Abstract
Stomata optimize land plants’ photosynthetic requirements and limit water vapor loss. So far, all of the molecular and electrical components identified as regulating stomatal aperture are produced, and operate, directly within the guard cells. However, a completely autonomous function of guard cells is inconsistent with anatomical and biophysical observations hinting at mechanical contributions of epidermal origins. Here, potassium (K+) assays, membrane potential measurements, microindentation, and plasmolysis experiments provide evidence that disruption of the Arabidopsis thaliana K+ channel subunit gene AtKC1 reduces pavement cell turgor, due to decreased K+ accumulation, without affecting guard cell turgor. This results in an impaired back pressure of pavement cells onto guard cells, leading to larger stomatal apertures. Poorly rectifying membrane conductances to K+ were consistently observed in pavement cells. This plasmalemma property is likely to play an essential role in K+ shuttling within the epidermis. Functional complementation reveals that restoration of the wild-type stomatal functioning requires the expression of the transgenic AtKC1 at least in the pavement cells and trichomes. Altogether, the data suggest that AtKC1 activity contributes to the building of the back pressure that pavement cells exert onto guard cells by tuning K+ distribution throughout the leaf epidermis.
Inactivation of the Arabidopsis K+ channel gene AtKC1 reveals that interactions and K+ shuttling between guard cells, pavement cells, and trichomes contribute to the non-autonomous stomatal responses.
IN A NUTSHELL.
Background: The water that plants absorb from the soil is mainly lost through evaporation at the leaf surface across microscopic pores called stomata. On the other hand, these pores also allow the diffusion of CO2 from the atmosphere to the internal tissues for photosynthesis. Stomata are surrounded by two specialized cells, called guard cells. Changes in guard cell turgor allow them to swell or shrink for enlarging or narrowing the pore. This control is aimed at preventing excessive water loss. Gaining knowledge on the underlying mechanisms will provide tools to optimize water use by plants.
Question: The epidermal cells surrounding the guard cells, called pavement cells, form an anatomical obstacle that limits the movement of the guard cells. Their turgidity exerts a counter-pressure on the guard cells that opposes stomatal opening. The molecular mechanisms underlying this non-autonomous control of stomatal aperture by guard cells are poorly understood.
Findings: We show that disruption of the Arabidopsis thaliana K+ channel subunit gene AtKC1 reduces pavement cell turgor, due to decreased K+ accumulation, without affecting guard cell turgor. This results in a decrease in the back-pressure of pavement cells onto guard cells. The atkc1 mutation increases the sensitivity of the pavement cell membrane electrical potential to external K+, revealing an impaired control of K+ transport properties. Restoration of the wild-type stomatal phenotype requires expression of AtKC1 in the pavement cells but also in another epidermal cell type, the trichomes. Thus, the whole epidermis appears to contribute to the non-autonomous control of transpirational water loss.
Next steps: The family of K+ channels to which AtKC1 belongs is strongly conserved in plants and comprises AtKC1 homologs in every species. Whether the present information is translatable to crops should be investigated. The role of trichomes in regulating stomatal aperture via AtKC1 also deserves further research.
Introduction
In land plants, the epidermis is covered by a non-permeable waxy cuticle, and the diffusion of CO2 from the atmosphere to inner photosynthetic tissues takes place through microscopic pores present on the leaf surface. Each of these pores is surrounded by a pair of osmocontractile cells, named guard cells, together forming a stoma. The physical continuum provided by the stomata between the leaf inner tissue and the atmosphere also enables transpiration, which has however to be tightly controlled to avoid desiccation.
The epidermis comprises three main types of clonally related cells: pavement cells, guard cells, and trichomes. Embedded within the epidermal cell layer, guard cells can be in direct contact with surrounding pavement cells (e.g. in A. thaliana; Supplemental Figure S1, left photo column), or associated with subsidiary cells (Nguyen et al., 2017) to form a stomatal complex (Gray et al., 2020). The molecular and osmotic machinery responsible for the changes in guard cell turgor that either open (Tominaga et al., 2001; Jammes et al., 2014) or close the stomatal pore has been deeply investigated (Jezek and Blatt, 2017). All components regulating stomatal movements, even the cell-to-cell mobile abscisic acid (ABA) stress hormone (Bauer et al., 2013), are produced and act directly within the guard cells. For instance, in response to low atmospheric or soil humidity, ABA initiates stomatal closing by binding to a subfamily of cytosolic receptors within the guard cells to activate a phosphorylation-based signaling cascade leading to reduced cell turgor by modulating interdependent H+, potassium (K+), and anion fluxes (Hedrich, 2012; Jezek and Blatt, 2017). In angiosperms, mature guard cells are thought to lack plasmodesmata with adjoining cells (Wille and Lucas, 1984; Palevitz and Hepler, 1985), reinforcing the notion of their self-sufficient functioning. Highly purified guard cell protoplasts have been extensively used to characterize in detail the ABA-induced events that include changes in ion transport activities (Jezek and Blatt, 2017) as well as transcriptomic (Leonhardt et al., 2004; Wang et al., 2011), proteomic (Zhao et al., 2008), and metabolomic profiles (Jin et al., 2013; Misra et al., 2015; Zhu and Assmann, 2017).
K+ is a major osmoticum in this machinery (Humble and Raschke, 1971; Talbott and Zeiger, 1996; Hedrich, 2012; Jezek and Blatt, 2017; Britto et al., 2021). K+ fluxes into, or out of, guard cells, resulting in stomatal opening or closure, respectively, involve voltage-gated K+ channels of the Shaker family (Blatt, 2000; Véry and Sentenac, 2003; Pandey et al., 2007; Kim et al., 2010; Hedrich, 2012; Véry et al., 2014). Gene expression studies, electrophysiological analyses, and reverse genetics approaches carried out in Arabidopsis have revealed that K+ influx into guard cells, leading to stomatal opening, is strongly dependent on expression of the inwardly rectifying hyperpolarization-activated Shaker K+ channels KAT1 and KAT2 (K+ channel in A. thaliana 1 and 2) (Lebaudy et al., 2008, 2010), while the efflux of K+ from guard cells, allowing stomatal closure, involves expression of the outwardly rectifying depolarization-activated Shaker K+ channel GORK (Hosy et al., 2003).
The large body of cellular and molecular information leads to the conclusion that guard cells possess all necessary molecular and electrical components in stomatal control. This understanding is, however, unmoored from the biophysical and anatomical approaches of stomatal regulation within its epidermal context, in which the embedded guard cells are subjected to mechanical and physiological exertions from their neighboring pavement/subsidiary cells (Jezek et al., 2019). For example, stomatal conductances can show considerable microheterogeneity in the leaf even when this organ is kept in a constant environment. This has been attributed to variable or unstable hydraulic interactions between guard cells with their surrounding pavement cells, usually within leaf sectors defined anatomically by vein patterns (Mott and Buckley, 2000). Also, at the cell level, when a series of reductions of turgor are experimentally imposed on both guard and pavement cells of epidermal strips, by increasing the concentration of an osmotically active solute in the external solution, the stomatal aperture does not narrow, as would be expected if guard cells responded independently of pavement cells. Rather, the pore aperture will widen in a first phase. When the concentration of the external solute is further increased, the stomatal aperture will then narrow in a more gradual second phase. In contrast, if the turgor of the pavement/subsidiary cell is selectively ablated, the stomatal aperture will simply narrow in a “monotonic” way with the increase in external solute concentration (MacRobbie, 1980). These observations suggest that, within an epidermal layer, the stomatal aperture is not autonomously regulated, but conjointly set by, at least, the relative turgor that opposes the guard cells with the surrounding pavement cells. Much is still unknown about the mechanisms that underlie the non-autonomous stomatal response, such as the molecular, cellular, and physiological bases of the interacting mechanisms, and the responsible epidermal cell types or their locations in the leaf. Here, we show that the Shaker channel gene AtKC1 (A. thaliana K+ channel 1) (accession number AT4G32650) contributes to these mechanisms.
Shaker channels, which dominate the plasma membrane conductance to K+ in most cell types, are encoded by a family of nine members in Arabidopsis (Véry et al., 2014). These channels are sensitive to voltage and activated by either membrane depolarization for K+ efflux (outwardly rectifying channels), or membrane hyperpolarization allowing K+ influx (inwardly rectifying channels). They are tetrameric proteins, and the four subunits that assemble to form a functional protein can be encoded by the same Shaker gene (giving rise to a homotetrameric channel) or different Shaker genes (heterotetrameric channel) (Daram et al., 1997; Urbach et al., 2000; Jegla et al., 2018). The Shaker subunit encoded by AtKC1 (At4G32650) has been termed a “silent” Shaker channel subunit (Reintanz et al., 2002) because it does not form functional channels on its own but only when in complex with other inwardly rectifying channel subunits to modulate the functional properties of the channel, including voltage sensitivity (Duby et al., 2008; Geiger et al., 2009; Honsbein et al., 2009; Jeanguenin et al., 2011; Zhang et al., 2015; Wang et al., 2016).
AtKC1 is expressed in roots and leaves (Reintanz et al., 2002; Pilot et al., 2003). In the root, it is expressed in the periphery cells, where it associates with the AKT1 inward Shaker subunit and thereby plays a role in channel-dependent K+ uptake from the soil (Geiger et al., 2009; Honsbein et al., 2009). In leaves, AtKC1 is expressed in the whole epidermal tissue, that is in trichomes, hydathodes, pavement cells, and guard cells (Pilot et al., 2003; Supplemental Figure S1), in contrast to the two other well-studied inward Shaker channel genes KAT1 and KAT2 whose expression pattern in the leaf epidermis is restricted to guard cells (Nakamura et al., 1995; Pilot et al., 2001). In this report, we show that AtKC1 contributes to stomatal aperture regulation by modulating conflicting turgors of guard cells and surrounding pavement cells.
Results
Disruption of AtKC1 impairs the control of stomatal aperture
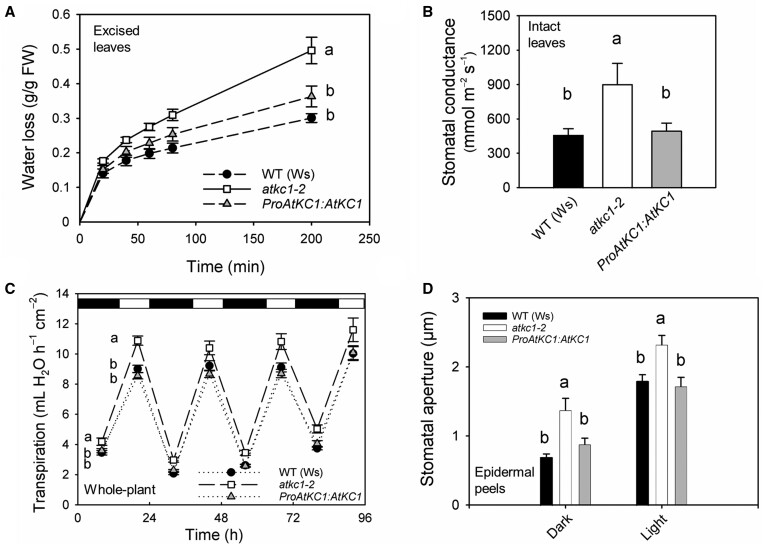
The role of AtKC1 in the leaf epidermis was investigated using a loss-of-function Arabidopsis line, atkc1–2, obtained in the Wassilewskija (Ws) ecotype (Jeanguenin et al., 2011). Leaves excised from atkc1–2 plants were found to lose more water than leaves excised from wild-type (WT) plants (Figure 1A). Furthermore, stomatal conductance measured in intact leaves (Figure 1B) and transpiration rates in whole-plant assays during both light and dark periods (Figure 1C) were larger in atkc1–2 than in WT plants. In agreement with these observations, in vitro measurements of stomatal aperture on leaf epidermal strips yielded larger values in atkc1–2 than in WT plants, regardless of dark or light conditions (stimuli of stomatal closure and opening, respectively) (Figure 1D). Stomatal density was not affected by the atkc1–2 mutation (Supplemental Figure S2). Transformation of the atkc1–2 mutant with a construct-allowing expression of AtKC1 under the control of its own promoter region led to a WT phenotype in each of these experiments (Figure 1, A–D), providing evidence that the stomatal defects of the atkc1–2 mutant plants resulted from the absence of AtKC1 functional expression.
Figure 1.
