Abstract
Atovaquone is a chemotherapeutic agent used to treat pneumonia caused by Pneumocystis carinii in some immunocompromised patients. A set of cyclic 1,4-diones were tested in vitro for ability to inhibit growth of P. carinii, including 22 variously substituted 1,4-naphthoquinones, one bis-1,4-naphthoquinone, and three other quinones. For comparison, the antipneumocystic primaquine and its 5-hydroxy-6-desmethyl metabolite were also tested. At 1.0 μg/ml, seven compounds inhibited growth by at least 39%, with atovaquone at 92%; of these seven, five are 2-hydroxy-1,4-naphthoquinones, while one is a 2-chloro- and another is a 2-methyl-1,4-naphthoquinone. At 0.1 μg/ml, however, the most active compound tested was the primaquine metabolite, which inhibited growth by more than 42% at this concentration. To ascertain a structure-activity relationship, all 1,4-naphthoquinones were compared conformationally by means of computer-based molecular modeling (Spartan) incorporating the Sybyl force field. Without exception, for all 21 monomers tested, the substituent at position 3 of the 1,4-naphthoquinone favored activity most strongly when it simultaneously occupied (i) space centered at about 3 Å from position 3, without projecting steric bulk from the area encompassed by atovaquone's cyclohexyl ring, and (ii) roughly planar space at about 7.3 Å from position 3, without projecting steric bulk perpendicularly. This structure-activity relationship may prove useful in the rational design of better antipneumocystis agents.
Pneumonia caused by Pneumocystis carinii (PCP) is a life-threatening opportunistic infection occurring in immunocompromised patients, especially those with AIDS. Although the combination of trimethoprim and sulfamethoxazole is the first-line prophylactic and therapeutic agent for PCP, pentamidine, trimetrexate, dapsone, primaquine-clindamycin, and atovaquone (ATQ) may be used when standard treatment is intolerable or ineffective (6, 21).
