in this regulation. However, how nitrate acts in the signalling pathway to control nodulation remains largely unknown.
Question: How do legume nitrate transporters control root nodule symbiosis in a nitrate-rich environment?
Findings: By analyzing mutants involved in the nitrate-mediated control of root nodule symbiosis, we showed that a defect in the nitrate transporter gene LjNRT2.1 results in the maintenance of nodulation in nitrate-rich environments. We also found that LjNLP1 directly regulated LjNRT2.1 expression. Furthermore, our data suggest that LjNRT2.1-mediated nitrate influx into the cell is relevant to nuclear localization of LjNLP4 and subsequent regulation of the expression of nodulation-related genes. Interestingly, LjNIN, a nodulation-specific transcription factor, controls nitrate uptake by interfering with LjNRT2.1 expression by LjNLP1. These findings enhance our understanding of the mechanisms of nitrogen acquisition that is unique to nodulating plants.
Next steps: In plant evolution, legumes that depend on nodules for their nitrogen source may have developed a unique mode of nitrogen acquisition. Comparative functional analysis of nitrate transporter genes, including NRT2.1, in various plants may be useful in testing this hypothesis.
Introduction
Nitrogen acquisition is critical for plant growth. Growth of most plant species depends on water-soluble forms of nitrogen nutrients such as nitrate and ammonia in the soil (Oldroyd and Leyser, 2020). Legumes can establish endosymbiotic relationships with nitrogen-fixing bacteria called rhizobia by forming root nodules; the rhizobia in the nodules fix atmospheric nitrogen, thus making it available to plants (Oldroyd et al., 2011; Roy et al., 2020). Root nodule symbiosis plays an important role in the growth and survival of symbiotic host plants in a nitrogen-deficient environment. This symbiosis, however, is not always beneficial for plants because photosynthetic products that could be used for plant growth need to be consumed as energy sources for nodule development and nitrogen fixation. To maintain a balance between gaining nitrogen and losing carbon during root nodule symbiosis, plants control root nodule symbiosis depending on nitrogen nutrient availability in the soil (Nishida and Suzaki, 2018a); in nitrate-sufficient conditions, plants negatively regulate several key processes in root nodule symbiosis, including rhizobial infection, nodule initiation and growth, and the nitrogen fixation process (Streeter and Wong, 1988; Carroll and Mathews, 1990; Nishida and Suzaki, 2018b).
Soybean (Glycine max) nitrate-tolerant symbiotic (nts) mutants are the first identified legume mutants that affect nitrate-mediated control of nodulation (Carroll et al., 1985). In addition to their hypernodulating phenotypes, the nts mutants are tolerant to high nitrate concentrations. The gene responsible for the nts-1 mutants encodes a shoot-acting receptor-like kinase, Nodule autoregulation receptor kinase (GmNARK; Searle et al., 2003). Defects in the GmNARK ortholog in two model legumes, Lotus japonicus HYPERNODULATION ABERRANT ROOT FORMATION 1 (LjHAR1) and Medicago truncatula SUPER NUMERIC NODULES, exhibit similar phenotypes to the Gmnark mutants (Krusell et al., 2002; Nishimura et al., 2002; Schnabel et al., 2005). In addition, the expression of CLAVATA3/ESR-related (CLE)-ROOT SIGNAL 2 (LjCLE-RS2), encoding a root-derived ligand of LjHAR1, is induced not only by rhizobial inoculation but also by nitrate treatment (Okamoto et al., 2009). In M. truncatula, rhizobia/nitrate-inducible MtCLE35 was recently identified as a functional counterpart of LjCLE-RS2 (Luo et al., 2021; Mens et al., 2021; Moreau et al., 2021). In nitrate-sufficient conditions, loss-of-function mutations in LjHAR1 or LjCLE-RS2 show tolerance to the nitrate-induced reduction in nodule number, not to other processes such as rhizobial infection and nodule growth (Nishida et al., 2018, 2020). These observations suggest the signaling pathway including these genes is responsible for modulating nodule number in the pleiotropic control of root nodule symbiosis by nitrate.
A recent genetic approach in L. japonicus identified nitrate unresponsive symbiosis 1 (nrsym1) and nrsym2 mutants that have a normal nodule number but attenuate nitrate-induced control of root nodule symbiosis (Nishida et al., 2018, 2021). NRSYM1 and NRSYM2 encode NODULE INCEPTION (NIN)-LIKE PROTEIN (NLP) transcription factor, LjNLP4 and LjNLP1, respectively. NLP is paralogous to NIN, a necessary and sufficient regulator of nodulation (Schauser et al., 1999, 2005; Soyano et al., 2013; Vernié et al., 2015). In addition to its positive role in nodulation, LjNIN/MtNIN negatively regulates nodulation via induction of LjCLE-RS1/2 and MtCLE13 (Soyano et al., 2014; Laffont et al., 2020). LjNLP4 shares common DNA-binding sites on the LjCLE-RS2 promoter with LjNIN, and LjNLP4 negatively regulates nodule number by directly inducing LjCLE-RS2 expression in response to nitrate (Nishida et al., 2018). Furthermore, the expression of LjNIN target genes with positive roles in rhizobial infection and nodule organogenesis are repressed by nitrate in LjNLP4- and LjNLP1-dependent manners (Nishida et al., 2021).
LjNLP4 localizes within nuclei in response to nitrate, as do the NLPs in other plants (Marchive et al., 2013; Liu et al., 2017; Lin et al., 2018; Nishida et al., 2018). Therefore, in the presence of high nitrate levels, nuclear-localized LjNLP4 can negatively regulate nodulation by bifunctional transcriptional regulation, inducing or repressing the expression of LjNIN target genes (Nishida et al., 2021). The induced genes include LjCLE-RS2, which has a negative role in nodulation, and the repressed genes include NUCLEAR FACTOR Y-A (LjNF-YA), LjNF-YB, EXOPOLYSACCHARIDE RECEPTOR 3 (LjEPR3), and RHIZOBIAL INFECTION RECEPTOR-LIKE KINASE 1, which have positive roles in nodulation (Okamoto et al., 2009; Soyano et al., 2013; Kawaharada et al., 2015, 2017; Li et al., 2019; Shrestha et al., 2021). In M. truncatula, MtNLP1 directly induces MtCLE35, a negative regulator of nodulation, and represses CYTOKININ RESPONSE 1, a positive regulator of nodulation and a direct target of MtNIN (Lin et al., 2018; Luo et al., 2021). Hence, it is likely that the mode-of-action for NLP-mediated bifunctional transcriptional regulation of symbiotic genes is conserved in L. japonicus and M. truncatula.
Generally, plants use two different nitrate transport systems depending on the nitrate concentration: a high-affinity transport system (HATS) and a low-affinity transport system (LATS) for low (<0.5 mM) and high (>0.5 mM) nitrate concentration ranges, respectively (Krapp et al., 2014). In Arabidopsis thaliana, the NITRATE TRANSPORTER 2 (NRT2) family is primarily responsible for HATS, whereas the NRT1 family is responsible for LATS (Wang et al., 1998; Filleur et al., 2001; Li et al., 2007). An exception is AtNRT1.1, which can switch between high- and low-affinity transport activities depending on the phosphorylation state of the AtNRT1.1 protein (Ho et al., 2009). Several studies on legume nitrate transporters suggest their potential roles in nodulation and nitrate-induced control of nodulation (Pellizzaro et al., 2017). In particular, the roles of some NITRATE PEPTIDE FAMILY (NPF) genes have been characterized. MtNPF1.7 positively regulates nodulation and is dispensable for nitrate-induced control of nodulation (Yendrek et al., 2010). LjNPF8.6 is involved in nitrogen fixation (Valkov et al., 2017). In addition, the Mtnpf7.6 mutants have nodules of reduced size and are tolerant to nitrate, suggesting that MtNPF7.6 is required for nodule growth and nitrate-induced control of nodulation (Wang et al., 2020).
Despite the accumulating examples of the roles of legume nitrate transporters, how the nitrate transport system plays a role in the signaling pathway to control nodulation under high nitrate conditions remains mostly elusive. This study shows that a mutation in LjNRT2.1 maintains nodulation under high nitrate concentrations. Phenotypic analysis indicates that LjNRT2.1 and LjNLP1 act in the nitrate uptake/transport process. In addition, LjNLP1 is necessary and sufficient to induce LjNRT2.1 expression, which is likely to be associated with the nitrate-dependent LjNLP4 nuclear localization and induction/repression of symbiotic genes. These data indicate that nitrate transport via LjNRT2.1 is a key process in the NLP signaling pathway to control nodulation. Furthermore, we show that LjNIN can counteract LjNLP1-dependent induction of LjNRT2.1. This mechanism may reflect a plant strategy in which the acquisition of nitrogen nutrients switches from depending on nitrogen in the soil to depending on symbiotic nitrogen fixation.
Results
LjNRT2.1 is required for nitrate-induced control of nodulation
In our previous screen for L. japonicus ethylmethane sulfonate (EMS) mutants involved in the nitrate response during nodulation (Nishida et al., 2018), we identified two new recessive mutants, nrsym3 and nrsym4. Treatment with a high nitrate concentration (10 mM) attenuated nodulation in the wild-type (WT), but the effect of nitrate was suppressed by the nrsym3 or the nrsym4 mutations (Figure 1A). An allelism test suggested that the nitrate-unresponsive phenotypes of nrsym3 and nrsym4 were caused by a mutation in an identical gene, as F1 plants obtained from a cross between the plants formed mature nodules under high nitrate conditions (Supplemental Figure S1). A genome-resequencing approach using the nrsym3 and nrsym4 mutants identified a point mutation that caused a missense mutation in LjNRT2.1 (Lj3g3v3069030; Criscuolo et al., 2012; Valkov et al., 2020), a gene that encodes a protein similar to Arabidopsis NRT2 proteins, including AtNRT2.1, AtNRT2.2, and AtNRT2.4 (Figure 1, B and C).
Figure 1.
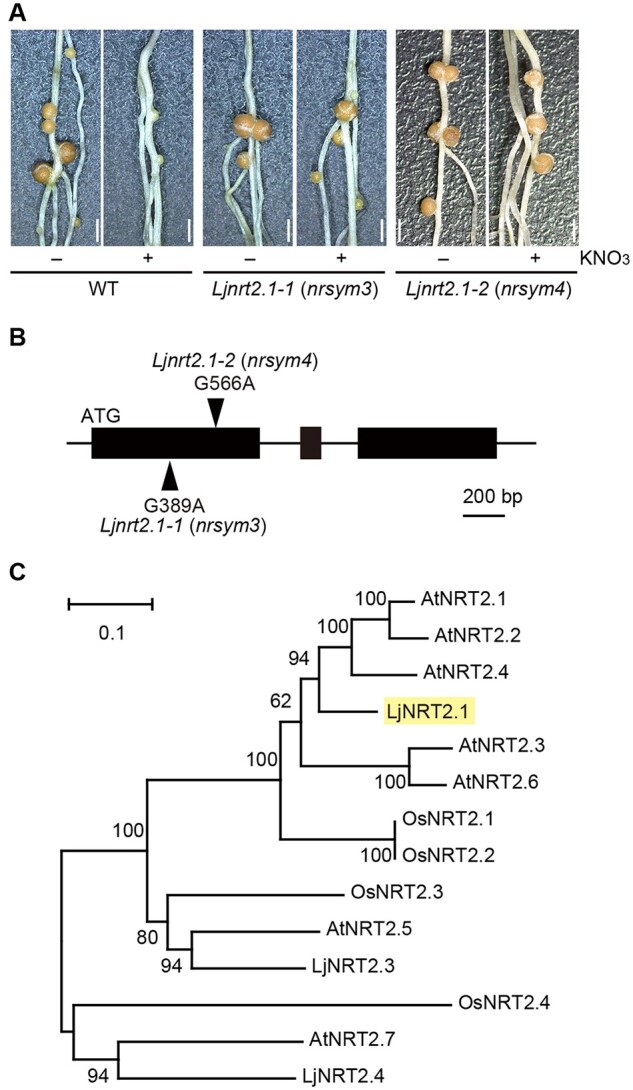
Identification of the LjNRT2.1 gene. A, Nodule phenotypes of the WT, the Ljnrt2.1-1, and the Ljnrt2.1-2 mutants grown with 0 (−) or 10 mM KNO3 (+) for 21 dai. Scale bars = 1 mm. B, Exon-intron structure of the LjNRT2.1 gene. The arrowheads indicate the locations of the Ljnrt2.1-1 and the Ljnrt2.1-2 mutations. C, A phylogenetic tree of the NRT2 gene family from A. thaliana, Oryza sativa, and L. japonicus generated using the Neighbor-Joining method. The nomenclature for LjNRT2 genes was based on previous studies (Criscuolo et al., 2012; Valkov et al., 2020). LjNRT2.2 was excluded from the phylogenetic tree, as this sequence is unlikely to be functional (Supplemental Figure S3). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown.
