Abstract
Introduction:
Delays from primary surgery to chemotherapy are associated with worse survival in ovarian cancer; however, the impact of delays from neoadjuvant chemotherapy to interval debulking surgery is unknown. We sought to evaluate the association of delays from neoadjuvant chemotherapy to interval debulking with survival.
Methods:
Patients with a diagnosis of stage III/IV ovarian cancer receiving neoadjuvant chemotherapy from July 2015 to December 2017 were included in our analysis. Delays from neoadjuvant chemotherapy to interval debulking were defined as time from last preoperative carboplatin to interval debulking>6 weeks. Fisher’s exact/Wilcoxon rank sum tests were used to compare clinical characteristics. Kaplan-Meier method, log-rank test and multivariate Cox Proportional-Hazards models were used to estimate progression-free and overall survival and examine differences by delay groups, adjusting for covariates.
Results:
Of the 224 women, 159 (71%) underwent interval debulking and 34 (21%) of these experienced delays from neoadjuvant chemotherapy to interval debulking. These women were older (median 68 vs. 65 years, p=0.05) and received more preoperative chemotherapy cycles (median 6 vs. 4, p=0.003). Delays from neoadjuvant chemotherapy to interval debulking were associated with worse overall survival (HR 2.4 95% CI 1.2–4.8, p=0.01); however, survival was not significantly shortened after adjusting for age, stage and complete gross resection, HR 1.66 95% CI 0.8–3.4, p=0.17. Delays from neoadjuvant chemotherapy to interval debulking were not associated with worse progression-free survival (HR 1.55 95% CI 0.97–2.5, p=0.062). Increase in number of preoperative cycles (p=0.005) and lack of complete gross resection (p<0.001) were the only variables predictive of worse progression-free survival.
Discussion:
Delays from neoadjuvant chemotherapy to interval debulking were not associated with worse overall survival after adjustment for age, stage and complete gross resection.
INTRODUCTION
Many studies have examined the time interval from primary debulking s gery to postoperative chemotherapy and have shown that delays greater than 6 weeks ar issociated with worse survival1,2. In one analysis, if the time to initiation of chemotherapy exceeded 25 days after primary debulking surgery, there was an increased risk of death.3 However, few studies have examined the impact of delays from neoadjuvant chemotherapy to interval debulking surgery. Given the increasing use of neoadjuvant chemotherapy in treating women with advanced ovarian cancer over the last decade, we consider this an important question to address.4,5,6
A few small studies suggest that delays, from neoadjuvant chemotherapy to interval debulking may affect survival. Le et al. found that in 97 women receiving neoadjuvant chemotherapy, increased time between neoadjuvant chemotherapy and surgery may adversely affect overall survival, but this association was only significant in women with sub-optimal debulking.7 Chen et al. studied l52 women with stage III/IV ovarian cancer and found that time from neoadjuvant chemotherapy to interval debulking >4 weeks predicted worse progression-free but not overall survival.8.
In addition, there are multiple circumstances for delays during neoadjuvant therapy, including delays from diagnosis to chemotherapy, chemotherapy to interval debulking, and surgery to postoperative chemotherapy. Lee et al. studied 220 women with newly diagnosed ovarian cancer receiving neoadjuvant therapy and found that the time interval between neoadjuvant and postoperative chemotherapy affected survival, and that longer time intervals resulted in poorer survival.9
Some hypothesize that delays in neoadjuvant chemotherapy are due to underlying patient comorbidity that may adversely influence surgical and survival outcomes.2,10 Although some studies have reported that delays in chemotherapy after primary debulking were more likely to occur in the older adult, the malnourished, and those with high Charlson comorbidity index scores2, others have found that comorbidity and systems delays may affect survival independently.10
As a result, it is unclear if delays in neoadjuvant therapy have an independent impact on outcomes, or if all associations are mediated through increased patient comorbidity and suboptimal surgeries. Given this, we sought to evaluate the association of delays from neoadjuvant chemotherapy to interval debulking with survival, adjusting for other clinical variables as well as other time intervals in therapy.
METHODS
This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center, protocol 17–430.
Patient Selection
From July 2015 until December 1, 2017, we identified 241 women with newly diagnosed, pathologically verified ovarian, fallopian tube or primary peritoneal cancer and were recommended to receive neoadjuvant chemotherapy. Patients were identified from a rospectively kept institutional ovarian database, which was started on July 1, 2015, and captures all women presenting to Memorial Sloan Kettering Cancer Center with an adnexal complaint. Women were subsequently excluded if they were still undergoing treatment at the end of data abstraction (n=4), had incomplete chemotherapy data (n=8) or a histology revealing germ cell (n=1), small cell (n=3) or mucinous adenocarcinoma (n=1), resulting in 224 women who were included in this study. Of these, 162 women underwent debulking surgery of which 1 59 had complete chemotherapy dates and were included in this analysis. (Figure S1).