Though not without side effects, ATQ (Table 1) is a promising candidate for further development in the chemotherapy of PCP. Recent studies have shown ATQ to be safe in human immunodeficiency virus-infected children (8) and effective against clinical isolates of P. carinii (5). In immunosuppressed rats, ATQ was effective against P. carinii in a formulation with surfactant (7) alone or in combination with other agents (21). In combination with proguanil, furthermore, ATQ is safe and effective in the prophylaxis of Plasmodium falciparum malaria (9, 15, 16). In this respect, ATQ is like primaquine and other 8-aminoquinolines, some of which are both antipneumocystic and antimalarial (4, 12).
TABLE 1.
Antipneumocystis activities of 1,4-naphthoquinone derivatives
| Compounda | Substituent (at position specified on above parent structureb)
|
% Inhibition at the following concn [μg/ml]):
|
||||
|---|---|---|---|---|---|---|
| 2Q | 3Q | 7Q | 0.1 | 1 | 10 | |
| ATQ | −OH | 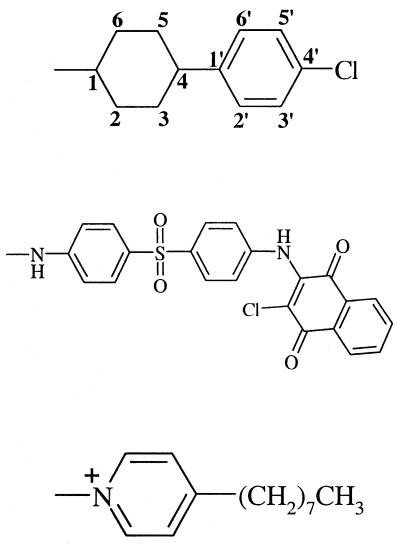 |
−H | 27 | 92 | −c |
| 1 (39355) | −Cl | −H | 42 | 88 | − | |
| 2 (142574) | −O− | −H | 42 | 79 | − | |
| 3 (278) | −OH | 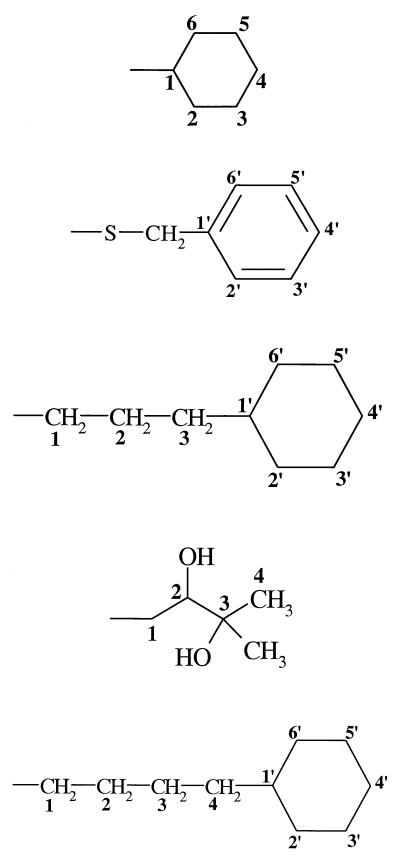 |
−H | 28 | 51 | − |
| 4 (66457) | −CH3 | −H | 27 | 43 | − | |
| 5 (109542) | −OH | −OCH3 | 25 | 39 | − | |
| 6 (100407) | −OH | −H | 11 | 39 | − | |
| 7 (31435) | −OH | −H | − | 28 | − | |
| 8 (359831) | −Cl | 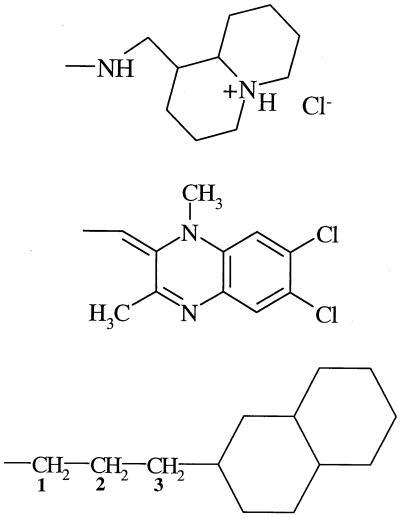 |
−H | − | 25 | − |
| 9 (295483) | −Cl | −H | − | 24 | − | |
| 10 (2035) | −OH | −H | − | 18 | − | |
| 11 (26695) | −OH | 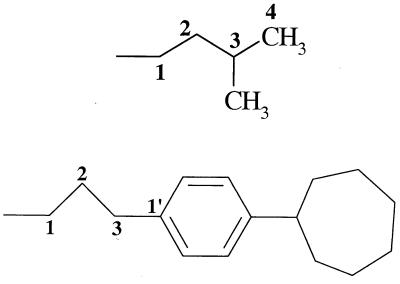 |
−H | − | 14 | − |
| 12 (113452) | −OH | −H | − | 13 | − | |
| 13 (92195) | −Cl | −H | − | 10 | − | |
| 14 (126771) | −OH | −H | − | 3 | − | |
| 15 (26654) | −OH | −H | − | − | 14 | |
| 16 (31855) | −OH | −H | − | − | 12 | |
| 17 (114930) | −OH | 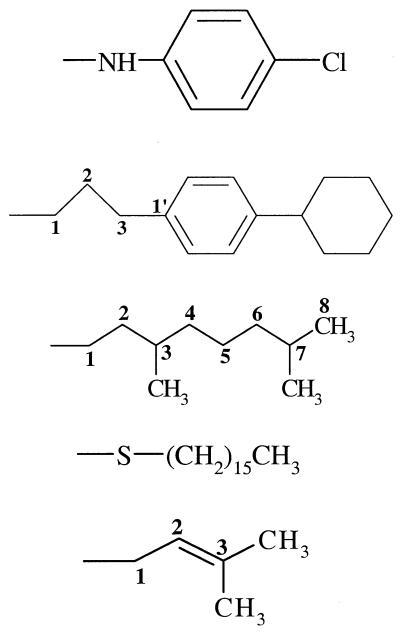 |
−H | − | − | 6 |
| 18 (113455) | −OH | −H | − | −5 | − | |
| 19 (138685) | −OH | −H | − | −15 | − | |
| 20 (247507) | −NH2 | −H | − | − | −23 | |
| 21 (629756) | −OH | −H | − | − | −23 | |
Numbers in parentheses are the National Cancer Institute NSC entry numbers.
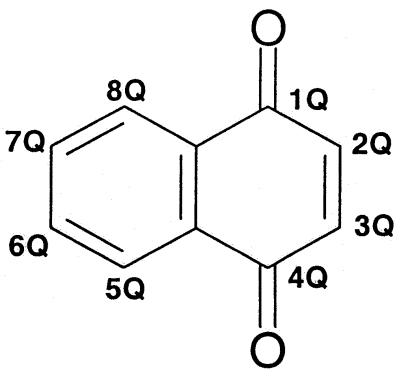
A Q is suffixed to each locant on the 1,4-naphthoquinone nucleus to distinguish it from the locant bearing the same numeral but identifying a position in the substituent.
