Abstract
Background
Demography is changing, with people living longer with comorbidities. In this nationwide population-based study, we investigated the serotype-specific invasive pneumococcal disease (IPD) risk in individuals with comorbidities, and effects of the pneumococcal conjugated vaccine (PCV) child immunization program.
Methods
Cases included 14 096 IPD episodes in Sweden during 2006–2015. Controls (n = 137 289), matched to cases by age, sex, region, and calendar time, were selected from the general population. Comorbidity data was obtained through health registers and grouped as immunocompromising (IC) or chronic medical conditions (CMC).
Results
The prevalence of CMC and IC among elderly cases was 33.9% and 39.4%. New risks identified for IPD were sarcoidosis, inflammatory polyarthropathies, systemic connective tissue, and neurological diseases. The odds ratio (OR) for IPD caused by non-PCV13 compared with PCV13 serotypes was higher in individuals with CMC/IC. Serotypes associated with the highest risk were 16F, 15C, 35F, 19F, and 23A (OR 3–5 for CMC, >10 for IC). Most comorbidities increased post-vaccination, and absolute increases of IPD caused by non-PCV13, PPV23–non-PCV13, and non-PCV13/non-PPV23 serotypes were higher in individuals with IC/CMC compared with healthy persons. Non-PCV13 serotypes 6C, 9N, 11A, 22F, 23A and 35F increased more in those with comorbidities. Mortality due to non-PCV13 serotypes increased in individuals with IC/CMC, while remaining stable in persons without comorbidities.
Conclusions
The PCV child immunization program associates with an increased disease burden of non-vaccine serotypes in individuals with comorbidities. These data are important for vaccine design and optimization of current vaccination strategies.
Keywords: immunodeficiency, invasive pneumococcal disease, pneumococcal conjugate vaccines, serotypes, Streptococcus pneumoniae
This nationwide study shows that pneumococcal nonvaccine-serotypes preferentially infect individuals with comorbidities. Pneumococcal conjugated vaccine introduction has shifted invasive pneumococcal disease caused by vaccine- to nonvaccine-serotypes in this patient group. The results are important for pneumococcal vaccination strategies.
Streptococcus pneumoniae contributed to 1.2 million deaths globally in 2016, with the highest burden in young children and the elderly [1]. Several comorbidities, particularly immunosuppression, are associated with higher risk for invasive pneumococcal disease (IPD) [2-5]. A 23-valent pneumococcal polysaccharide vaccine (PPV23) is available for immunization of the elderly and individuals with comorbidities [6-8]. Pneumococcal conjugate vaccines (PCVs) are included in child immunization programs globally. First, a 7-valent vaccine (PCV7) was launched around 2010; it was replaced by a 10-valent (PCV10) or 13-valent (PCV13) vaccine. PCV13 is also licensed for use in adults [9]. PCV introduction has resulted in reductions of IPD caused by vaccine serotypes both in vaccinated children and nonvaccinated adults [10-12]. In most countries, there has been a concomitant increase in nonvaccine serotypes, hampering the impact of PCVs [10-14]. In Sweden, we reported a decrease in IPD caused by vaccine serotypes in all age groups after PCV introduction, but the overall impact in the elderly was limited due to the increase in nonvaccine serotypes [15].
In many countries, life expectancy rises, reflecting improved survival among healthy individuals and those with underlying diseases [16]. Associations have been found between comorbidities and pneumococcal serotypes in patients with IPD [17, 18]. However, studies assessing the impact of PCV introduction on serotype-specific IPD in individuals with comorbidities are hampered by study designs that might cause differential misclassification, by assessing only subgroups of immunosuppressed individuals, or by grouping all individuals with comorbidities together [9, 19-21]. To guide future pneumococcal vaccine development and immunization policies, it is important to assess the impact of pneumococcal childhood immunization on serotype-specific IPD in individuals with underlying diseases in population-based studies with high validity.
In Sweden, the national PCV child immunization program started generally in 2009 using PCV7 followed by a change to PCV10 or PCV13 around 2010 in a 2 + 1 schedule [15]. The coverage among children aged 2 years was 96.5%–97.6% during 2011–2017 [22]. PCV13 was not recommended nationally for adult risk groups until 2016. Adult risk groups have been recommended PPV23 since the 1990s, but the coverage of PPV23 in the elderly has been low at <30% [22]. All Swedish citizens have unique personal identification numbers that enable linkage to comprehensive national health registers with high validity that include information about hospital care, drug prescriptions, migration, and mortality [23].