Impaired control of stomatal aperture and transpirational water loss in atkc1–2-mutant plants. A, Transpirational water loss from excised leaves. The second leaf was excised from WT (Ws ecotype), atkc1–2, and ProAtKC1:AtKC1-complemented atkc1–2 plants. Excised leaf water loss was deduced from the decrease in leaf weight. B, Leaf water conductance measured on intact leaves with a porometer. C, Transpiration rates in whole-plant assays. D, Stomatal aperture in WT, atkc1–2, and ProAtKC1:AtKC1-complemented atkc1–2 plants. Before stomatal aperture measurements, epidermal strips were kept in the dark for 2 h (dark treatment) or in dark for 2 h, followed by 2 h in the light (light treatment) in a 40 mM K+ solution. A–D, Means ± se. In (A)–(C), n = 5, 9, and 11, respectively; in (D), n=6 values, each value corresponding to ∼100 stomata. Letters depict significant group values after ANOVA and Tukey’s post hoc test. In C, for the statistical analysis, the data obtained during the four consecutive days were pooled, taking into account the corresponding day cycle.
Patch-clamp analyses of the membrane conductance to K+ in epidermal cells
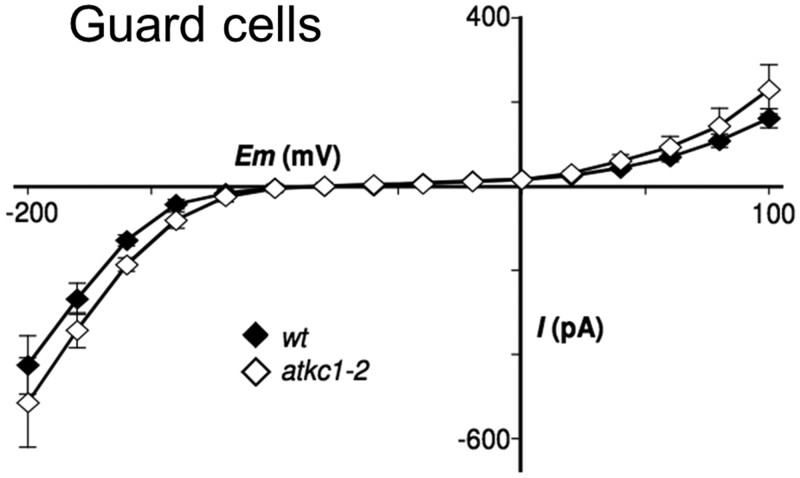
The patch-clamp technique was used to investigate the membrane conductance to K+ in protoplasts enzymatically obtained from WT and atkc1–2 epidermal strips. The electrophysiological recordings carried out in WT guard cell protoplasts yielded a classical current–voltage (I–V) curve, displaying the typical strong rectification of both the inward and outward K+ currents (Figure 2) in agreement with literature data (Schroeder et al., 1987; Hosy et al., 2003; Lebaudy et al., 2008). This rectification results from the fact that the K+ channels mediating K+ transport across the guard cell membrane are either activated by membrane hyperpolarization and dedicated to K+ influx, or activated by membrane depolarization and then dedicated to K+ efflux. Within a large range of voltages, from about −150 to 0 mV in the experiment described in Figure 2, the two populations of channels are inactive and the membrane is almost impermeable to K+. Such channels are said to be “rectifiers”: they mediate a K+ current in only one direction, either into or out from the cell. Very similar I–V curves were obtained in the WT and in the atkc1–2 mutant (Figure 2), which led to the conclusion that the absence of AtKC1 expression had no significant impact on the membrane conductance to K+ in guard cells.
Figure 2.
Shaker-like K+ channel activity in guard cells from WT and atkc1–2-mutant plants (Ws ecotype). Guard cell protoplast current/voltage relationships. Means ± se; n = 8 and 10 for the WT and mutant genotypes, respectively. External K-glutamate concentration was 100 mM.
No patch-clamp analysis of pavement cell protoplasts has been reported in Arabidopsis to our knowledge. Protoplasts from pavement cells were obtained by shorter enzymatic cell-wall digestion compared with guard cell protoplasts. Pavement cell protoplasts could be distinguished from guard cell protoplasts based on their larger size and from contaminating mesophyll protoplasts (if any in the preparation) based on the absence of chloroplasts.
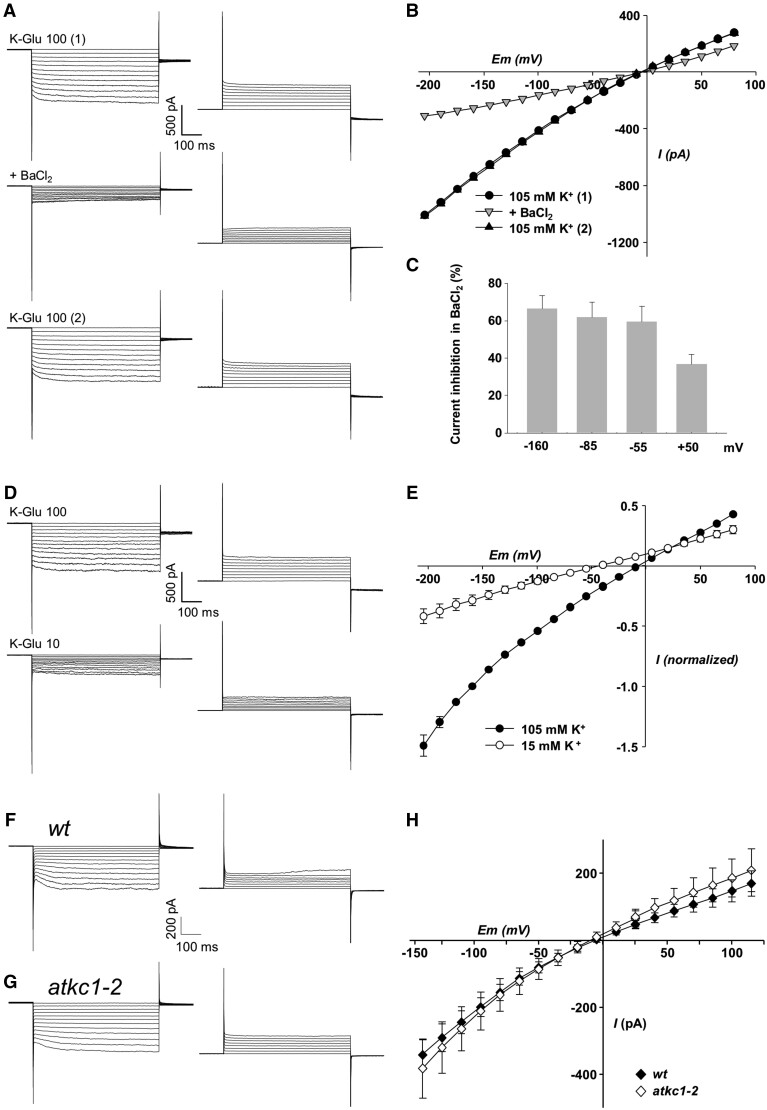
Different types of current traces could be distinguished in the WT pavement cell protoplasts (Figure 3 and Supplemental Figure S3, A–C). The recorded traces/protoplasts were operationally sorted into two major categories, according to the presence (Figure 3) or absence (see below, Supplemental Figure S3, A–C) of an inward current component displaying a time-dependent activation, reminiscent of a Shaker-type slowly activating conductance (Véry and Sentenac, 2002).
Figure 3.
Weakly inwardly-rectifying K+ channel activity in pavement cells from WT and atkc1–2-mutant plants (Ws ecotype). A–C, Typical weakly inwardly rectifying K+ currents recorded in pavement cell protoplasts and their blockage by 10 mM external BaCl2. A, Example of inward and outward current traces (right and left panels, respectively), recorded in the presence of 100 mM K-glutamate (total K+ concentration: 105 mM) and successively before BaCl2 addition (top panels), in the presence of BaCl2 (middle panel), and after BaCl2 rinse (lower panels). B, Corresponding current/voltage relationships. C, Current inhibition in the presence of BaCl2 at negative and positive voltages. Means ± se; n = 7. D and E, Effect of change in external K-glutamate concentration on the weakly inwardly rectifying currents in pavement cell protoplasts. D, Example Figure 3: (continued) of inward and outward current traces (right and left panels, respectively) recorded successively in 100 mM K-glutamate (top panels) and 10 mM K-glutamate (lower panels; total K+ concentration: 15 mM). E, Current/voltage relationships in the two external K-glutamate conditions. Currents were normalized in each protoplast by the current value obtained in 100 mM K-glutamate at −160 mV. Means ± se; n = 7. F and G, Representative inward and outward (right and left panels, respectively) Shaker-like K+ current traces in WT (F) and atkc1–2 (G) pavement cell protoplasts. H, Pavement cell protoplast Shaker-like current/voltage relationship in WT and atkc1–2-mutant plants. External K-glutamate concentration: 100 mM. Means ± se; n = 8 for both the WT and the mutant genotypes. The concentration of K+ (essentially as glutamate salt) in the pipette solution and in the bath solution was 140 and 105 mM, respectively, which results in a K+ equilibrium potential of close to −7 mV.
The membrane conductance to K+ of WT protoplasts displaying the Shaker-type slowly activating currents was analyzed in more detail. The I–V curve obtained for this type of protoplast in the presence of 105 mM K+ in the external solution (Figure 3, B and E, black symbols) crosses the x-axis close to the K+ equilibrium potential, estimated to be close to –7 mV (the K+ concentration of the pipette solution and external bath being close to 140 and 105 mM, respectively), as expected since K+ was the single permeable ion present at a high concentration in these solutions. A major result is that these I-V curves reveal a rather low level of rectification (Figure 3, B and E), when compared with that displayed by the guard cell I–V curve (Figure 2). Adding 10 mM Ba2+ (a classical K+ channel blocker: Schroeder et al., 1987; Wegner et al., 1994; Roelfsema and Prins, 1997; Pilot et al., 2001; Su et al., 2005; Rohaim et al., 2020) resulted in a strong inhibition of the recorded currents (Figure 3, A–C), the magnitude of the inhibition appearing to be slightly voltage-dependent (Figure 3C). Decreasing the external concentration of K+ from 105 to 15 mM shifted the current reversal potential by about −40 mV (Figure 3, D and E; theoretical shift by ca. −49 mV expected for a membrane permeable to K+ only). Altogether, these results indicated that the currents were mainly channel mediated and carried by K+ ions.
Patch-clamp recordings were carried out in parallel experiments (alternating measurements on WT and mutant plants grown simultaneously) to compare the electrical properties of pavement cell protoplasts from WT and atkc1–2 plants. Among 28 protoplasts from WT pavement cells, 10 (ca. 36%) belonged to the first category, that is displaying a Shaker-type time-dependent activation of inward currents (as shown in Figure 3F). In agreement with the data shown by Figure 3, B and E, the I–V curve obtained from these 10 protoplasts displays a low level of rectification (Figure 3H, black symbols). In the second category of protoplasts, that is characterized by the absence of an inward current component displaying a time-dependent activation (18 protoplasts out of the 28 ones), at least three patterns of current traces could be identified (Supplemental Figure S3, A–C). The I–V curves corresponding to these recordings also displayed a rather weak level of rectification (lower panels in Supplemental Figure S3, A–C), when compared with that observed in guard cell protoplasts (Figure 2). The current recordings obtained in the atkc1–2 mutant protoplasts could be sorted into the same categories as those defined for the WT protoplasts, according to the presence or absence of a detectable time-dependent inward Shaker-type component. From 32 protoplasts, 8 (25%) displayed such a component (Figure 3G), which was also characterized by a low level of rectification (Figure 3H, open symbols), and 24 protoplasts belonged to the other category (Supplemental Figure S3, D–F). No significant impairment of the membrane conductance to K+ was detected in the atkc1–2 protoplasts classified as belonging to the former category, that is displaying the Shaker-type component, when compared with the corresponding WT protoplasts (Figure 3H). In the other category, each of the different types of current patterns that were recorded in the atkc1–2 pavement cell protoplasts seemed to have a counterpart among the current patterns observed in the corresponding WT protoplasts (Supplemental Figure S3). This whole set of data did not provide evidence that the atkc1–2 mutation affected the membrane conductance to K+ in every pavement cell.