−, not tested.
Many series of compounds, including 8-aminoquinolines, have been evaluated against P. carinii (13, 17), and some structure-activity trends have been noted (12). The importance of structural complementarity in the binding of quinones to their putative receptor, cytochrome b, has become clear through the discovery of mutations in a ubiquinone binding site on that protein in P. carinii from patients for whom ATQ prophylaxis failed (19). These mutations, unfortunately, portend the development of resistance to ATQ in this organism. For P. falciparum, furthermore, the structural uniqueness of the quinone binding site in cytochrome b may account for that species' sensitivity to both 8-aminoquinolines and hydroxynaphthoquinones (18). Marked differences in toxicity have been traced to comparatively small differences in structure. For 2-hydroxy-1,4-naphthoquinones, 3-alkylation decreased toxicity, although the 3-methyl, -ethyl, and -propyl derivatives were still hemolytic and nephrotoxic. By contrast, the 3-butyl and -pentyl derivatives showed no toxicity (10).
Thus, in view of (i) the growing importance of ATQ as a chemotherapeutic agent, (ii) the influence of the 3-substitutent on biological activity, and (iii) the emerging possibility of drug resistance, we tested a series of cyclic 1,4-diones in vitro for ability to inhibit growth of P. carinii for the ultimate purpose of deducing a structure-activity relationship that may facilitate the rational and systematic improvement of antipneumocystis agents in the future.
Because of the large structural differences among the active, marginally active, and inactive derivatives of 1,4-naphthoquinone in this study, an efficient approach with which to begin a structure-activity study is to search conformational space for structural elements common to active derivatives and absent from inactive derivatives. This approach attempts to assess the ability of a given molecule, whether active or inactive, to occupy the same space as another active molecule and to derive from such a comparison of several molecules a structure-activity relationship—i.e., to fit a test molecule to an active molecule in a fixed conformation and to identify three-dimensional structural elements that may explain activity. In the absence of information about the conformation in which a 1,4-naphthoquinone binds to its receptor, the strategy of this approach, steric fitting, obviates calculations that return molecular descriptors (e.g., electrostatic potential) that depend strongly on conformation.
MATERIALS AND METHODS
Growth of P. carinii in vitro.
Compounds were tested for antipneumocystis activity in vitro by an established method using inocula from P. carinii-infected rat lung and human embryonic lung (HEL) fibroblasts (1, 14). In 24-well plates, HEL cells were grown to confluency in minimum essential medium. They were then inoculated with P. carinii from lung homogenate to give 7 × 105 viable organisms per ml in each well. The inoculum was prepared by homogenizing P. carinii-infected rat lung in minimum essential medium, followed by low-speed (250 × g) centrifugation to pellet debris. Obtained from the National Cancer Institute, compounds were selected from a large pool of naphthoquinones by means of computerized substructure searching (11) on the basis of potential relevance, interest reflected in the literature, and existing active leads. Compounds to be tested (Tables 1 to 3) were dissolved in dimethyl sulfoxide and added to the medium in the wells. The negative-treatment control consisted of wells inoculated with P. carinii but without drug, whereas wells for the positive-treatment control contained inoculum and trimethoprim-sulfamethoxazole (TMP-SMX) at 50 and 25 μg/ml, respectively, a standard chemotherapeutic agent for PCP.
TABLE 3.
Antipneumocystis activities of primaquine and its known metabolite
Plates were incubated for 7 days at 35°C in 5% O2, 10% CO2, and 85% N2. After the incubation period, cells were washed with medium, and organisms of P. carinii were counted in 10-μl aliquots on 1-cm2 areas of glass slides. Aliquots on the slides were air dried, fixed in methanol, and Giemsa stained, and trophozoites were counted in 10 oil immersion fields on each slide.
Each compound was first evaluated at 1 and/or 10 μg/ml; those compounds that inhibited growth by an average of >40% at 1 μg/ml were tested further at 0.1 μg/ml. Trophozoites were counted in treated and untreated quadruplicate wells on days 5 and 7 of incubation. The percent inhibition of growth for each treatment was calculated relative to the negative-treatment wells. For each drug at each concentration, percent inhibition for days 5 and 7 was averaged. For all quadruplicate measurements, the standard deviation ranged from 1 to 27% of the mean.
Computer-based molecular modeling.
