Abstract
Context
Continuous glucose monitoring (CGM) is increasingly being used both for day-to-day management in patients with diabetes and in clinical research. While data on glycemic profiles of healthy, nondiabetic individuals exist, data on nondiabetic very young children are lacking.
Objective
This work aimed to establish reference sensor glucose ranges in healthy, nondiabetic young children, using a current-generation CGM sensor.
Methods
This prospective observational study took place in an institutional practice with healthy, nondiabetic children aged 1 to 6 years with normal body mass index. A blinded Dexcom G6 Pro CGM was worn for approximately 10 days by each participant. Main outcome measures included CGM metrics of mean glucose, hyperglycemia, hypoglycemia, and glycemic variability.
Results
Thirty-nine participants were included in the analyses. Mean average glucose was 103 mg/dL (5.7 mmol/L). Median percentage time between 70 and 140 mg/dL (3.9-7.8 mmol/L) was 96% (interquartile range, 92%-97%), mean within-individual coefficient of variation was 17 ± 3%, median time spent with glucose levels greater than 140 mg/dL was 3.4% (49 min/day), and median time less than 70 mg/dL (3.9 mmol/L) was 0.4% (6 min/day).
Conclusion
Collecting normative sensor glucose data and describing glycemic measures for young children fill an important informational gap and will be useful as a benchmark for future clinical studies.
Keywords: continuous glucose monitoring, mean glucose, time in range, pediatric diabetes
Continuous glucose monitoring (CGM) has become an extremely valuable tool for diabetes management and clinical research. CGM is increasingly being relied on for day-to-day management decisions and treatment adjustments in type 1 diabetes (T1D) [1]. CGM provides an abundance of information on glycemic indices that helps patients and providers optimize glycemic control without increasing risk for hypoglycemia. Moreover, CGM metrics are often used to assess the effectiveness and safety of new therapeutic agents and modern technologies in clinical diabetes research [2].
Understanding CGM metrics in healthy individuals without diabetes is needed to serve as a benchmark for clinical research studies to define impaired glycemic status as well as entry criteria and end point into clinical trials as prevention therapies for T1D are emerging [3]. For instance, CGM has been shown to be a valuable and accurate tool for monitoring autoantibody-positive individuals with stage 2 T1D who are at risk for developing stage 3 T1D [4], as described in 2015 by a joint statement from the American Diabetes Association, JDRF, and the Endocrine Society [5]. Determining the frequency of sensor-derived, interstitial glucose concentrations that are less than 70 mg/dL (< 3.9mmol/L) in healthy participants using current-generation sensors are particularly important in assessing the ability of closed-loop systems to prevent hypoglycemia in patients with T1D.
A recent multicenter study with the Dexcom G6 was conducted to collect normative CGM data on healthy individuals aged 7 and older [6]. However, no data currently exist on children younger than 7 years. This study was undertaken to provide normative CGM sensor glucose data in healthy, nondiabetic young children.
Materials and Methods
This study was conducted at the Barbara Davis Center at the University of Colorado after approval by the institutional review board. Written informed consent was obtained from a parent or legal guardian before study participation. Participants were healthy, nondiabetic children, primarily recruited from family, friends, and neighbors of patients seen in the diabetes clinic. Major eligibility criteria included age 1 to younger than 7 years at time of screening; body mass index between the 10th and 90th percentile for age and sex; no chronic illness or medications that might affect glucose metabolism; and point-of-care glycated hemoglobin A1c (HbA1c) less than 5.7% (< 39 mmol/mol).
At the initial visit, blood samples were obtained to measure HbA1c (University of Colorado Hospital Clinical and Reference Laboratory) and autoantibodies to glutamic acid decarboxylase, islet antigen 2, insulin antibodies, and zinc transporter-8 (Immunogenetics Laboratory at the Barbara Davis Center for Diabetes). Only participants with no islet autoantibodies and a laboratory HbA1c level less than 5.7% (< 39 mmol/mol) were included in the analysis.
Participants were provided a Dexcom G6 Pro CGM (Dexcom Inc) in blinded mode (ie, the participant was unable to see the glucose values) to wear for up to 10 days. Calibrations were not required. The Dexcom G6 CGM sensor measures interstitial glucose concentrations every 5 minutes. Approximately 10 days after the blinded CGM sensor was placed, the participant returned to have the sensor removed, and the CGM data were downloaded. If less than 72 hours of data were recorded, participants were asked to complete an additional sensor wear.
