Abstract
Background
Age-related comorbidities accumulate faster in people with HIV (PWH) than in those without HIV. We evaluated whether a validated multimorbidity scale, the Charlson index, predicted neurocognitive trajectories in PWH.
Methods
Scaled scores of a comprehensive neuropsychological battery were averaged across all visits. Multilevel modeling examined between- and within-person predictors of global neurocognition. At the between-person level, averaged Charlson scores were examined as a predictor of neurocognitive change rate, covarying for HIV disease characteristics. Within-persons, visit-specific Charlson index was used to predict fluctuations in global neurocognition at the same and next visit, covarying for disease measures.
Results
Participants were 1195 PWH (mean baseline age: 43.0; SD: 9.7 years) followed for a mean of 7.1 years (range: 0.5–20.5). At the between-person level, more rapid neurocognitive worsening correlated with higher (worse) average Charlson scores (standardized β: −0.062; SE: 0.015; P = .001) and lower CD4 nadir (standardized β: 0.055; SE: 0.021; P = .011), but not viral suppression or average CD4+ lymphocytes (P > .05). At the within-person level, poorer visit-specific neurocognition was related to worse concurrent, but not preceding, Charlson scores (standardized β: −0.046; SE: 0.015; P = .003), detectable HIV viral load (standardized β: 0.018; SE: 0.006; P = .001), and higher CD4+ (standardized β: 0.043; SE: 0.009; P < .001).
Conclusions
The impact of comorbidities on neurocognitive decline exceeded that of HIV disease factors. Although correlative, the temporal relationships suggested that treatment of comorbidities might improve neurocognitive prognosis for PWH.
Keywords: comorbidities, HIV, neurocognitive
Medical multimorbidities in this cohort of people with human immunodeficiency virus (PWH) accumulated at a high rate and were strongly associated with faster rates of neurocognitive decline. Findings suggest that interventions to prevent or ameliorate comorbidities may improve neurocognitive prognosis for PWH.
Comorbidities in people with human immunodeficiency virus (PWH) are common [1, 2] and linked to frailty [3], neurocognitive impairment [4], poor quality of life [5], and early death [6]. Comorbidities tend to appear at younger ages in PWH than in the general population and accumulate at a faster rate as PWH age, phenomena often referred to as premature and accelerated aging [7]. Some have even argued that comorbidities now are the chief source of neurocognitive impairment in human immunodeficiency virus (HIV), rather than HIV disease itself [8]. However, previous observations, because they are cross-sectional and may confound HIV and comorbidity effects, may inaccurately estimate the cumulative impact of comorbidities versus HIV disease factors over the decades that PWH now may live while suppressed on antiretroviral therapy (ART). We sought to delineate (1) how neurocognitive trajectories were influenced by the cumulative burden of comorbidities over time and (2) what was the relative impact of comorbidities as compared to HIV disease factors.
METHODS
Design
This was a longitudinal, observational cohort study of PWH prospectively recruited from community sources who agreed to undergo comprehensive neuromedical and neurobehavioral assessments for National Institutes of Health–funded studies at the HIV Neurobehavioral Research Program at the University of California San Diego [9] (HNRP; https://hnrp.hivresearch.ucsd.edu). Study visits took place between May 1999 and March 2020.
Participants
A total of 1195 participants with serology-confirmed HIV were included in this study. Exclusion criteria were severe or complex developmental, psychiatric, or neuromedical histories that confounded the interpretation of neuropsychological test data and their association with HIV disease and comorbidity burden. These exclusionary conditions included history of severe learning disability; presence of an active, major psychiatric condition with current psychotic features such as schizophrenia; major neurological conditions or active central nervous system (CNS) opportunistic infection (eg, CNS toxoplasmosis); and an active substance-use disorder or positive urine toxicology screen for substance use (except marijuana) or breathalyzer test for alcohol on the day of testing. Major confounding neurologic conditions such as active opportunistic disease were excluded based on history and clinical neurological examination obtained by a trained clinician (MD, NP, or RN). The University of California San Diego’s Human Research Protections Program (irb.ucsd.edu) approved all study procedures, and all participants provided written informed consent.