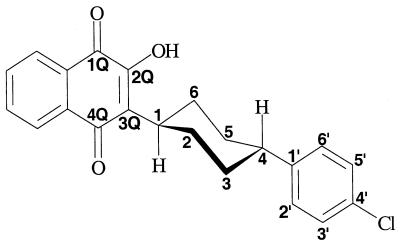
All molecules were built, manipulated, and analyzed in Spartan (version 5.0; Wavefunction, Inc., Irvine, Calif.). The Sybyl force field in the mechanics module of Spartan was used to calculate equilibrium geometry, compute strain energy, and provide standard van der Waals radii, bond distances, bond angles, and dihedral angles. Molecular mechanics treats the total energy of a molecule as the sum of the energies arising from bond distance (summed over all bonds), bond angle (summed over all bond angles), dihedral angle (summed over all dihedral angles), and nonbonding interactions (summed over all pairs of nonbonded atoms). The energies of bond distance, bond angle, and dihedral angle contain stretch, bend, and torsion terms, respectively, while the energy of nonbonding interactions includes terms for van der Waals and electrostatic forces. From the set of all final geometries calculated iteratively from several starting conformations, the equilibrium geometry of ATQ (Table 4) was identified as that with the lowest strain energy. Starting conformations included those with the cyclohexyl ring in the chair arrangement and (i) the 2-hydroxy substituent in the plane of the naphthoquinone, directing its hydrogen atom either toward or away from the 1-oxo, (ii) the naphthquinonyl and phenyl rings either ax/ax or eq/eq on the cyclohexyl ring, and (iii) the hydrogen of C1 of the cyclohexyl ring either anti to or eclipsing C2 of the naphthoquinone, with respect to the C1-C3Q bond.
TABLE 4.
ATQ in its equilibrium geometry, as determined by the Sybyl force field in the mechanics module of Spartana
| Descriptorb | Value |
|---|---|
| Strain energy (kcal/mol) | 4.9 |
| r (Å) | |
| C3Q-centroid of cyclohexyl | 3.0 |
| C3Q-centroid of phenyl | 7.3 |
| C3Q-C1′ | 5.9 |
| C4-centroid of phenyl | 3.0 |
| ∠ (°) | |
| C1-C2-C3 | 110.5 |
| C2-C3-C4 | 110.4 |
| C3-C4-C5 | 110.8 |
| C4-C5-C6 | 110.4 |
| C5-C6-C1 | 110.5 |
| C6-C1-C2 | 111.1 |
| θ(°) | |
| C2Q-C3Q-C1-H | 180.0 |
| C2′-C1′-C4-H | 180.0 |
A Q is suffixed to each locant of the naphthoquinone rings to distinguish it from the locant bearing the same numeral but referring to the substituent.
Measurements are reported for the C3Q substituent, which is the focus of the structure-activity analysis, rather than for the naphthoquinone rings.
RESULTS
At 1 μg/ml, seven 1,4-naphthoquinone derivatives inhibited growth by at least 39%, with ATQ at 92% (Table 1). Of these seven with various substituents at C3Q (Table 1), one (no. 1) is a dimeric 2-chloro-1,4-naphthoquinone, one (no. 4) is a 2-methyl-1,4-naphthoquinone, and five (ATQ, 2, 3, 5, and 6) are 2-hydroxy-1,4-naphthoquinones, of which one (no. 5) is also a 7-methoxy derivative. Activity was observed for C3Q substituents with a wide variety of compositions, configurations, and electron distributions: solely aliphatic (3, 5, and 6), both aliphatic and aromatic (ATQ, 4, and 2), highly π conjugated (no. 1), positively charged (no. 2), cyclic (ATQ and 3), acyclic (no. 6), and both cyclic and acyclic (2, 4, and 5). Furthermore, in three (1, 2, and 4) of the seven most active compounds, a heteroatom links the C3Q substituent to the naphthoquinone moiety.
At 1 μg/ml, only three compounds (ATQ, 1, and 2) inhibited growth more than 75%, while four compounds (3, 4, 5 and 6) showed activities approximately within the range of 40 to 50%, though one (no. 6) inhibited growth markedly less than the others at 0.1 μg/ml. Thus, in order of descending activity, the compounds may be ranked ATQ = 1 = 2 > 3 = 4 = 5 > 6. Inhibiting growth by 24 to 31%, four compounds with various substituents at C3Q were marginally active: one 2-hydroxy- (no. 7) and two 2-chloro-1,4-naphthoquinones (9 and 8) (Table 1), along with a dimeric, ubiquinone-like, cyclic 1,4-dione (no. 22) (Table 2). All other naphthoquinones were inactive, with the greatest inhibition of growth being only 18%.
TABLE 2.