To test whether LjNRT2.1 could complement the nrsym3 mutant, a 6.4-kb genomic fragment, including the promoter (3.2 kb) and the terminator (1.2 kb) regions of LjNRT2.1, was introduced into the nrsym3 mutants by Agrobacterium rhizogenes-mediated hairy root transformation. Nodulation on the mutant roots expressing the complementation construct was inhibited by nitrate (Supplemental Figure S2, A and B). This result indicates that Lj3g3v3069030 is responsible for the nrsym3 mutation.
Hereafter, we unified the nomenclature by re-naming nrsym3 and nrsym4 as Ljnrt2.1-1 and Ljnrt2.1-2, respectively. The typical structure of NRT2 family proteins has 12 transmembrane regions. Mutations in the Ljnrt2.1-1 and Ljnrt2.1-2 mutants are located in the third (G130D) and fifth (G189E) transmembrane regions, respectively. Previous studies showed that the L. japonicus genome has four genes in the NRT2 family, including LjNRT2.1, LjNRT2.2 (Lj3g3v3069010, Lj3g3v3069020, Lj3g3v3069040, Lj3g3v3069050), LjNRT2.3 (Lj4g3v1085060), and LjNRT2.4 (Lj1g3v3646440) (Criscuolo et al., 2012; Valkov et al., 2020). As the coding sequence (CDS) of LjNRT2.2 available from the L. japonicus genome database seemed to be partial and possibly inaccurate, we determined the full-length CDS of LjNRT2.2. We found that the structure of LjNRT2.2 in two WT ecotypes, MG-20 and Gifu, contains a premature stop codon, thus encoding a truncated version of protein relative to LjNRT2.1 (Supplemental Figure S3). Therefore, it is likely that LjNRT2.2 is nonfunctional in some L. japonicus ecotypes.
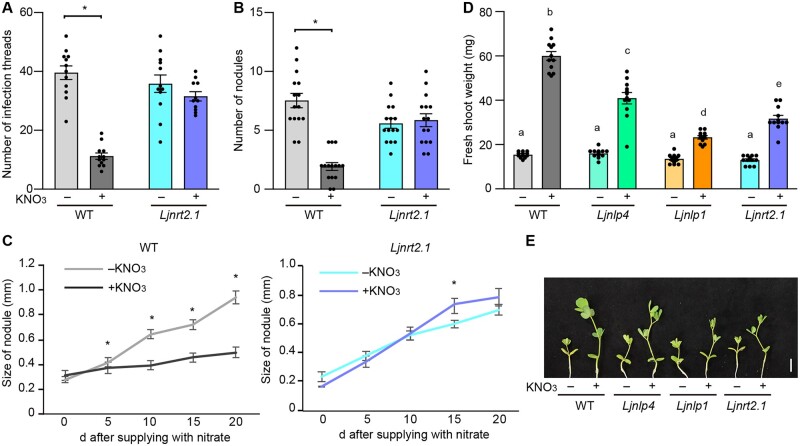
Nitrate has pleiotropic effects on key events of nodulation, including rhizobial infection, nodule initiation, and nodule growth (Streeter and Wong, 1988; Carroll and Mathews, 1990; Nishida and Suzaki, 2018b). We, therefore, investigated whether the Ljnrt2.1 mutation influenced these nitrate-affected nodulation processes. In the WT, the formation of infection threads, an indicator of rhizobial infection foci (Murray, 2011), was attenuated by nitrate; however, the nitrate-induced reduction in infection thread number was not observed in the Ljnrt2.1 mutants (Figure 2A). In addition, the number of nodules formed on Ljnrt2.1 roots in the presence of nitrate was comparable to that in the absence of nitrate (Figure 2B). Next, to focus on nodule growth, the WT and Ljnrt2.1 plants were inoculated for 7 days in nitrate-free agar plates. After the formation of nodule primordia, the plants were transferred to new plates containing nitrate, and the successive changes in nodule size were measured. WT nodule growth was halted by nitrate, but the nitrate effect was largely abolished in the Ljnrt2.1 mutants (Figure 2C). Therefore, these results indicate that LjNRT2.1 is required for nitrate-mediated control of rhizobial infection, nodule initiation, and growth.
Figure 2.
Nitrate effects on nodulation and plant growth in the Ljnrt2.1 mutants. A–C, Nitrate effects on nodulation in the WT and Ljnrt2.1-1 mutants. A, The number of infection threads in plants growing in 0 (−) or 10 mM KNO3 (+) at 7 dai with rhizobia that constitutively express DsRED (n = 11–12 plants). B, The number of nodules in the presence of 0 (−) or 10 mM KNO3 (+) for 21 dai (n = 15 plants). C, Nodule size of the WT and Ljnrt2.1-1 mutants (n = 19–22 nodules). The WT and Ljnrt2.1-1 plants were inoculated for 7 days in nitrate-free agar plates. After the formation of nodule primordia, the plants were transferred to new plates containing 0 or 10 mM KNO3. Individual nodule size was measured at 0, 5, 10, 15, and 20 days after the transfer. Error bars indicate standard error of the mean (SEM). *P < 0.05 by a two-sided Welch’s t test. D and E, Fresh shoot weight (D) and shoot growth (E) of the WT, Ljnlp4-1, Ljnlp1, and the Ljnrt2.1-1 mutants grown in 0 (−) or 10 mM KNO3 (+) in the absence of rhizobia for 13 days (n = 12 plants). Error bars indicate sem. Different letters indicate statistically significant differences (P < 0.05, one-way analysis of variance (ANOVA) followed by multiple comparisons). Scale bar = 1 cm.
The nodulation phenotypes of the Ljnlp4 and the Ljnlp1 mutants under high nitrate conditions are similar to those of the Ljnrt2.1 mutants (Nishida et al., 2018, 2021). We then created multiple mutants and investigated the potential genetic relationships between the corresponding genes. Single, double, and triple mutants formed similar numbers of mature nodules that were indistinguishable from each other under high nitrate conditions (Supplemental Figure S4), suggesting that LjNLP4, LjNLP1, and LjNRT2.1 act in the same genetic pathway at least in the nitrate-induced control of nodulation.
We next tested the effect of nitrate on plant growth in the absence of rhizobia. Promotion of shoot growth by nitrate was diminished in each mutant relative to the WT, and the severity of the phenotype was strongest in Ljnlp1, weakest in Ljnlp4, and intermediate in Ljnrt2.1 (Figure 2, D and E). The defect in nitrate-promoted shoot growth in the Ljnrt2.1 plants was rescued by the introduction of a complementation construct (Supplemental Figure S2C), suggesting that LjNRT2.1 is responsible for the phenomenon.
LjNRT2.1 and LjNLP1 mediate nitrate uptake/transport
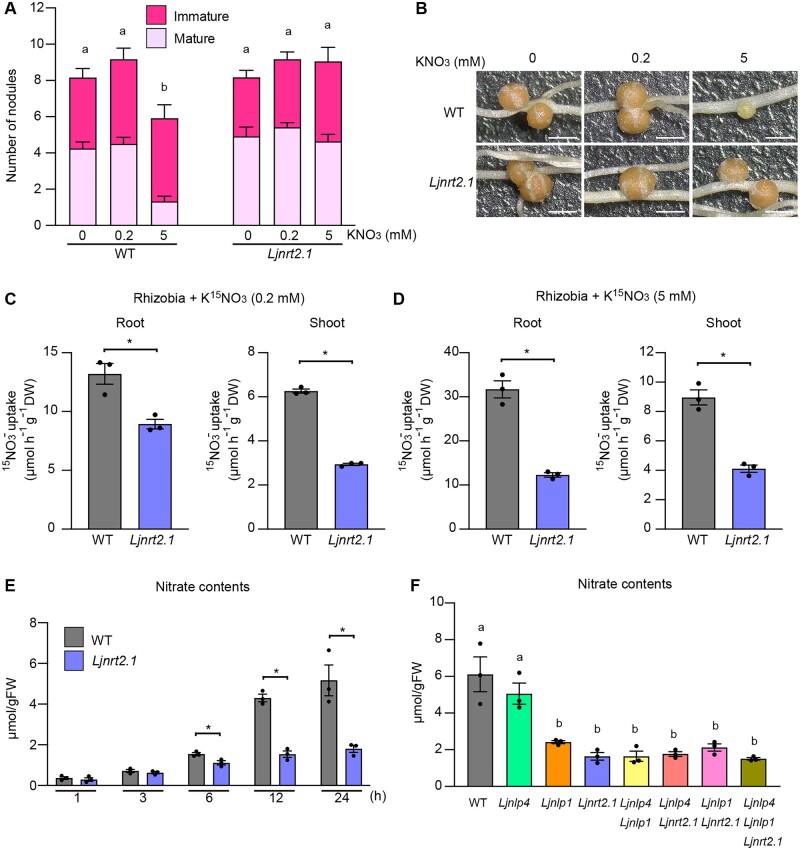
In WT, nitrate-induced inhibition of nodulation occurred in a dose-dependent manner: treatment with a high nitrate concentration (5 mM) reduced nodulation, whereas treatment with a low nitrate concentration (0.2 mM) did not affect nodulation (Figure 3, A and B; Nishida et al., 2018). The effect of nitrate (5 mM) was suppressed by the Ljnrt2.1 mutations. As LjNRT2.1 belongs to the NRT2 family and AtNRT2 genes are primarily responsible for HATS (Wang et al., 1998; Filleur et al., 2001; Li et al., 2007), we suspected that LjNRT2.1 might be involved in nitrate uptake/transport. To examine this possibility, the WT and Ljnrt2.1 plants were treated with 0.2- or 5-mM nitrate solution that included a 15N stable isotope in the presence of rhizobia, and the amount of 15N in planta was measured. The nitrate uptake capacity in the root and shoot of the Ljnrt2.1 mutants was significantly reduced compared with WT regardless of the exogenous nitrate concentration (Figure 3, C and D). In terms of nitrate uptake, noninoculated roots of the Ljnrt2.1 mutants had a similar defect: the Ljnrt2.1 mutations attenuated nitrate uptake in both low and high nitrate conditions (Supplemental Figure S5, A and B). These results suggest that LjNRT2.1 is required for nitrate uptake/transport.
Figure 3.