Here, we performed a nationwide, population-based study to investigate the risk of serotype-specific IPD in individuals with underlying diseases and the effect of underlying diseases on the IPD incidence after PCV introduction.
METHODS
Case and Control Groups
Cases consisted of citizens with IPD reported to the Public Health Agency of Sweden between 2006 and 2015. An IPD case was defined as pneumococcal isolation from sterile locations (eg, blood, cerebrospinal fluid) [15]. For each case, up to 10 controls were randomly selected by incidence density sampling from the general population using the population register at Statistics Sweden. The controls were matched to cases by age (±1 year), sex, calendar time, and geographical region (exact matching). The study was approved by the Ethical Committee in Stockholm.
Comorbidities
Data on comorbidities were obtained through linkage to the patient register, which contains International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) diagnoses for all hospital care administered in Sweden. Comorbidities were defined as present if there was a diagnosis within the last 5 years prior to the study index date. Dispensation of medical drugs within the year before that index date was obtained through linkage to the Prescribed Drug Register that contains data on dispensed drugs from pharmacies in Sweden. Comorbidity categories were defined according to ICD-10 diagnoses and/or dispensed medical drugs (Supplementary Table 1). In accordance with the Advisory Committee on Immunization Practices (ACIP) guidelines for pneumococcal vaccination, individuals were grouped as individuals with immunocompromising conditions (ICs), which included immunodeficiency, human immunodeficiency virus (HIV), hematological/solid tumor malignancy, and chronic renal failure, and individuals with chronic medical conditions (CMC), which included diabetes and heart, lung, and liver disease, without having diagnosis belonging to the IC group [24].
Serotyping of Isolates
Serotyping was performed at the Public Health Agency of Sweden by gel diffusion using antisera (SSI, Denmark), as previously described [25]. The pneumococcal serotypes were grouped into PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F), non-PCV13 (not included in PCV13), PPV23–non-PCV13 (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, 33F), and non-PCV13/non-PPV23 serotypes (not included in PCV13 or PPV23).
Statistical Analyses
IPD episodes without adequate serotyping data (951 of 14096, 6.7%) were imputed using multivariate imputations by chained equations (Supplementary Methods). Conditional logistic regression was used to estimate the risk of IPD for individuals with comorbidities in the periods before (2006–2007) and after (2014–2015) PCV introduction that included an interaction term (ratio of odds ratios [ORs]) of comorbidity with period. Stratified analyses were performed by age group, serotype group, and for serotypes with at least 100 cases on average from all imputed datasets. Population attributable proportions of comorbidities were calculated based on estimates of the post-vaccine period [26]. Logistic regression was used to estimate the association between serotype and 30-day mortality adjusted for age, sex, year, and comorbidity.
The yearly incidence and mortality rates of IPD during 2006–2015 were estimated using Poisson regression. Population size was obtained from Statistics Sweden. The proportion of people in the population with underlying conditions was estimated from the population-based controls where a logistic regression was applied to every age group–sex combination, having year as the covariate (Supplementary Methods) [22]. Absolute risk differences (RDs) and risk ratios (RRs) compared 2014–2015 with 2006–2007. The IPD incidence was stratified by comorbidity for serotypes 3, 6C, 9N, 11A, 19A, 22F, 23A, and 35F (these serotypes increased post-vaccine introduction [15]) and for nonvaccine serotypes with >250 cases during the study period. Changes in the proportion of comorbidities among cases and controls in the post-vaccine compared with the pre-vaccine period were assessed using logistic regression models having different comorbidities as outcome and estimating the effect of period after adjustment for age group, sex, and county. Firth’s logistic regression was used to deal with separation problems [27]. Two-sided P values < .05 were considered statistically significant, and data were presented with 95% confidence intervals. Analyses were performed using R (www.r-project.org).