Data Collection
Clinical data were abstracted and verified from the electronic medical record for these patients from July 1, 2018 to December 1, 2018 by two independent reviewers (YL and OF). Patients were identified via the center’s Ovarian Database, which tracks all patients seen with an ovarian complaint from the time of initial visit. Age was defined in years from the date of pathological diagnosis. Stage was defined at pathological diagnosis using the 2014 International Federation of Gynecology and Obstetrics (FIGO) staging.11 The Charlson comorbidity score (a composite score measuring comorbidity in 12 areas, which has been shown to be predictive of mortality) 12,13 was calculated based on medical conditions present at pathological diagnosis. CA125 levels, serum albumin levels, and Karnofsky performance score14 were collected at the time of first chemotherapy treatment from the medical record. Body mass index, calculated as weight (kg) divided by height2 (m2), was collected at the time of first chemotherapy. BRCA testing status and results were abstracted from the medical record.
Surgical and medical oncology notes were reviewed to determine the indication for neoadjuvant therapy and were categorized as follows: 1) extent of disease not amenable to sufgery either by a predictive model15 or by radiologic evidence of stage IV disease; 2) patient comorbidity preventing surgery; 3) both extent of disease and patient comorbidity; 4) other, which was mostly due to venous thromboembolic disease. Optimal debulking was defined as residual disease <1cm. A complete gross resection was defined as no visible residual disease at the conclusion of surgery.
Chemotherapy regimens/doses were documented. Institutional practice guidelines al for an individualized decision between weekly or every 3 week administration of neoadjuvant chemotherapy. Dose reductions were defined as any reductions in chemotherapy occurring before debulking surgery and included any of the following: 1) carboplatin are a under the curve (AUC)<5; 2) weekly paclitaxel <80 mg/m2; 3) every 3-week paclitaxel<175mlg/m2; 4) any single-agent treatment for one or more treatment cycles. These we re further categorized as baseline dose reductions or dose reductions occurring after the first cycle but before surgery. The use of bevacizumab (monotherapy or combined with other chemotherapy) and PARP inhibitors at any time during treatment was documented. at any time during treatment was documented.
Defining Neoadjuvant Time Intervals
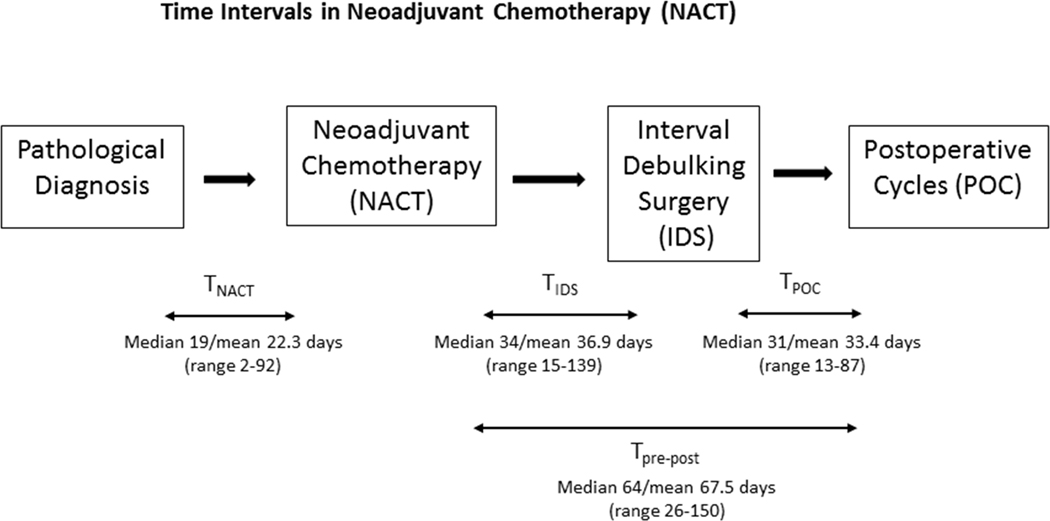
TNACT was defined as the time from pathological diagnosis to the start of chemotherapy. The period from the last preoperative carboplatin treatment to the first postoperative chemotherapy treatment was divided into two time periods: a. the time from last preoperative carboplatin to interval debulking; and b. time from interval debulking to first post-operative chemotherapy (Figure 1). Delay was defined as time from last preoperative carboplatin to interval debulking >6 weeks, based on the EORTC study.6 In patients with delays in time from last preoperative carboplatin to interval debulking, detailed chart review was performed to determine the reason(s) for delays.
Figure 1.
Time Intervals in Neoadjuvant Chemotherapy (NACT) Time from pathological diagnosis to preoperative chemotherapy cycles was defined as TNACT.Time from last preoperative carboplatin to interval debulking surgery was defined as TIDS. Time from interval debulking to postoperative chemotherapy was defined as TPOC. Time from last preoperative carboplatin to first postoperative chemotherapy was defined as TPre-Post.