The capacity for nitrate uptake in the Ljnrt2.1 mutants. A and B, Nodule number and nodulation phenotypes of the WT and Ljnrt2.1-1 mutants in the presence of 0, 0.2, or 5 mM KNO3 at 21 dai (n = 11–12 plants). Scale bars = 1 mm. C and D, The capacity for nitrate uptake by the WT and Ljnrt2.1-1 mutants root and shoot in 0.2 (C) and 5 mM (D) KNO3 in the presence of rhizobia. The plants were grown with 0.2 or 5 mM KNO3 for 12 days in the presence of rhizobia. They were then treated with same concentration of K15NO3 for 24 h (n = 3 independent pools of roots derived from 12 plants). E, Temporal changes in the nitrate contents of noninoculated roots of the WT and Ljnrt2.1-1 mutants after treatment with 10 mM KNO3 (n = 3 independent pools of roots derived from seven plants). F, The nitrate contents of noninoculated roots of the WT and seven respective mutants 24 h after treatment with 10 mM KNO3 (n = 3 independent pools of roots derived from seven plants). Error bars indicate sem. *P < 0.05 by a two-sided Welch’s t test (C–E). Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons) (A) and (F).
We next analyzed the temporal changes in nitrate contents after nitrate treatment in noninoculated roots. Although the nitrate contents at relatively shorter time points (1 and 3 h) was unaffected in the Ljnrt2.1 mutants, the reduction in nitrate contents become evident 6 h after nitrate treatment (10 mM; Figure 3E). Retarded shoot growth of the Ljnlp4 and Ljnlp1 mutants in the presence of nitrate implied that the mutants might be impaired in nitrate transport and/or utilization (Figure 2, D and E). We then investigated the nitrate contents 24 h after nitrate treatment (10 mM) in single, double, and triple mutants of Ljnlp4, Ljnlp1, and Ljnrt2.1. The nitrate contents were unaffected in the Ljnlp4 mutants but were reduced in the Ljnlp1 mutants to the same extent as in the Ljnrt2.1 mutants (Figure 3F). The defect in nitrate uptake in the Ljnlp1 mutants was also observed in the nitrate uptake assay using 15N stable isotope (Supplemental Figure S5B). Therefore, these results suggest that LjNLP1 and LjNRT2.1 have roles in nitrate uptake/transport. LjNLP1 and LjNRT2.1 likely regulate nitrate uptake/transport in the same genetic pathway, as the nitrate contents in the Ljnlp1 Ljnrt2.1 double mutants were equivalent to that of the Ljnlp1 or the Ljnrt2.1 single mutants. The relatively stronger defects in shoot growth in the Ljnlp1 and the Ljnrt2.1 mutants compared to the Ljnlp4 mutants (Figure 2, D and E) were consistent with the defects in nitrate uptake in these mutants.
To verify the relationship between nitrate uptake/transport and gene expression, the expression of two nitrate-inducible genes, NITRATE REDUCTASE (LjNIA) and LjCLE-RS2, was analyzed in noninoculated roots by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Whereas the expression of LjNIA was rapidly (<0.5 h) induced, it took 6 h for the expression of LjCLE-RS2 to be induced after nitrate treatment in the WT (Supplemental Figure S5, C and D). Transcript abundance of both genes was significantly reduced at later time points (>6 h) in the Ljnrt2.1 mutants (Supplemental Figure S5, C and D), a result consistent with the observation that the Ljnrt2.1 mutation attenuated nitrate uptake by 6 h and later (Figure 3E).
LjNLP1 is necessary and sufficient to induce LjNRT2.1 expression in response to nitrate
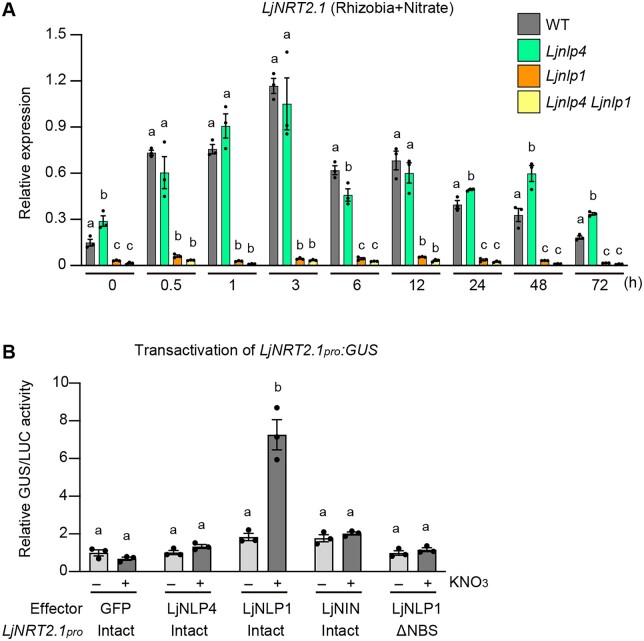
We next analyzed the temporal expression pattern of LjNRT2.1 in inoculated roots after nitrate treatment (10 mM) and the potential implications of LjNLP4 and LjNLP1 in LjNRT2.1 expression. RT-qPCR analysis showed that, in the timescale tested, LjNRT2.1 expression was inducible by nitrate treatment, and the expression level was highest at 3 h in the WT (Figure 4A). LjNRT2.1 expression in the Ljnlp4 mutants was largely comparable to that of the WT at least earlier time points (<3 h). At later time points (>24 h), the LjNRT2.1 expression in the Ljnlp4 mutants was constantly higher than WT. Of note, the LjNRT2.1 expression was abolished in the Ljnlp1 mutants at every time point. The LjNRT2.1 expression pattern in the Ljnlp4 Ljnlp1 double mutants was indistinguishable from that of Ljnlp1 (Figure 4A).
Figure 4.
Temporal expression of LjNRT2.1 and transactivation of LjNRT2.1. A, RT-qPCR analysis of temporal LjNRT2.1 expression of the WT, Ljnlp4-1, Ljnlp1, and Ljnlp4-1 Ljnlp1 mutants after simultaneous treatment with 10mM KNO3 and rhizobia. Each cDNA sample was prepared from total RNA derived from inoculated roots treated with 10mM KNO3 (n = 3 independent pools of roots derived from four plants). The expression of LjUBQ was used as the reference. Error bars indicate sem. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons). Data for each time point were used in the statistical analysis. B, Transactivation of LjNRT2.1pro:GUS in L. japonicus mesophyll protoplasts transformed with respective constructs (n = 3 independent pools of protoplasts). GFP, LjNLP4, LjNLP1, and LjNIN were used as effectors. Two types of LjNRT2.1pro:GUS reporter constructs were used, one with an intact LjNRT2.1 promoter and one with a modified LjNRT2.1 promoter in which the NBS (Soyano et al., 2015) was specifically deleted (ΔNBS) (Supplemental Figure S6). Transformed protoplasts were incubated with 0 (−) or 10 mM (+) KNO3. GUS activity was measured relative to 35Spro:LUC activity. Transactivation data were normalized to the condition in which GFP was expressed in the absence of KNO3. Error bars indicate sem. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons).
We next investigated whether LjNLP1 was sufficient to induce LjNRT2.1 expression by a transactivation assay using mesophyll protoplasts of L. japonicus. Although LjNLP4 or LjNIN did not affect LjNRT2.1 expression, LjNLP1 did induce LjNRT2.1 expression in a nitrate-dependent manner (Figure 4B). The 3.2-kb LjNRT2.1pro region used in the transactivation assay had an LjNIN-binding sequence (NBS; Soyano et al., 2015). Given the similarity of the DNA-binding sites between NLP and NIN (Soyano et al., 2015; Nishida et al., 2021), we assumed that the NBS in the LjNRT2.1pro might be relevant to LjNLP1-mediated LjNRT2.1 expression. LjNLP1 failed to express the GUS reporter gene under the control of a modified LjNRT2.1pro in which the NBS was specifically deleted (Figure 4B; Supplemental Figure S6A). Taken together with the observation of abolished expression of LjNRT2.1 in the Ljnlp1 mutants (Figure 4A), the results from the transactivation assay indicate that LjNLP1 is necessary and sufficient to induce LjNRT2.1 expression in response to nitrate.
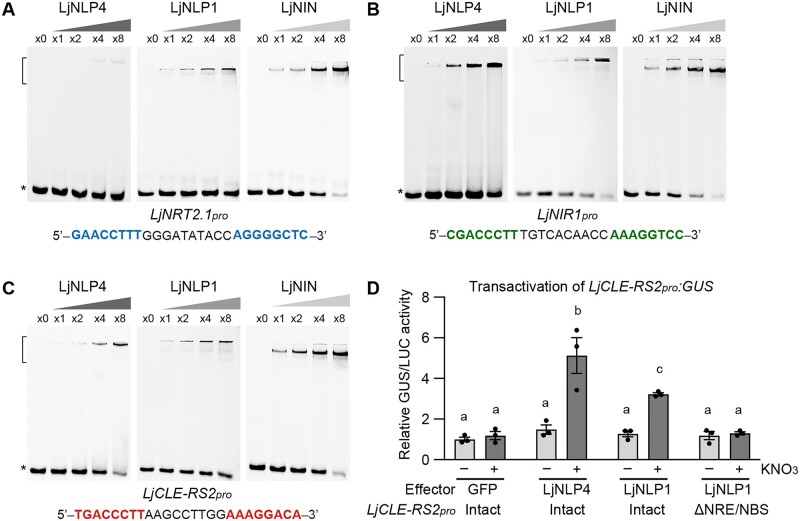
To verify the protein–DNA interaction in more detail, we carried out an electrophoretic mobility shift assay (EMSA). The recombinant LjNLP1 and LjNIN proteins, consisting of an RWP-RK and a PB1 domain, bound to cis-elements of the LjNRT2.1 promoter (Figure 5A). In contrast, the recombinant LjNLP4 protein with similar domains showed much weaker binding to the cis-elements (Figure 5A), which was consistent with the observation that LjNLP4 was not involved in the regulation of LjNRT2.1 expression (Figure 4). As previously shown, LjNLP4 and LjNIN bound to cis-elements of NITRITE REDUCTASE 1 (LjNIR1) and LjCLE-RS2 promoters (Figure 5, B and C; Nishida et al., 2021). LjNLP1 also bound to the same regions (Figure 5, B and C). Transactivation assay showed that LjNLP1, as well as LjNLP4, could activate LjCLE-RS2 expression through direct binding to the cis-element in a nitrate-dependent manner (Figure 5D; Supplemental Figure S6B).
Figure 5.
Protein–DNA interaction of LjNLP4, LjNLP1, and LjNIN. A–C, EMSA showing LjNLP4, LjNLP1, or LjNIN binding to the cis-elements on the LjNRT2.1pro (A), LjNIR1pro (B), and LjCLE-RS2pro (C). MBP-LjNLP4 (564–976), MBP-LjNLP1 (573‒903), or MBP-LjNIN (551–878) recombinant proteins, consisting of an RWP-RK and a PB1 domain, were incubated with the FAM-labeled DNA probe (Supplemental Data Set 1). Blue, green, and red nucleotides indicate conserved motifs among the DNA fragments. Brackets and asterisks, respectively, indicate the position of shifted bands showing protein–DNA interaction and of free probes that did not interact with proteins. D, Transactivation of LjCLE-RS2pro:GUS in L. japonicus mesophyll protoplasts transformed with respective constructs (n = 3 independent pools of protoplasts). GFP, LjNLP4, and LjNLP1 used as effectors. Two types of LjCLE-RS2pro:GUS reporter constructs were used, one with an intact LjCLE-RS2 promoter and one with a modified LjCLE-RS2 promoter in which the NRE/NBS (Figure 5C; Nishida et al., 2021) was specifically deleted (ΔNRE/NBS) (Supplemental Figure S6). Transformed protoplasts were incubated with 0 (−) or 10 mM (+) KNO3. GUS activity was measured relative to 35Spro:LUC activity. Transactivation data were normalized to the condition in which GFP was expressed in the absence of KNO3. Error bars indicate sem. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons).