RESULTS
Increased Risk for IPD in Individuals With Chronic and Immunocompromising Conditions
A total of 14266 IPD cases were reported in Sweden during 2006–2015; 166 did not have an identification number and 4 were excluded due to more than 1 pneumococcal serotype. Thus, the study population consisted of 14096 IPD cases (50.2% females): 410 aged 0–4 years, 5596 aged 5–64 years, and 8090 aged ≥65 years. Cases were matched to 137289 controls. The prevalence of CMC and IC among IPD cases was 15.1% and 9.3% among those aged 0–4 years, 20.8% and 23.6% among those aged 5–64 years, and 33.9% and 39.4% among those aged ≥65 years. After adjustment for age, sex, and region, the proportion of most comorbidities increased post-vaccination compared with pre-vaccination both among cases and controls (Supplementary Table 2). The median age among cases increased from 65 years (interquartile range [IQR], 52–79) in 2006–2007 to 70 years (IQR, 59–80) in 2014–2015.
The OR for IPD was 2.50 (95% CI 2.39 to 2.62) for individuals with CMC and 5.01 (95% CI 4.78 to 5.25) for those with IC compared with individuals without these conditions (Table 1). Comorbidities associated with the highest OR were hematological malignancies (95% CI 9.16 to 11.16) and HIV (12.31; [95% CI 8.24 to 18.37]; Supplementary Table 3A–3C). After adjustment for other comorbidities and immunosuppressive treatment, the following diagnoses, not currently included in ACIP recommendations for pneumococcal vaccination, were associated with IPD: sarcoidosis, inflammatory polyarthropathies, or systemic connective tissue disorders (OR, 1.26; [95% CI 1.17 to 1.36]), and neurological diseases (OR, 1.26; [95% CI 1.20 to 1.34]).
Table 1.
Risk of Invasive Pneumococcal Disease Associated With Comorbidity Groups in the Pre- and Post-Vaccine Periods
| All Years (2006–2015) | Pre-Vaccine Period (2006–2007) | Post-Vaccine Period (2014–2015) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases % (n) | Controls % (n) | OR (95% CI) | Cases % (n) | Controls % (n) | OR (95% CI) | Cases % (n) | Controls % (n) | OR (95% CI) | Ratio of ORs (95% CI) | Population Attributable Proportion a (95% CI) | |
| All data | (N = 14096) | (N = 137289) | (N = 2815) | (N = 27183) | (N = 2411) | (N = 23664) | |||||
| All ages | |||||||||||
| No comorbidities | 39.6 | 64.7 | 1 (Ref) | 43.6 | 67.6 | 1 (Ref) | 34.8 | 61.0 | 1 (Ref) | 1 (Ref) | Ref |
| (5577) | (88778) | (1228) | (18363) | (839) | (14437) | ||||||
| CMC | 28.2 | 22.1 | 2.50 | 27.4 | 20.7 | 2.45 | 28.8 | 23.5 | 2.60 | 1.06 | 17.72 |
| (3973) | (30374) | (2.39–2.62) | (772) | (5619) | (2.21–2.71) | (695) | (5554) | (2.32–2.91) | (0.91–1.24) | (15.46–19.92) | |
| IC | 32.3 | 13.2 | 5.01 | 29.0 | 11.8 | 4.73 | 36.4 | 15.5 | 5.19 | 1.10 | 29.39 |