The parent or caregiver was asked to record the participant’s bedtime and morning wake time in a daily log. These logs were used to identify the periods of sensor data when participants were awake vs asleep.
Once all participants had completed the 10-day CGM wear, glucose data were reviewed by the study group. Some participants were observed to have higher CGM-measured mean glucose than would be expected for individuals with HbA1c less than 5.7% (< 39 mmol/mol) and negative islet autoantibodies (defined as mean sensor glucose ≥ 110 mg/dL [≥ 6.1 mmol/L]). These participants were asked if they would wear a second blinded CGM sensor for 10 days to confirm the accuracy of the first sensor, which was agreed to by 11 out of 12 such participants. After reviewing the glucose data from all wear periods, one CGM lot number was identified as having substantially higher mean glucose than the others. Therefore, all sensor readings associated with this lot number were excluded from the analysis. In addition, one sensor wear period during which the mean glucose was 151 mg/dL (8.4 mmol/L) was excluded. After excluding the aforementioned unusable data, participants with less than 72 total hours of CGM data (or < 24 hours overnight) also were excluded from the analysis.
Statistical Methods
Glucose outcomes were summarized as mean ± SD or median (interquartile range) depending on the variable distribution. Each CGM glucose reading was counted as a data point and was summarized in the glucose outcomes on either a participant or event level. If a participant had usable data from 2 different wear periods (n = 6), glucose outcomes were calculated by pooling data across both wear periods. Percentage of time spent within a threshold (eg, % time < 70 mg/dL [3.9 mmol/L]) was calculated as the number of CGM glucose readings that fell within the threshold divided by the total number of CGM glucose readings from the participant, represented as a percentage. A hypoglycemic event was defined as at least 2 sensor values less than 54 mg/dL (< 3.0 mmol/L) that were 15 or more minutes apart with no intervening values greater than 54 mg/dL (> 3.0 mmol/L). At least 2 sensor values greater than 70 mg/dL (> 3.9 mmol/L) that were 15 or more minutes apart with no intervening values less than 70 mg/dL (< 3.9 mmol/L) were required to end a hypoglycemic event. Glucose outcomes were assessed for the 24-hour, daytime, nighttime, awake, and asleep time periods. Daytime was defined as 6 am to 9:59 pm and nighttime as 10 pm to 5:59 am. Periods of awake and asleep were identified based on the bedtimes and wake times recorded in the daily log by the parent or caregiver.
A sensitivity analysis was performed to assess glucose outcomes after removing potentially inaccurate or implausible values. In this analysis, the following data were excluded from the calculation of glucose outcomes: any readings the first day of sensor wear; any readings on days with more than 20% missing data; any readings less than 50 mg/dL (2.8 mmol/L) while asleep (in which case participant was likely to be lying on the CGM sensor); any single reading or strings of readings less than 50 mg/dL (< 2.8 mmol/L) flanked by readings greater than or equal to 80 mg/dL (4.4 mmol/L) within 10 minutes before or after the string; and any single reading or string of readings greater than 200 mg/dL (11.1 mmol/L) flanked by readings less than 70 mg/dL (< 3.9 mmol/L) within 10 minutes before or after the string.
All analyses were performed using SAS 9.4 (SAS Institute).
Results
A flowchart of study participants is given in Supplementary Fig. 1 [7]. A total of 54 participants were screened for the study. Nine participants failed the screening because they did not meet the body mass index eligibility criteria. A further 6 individuals were excluded from the analysis for having a laboratory HbA1c greater than or equal to 5.7% (≥ 3.9 mmol/mol), positive diabetes autoantibodies, or for having an insufficient number of usable CGM hours. Therefore, the analysis cohort included 39 participants. Baseline characteristics of the analysis cohort are shown in Table 1. Mean age was 4.4 ± 1.4 years and laboratory HbA1c was 5.0 ± 0.2% (31 ± 2.2 mmol/mol). Ninety-two percent of participants had a first-degree relative with T1D.
Table 1.