Participants underwent comprehensive neuromedical assessments at each visit, approximately annually. Comorbidities were summarized using the Charlson Comorbidity Index (CCI), a validated comorbidity burden scale, which was calculated at each visit [10]. The CCI accounts for 19 comorbidities, each assigned a weight based on the adjusted 1-year mortality. Comorbid conditions were ascertained based on a structured interview performed by a trained clinician using self-reported medical history, medical records if available, and a review of laboratory studies that included liver function testing, complete blood counts, and a comprehensive metabolic panel. Most conditions are rated as either present or absent. However, some conditions have a severity rating: for example, severe liver disease = cirrhosis and portal hypertension with variceal bleeding history, moderate = cirrhosis and portal hypertension but no variceal bleeding history, mild = chronic hepatitis (or cirrhosis without portal hypertension). Historically, dementia was included as a comorbid condition in the Charlson index; however, no participant was diagnosed with HIV-associated dementia at baseline and dementia did not factor into our index calculations. HIV disease was diagnosed by enzyme-linked immunosorbent assay with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cells (flow cytometry) were performed. Levels of HIV viral load in plasma were measured using reverse transcriptase–polymerase chain reaction (Amplicor; Roche Diagnostics, Indianapolis, IN), with a lower limit of quantitation (LLQ) of 50 copies/mL. HIV viral load was dichotomized as detectable versus undetectable at the LLQ of 50 copies/mL. Detailed medical and antiretroviral (ARV) drug exposure history was captured via a structured, clinician-administered questionnaire. Because efavirenz (EFV) has been associated with neurocognitive impairment in some prior studies [11], we evaluated the relationship of this specific ARV to neurocognitive decline.
Neurocognitive function was assessed using a comprehensive, standardized battery described in detail previously [12]. The battery covered 7 cognitive domains known to be commonly affected by HIV-associated CNS dysfunction (ie, verbal fluency, executive functioning, processing speed, learning, delayed recall, working memory, and complex motor skills). Raw scores from each test were transformed into practice-effect–corrected scaled scores (mean = 10; SD = 3 in normative sample), which subtract a median practice effect from the observed scaled score based on the number of testings [13]. Scaled scores were then averaged to create a composite global scaled score.
Statistical Analyses
Multilevel modeling was used to examine between- and within-person predictors of global neurocognitive performance. The effect of time (ie, years since baseline) was modeled as a random slope, allowing the relationship between time and global scaled score to vary by participant (ie, modeling a cognitive change trajectory for each person), which allows us to appropriately examine average CCI score as a between-person predictor of this random slope. Thus, at the between-person level of the model, average CCI score was examined as a moderator of the relationship between global neurocognition and time. HIV disease characteristics (ie, proportion of visits virally suppressed, average CD4) were also included as predictors of the random slope. Average CCI score, average age, sex, baseline education, race/ethnicity (non-Hispanic White vs other), proportion of visits on ARV therapy, and proportion of visits virally suppressed also predicted average global neurocognition (ie, global scaled score averaged over each participant’s visits) at the between-person level. At the within-person level of the multilevel model, concurrent-visit CCI score and previous-visit CCI score predicted global neurocognitive performance at each visit, covarying for time-varying markers of HIV disease severity (ie, CD4 count and HIV viral load detectability). Participant-specific random intercepts were specified. This statistical model was repeated for each neurocognitive domain outcome to explore domain-specific effects. Three additional analyses were conducted to support our interpretation of the data. First, the analyses were conducted in a subsample of participants who were virally suppressed at every visit (n = 312). Second, the analyses were repeated again in the entire sample using a modified version of the CCI that does not include AIDS status as a factor. Third, contributions from individual components of the CCI were explored by examining each as an independent predictor of global neurocognitive change in separate multilevel models, covarying only for demographics (ie, age, sex, education, race/ethnicity). Unstandardized and standardized βs are reported. All analyses were conducted in R, version 3.5.0 (R Foundation for Statistical Computing). Multilevel models were conducted using the “lme4” package [14].