Loss of AtKC1 expression in guard cells does not underlie the atkc1–2 mutant stomatal phenotype
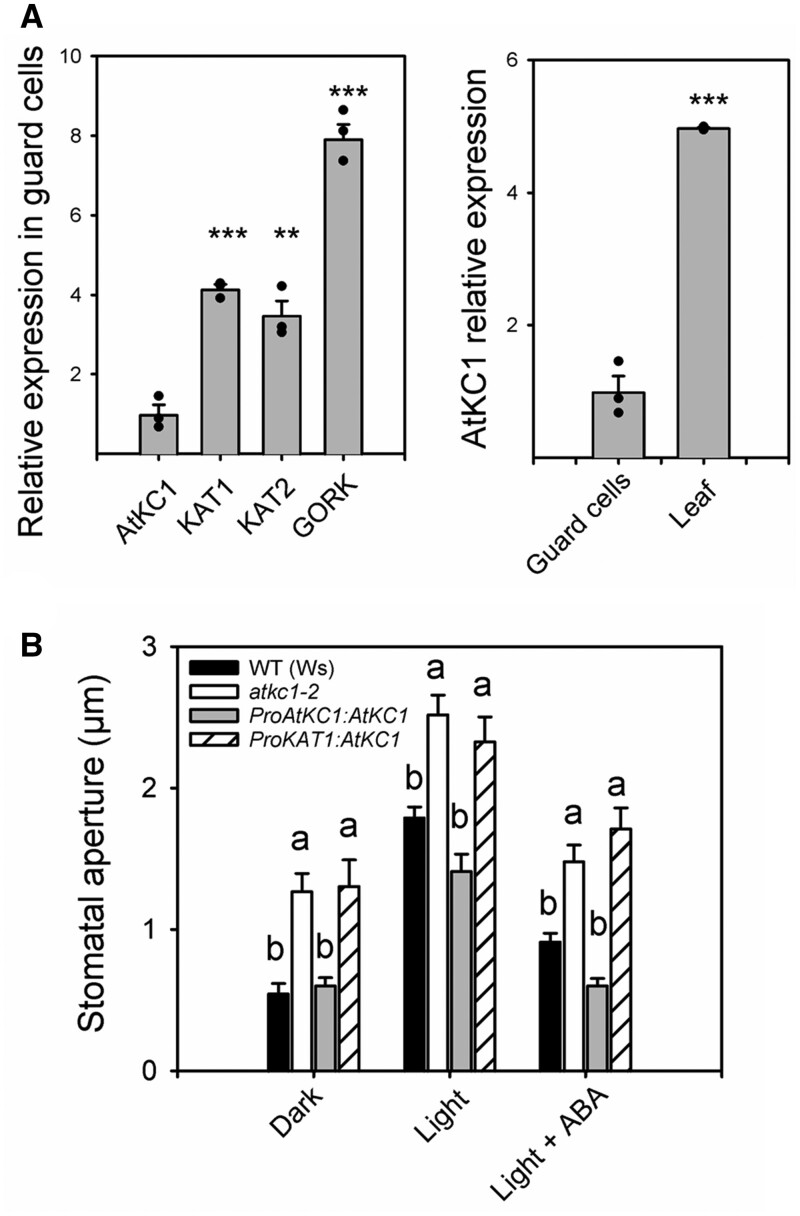
AtKC1 transcripts were found to be at higher levels, by about five times, in whole leaf extracts than in guard cells (Figure 4A, right panel). Furthermore, AtKC1 transcripts in guard cell protoplasts were at lower levels than those of KAT1 and KAT2 (Figure 4A, left panel), the major contributors to the Shaker inward conductance in guard cells (Lebaudy et al., 2008, 2010; Hedrich, 2012).
Figure 4.
The defect in stomatal aperture displayed by the atkc1–2 mutant does not result from loss of AtKC1 expression in guard cells. A, Relative expression of AtKC1 compared with that of other Shaker channels in guard cells (left panel) and relative expression of AtKC1 in guard cells compared with that in leaves (right panel). Expression levels determined by RT-qPCR experiments. B, Stomatal aperture in WT plants, in atkc1–2-mutant plants, and in atkc1–2-mutant plants transformed with either the complementing ProAtKC1:AtKC1 construct (see Figure 1) or with a construct, ProKAT1:AtKC1, rendering AtKC1 expression dependent on the activity of the promoter of KAT1, a Shaker channel gene whose expression in guard cells is specific of this cell type in leaf epidermis (see also Supplemental Figure S4). “Dark” and “light” treatments: stomatal aperture was measured under dark or light as described in Figure 1D. “Light + ABA” treatment: 10 μM ABA was applied for 2 h to light-treated strips before stomatal aperture measurement. A and B, Means ± se. For (A), n = 3 pools of 5–6 plants, and ** and *** denote P < 0.01 and <0.001 in a two-tailed Student’s t test (comparison AtKC1 expression to that of KAT1, KAT2, or GORK, left panel, and AtKC1 expression in guard cells versus AtKC1 expression in leaves, right panel). For (B), n = 6–10 values, each value corresponding to ∼60 stomata. Letters depict significant group values after ANOVA and Tukey’s post hoc test.
In the epidermis, the KAT1 promoter (ProKAT1) is specifically active in guard cells (Nakamura et al., 1995). A ProKAT1:AtKC1 construct introduced into atkc1–2 mutant plants did not rescue the stomatal phenotype of atkc1–2 in the dark, in the light, and after a treatment with the stress hormone ABA, well known to induce stomatal closure (Figure 4B). Detection of AtKC1 transcripts in leaves of atkc1–2-mutant plants transformed with this ProKAT1:AtKC1 construct (Supplemental Figure S4A) provided first evidence that the absence of complementation was not due to expression issues. A crucial objective was then to check whether the ProKAT1 promoter was actually active and allowed expression of AtKC1 subunits in guard cells of the atkc1–2 mutant in the experimental conditions that had previously allowed the defect in stomatal aperture control to be observed in the atkc1–2 mutant (Figure 1). In planta, we did not succeed in detecting the fluorescence of AtKC1-GFP translational fusions expressed under the control of the ProKAT1 promoter (or under control of any of the promoters described below when stably expressed in Arabidopsis transgenic plants). So far, to our knowledge, translational AtKC1-GFP fluorescence in plant cells has only been observed with strong constitutive promoters such as that from the gene of an H+-ATPase (Duby et al., 2008; Jeanguenin et al., 2011; Nieves-Cordones et al., 2014) or 35S (Honsbein et al., 2009). We thus developed an alternative strategy by taking advantage of the fact that AtKC1 can associate with the guard cells KAT1 and KAT2 inward Shaker channel subunits and thereby form heteromeric channels (Jeanguenin et al., 2011) to develop a dominant negative approach as described by Lebaudy et al. (2008). A dominant-negative form of AtKC1, AtKC1–DN, was substituted for AtKC1 in the previous ProKAT1:AtKC1 construct. AtKC1–DN encodes a mutated channel subunit (obtained by site-directed mutagenesis) in which large and positive residues (R) are present in the pore region (Jeanguenin et al., 2011). These residues plug the channel permeation pathway when AtKC1-DN subunits associate with other inwardly rectifying Shaker subunits, including KAT1 and KAT2 (Jeanguenin et al., 2011). After introduction into the atkc1–2 mutant, the new construct, ProKAT1:AtKC1–DN, was found to reduce stomatal aperture (Supplemental Figure S4B), providing evidence that AtKC1–DN was expressed in atkc1–2-mutant guard cells, inhibiting inward channel activity, and thus that ProKAT1 was actually active in atkc1–2 guard cells under our experimental conditions. Altogether, these results provided the first indication that the absence of AtKC1 expression in guard cells alone could not be considered as the main cause of the atkc1–2-mutant stomatal phenotype.
Disruption of AtKC1 results in decreased K+ accumulation in leaf epidermis and reduced turgor pressure in pavement cells
K+ contents were measured in whole leaves, in leaf margins (isolated 2-mm-width strips) enriched with hydathodes and in epidermal strips. Compared with the WT, the overall K+ status of atkc1–2 was not substantially altered in whole leaves (Figure 5), in agreement with previous analyses (Jeanguenin et al., 2011), nor was it altered in leaf margins (Figure 5). In contrast, K+ contents in epidermal strips were significantly lower, by 42 mM, in the mutant than in WT plants, when compared on a fresh weight basis (from Figure 5, the FW/DW ratio being 9.3 ± 0.3, n = 12). Such a difference in K+ content between atkc1–2 and WT plants could hardly be ascribed to guard cells alone but rather to pavement cells, because of the relatively lower abundance and volume of guard cells in the leaf epidermis (see Supplemental Figure S1).
Figure 5.
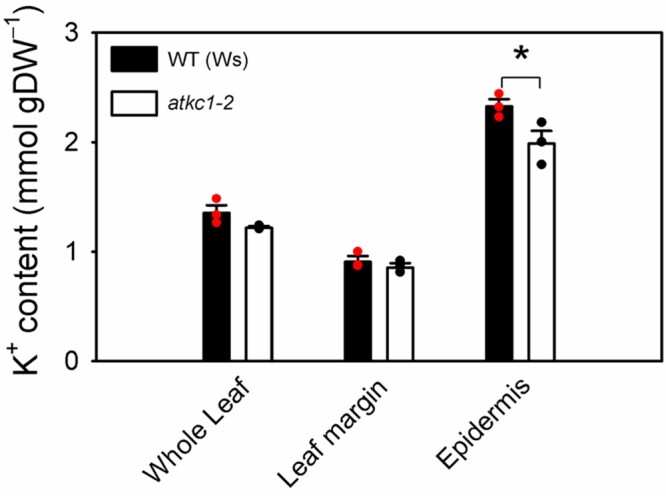
Disruption of AtKC1 leads to reduced K+ contents in leaf epidermis. K+ contents in whole leaf, leaf margin, and leaf epidermis in WT and atkc1–2-mutant plants. Means ± se; n = 3 pools, each one obtained from nine leaves (*P < 0.05, using two-tailed Student’s t test).
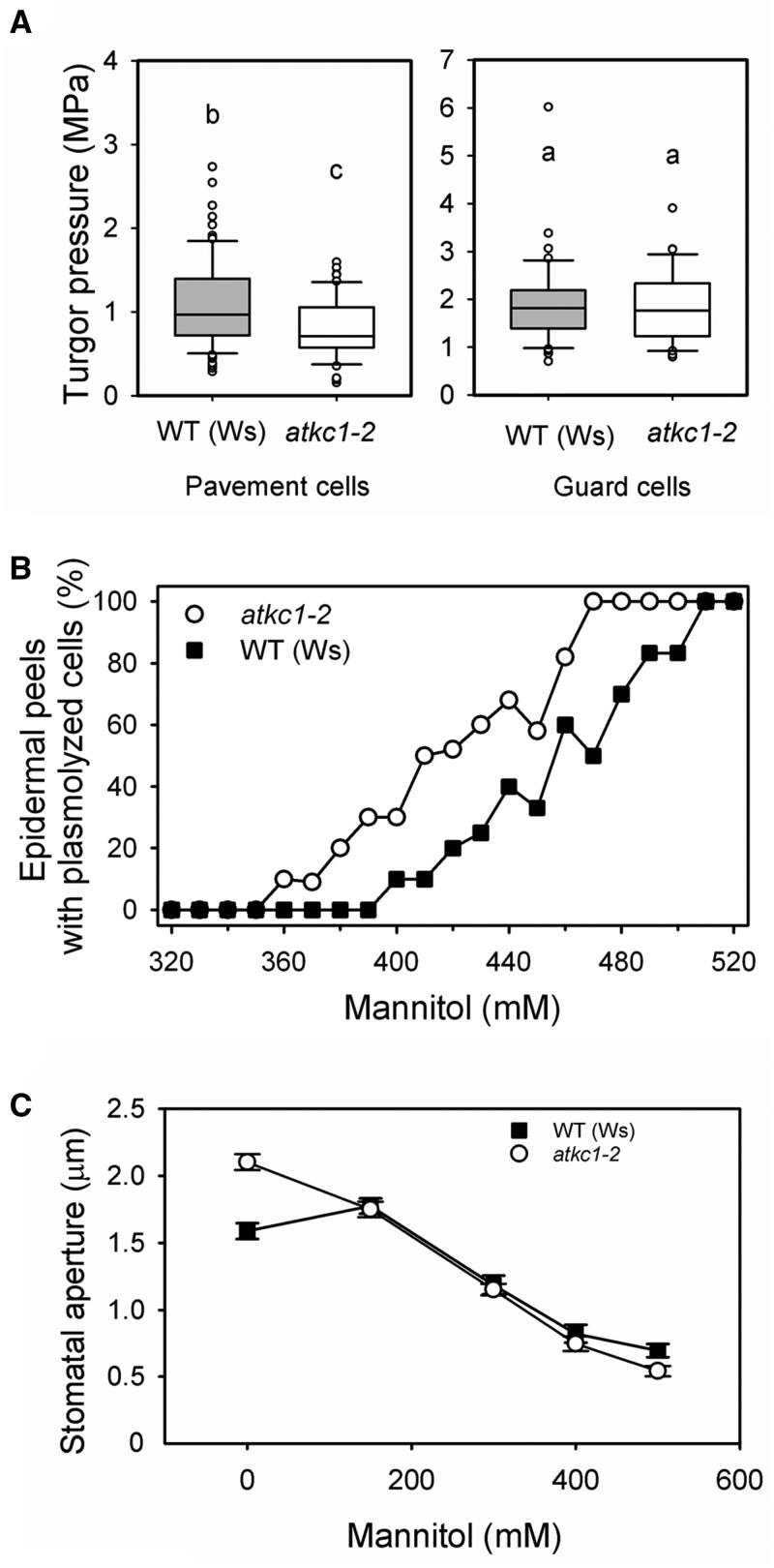
The hypothesis that reduced K+ contents in atkc1–2 pavement cells would decrease the turgor of these cells was then checked via three independent experimental approaches. First, recent improvements in atomic force microscopy (AFM) allowed us to quantify turgor pressure in living plant cells (Beauzamy et al., 2015). Data obtained under similar conditions as those used for in vitro measurements of stomatal aperture in epidermal strips (under light and in the presence of stomatal aperture solution; Figures 1D and 4B) showed that the pavement cell turgor pressure was weaker (by ∼0.15 MPa) in atkc1–2 than in WT plants (Figure 6A, left panel). In contrast, measurements performed in parallel on the same leaves in the same experimental conditions did not reveal any significant difference in guard cell turgor between the WT and atkc1–2 plants (Figure 6A, right panel), providing further support to the hypothesis that AtKC1 plays a role in regulating stomatal aperture from another cell type than guard cells. During these measurements, we have also noted that within the same WT leaf, the turgor pressure was higher, on average by about two times, in the guard cells than in the pavement cells (Figure 6A; note the difference in y-axis scale between the left and right panels).
