Abstract
The antimicrobial activity of BMS 284756, a novel des-F(6)-quinolone, was comparatively evaluated against 257 Streptococcus pneumoniae, 198 Haemophilus influenzae, and 88 Moraxella catarrhalis strains isolated in Latin America between July and September of 1999 as part of the SENTRY Antimicrobial Surveillance Program. Nearly 28.0% of S. pneumoniae strains were nonsusceptible to penicillin. The rank order of quinolone potency versus S. pneumoniae was BMS 284756 (MIC at which 90% of isolates were inhibited [MIC90], 0.12 μg/ml) > trovafloxacin (MIC90, 0.25 μg/ml) > gatifloxacin (MIC90, 0.5 μg/ml) > levofloxacin and ciprofloxacin (MIC90, 1 to 2 μg/ml). All S. pneumoniae strains that were not susceptible to other quinolones were inhibited by BMS 284756 at ≤2 μg/ml. The overall prevalence of β-lactamase production was 15.2% in H. influenzae and 98.9% in M. catarrhalis. BMS 284756 showed excellent potency and spectrum against this group of pathogens, inhibiting all isolates at ≤0.12 μg/ml. BMS 284756 exhibited activity similar to those displayed by the new fluoroquinolones, such as levofloxacin, trovafloxacin, or gatifloxacin, and could be a therapeutic option for empirical treatment of community-acquired respiratory tract infections.
The introduction of the fluoroquinolones ofloxacin and ciprofloxacin represented a great advance in the treatment of a wide range of infections caused by gram-negative organisms by oral and parenteral routes. However, these compounds possess limited activity against some gram-positive pathogens and anaerobes. The increased importance of gram-positive organisms, such as penicillin-resistant Streptococcus pneumoniae, and the emergence of ciprofloxacin-resistant isolates has led to a synthesis and development of several newer fluoroquinolones in the last decade (1, 2; K. Hayashi, Y. Todo, S. Hamamoto, K. Ojima, M. Yamada, T. Kito, M. Takahata, Y. Watanabe, and H. Narita, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F158, p. 173, 1997).
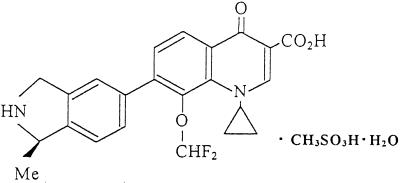
BMS 284756 (formerly T-3811ME), 1-cyclopropyl-8-(difluoromethoxy)-7-[(1R)-(1-methyl-2,3-dihydro-1H-5-isoindolyl)]-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid methanesulfonate monohydrate, is a novel des-F(6)-quinolone (Fig. 1) that has shown excellent potency against a broad spectrum of pathogens, including some atypical pathogens, such as Mycobacterium, Mycoplasma, and Chlamydia (7; Hayashi et al., 37th ICAAC). The purpose of this study was to comparatively evaluate and confirm (7; Hayashi et al., 37th ICAAC; R. Hori, M. Takahta, M. Shimakura, H. Sugiyama, M. Yonezawa, Y. Todo, S. Minami, Y. Watanabe, and H. Narita, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-78, 1998; A. Nagai, M. Takahata, M. Miyazaki, Y. Kawamura, T. Kodama, Y. Todo, Y. Watanabe, H. Narita, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F162, p. 173, 1997; M. Takahata, J. Mitsuyama, Y. Yamshiro, H. Araki, H. Yamada, H. Hayakawa, Y. Todom, S. Minami, Y. Watanabe, and H. Narita, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F160, p. 173, 1997) the antimicrobial activity of BMS 284756 against S. pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the process of monitoring emerging resistance among the most frequent causes of community-acquired lower respiratory tract infections (SENTRY Antimicrobial Surveillance Program, involving 10 Latin American medical centers).
FIG. 1.
Chemical structure of BMS 284756 (T-3811).
MATERIALS AND METHODS
Bacterial strains.