| (4546) | (18137) | (4.78–5.25) | (815) | (3201) | (4.25–5.27) | (877) | (3673) | (4.63–5.80) | (0.94–1.28) | (27.15–31.55) | |
| Age group 5–64 | |||||||||||
| No comorbidities | 55.6 | 82.2 | 1 (Ref) | 57.2 | 82.9 | 1 (Ref) | 51.9 | 81.0 | 1 (Ref) | 1 (Ref) | Ref |
| (3112) | (45844) | (696) | (10038) | (429) | (6680) | ||||||
| CMC | 20.8 | 12.4 | 2.72 | 20.5 | 11.6 | 2.80 | 21.8 | 13.3 | 2.83 | 1.01 | 14.10 |
| (1165) | (6889) | (2.53–2.93) | (249) | (1403) | (2.39–3.29) | (180) | (1098) | (2.34–3.43) | (0.79–1.30) | (10.85–17.22) | |
| IC | 23.6 | 5.4 | 7.31 | 22.4 | 5.5 | 6.80 | 26.3 | 5.7 | 8.35 | 1.23 | 23.15 |
| (1319) | (3026) | (6.76–7.91) | (272) | (662) | (5.73–8.07) | (217) | (467) | (6.83–10.21) | (0.94–1.60) | (19.93–26.25) | |
| Age group 65+ | |||||||||||
| No comorbidities | 26.6 | 50.7 | 1 (Ref) | 29.1 | 51.6 | 1 (Ref) | 24.4 | 49.2 | 1 (Ref) | 1 (Ref) | Ref |
| (2155) | (39228) | (425) | (7059) | (376) | (7376) | ||||||
| CMC | 33.9 | 29.8 | 2.23 | 34.4 | 29.9 | 2.08 | 33.3 | 29.5 | 2.36 | 1.13 | 19.19 |
| (2746) | (23109) | (2.10–2.36) | (501) | (4088) | (1.82–2.39) | (514) | (4418) | (2.05–2.71) | (0.93–1.38) | (16.00–22.26) | |
| IC | 39.4 | 19.5 | 4.00 | 36.5 | 18.5 | 3.61 | 42.3 | 21.4 | 4.18 | 1.16 | 32.18 |
| (3189) | (15095) | (3.77–4.25) | (532) | (2535) | (3.14–4.14) | (653) | (3205) | (3.64–4.79) | (0.95–1.41) | (29.07–35.15) | |
| PCV13 serotypes | (N = 8627) b | (N = 84089) b | (N = 2224) b | (N = 21479) b | (N = 859) b | (N = 8440) b | |||||
| All ages | |||||||||||
| No comorbidities | 43.6 | 66.3 | 1 (Ref) | 45.4 | 68.3 | 1 (Ref) | 40.0 | 62.2 | 1 (Ref) | 1 (Ref) | Ref |
| (343) | (5254) | ||||||||||
| (3762) | (55762) | (1011) | (14671) | ||||||||
| CMC | 28.0 | 21.3 | 2.36 | 27.2 | 20.2 | 2.39 | 29.5 | 22.8 | 2.34 | 0.98 | 16.91 |
| (1921) | (1.95–2.82) | (0.79–1.22) | (12.88–20.76) | ||||||||
| (2413) | (17926) | (2.23–2.50) | (606) | (4341) | (2.12–2.69) | (253) | |||||
| IC | 28.4 | 12.4 | 4.31 | 27.3 | 11.5 | 4.38 | 30.5 | 15.0 | 3.83 | 0.87 | 22.53 |
| (1265) | (3.16–4.63) | (0.70–1.10) | (18.81–26.08) | ||||||||
| (2452) | (10401) | (4.04–4.59) | (607) | (2467) | (3.86–4.96) | (262) | |||||
| Age group 5–64 | |||||||||||
| No comorbidities | 59.1 | 82.7 | 1 (Ref) | 59.0 | 83.2 | 1 (Ref) | 56.8 | 80.7 | 1 (Ref) | 1 (Ref) | Ref |
| (2595) | |||||||||||
| (2178) | (30358) | (571) | (7993) | (183) | |||||||
| CMC | 20.5 | 12.1 | 2.58 | 20.3 | 11.4 | 2.76 | 23.9 | 13.6 | 2.77 | 1.00 | 15.28 |
| (438) | (2.05–3.74) | (0.71–1.43) | (9.68–20.54) | ||||||||
| (757) | (4440) | (2.35–2.83) | (196) | (1094) | (2.29–3.31) | (77) | |||||
| IC | 20.3 | 5.2 | 6.19 | 20.6 | 5.4 | 6.12 | 19.2 | 5.7 | 5.37 | 0.88 | 15.65 |
| (184) | (3.79–7.59) | (0.59–1.31) | (10.87–20.18) | ||||||||