Participant characteristics
| Overall (N = 39) | Aged 1-3 y (n = 10) | Aged 4-6 y (n = 29) | |
|---|---|---|---|
| Age at enrollment mean ± SD, y | 4.4 ± 1.4 | 2.4 ± 0.7 | 5.1 ± 0.9 |
| Sex, No. (%) | |||
| Female | 20 (51%) | 7 (70%) | 13 (45%) |
| Male | 19 (49%) | 3 (30%) | 16 (55%) |
| Race/Ethnicity, No. (%) | |||
| White Non-Hispanic | 34 (87%) | 8 (80%) | 26 (90%) |
| Black Non-Hispanic | 2 (5%) | 2 (20%) | – |
| Hispanic or Latino | 2 (5%) | – | 2 (7%) |
| > 1 Race | 1 (3%) | – | 1 (3%) |
| First-degree family member with T1D, No. (%) | 36 (92%) | 10 (100%) | 26 (90%) |
| Parent, No. (%) | 14 (36%) | 6 (60%) | 8 (28%) |
| Sibling, No. (%) | 21 (54%) | 4 (40%) | 17 (59%) |
| Parent and sibling, No. (%) | 1 (3%) | 0 (0%) | 1 (3%) |
| BMI percentile, median (quartiles)a | 51 (41-77) | 68 (44-86) | 48 (41-74) |
| Range | 10-89 | 10-89 | 11-85 |
| POC HbA 1c , mean ± SD | 5.0 ± 0.2 | 5.0 ± 0.2 | 5.0 ± 0.2 |
| Range | 4.6-5.4 | 4.7-5.3 | 4.6-5.4 |
| Lab HbA 1c , mean ± SD | 5.0 ± 0.2 | 5.1 ± 0.2 | 5.0 ± 0.2 |
| Range | 4.6-5.4 | 4.6-5.4 | 4.6-5.3 |
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin A1c; POC, point of care; T1D, type 1 diabetes.
a One participant aged 1 year had missing BMI percentile. (Weight per length percentile chart was used to assess eligibility.)
Glucose outcomes over 24 hours and by time of day are given in Table 2. Over 24 hours, mean individual average glucose was 103 ± 8 mg/dL (5.7 ± 0.4 mmol/L) and mean within-individual coefficient of variation, a measure of glucose variability, was 17 ± 3%. Median percentage of time spent between 70 and 140 mg/dL (3.9-7.8 mmol/L) and median percentage of time spent between 60 and 140 mg/dL (3.3-7.8 mmol/L) were both 96%. Median percentage of time spent above 140 mg/dL (7.8 mmol/L) was 3.4% and below 70 mg/dL (3.9 mmol/L) was 0.4%. Twenty-three percent of participants had at least one hypoglycemic event.
Table 2.
Glucose outcomes overall and by time of day
| 24 H (n = 39) |
Daytimea (n = 39) |
Nighttimea (n = 39) |
Awakeb (n = 38) |
Asleep overnightb (n = 38) |
|
|---|---|---|---|---|---|
| H of CGM data, mean ± SD | 205.6 ± 68.6 | 136.2 ± 45.6 | 69.4 ± 23.1 | 102.5 ± 37.3 | 87.2 ± 28.3 |
| Range | 79.5-425.1 | 55.5-281.1 | 24.0-144.0 | 37.2-232.7 | 30.2-189.8 |
| Overall glucose distribution, mean ± SD | |||||
| Mean glucose, mg/dL | 103 ± 8 | 106 ± 9 | 97 ± 8 | 107 ± 9 | 99 ± 8 |
| Glucose SD, mg/dL | 17 ± 3 | 18 ± 3 | 12 ± 3 | 18 ± 3 | 14 ± 3 |
| Glucose coefficient of variation, % | 17% ± 3% | 17% ± 3% | 12% ± 3% | 17% ± 3% | 14% ± 3% |
| % of sensor values, median (IQR) | |||||
| % time in range 70-120 mg/dL | 86% (75%-89%) |
81% (70%-86%) |
93% (88%-97%) |
80% (68%-87%) |
90% (86%-94%) |
| % time in range 70-140 mg/dL | 96% (92%-97%) |
94% (90%-96%) |
98% (96%-99%) |
94% (90%-96%) |
97% (95%-99%) |