Statistical Analysis
Summary statistics were used to define each of the neoadjuvant time intervals. Fisher’s exact and Wilcoxon rank sum tests were used to compare clinical characteristics based on delays in time from last preoperative carboplatin to interval debulking. Progression-free survival was derived from date of surgery to date of disease progression, defined as clinical recurrence via pathologically confirmed biopsy (when available) or imaging showing disease recurrence per the clinical provider, or death in patients without recurrence, or last follow-up in those without recurrence or death. Overall survival was derived from date of interval debulking to date of death from all causes, or last follow-up in those without death. The Kaplan-Meier method was used to estimate median survival and the survival rate at 1 year. The log-rank test and Cox Proportional- Hazards (CoxPH) model were used to assess the relationship of covariates to outcome. Landmark analysis was used for the time-dependent variable time from interval debulking to first post-operative chemotherapy, and only patients who received postoperative treatments were included in these analyses.
RESULTS
Patient Characteristics
Median age of the cohort was 66 years (range; 43–87), and most patients had high-grade serous ovarian cancer (96% ). Most had stage IV disease (69%) and were referred for neoadjuvant therapy due to extent of disease (70%). Twenty-eight women (18%) had BRCA 1/2 mutation s, but 30 ha d not yet undergone testing. Median body mass index was 25.5 kg/m2 s, (range; 15.4– 60.1), and median CA125 level was 1170 U/mL (range; 4–38,600). At start of chemotherapy, median Karnofsky performance score was 80% (range; 50–100%), Charlson score was 8 (range; 6–12), and albumin was 3.8 g/dL (range; 2.3–4.9). Most women received weekly paclitaxel and carboplatin (76%), and only 11% required a dose reduction. Patients received a median of 4 preoperative cycles (range; 2–7); 90% of patients received postoperative chemotherapy, with a median of 3 postoperative cycles (range; 1–8). In addition to chemotherapy, 8 women received bevacizumab, and 14 received PARP inhibitor or placebo on protocol. Of the 159 women, 139 (87%) underwent optimal debulking and 106 (67%) achieved complete gross resection (Table 1).
Table 1:
Patient characteristics, overall and by TIDS delays
| All Patients N= 159 | TIDS Delay 34(21.4%) | No Delay 125(78.6%) | p-value* | |
|---|---|---|---|---|
|
| ||||
| Age | ||||
| Median(Mean) | 66(65.8) | 67.5(68.8) | 65(64.9) | 0.05 |
| Range | 43–87 | 44–86 | 43–87 | |
|
| ||||
| BRCA Mutations | ||||
| No mutation | 101(63.5%) | 24(70.6%) | 77(61.6%) | 0.67 |
| BRCA mut/VUS | 28(17.6%) | 5(14.7%) | 23(18.4%) | |
| missing | 30(18.9%) | 5(14.7%) | 25(20%) | |
|
| ||||
| Stage at Diagnosis | ||||
| III | 50(31.4%) | 7(20.6%) | 43(34.4%) | 0.148 |
| IV | 109(68.6%) | 27(79.4%) | 82(65.6%) | |
|
| ||||
| Histology | ||||
| High grade serous | 153(96.2%) | 33(97.1%) | 120(96%) | 1 |
| Other | 6(3.8%) | 1(2.9%) | 5(4%) | |
|
| ||||
| BMI (6 missing) | ||||
| Median(Mean) | 25.5(27.5) | 24.1(27.3) | 25.9(27.6) | 0.239 |
| Range | 15.4–60.1 | 18.4–46.8 | 15.4–60.1 | |
|
| ||||
| CA125 Cycle 1 (46 missing) | ||||
| Median(Mean) | 1170(3203.3) | 1043(3700.9) | 1182.5(3061.9) | 0.939 |
| Range | 4–38600 | 95–38600 | 4–29680 | |
|
| ||||
| CA125 Pre-surgery (80 missing) | ||||
| Median(Mean) | 42(163.1) | 44.5(174.1) | 41(132.9) | 0.571 |
| Range | 1–1901 | 1–1901 | 4–749 | |
|
| ||||
| Charlson Score | ||||
| Median(Mean) | 8(8.5) | 9(8.8) | 8(8.4) | 0.102 |
| Range | 6–12 | 6–12 | 6–12 | |
|
| ||||
| Albumin (1 missing) | ||||
| Median(Mean) | 3.8(3.7) | 3.8(3.7) | 3.8(3.7) | 0.872 |
| Range | 2.3–4.9 | 2.3–4.8 | 2.3–4.9 | |
|
| ||||
| KPS (47 missing) | ||||
| Median(Mean) | 80(80.6) | 80(79.5) | 80(80.9) | 0.874 |
| Range | 50–100 | 50–90 | 50–100 | |
|
| ||||
| Reason for Neoadjuvant Chemotherapy | ||||
| Disease Extent | 111(69.8%) | 22(64.7%) | 89(71.2%) | 0.724 |
| Comorbidity | 8(5%) | 1(2.9%) | 7(5.6%) | |