Figure 6.
Disruption of AtKC1 leads to reduced turgor pressure in pavement cells but not in guard cells. A, Boxplots depicting turgor pressure values obtained with AFM in WT and atkc1–2 pavement cells (left panel) and guard cells (right panel). Upper and lower whiskers: 1.5 times the IQR (first to third interquartile range); border of the boxes: first and third quartile; central line: median. Letters depict different group values after Student’s t test (P < 0.05). For guard cells, n = 46 for the WT genotype and 32 for the atkc1–2-mutant genotype. For pavement cells, n=86 for the WT and 51 for the mutant genotype. B, Disruption of AtKC1 results in decreased osmotic pressures in leaf epidermis as deduced from plasmolysis curves obtained by measuring the percentage of epidermal strips displaying plasmolyzed cells when bathed for 5 min in the presence of mannitol. Ten–twelve strips were examined for each genotype and mannitol concentration. C, Effect on stomatal aperture of adding mannitol to the solution bathing epidermal strips from WT or atkc1–2-mutant plants. n = 92–120 from six leaves for each mannitol concentration and genotype.
In a second series of experiments, we assessed the effects of increasing the concentration of mannitol in the solution bathing epidermal strips on pavement cell plasmolysis. Relative to the WT, 40–60 mM less mannitol was needed to plasmolyze 50% of atkc1–2 pavement cells (Figure 6B), which indicated reduced osmotically active solute contents in atkc1–2 pavement cells.
The third series of experiments was inspired by classical analyses of the effects of external medium osmolarity on stomatal aperture. MacRobbie (1980) showed that stomatal aperture in epidermal strips responded differently to increasing the external osmolarity depending on whether the surrounding pavement cells were dead (killed by acid treatment) or alive (see “Introduction”). We investigated the relationship between stomatal aperture and external medium osmolarity, this time independently of the alive/dead status of the pavement cells, but rather in the presence or absence of AtKC1 expression. Stomatal aperture was measured in epidermal strips bathed in standard medium (as in Figure 1D experiment) supplemented with mannitol at increasing concentrations in order to raise the external osmolarity. In atkc1–2 epidermal strips, the stomatal aperture decreased monotonically (Figure 6C). Conversely, in WT epidermal strips, the stomatal aperture displayed a slight increase in a first step and then decreased when the mannitol concentration was further increased (Figure 6C). Such a non-monotonic relationship between extracellular osmotic potential and stomatal aperture is reminiscent of the results of MacRobbie (1980) discussed above. It can be classically explained as follows. Increasing the external osmolarity decreases the turgor of both the guard cells and the pavement cells by the same amount. The resulting turgor reduction in pavement cells tends to increase the stomatal aperture; while in guard cells, it tends to reduce this aperture. The balance of these opposite effects determines the final stomatal aperture at a given external osmolarity. Thus, the relation between stomatal aperture and the external osmolarity can be non-monotonic. Reciprocally, such a non-monotonic response provides evidence that guard cell turgor is not the only determinant of stomatal aperture and that the turgor of the surrounding pavement cells exerts a back pressure onto guard cells, thereby playing a role in the control of stomatal aperture. The results displayed in Figure 6C therefore indicate that a back pressure was exerted on guard cells by surrounding pavement cells in WT epidermal strips, but that this phenomenon did not occur in atkc1–2 epidermal strips.
Altogether, these three series of experiments indicated that reduced K+ contents decreased the turgor in atkc1–2 pavement cells and thereby the back pressure that these cells can exert onto guard cells. They thus provided evidence that AtKC1 contributes to control of stomatal aperture from the surrounding pavement cells.
Membrane potential measurements in pavement cells
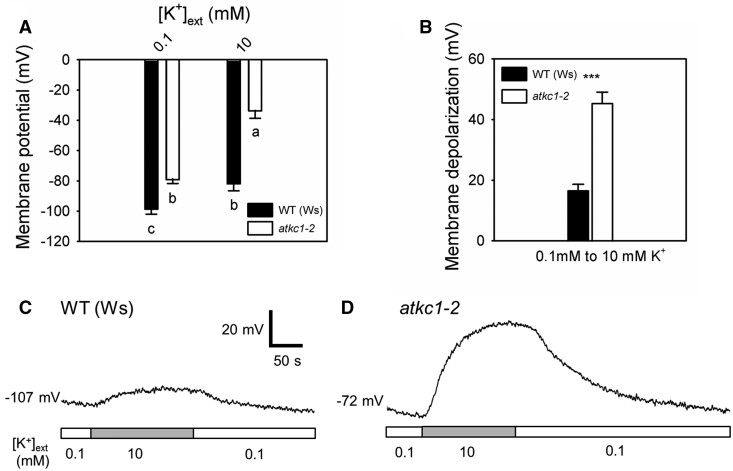
Electrical consequences of the atkc1–2 mutation in pavement cells were looked for in planta by recording the resting membrane potentials (MPs) successively at two different external K+ concentrations, 0.1 and 10 mM, using the microelectrode impalement technique. Significantly less negative MP values were recorded in atkc1–2-mutant plants, when compared with WT control plants, by ca. 20 and 48 mV at 0.1 mM and 10 mM K+, respectively (Figure 7A). The observation of less-negative MPs in pavement cells of the mutant plants is consistent with the lower K+ content of the epidermis displayed by these plants, when compared with the WT control plants (Figures 5 and 6B). In such experiments, the magnitude of the membrane depolarization induced by an increase in external K+ is classically interpreted as reflecting the relative K+ permeability of the membrane (Spalding et al., 1999). The depolarization induced in pavement cells by the increase in K+ concentration from 0.1 to 10 mM was significantly smaller in the WT than in atkc1–2 pavement cells (Figure 7B–D), revealing a higher relative K+ permeability in the atkc1–2-mutant cells, which is consistent with the role of AtKC1 as a negative regulator of Shaker inward K+ channels (Jeanguenin et al. 2011; see “Discussion”).
Figure 7.
The atkc1–2 mutation results in membrane depolarization in pavement cells and in an increased sensitivity of the MP to the external concentration of K+. A, MPs recorded in WT and atkc1–2 pavement cells bathed in 0.1 mM or 10 mM K+. B, Membrane depolarizations induced by the increase in external K+ concentration from 0.1 to 10 mM. Each value corresponded to the difference in the MP that was observed when the external K+ concentration was increased from 0.1 mM to 10 mM K+ within the same cell. C, Representative trace of a WT pavement cell showing membrane depolarization and repolarization due to changes in external K+ concentration. D, Representative trace of an atkc1–2 pavement cell subjected to the same protocol as in (C). White and gray bars depict the periods where the external K+ concentration was 0.1 and 10 mM, respectively. In (A) and (B), means ± se are shown. n = 14 cells from five different plants for WT and n = 14 cells from three different plants for atkc1–2. Letters depict significant group values after ANOVA and Tukey’s post hoc test. *** denotes P < 0.001 in a two-tailed Student’s t test.
Expression of AtKC1 in several epidermal cell types is required to complement the atkc1–2-mutant stomatal phenotype
AtKC1 was expressed in atkc1–2-mutant plants using different promoters with overlapping epidermal cell-specificity to determine further cell types, besides pavement cells, in which AtKC1 would affect stomatal aperture control: ProCER5 (At1G51500) (Pighin et al., 2004), ProOCT3 (At1G16390) (Kufner and Koch, 2008), ProGL2 (At1G79840) (Szymanski et al., 1998), promoter of the Uncharacterized Protein Kinase gene At1G66460 (Jakoby et al., 2008), ProFMO1 (At1G19250) (Olszak et al., 2006), ProCYP96A4 (At5G52320), and ProKCS19 (At5G04530). The expression patterns of these promoters were experimentally confirmed in transgenic Arabidopsis by fusing them to the glucuronidase (GUS) reporter gene. These observed patterns (Supplemental Figure S1 and Table 1; see description below) were entirely consistent with the eFP Browser data (Supplemental Table S1).
Table 1.
Summary of the results presented in Supplemental Figure S1 (expression pattern) and Figure 8A (stomatal aperture)
| AtKC1 expression in | Promoter |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pro | Pro | Pro | Pro | Pro | Pro | Pro | Pro | Pro | |
| AtKC1 | CER5 | CYP96A4 | KCS19 | OCT3 | KAT1 | GL2 | At1G66460 | FMO1 | |
| Guard cells | + | + | + | + | + | + | − | − | − |
| Pavement cells | + | + | + | + | + | − | − | − | − |
| Trichomes | + | + | + | − | − | − | + | + | − |
| Hydathodes | + | + | − | − | + | − | − | − | + |
| Stomatal aperture similar to that in WT plants | Yes | Yes | Yes | No | No | No | No | No | No |
Each of these seven promoters was used to direct transgenic expression of AtKC1 in the atkc1–2-mutant tissues. The capacity of the transgenes to complement the mutant phenotype was checked in a first series of experiments by measuring stomatal aperture in the transformed plants (T3 homozygous transgenic lines) in light conditions as previously performed for the complementing construct ProAtKC1:AtKC1 and the non-complementing one ProKAT1:AtKC1 (Figure 4B).
Five of the seven constructs were found not to complement the mutant stomatal phenotype (Figure 8A and Table 1). These were ProKCS19:AtKC1, ProOCT3:AtKC1, ProGL2:AtKC1, ProAt1G66460:AtKC1, and ProFMO1:AtKC1 (Figure 8A). The results in Supplemental Figure S1 indicate that ProKCS19 and ProOCT3 are active in both guard cells and pavement cells, and for the latter, in hydathodes as well. ProGL2 and ProAt1G66460 are active only in trichomes, and ProFMO1 is active only in hydathodes (Supplemental Figure S1).
Figure 8.