RESULTS
At baseline, participants were 1195 PWH, with a mean age of 43.0 (SD: 9.7) years; 17.4% (n = 208) were female, 53.1% were non-Hispanic White, with 13.0 (3.0) years of education. Their median current CD4 was 366 cells/μL (interquartile range [IQR]: 183, 583), median nadir CD4 was 170 cells/μL (IQR: 35, 323), and 44.4% had undetectable plasma HIV RNA. Over the follow-up period, the mean (SD) proportion of visits at which participants were virally suppressed was 0.816 (0.193) and the mean proportion of visits at which participants were on ART was 0.830 (0.227).
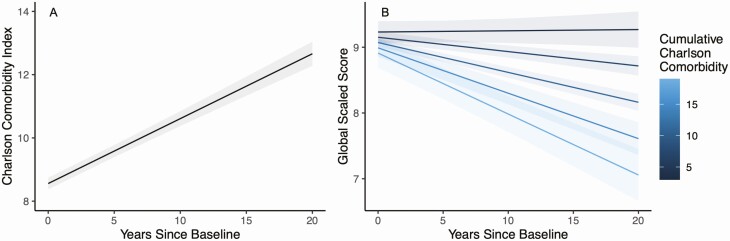
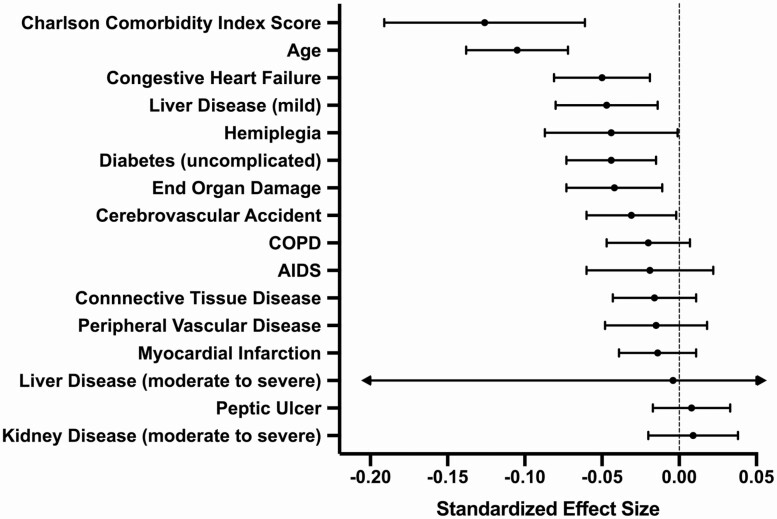
Over an average 7.1 years of follow-up (SD = 5.0 years; minimum = 0.5, maximum = 20.5), the Charlson score grew at an average rate of 0.205 points per year within persons (P < .001) (Figure 1A). Results of the multilevel model examining between- and within-person predictors of global neurocognition are presented in Table 1. At the between-person level of the model, higher average Charlson scores were associated with faster rates of decline in global neurocognitive performance (standardized β = −0.062, SE = 0.015; P = .001) (Figure 1B). Also at the between-person level, lower CD4 nadir was associated with worsening neurocognition over time (standardized β = 0.055, SE = 0.021; P = .011). However, other measures of HIV disease severity or treatment success (ie, proportion of visits virally suppressed and average CD4) were not significant (P values > .05). At the within-person level, higher visit-specific CCI score was related to lower concurrent global neurocognitive performance within persons (standardized β = −0.046, SE = 0.015; P = .003); however, previous-visit CCI score was not associated with neurocognition (P = .562). Also at the within-person level undetectable HIV viral load (standardized β = 0.019, SE = 0.006; P = .001) and higher concurrent CD4+ lymphocytes (standardized β = 0.046, SE = 0.010; P < .001) were independently associated with better concurrent global neurocognitive performance. Exploratory analyses examining each neurocognitive domain-specific outcome showed that the effect of average CCI score on global neurocognitive decline was driven by effects in the cognitive domains of executive functioning (P = .001) and working memory (P = .007). Additional analyses showed that the patterns of results held when (1) examining only the subset of 312 participants who were virally suppressed at every visit and (2) using a modified version of the Charlson index that does not include AIDS status. The additional analysis examining individual contributions from each component of the Charlson index showed that age was the strongest individual predictor of neurocognitive decline, followed by congestive heart failure (CHF), liver disease, and so on (see Figure 2). Importantly, the Charlson index was a stronger predictor of neurocognitive decline than any of its component parts.