| Both | 32(20.1%) | 9(26.5%) | 23(18.4%) | |
| Other | 8(5%) | 2(5.9%) | 6(4.8%) | |
|
| ||||
| Chemotherapy Regimen | ||||
| Weekly Paclitaxel/Carboplatin | 121(76.1%) | 25(73.5%) | 96(76.8%) | 0.658 |
| Others | 38(23.9%) | 9(26.5%) | 29(23.2%) | |
|
| ||||
| Preoperative Cycles | ||||
| Median(Mean) | 4(4.2) | 6(4.9) | 4(4) | 0.003 |
| Range | 2–7 | 3–6 | 2–7 | |
|
| ||||
| Dose Reduction | ||||
| No | 142(89.3%) | 30(88.2%) | 112(89.6%) | 0.762 |
| Yes | 17(10.7%) | 4(11.8%) | 13(10.4%) | |
|
| ||||
| TIDS (days) | ||||
| Median(Mean) | 34(36.9) | 48(56.7) | 32(31.4) | - |
| Range | 15–139 | 43–139 | 15–42 | |
|
| ||||
| TNACT (days) | ||||
| Median(Mean) | 19(22.3) | 22(28.8) | 17(20.6) | 0.014 |
| Range | 2–92 | 6–77 | 2–92 | |
|
| ||||
| TPOC (days) † | ||||
| Median(Mean) | 31(33.4) | 37(39.4) | 30(32.2) | 0.01 |
| Range | 13–87 | 15–84 | 13–87 | |
|
| ||||
| Complete Gross Resection | ||||
| No | 53(33.3%) | 11(32.4%) | 42(33.6%) | 1 |
| Yes | 106(66.7%) | 23(67.6%) | 83(66.4%) | |
|
| ||||
| Optimal Debulking | ||||
| No | 20(12.6%) 4 | 7(20.6%) | 13(10.4%) | 0.143 |
| Yes | 139(87.4%) | 27(79.4%) | 112(89.6%) | |
p value is obtained by using Fisher-exact test for categorical variables and Wilcoxon rank sum test for continuous variables
Only for the patients who received postoperative chemotherapy
Neoadjuvant Time Intervals and Delays
Time from last preoperative carboplatin to interval debulking ranged from 15–139 days, with a median of 34 days and mean of 36.9 days. Time from pathological diagnosis to preoperative chemotherapy ranged from 2–92 days, with a median of 19 and mean of 22.3 days. In those who were diagnosed at Memorial Sloan Kettering (n=69, 43%), the time from pathological diagnosis to preoperative chemotherapy was significantly shorter (p<0.001) with a median of 10 days (range; 2–37) compared to a median of 26 days (range; 2–92) in those diagnosed elsewhere (n=90, 57%). The period from the last preoperative carboplatin treatment to the first postoperative chemotherapy treatment ranged from 26–150 days, with a median of 64 and mean of 67.5 days. The time from interval debulking to first post-operative chemotherapy ranged from 13–87 days, with a median of 31 and mean of 33.4 days.
Of the 159 women who received neoadjuvant chemotherapy and underwent interval debulking, 34 (21%) had delays in time from neoadjuvant chemotherapy to interval debulking. In those with delays, median time from neoadjuvant chemotherapy to interval debulking was 48 days (range; 43–139) compared to a median of 32 days (range; 15–42) in those without delays. Patients with delays from neoadjuvant chemotherapy to interval debulking were older (median age 67.5 vs 65 years, p=0.05) and received more preoperative cycles (median 6 vs. 4, p=0.003) compared to those without delays. Patients with delays from neoadjuvant chemotherapy to interval debulking delays also had longer time from pathological diagnosis to preoperative chemotherapy (median 22 vs. 17 days, p=0.014) and longer time from interval debulking to first post-operative chemotherapy (median 37 vs. 30 days, p=0.01) compared to those without delays. There were no differences in other clinical variables including stage, histology, Charlson score, or rates of optimal debulking and complete gross resection (Table 1).
For the 34 women with delays from neoadjuvant chemotherapy to interval debulking, the most common reasons for delays were logistical 13 (38%), primarily due to delays in scheduling. Nine patients (26%) were delayed due to a prolonged period required for pre-surgical clearance. Four patients (12%) were delayed due to medical comorbidity or acute hospitalizations, and 1 patient (3%) was delayed due to persistent anemia/neutropenia. Three patients (9%) were delayed because they initially deferred surgery. One patient (3%) was delayed because her case required discussion at a multidisciplinary treatment planning conference. In 3 patients (9%) surgery was aborted due to extent of disease, which was felt to be unresectable after assessment in the operating room (Table S1).