LjNRT2.1, LjNLP4, and LjNLP1 have overlapping expression patterns during nodulation
To determine spatial expression pattern of LjNRT2.1 and its relevance to those of LjNLP4 and LjNLP1, we conducted promoter-GUS reporter analysis. We first identified the functional promoter that could rescue the corresponding mutants to the same extent as constructs using the LjUBQ promoter, when used to express each gene (Supplemental Figure S7, A–C). The promoter fragments LjNRT2.1pro (3.2 kb), LjNLP4pro (2.3 kb) and LjNLP1pro (4.0 kb), harbored same regions as genomic fragments used in complementation assays (Supplemental Figure S2; Nishida et al., 2018, 2021). At initial developmental stages of nodulation, the LjNRT2.1pro:GUS construct showed that LjNRT2.1 was expressed within dividing cortical cells, in an overlapping pattern with LjNLP4 and LjNLP1 (Figure 6). At later stages of nodulation, promoter-GUS constructs of each gene showed strong GUS activity in the outer regions of nodules, including epidermis. In addition to the expression in nodulation cell lineage, LjNRT2.1 was expressed in the region of the root closer to the root base than the tip (Supplemental Figure S7D). Transverse sections of the region indicated that the LjNRT2.1 was predominantly expressed in the epidermis (Supplemental Figure S7E). The epidermal LjNRT2.1 expression might be insufficient to ensure the full LjNRT2.1 function, as the epidermis-specific expression of LjNRT2.1 using the Epi promoter (Hayashi et al., 2014) failed to rescue the Ljnrt2.1 phenotype (Supplemental Figure S7A).
Figure 6.
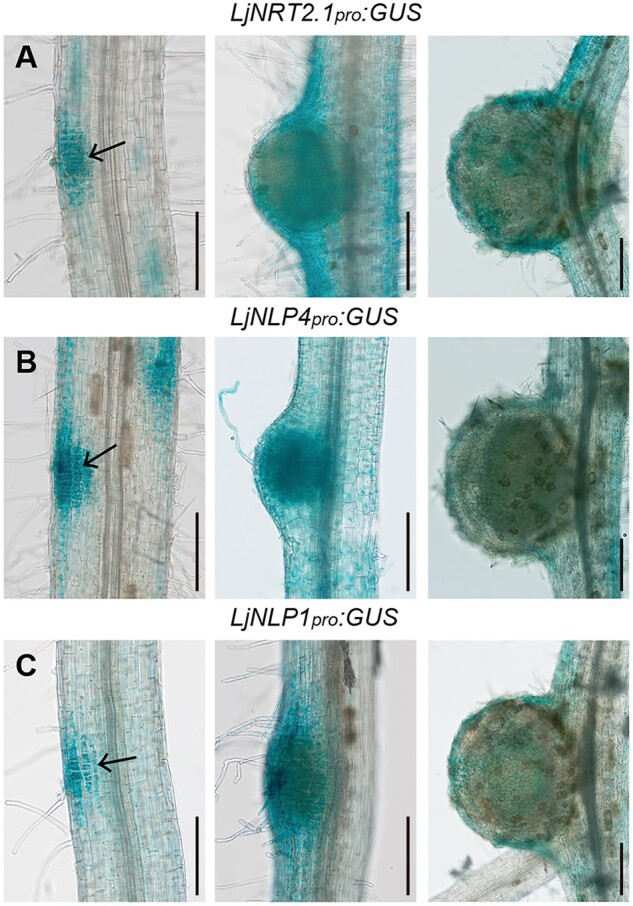
Spatial expression of LjNRT2.1, LjNLP4, and LjNLP1 during nodulation. A–C, GUS activities in nodulating WT transgenic hairy roots transformed with the LjNRT2.1pro:GUS (A), LjNLP4pro:GUS (B), and LjNLP1pro:GUSplus (C) constructs. Arrows indicate dividing cortical cells. Transgenic roots were identified by GFP fluorescence. Scale bars = 200 µm.
Our data have shown that LjNLP1–LjNRT2.1 pathway has a role in nitrate-induced control of nodulation. We then asked if the expression of LjNRT2.1 could compensate for LjNLP1 function. Constitutive LjNRT2.1 expression from the LjUBQ promoter could not rescue the Ljnlp1 mutation (Supplemental Figure S7C). Therefore, the expression of LjNRT2.1 alone might be insufficient for nitrate-induced control of nodulation in the Ljnlp1 mutants.
LjNRT2.1 function is linked to the nitrate-dependent regulation of LjNLP4 nuclear localization and control of symbiotic gene expression
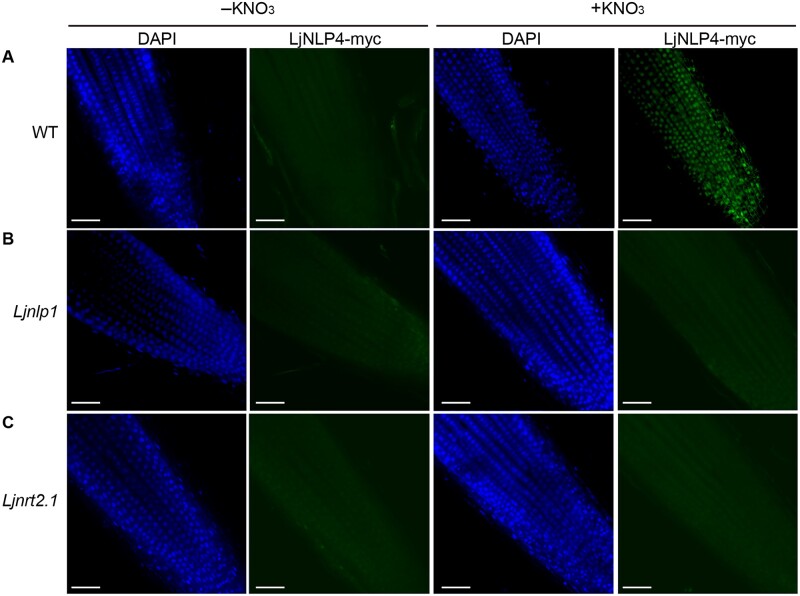
One of the features of LjNLP4 is that its nuclear localization is regulated by nitrate (Nishida et al., 2018). Given the involvement of LjNRT2.1 and LjNLP1 in the nitrate-induced control of nodulation and the nitrate uptake/transport process, we verified that these proteins might be involved in regulating the subcellular localization of LjNLP4. In agreement with a previous study (Nishida et al., 2018), immunohistochemistry using an LjNLP4–myc fusion protein showed that LjNLP4 was localized within nuclei in response to nitrate in the WT (Figure 7A; Supplemental Figure S8A). In contrast, a strong signal for LjNLP4 was not evident in the nuclei of the Ljnlp1 and the Ljnrt2.1 mutants even in the presence of nitrate (Figure 7, B and C; Supplemental Figure S8A). RT-qPCR and immunoblot analysis indicated that the loss of nuclear LjNLP4 signals in the Ljnlp1 and Ljnrt2.1 mutants was not due to reduced LjNLP4 expression nor LjNLP4 stability (Supplemental Figure S8, B and C). Therefore, our observations suggest that LjNLP1 and LjNRT2.1 act upstream of the nitrate-dependent LjNLP4 nuclear localization; LjNLP1-mediated activation of LjNRT2.1 expression and subsequent nitrate transport may be required for LjNLP4 nuclear localization.
Figure 7.
Subcellular localization of LjNLP4. A–C, Immunohistochemistry of the LjNLP4-myc protein in root apical cells of WT, Ljnlp1, and Ljnrt2.1-1 mutants. A monoclonal anti-myc antibody and an antibody conjugated to Alexa Fluor 488 Plus (right: green signal) were used as the primary and secondary antibodies. Nuclei were visualized with DAPI (left: blue signal). Plants with transgenic hairy roots transformed with the LjUBQpro:LjNLP4-myc construct were grown on a nitrogen-free medium for 3 days and then supplied with 0 (−) or 10 mM KNO3 (+) for 1 h. Representative images of at least five independent locations are shown for each condition. Scale bars = 50 µm.
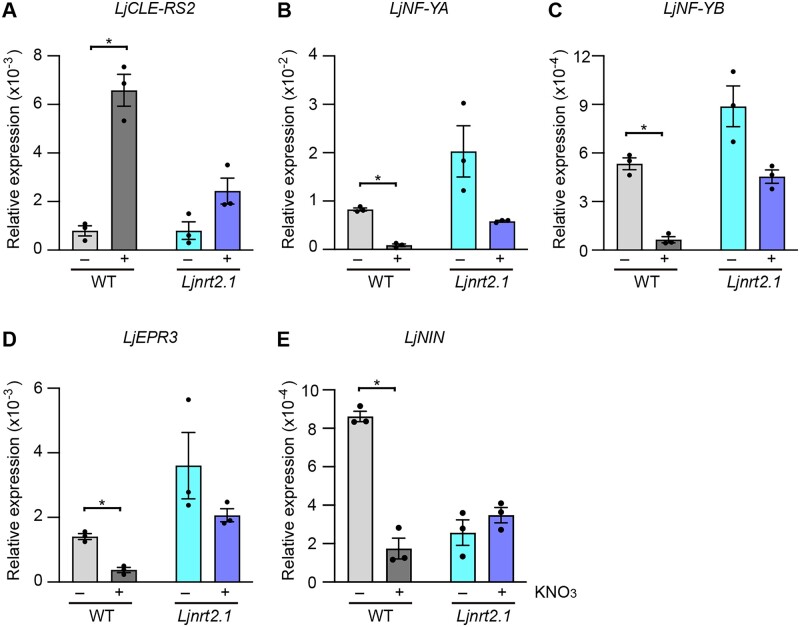
To further characterize the involvement of LjNRT2.1 in the nitrate-induced control of nodulation, we focused on the expression of symbiotic genes whose expression was affected by nitrate. Whereas nitrate upregulates the expression of LjCLE-RS2, nitrate downregulates the expression of LjNF-YA, LjNF-YB, and LjEPR3 in an LjNLP4- and LjNLP1-dependent manner (Nishida et al., 2021). For RT-qPCR analysis, the WT and Ljnrt2.1 plants were pretreated with nitrate for 24 h before rhizobial inoculation. Roots were collected at 3 days after inoculation (dai) with continuous nitrate treatment. Consequently, we found that the nitrate-induced level of LjCLE-RS2 expression was lower in the Ljnrt2.1 mutants than the WT (Figure 8A). In contrast, nitrate-repressed level of LjNF-YA, LjNF-YB, and LjEPR3 was lower in the Ljnrt2.1 mutants relative to the WT (Figure 8, B-D). These nitrate-induced/repressed expression patterns of the symbiotic genes in the Ljnrt2.1 mutants resembled those in the Ljnlp4 and the Ljnlp1 mutants (Nishida et al., 2021). Furthermore, we found that the LjNIN expression was nitrate-repressible in WT and the nitrate-mediated reduction in LjNIN expression was abolished in the Ljnrt2.1 mutants (Figure 8E).
Figure 8.
Symbiotic gene expression in the Ljnrt2.1 mutants. A–E, RT-qPCR analysis of LjCLE-RS2 (A), LjNF-YA (B), LjNF-YB (C), LjEPR3 (D), and LjNIN (E) expression in the WT and Ljnrt2.1-1 mutants. Plants were pretreated with 0 (−) or 10 mM KNO3 (+) for 24 h before rhizobial inoculation. Roots at 3 dai with continuous treatment of 0 or 10 mM KNO3 were collected (n = 3 independent pools of roots derived from three plants), and total RNA from the 3 dai roots was isolated for cDNA synthesis. The expression of LjUBQ was used as the reference. Error bars indicate sem. *P < 0.05 by a two-sided Welch’s t test.