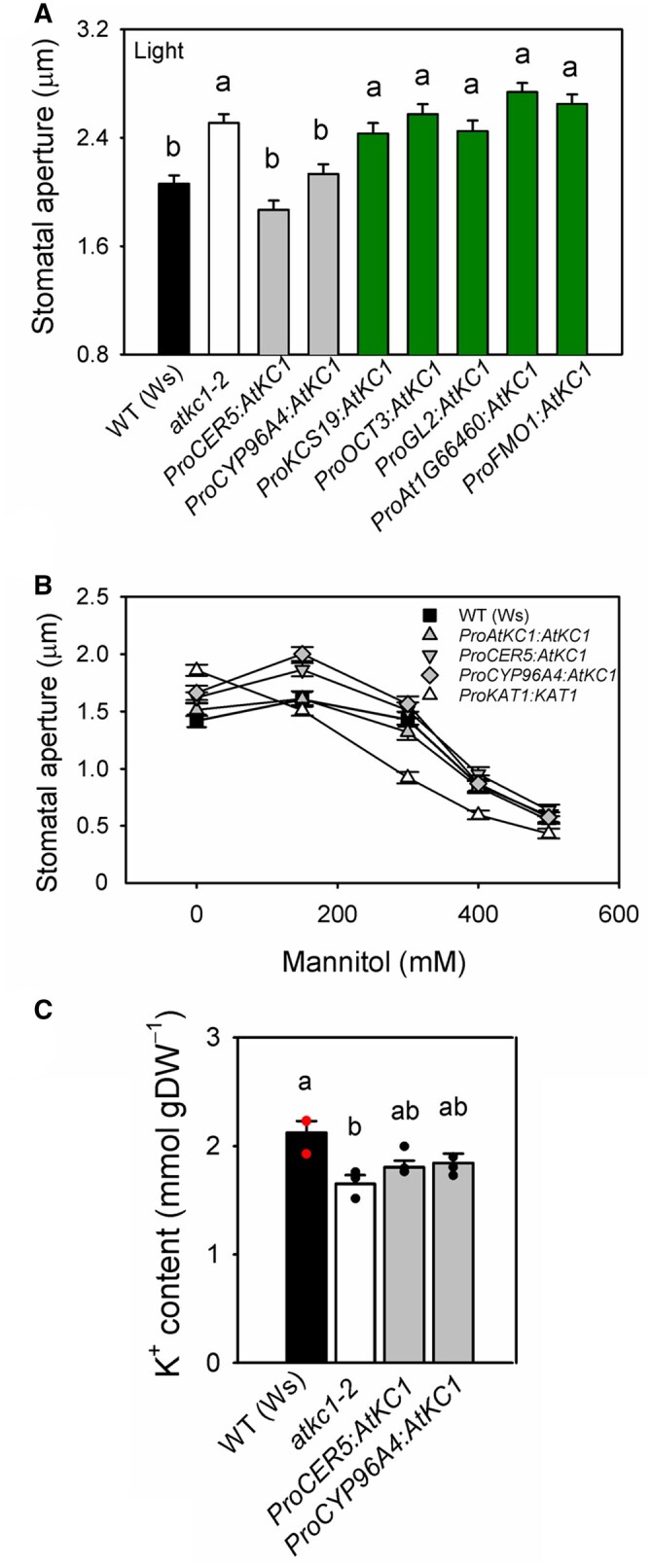
Restoration of WT stomatal features in the atkc1–2 mutant requires AtKC1 expression in pavement cells and trichomes. A, Stomatal aperture under light in WT Arabidopsis plants (Ws ecotype, black bar), in atkc1–2-mutant plants (white bar), and in atkc1–2-mutant plants transformed with a construct allowing expression of AtKC1 under control of one of the following promoters: ProCER5, ProCYP96A4, ProKCS19, ProOCT3, ProGL2, ProAt1G66460, and ProFMO1 (expression patterns of these promoters: see Table 1 and Figure 8: (continued) Supplemental Figures S1 and S5). Gray bars and dark green bars: transformed plants with rescued or non-rescued stomatal phenotype, respectively. Stomatal aperture was measured following the same procedure as in Figure 1D. B, Stomatal aperture in epidermal strips bathed in mannitol solutions. Transformed lines identified in (A) as displaying stomatal aperture values similar to that of WT plants (transforming constructs: ProAtKC1:AtKC1, ProCER5:AtKC1, and ProCYP96A4:AtKC1) also behaved like WT plants in response to added mannitol (showing a non-monotonous sensitivity to mannitol concentration). In contrast, the transgenic line ProKAT1:AtKC1, shown in (A) to display a stomatal aperture similar to that of atkc1–2-mutant plants, also displayed a monotonous decrease in stomatal aperture in response to increased mannitol concentration, and thus behaved like atkc1–2-mutant plants (see Figure 6C). C, Leaf epidermis K+ content in WT plants, in atkc1–2-mutant plants and in atkc1–2-mutant plants transformed with the ProCER5:AtKC1 and ProCYP96A4:AtKC1 complementing constructs. A–C, Means ± se. In (A) and (B), n = 94–131 stomata from six leaves. In (C), n = 3 pools of samples, each one obtained from nine leaves. In (A) and (C), letters depict significant group values after ANOVA and Tukey’s post hoc test.
In contrast to the above, two constructs complemented the phenotype as efficiently as ProAtKC1:AtKC1 (Figure 4B). These were ProCER5:AtKC1 and ProCYP96A4:AtKC1 (Figure 8A). The GUS staining data displayed in Supplemental Figure S1 indicate that ProCER5 (shown to be epidermis-specific; Pighin et al., 2004) is active in guard cells, pavement cells, hydathodes, and trichomes (abaxial and adaxial sides). ProCYP96A4 (also leaf epidermis-specific as shown by Mustroph et al., 2009) is active in guard cells, pavement cells, and trichomes (abaxial and adaxial sides) but not in hydathodes. It should be noted that leaves of Ws ecotype plants harbor trichomes at both the abaxial and adaxial faces (Telfer et al., 1997; Supplemental Figure S5) and that the atkc1–2 mutation did not affect trichome density on either face (Supplemental Figure S6).
In a second series of experiments, both the ProCER5:AtKC1 and ProCYP96A4:AtKC1 constructs were found to also restore the stomatal aperture to the WT non-monotonic mode in responding to rising external mannitol concentration—in contrast to ProKAT1:AtKC1 (Figure 8B compared with Figure 6C)—and the level of K+ accumulation in leaf epidermis (Figure 8C compared with Figure 5).
By cross-comparing these data, complementation of the defect in stomatal aperture control of atkc1–2 plants required AtKC1 expression not only in surrounding pavement cells but, unexpectedly, also in trichomes. However, targeted transgenic expression in only trichomes by two different specific promoters (ProGL2 and ProAt1G66460) did not rescue the atkc1–2 phenotype.
Discussion
Relative to autonomous stomatal control, the non-autonomous regulatory mechanism is conceptually more abstract, as there had been a paucity of functionally defined genetic or molecular components. Neither had there been detailed knowledge on the precise cell types from which these components operated, nor their physiological modes of action. We report here that the inactivation of AtKC1 results in larger stomatal apertures and increased transpirational water loss. AtKC1 encodes a silent Shaker channel subunit because it does not form functional K+ channels on its own (see below). The atkc1–2 mutation was not compensated by transgenic expression of AtKC1 only in guard cells within the leaf epidermis using the promoter of the Shaker channel gene KAT1 (Figure 4B). The dominant-negative approach by expressing ProKAT1:AtKC1–DN (Supplemental Figure S4) proved that ProKAT1 remained active in the guard cells throughout our experiment. Altogether, these results suggested that AtKC1 does not control stomatal aperture from within the guard cells, but that it contributes to the non-autonomous mechanism that opposes the guard cells’ outward push.
Turgor pressures of the guard cell and surrounding pavement cells have rarely been directly measured. Due to their small size, Arabidopsis guard cells are not easily amenable to investigations using the classical pressure probe methodology (Franks et al., 1995, 1998, 2001). We have therefore used an atomic force microscope (Beauzamy et al., 2015) to assess the amount of hydrostatic pressure required to cause indentation on guard cells and pavement cells. This methodology, which is not destructive, basically applies a non-penetrative indentation with an elastic probe on the sample surface (Beauzamy et al., 2015). The applied force can be linearly deduced from the measured probe deformation, while the mechanical properties of the sample can be deduced from the applied force and sample surface deformation due to indentation. Turgor pressure is further deduced using established continuum mechanics equations of the inflated shell model (Beauzamy et al., 2015). This approach has been applied to epidermal cells in cotyledon (Verger et al., 2018), a system histologically similar to leaves, and in shoot apical meristem (Long et al., 2020). Following these two studies, we deduced turgor pressure using forces at depth ranges that minimize the influence of neighboring cells and of underlying cell layers or cavities (Malgat et al., 2016; Long et al., 2020).
The turgor pressure values deduced in the present study for WT Arabidopsis guard cells and pavement cells (close to 2 and 1 MPa, respectively, in open stomata; Figure 6A) are within the range of values previously obtained by pressure probe applied to Vicia faba and Tradescantia virginiana, which ranged from 1 to 5 MPa for guard cells, and from 0.6 to 1 MPa for pavement cells (Franks et al., 1995, 1998). In all of these studies, pavement cells exhibited lower turgor pressure than guard cells. In Arabidopsis, the difference in turgor between guard cells and pavement cells observed in our experimental conditions is close to 0.8 MPa, which indicates that the osmolyte content of pavement cells was significantly lower than that of guard cells, by about ∼330 mOsm L-1.
The AFM data did not reveal any significant difference in guard cell turgor between the WT and atkc1–2. In contrast, the turgor pressure of pavement cells was weaker in atkc1–2 than in the WT, by about 0.15 MPa, that is by ca. 20% (Figure 6A). This decrease in turgor corresponds to a decrease in osmoticum concentration by about 60 mOsm L−1. Such a difference is supported by the 40–60 mOsm L−1 difference in mannitol concentration required to induce epidermal cell plasmolysis in WT and atkc1–2 plants as deduced from Figure 6B (the curves of the WT and atkc1–2-mutant plants being shifted from each other by about 40–60 mOsm L−1). Such differences are also consistent with the observation that the internal concentration of K+ in epidermal strips was about 42–58 mM lower in the mutant plants (as computed from Figures 5 and 8C, respectively, FW/DW ratio = 9). Thus, the decrease in pavement cell turgor revealed by microindentation in the atkc1–2 mutant can be mainly ascribed to lower K+ accumulation in these cells.
Because AtKC1 is a member of the Shaker K+ channel family, its absence may disturb the steady-state accumulation of K+ in diverse tissue types. The atkc1–2 mutation has been found to affect neither whole root, whole shoot nor whole leaf K+ contents (Jeanguenin et al., 2011; Figure 5). Thus, the decrease in leaf epidermal strip K+ contents resulting from this mutation appears to be limited to this tissue. The observation that the ProCER5:AtKC1 and ProCYP96A4:AtKC1 constructs complemented the mutant defect in epidermal strip K+ content (Figure 8C) and in stomatal aperture control (Figure 8A and B) while the promoters ProCER5 and ProCYP96A4 are known to be essentially active in leaf epidermis (Pighin et al., 2004; Mustroph et al., 2009; Supplemental Figure S1) provides further evidence that both defects have their origin in the leaf epidermis and not in another plant tissue.
MP measurements indicated that the atkc1–2 mutation resulted in a significant depolarization of pavement cells, by about 20 or 48 mV when the external solution contained 0.1 or 10 mM K+, respectively (Figure 7A). The magnitude of the depolarization induced by the 100-fold increase in the external K+ concentration was thus much larger in the mutant than in the WT pavement cells (Figure 7, B–D). Altogether, these results provide evidence that the absence of AtKC1 functional expression impacted electrical features within the leaf epidermis. The decrease in membrane polarization resulting from the atkc1–2 mutation is consistent with—and could result from or contribute to—the lower K+ content of mutant pavement cells (Figures 5, 6, and 8C). The increase in the sensitivity of the MP to K+ external concentration, which indicates an increase in the membrane conductance to K+ in the mutant, when compared with the WT, is consistent with the fact that AtKC1 behaves as a negative regulator of inward Shaker channels (Jeanguenin et al., 2011). Indeed, AtKC1 does not form homotetrameric channels on its own, as indicated above, but can form heteromeric channels upon interaction with co-expressed inwardly rectifying Shaker channel subunits, leading to increased diversity in channel functional properties (Reintanz et al., 2002; Duby et al., 2008; Geiger et al., 2009; Honsbein et al., 2009; Jeanguenin et al., 2011; Zhang et al., 2015; Wang et al., 2016). The activation potential of heteromeric channels associating AtKC1 to KAT1, KAT2, or AKT2 is shifted toward more negative values, when compared with KAT1, KAT2, or AKT2 homomeric channels (Duby et al., 2008; Jeanguenin et al., 2011). Such a negative regulation has been proposed to prevent K+ efflux (loss) when the MP is less negative than the K+ equilibrium potential (EK) but more negative than the (homomeric) channel-activation potential (Duby et al., 2008; Jeanguenin et al., 2011).
Patch-clamp analysis revealed different types of current patterns among protoplasts derived from pavement cells recognizable by their size and shape, and in particular the fact that they did not possess chloroplasts. Thus, this analysis provides evidence that, within the leaf epidermis, cells that are neither guard cells nor trichomes (the latter cells being not digested by the enzyme cocktail in our experimental conditions) do not form a homogeneous tissue in terms of plasma membrane electrical properties. Evidence is available at the molecular level that the generic term of “pavement cells” actually belies a functionally heterogeneous population of cells, based on the criterion of gene expression markers. For instance, PATROL1 is expressed in guard cells and only in the smallest of the immediately adjacent pavement cells. The other two surrounding pavement cells do not express this gene to detectable levels. PATROL1 directs trafficking of certain proteins, including AHA1/OST2, a proton pump that is important for hyperpolarization of the plasma membrane (Merlot et al., 2007; Higaki et al., 2014). Moreover, single-cell gene transcriptomic profiling in the epidermis of Arabidopsis has revealed differences between pavement cells and basal trichome cells (also named socket or skirt cells) (Lieckfeldt et al., 2008; Schliep et al., 2010; Zhou et al., 2017). This diversity in gene expression, as well as the diversity in membrane electrical properties revealed by our patch-clamp recordings, might be related to the positional information sensed by the epidermal cells with respect to veins, trichomes, and/or stomata.