A total of 257 S. pneumoniae, 198 H. influenzae, and 88 M. catarrhalis strains were tested, each isolated from patients with community-acquired infections in Latin America between July and September of 1999 as part of the SENTRY Antimicrobial Surveillance Program (3). Only one isolate per patient was included. The participating medical centers were distributed throughout six countries (nine cities), including São Paulo, Florianópolis, and Rio Grande do Sul in Brazil; Buenos Aires and San Isidro in Argentina; Santiago (two sites) in Chile; Medellin in Colombia; Caracas in Venezuela; and Mexico City in Mexico. The isolates were transported to the University of Iowa College of Medicine (Iowa City, Iowa), the monitoring laboratory, where the identification of isolates was confirmed by using conventional methods described earlier (3).
Susceptibility testing.
The MICs were determined and interpreted using the reference broth microdilution method as described by the National Committee for Clinical Laboratory Standards (NCCLS) (5, 6). Antimicrobial agents were obtained from their respective manufacturers and included penicillins (ampicillin and penicillin), β-lactamase inhibitor combinations (amoxicillin-clavulanic acid), cephalosporins (cefaclor, cefixime, cefpodoxime, and cefuroxime), macrolides (azithromycin, clarithromycin, and erythromycin), fluoroquinolones (ciprofloxacin, gatifloxacin, levofloxacin, trovafloxacin, and BMS 284756), vancomycin, and other widely marketed compounds in the region, such as chloramphenicol, clindamycin, tetracycline, and trimethoprim-sulfamethoxazole. H. influenzae ATCC 49247, S. pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, and Escherichia coli ATCC 25922 were utilized as quality control strains (5). The production of β-lactamase was assessed by using chromogenic cephalosporin disks (Becton Dickinson Microbiology Systems, Cockeysville, Md.).
RESULTS AND DISCUSSION
The activities of 14 orally administered antimicrobial agents against S. pneumoniae are summarized in Table 1. Among 257 S. pneumoniae strains recovered from the Latin American medical centers, 72.4% were susceptible to penicillin (MICs, ≤0.06 μg/ml) and 13.2% had high-level resistance to penicillin (MICs, ≥ 2 μg/ml). Among the penicillin-susceptible S. pneumoniae strains, all β-lactam antimicrobial agents demonstrated excellent activity and isolates showed near complete susceptibility to them (except cefaclor; isolates showed 86.6% susceptibility). The glycopeptide and a streptogramin (data not shown) along with the newer fluoroquinolones were the only agents or classes of antimicrobials that inhibited all penicillin-resistant S. pneumoniae strains. Independent of the penicillin susceptibility, BMS 284756 (MIC at which 50% of isolates were inhibited [MIC50], 0.06 μg/ml) was twofold and fourfold more potent than trovafloxacin (MIC50, 0.12 μg/ml) and gatifloxacin (MIC50, 0.25 μg/ml) against the pneumococci, respectively (2, 3; Hori et al., 38th ICAAC). Among all the antimicrobial drugs tested, BMS 284756 was the most potent compound and was 8- to 16-fold more active than vancomycin and cefotaxime (data not shown), both of which are currently the parenteral drugs of choice for treatment of serious penicillin-resistant S. pneumoniae infections (Hori et al., 38th ICAAC).
TABLE 1.