Progression-free Survival Analysis
Among the 159 women, there were 113 events of progression (108 clinical recurrences and 5 deaths without recurrence). The median follow-up time in those without progression was 12.3 months (range; 1.6–32.9). On univariate analysis, delays from neoadjuvant chemotherapy to interval debulking were not associated with worse progression-free survival (HR 1.55 95% CI 0.97–2.47, p=0.062). In those with delays from neoadjuvant chemotherapy to interval debulking, n progression-free survival was 10.0 months (95% CI 6.4–10.8) vs. 11.5 months (95% CI 0.1–14.5) in those without delays. Presence of BRCA mutations (HR 0.61 95% CI 0.37–0.98, p=0.038), optimal debulking (HR 0.48 95% CI 0.29–0.78, p=0.003) and complete gross resection (HR 0.45 95% CI 0.31–0.66, p<0.001) were associated with improved progression-free survival, while increased number of preoperative cycles was associated with worse progression-free survival (HR 1.29 95% CI 1.1–1.51, p=0.002). Neither time from pathological diagno sis to preoperative chemotherapy nor time from interval debulking to first post-operative chemotherapy were associated with progression-free survival on univariate analysis (Table S2).
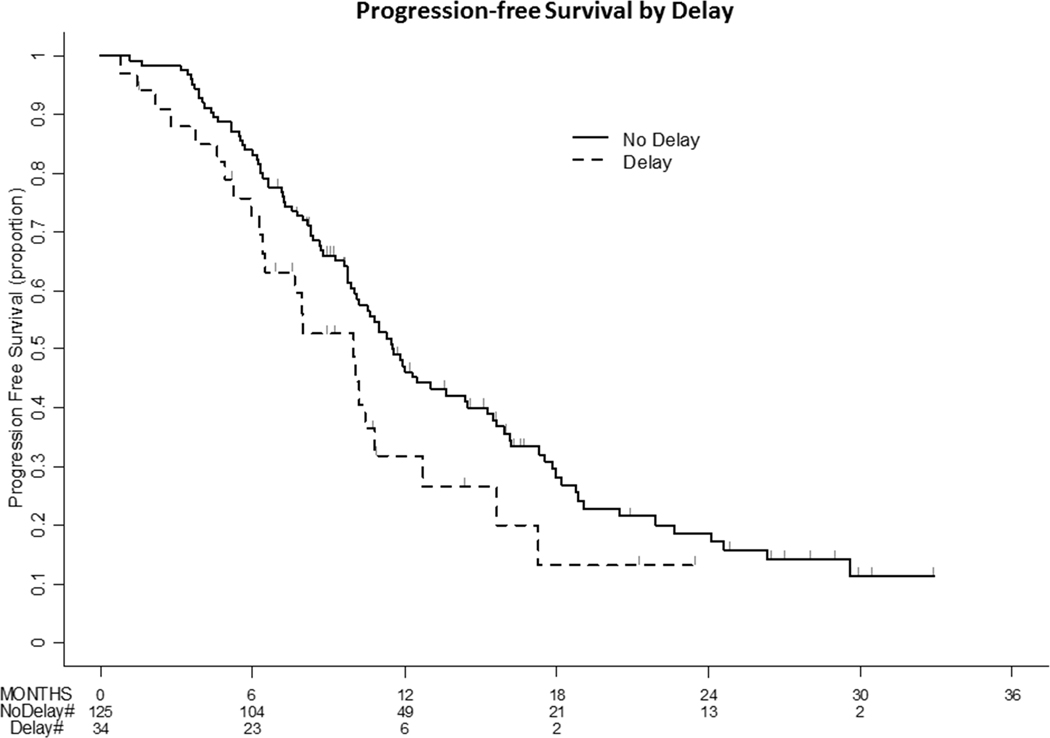
In multivariate models, delays from neoadjuvant chemotherapy to interval debulking were not associated with progression-free survival (HR 1.52 95% CI 0.94–2.45, p=0.089), even after adjustment for preoperative cycles and complete gross resection (Table 2 and Figure 2).
Table 2:
Multivariate Model of Progression-Free and Survival (PFS) and Overall Survival(OS)
| Progression-free Survival | ||||
|---|---|---|---|---|
|
| ||||
| Variable | HR | Lower 95%CI | Upper 95%CI | p-value |
|
| ||||
| TIDS Delay (Yes vs. No) | 1.516 | 0.938 | 2.451 | 0.089 |
| Preoperative Cycles (Continuous, increase by 1) | 1.256 | 1.073 | 1.47 | 0.005 |
| Complete Gross Resection: Yes vs. No | 0.432 | 0.295 | 0.633 | <0.001 |
|
| ||||
| Overall Survival | ||||
|
| ||||
| Variable | HR | Lower 95%CI | Upper 95%CI | p-value |
|
| ||||
| TIDS Delay (Yes vs. No) | 1.659 | 0.799 | 3.442 | 0.174 |
| Age (Continuous, increase by 5 years) | 1.185 | 1.006 | 1.396 | 0.043 |
| Stage: IV vs. III | 2.683 | 1.082 | 6.651 | 0.033 |
| Complete Gross Resection: Yes vs. No | 0.469 | 0.244 | 0.902 | 0.023 |
Figure 2:
Progression-Free Survival (PFS) by TIDS Delays TIDS delays were not significantly associated with progression-free survival on univariate analysis.