LjNLP1 can substitute for LjNLP4 function
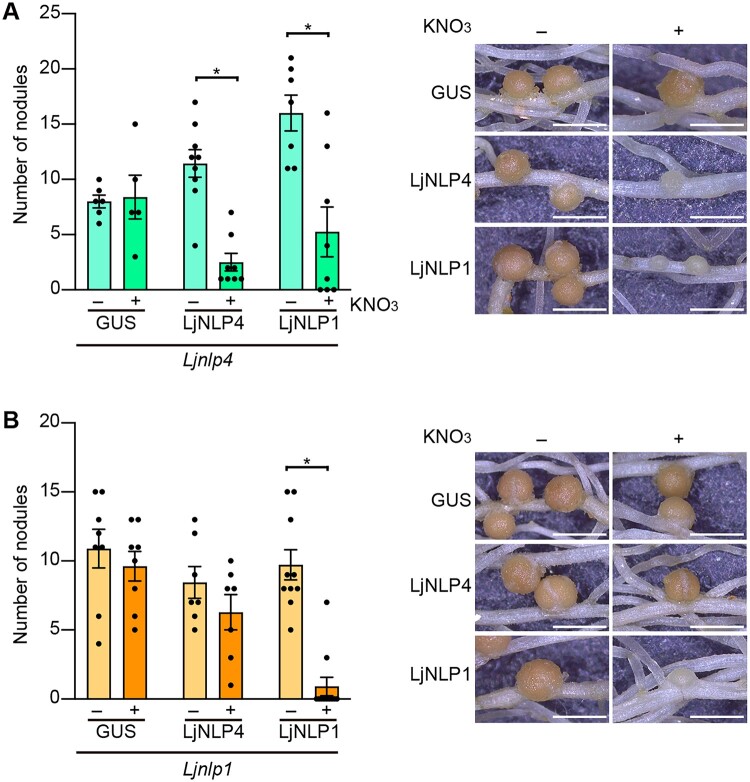
In addition to the phenotypic similarity of the mutants, our previous RNA-seq analysis indicated that LjNLP4 and LjNLP1 had mostly similar downstream genes (Nishida et al., 2021). Indeed, LjCLE-RS2 was identified as a common direct target of LjNLP4 and LjNLP1 (Figure 5). These findings led us to postulate that the two NLPs might have overlapping functions. To examine the functional relationships of LjNLP4 and LjNLP1, we expressed one gene in mutants of the other gene. The nodulation phenotype of the Ljnlp4 mutants in the presence of nitrate was rescued by constitutive expression of LjNLP1 (Figure 9A). In contrast, nodulation of the Ljnlp1 mutants was unaffected by LjNLP4 expression (Figure 9B). These results suggest that LjNLP1 can functionally substitute for LjNLP4.
Figure 9.
Reciprocal complementation assays of LjNLP4 and LjNLP1. A and B, Nodule number and nodulation phenotypes of transgenic hairy roots of the Ljnlp4-1 (A) and Ljnlp1 (B) mutants transformed with either the LjUBQpro:GUS, LjUBQpro:LjNLP4, or LjUBQpro:LjNLP1 constructs in the presence of 0 (−) or 10 mM KNO3 (+) at 21 dai (n = 6–11 plants). Transgenic roots were identified by GFP fluorescence. Error bars indicate sem. *P < 0.05 by a two-sided Welch’s t test. Scale bars = 1 mm.
LjNIN counteracts LjNLP1 function
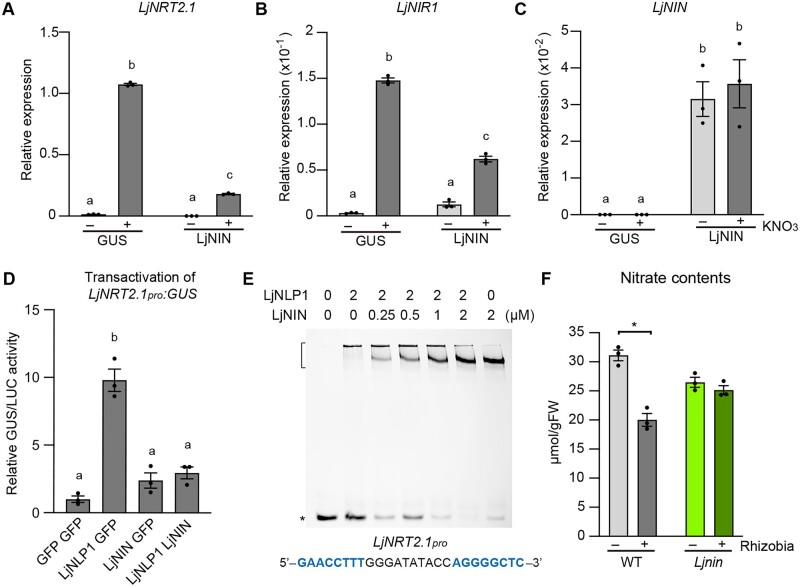
A previous study showed that LjNIN inhibits the nitrate-inducible expression of LjNRT2.1 and LjNIR1 (Soyano et al., 2015). We sought to elucidate a more detailed mechanism underlying this LjNIN-mediated repression of nitrate-inducible gene expression. In transgenic hairy roots, LjNIN overexpression reduced the nitrate-induced levels of LjNRT2.1 and LjNIR1 (Figure 10, A–C), confirming a previous finding (Soyano et al., 2015). Since LjNLP1 has now been identified as a transcription factor that induces LjNRT2.1 expression, and the NBS within the LjNRT2.1pro was required for the LjNLP1-dependent LjNRT2.1 expression (Figures 4, B and 5, A), we reasoned that LjNIN might interfere with LjNLP1 function via protein–DNA interaction, thereby downregulating LjNRT2.1 expression. To test this hypothesis, we first co-expressed LjNLP1 and LjNIN in L. japonicus protoplasts and measured the expression of LjNRT2.1. The co-expression of LjNLP1 and LjNIN significantly reduced the relative expression level of LjNRT2.1 compared to the case in which LjNLP1 alone was expressed (Figure 10D). In addition, EMSA showed LjNLP1 and LjNIN bound competitively to the cis-element of LjNRT2.1 promoter (Figure 10E).
Figure 10.
The regulation of LjNRT2.1 expression by LjNIN. A–C, RT-qPCR analysis of LjNRT2.1 (A), LjNIR1 (B), and LjNIN (C) expression in WT transgenic hairy roots transformed with the LjUBQpro:GUS or LjUBQpro:LjNIN constructs. Each cDNA sample was prepared from total RNA derived from noninoculated roots pretreated with 0 (−) or 10 mM KNO3 (+) for 24 h (n = 3 independent pools of roots derived from 10 plants). Transgenic roots were identified by GFP fluorescence. The expression of LjUBQ was used as the reference. Error bars indicate sem. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons). D, Transactivation of LjNRT2.1pro:GUS in L. japonicus mesophyll protoplasts transformed with three respective constructs (n = 3 independent pools of protoplasts). Transformed protoplasts were incubated with 10 mM KNO3. GUS activity was measured relative to 35Spro:LUC activity. Transactivation data are normalized using the condition in which GFP is expressed. Different letters indicate statistically significant differences (P < 0.05, one-way ANOVA followed by multiple comparisons). E, EMSA showing LjNLP1 or LjNIN binding to the cis-elements on the LjNRT2.1pro. MBP-LjNLP1 (573‒903) or MBP-LjNIN (551–878) recombinant proteins, consisting of an RWP-RK and a PB1 domain, were incubated with the FAM-labeled DNA probe (Figure 5A; Supplemental Data Set 1). Concentration of the proteins is shown. Bracket and asterisk, respectively, indicate the position of shifted bands showing protein–DNA interaction and of free probes that did not interact with proteins. F, Nitrate contents in inoculated (7 dai) (+) and noninoculated (−) roots of the WT and Ljnin-9 mutants (n = 3 independent pools of roots derived from four plants). The plants were first grown in each condition without KNO3, incubated with 10 mM KNO3 for 24 h, followed by measurement of the root nitrate contents. Error bars indicate sem. *P < 0.05 by a two-sided Welch’s t test.
Lastly, we investigated if rhizobial inoculation could influence nitrate uptake. To test this possibility, we measured the nitrate contents using inoculated and noninoculated WT plants grown without nitrate and then fed nitrate. The nitrate content in inoculated roots (7 dai) 24 h after nitrate treatment (10 mM) was lower than noninoculated roots of the same age (Figure 10F). Of note, the rhizobia-dependent reduction in nitrate uptake was not observed in the Ljnin mutants (Figure 10F). Therefore, these data suggest that LjNIN has a role in modulating nitrate uptake by counteracting the LjNLP1 function that activates LjNRT2.1 expression.
Discussion
The role of nitrate transporter genes in the nitrate-induced control of nodulation has been largely unknown. A recently identified gene, MtNPF7.6, is expressed in nodule transfer cells and mediates nitrate transport in nodules; Mtnpf7.6 mutations affect the nitrate-induced control of nodulation (Wang et al., 2020). In our study, we showed that the Ljnrt2.1 mutants maintained nodulation in high nitrate conditions. Although the MtNPF7.6 function appears to be restricted to nodules, LjNRT2.1 has a more general role in nitrate transport since it is involved in nitrate transport even in the absence of rhizobia.
In L. japonicus, LjNLP4 and LjNLP1 are known to have pivotal roles in transcriptional regulation in response to nitrate (Nishida et al., 2021); however, details about the genetic relationship of LjNLP4 and LjNLP1 have been incomplete. Here, we identified a critical functional difference between LjNLP4 and LjNLP1; LjNLP1 has an ability to induce LjNRT2.1 expression perhaps because LjNLP1 can bind to a cis-element of the LjNRT2.1 promoter more strongly than LjNLP4. MtNRT2.1 expression was compromised by the mutation of MtNLP1, which is orthologous to LjNLP1 (Lin et al., 2018). Therefore, the NLP1-NRT2.1 regulatory module may be conserved between L. japonicus and M. truncatula.
The nitrate uptake/transport process was attenuated not only by the Ljnrt2.1 mutations but also by the Ljnlp1 mutations. Nitrate-promoted shoot growth was more severely affected in the Ljnlp1 or the Ljnrt2.1 mutants than the Ljnlp4 mutants, an observation that may result from defects in nitrate uptake/transport. Phenotypic analysis using multiple mutants suggested that LjNRT2.1 acts in the same genetic pathway as LjNLP4 and LjNLP1 in the nitrate-induced control of nodulation. Furthermore, the nuclear localization of nitrate-dependent LjNLP4, a feature related to NLPs activity, was diminished by either the Ljnlp1 or the Ljnrt2.1 mutations.
Based on these observations and previous findings, we propose a signaling pathway model that acts in the nitrate-induced control of nodulation (Figure 11). In the model, LjNLP1 induces LjNRT2.1 expression in the presence of exogenous nitrate. The activated LjNRT2.1 may enhance nitrate transport, which ultimately triggers LjNLP4 nuclear localization. Then, the expression of symbiotic genes is induced or repressed by LjNLP4 depending on the nature of their regulation; LjNLP4 acts synergistically to inhibit nodulation. Since the expression of LjNRT2.1 was insufficient to rescue the Ljnlp1 mutations, LjNLP1 likely has different downstream pathway from that includes LjNRT2.1 to control nodulation. Indeed, we demonstrated that LjNLP1 can directly induce LjCLE-RS2 expression in response to nitrate. The NLP1-CLE regulatory module is also identified in M. truncatula (Luo et al., 2021). Considering LjNLP1’s specific role in response to nitrate, LjNLP1 must be activated in some way by nitrate. Generally, there are two types of nitrate transporters in terms of gene expression pattern: whose expression is either constitutively expressed or is affected by nitrate (Cerezo et al., 2001; Krapp et al., 2014). An unknown constitutively expressed nitrate transporter independent of LjNRT2.1 may mediate the first step of nitrate uptake/transport to activate LjNLP1. Such a transporter may function similarly to AtNRT1.1, which is thought to act upstream of AtNLP6/7-mediated nitrate signaling (Liu et al., 2017). Our results also show that the expression of LjNLP1 substituted for the LjNLP4 function. Although the results need to be carefully interpreted, as a constitutive promoter (LjUBQpro) was used in the assay, it is possible that LjNLP1 per se can regulate the expression of symbiotic genes in the same way as LjNLP4. Meanwhile, LjNLP4 was insufficient to replace LjNLP1’s function, which is likely to reflect the fact that LjNLP4 could not induce LjNRT2.1 expression. Elucidating the details of the functional overlap between LjNLP4 and LjNLP1 is a future challenge.