Figure 1.
A, Longitudinal increase in Charlson Comorbidity Index within persons. B, Higher average Charlson score across visits was related to steeper declines in neurocognitive performance, after adjusting for relevant covariates including demographics and HIV disease factors. Each line represents a quintile of the distribution of the average Charlson score. Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Multilevel Model Results
| Unstandardized Estimate (SE) | Standardized Estimate (SE) | P | |
|---|---|---|---|
| Between-person level | |||
| Outcome: random slope (global SS on years) | |||
| Intercepta | −0.039 (0.006) | −0.071 (0.010) | <.001 |
| Proportion of visits virally suppressed | 0.025 (0.019) | 0.020 (0.016) | .195 |
| Average CD4 | <0.0001 (<0.0001) | −0.011 (0.020) | .593 |
| Nadir CD4 | 0.0001 (<0.001) | 0.055 (0.021) | .011 |
| Baseline duration of HIV infection | −0.001 (0.0009) | −0.021 (0.015) | .164 |
| Average Charlson index score | −0.007 (0.002) | −0.062 (0.019) | <.001 |
| Outcome: average global SS | |||
| Average Charlson index score | −0.026 (0.028) | −0.036 (0.038) | .337 |
| Average age (years) | −0.054 (0.007) | −0.236 (0.030) | <.001 |
| Sex (ref: male) | −0.368 (0.156) | −0.061 (0.026) | .019 |
| Education (years) | 0.303 (0.021) | 0.392 (0.027) | <.001 |
| Race/ethnicity (ref: non-Hispanic White) | −0.675 (0.122) | −0.145 (0.026) | <.001 |
| Proportion of visits virally suppressed | 0.844 (0.228) | 0.117 (0.032) | <.001 |
| Average CD4 | 0.0001 (0.0002) | 0.008 (0.035) | .812 |
| Nadir CD4 | 0.0008 (0.0006) | 0.050 (0.036) | .169 |
| Baseline duration of HIV infection | −0.018 (0.009) | −0.051 (0.027) | .054 |
| Proportion of visits on ART | −0.899 (0.268) | −0.095 (0.028) | <.001 |
| Within-person level | |||
| Outcome: global SS | |||
| Concurrent-visit Charlson Index Score | −0.032 (0.011) | −0.047 (0.016) | .004 |
| Previous-visit Charlson Index Score | 0.006 (0.010) | 0.009 (0.015) | .562 |
| Undetectable HIV plasma viral load (ref: no) | 0.093 (0.028) | 0.019 (0.006) | .001 |
| Current CD4 count | 0.0003 (0.0001) | 0.046 (0.010) | <.001 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; ref, reference; SS, scaled score.
aRepresents the effect of time when the proportion of visits virally suppressed, average CD4, and average Charlson index score are all at their grand mean.
Figure 2.