Overall Survival Analysis
Among the 159 women, there were 38 deaths observed. The median follow-up time in survivors was 18.0 months (range; 1.6–36.4). On univariate analysis, delays from neoadjuvant chemotherapy to interval debulking were associated with worse overall survival (HR 2.4 95% CI 1.2–4.77, p=0.01). In those with delays from neoadjuvant chemotherapy to interval debulking, median overall survival was 172Sowhs (95% CI 14.4 - not estimable), and the 1-year overall survival rate was 86% (95% CI 66.3–94.6). In those without delays, median survival was not reached, and the 1-year overall survival rate was 89.7% (95% CI 82.5–94). Presence of BRCA mutations (HR 0.31 95% CI 0.09–1.02, p=0.041) and complete gross resection (HR 0.41 95% CI 0.22–0.77, p=0.004) were associated with improved overall survival, while stage IV disease (HR 2.53 95% CI 1.06–6.06, p=0.031) was associated with worse overall survival. Neither time from pathological diagnosis to preoperative chemotherapy nor time from interval debulking to first post-operative chemotherapy were associated with overall survival on univariate analysis (Table S3).
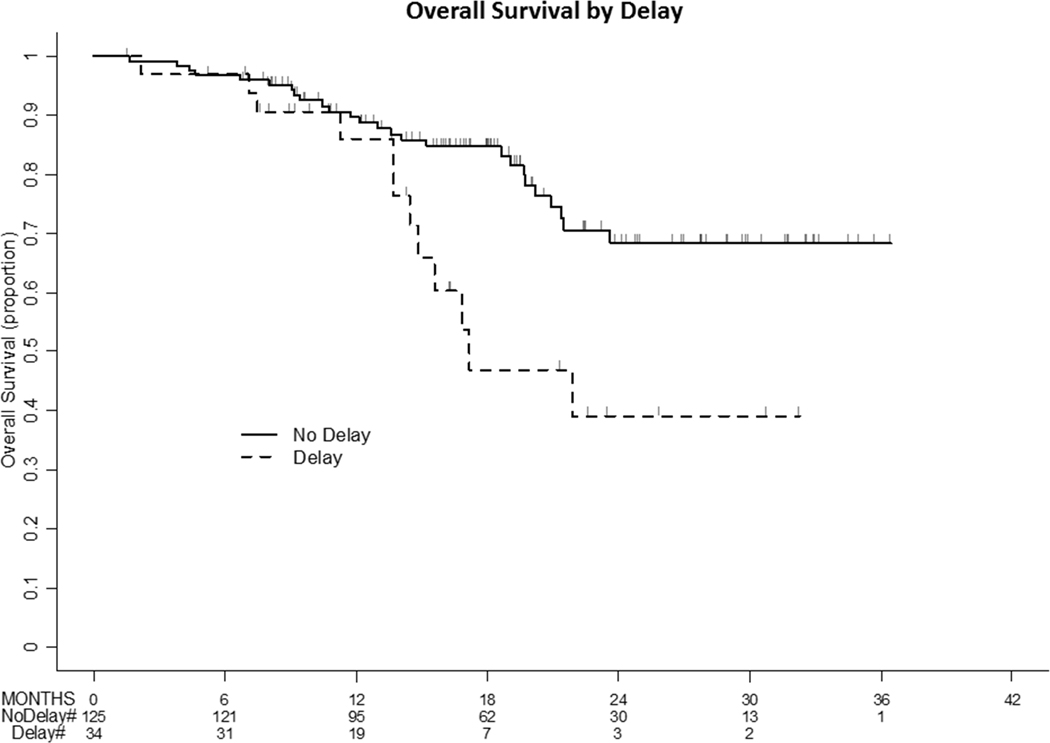
In multivariate models, delays from neoadjuvant chemotherapy to interval debulking were not associated with overall survival after adjustment for age, stage at diagnosis and complete gross resection (HR 1.66 95% 0.80–3.44, p=0.174). Only increasing age, stage IV disease, and failure to achieve complete gross resection were significantly associated with worse overall survival (Table 2 and Figure 3). overall survival (Table 2 and Figure 3).
Figure 3:
Overall Survival (OS) by TIDS Delays Although TIDS delays were significantly associated with overall survival on univariate analysis, this was attenuated after adjustment for age, stage and complete gross resection. TIDS delays are not independently associated with overall survival.
DISCUSSION
Delays from neoadjuvant chemotherapy to interval debulking occurred in 21% of women. Those with delays from neoadjuvant chemotherapy to interval debulking were older and received more preoperative cycles. Although delays from neoadjuvant chemotherapy to interval debulking were associated with worse overall survival on univariate analysis, this association disappeared after adjustment for age, stage and complete gross resection. In multivariate models, only a greater number of preoperative cycles and lack of complete gross resection were associated with worse progression-free survival. Time from pathological diagnosis to preoperative chemotherapy and time from interval debulking to first post-operative chemotherapy were not associated with survival.