Figure 11.
Model for the nitrate-induced control of nodulation in L. japonicus. A, A model of the LjNLP1-LjNRT2.1-LjNLP4 signaling pathway, which controls nodulation in response to nitrate. Nitrate taken up from the soil leads to activation of LjNLP1, thereby enabling LjNLP1 to induce the expression of LjNRT2.1. LjNRT2.1 then plays a role in enhancing nitrate uptake/transport. LjNLP4 nuclear localization is triggered by the enhanced influx of nitrate. LjNLP4 subsequently induces the expression of LjCLE-RS2, a negative regulator of nodulation. LjNLP4 also represses the expression of genes for positive regulators of nodulation, including LjNF-YA, LjNF-YB, and LjEPR3, by interfering with LjNIN’s role in inducing these genes (Nishida et al., 2021). The resultant altered expression of these symbiotic genes acts synergistically to negatively regulate nodulation. B, A simplified model. In addition to the pathway including LjNRT2.1, LjNLP1 has a different downstream pathway to negatively regulate nodulation. Expression of LjNIN, a positive regulator of nodulation, is induced by rhizobial infection, and LjNIN has a role to block the LjNLP1-dependent expression of LjNRT2.1. Red indicates nitrate-related regulation.
The temporal expression pattern of LjNRT2.1 after nitrate treatment showed that nitrate-induced LjNRT2.1 activation is transient, suggesting there is a feedback mechanism to control LjNRT2.1 expression. Notably, AtNRT2.1 expression is downregulated by nitrate assimilation products (Lejay et al., 1999). In the Ljnlp4 mutants, the expression of LjNIA and LjNIR1 genes was reduced (Nishida et al., 2018). Therefore, the higher levels of LjNRT2.1 expression at later time points in the Ljnlp4 mutants relative to the WT may be due to failed feedback regulation of LjNRT2.1 by nitrate assimilation products. Although expression of the nitrate assimilation genes was also low in the Ljnlp1 mutants (Nishida et al., 2021), LjNRT2.1 expression was also compromised. Our interpretation of these results is that the defect in the induction of LjNRT2.1 expression in the Ljnlp1 mutants precedes that responsible for its feedback regulation by nitrate assimilation products.
How physiological processes in plants are regulated during root nodule symbiosis is poorly understood at the molecular level. Here, we provide a dataset that suggests that the nitrate uptake process can be modulated during nodulation. LjNIN, whose expression is induced specifically by rhizobial infection (Schauser et al., 1999; Suzaki et al., 2013), counteracts LjNLP1-dependent induction of LjNRT2.1 expression (Figure 11). LjNIN and LjNLP1 have common cis-elements on the LjNRT2.1 promoter. Importantly, the LjNIN–DNA interaction does not result in gene expression; instead, the interaction seems to block LjNRT2.1 expression by LjNLP1. A previous study that supports our notion indicated that the expression of LjNRT2.1 is downregulated by rhizobial inoculation (Criscuolo et al., 2012). Indeed, the negative regulation of LjNRT2.1 by LjNIN is associated with a reduction in nitrate uptake. The physiological significance of this mechanism needs to be elucidated in the future; however, an attractive hypothesis is that this mechanism may be relevant to a switch in the plant’s strategy concerning nitrogen acquisition. As nodule development progresses, legumes may switch from depending on nitrogen levels in the soil to symbiotic nitrogen fixation, thereby reducing soil nitrate uptake.
In Arabidopsis, the AtNRT2 family proteins are thought to play exclusive roles in HATS (Kiba et al., 2012; Krapp et al., 2014). AtNRT2.1 is responsible for 72% of HATS but is not involved in LATS (Wang et al., 1998; Filleur et al., 2001; Li et al., 2007). In L. japonicus, the effect of the Ljnrt2.1 mutation was pronounced in the presence of high nitrate (10 mM). As far as we examined, WT and Ljnrt2.1 mutants showed no obvious nodulation phenotypes at low nitrate concentrations. In addition, LjNRT2.1 is required for nitrate transport irrelevant to the exogenous nitrate concentration. Hence, it is possible that the biochemical function of LjNRT2.1 differs from that of AtNRT2.1.
Many current research efforts to learn about plant adaptive strategies in nitrogen-deficient environments are primarily focused on Arabidopsis, a nonnodulating plant. Arabidopsis seems to have developed HATS to acquire low concentrations of soil nitrogen successfully (Kiba and Krapp, 2016; Oldroyd and Leyser, 2020). In contrast, it is enigmatic how nodulating plants such as legumes have adapted to nitrogen-deficient environments when they do not produce nodules. Given that coexistence with rhizobia is a prerequisite in nature, we propose that legumes have always depended on symbiotic nitrogen fixation and might not have needed to develop HATS. An observation potentially related to this hypothesis is that the number of NRT2 family genes in legumes is lower than in Arabidopsis; there are three NRT2 family genes in L. japonicus and M. truncatula, five members in soybean, and seven NRT2 family genes in Arabidopsis (Valkov et al., 2020). A comparative functional analysis of NRT2 family genes in the future may provide clues for elucidating the conserved and diverse roles of nitrate transport systems in nodulating and nonnodulating plants.
Materials and methods
Plant materials and growth conditions
The Miyakojima MG-20 ecotype of L. japonicus was used as the WT plant (Kawaguchi, 2000). The nrsym3 and nrsym4 mutants were isolated in a previous screening for EMS mutants involved in the nitrate response during nodulation (Nishida et al., 2018). The Ljnrt2.1-1 mutants were backcrossed with WT once and descendent Ljnrt2.1-1 mutants were used for all analyses in this study. A description of Ljnlp4-1, Ljnlp1, and Ljnin-9 plants was published previously (Yoro et al., 2014; Nishida et al., 2018, 2021). Plants were grown with or without Mesorhizobium loti MAFF 303099 in autoclaved vermiculite or on 1% agar plates with Broughton and Dilworth (B&D; Broughton and Dilworth, 1971) solution under a 16-h light/8-h dark cycle at 24°C.
Genome-resequencing of the nrsym3 and nrsym4 mutants
The nrsym3 and nrsym4 mutants were crossed with MG-52, and F2 progeny displaying nitrate-tolerant phenotype were screened. Genomic DNA was extracted from pools of leaves derived from 20 plants using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Libraries were constructed using a TruSeq DNA Sample Prep Kit (Illumina, San Diego, CA, USA) and sequenced using a NextSeq 500 (Illumina) instrument with an 86-bp single-end sequencing protocol. Reads were mapped against the L. japonicus genome version 3.0 (http://www.kazusa.or.jp/lotus/) by Bowtie-0.12.9 (Langmead et al., 2009). Single-nucleotide polymorphism candidates were identified using the Mitsucal program (Suzuki et al., 2018).
Phylogenetic analysis
Phylogenetic analysis was conducted in MEGA X. The phylogenetic tree was built using the Neighbor-Joining method. The optimal tree with the sum of branch length = 2.47369594 was shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and were in the units of the number of amino acid substitutions per site. All positions containing gaps and missing data were eliminated (complete deletion option). Sequence alignment and machine-readable tree files are provided as Supplemental Files S1 and S2, respectively.
Constructs
The primers used for PCR are shown in Supplemental Data Set 1. For the complementation analysis of the nrsym3 mutants, a 6.4-kb genomic DNA fragment including the LjNRT2.1 candidate gene was amplified by PCR from WT genomic DNA. This fragment, including a 3.2-kb sequence directly upstream of the initiation codon, was cloned into pCAMBIA1300-GFP (Suzaki et al., 2019). For promoter-GUS analysis using L. japonicus hairy roots, a 3.2-kb fragment of the LjNRT2.1 promoter region was amplified by PCR from WT genomic DNA and cloned upstream of the GUS gene in the pCAMBIA1300-GUS-GFP-LjLTI6b vector (Nishida et al., 2016). For making LjNLP1pro:GUSplus:LjNLP1ter, LjNLP1pro:LjNLP1:LjNLP1ter, and LjNLP4pro:LjNLP4-myc constructs, each DNA fragment was amplified by PCR from WT genomic DNA, cDNA, or original vectors (Nishida et al., 2018; Suzaki et al., 2019) and inserted in pCAMBIA1300-GFP by the In-Fusion (Clontech, Mountain View, CA, USA) reaction. LjNLP4pro:GUS construct was previously created (Nishida et al., 2018). For epidermis-specific expression, first DNA fragments of Epi308pro and NOSter were amplified from an original vector (Hayashi et al., 2014) and inserted in pCAMBIA1300-GFP by the In-Fusion reaction. Then, the LjNRT2.1 CDS was inserted downstream of Epi308pro by the In-Fusion reaction. To make the constructs for recombinant protein expression in Escherichia coli, a gene encoding maltose-binding protein (MBP) and a portion of LjNLP1 (573‒903) was fused in the expression vector pMAL-c2X (New England Biolabs, Ipswich, MA, USA) by the In-Fusion reaction. MBP-LjNLP4 (564‒976) and MBP-LjNIN (551‒878) were previously created (Nishida et al., 2021). For the transactivation assay using protoplasts, LjNLP1 CDS was amplified by PCR from template cDNA prepared from WT roots and inserted downstream of LjUBQpro in the vector with pUC19 backbone (Nishida et al., 2021) by the In-Fusion reaction. A 3.2-kb fragment of the LjNRT2.1 promoter region was amplified by PCR from WT genomic DNA and inserted upstream of GUS-RBCSter cassette in the vector with pUC19 backbone (Nishida et al., 2021) by the In-Fusion reaction. To make the LjNRT2.1(ΔNBS)pro:GUS construct, the upstream and downstream regions across the LjNIN-binding site on the LjNRT2.1 promoter were, respectively, amplified by PCR and ligated using PstI site and inserted upstream of GUS-RBCSter cassette in the vector with pUC19 backbone (Nishida et al., 2021) by the In-Fusion reaction. The DNA fragments of a part of LjCLE-RS2pro fused with 35S minimal promoter with or without LjNLP4/LjNIN-binding sequence (NRE/NBS) were artificially synthesized based on previous finding (Soyano et al., 2014). They were amplified by PCR and inserted upstream of GUS-RBCSter cassette in the vector with pUC19 backbone (Nishida et al., 2021) by the In-Fusion reaction. Other constructs, LjUBQpro:GFP, LjUBQpro:LjNLP4, LjUBQpro:LjNIN, and 35Spro:LUC, were previously created (Nishida et al., 2021). A previously created LjUBQpro:LjNLP4-myc construct (Nishida et al., 2018) was used for immunohistochemistry. For LjNRT2.1, LjNLP4, or LjNLP1 overexpression in L. japonicus hairy roots, CDS of each gene was amplified by PCR from template cDNA prepared from WT roots and was cloned into the pENTR/D-TOPO vector (Invitrogen, Waltham, MA, USA). The insert was transferred into pUB-GW-GFP (Maekawa et al., 2008) by the LR recombination reaction. The LjUBQpro:GUS or LjUBQpro:LjNIN constructs for hairy roots transformation were described previously (Suzaki et al., 2012; Nishida et al., 2018).