| (749) | (1916) | (5.58–6.85) | (199) | (522) | (5.01–7.48) | (62) | |||||
| Age group 65+ | |||||||||||
| No comorbidities | 29.2 | 51.2 | 1 (Ref) | 30.1 | 52.1 | 1 (Ref) | 28.8 | 50.2 | 1 (Ref) | 1 (Ref) | Ref |
| (2564) | |||||||||||
| (1357) | (22786) | (340) | (5516) | (151) | |||||||
| CMC | 34.6 | 29.7 | 2.09 | 34.5 | 29.6 | 2.05 | 33.4 | 28.7 | 2.10 | 1.02 | 17.46 |
| (1464) | (1.66–2.66) | (0.77–1.36) | (11.61–22.93) | ||||||||
| (1610) | (13225) | (1.94–2.26) | (390) | (3128) | (1.75–2.40) | (175) | |||||
| IC | 36.2 | 19.0 | 3.45 | 35.3 | 18.3 | 3.40 | 37.9 | 21.1 | 3.26 | 0.96 | 26.24 |
| (1080) | (2.59–4.10) | (0.72–1.27) | (20.76–31.35) | ||||||||
| (1686) | (8470) | (3.18–3.74) | (399) | (1941) | (2.89–3.99) | (199) | |||||
| Non-PCV13 serotypes | (N = 5469) b | (N = 53200) b | (N = 591) b | (N = 5704) b | (N = 1552) b | (N = 15224) b | |||||
| All ages | |||||||||||
| No comorbidities | 33.2 | 62.1 | 1 (Ref) | 36.8 | 64.7 | 1 (Ref) | 31.9 | 60.3 | 1 (Ref) | 1 (Ref) | Ref |
| (9183) | |||||||||||
| (1815) | (33016) | (217) | (3692) | (496) | |||||||
| CMC | 28.5 | 23.4 | 2.78 | 28.1 | 22.4 | 2.71 | 28.5 | 23.9 | 2.78 | 1.03 | 18.23 |
| (3633) | (2.41–3.22) | (0.76–1.39) | (15.41–20.96) | ||||||||
| (1560) | (12448) | (2.57–3.01) | (166) | (1278) | (2.09–3.52) | (442) | |||||
| IC | 38.3 | 14.5 | 6.26 | 35.1 | 12.9 | 6.26 | 39.6 | 15.8 | 6.13 | 0.98 | 33.16 |
| (2408) | (5.31–7.07) | (0.73–1.31) | (30.30–35.90) | ||||||||
| (2094) | (7736) | (5.78–6.78) | (208) | (734) | (4.84–8.11) | (615) | |||||
| Age group 5–64 | |||||||||||
| No comorbidities | 48.8 | 81.3 | 1 (Ref) | 50.0 | 82.0 | 1 (Ref) | 48.8 | 81.2 | 1 (Ref) | 1 (Ref) | Ref |
| (4085) | |||||||||||
| (934) | (15486) | (125) | (2045) | (246) | |||||||
| CMC | 21.4 | 12.9 | 3.03 | 21.0 | 12.4 | 2.98 | 20.4 | 13.1 | 2.88 | 0.96 | 13.32 |
| (660) | (2.23–3.71) | (0.61–1.53) | (9.25–17.22) | ||||||||
| (408) | (2449) | (2.65–3.47) | (53) | (309) | (2.02–4.41) | (103) | |||||
| IC | 29.8 | 5.8 | 9.63 | 29.0 | 5.6 | 9.73 | 30.8 | 5.6 | 10.71 | 1.10 | 27.90 |
| (283) | (8.29–13.84) | (0.69–1.75) | (23.50–32.04) | ||||||||
| (570) | (1110) | (8.43–11.00) | (73) | (140) | (6.59–14.38) | (155) | |||||
| Age group 65+ | |||||||||||
| No comorbidities | 23.2 | 49.9 | 1 (Ref) | 25.7 | 49.8 | 1 (Ref) | 22.1 | 48.7 | 1 (Ref) | 1 (Ref) | Ref |
| (4812) | |||||||||||
| (798) | (16442) | (85) | (1543) | (225) | |||||||
| CMC | 33.1 | 30.0 | 2.46 | 33.8 | 31.0 | 2.22 | 33.3 | 29.9 | 2.54 | 1.14 | 20.16 |
| (2954) | (2.12–3.04) | (0.78–1.66) | (16.19–23.94) | ||||||||
| (1136) | (9884) | (2.23–2.71) | (111) | (960) | (1.60–3.08) | (339) | |||||
| IC | 43.7 | 20.1 | 4.92 | 40.5 | 19.2 | 4.46 | 44.6 | 21.5 | 4.78 | 1.07 | 35.29 |