Antimicrobial activity of BMS 284756 and 13 other orally administered agents tested against 257 S. pneumoniae isolates in 10 participant centers in Latin America (SENTRY Antimicrobial Surveillance Program, 1999)
| Antimicrobial agent | Penicillin susceptibility category (no. of strains)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Susceptible (186)
|
Intermediate (37)
|
Resistant (34)
|
|||||||
| MIC (μg/ml)
|
% Susceptible | MIC (μg/ml)
|
% Susceptible | MIC (μg/ml)
|
% Susceptible | ||||
| 50% | 90% | 50% | 90% | 50% | 90% | ||||
| BMS 284756 | 0.06 | 0.12 | 100.0 (≤4) | 0.06 | 0.12 | 100.0 (≤4) | 0.06 | 0.12 | 100.0 (≤4) |
| Ciprofloxacin | 1 | 2 | 67.5 (≤1) | 1 | 2 | 64.9 (≤1) | 1 | 2 | 76.5 (≤1) |
| Gatifloxacin | 0.25 | 0.5 | 99.5 | 0.25 | 0.5 | 100.0 | 0.25 | 0.5 | 100.0 |
| Levofloxacin | 1 | 1 | 99.5 | 1 | 1 | 100.0 | 1 | 1 | 100.0 |
| Trovafloxacin | 0.12 | 0.25 | 99.5 | 0.12 | 0.25 | 100.0 | 0.12 | 0.25 | 100.0 |
| Amoxicillin-clavulanate | ≤0.25 | ≤0.25 | 100.0 | ≤0.25 | 1 | 100.0 | 2 | 2 | 91.2 |
| Cefaclor | 1 | 2 | 86.6 | 2 | >32 | 32.4 | >32 | >32 | 0.0 |
| Cefuroxime | 0.06 | 0.12 | 100.0 | 0.5 | 4 | 83.8 | 8 | 8 | 0.0 |
| Cefixime | 0.25 | 0.5 | 2 | >4 | >4 | >4 | |||
| Cefpodoxime | ≤0.03 | 0.06 | 100.0 | 0.25 | 1 | 81.1 | 2 | 4 | 0.0 |
| Erythromycin | ≤0.25 | ≤0.25 | 91.9 | ≤0.25 | 16 | 78.4 | ≤0.25 | 4 | 67.6 |
| Clindamycin | ≤0.25 | ≤0.25 | 97.3 | ≤0.25 | ≤0.25 | 91.9 | ≤0.25 | ≤0.25 | 94.1 |
| Tetracycline | ≤2 | >16 | 79.6 | ≤2 | >16 | 70.3 | 2 | 16 | 85.3 |
| Trimethoprim-sulfamethoxazole | ≤0.5 | 4 | 75.8 | 1 | >4 | 48.6 | >4 | >4 | 0.0 |
Although the mechanisms of resistance to macrolides are completely distinct from those causing penicillin or β-lactam resistance, non-penicillin-susceptible S. pneumoniae isolates are usually less susceptible to macrolides. Among these isolates, the mechanism of resistance to macrolides appears to be the presence of a noninducible, macrolide-specific efflux pump, since most macrolide-resistant S. pneumoniae strains remain susceptible to clindamycin (overall susceptibility rate, 96.0%) and represent the so-called “M phenotype.”
Among the H. influenzae strains, the overall prevalence of β-lactamase production was 15.2%. The antimicrobial activities of BMS 285756 and several other compounds are depicted in Table 2. BMS 284756 (MIC90, ≤0.03 μg/ml; 100.0% susceptible) demonstrated potency and susceptibility rates indistinguishable from those of ciprofloxacin (MIC90, ≤0.015 μg/ml; 100.0% susceptible), gatifloxacin (MIC90, ≤0.015 μg/ml; 100.0% susceptible), trovafloxacin (MIC90, ≤0.03 μg/ml; 100.0% susceptible), and levofloxacin (MIC90, ≤0.03 μg/ml; 100.0% susceptible) (1–4, 7; Hayashi et al., 37th ICAAC). All the cephalosporins tested inhibited 100.0% of H. influenzae isolates, except for cefaclor, which showed a susceptibility rate of 97.0%. Azithromycin (MIC90, 1 μg/ml) was the most active macrolide, and isolates exhibited the highest rate of susceptibility to it (100.0%). Resistance to non-β-lactam antimicrobial agents such as chloramphenicol (1.0% with documented chloramphenicol acetyltransferase enzyme) and tetracycline (1.0%) was also very uncommon. In contrast, 31.3% of H. influenzae isolates were resistant to trimethoprim-sulfamethoxazole.
TABLE 2.