| % time in range 60-140 mg/dL | 96% (93%-98%) |
95% (91%-97%) |
99% (98%-100%) |
95% (90%-97%) |
98% (97%-99%) |
| % time < 70 mg/dL | 0.44% (0.13%-1.02%) |
0.21% (0.07%-0.78%) |
0.65% (0.00%-1.53%) |
0.18% (0.00%-0.88%) |
0.52% (0.00%-1.18%) |
| % time < 60 mg/dL | 0.10% (0.00%-0.22%) | 0.00% (0.00%-0.13%) |
0.15% (0.00%-0.54%) |
0.00% (0.00%-0.00%) |
0.14% (0.00%-0.47%) |
| % time < 54 mg/dL | 0.02% (0.00%, 0.15%) | 0.00% (0.00%-0.00%) |
0.00% (0.00%-0.40%) |
0.00% (0.00%-0.00%) |
0.00% (0.00%-0.33%) |
| % time > 120 mg/dL | 13.0% (10.1%-23.1%) |
17.5% (12.7%-30.2%) |
4.9% (1.4%-9.1%) |
19.1% (12.7%-31.4%) |
8.1% (3.9%-13.2%) |
| % time > 140 mg/dL | 3.35% (2.20%-6.15%) |
5.05% (2.68%-8.56%) |
0.58% (0.00%-1.63%) |
5.50% (2.88%-9.17%) |
1.70% (0.35%-3.37%) |
| % time > 160 mg/dL | 0.79% (0.40%-1.79%) |
1.16% (0.54%-2.68%) |
0.00% (0.00%-0.00%) |
1.19% (0.46%-2.50%) |
0.20% (0.00%-0.55%) |
| % time > 180 mg/dL | 0.14% (0.00%-0.49%) |
0.21% (0.00%-0.75%) |
0.00% (0.00%-0.00%) |
0.21% (0.00%-0.78%) |
0.00% (0.00%-0.08%) |
| Participants with ≥ 1 hypoglycemic event c | 9 (23%) | 0 (0%) | 9 (23%) | 0 (0%) | 9 (24%) |
| Duration of hypoglycemic events for participants with ≥ 1 hypoglycemic event, min c , d | n = 13 events |
n = 0 events |
n = 13 events |
n = 0 events |
n = 12 events |
| Median (IQR) | 45 (35-50) | – | 45 (35-50) | – | 43 (35-50) |
Abbreviations: CGM, continuous glucose monitoring; IQR, interquartile range.
a Daytime is from 6 am to 9:59 pm. Nighttime is from 10 pm to 5:59 am.
b The parent or caregiver was asked to record the participant’s bedtime and morning wake time in a daily log. These logs were used to identify the periods of sensor data when participants were awake vs asleep overnight. Nap times are considered awake.
c A hypoglycemic event is defined as at least 2 sensor values less than 54 mg/dL that are 15 or more minutes apart with no intervening values greater than 54 mg/dL. An event ends when there are at least 2 sensor values greater than 70 mg/dL that are 15 or more minutes apart with no intervening values less than 70 mg/dL. Participant becomes eligible for a new event as soon as the aforementioned criteria for ending the previous event have been met.
d Based on an event level.
Mean average glucose was higher during the daytime (106 ± 9 mg/dL [5.9 ± 0.5 mmol/L]) compared with nighttime (97 ± 8 mg/dL [5.4 ± 0.4 mmol/L]). Similarly, glucose variability was higher during the daytime compared to nighttime (mean coefficient of variation 17 ± 3% and 12 ± 3%, respectively). Median percentage of time spent between 70 and 140 mg/dL was 94% during the daytime and 98% during the nighttime. All hypoglycemia events occurred during the nighttime. Glucose outcomes for the awake and asleep time periods were similar to the daytime and nighttime periods.