Hairy root transformation of L. japonicus
Respective constructs were introduced into A. rhizogenes AR1193 strain. Hairy root transformation of L. japonicus was conducted based on the method (Okamoto et al., 2013).
Measurement of nitrate uptake and contents
For the analysis using an 15N stable isotope, samples were prepared based on the method described previously (Tabata et al., 2014). In inoculated conditions, plants were grown with 0.2 or 5mM KNO3 in the presence of rhizobia for 12 days, and plants were then treated with 0.2 or 5mM K15NO3 for 24 h. In noninoculated conditions, 12-day-old noninoculated seedlings grown on B&D medium without KNO3 were transferred to new B&D medium with 0.2 or 10mM KNO3 for 12 h. Then, they were washed by 0.1mM CaSO4 for 1 min and transferred to B&D medium with 0.2 or 10mM K15NO3 for 5 min. At the end of the 15N labeling, roots were washed for 1 min in 0.1mM CaSO4 and were separated from shoots. Each sample was dried for 2 days at 75°C and analyzed for total N and 15N contents by elemental analysis–isotope ratio mass spectrometry (Flash 2000-DELTA plus Advantage ConFlo III System; Thermo Fisher Scientific, Waltham, MA, USA).
Nitrate contents were measured based on the method previously described (Hachiya and Okamoto, 2017). Seedlings grown under each experimental condition were treated with 10mM KNO3. Then, they were washed by sterilized water and frozen by liquid nitrogen. Each sample was crushed using a TissueLyser II (Qiagen), and 10 μL of sterilized water at 80°C was added per 1 mg of sample weight, and vortexing was performed every 5 min for 20 min at 100°C. The sample was then chilled on ice and spun down. The supernatant was stored as extraction solution at −80°C. About 20 μL of 0.05% salicylic acid in sulfuric acid or sulfuric acid was added to 5 μL of extraction solution in a tube, which was vortexed, spun down, and left at room temperature for 20 min. Then, 500 μL of 8% NaOH in sterilized water was added and the mixture was vortexed until it became clear. Nitrate content in the solution was determined by absorbance at 410 nm using a Multiskan GO (Thermo Fisher Scientific) or a Synergy LX (Biotek, Winooski, VT, USA).
EMSA
Recombinant proteins were prepared based on the method previously described (Nishida et al., 2021). For preparing the probes, DNA fragments (Supplemental Data Set 1) were labeled with carboxyfluorescein (FAM). The labeled DNA fragments were purified on the Superdex 200 10/300 GL column (GE Healthcare, Chicago, IL, USA). The purified DNA fragments (0.25 µM) and poly (dI-dC) (50 ng/µL) were mixed with the purified proteins in buffer D (10mM Tris–HCl pH 7.5, 100mM KCl, 100mM NaCl, 1mM DTT, 2.5% glycerol, and 5mM MgCl2), and incubated at 25°C for 30 min. The mixtures were loaded on a 10% polyacrylamide gel, and fluorescence was detected using LuminoGraph III WSE-6300 (ATTO, Tokyo, Japan).
Gene expression analysis
The primers used for PCR are shown in Supplemental Data Set 1. Total RNA was isolated from whole roots using the PureLink Plant RNA Reagent (Invitrogen) or the Plant Total RNA Mini Kit (Favorgen Biotech, Ping-Tung, Taiwan). First-strand cDNA was prepared using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan). RT-qPCR was performed using a 7900HT Real-Time PCR system (Applied Biosystems Waltham, MA, USA) with a THUNDERBIRD SYBR qPCR Mix (Toyobo) following the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemistry was conducted based on the method (Nishida et al., 2018). A monoclonal anti-myc antibody and an antibody conjugated to Alexa Fluor 488 Plus anti-mouse IgG-Alexa Fluor Plus 488 (Invitrogen) were used for detecting the signal derived from LjNLP4-myc. Before observing the signal, the roots were stained with 5μg mL−1 4', 6-diamidino-2-phenylindole (DAPI; Dojindo, Kumamoto, Japan) for 15 min. Fluorescent images were obtained using an LSM700 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany) equipped with ZEN software (Carl Zeiss). The obtained images were analyzed using ImageJ; first, the threshold of the green signals derived from LjNLP4-myc was set equally among the images, and then the ratio of the number of the nuclei with green signals was quantified against the number of the total, namely DAPI-stained, nuclei in every image.
Transactivation using L. japonicus protoplasts
The isolation of L. japonicus mesophyll protoplasts and the transactivation assay were conducted based on the method (Nishida et al., 2021). To exclude the effect of endogenous nitrate on gene expression, plants were grown without nitrate but with 10mM NH4Cl for 16 days. Fluorescence and luminescence were measured using a Synergy LX (Biotek). For transformation of protoplasts, equal amount of DNA (10 µg each) of effector, reporter and internal control plasmids were used. 35Spro:LUC was used as the internal control plasmid.
Immunoblot analysis
The plants with transgenic hairy roots were treated with 10mM KNO3 for 1 h, and their roots (150 mg), which were derived from five plants, were ground by bead beating after freezing with liquid nitrogen. Lysis buffer (50mM Tris–HCl, pH 8.0, 120mM NaCl, 0.2mM sodium orthovanadate, 100mM NaF, 10% glycerol, 0.2% Triton X-100, 5mM DTT, 5mM EDTA, 1mM PMSF, and 1× protein inhibitor cocktail [Nacalai Tesque, Kyoto, Japan]) were added. The ground roots and buffer were thoroughly mixed by bead beating, and then sonicated on ice. The suspension was centrifuged at 20,000 g for 20 min at 4°C. The supernatant was collected and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 4%–15% Mini-PROTEAN TG Precast Protein Gels (Bio-Rad, Hercules, CA, USA). The separated proteins were electrically transferred onto a PVDF membrane (Amersham Hybond P PVDF; GE Healthcare). A primary antibody against c-Myc (Santa Crux Biotechnology, Dallas, TX, USA; catalog no. c-789) diluted in blocking buffer (1% bovine standard albumin and 1% polyvinylpyrrolidone in TBST buffer [Tris-buffered saline, 0.1% Tween-20]) was applied to the membrane for overnight incubation at 4°C. After extensive washing, a Horseradish Peroxidase (HRP)-conjugated secondary antibody (Rockland; catalog no. 18-8816-31) diluted in the same blocking buffer was applied for 1 h at room temperature. Immobilon Forte Western HRP substrate (Merck, Kenilworth, NJ, USA) chemiluminescence reagent was used to detect antibody binding. Signals were detected using LuminoGraph III WSE-6300 (ATTO). For antibody stripping, the membrane was incubated in stripping buffer (6.4mM Tris–HCl, pH 6.8, 2% SDS, and 10mM DTT) for 45 min at 50°C. After being washed by the TBST buffer, the membrane was incubated with a primary antibody against GFP (Invitrogen; catalog no. A11122) diluted in blocking buffer. The antibody binding was detected in the same way as above.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA). Normality was checked using the Shapiro-Wilk test and P > 0.05 was considered as normal distribution. The F-test was used to test if the variances of two populations were equal or not. Appropriate methods were chosen according to the nature of the data. The criterion of P < 0.05 means statistically significant difference in this study. The results of the statistical analyses are shown in Supplemental Data Set 2.
Accession number
Data from the short reads from genome-resequencing of nrsym3 and nrsym4 were deposited in the DNA Data Bank of Japan Sequence Read Archive under the accession number DRA011845.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Allelism test of the nrsym3 and the nrsym4 mutants (Supports Figure 1).
Supplemental Figure S2. Complementation of the nrsym3 phenotypes (Supports Figure 1).
Supplemental Figure S3. Comparison of LjNRT2.1 and LjNRT2.2 (Supports Figure 1).
Supplemental Figure S4. Nitrate effects on nodulation of multiple combinations of mutants among Ljnlp4, Ljnlp1, and Ljnrt2.1 (Supports Figure 2).
Supplemental Figure S5. Effects of the Ljnrt2.1 mutations on nitrate uptake and gene expression (Supports Figure 3).
Supplemental Figure S6. A schematic diagram of the promoter-GUS constructs used in transactivation assay (Supports Figures 4 and 5).
Supplemental Figure S7. Complementation of the Ljnrt2.1, Ljnlp4, and Ljnlp1 phenotypes and LjNRT2.1pro:GUS (Supports Figure 6).
Supplemental Figure S8. The ratio of LjNLP4-accumulating nuclei in immunohistochemistry (Supports Figure 7).
Supplemental Data Set 1. Primers used in this study.
Supplemental Data Set 2. Statistical analysis data.
Supplemental File S1. Sequence alignment of NRT2s in fasta format.
Supplemental File S2 . Phylogenetic tree in newick format.
Supplementary Material
Acknowledgments
We thank Yoshikatsu Matsubayashi and Takatoshi Kiba for valuable suggestions; Takushi Hachiya and Kazuko Ito for technical support; Haruko Imaizumi-Anraku for providing Epipro vector.
Funding
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (JP19H03239 and JP20H05908), by Japan Science Technology Agency (JST) Exploratory Research for Advanced Technology (ERATO) (JPMJER1502).
Conflict of interest statement. The authors declare no conflict of interests.
Contributor Information
Fumika Misawa, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Momoyo Ito, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Shohei Nosaki, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan; Tsukuba Plant-Innovation Research Center, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Hanna Nishida, Institute of Agrobiological Sciences, National Agriculture and Food Research Organization, Tsukuba, Ibaraki, Japan.
Masahiro Watanabe, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Takamasa Suzuki, College of Bioscience and Biotechnology, Chubu University, Kasugai, Aichi, Japan.
Kenji Miura, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan; Tsukuba Plant-Innovation Research Center, University of Tsukuba, Tsukuba, Ibaraki, Japan.
Masayoshi Kawaguchi, National Institute for Basic Biology, Okazaki, Aichi, Japan; School of Life Science, Graduate University for Advanced Studies, Okazaki, Aichi, Japan.
Takuya Suzaki, Faculty of Life and Environmental Sciences, University of Tsukuba, Tsukuba, Ibaraki, Japan; Tsukuba Plant-Innovation Research Center, University of Tsukuba, Tsukuba, Ibaraki, Japan.
These authors contributed equally (F.M. and M.I.).
T.Suza. conceived the project. F.M., M.I., H.N., M.K., and T.Suza. designed the experiments. F.M., M.I., S.N., H.N., M.W., K.M., T.Suzu., and T.Suza. performed experiments and analyzed the data. T.Suza. wrote the manuscript, which was approved by all authors.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) is: Takuya Suzaki (suzaki.takuya.fn@u.tsukuba.ac.jp).