Forest plot showing the relative effect sizes of the predictor variables, including individual components of the Charlson index, as related to the outcome of neurocognitive slopes of decline (change over time). The overall Charlson score was the strongest predictor. Each of the predictors below is person-averaged (ie, at the between-person level). These mixed-effect models covary for demographics (age, sex, education, and race/ethnicity). Abbreviation: COPD, chronic obstructive pulmonary disease.
Of the 1195 participants in our sample, 364 reported taking efavirenz, an ARV drug associated with neurocognitive impairment in previous reports [11], during at least 1 study visit. Among these 364 individuals, they were on EFV for an average of 50% of study visits (SD = 29%, range = 5–100%). We added the proportion of visits on EFV into the full multilevel model as another potential between-person predictor of neurocognitive change over time (ie, the random slope). However, this did not significantly predict cognitive change (P = .843).
DISCUSSION
These findings indicate that the accumulation of comorbidities, many of which are related to HIV and ART [15–18], contribute to trajectories of neurocognitive decline more so than cumulative measures of HIV disease itself (proportion of visits virally suppressed, nadir CD4, average CD4 across visits). We report the magnitude of effects of these variables on neurocognitive decline as standardized β-coefficients. These coefficients are sensitive to scaling, meaning that a small value does not necessarily indicate a small clinical effect. In this case, a 1-unit increase in CCI score yielded a 0.062-unit worsening in the rate of neurocognitive decline. In comparison, a 1-unit drop in nadir CD4 yielded a 0.055-unit worsening. Relative to its standard error, the coefficient for the Charlson (−0.062/0.019 = −3.26) is much larger than that for the nadir CD4 (= −0.0001/0.055 = −0.0018), and its P value is smaller, suggesting that the magnitude of the Charlson effect is considerably greater than that of the more familiar nadir CD4 effect. Our observations, in combination with the known high prevalence of multimorbidity in PWH [1, 2] and previous findings that neurocognitive decline predicts frailty [3, 4], poor quality of life [5], and early death [6], point to the possibility that aggressive treatment of comorbidities will benefit long-term outcomes. There is abundant evidence in other clinical situations, such as normal aging, that successful treatment of comorbidities does indeed benefit neurocognition [19, 20]. Although in the between-persons models, HIV disease factors did not significantly predict declines in neurocognition, HIV disease factors still appeared to co-fluctuate with cognitive performance within persons, suggesting that treatment of HIV itself—viral suppression—is also critical for maintaining normal neurocognitive functioning in PWH.
Our findings are in general agreement with the literature, but represent a substantial advance with respect to the length of follow-up, the large size of the cohort, and the sophistication of the statistical analyses applied. Additionally, the breadth of comorbidities examined was larger than in previous studies, most of which focused on cardiovascular risk [8, 21]. Whereas previous reports of the relationship of comorbidities to neurocognitive impairment have been published [8, 21, 22], these were cross-sectional. Ours is the largest longitudinal study of the impact of comorbidities on neurocognitive decline.
Because many of the comorbidities that comprise the Charlson index are known to have downstream effects on neurocognition, we favor the interpretation that accumulating comorbidities are responsible for the observed neurocognitive decline. For example, individuals with diabetes [23–25], CHF [26, 27], and stroke [28, 29] decline faster than those without these conditions. However, an alternative explanation for the associations is that neurocognitive impairment interferes with the ability of PWH to engage in and benefit from the best medical care, resulting in increased comorbidity burden among those who are impaired. Under this interpretation, engagement in care is essential for protecting neurocognitive health. Alternatively, both comorbidities and neurocognitive decline might be determined by a third, unobserved variable such as persistent inflammation or metabolic derangements.
Both comorbidities and neurocognitive impairment in PWH are closely linked to viral control, immune suppression, and even ARV drugs. For example, persistent inflammation in virally suppressed PWH, as indicated by circulating T cells expressing markers of activation and inflammatory cytokine levels, correlated independently with pulmonary function abnormalities [30]. Among the commonly used ARVs, tenofovir is a well-recognized cause of kidney disease [31], osteoporosis [32, 33], and increased fracture risk [34]. Antiretroviral drugs also can be neurotoxic, contributing to cognitive impairment [35–37]. This is not to diminish the importance of successful ARV therapy, which has been transformative in the management of HIV infection. It is only because of ART and successful viral suppression that comorbidities have become so important.