Antipneumocystis activities of selected cyclic 1,4-diones
| Compound no.a | Structure | % Inhibition at 1 μg/ml |
|---|---|---|
| 22 (87413) | 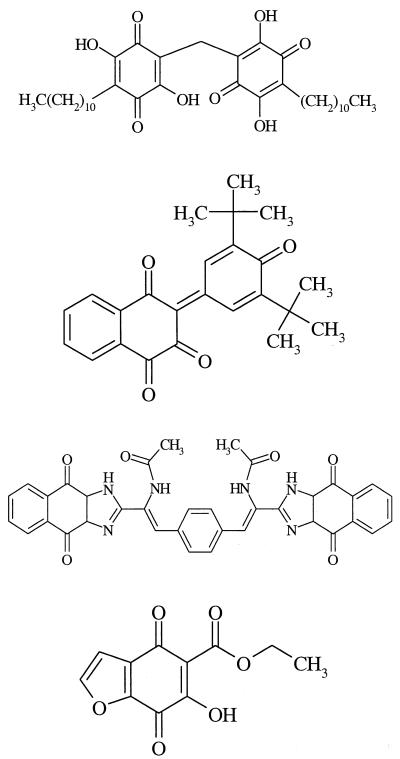 |
31 |
| 23 (652596) | −3 | |
| 24 (624434) | −15 | |
| 25 (651210) | −31 |
Numbers in parentheses are the National Cancer Institute NSC entry numbers.
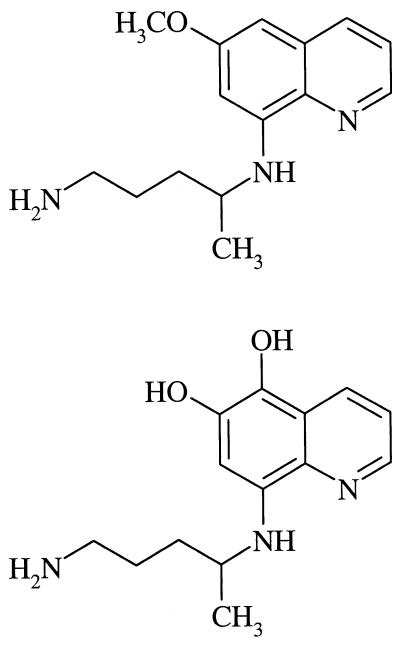
The two 8-aminoquinolines (Table 3) were comparable in activity to the seven most active 1,4-naphthoquinones (Table 1). At 0.1 μg/ml, the primaquine metabolite 5-hydroxy-6-desmethylprimaquine inhibited growth by 60%, more than any other compound in this study.
With a theoretical strain energy of 4.93 kcal/mol, the equilibrium geometry of ATQ (Table 4) is not surprising. The naphthoquinone rings and the plane of the cyclohexyl ring, which occupies a chair conformation, are orthogonal, placing the hydrogen of C1 anti to C2Q with respect to the C1-C3Q bond. The phenyl ring, by being perpendicular to the plane of the cyclohexyl ring, lies consequently parallel with the naphthoquinone rings.
DISCUSSION
The goal of this study was to identify three-dimensional structural similarities and dissimilarities that may account for the antipneumocystis activities of 1,4-naphthoquinone derivatives. Conformationally fitting one molecule to another by rotation about single bonds is the heart of the approach in this study to developing a reasonable structure-activity hypothesis. In deriving this relationship, we chose to focus on (i) the substituent at C3Q, because the largest structural differences occur there in our 1,4-naphthoquinones, (ii) monomeric derivatives, because of the (currently unconfirmed) possibility that dimers undergo metabolic cleavage into two monomers of equal or unequal activities, and (iii) derivatives with intact 1,4-naphthoquinone nuclei. The structural hypothesis that we develop herein accounts for activity without invoking the other substituents at C2Q and C7Q.
Relative to ATQ, in which the C3Q substituent is trans-4-(p-chlorophenyl)-1-cyclohexyl, hydrocarbon chains at C3Q abolished activity in nearly every instance (Table 1). By contrast, a cyclohexyl ring alone attached to C3Q (no. 3) diminished activity only 44% relative to ATQ. Thus, both the cyclohexyl and phenyl rings may be important for activity.