| (2125) | (4.03–5.68) | (0.74–1.55) | (31.41–38.95) | ||||||||
| (1503) | (6625) | (4.46–5.42) | (133) | (594) | (3.21–6.19) | (454) | |||||
| PPV23–non-PCV13 serotypes | (N = 3467) b | (N = 33844) b | (N = 402) b | (N = 3886) b | (N = 900) b | (N = 8859) b | |||||
| All ages | |||||||||||
| No comorbidities | 36.8 | 63.6 | 1 (Ref) | 40.4 | 66.2 | 1 (Ref) | 34.7 | 62.1 | 1 (Ref) | 1 (Ref) | Ref |
| (5500) | |||||||||||
| (1276) | (21526) | (162) | (2573) | (312) | |||||||
| CMC | 28.7 | 22.7 | 2.60 | 28.5 | 21.8 | 2.58 | 30.2 | 23.1 | 2.86 | 1.11 | 19.65 |
| (2048) | (2.37–3.45) | (0.78–1.57) | (15.82–23.31) | ||||||||
| (994) | (7677) | (2.37–2.87) | (115) | (846) | (1.92–3.47) | (272) | |||||
| IC | 34.5 | 13.7 | 5.38 | 31.1 | 12.0 | 5.33 | 35.1 | 14.8 | 5.39 | 1.01 | 28.58 |
| (1311) | (4.46–6.51) | (0.71–1.45) | (24.92–32.07) | ||||||||
| (1197) | (4640) | (4.88–5.94) | (125) | (467) | (3.93–7.23) | (316) | |||||
| Age group 5–64 | |||||||||||
| No comorbidities | 52.3 | 81.3 | 1 (Ref) | 54.0 | 82.7 | 1 (Ref) | 52.7 | 81.2 | 1 (Ref) | 1 (Ref) | Ref |
| (2700) | |||||||||||
| (710) | (10997) | (97) | (1474) | (176) | |||||||
| CMC | 22.6 | 12.8 | 2.98 | 20.5 | 11.7 | 2.85 | 22.2 | 13.1 | 2.87 | 1.01 | 14.49 |
| (437) | (2.12–3.89) | (0.59–1.73) | (9.22–19.46) | ||||||||
| (307) | (1727) | (2.56–3.47) | (37) | (208) | (1.81–4.49) | (74) | |||||
| IC | 25.2 | 5.9 | 7.33 | 25.5 | 5.6 | 7.79 | 25.0 | 5.7 | 7.92 | 1.02 | 21.87 |
| (190) | (5.72–10.96) | (0.58–1.78) | (16.75–26.68) | ||||||||
| (342) | (801) | (6.25–8.60) | (46) | (99) | (4.91–12.36) | (83) | |||||
| Age group 65+ | |||||||||||
| No comorbidities | 25.7 | 50.8 | 1 (Ref) | 28.3 | 50.9 | 1 (Ref) | 22.7 | 49.5 | 1 (Ref) | 1 (Ref) | Ref |
| (2675) | |||||||||||
| (528) | (10063) | (61) | (1036) | (126) | |||||||
| CMC | 33.1 | 29.8 | 2.27 | 35.2 | 31.1 | 2.15 | 35.7 | 29.7 | 2.70 | 1.26 | 22.48 |
| (1604) | (2.12–3.43) | (0.80–1.98) | (16.89–27.70) | ||||||||
| (681) | (5900) | (2.01–2.57) | (76) | (633) | (1.46–3.16) | (198) | |||||
| IC | 41.2 | 19.4 | 4.40 | 36.5 | 18.0 | 3.93 | 41.5 | 20.8 | 4.47 | 1.14 | 32.26 |
| (1121) | (3.54–5.66) | (0.71–1.83) | (27.02–37.12) | ||||||||
| (848) | (3838) | (3.88–4.98) | (79) | (367) | (2.60–5.95) | (230) | |||||
| Non-PCV13/non-PPV23 serotypes | (N = 2003) b | (N = 19357) b | (N = 188) b | (N = 1818) b | (N = 653) b | (N = 6365) b | |||||
| All ages | |||||||||||
| No comorbidities | 26.9 | 59.4 | 1 (Ref) | 29.0 | 61.6 | 1 (Ref) | 28.1 | 57.9 | 1 (Ref) | 1 (Ref) | Ref |
| (3683) | |||||||||||
| (539) | (11490) | (55) | (1119) | (183) | |||||||
| CMC | 28.3 | 24.6 | 3.22 | 27.3 | 23.7 | 3.10 | 26.0 | 24.9 | 2.68 | 0.86 | 16.32 |
| (1585) | (2.11–3.39) | (0.49–1.51) | (11.99–20.43) | ||||||||
| (567) | (4771) | (2.80–3.69) | (51) | (432) | (1.88–5.13) | (170) | |||||