Of particular importance among the comorbidities were CHF, liver disease, diabetes mellitus, and cerebrovascular disease. Notably, most of these are treatable conditions. These comorbidities are known to influence brain function and neurocognitive performance. For example, new-onset CHF is known to be followed by neurocognitive decline [2, 26, 38, 39], likely due to white matter hyperintensities, lacunar infarcts, and generalized volume loss [40–42]. The domains that drove global neurocognitive change in this study were executive function and working memory. These 2 domains reflect fronto-striatal system neuronal dysfunction and have been shown to be differentially vulnerable to HIV, but also to some comorbidities, such as vascular disease (cerebral small vessel disease, for which risk factors are diabetes, hypertension, and hyperlipidemia) [43, 44].
As demonstrated previously, higher education was protective with respect to average neurocognitive performance, a phenomenon known as cognitive reserve [45]. Thus, the protective effect of higher education might be also leveraged to benefit the population of aging PWH.
Limitations of this study include the relatively low proportion of visits at which participants had achieved viral suppression and the relatively small numbers of women. Yet, the influence of comorbidities remained after statistically adjusting for these variables. We did not systematically assess the impact of some well-recognized comorbidities such as depression, which has been shown to adversely influence neurocognitive function [46]. Our assessment of comorbidities, done through history-taking and severity estimation, was crude; a more accurate and precise assessment of comorbidities and their severity could be captured through measures such as laboratory testing, pulmonary function measures, and cardiovascular fitness evaluations. We did not evaluate individuals without HIV, so could not assess whether comorbidities accumulated at a faster rate in PWH or whether neurocognitive decline was related to different predictors in the 2 groups.
Future studies should investigate the mechanisms that link multimorbidity to neurocognition in HIV. Furthermore, the potential protective impact for neurocognition of an intensive strategy for managing comorbidities in PWH would have the potential to greatly influence standard clinical management strategies.
Notes
Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the US government. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of the National NeuroAIDS Tissue Consortium (NNTC) or National Institutes of Health.
Financial support. This work was supported by the National Institute of Mental Health (NIMH) and National Institute of Neurological Disorders and Stroke (NINDS) by the following grants: HIV Neurobehavioral Research Center (HNRC): P30 MH62512; Manhattan HIV Brain Bank (MHBB): U24MH100931; Texas NeuroAIDS Research Center (TNRC): U24MH100930; National Neurological AIDS Bank (NNAB): U24MH100929; California NeuroAIDS Tissue Network (CNTN): U24MH100928; Data Coordinating Center (DCC): U24MH100925; and Translational Methamphetamine AIDS Research Center (TMARC): P50 DA026306. The HNRC is supported by Center award P30MH062512 from the NIMH.