At 1.0 μg/ml, compound 5, whose substituent at C3Q is 3-cyclohexylpropyl, can conform to superimpose its atoms C1 to C3 upon those of ATQ, but in so doing it either (i) projects its cyclohexyl ring as steric bulk outward into space that is vacant in ATQ, (ii) fails to fill the entire volume of ATQ's cyclohexyl or phenyl ring, or (iii) superimposes a nonplanar aliphatic ring over a planar phenyl ring. Thus, what may be critical for activity is not only the rings of ATQ, but also the vacancy of the conformational space around them. These structural observations may account for the intermediacy of the activity of 5. It is not surprising, then, that although the conformations accessible to 5 are also accessible to 10, the additional four methylenes of the decahydronaphthalenyl system of 10 lower activity further by enlarging the steric projection of compound 5.
Compound 11 further evinces the importance of the space centered at about 3 Å from C3Q, corresponding to ATQ's cyclohexyl ring, and at about 7.3 Å from C3Q, corresponding to ATQ's phenyl ring (Table 4). Although the atoms C1 to C4 of ATQ and of the 3-methylbutyl substituent of 11 can occupy the same space, 11 projects a methyl group as steric bulk and, unlike 5, fails to occupy any of ATQ's phenyl space. Although it has the same carbon skeleton as 11, compound 6 is more hydrophilic and may consequently be more soluble, partly offsetting the negative factors. Although this possibility is reasonable, the role of solubility in this study is unclear; across the six most active compounds, activity does not parallel hydrophilicity. Even less active than 11, however, was 15, which occupies less space centered at 3 Å from C3Q (ATQ's cyclohexyl ring) and projects methyl bulk from C2.
In the 4-(cyclohexyl)butyl substituent at C3Q of 7, atoms C1 to C4 can adopt a chair conformation, while the two atoms C1′ of 7 and ATQ superimpose themselves on each other. In the resulting arrangement of atoms, because C1′ of 7 is tetrahedral, the cyclohexyl ring of 7 never occupies space at about 7.3 Å from C3Q (ATQ's phenyl ring). Furthermore, for the entire 360° rotation about the C4-C1′ bond of 7, the cyclohexyl ring always places steric bulk beyond the planar space of ATQ's phenyl ring.
The other inactive and marginally active monomers are consistent with this structure-activity hypothesis. Compound 17 cannot adopt a conformation that superimposes atoms on those of ATQ's cyclohexyl ring and altogether fails to occupy space at 7.3 Å from C3Q, corresponding to ATQ's phenyl ring. Compounds 12 and 18 and ATQ can mutually superimpose their first four substituent atoms in conformational space. Neither 12 nor 18, however, can orient its phenyl ring into ATQ's phenyl space, regardless of the dihedral angles involving C1, C2, and C3. Moreover, the phenyl, cycloheptyl, and cyclohexyl rings of these two compounds, along with the inactive derivatives 8, 9, 13, 19, and 20, all project significant steric bulk into space that is vacant in the most active compounds, ATQ and 2 to 6.
For the inactive compound 16, the presence of only one methylene spacer restricts the conformational space accessible to the isoxazinyl ring, preventing it from filling the cyclohexyl space of ATQ and causing it to project steric bulk equatorially. Like 11, the inactive compounds 14 and 21 occupy little of the space centered at 3 Å from C3Q and project steric bulk either as a chlorine (no. 14) or as a methyl (no. 21).
In contrast to these active and inactive compounds, the other active compounds 4 and 2 each have a heteroatom at the position occupied by C1 of ATQ's cyclohexyl ring. In 4, with ∠C3Q-S-C being 95.5°, the benzylthio substituent sits neatly at about 3 Å from C3Q without projecting steric bulk, while orienting its phenyl ring parallel with that of ATQ. Compound 2, with its 4-octylpyridinium substituent at C3Q, places all but the last three atoms of its octyl chain in space occupied by both ATQ and 4. Neither 4 nor 2 projects steric bulk from the space centered at 3 Å or from that at 7.3 Å from C3Q.
Thus, without exception, for all the monomeric derivatives in Table 1, the substituent at C3Q favors activity most strongly when it simultaneously (i) occupies space centered at about 3 Å from C3Q, without projecting steric bulk beyond the area encompassed by ATQ's cyclohexyl ring, and (ii) occupies roughly planar space at about 7.3 Å from C3Q, without projecting steric bulk perpendicularly. Even though this hypothesis considers only stericity, the future availability of more derivatives might allow refinement in terms of electron distribution and aromaticity.