Results of testing GMS 284756 and 16 other orally administered agents against 198 strains of H. influenzae isolated from Latin American laboratories participating in the SENTRY Antimicrobial Surveillance Program, 1999
| Antimicrobial agent | MIC (μg/ml)
|
% Susceptiblea | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| BMS 284756 | ≤0.03 | ≤0.03 | ≤0.03–0.06 | 100.0 (≤4) |
| Ciprofloxacin | ≤0.015 | ≤0.015 | ≤0.015–0.003 | 100.0 |
| Gatifloxacin | ≤0.015 | ≤0.015 | ≤0.015–0.03 | 100.0 |
| Levofloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.06 | 100.0 |
| Trovafloxacin | ≤0.03 | ≤0.03 | ≤0.03 | 100.0 |
| Ampicillinb | ≤0.5 | >4 | ≤0.5–>4 | 85.4 |
| Amoxicillin-clavulanate | 0.5 | 1 | ≤0.25–2 | 100.0 |
| Cefaclor | 2 | 8 | ≤0.25–32 | 97.0 |
| Cefuroxime | 1 | 2 | ≤0.06–4 | 100.0 |
| Cefixime | ≤0.03 | 0.06 | ≤0.03–0.25 | 100.0 |
| Cefpodoxime | 0.06 | 0.12 | ≤0.03–0.25 | 100.0 |
| Azithromycin | 0.5 | 1 | ≤0.12–2 | 100.0 |
| Clarithromycin | 4 | 8 | ≤0.25–16 | 97.5 |
| Chloramphenicol | ≤2 | ≤2 | ≤2–16 | 99.0 |
| Tetracycline | ≤2 | ≤2 | ≤2–16 | 99.0 |
| Trimethoprim-sulfamethoxazole | ≤0.5 | >4 | ≤0.5–>4 | 62.0 |
| Rifampin | ≤1 | ≤1 | ≤1 | 100.0 |
Since specific guidelines for the interpretation of MICs for M. catarrhalis have not been described by NCCLS (6), the antimicrobial susceptibility rates were interpreted using criteria generally used for H. influenzae or as listed in Table 3. The penicillins showed poor antimicrobial activity (MIC90s, ≥4 μg/ml) since 98.9% of M. catarrhalis isolates were β-lactamase producers. Based on MIC50, MIC90, and susceptibility rates, BMS 284756 had a high activity similar to those displayed by other quinolones (1–4). One macrolide-resistant M. catarrhalis isolate was detected, but the mechanism of resistance was undetermined.
TABLE 3.
Results of testing BMS 284756 and 15 comparison orally administered agents against 88 M. catarrhalis strains isolated from SENTRY Antimicrobial Surveillance Program sites in Latin America, 1999
| Antimicrobial agent | MIC (μg/ml)
|
% Susceptibilea | ||
|---|---|---|---|---|
| 50% | 90% | Range | ||
| BMS 284756 | ≤0.03 | ≤0.03 | ≤0.03–0.12 | 100.0 (≤4) |
| Ciprofloxacin | 0.03 | 0.03 | ≤0.015–0.25 | 100.0 (≤1) |
| Gatifloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.25 | 100.0 (≤0.5) |
| Levofloxacin | ≤0.03 | 0.06 | ≤0.03–0.5 | 100.0 (≤2) |
| Trovafloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.25 | 100.0 (≤1) |
| Penicillin | 4 | >4 | ≤0.03–>4 | 1.1 (BL) |
| Amoxicillin-clavulanate | ≤0.25 | ≤0.25 | ≤0.25 | 100.0 (≤2) |
| Cefaclor | 0.5 | 1 | ≤0.25–8 | 100.0 (≤8) |
| Cefuroxime | 1 | 2 | ≤0.06–4 | 100.0 (≤4) |
| Cefixime | 0.12 | 0.25 | ≤0.03–2 | 98.9 (≤1) |
| Cefpodoxime | 0.5 | 1 | 0.06–1 | 100.0 (≤2) |
| Erythromycin | ≤0.25 | ≤0.25 | ≤0.25–4 | 98.9 (≤0.5) |
| Chloramphenicol | ≤2 | ≤2 | ≤2–4 | 100.0 (≤4) |
| Tetracycline | ≤2 | ≤2 | ≤2 | 100.0 (≤2) |
| Trimethoprim-sulfamethoxazole | ≤0.5 | ≤0.5 | ≤0.5–2 | 97.7 (≤0.5) |
| Rifampin | ≤1 | ≤1 | ≤1 | 100.0 (≤1) |
Susceptibility criteria have not been published for this species (6). β-Lactamase (BL) production indicated resistance for penicillins, and other drugs were judged according to the criteria in parentheses.