The San Diego HNRC group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes the following members—Director: Robert K. Heaton, PhD; Co-Director: Igor Grant, MD; Associate Directors: J. Hampton Atkinson, MD, Ronald J. Ellis, MD, PhD, and Scott Letendre, MD; Center Manager: Jennifer Iudicello, PhD, Donald Franklin, Jr, Melanie Sherman; NeuroAssessment Core: Ronald J. Ellis, MD, Ph.D. (Principal Investigator [PI]), Scott Letendre, MD, Thomas D. Marcotte, PhD, Christine Fennema-Notestine, PhD, Debra Rosario, MPH, Matthew Dawson; NeuroBiology Core: Cristian Achim, MD, PhD (PI), Ana Sanchez, PhD, Adam Fields, PhD; NeuroGerm Core: Sara Gianella Weibel, MD (PI), David M. Smith, MD, Rob Knight, PhD, Scott Peterson, PhD; Developmental Core: Scott Letendre, MD (PI), J. Allen McCutchan; Participant Accrual and Retention Unit: J. Hampton Atkinson, MD (PI), Susan Little, MD, Jennifer Marquie-Beck, MPH; Data Management and Information Systems Unit: Lucila Ohno-Machado, PhD (PI), Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD (Co-PI), Anya Umlauf, MS, Bin Tang, MS.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. ; HIV Neurobehavioral Research Program HNRP Group. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS 2013; 27:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allavena C, Hanf M, Rey D, et al. ; Dat’AIDS Study Group. Antiretroviral exposure and comorbidities in an aging HIV-infected population: The challenge of geriatric patients. PLoS One 2018; 13:e0203895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgello S, Gensler G, Sherman S, et al. ; National NeuroAIDS Tissue Consortium (NNTC). Frailty in medically complex individuals with chronic HIV. AIDS 2019; 33:1603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tedaldi EM, Minniti NL, Fischer T. HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int 2015; 2015:641913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ijoma UN, Unaogu NN, Onyeka TI, et al. . Health-related quality of life in people with chronic diseases managed in a low-resource setting—a study from South East Nigeria. Niger J Clin Pract 2019; 22:1180–8. [DOI] [PubMed] [Google Scholar]
- 6. Duffau P, Ozanne A, Bonnet F, et al. . Multimorbidity, age-related comorbidities and mortality: association of activation, senescence and inflammation markers in HIV adults. AIDS 2018; 32:1651–60. [DOI] [PubMed] [Google Scholar]
- 7. Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H. Premature and accelerated aging: HIV or HAART? Front Genet 2012; 3:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker JT, Kingsley L, Mullen J, et al. ; Multicenter AIDS Cohort Study. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology 2009; 73:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heaton RK, Franklin DR, Ellis RJ, et al. ; CHARTER Group; HNRC Group. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 11. Ma Q, Vaida F, Wong J, et al. ; CHARTER Group. Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neurovirol 2016; 22:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heaton RK, Clifford DB, Franklin DR Jr, et al. ; CHARTER Group. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cysique LA, Franklin D Jr, Abramson I, et al. ; CHARTER Group; HNRC Group. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol 2011; 33:505–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2014; 67:1–48. [Google Scholar]
- 15. Gallant J, Hsue PY, Shreay S, Meyer N. Comorbidities among US patients with prevalent HIV infection—a trend analysis. J Infect Dis 2017; 216:1525–33. [DOI] [PubMed] [Google Scholar]
- 16. Lerner AM, Eisinger RW, Fauci AS. Comorbidities in persons with HIV: the lingering challenge. JAMA 2019; 323:19–20. [DOI] [PubMed] [Google Scholar]
- 17. Weiss JJ, Osorio G, Ryan E, Marcus SM, Fishbein DA. Prevalence and patient awareness of medical comorbidities in an urban AIDS clinic. AIDS Patient Care STDS 2010; 24:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lorenc A, Ananthavarathan P, Lorigan J, Jowata M, Brook G, Banarsee R. The prevalence of comorbidities among people living with HIV in Brent: a diverse London borough. London J Prim Care (Abingdon) 2014; 6:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bliss ES, Wong RH, Howe PR, Mills DE. Benefits of exercise training on cerebrovascular and cognitive function in ageing. J Cereb Blood Flow Metab 2021; 41:447–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yurko-Mauro K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr Alzheimer Res 2010; 7:190–6. [DOI] [PubMed] [Google Scholar]