None of the different types of current patterns displaying no time-dependent slowly activating component (Supplemental Figure S3) is reminiscent of the activity of a cloned and functionally characterized ion channel. The situation is different for the protoplasts displaying a Shaker-like time-dependent activation (Figure 3). Indeed, the available transcriptome data (EMBL-EBI expression atlas) as well as GUS reporter gene analysis (Lacombe et al., 2000) indicate that, together with AtKC1, the Shaker gene AKT2 is expressed in pavement cells. Thus, the Shaker-like slowly activating weakly inwardly rectifying current pattern (Figure 3) that was observed in about one-third or one-quarter (in the WT and the mutant, respectively) of the pavement cell protoplasts suggests that a significant part of the inward and outward currents was mediated by AKT2 homomeric and heteromeric channels comprising, in WT plants, AtKC1 subunits since both AKT2 and AKT2-AtKC1 channels have been shown to be weakly rectifying (Jeanguenin et al., 2011). The weak rectification of AKT2 results from coexistence in the membrane of two populations of channels, one displaying activation by increasingly negative voltages and the other displaying an instantaneously activated non-rectifying (“leak-like”) behavior, depending on the channel phosphorylation status (Michard et al., 2005a, 2005b). Such phosphorylation-controlled variations of the channel gating properties could also contribute to the diversity of plasma membrane electrical behavior among pavement cell protoplasts.
Comparison of the patch-clamp recordings in WT and atkc1–2 pavement cell protoplasts did not provide evidence that the pavement cell diversity in plasma membrane electrical features was reduced by the mutation (Figure 3 and Supplemental Figure S3). The I–V curves derived for the WT and atkc1–2-mutant protoplasts displaying a time-dependent slowly activating AKT2-like component are quite similar (Figure 3H). This suggests that it is not by affecting the time-dependent AKT2-like conductance that the atkc1–2 mutation alters the pavement cell K+ content (Figures 5, 6, and 8) and the sensitivity of pavement cell MP to K+ (Figure 7).
Altogether, these patch-clamp data leave the actual impact of the mutation on the K+ conductance of (the different types of) pavement cells still elusive. Patch-clamp measurements on protoplasts provide information about individual cell (protoplast) properties, while MP measurements give access to data reflecting in situ (in the leaf apoplastic solution) integrated (within the leaf epidermis as a whole due to electrical connection through plasmodesmata) electrical properties. Such a difference, together with the large diversity in K+ conductance among pavement cells and the fact that the present patch-clamp analysis has essentially taken into account the protoplasts whose membrane inward conductance appeared to be dominated by a time-dependent slowly activating conductance (Figure 3), might explain that no significant difference between atkc1–2 mutant and WT pavement cell protoplasts has been evidenced by this analysis.
It should also be noted that AtKC1 is known to play a role in exocytosis, besides its contribution to the regulation of inwardly rectifying Shaker channel activity. It interacts with the SNARE AtSYP121 (Honsbein et al., 2009), a vesicle-trafficking protein active at the plasma membrane and mediating vesicle fusion required for cellular homeostasis and growth (Geelen et al., 2002). Formation of tripartite complexes associating AtSYP121 to AtKC1, itself associated to the other Shaker subunit of the heteromeric channel, has been shown to confer voltage sensitivity to the contribution of AtSYP121 to vesicle fusion at the plasma membrane, rendering the secretion voltage dependent, a process proposed to couple K+ uptake to exocytosis and to maintain turgor pressure in growing plant cells (Honsbein et al., 2009; Grefen et al., 2015). Finally, screening tests using a split ubiquitin derived system suggest that AtKC1 might also interact with a ROP protein (Rho-of-Plant, a Rho GTPase) as well as a nitrate transporter (Obrdlik et al., 2004).
Fused to the AtKC1 coding sequence, cell-type-specific promoters directing expression in guard cells, or in both guard cells and pavement cells, or in trichomes only, did not complement the atkc1-mutant stomatal phenotype, while complementation was observed with promoters directing expression in these three cell types together (Figure 8). Considering the whole set of observations, the simplest hypothesis is that AtKC1 contributes to non-autonomous guard cell control of stomatal aperture and that this contribution involves pavement cells and trichomes.
A salient finding from the patch-clamp recordings in pavement cell protoplasts is that, despite the observed diversity in cell membrane electrical properties (Figure 3 and Supplemental Figure S3), pavement cells possess in common a rather weak level of rectification when compared with that displayed by guard cells (Figure 2). The model suggested by these results is thus that guard cells, with strong rectification of both inward and outward K+ conductances, are embedded in a layer of cells mostly displaying weak rectification. It is tempting to assume that this functional differentiation between pavement cells and guard cells renders the exchanges of K+ between these two types of cells immediately dependent on the guard cell membrane transport activity. The quasi-linearity of the I–V curve of pavement cells would allow that any change in K+ apoplastic concentration due to uptake of this cation by—or release from—guard cells could modulate the efflux of K+ from—or influx into—pavement cells. In other words, due to their low level of rectification, pavement cells could be a permanent and immediately available K+ source or sink, depending on the demand of guard cells, in agreement with the model that guard cells play the dominant motor role in stomatal movements. Finally, the low level of rectification of pavement cells, which allows K+ exchanges in the whole range of MPs, can also be hypothesized to facilitate K+ exchange/shuttling among the pavement cells themselves.
The mutant defect in stomatal movements observed in planta (Figure 1C) is not likely to directly result from altered control of K+ availability in the external solution (i.e. in the leaf epidermis apoplast) since impaired control of stomatal aperture was also observed in vitro in epidermal strips bathed in a solution containing a high concentration of K+ (Figures 1D, 4B, and 8A), like that used in microindentation experiments (Figure 6A). Our results suggest that, when AtKC1 is functional, trichomes cooperate with adjacent epidermal cells in K+ homeostasis. ProAtKC1, as well as ProCER5 and ProCYP96A4, which complemented the atkc1–2 mutant, are all expressed in the ring of basal cells skirting the base of the trichome. The major class of transcripts detected in trichomes, basal, and epidermal cells belongs to transport and transport-associated proteins (Lieckfeldt et al., 2008), suggesting that these cells are particularly active in intra- and intercellular movements of solutes. Absence of AtKC1 functional expression might affect K+ distribution between trichomes, basal cells, and pavement cells, resulting in a reduction of K+ accumulation in the latter cells.
In conclusion, the whole set of results supports the following causal chain: absence of AtKC1 functional expression leads to a reduced steady-state K+ accumulation in pavement cells and thereby in a decrease in the turgor of these cells. The weakened backpressure of the epidermal cells therefore surrenders to the opposing guard cell turgor, constitutively resulting in more open stomata. The present data provide genetic, molecular, and electrophysiological evidence that complex K+ distribution among several epidermal cell types contributes to stomatal aperture outcome. In conclusion, these data support the view that the entire epidermis should be regarded as a dynamic filter controlling stomatal aperture.
Materials and methods
Plant culture
Arabidopsis thaliana (Ws) plants were grown in a growth chamber, at 20°C, with a 8-/16-h light/dark photoperiod (300 μmol photons m-2 s-1, white light from fluorescent tubes), at 70% RH (RH = relative air humidity), in commercial compost. They were used for experiments when they were 6-weeks old and still not bolting.
Stomatal aperture and transpiration measurements
Rosette transpirational water loss, preparation of leaf epidermal strips, and measurements of stomatal aperture (in 30 mM KCl and 10 mM KOH-MES, pH 6.5) were performed as previously described (Hosy et al., 2003; Nieves-Cordones et al., 2012). Stomatal aperture measurements were performed in triplicate on at least six epidermal strips from six different plants. To study the effect of increased mannitol concentration on stomatal aperture, epidermal strips were incubated in stomatal opening buffer containing 30 mM KCl and 10 mM KOH-MES, pH 6.5, under light for 2 h and then transferred into dishes containing the same solution plus different concentrations of mannitol. Images were taken within 5 min incubation under a microscope (Olympus BH2) coupled to a color camera (Olympus Color View II). Displayed data are mean of at least 100 values per treatment and per mannitol concentration (when stated) for each plant genotype. All experiments were conducted in blind, that is genotypes unknown to the experimenter until data had been analyzed. Vital staining with neutral red at 0.02% (w/v) was performed to confirm the viability of guard cells and other epidermal cells in epidermal strips. For whole-plant transpiration assays, pots containing individually grown 6-week-old plants subjected to the same watering regime were sealed with a plastic film to prevent water loss from the substrate. The soil water content was initially adjusted to 2.5 g of H2O per g of dry soil. Evapo-transpirational water loss was then compensated by addition of equivalent amounts of water in order to maintain the water content at its initial value over a four-day period. Pots were weighed twice a day, at dusk and at dawn, for determination of transpirational water loss (in milliliters H2O per square centimeter of leaf and per hour). Foliar area was measured with ImageJ from images of rosettes. Stomatal conductance was measured on intact leaves with a diffusion porometer (AP4; Delta-T Devices).
Patch-clamp recordings
WT and atkc1–2 A. thaliana Ws plants were grown for 6 weeks in compost (individual containers) in a growth chamber (20°C, 65% relative humidity, 8-h/16-h light/dark, 250 µmol m2 s1). Electrophysiological analyses on guard cell protoplasts were performed as previously described (Hosy et al., 2003; Lebaudy et al., 2008). Epidermal cell protoplasts were isolated by enzymatic digestion of leaf epidermal strips in darkness. The digestion solution contained 1-mM CaCl2, 2-mM ascorbic acid, 1-mM MES-KOH (pH 5.5), Onozuka RS cellulase (1% w/v, Duchefa Biochemie, Haarlem, Netherlands), and Y-23 pectolyase (0.1% w/v, Seishin Pharmaceutical, Tokyo, Japan). The osmolarity was adjusted to 500 mosM with d-mannitol. The epidermal strips were digested for 35 min at 27°C. Filtration through 50-µm mesh allowed recovery of protoplasts. The filtrate was rinsed four times with two volumes of conservation buffer: 100-mM potassium glutamate, 10-mM CaCl2, 10-mM HEPES, the osmolarity being adjusted to 520 mOsm with d-mannitol, and the pH to 7.5 with KOH. The protoplast suspension was allowed to sediment and then kept on ice in darkness in the conservation buffer, which was also used as external solution for the sealing step. Patch-clamp pipettes were pulled (P07, DMZ-Universal Puller, Zeitz-Instruments, Germany) from borosilicate capillaries (GC150TF-7.5, Phymep, France). The pipette solution contained 1-mM CaCl2, 5-mM EGTA, 0.5-mM MgCl2, 100-mM potassium glutamate, 2-mM Mg-ATP, and 20-mM HEPES. The osmolarity of the solution was adjusted to 540 mOsm with d-mannitol and the pH was adjusted to 7.5 with KOH (final K+ concentration assayed by flame spectrophotometry: ca. 140 mM). Under these conditions, the pipette resistance was about 18 MΩ. Seals with resistance >1 GΩ were used for electrophysiological analyses. The bath solution contained, except when otherwise mentioned, 100-mM potassium glutamate, 0.1-mM CaCl2, 10-mM HEPES, the osmolarity being adjusted to 520 mOsm with d-mannitol, and the pH to 7.5 with KOH (final K+ concentration: 105 mM, assayed by flame spectrophotometry). Whole-cell recordings were obtained using an Axon Instruments Axopatch 200B amplifier. pCLAMP 8.2 software (Axon Instruments, Foster City, CA, USA) was used for voltage pulse stimulation, online data acquisition, and data analysis. The voltage protocol consisted of stepping the MP from −40 mV (holding potential) to +80 mV or −205 mV, or from +25 mV (holding potential) to either +130 mV or −140 mV, in 15 mV steps. Liquid junction potentials at the pipette/bath interface were measured and corrected.
MP recordings in pavement cells
Rosette leaves from WT and atkc1–2-mutant plants grown in hydroponics for 3 weeks (1/5 Hoagland solution) were excised and immobilized in a 1-mL chamber. The external solution contained 5-mM MES (2-(N-Morpholino) ethanesulfonic acid), 0.1-mM KCl, 0.1-mM CaCl2, and 0.1-mM NaCl, brought to pH 6.0 with Ca(OH)2. The leaf was bathed for at least 30 min in the perfusion solution before cell impalement. Impalement microelectrodes were pulled from borosilicate glass capillaries (1B120F-4, World precision instruments, http://www.wpiinc.com) and showed a diameter of approximately 0.5 µm at the tip. Glass microelectrodes were fixed to electrode holders containing an Ag/AgCl pellet and connected to a high-impedance amplifier (model duo 773; World precision instruments). Impalement and reference electrodes were filled with 200-mM KCl. To impale leaf pavement cells, the microelectrode was approached to the leaf surface with a motorized micromanipulator (Narishige MM-89, http://narishige-group.com) and impalements were carried out with a one-axis oil hydraulic micromanipulator (Narishige MO-10). The precise penetration of the microelectrode into pavement cells was visually followed with an inverted microscope.