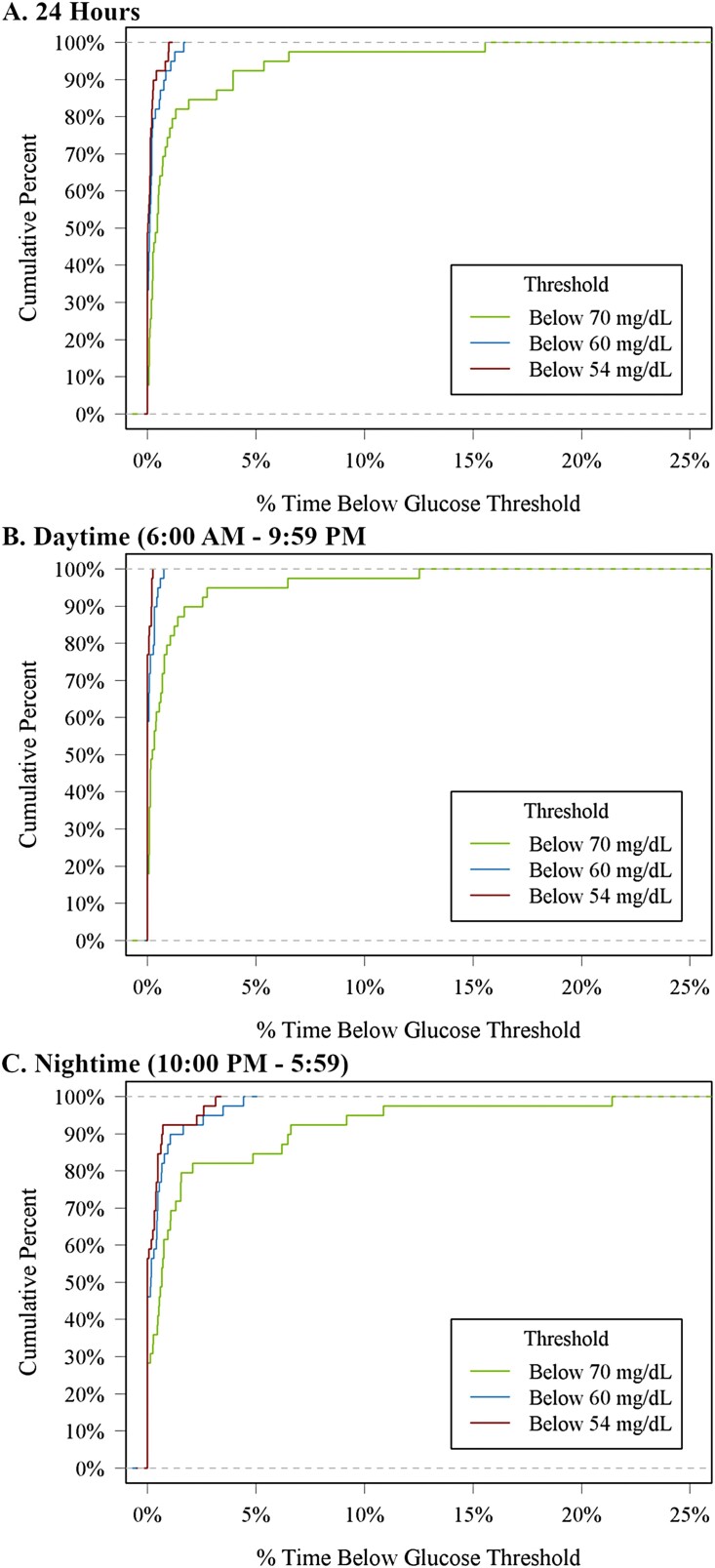
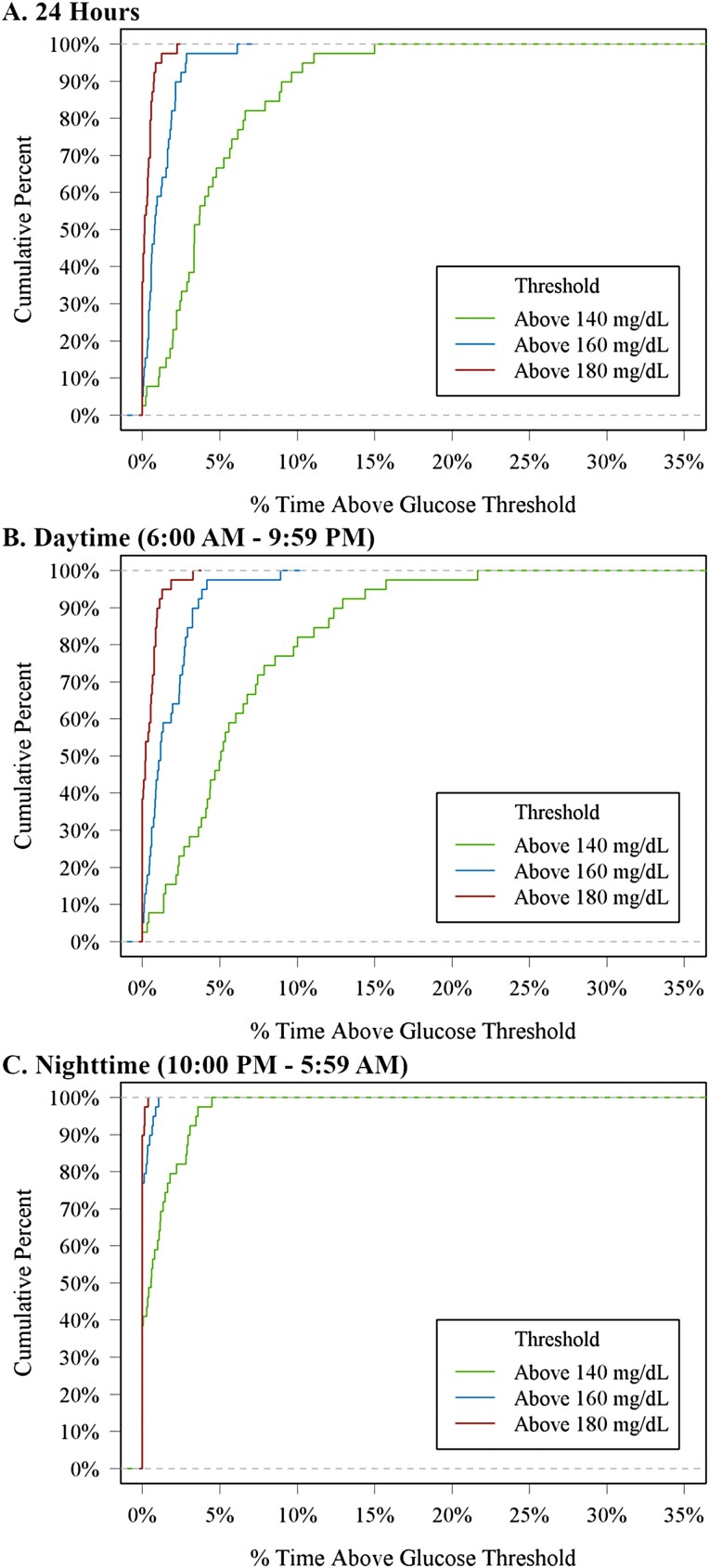
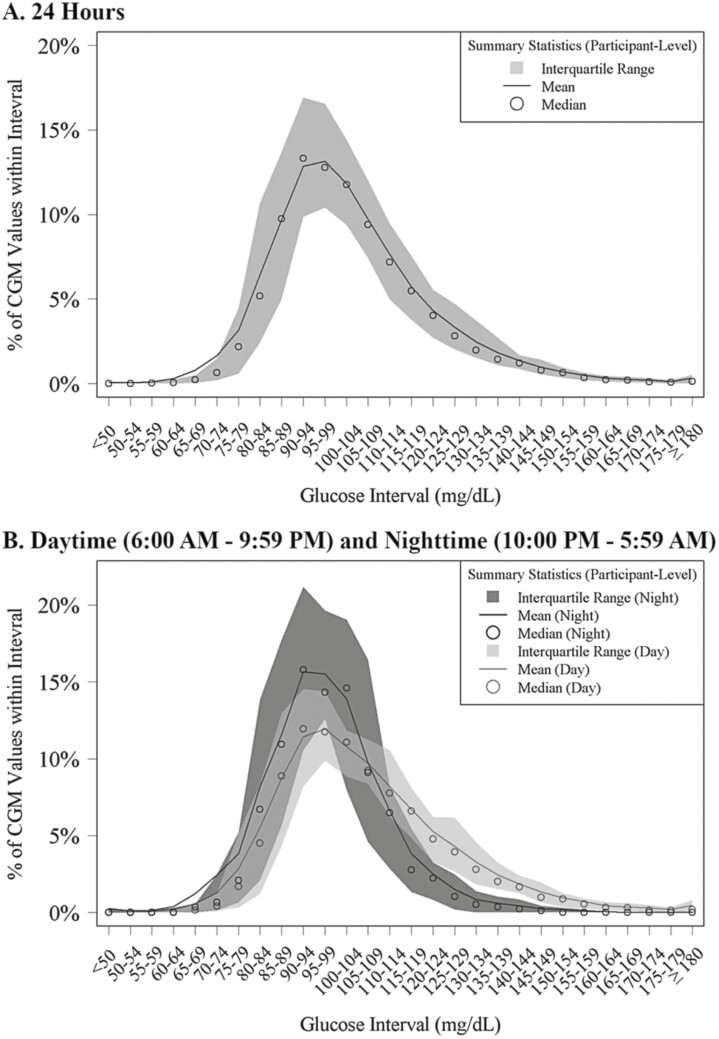
The cumulative frequency distributions of 24-hour, daytime, and nighttime hypoglycemia and hyperglycemia are shown in Figs. 1 and 2 and the distributions of CGM glucose values are displayed in Fig. 3. Mean glucose by hour of day is shown in Supplementary Fig. 2 [7]. In a sensitivity analysis removing potentially inaccurate or implausible data, mean glucose, glycemic variability measured by coefficient of variation, and percentage of time spent in glucose ranges were similar to the main analysis (Supplementary Table 1) [7]. However, only 1 participant (3%) had a hypoglycemic event after removing implausible data compared with 9 participants (23%) for the main analysis.