This is one of the first studies to examine multiple time periods during neoadjuvant therapy and reasons for delays. Most delays from neoadjuvant chemotherapy to interval debulking particularly those in the range of 43–60 days, were due to logistical constraints and prolonged pre-surgical clearance for complicated medical patients. This suggests the need for multidisciplinary collaboration and systems-level processes to facilitate a timely transition from chemotherapy to surgery. Delays lasting >60 days were primarily due to patient comorbidity, rolonged recovery from chemotherapy, and patient choices. Of note, the patient with the longest delay (139 days) had low-grade serous histology and initially refused surgery but then requested surgery. Although histologies other than high-grade serous were rare in this study (n=6), there may be differences in underlying biology that affect delays, which should be investigated in larger studies. Finally, in 3 patients (9%) surgery was aborted due to extent of disease, suggesting that there may be a group of patients experiencing significant delays from neoadjuvant chemotherapy to interval debulking who may not benefit from surgery.
Our study found that delays from neoadjuvant chemotherapy to interval debulking were associated with overall survival on univariate analysis, while other time periods (time from is to preoperative chemotherapy and time from interval debulking to first post-operative chemotherapy) were not predictive of survival. This contrasts with studies of primary debulking demonstrating the importance of time from interval debulking to first post-operative chemotherapy and the role of delays from surgery to postoperative chemotherapy on survival. This may be a function of receiving neoadjuvant therapy and should be investigated further. It is possible that time from interval debulking to first post-operative chemotherapy plays less of a role in these patients because of the systemic effects of neoadjuvant chemotherapy; the biological basis should be investigated further. Interestingly, being diagnosed at an outside institution was associated with longer time from diagnosis to initiation of chemotherapy, suggesting that logistical factors such as obtaining pathology slides and records may play a role.
Unlike other studies, we did not find an independent effect of delays from neoadjuvant chemotherapy to interval debulking on survival. Instead, we found that any influence on survival was accounted for by other clinical factors such as age, stage, preoperative cycles and complete gross section, which have been well described.16–18 Our group has long emphasized the importance of maximal cytoreduction leaving as little residual tumor as possible to improve survival.19
Our overall survival curve shows a sharp drop-off in patients with delays, suggesting aggressive underlying disease in the 12 women who experienced delays from neoadjuvant chemotherapy to interval debulking and deaths. Eleven of these 12 women had stage IV disease, and all had high-grade serous histology. Nine of the 12 were platinum resistant, and 2 of the platinum-sensitive patients progressed between 6–7 months from the time of their last platinum treatment.
The strengths of our study include its utilization of a prospective, comprehensive Ovarian Database to identify patients, evaluation of multiple time periods during neoadjuvant therapy, and inclusion of multiple clinical variables. Limitations include the small sample size and limited follow-up. As the prospective ovarian database grows with time, such a study could be repeated with a larger sample size and longer follow-up to better explore these relationships. In addition, utilization of neoadjuvant therapy may not be based solely on clinical factors such as stage and comorbidity but may equally depend on non-clinical factors such as race, income, geography and insurance,20 which should be investigated further.
Our study also identified an increasing number of preoperative cycles as a predictor of worse survival. A meta-analysis of 835 patients undergoing neoadjuvant therapy for ovarian cancer found that each incremental increase in preoperative cycles was associated with a median decrease in survival of 4.1 months.21 Bogani et al. found that increasing preoperative cycle number,.4 vs. 3, may adversely affect survival. This should also be investigated further in future studies.
In summary, delays from neoadjuvant chemotherapy to interval debulking occur in approximately 1 in 5 women undergoing neoadjuvant therapy for newly diagnosed ovarian cancer. These women are usually older and receive more preoperative cycles. After considering other clinical variables such as age, stage and surgical outcomes, we found that delays from from neoadjuvant chemotherapy to interval debulking do not independently affect survival. This emphasizes the importance of maximizing cytoreduction to improve survival
Supplementary Material
Figure S1: Patient Selection
Table S1: Reasons for TIDS Delays
Table S2: Univariate Progression-free Survival Analysis
Table S3: Univariate Overall Survival Analysis
ACKNOWLEDGEMENTS
We would like to thank the Ovarian Database team, and all the women who underwent treatment at Memorial Sloan Kettering Cancer Center.
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Disclosures:
Dr. Iasonos reports personal fees from Mylan, outside the submitted work.
Dr. Chi reports personal fees from Bovie Medical Co. (medical advisory board and stock options), Verthermia Inc. (medical advisory board and stock options), C Surgeries (shareholder), and Intuitive Surgical Inc (stock ownership), outside the submitted work.