Atomic force microscopy
AFM determination of turgor pressure in the leaf epidermis was performed as in Beauzamy et al. (2015) with modifications. Specifically, 1 × 1 cm leaf segments were fixed in Petri-dishes by double-sided tape and microtube tough-tags (Diversified Biotech) with the abaxial face up. Adaxial trichomes were removed by tweezers to facilitate tape fixation. Leaf segments were incubated in the stomata opening buffer (see above) under light for at least 2 h before being mounted onto a BioScope Catalyst AFM (Bruker). A spherical-tipped AFM cantilever with 400 nm tip radius and 42 N/m spring constant was used for the measurements (SD-SPHERE-NCH-S-10, Nanosensors); a spherical tip was used to avoid the cell wall puncture that often occurs upon usage of a more standard sharp pyramidal tip. One to 2-μm-deep indentations were made along the topological skeletons of epidermal cells to ensure relative normal contact between the probe and sample surface. At least three indentation positions were chosen for each cell, with each position consecutively indented three times, making at least nine indentation force curves per cell. Cell recordings of AFM force curves were performed with the NanoIndentation plugin for ImageJ (https://fiji.sc/) as described in Long et al. (2020). Parameters for turgor deduction were generated as follows. The cell wall elastic modulus and apparent stiffness were calculated from each force curve following Beauzamy et al. (2015). To minimize the effect of neighboring and underlying cells (Malgat et al., 2016; Long et al., 2020), we used a force range of 1%–10% of maximal force for modulus and 75%–99% of maximal force for cell stiffness, which typically correspond to depths in the ranges 10–100 and 400–500 nm, respectively. Cell surface curvature was estimated from AFM topographic images, with the curvature radii fitted to the long and short axes of small cells or along and perpendicular to the most prominent topological skeleton of heavily serrated pavement cells. Turgor pressure was further deduced from each force curve (four iterations) with the simplified hypothesis that the surface periclinal cell walls of leaf epidermis have a constant thickness, at 200 nm, and cell-specific turgor pressure is retrieved by averaging all turgor deductions per cell.
Plasmolysis assays
Epidermal strips were peeled, fixed on glass slides, and bathed in solutions differing in mannitol concentration. The percentage of strips displaying plasmolysis within 5 min incubation was determined using a microscope.
Tissue K+ content
Leaf margins were isolated by obtaining 2-mm razor-cut bands, which were enriched for hydathodes. Leaf epidermis was obtained by peeling abaxial epidermis with forceps. K+ contents were determined in dried samples by flame spectrometry (SpectrAA 220 FS, Varian, http://www.varianinc.com/), after ionic extraction (sample incubation for 2 days in 0.1 N HCl).
Complementation of atkc1–2-mutant plants and promoter analyses
Mutant isolation and generation of transgenic plants expressing AtKC1 under its native promoter has been described elsewhere (Jeanguenin et al., 2011). For guard cell-specific complementation of atkc1–2-mutant plants, AtKC1 and AtKC1–DN cDNAs were expressed under the KAT1 promoter in pCambia1301 vector (Hajdukiewicz et al., 1994). AtKC1-DN has been described previously (Jeanguenin et al., 2011) and contained two pore residue mutations (G291R and Y292R) that rendered it a dominant-negative channel subunit. For other indicated cell-specific expression of AtKC1, we cloned the previously characterized genomic regions upstream of the first ATG from the loci CER5, OCT3, GL2, At1G66460, and FMO1 in the pCambia1301 vector to drive AtKC1 expression. For expression pattern analyses, the same upstream regions were also cloned in pGWB3 using Gateway cloning (Nakagawa et al., 2007) to drive GUS expression in WT transformed plants, except ProOCT3:GUS lines that were kindly gifted by Isabell Kufner and described elsewhere (Kufner and Koch, 2008). For previously uncharacterized promoters (ProCYP96A4 and ProKCS19), the inter-genomic regions located between the first ATG and the 3′-end of the corresponding upstream loci were amplified. Floral dip method was used to transfect Arabidopsis plants (Clough and Bent, 1998). Transformed lines were verified by RT-PCR on RNA extracted from leaves of individual T1 plants, and T2 progeny homozygous for the transgene were selected based on true segregation of the linked hygromycin resistance marker of pCambia1301. Experiments were conducted on T3 homozygous plants.
Guard cell protoplast preparation for gene expression analysis
About 30–35 fully expanded rosette leaves were kept in cold water and in the dark. Main veins of leaves were removed using a scalpel. Leaf pieces were blended three times for 45 s at full speed, and the yielded mixture was put over a nylon mesh and rinsed with cold distilled water. The epidermis fragments recovered from the 75-µm nylon mesh were digested for 30–45 min at 25°C with gentle shaking (140 rpm) in an enzyme solution (0.7% Calbiochem cellulysin, 0.1% PVP 40, 0.25% BSA, 0.5 mM ascorbic acid, 45% distilled water, and 55% solution containing sorbitol 560 mmol/kg, 5 mM MES, 0.5 mM CaCl2, 0.5 mM MgCl2, 0.5 mM ascorbic acid, pH 5.5 with Tris). Translation inhibitor (100 mg/L Cordycepin, C3394-Sigma) and transcription inhibitor (33 mg/L Actinomycin D, A1410- Sigma) were also added to the digestion mixture. The digestion process was followed under a microscope (Olympus BH2), to check that “intact” guard cells were still present in situ in the digested epidermis at the end of the enzymatic treatment. The undigested fraction was recovered by filtration through 40-μm nylon mesh, rinsed with basic solution, and stored at −80°C.
Gene expression analysis by RT-qPCR
Total RNA extraction, synthesis of first-strand cDNAs, and quantitative RT–PCR procedures were performed as described elsewhere (Cuellar et al., 2010). Primers used for real time qRT-PCR were designed using PRIMER3 (http://frodo.wi.mit.edu) (Supplemental Table S2). All amplification plots were analyzed with an Rn threshold (normalized reporter) of 0.2 to obtain CT (threshold cycle) values. Standard curves for AtKC1, KAT1, KAT2, and GORK were obtained from dilution series of known quantities of corresponding cDNA fragments used as templates. Standard curves were used to calculate the absolute numbers of tested cDNA molecules in each cDNA sample and these values were then normalized against corresponding housekeeping gene signals. Four housekeeping genes EF1alpha, TIP41, PDF2, and MXC9.20 (Czechowski et al., 2005) were used to calculate a normalization factor with the online algorithm “geNorm” (https://genorm.cmgg.be/).
Expression analyses by GUS staining
GUS staining of leaves from 6-week-old transgenic plants expressing the β-GUS reporter gene under the control of the promoters listed in Supplemental Figure S1 was performed as described elsewhere (Lagarde et al., 1996). Similar expression patterns were obtained in three independent transgenic lines for each promoter.
Statistical analysis
Statistical analysis was performed using the two-tailed Student’s t test, analysis of variance (ANOVA), and Tukey’s post hoc test as indicated with Statistix V.8 software for Windows. The results are shown in Supplemental Data Set S1.
Accession numbers
Sequence data from this article can be found in the TAIR (Arabidopsis) database under accession numbers: AtKC1 (At4G32650), KAT1 (AT5G46240), CER5 (At1G51500), OCT3 (At1G16390), GL2 (At1G79840), Uncharacterized Protein Kinase gene (At1G66460), FMO1 (At1G19250), CYP96A4 (At5G52320), and ProKCS19 (At5G04530).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression patterns driven by the AtKC1 promoter and other selected cell-specific promoters in leaf epidermis.
Supplemental Figure S2. Stomatal density in WT and atkc1–2 leaves (abaxial side).
Supplemental Figure S3. Example of non-Shaker-like channel activities in pavement cell protoplasts from A. thaliana WT and atkc1–2-mutant plants (Ws ecotype).
Supplemental Figure S4. Expression of AtKC1 and AtKC1–DN under the KAT1 promoter.
Supplemental Figure S5. Expression patterns driven by AtKC1 promoter and other selected cell-specific promoters in abaxial leaf epidermis and trichomes.
Supplemental Figure S6. The atkc1–2 mutation does not affect trichome density in WT and atkc1–2 plants.
Supplemental Table S1. Cell-specific expression level of the genes selected in complementation experiments (Figure 8) obtained from eFP browser site (supports Figure 8).
Supplemental Table S2. Primer list.
Supplemental Data Set S1. Statistical analysis results.
Supplementary Material
Acknowledgments
We thank Isabell Kufner for providing ProOCT3:GUS lines.
Funding
This work was supported by the Alfonso Martin Escudero Foundation (to M.N.-C.), the Marie Curie Programme (Grant No. 272390 FP7-IEF to M.N.-C.), and the European Research Council (PhyMorph #307387 to A.B.).
Conflict of interest statement. None declared.
Contributor Information
Manuel Nieves-Cordones, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Farrukh Azeem, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Yuchen Long, Laboratoire Reproduction et Développement des Plantes, Univ Lyon, ENS de Lyon, UCB Lyon 1, CNRS, INRA, F-69342 Lyon, France.
Martin Boeglin, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Geoffrey Duby, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Karine Mouline, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Eric Hosy, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Alain Vavasseur, CEA Cadarache DSV DEVM LEMS UMR 163, CNRS/CEA, F-13108 St Paul Lez Durance, France.
Isabelle Chérel, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Thierry Simonneau, INRA Laboratoire d’Ecophysiologie des Plantes sous Stress Environnementaux, Place Viala, 2, F-34060 Montpellier Cedex 1, France.
Frédéric Gaymard, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Jeffrey Leung, Université Paris-Saclay, INRAE, AgroParisTech, CNRS, Institut Jean-Pierre Bourgin (IJPB), 78000 Versailles, France.
Isabelle Gaillard, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Jean-Baptiste Thibaud, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France; Institut des biomolécules Max Mousseron (UMR 5247 CNRS-UM-ENSCM) Campus CNRS, 1919 route de Mende, F-34293 Montpellier Cedex 05, France.
Anne-Aliénor Véry, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
Arezki Boudaoud, Laboratoire Reproduction et Développement des Plantes, Univ Lyon, ENS de Lyon, UCB Lyon 1, CNRS, INRA, F-69342 Lyon, France.
Hervé Sentenac, Biochimie et Physiologie Moléculaire des Plantes, UMR BPMP, Univ Montpellier, CNRS, INRAE, Montpellier SupAgro, Montpellier 34060, France.
M.N.-C. carried out the mutant phenotyping, cell-specific complementation experiments, and MP recordings by microelectrode impalement. F.A. performed the gene expression and guard cell complementation analyses. G.D. carried out the mutant phenotyping experiments and the epidermal plasmolysis assays. K.M., A.V., T.S., and F.G. identified the mutant phenotype and conducted the first transpiration analyses. M.B., E.H., and A.-A.V. carried out the patch-clamp analyses on guard cell and pavement cell protoplasts. M.N.-C., A.V., and I.G. performed the stomatal aperture measurements. I.C., J.L., and T.S. contributed to the project conception. I.G. supervised the gene expression, mutant phenotyping, and complementation experiments. Y.L. performed the AFM measurements. Y.L. and A.B. designed the AFM experiments and analyzed the AFM data. H.S. and J.-B.T. jointly supervised the whole project. M.N.-C., H.S., J.-B.T., J.L., and A.-A.V. wrote the manuscript.
The author(s) responsible for the distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plcell) are: Manuel Nieves-Cordones (mncordones@cebas.csic.es) and Anne-Aliénor Véry (anne-alienor.very@cnrs.fr).