Figure 1.
Cumulative distribution function of hypoglycemia.
Figure 2.
Cumulative distribution function of hyperglycemia.
Figure 3.
Distribution of glucose values.
Glucose outcomes over 24 hours were similar across age groups (aged 1-3 years and 4-6 years; Supplementary Table 2) [7].
Discussion
In this study, we report CGM profiles in very young children (aged 1-6 years) who are healthy, with normal HbA1c and negative islet autoantibodies. To our knowledge, this is the largest study to date to report CGM metrics in healthy, nondiabetic young children. These children had an overall mean glucose of 103 mg/dL (5.7 mmol/L). This compares with a mean glucose of 98 to 99 mg/dL (5.4-5.5 mmol/L) in individuals aged 7 to 59 years and 104 mg/dL (5.8 mmol/L) in individuals aged 60 years and older [6]. Overall median time spent in a narrow target range of 70 to 140 mg/dL (3.9-7.8 mmol/L) was 96% and between 60 and 140 mg/dL (3.3-7.8 mmol/L) was 96%. The percentage of time between 70 and 140 mg/dL (3.9-7.8 mmol/L) was quite different for daytime vs nighttime (94% vs 98%, respectively), similar to those aged 60 and older from the previous study. Glycemic profiles were similar for children aged 1 to 3 years compared with those aged 4 to 6 years.
International efforts are underway to screen general-population children for T1D risk, including programs in the United States (ASK, T1Detect), in Europe (FR1da), as well as in Israel and Australia [8-10]. To follow these children at high risk for progression to T1D, accurate and feasible methods of monitoring are needed. Current American Diabetes Association criteria for T1D staging include HbA1c and oral glucose tolerance test measures. HbA1c is specific but not very sensitive in children and may miss acute evolution [11, 12]. Oral glucose tolerance tests are highly predictive of progression, especially when C-peptide dynamics are incorporated into risk score [13-16], but they are time-consuming and therefore unlikely to become part of routine monitoring in clinic. On the other hand, CGM has been shown to be well tolerated by children and their parents, with more than 10% time above 140 mg/dL (7.8 mmol/L) associated with a high risk of progression to clinical diabetes within the next year in autoantibody-positive children [4]. In the present study, we provide data for how much time young healthy children spend at various CGM ranges, which is important information needed both for monitoring children at risk for dysglycemia as well as therapies targeting tight glycemic control (advanced closed-loop systems) in patients with T1D and prevention strategies in those at risk for T1D.
Strengths of our study include the recruitment of very young children, use of the latest-generation technology, and up to 10 days of CGM wear time. While there were some participants who had unexpectedly high glucose values considering their normal HbA1c values and negative islet autoantibodies, almost all returned to rewear another sensor and we were able to exclude data from a potentially inaccurate sensor batch. Of note, all these participants had normal HbA1c (mean 5.1 ± 0.2% [32 ± 2.2 mmol/mol]) when coming back for the second CGM wear (mean 6 months later). Nevertheless, despite the normal HbA1c and negative autoantibodies, since most participants had a first-degree relative with T1D, we cannot completely rule out the possibility that the low frequency of hyperglycemia observed in a small number of participants was reflective of impaired β-cell function. As all hypoglycemic events happened at night, it is likely that some CGM readings were inaccurate because of pressure on the sensor during sleep. While it is not possible to confirm which readings may have been inaccurate because of movement or position during sleep, it is noteworthy that, after removing implausible data, glycemic metrics including mean glucose, time in target range, and time spent hypoglycemic remained similar, but the percentage of participants with a hypoglycemic event was much lower in the sensitivity analyses, with only one participant having a hypoglycemic event.