The in vitro results observed in this study, in which contemporary isolates were tested by a reference method, agree with those of previous studies (7; Hayashi et al., 37th ICAAC; Hori et al., 38th ICAAC), where the activity of BMS 284756 was tested against smaller numbers of S. pneumoniae, H. influenzae, and M. catarrhalis isolates collected between 1994 and 1997. Only one fluoroquinolone-resistant S. pneumoniae isolate was detected during this study, the first found in Latin America. The activity of BMS 284756 against S. pneumoniae carrying a parC single mutation has been demonstrated (Hori et al., 38th ICAAC). Since all the quinolones tested showed similar activity against H. influenzae and M. catarrhalis, the activity against S. pneumoniae (including penicillin-resistant strains), toxicity profiles, pharmacokinetics properties (Takahata et al., 37th ICAAC), and cost may determine which quinolone will be more useful for the usually empirical treatment of community-acquired respiratory infections. BMS 284756 represents a promising novel antimicrobial agent for clinical use, since it possesses the most potent activity against the penicillin-resistant pneumococci tested and has previously demonstrated minimal in vivo toxicity (Nagai et al., 37th ICAAC).
ACKNOWLEDGMENTS
We express our sincere thanks to the many technologists and participants referring strains to the SENTRY Program. Also, we appreciate the efforts of the following individuals in the preparation of the manuscript and processing of the isolates: K. L. Meyer, D. J. Biedenbach, D. M. Johnson, M. L. Beach, M. Stilwell, and M. A. Pfaller.
The SENTRY Antimicrobial Surveillance Program, and this study were made possible by an educational and research grant from Bristol-Myers Squibb.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones BAY 12-8039, gatifloxacin (AM1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande L M, Diekema D J, Jones R N. Comparative activity of clinafloxacin and nine other compounds tested against 2000 contemporary clinical isolates from patients in United States hospitals. Diagn Microbiol Infect Dis. 1999;35:81–88. doi: 10.1016/s0732-8893(99)00020-6. [DOI] [PubMed] [Google Scholar]
- 3.Doern G V, Jones R N, Pfaller M A, Kugler C The Sentry Participants Group. Haemophilus influenzae and Moraxella catarrhalis from patients with community-acquired respiratory tract infections: antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (United States and Canada, 1997) Antimicrob Agents Chemother. 1999;43:385–389. doi: 10.1128/aac.43.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones R N, Biedenbach D J, Erwin M E, Beach M L, Pfaller M A The Quality Control Study Group. Activity of gatifloxacin against Haemophilus influenzae and Moraxella catarrhalis, including susceptibility test development, E-test comparisons, and quality control guidelines for H. influenzae. J Clin Microbiol. 1999;37:1999–2002. doi: 10.1128/jcm.37.6.1999-2002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial tests for bacteria that grow aerobically. Approved standard M7–A5. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Supplement tables, M100–S10. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [Google Scholar]
- 7.Takahata M, Mitsuyama J, Yamashiro Y, Yonezawa M, Araki H, Todo Y, Minami S, Watanabe Y, Narita H. In vitro and in vivo antimicrobial activites of T-3811ME, a novel des-F(6)-quinolone. Antimicrob Agents Chemother. 1999;43:1077–1084. doi: 10.1128/aac.43.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]