References
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KA, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al. (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Beauzamy L, Derr J, Boudaoud A (2015) Quantifying hydrostatic pressure in plant cells by using indentation with an atomic force microscope. Biophys J 108: 2448–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 [DOI] [PubMed] [Google Scholar]
- Britto DT, Coskun D, Kronzucker HJ (2021) Potassium physiology from Archean to Holocene: A higher-plant perspective. J Plant Physiol 262: 153432. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cuellar T, Pascaud F, Verdeil JL, Torregrosa L, Adam-Blondon AF, Thibaud JB, Sentenac H, Gaillard I (2010) A grapevine Shaker inward K+ channel activated by the calcineurin B-like calcium sensor 1-protein kinase CIPK23 network is expressed in grape berries under drought stress conditions. Plant J 61: 58–69 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daram P, Urbach S, Gaymard F, Sentenac H, Chérel I (1997) Tetramerization of the AKT1 plant potassium channel involves its C-terminal cytoplasmic domain. EMBO J 16: 3455–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duby G, Hosy E, Fizames C, Alcon C, Costa A, Sentenac H, Thibaud JB (2008) AtKC1, a conditionally targeted Shaker-type subunit, regulates the activity of plant K+ channels. Plant J 53: 115–123 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21: 94–100 [Google Scholar]
- Franks PJ, Buckley TN, Shope JC, Mott KA (2001) Guard cell volume and pressure measured concurrently by confocal microscopy and the cell pressure probe. Plant Physiol 125: 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Tyerman SD, Cleary AL, Lloyd J, Farquhar GD (1995) Guard-cell pressure aperture characteristics measured with the pressure probe. Plant Cell Environ 18: 795–800 [Google Scholar]
- Geelen D, Leyman B, Batoko H, Di Sansebastiano GP, Moore I, Blatt MR (2002) The abscisic acid-related SNARE homolog NtSyr1 contributes to secretion and growth: Evidence from competition with its cytosolic domain. Plant Cell 14: 387–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Becker D, Vosloh D, Gambale F, Palme K, Rehers M, Anschuetz U, Dreyer I, Kudla J, Hedrich R (2009) Heteromeric AtKC1·AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J Biol Chem 284: 21288–21295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Liu L, Facette M (2020) Flanking support: How subsidiary cells contribute to stomatal form and function. Front Plant Sci 11: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen C, Karnik R, Larson E, Lefoulon C, Wang Y, Waghmare S, Zhang B, Hills A, Blatt MR (2015) A vesicle-trafficking protein commandeers Kv channel voltage sensors for voltage-dependent secretion. Nat Plants 1: 15108. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Hedrich R (2012) Ion channels in plants. Physiol Rev 92: 1777–1811 [DOI] [PubMed] [Google Scholar]
- Higaki T, Hashimoto-Sugimoto M, Akita K, Iba K, Hasezawa S (2014) Dynamics and environmental responses of PATROL1 in Arabidopsis subsidiary cells. Plant Cell Physiol 55: 773–780 [DOI] [PubMed] [Google Scholar]
- Honsbein A, Sokolovski S, Grefen C, Campanoni P, Pratelli R, Paneque M, Chen Z, Johansson I, Blatt MR (2009) A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell 21: 2859–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, Boucherez J, Lebaudy A, Bouchez D, Véry A-A, et al. (2003) The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc Natl Acad Sci USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble GD, Raschke K (1971) Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol 48: 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoby MJ, Falkenhan D, Mader MT, Brininstool G, Wischnitzki E, Platz N, Hudson A, Hulskamp M, Larkin J, Schnittger A (2008) Transcriptional profiling of mature Arabidopsis trichomes reveals that NOECK encodes the MIXTA-like transcriptional regulator MYB106. Plant Physiol 148: 1583–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F, Leonhardt N, Tran D, Bousserouel H, Véry A-A, Renou J-P, Vavasseur A, Kwak JM, Sentenac H, Bouteau F, et al. (2014) Acetylated 1,3-diaminopropane antagonizes abscisic acid-mediated stomatal closing in Arabidopsis. Plant J 79: 322–333 [DOI] [PubMed] [Google Scholar]
- Jeanguenin L, Alcon C, Duby G, Boeglin M, Chérel I, Gaillard I, Zimmermann S, Sentenac H, Véry A-A (2011) AtKC1 is a general modulator of Arabidopsis inward Shaker channel activity. Plant J 67: 570–582 [DOI] [PubMed] [Google Scholar]
- Jegla T, Busey G, Assmann SM (2018) Evolution and structural characteristics of plant voltage-gated K+ Channels. Plant Cell 30: 2898–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Hills A, Blatt MR, Lew VL (2019) A constraint-relaxation-recovery mechanism for stomatal dynamics. Plant Cell Environ 42: 2399–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Wang RS, Zhu M, Jeon BW, Albert R, Chen S, Assmann SM (2013) Abscisic acid-responsive guard cell metabolomes of Arabidopsis wild-type and gpa1 G-protein mutants. Plant Cell 25: 4789–4811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI (2010) Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufner I, Koch W (2008) Stress regulated members of the plant organic cation transporter family are localized to the vacuolar membrane. BMC Res Notes 1: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB (2000) A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell 12: 837–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde D, Basset M, Lepetit M, Conejero G, Gaymard F, Astruc S, Grignon C (1996) Tissue-specific expression of Arabidopsis AKT1 gene is consistent with a role in K+ nutrition. Plant J 9: 195–203 [DOI] [PubMed] [Google Scholar]
- Lebaudy A, Vavasseur A, Hosy E, Dreyer I, Leonhardt N, Thibaud JB, Véry A-A, Simonneau T, Sentenac H (2008) Plant adaptation to fluctuating environment and biomass production are strongly dependent on guard cell potassium channels. Proc Natl Acad Sci USA 105: 5271–5276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebaudy A, Pascaud F, Véry A-A, Alcon C, Dreyer I, Thibaud J-B, Lacombe B (2010) Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J Biol Chem 285: 6265–6274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt N, Kwak JM, Robert N, Waner D, Leonhardt G, Schroeder JI (2004) Microarray expression analyses of Arabidopsis guard cells and isolation of a recessive abscisic acid hypersensitive protein phosphatase 2C mutant. Plant Cell 16: 596–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieckfeldt E, Simon-Rosin U, Kose F, Zoeller D, Schliep M, Fisahn J (2008) Gene expression profiling of single epidermal, basal and trichome cells of Arabidopsis thaliana. J Plant Physiol 165: 1530–1544 [DOI] [PubMed] [Google Scholar]
- Long Y, Cheddadi I, Mosca G, Mirabet V, Dumond M, Kiss A, Traas J, Godin C, Boudaoud A (2020) Cellular heterogeneity in pressure and growth emerges from tissue topology and geometry. Curr Biol 30: 1504–1516.e8 [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC (1980) Osmotic measurements on stomatal cells of Commelina communis L. J Membr Biol 53: 189–198 [Google Scholar]
- Malgat R, Faure F, Boudaoud A (2016) A mechanical model to interpret cell-scale indentation experiments on plant tissues in terms of cell wall elasticity and turgor pressure. Front Plant Sci 7: 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Leonhardt N, Fenzi F, Valon C, Costa M, Piette L, Vavasseur A, Genty B, Boivin K, Muller A, et al. (2007) Constitutive activation of a plasma membrane H+-ATPase prevents abscisic acid-mediated stomatal closure. EMBO J 26: 3216–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Dreyer I, Lacombe B, Sentenac H, Thibaud JB (2005a) Inward rectification of the AKT2 channel abolished by voltage-dependent phosphorylation. Plant J 44: 783–797 [DOI] [PubMed] [Google Scholar]
- Michard E, Lacombe B, Porée F, Mueller-Roeber B, Sentenac H, Thibaud JB, Dreyer I (2005b) A unique voltage sensor sensitizes the potassium channel AKT2 to phosphoregulation. J Gen Physiol 126: 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra BB, Acharya BR, Granot D, Assmann SM, Chen S (2015) The guard cell metabolome: Functions in stomatal movement and global food security. Front Plant Sci 6: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Buckley TN (2000) Patchy stomatal conductance: Emergent collective behaviour of stomata. Trends Plant Sci 5: 258–262 [DOI] [PubMed] [Google Scholar]
- Mustroph A, Zanetti ME, Jang CJ, Holtan HE, Repetti PP, Galbraith DW, Girke T, Bailey-Serres J (2009) Profiling translatomes of discrete cell populations resolves altered cellular priorities during hypoxia in Arabidopsis. Proc Natl Acad Sci USA 106: 18843–18848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR (1995) Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol 109: 371–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TH, Huang S, Meynard D, Chaine C, Michel R, Roelfsema MRG, Guiderdoni E, Sentenac H, Véry A-A (2017) A dual role for the OsK5.2 ion channel in stomatal movements and K+ loading into Xylem Sap. Plant Physiol 174: 2409–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieves-Cordones M, Caballero F, Martinez V, Rubio F (2012) Disruption of the Arabidopsis thaliana inward-rectifier K+ channel AKT1 improves plant responses to water stress. Plant Cell Physiol 53: 423–432 [DOI] [PubMed] [Google Scholar]
- Nieves-Cordones M, Chavanieu A, Jeanguenin L, Alcon C, Szponarski W, Estaran S, Chérel I, Zimmermann S, Sentenac H, Gaillard I (2014) Distinct amino acids in the C-linker domain of the Arabidopsis K+ channel KAT2 determine its subcellular localization and activity at the plasma membrane. Plant Physiol 164: 1415–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak B, Malinovsky FG, Brodersen P, Grell M, Giese H, Petersen M, Mundy J (2006) A putative flavin-containing mono-oxygenase as a marker for certain defense and cell death pathways. Plant Sci 170: 614–623 [Google Scholar]
- Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, Ellerbrok H, Kamuzinzi R, Ledent V, Blaudez D, et al. (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevitz BA, Hepler PK (1985) Changes in dye coupling of stomatal cells of Allium and Commelina demonstrated by microinjection of Lucifer yellow. Planta 164: 473–479 [DOI] [PubMed] [Google Scholar]
- Pandey S, Zhang W, Assmann SM (2007) Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett 581: 2325–2336 [DOI] [PubMed] [Google Scholar]
- Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, Kunst L, Samuels AL (2004) Plant cuticular lipid export requires an ABC transporter. Science 306: 702–704 [DOI] [PubMed] [Google Scholar]
- Pilot G, Gaymard F, Mouline K, Chérel I, Sentenac H (2003) Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol Biol 51: 773–787 [DOI] [PubMed] [Google Scholar]
- Pilot G, Lacombe B, Gaymard F, Chérel I, Boucherez J, Thibaud JB, Sentenac H (2001) Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J Biol Chem 276: 3215–3221 [DOI] [PubMed] [Google Scholar]
- Reintanz B, Szyroki A, Ivashikina N, Ache P, Godde M, Becker D, Palme K, Hedrich R (2002) AtKC1, a silent Arabidopsis potassium channel alpha-subunit modulates root hair K+ influx. Proc Natl Acad Sci USA 99: 4079–4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Prins HBA (1997) Ion channels in guard cells of Arabidopsis thaliana (L.) Heynh. Planta 202: 18–27 [DOI] [PubMed] [Google Scholar]
- Rohaim A, Gong LD, Li J, Rui H, Blachowicz L, Roux B (2020) Open and closed structures of a barium-blocked potassium channel. J Mol Biol 432: 4783–4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep M, Ebert B, Simon-Rosin U, Zoeller D, Fisahn J (2010) Quantitative expression analysis of selected transcription factors in pavement, basal and trichome cells of mature leaves from Arabidopsis thaliana. Protoplasma 241: 29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E (1987) Voltage dependence of K+ channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Hirsch RE, Lewis DR, Qi Z, Sussman MR, Bryan DL (1999) Potassium uptake supporting plant growth in the absence of AKT1 channel activity. J Gen Physiol 113: 909–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, North H, Grignon C, Thibaud JB, Sentenac H, Véry AA (2005) Regulation by external K+ in a maize inward shaker channel targets transport activity in the high concentration range. Plant Cell 17: 1532–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski DB, Jilk RA, Pollock SM, Marks MD (1998) Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125: 1161–1171 [DOI] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E (1996) Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol 111: 1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poethig RS (1997) Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124: 645–654 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kinoshita T, Shimazaki K-I (2001) Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant Cell Physiol 42: 795–802 [DOI] [PubMed] [Google Scholar]
- Urbach S, Chérel I, Sentenac H, Gaymard F (2000) Biochemical characterization of the Arabidopsis K+ channels KAT1 and AKT1 expressed or co-expressed in insect cells. Plant J 23: 527–538 [DOI] [PubMed] [Google Scholar]
- Verger S, Long Y, Boudaoud A, Hamant O (2018) A tension-adhesion feedback loop in plant epidermis. Elife 7: 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2002) Cation channels in the Arabidopsis plasma membrane. Trends Plant Sci 7: 168–175 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Véry A-A, Nieves-Cordones M, Daly M, Khan I, Fizames C, Sentenac H (2014) Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J Plant Physiol 171: 748–769 [DOI] [PubMed] [Google Scholar]
- Wang RS, Pandey S, Li S, Gookin TE, Zhao Z, Albert R, Assmann SM (2011) Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC Genomics 12: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Chen LM, Liu WX, Shen LK, Wang FL, Zhou Y, Zhang Z, Wu WH, Wang Y (2016) AtKC1 and CIPK23 synergistically modulate AKT1-mediated low-potassium stress responses in Arabidopsis. Plant Physiol 170: 2264–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner LH, De Boer AH, Raschke K (1994) Properties of the K+ inward rectifier in the plasma membrane of xylem parenchyma cells from barley roots: Effects of TEA+, Ca2+, Ba2+ and La3+. J Membr Biol 142: 363–379 [DOI] [PubMed] [Google Scholar]
- Wille AC, Lucas WJ (1984) Ultrastructural and histochemical studies on guard cells. Planta 160: 129–142 [DOI] [PubMed] [Google Scholar]
- Zhang B, Karnik R, Wang Y, Wallmeroth N, Blatt MR, Grefen C (2015) The Arabidopsis R-SNARE VAMP721 interacts with KAT1 and KC1 K+ channels to moderate K+ current at the plasma membrane. Plant Cell 27: 1697–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM (2008) Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 20: 3210–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LH, Liu SB, Wang PF, Lu TJ, Xu F, Genin GM, Pickard BG (2017) The Arabidopsis trichome is an active mechanosensory switch. Plant Cell Environ 40: 611–621 [DOI] [PubMed] [Google Scholar]
- Zhu M, Assmann SM (2017) Metabolic signatures in response to abscisic acid (ABA) treatment in Brassica napus Guard cells revealed by metabolomics. Sci Rep 7: 12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.