References
- Broughton WJ, Dilworth MJ (1971) Control of leghaemoglobin synthesis in snake beans. Biochem J 125: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Mathews A (1990) Nitrate inhibition of nodulation in legumes. In Gresshof PM, ed, The Molecular Biology of Symbiotic Nitrogen Fixation, CRC Press, Boca Raton, FL, pp 159–180 [Google Scholar]
- Carroll BJ, McNeil DL, Gresshoff PM (1985) A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol 78: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M, Tillard P, Filleur S, Munos SP, Daniel-Vedele FO, Gojon A (2001) Major alterations of the regulation of root uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol 127: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuolo G, Valkov VT, Parlati A, Alves LM, Chiurazzi M (2012) Molecular characterization of the Lotus japonicus NRT1(PTR) and NRT2 families. Plant Cell Environ 35: 1567–1581 [DOI] [PubMed] [Google Scholar]
- Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F (2001) An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett 489: 220–224 [DOI] [PubMed] [Google Scholar]
- Hachiya T, Okamoto Y (2017) Simple spectroscopic determination of nitrate, nitrite, and ammonium in Arabidopsis thaliana. Bio-protocol 7: e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Shimoda Y, Sato S, Tabata S, Imaizumi-Anraku H, Hayashi M (2014) Rhizobial infection does not require cortical expression of upstream common symbiosis genes responsible for the induction of Ca2+ spiking. Plant J 77: 146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF (2009) CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Kawaguchi M (2000) Lotus japonicus `Miyakojima' MG-20: an early-flowering accession suitable for indoor handling. J Plant Res 113: 507–509 [Google Scholar]
- Kawaharada Y, Kelly S, Nielsen MW, Hjuler CT, Gysel K, Muszynski A, Carlson RW, Thygesen MB, Sandal N, Asmussen MH, et al. (2015) Receptor-mediated exopolysaccharide perception controls bacterial infection. Nature 523: 308–312 [DOI] [PubMed] [Google Scholar]
- Kawaharada Y, Nielsen MW, Kelly S, James EK, Andersen KR, Rasmussen SR, Füchtbauer W, Madsen LH, Heckmann AB, Radutoiu S, et al. (2017) Differential regulation of the Epr3 receptor coordinates membrane-restricted rhizobial colonization of root nodule primordia. Nat Commun 8: 14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Feria-Bourrellier AB, Lafouge F, Lezhneva L, Boutet-Mercey S, Orsel M, Bréhaut V, Miller A, Daniel-Vedele F, Sakakibara H, et al. (2012) The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. Plant Cell 24: 245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Krapp A (2016) Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol 57: 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, David LC, Chardin C, Girin T, Marmagne A, Leprince AS, Chaillou S, Ferrario-Méry S, Meyer C, Daniel-Vedele F (2014) Nitrate transport and signalling in Arabidopsis. J Exp Bot 65: 789–798 [DOI] [PubMed] [Google Scholar]
- Krusell L, Madsen LH, Sato S, Aubert G, Genua A, Szczyglowski K, Duc G, Kaneko T, Tabata S, de Bruijn F, et al. (2002) Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 420: 422–426 [DOI] [PubMed] [Google Scholar]
- Laffont C, Ivanovici A, Gautrat P, Brault M, Djordjevic MA, Frugier F (2020) The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat Commun 11: 3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two uptake systems by N- and C-status of Arabidopsis plants. Plant J 18: 509–519 [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, Glass ADM (2007) Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol 143: 425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zheng Z, Kong X, Xu J, Qiu L, Sun J, Reid D, Jin H, Andersen SU, Oldroyd GED, Stougaard J, Downie JA, et al. (2019) Atypical receptor kinase RINRK1 required for rhizobial infection but not nodule development in Lotus japonicus. Plant Physiol 181: 804–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Li X, Luo Z, Mysore KS, Wen J, Xie F (2018) NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat Plants 4: 942–952 [DOI] [PubMed] [Google Scholar]
- Liu KH, Niu Y, Konishi M, Wu Y, Du H, Sun Chung H, Li L, Boudsocq M, McCormack M, Maekawa S, Ishida T, Zhang C, et al. (2017) Discovery of nitrate–CPK–NLP signalling in central nutrient–growth networks. Nature 545: 311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Lin JS, Zhu Y, Fu M, Li X, Xie F (2021) NLP1 reciprocally regulates nitrate inhibition of nodulation through SUNN-CRA2 signaling in Medicago truncatula. Plant Commun 2: 100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Kusakabe M, Shimoda Y, Sato S, Tabata S, Murooka Y, Hayashi M (2008) Polyubiquitin promoter-based binary vectors for overexpression and gene silencing in Lotus japonicus. Mol Plant-Microbe Interact 21: 375–382 [DOI] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A (2013) Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nat Commun 4: 1713. [DOI] [PubMed] [Google Scholar]
- Mens C, Hastwell AH, Su H, Gresshoff PM, Mathesius U, Ferguson BJ (2021) Characterisation of Medicago truncatula CLE34 and CLE35 in nitrate and rhizobia regulation of nodulation. New Phytol 229: 2525–2534 [DOI] [PubMed] [Google Scholar]
- Moreau C, Gautrat P, Frugier F (2021) Nitrate-induced CLE35 signaling peptides inhibit nodulation through the SUNN receptor and miR2111 repression. Plant Physiol 185: 1216–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD (2011) Invasion by invitation: rhizobial infection in legumes. Mol Plant-Microbe Interact 24: 631–639 [DOI] [PubMed] [Google Scholar]
- Nishida H, Handa Y, Tanaka S, Suzaki T, Kawaguchi M (2016) Expression of the CLE-RS3 gene suppresses root nodulation in Lotus japonicus. J Plant Res 129: 909–919 [DOI] [PubMed] [Google Scholar]
- Nishida H, Ito M, Miura K, Kawaguchi M, Suzaki T (2020) Autoregulation of nodulation pathway is dispensable for nitrate-induced control of rhizobial infection. Plant Signal Behav 15: 1733814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Nosaki S, Suzuki T, Ito M, Miyakawa T, Nomoto M, Tada Y, Miura K, Tanokura M, Kawaguchi M, et al. (2021) Different DNA-binding specificities of NLP and NIN transcription factors underlie nitrate-induced control of root nodulation. Plant Cell 33: 2340–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H, Suzaki T (2018a) Two negative regulatory systems of root nodule symbiosis: how are symbiotic benefits and costs balanced? Plant Cell Physiol 59: 1733–1738 [DOI] [PubMed] [Google Scholar]
- Nishida H, Suzaki T (2018b) Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol 44: 129–136 [DOI] [PubMed] [Google Scholar]
- Nishida H, Tanaka S, Handa Y, Ito M, Sakamoto Y, Matsunaga S, Betsuyaku S, Miura K, Soyano T, Kawaguchi M, et al. (2018) A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat Commun 9: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R, Hayashi M, Wu GJ, Kouchi H, Imaizumi-Anraku H, Murakami Y, Kawasaki S, Akao S, Ohmori M, Nagasawa M, et al. (2002) HAR1 mediates systemic regulation of symbiotic organ development. Nature 420: 426–429 [DOI] [PubMed] [Google Scholar]
- Okamoto S, Ohnishi E, Sato S, Takahashi H, Nakazono M, Tabata S, Kawaguchi M (2009) Nod factor/nitrate-induced CLE genes that drive HAR1-mediated systemic regulation of nodulation. Plant Cell Physiol 50: 67–77 [DOI] [PubMed] [Google Scholar]
- Okamoto S, Yoro E, Suzaki T, Kawaguchi M (2013) Hairy root transformation in Lotus japonicus. Bio-protocol 3: e795 [Google Scholar]
- Oldroyd GE, Murray JD, Poole PS, Downie JA (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45: 119–144 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Leyser O (2020) A plant’s diet, surviving in a variable nutrient environment. Science 368: eaba0196. [DOI] [PubMed] [Google Scholar]
- Pellizzaro A, Alibert B, Planchet E, Limami AM, Morère-Le Paven MC (2017) Nitrate transporters: an overview in legumes. Planta 246: 585–595 [DOI] [PubMed] [Google Scholar]
- Roy S, Liu W, Nandety RS, Crook A, Mysore KS, Pislariu CI, Frugoli J, Dickstein R, Udvardi MK (2020) Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32: 15–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402: 191–195 [DOI] [PubMed] [Google Scholar]
- Schauser L, Wieloch W, Stougaard J (2005) Evolution of NIN-like proteins in Arabidopsis, rice, and Lotus japonicus. J Mol Evol 60: 229–237 [DOI] [PubMed] [Google Scholar]
- Schnabel E, Journet EP, de Carvalho-Niebel F, Duc G, Frugoli J (2005) The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol Biol 58: 809–822 [DOI] [PubMed] [Google Scholar]
- Searle IR, Men AE, Laniya TS, Buzas DM, Iturbe-Ormaetxe I, Carroll BJ, Gresshoff PM (2003) Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 299: 109–112 [DOI] [PubMed] [Google Scholar]
- Shrestha A, Zhong S, Therrien J, Huebert T, Sato S, Mun T, Andersen SU, Stougaard J, Lepage A, Niebel A, et al. (2021) Lotus japonicus Nuclear Factor YA1, a nodule emergence stage-specific regulator of auxin signalling. New Phytol 229: 1535–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Hirakawa H, Sato S, Hayashi M, Kawaguchi M (2014) NODULE INCEPTION creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc Natl Acad Sci USA 111: 14607–14612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Kouchi H, Hirota A, Hayashi M (2013) NODULE INCEPTION directly targets NF-Y subunit genes to regulate essential processes of root nodule development in Lotus japonicus. PLoS Genet 9: e1003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T, Shimoda Y, Hayashi M (2015) NODULE INCEPTION antagonistically regulates gene expression with nitrate in Lotus japonicus. Plant Cell Physiol 56: 368–376 [DOI] [PubMed] [Google Scholar]
- Streeter J, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. Crit Rev Plant Sci 7: 1–23 [Google Scholar]
- Suzaki T, Kim CS, Takeda N, Szczyglowski K, Kawaguchi M (2013) TRICOT encodes an AMP1-related carboxypeptidase that regulates root nodule development and shoot apical meristem maintenance in Lotus japonicus. Development 140: 353–361 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Takeda N, Nishida H, Hoshino M, Ito M, Misawa F, Handa Y, Miura K, Kawaguchi M (2019) LACK OF SYMBIONT ACCOMMODATION controls intracellular symbiont accommodation in root nodule and arbuscular mycorrhizal symbiosis in Lotus japonicus. PLoS Genet 15: e1007865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Yano K, Ito M, Umehara Y, Suganuma N, Kawaguchi M (2012) Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 139: 3997–4006 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kawai T, Takemura S, Nishiwaki M, Suzuki T, Nakamura K, Ishiguro S, Higashiyama T (2018) Development of the Mitsucal computer system to identify causal mutation with a high-throughput sequencer. Plant Reprod 31: 117–128 [DOI] [PubMed] [Google Scholar]
- Tabata R, Sumida K, Yoshii T, Ohyama K, Shinohara H, Matsubayashi Y (2014) Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science 346: 343–346 [DOI] [PubMed] [Google Scholar]
- Valkov VT, Rogato A, Alves LM, Sol S, Noguero M, Léran S, Lacombe B, Chiurazzi M (2017) The nitrate transporter family protein LjNPF8.6 controls the N-fixing nodule activity. Plant Physiol 175: 1269–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkov VT, Sol S, Rogato A, Chiurazzi M (2020) The functional characterization of LjNRT2.4 indicates a novel, positive role of nitrate for an efficient nodule N2-fixation activity. New Phytol 228: 682–696 [DOI] [PubMed] [Google Scholar]
- Vernié T, Kim J, Frances L, Ding Y, Sun J, Guan D, Niebel A, Gifford ML, de Carvalho-Niebel F, Oldroyd GED (2015) The NIN transcription factor coordinates diverse nodulation programs in different tissues of the Medicago truncatula root. Plant Cell 27: 3410–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Huang Y, Ren Z, Zhang X, Ren J, Su J, Zhang C, Tian J, Yu Y, Gao GF, et al. (2020) Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat Plants 6: 800–808 [DOI] [PubMed] [Google Scholar]
- Wang R, Liu D, Crawford NM (1998) The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA 95: 15134–15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendrek CR, Lee YC, Morris V, Liang Y, Pislariu CI, Burkart G, Meckfessel MH, Salehin M, Kessler H, Wessler H, et al. (2010) A putative transporter is essential for integrating nutrient and hormone signaling with lateral root growth and nodule development in Medicago truncatula. Plant J 62: 100–112 [DOI] [PubMed] [Google Scholar]
- Yoro E, Suzaki T, Toyokura K, Miyazawa H, Fukaki H, Kawaguchi M (2014) A positive regulator of nodule organogenesis, NODULE INCEPTION, acts as a negative regulator of rhizobial infection in Lotus japonicus. Plant Physiol 165: 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.