Dr. O’Cearbhaill reports personal fees from Clovis (medical advisory board) and Tesaro (medical advisory board), outside the submitted work.
Dr. Konner reports personal fees from Clovis (guest speaker), AstraZeneca (medical advisory board), and Immunogen (medical advisory board), outside the submitted work.
Dr. Aghajanian reports personal fees from Tesaro (medical advisory board), Immunogen (medical advisory board), Mateon Therapeutics (steering committee) and Cerulean Pharma (medical advisory board), grants and personal fees from Clovis (medical advisory board) and Genentech (steering committee), and grants from AbbVie (steering committee) and Astra Zeneca, outside the submitted work.
Dr. Long Roche reports personal fees from Intuitive Surgical (travel), outside the submitted work.
Footnotes
Conflict of Interest Statement: None declared.
REFERENCES
- 1.Seagle BL, Butler SK, Strohl AE, Nieves-Neira W, Shahabi S. Chemotherapy delay after primary debulking surgery for ovarian cancer. Gynecologic oncology. 2017;144(2):260–265. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Guetzko M, Resnick K. Preoperative predictors of delay in initiation of adjuvant chemotherapy in patients undergoing primary debulking surgery for ovarian cancer. Gynecologic oncology. 2016;143(2):241–245. [DOI] [PubMed] [Google Scholar]
- 3.Tewari KS, Java JJ, Eskander RN, Monk BJ, Burger RA. Early initiation of chemotherapy following complete resection of advanced ovarian cancer associated with improved survival: NRG Oncology/Gynecologic Oncology Group study. Annals of oncology : official journal of the European Society for Medical Oncology. 2016;27(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mueller JJ, Zhou QC, Iasonos A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecologic oncology. 2016;140(3):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England). 2015;386(9990):249–257. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. The New England journal of medicine. 2010;363(10):943–953. [DOI] [PubMed] [Google Scholar]
- 7.Le T, Fathi KA, Hopkins L, Faught W, Fung-Kee-Fung M. The significance of duration of chemotherapy interruptions due to interval surgery in ovarian cancer patients treated with neoadjuvant chemotherapy. J Obstet Gynaecol Can. 2009;31(2):161–166. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Chen Z, Xu M, et al. Impact of the Time Interval from Neoadjuvant Chemotherapy to Surgery in Primary Ovarian, Tubal, and Peritoneal Cancer Patients. Journal of Cancer. 2018;9(21):4087–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YJ, Chung YS, Lee JY, et al. Impact of the time interval from completion of neoadjuvant chemotherapy to initiation of postoperative adjuvant chemotherapy on the survival of patients with advanced ovarian cancer. Gynecologic oncology. 2017. [DOI] [PubMed] [Google Scholar]
- 10.Noer MC, Sperling CD, Ottesen B, Antonsen SL, Christensen IJ, Hogdall C. Ovarian Cancer and Comorbidity: Is Poor Survival Explained by Choice of Primary Treatment or System Delay? International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2017;27(6):1123–1133. [DOI] [PubMed] [Google Scholar]
- 11.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 14.Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002–2007. [DOI] [PubMed] [Google Scholar]
- 15.Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecologic oncology. 2014;134(3):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suidan RS, Leitao MM Jr., Zivanovic O, et al. Predictive value of the Age-Adjusted Charlson Comorbidity Index on perioperative complications and survival in patients undergoing primary debulking surgery for advanced epithelial ovarian cancer. Gynecologic oncology. 2015;138(2):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tinquaut F, Freyer G, Chauvin F, Gane N, Pujade-Lauraine E, Falandry C. Prognostic factors for overall survival in elderly patients with advanced ovarian cancer treated with chemotherapy: Results of a pooled analysis of three GINECO phase II trials. Gynecologic oncology. 2016;143(1):22–26. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Liu N, Zhang A, Bao X. Potential risk factors associated with prognosis of neoadjuvant chemotherapy followed by interval debulking surgery in stage IIIc-IV high-grade serous ovarian carcinoma patients. J Obstet Gynaecol Res. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecologic oncology. 2006;103(2):559–564. [DOI] [PubMed] [Google Scholar]
- 20.Hinchcliff E, Melamed A, Bregar A, et al. Factors associated with delivery of neoadjuvant chemotherapy in women with advanced stage ovarian cancer. Gynecologic oncology. 2017. [DOI] [PubMed] [Google Scholar]
- 21.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecologic oncology. 2006;103(3):1070–1076. [DOI] [PubMed] [Google Scholar]
- 22.Bogani G, Matteucci L, Tamberi S, et al. The Impact of Number of Cycles of Neoadjuvant Chemotherapy on Survival of Patients Undergoing Interval Debulking Surgery for Stage IIIC-IV Unresectable Ovarian Cancer: Results From a Multi-Institutional Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2017;27(9):1856–1862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Patient Selection
Table S1: Reasons for TIDS Delays
Table S2: Univariate Progression-free Survival Analysis
Table S3: Univariate Overall Survival Analysis