As sensor glucose profiles are increasingly used to assess diabetes outcomes both in clinical practice and clinical research trials [17], there is a critical need for a repository of “normative” CGM data with the most up-to-date and widely used devices. Our study has been able to fill the current gap in the data, to describe glycemic metrics of healthy, very young children. It will be important to update the results in nondiabetic individuals across all ages periodically as the accuracy of CGM devices improves further.
Acknowledgments
The authors of this work wish to thank the Helmsley Charitable Trust and all the study participants.
Glossary
Abbreviations
- CGM
continuous glucose monitoring;
- HbA1c
glycated hemoglobin A1c;
- T1D
type 1 diabetes
Financial Support
This work was supported by the Leona M. and Harry B. Helmsley Charitable Trust.
Author Contributions
S.N.B. wrote/edited the manuscript. L.G.K. performed statistical analyses and wrote/edited the manuscript. B.B., M.S., R.W.B., and A.K.S. researched data, contributed to discussion, and reviewed/edited the manuscript.
Disclosures
The authors have nothing to disclosure.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or are publicly available at https://public.jaeb.org/datasets.
References
- 1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battelino T, Bergenstal RM. Continuous glucose monitoring-derived data report—simply a better management tool. Diabetes Care. 2020;43(10):2327-2329. [DOI] [PubMed] [Google Scholar]
- 3. Herold KC, Bundy BN, Long SA, et al. ; Type 1 Diabetes TrialNet Study Group. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steck AK, Dong F, Geno Rasmussen C, et al. CGM metrics predict imminent progression to type 1 diabetes: autoimmunity screening for kids (ASK) study. Diabetes Care. 2022;45(2):365-371. [DOI] [PubMed] [Google Scholar]
- 5. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DuBose S. Continuous glucose monitoring profiles in healthy, nondiabetic young children. Published online March 25, 2022. doi: 10.5281/zenodo.6384827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McQueen RB, Geno Rasmussen C, Waugh K, et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care. 2020;43(7):1496-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sims EK, Besser REJ, Dayan C;. NIDDK Type 1 Diabetes TrialNet Study Group. Screening for type 1 diabetes in general population: a status report and perspective. Diabetes. 2022;71(4):610-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziegler AG, Kick K, Bonifacio E, et al. Fr1da Study Group. Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA. 2020;323(4):339-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barker JM, Goehrig SH, Barriga K, et al. DAISY Study. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399-1404. [DOI] [PubMed] [Google Scholar]
- 12. Vehik K, Cuthbertson D, Boulware D, et al. ; TEDDY, TRIGR, Diabetes Prevention Trial–Type 1, and Type 1 Diabetes TrialNet Natural History Study Groups. Performance of HbA1c as an early diagnostic indicator of type 1 diabetes in children and youth. Diabetes Care. 2012;35(9):1821-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathan BM, Boulware D, Geyer S, et al. ; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups. Dysglycemia and Index60 as prediagnostic end points for type 1 diabetes prevention trials. Diabetes Care. 2017;40(11):1494-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simmons KM, Sosenko JM, Warnock M, et al. One-hour oral glucose tolerance tests for the prediction and diagnostic surveillance of type 1 diabetes. J Clin Endocrinol Metab. 2020;105(11):e4094-e4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sosenko JM, Krischer JP, Palmer JP, et al. ; Diabetes Prevention Trial–Type 1 Study Group. A risk score for type 1 diabetes derived from autoantibody-positive participants in the diabetes prevention trial-type 1. Diabetes Care. 2008;31(3):528-533. [DOI] [PubMed] [Google Scholar]
- 16. Sosenko JM, Skyler JS, Mahon J, et al. ; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial–Type 1 Study G. The application of the diabetes prevention trial-type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care. 2012;35(7):1552-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017;40(8):994-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or are publicly available at https://public.jaeb.org/datasets.