Not surprisingly, the active dimeric compound 1 (Table 1) does not accord with the structure-activity hypothesis developed herein for the monomers. With respect to such descriptors as bond length, bond angle, and dihedral angle, the aminophenyl moiety of its C3Q substituent is quite similar to that of the inactive compound 17 (vide supra). In the absence of metabolism data, however, it remains possible that the dimer undergoes cleavage into two monomers of equal or unequal activities. Moreover, the dimer may bind to its receptor at a site distinct from that at which the monomers bind. Likewise, the marginally active compound 22 and the inactive compound 24 are both dimeric (Table 2). Although the inactive monomer 23 can adopt a conformation similar to that of 2, it projects two t-butyl groups and fails to occupy the planar space at 7.3 Å from C3Q. Compound 25, another inactive monomer, lacks an intact naphthalene nucleus, having instead a benzofuran system.
Although each 8-aminoquinoline was highly effective at 1 μg/ml, primaquine was less active than its metabolite, 5-hydroxy-6-desmethylprimaquine, at 0.1 μg/ml (Table 3). This metabolite was also more effective than primaquine against Plasmodium bergheii, probably killing the organism by generating free radicals (2).
Atomic models of most protein components of bovine cytochrome bc1 were constructed recently from X-ray diffraction data, and the binding sites of two electron transport inhibitors were partially elucidated both structurally and functionally (22). Despite the mounting evidence for the role of cytochrome b in the mechanism of action of naphthoquinones against both Pneumocystis (19) and Plasmodium (18) spp., not all the naphthoquinones in this study have been proven to bind that particular protein. Nonetheless, such binding seems likely, and hypotheses relating structure to activity may facilitate the rational design of new agents for prophylaxis and treatment of infection by microbes of these genera.
ACKNOWLEDGMENTS
This work was supported by grant R01 AI38708 from the National Institutes of Health.
We thank the Keck Imaging Laboratory of Rose-Hulman Institute of Technology for hosting the computational chemistry software and for permitting the use of their workstations.
REFERENCES
- 1.Bartlett M S, Edlind T D, Durkin M M, Shaw M M, Queener S F, Smith J W. Antimicrotubule benzimidazoles inhibit in vitro growth of Pneumocystis carinii. Antimicrob Agents Chemother. 1992;36:779–782. doi: 10.1128/aac.36.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates M D, Meshnick S R, Sigler C I, Leland P, Hollingdale M R. In vitro effects of primaquine and its metabolites on exoerythrocytic stages of Plasmodium berghei. Am J Trop Med Hyg. 1990;42:532–537. doi: 10.4269/ajtmh.1990.42.532. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharjee A K, Pundlik S S, Gadre S R. Conformational and electrostatic properties of naphthazarin, juglone, and naphthoquinone: an ab initio theoretical study. Cancer Investig. 1997;15:531–541. doi: 10.3109/07357909709047594. [DOI] [PubMed] [Google Scholar]
- 4.Brueckner R P, Coster T, Wesche D L, Shmuklarsky M, Schuster B G. Prophylaxis of Plasmodium falciparum infection in a human challenge model with WR238605, a new 8-aminoquinoline antimalarial. Antimicrob Agents Chemother. 1998;42:1293–1294. doi: 10.1128/aac.42.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cirioni O, Giacometti A, Scalise G. In-vitro activity of atovaquone, sulphamethoxazole and dapsone alone and combined with inhibitors of dihydrofolate reductase and macrolides against Pneumocystis carinii. J Antimicrob Chemother. 1997;39:45–51. doi: 10.1093/jac/39.1.45. [DOI] [PubMed] [Google Scholar]
- 6.El-Sadr W M, Murphy R L, Yurik T M, Luskin-Hawk R, Cheung T W, Balfour H H, Jr, Eng R, Hooton T M, Kerkering T M, Schutz M, van der Horst C, Hafner R. Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. N Engl J Med. 1998;339:1889–1895. doi: 10.1056/NEJM199812243392604. [DOI] [PubMed] [Google Scholar]
- 7.Hughes W T, Sillos E M, LaFon S, Rogers M, Woolley J L, Davis C, Studenberg S, Pattishall E, Freeze T, Snyder G, Staton S. Effects of aerosolized synthetic surfactant, atovaquone, and the combination of these on murine Pneumocystis carinii pneumonia. J Infect Dis. 1998;177:1046–1056. doi: 10.1086/515252. [DOI] [PubMed] [Google Scholar]