- 21. Wright EJ, Grund B, Robertson K, et al. ; INSIGHT SMART Study Group. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology 2010; 75:864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siangphoe U, Archer KJ, Nguyen C, Lee KR. Associations of antiretroviral therapy and comorbidities with neurocognitive outcomes in HIV-1-infected patients. AIDS 2020; 34:893–902. [DOI] [PubMed] [Google Scholar]
- 23. McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012; 379:2291–9. [DOI] [PubMed] [Google Scholar]
- 24. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group, Jacobson AM, Musen G, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356(18):1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shalimova A, Graff B, Gąsecki D, et al. . Cognitive dysfunction in type 1 diabetes mellitus. J Clin Endocrinol Metab 2019; 104:2239–49. [DOI] [PubMed] [Google Scholar]
- 26. Hammond CA, Blades NJ, Chaudhry SI, et al. . Long-term cognitive decline after newly diagnosed heart failure: longitudinal analysis in the CHS (Cardiovascular Health Study). Circ Heart Fail 2018; 11:e004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Witt LS, Rotter J, Stearns SC, et al. . Heart failure and cognitive impairment in the Atherosclerosis Risk in Communities (ARIC) Study. J Gen Intern Med 2018; 33:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine DA, Galecki AT, Langa KM, et al. . Trajectory of cognitive decline after incident stroke. JAMA 2015; 314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng F, Yan L, Zhong B, Yang Z, Xie W. Progression of cognitive decline before and after incident stroke. Neurology 2019; 93:e20–8. [DOI] [PubMed] [Google Scholar]
- 30. Fitzpatrick ME, Singh V, Bertolet M, et al. . Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS 2014; 28:2505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Venter WDF, Fabian J, Feldman C. An overview of tenofovir and renal disease for the HIV-treating clinician. South Afr J HIV Med 2018; 19:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Güerri-Fernández R, Molina-Morant D, Villar-García J, et al. . Bone density, microarchitecture, and tissue quality after long-term treatment with tenofovir/emtricitabine or abacavir/lamivudine. J Acquir Immune Defic Syndr 2017; 75:322–7. [DOI] [PubMed] [Google Scholar]
- 33. Negredo E, Diez-Pérez A, Bonjoch A, et al. . Switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: changes in bone turnover markers and circulating sclerostin levels. J Antimicrob Chemother 2015; 70:2104–7. [DOI] [PubMed] [Google Scholar]
- 34. Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 2012; 26:825–31. [DOI] [PubMed] [Google Scholar]
- 35. Underwood J, Robertson KR, Winston A. Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 2015; 29:253–61. [DOI] [PubMed] [Google Scholar]
- 36. Lanman T, Letendre S, Ma Q, Bang A, Ellis R. CNS neurotoxicity of antiretrovirals. J Neuroimmune Pharmacol 2021; 16:130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bertrand L, Velichkovska M, Toborek M. Cerebral vascular toxicity of antiretroviral therapy. J Neuroimmune Pharmacol 2021; 16:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol 2014; 11:316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cannon JA, Moffitt P, Perez-Moreno AC, et al. . Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail 2017; 23:464–75. [DOI] [PubMed] [Google Scholar]
- 40. Ye S, Dong S, Tan J, et al. . White-matter hyperintensities and lacunar infarcts are associated with an increased risk of Alzheimer’s disease in the elderly in China. J Clin Neurol 2019; 15:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iadecola C, Duering M, Hachinski V, et al. . Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol 2019; 73:3326–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Breteler MM, van Swieten JC, Bots ML, et al. . Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology 1994; 44:1246–52. [DOI] [PubMed] [Google Scholar]
- 43. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol 2016; 131:659–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reed BR, Mungas DM, Kramer JH, et al. . Profiles of neuropsychological impairment in autopsy-defined Alzheimer’s disease and cerebrovascular disease. Brain 2007; 130:731–9. [DOI] [PubMed] [Google Scholar]
- 45. Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I; HIV Neurobehavioral Research Program (HNRP) Group. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS Behav 2012; 16:2279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamilton JL, Brickman AM, Lang R, et al. . Relationship between depressive symptoms and cognition in older, non-demented African Americans. J Int Neuropsychol Soc 2014; 20:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]