- 8.Hughes W, Dorenbaum A, Yogev R, Beauchamp B, Xu J, McNamara J, Moye J, Purdue L, van Dyke R, Rogers M, Sadler B. Phase I safety and pharmacokinetics study of micronized atovaquone in human immunodeficiency virus-infected infants and children. Pediatric AIDS Clinical Trials Group. Antimicrob Agents Chemother. 1998;42:1315–1318. doi: 10.1128/aac.42.6.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lell B, Luckner D, Ndjave M, Scott T, Kremsner P G. Randomised placebo-controlled study of atovaquone plus proguanil for malaria prophylaxis in children. Lancet. 1998;351:709–713. doi: 10.1016/S0140-6736(97)09222-2. [DOI] [PubMed] [Google Scholar]
- 10.Munday R, Smith B L, Munday C M. Comparative toxicity of 2-hydroxy-3-alkyl-1,4-naphthoquinones in rats. Chem Biol Interact. 1995;98:185–192. doi: 10.1016/0009-2797(95)03645-8. [DOI] [PubMed] [Google Scholar]
- 11.Nasr M, Paull K D, Narayanan V L. Computer assisted structure-activity correlations. Adv Pharmacol Chemother. 1984;20:123–190. doi: 10.1016/s1054-3589(08)60266-5. [DOI] [PubMed] [Google Scholar]
- 12.Queener S F, Bartlett M S, Nasr M, Smith J W. 8-Aminoquinolines effective against Pneumocystis carinii in vitro and in vivo. Antimicrob Agents Chemother. 1993;37:2166–2172. doi: 10.1128/aac.37.10.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Queener S F. New drug developments for opportunistic infections in immunosuppressed patients: Pneumocystis carinii. J Med Chem. 1995;38:4739–4759. doi: 10.1021/jm00024a001. [DOI] [PubMed] [Google Scholar]
- 14.Queener S F, Dean R A, Bartlett M S, Milhous W K, Berman J D, Ellis W Y, Smith J W. Efficacy of intermittent dosage of 8-aminoquinolines for therapy or prophylaxis of Pneumocystis pneumonia in rats. J Infect Dis. 1992;165:764–768. doi: 10.1093/infdis/165.4.764. [DOI] [PubMed] [Google Scholar]
- 15.Sabchareon A, Attanath P, Phanuaksook P, Chanthavanich P, Poonpanich Y, Mookmanee D, Chongsuphajaisiddhi T, Sadler B M, Hussein Z, Canfield C J, Hutchinson D B. Efficacy and pharmacokinetics of atovaquone and proguanil in children with multidrug-resistant Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1998;92:201–206. doi: 10.1016/s0035-9203(98)90749-0. [DOI] [PubMed] [Google Scholar]
- 16.Shanks G D, Gordon D M, Klotz F W, Aleman G M, Oloo A J, Sadie D, Scott T R. Efficacy and safety of atovaquone/proguanil as suppressive prophylaxis for Plasmodium falciparum malaria. Clin Infect Dis. 1998;27:494–499. doi: 10.1086/514710. [DOI] [PubMed] [Google Scholar]
- 17.Shaw M M, McChesney J D, Nanayakkara D, Dias L R S, Lee C-H, Durant P J, Ball M D, Smith J W, Bartlett M S. Modified 8-aminoquinolines cure mouse Pneumocystis carinii infection. J Eukaryot Microbiol. 1997;44:40S. doi: 10.1111/j.1550-7408.1997.tb05764.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya A B, Lashgari M S, Pologe L G, Morrisey J. Structural features of Plasmodium cytochrome b that may underlie susceptibility to 8-aminoquinolines and hydroxynaphthoquinones. Mol Biochem Parasitol. 1993;58:33–42. doi: 10.1016/0166-6851(93)90088-f. [DOI] [PubMed] [Google Scholar]
- 19.Walker D J, Wakefield A E, Dohn M N, Miller R F, Baughman R P, Hossler P A, Bartlett M S, Smith J W, Kazanjian P, Meshnick S R. Sequence polymorphisms in the Pneumocystis carinii cytochrome b gene and their association with atovaquone prophylaxis failure. J Infect Dis. 1998;178:1767–1775. doi: 10.1086/314509. [DOI] [PubMed] [Google Scholar]
- 20.Walzer P D, Runck J, Orr S, Foy J, Steele P, White M. Clinically used antimicrobial drugs against experimental pneumocytosis, singly and in combination: analysis of drug interactions and efficacies. Antimicrob Agents Chemother. 1997;41:242–250. doi: 10.1128/aac.41.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren E, George S, You J, Kazanjian P. Advances in the treatment and prophylaxis of Pneumocystis carinii pneumonia. Pharmacotherapy. 1997;17:900–916. [PubMed] [Google Scholar]
- 22.Xia D, Chang-An Y, Hoeon K, Xia J-Z, Kachurin A M, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]