| IC | 44.8 | 16.0 | 8.20 | 43.7 | 14.7 | 8.83 | 45.9 | 17.2 | 7.22 | 0.82 | 39.52 |
| (1097) | (5.78–9.02) | (0.48–1.40) | (34.88–43.83) | ||||||||
| (897) | (3096) | (7.18–9.38) | (82) | (267) | (5.41–14.39) | (299) | |||||
| Age group 5–64 | |||||||||||
| No comorbidities | 40.5 | 81.3 | 1 (Ref) | 40.3 | 80.2 | 1 (Ref) | 41.1 | 81.4 | 1 (Ref) | 1 (Ref) | Ref |
| (1385) | |||||||||||
| (224) | (4489) | (29) | (571) | (70) | |||||||
| CMC | 18.3 | 13.1 | 3.20 | 22.2 | 14.1 | 3.43 | 16.9 | 13.1 | 2.83 | 0.82 | 10.93 |
| (223) | (1.75–4.57) | (0.34–1.98) | (4.35–17.06) | ||||||||
| (101) | (723) | (2.45–4.18) | (16) | (101) | (1.66–7.10) | (29) | |||||
| IC | 41.2 | 5.6 | 17.89 | 37.5 | 5.7 | 17.24 | 42.0 | 5.4 | 17.79 | 1.03 | 39.64 |
| (93) | (11.45–27.64) | (0.42–2.56) | (31.29–46.98) | ||||||||
| (228) | (309) | (13.95–22.95) | (27) | (40) | (7.81–38.05) | (72) | |||||
| Age group 65+
|
|||||||||||
| No comorbidities | 19.5 | 48.5 | 1 (Ref) | 20.8 | 47.8 | 1 (Ref) | 21.4 | 47.6 | 1 (Ref) | 1 (Ref) | Ref |
| (2137) | |||||||||||
| (270) | (6379) | (23) | (507) | (99) | |||||||
| CMC | 33.0 | 30.3 | 2.82 | 30.9 | 30.8 | 2.43 | 30.4 | 30.1 | 2.35 | 0.97 | 17.43 |
| (1350) | (1.78–3.09) | (0.48–1.93) | (11.58–22.89) | ||||||||
| (455) | (3984) | (2.39–3.33) | (35) | (327) | (1.29–4.59) | (141) | |||||
| IC | 47.5 | 21.2 | 5.90 | 48.3 | 21.4 | 5.69 | 48.3 | 22.4 | 5.17 | 0.91 | 38.93 |
| (1004) | (4.00–6.70) | (0.48–1.72) | (32.93–44.40) | ||||||||
| (655) | (2786) | (5.03–6.92) | (54) | (227) | (3.16–10.25) | (225) | |||||
Abbreviations: CI, confidence interval; CMC, chronic medical condition; IC, immunocompromised; OR, odds ratio; Ref, reference.
Population attributable proportion based on data from 2014–2015.
The numbers represent the average of the imputed datasets.
Nonvaccine Serotypes Preferentially Infect Individuals With Immunocompromising Conditions
The OR for IPD caused by non-PCV13 serotypes and non-PCV13/non-PPV23 serotypes compared with PCV13 serotypes was higher in individuals with CMC: 2.78 (95% CI 2.57 to 3.01) and 3.22 (95% CI 2.80 to 3.69) compared with 2.36 (95% CI 2.23 to 2.50), and in individuals with IC: 6.26 (95% CI 5.78 to 6.78) and 8.20 (95% CI 7.18 to 9.38) compared with 4.31 (95% CI 4.04 to 4.59; Table 1). The same pattern was observed in stratified analyses according to age groups, where the largest differences were observed for individuals with IC aged 5–64 years: OR for PCV13 serotypes 6.19 (95% CI 5.58 to 6.85), for non-PCV13 serotypes 9.63 (95% CI 8.43 to 11.0), and for non-PCV13/non-PPV23 serotypes 17.89 (95% CI 13.95 to 22.95).
The population attributable proportions (PAPs) of CMC and IC were 17.7% (15.5–19.9) and 29.4% (27.2–31.6), respectively, in 2014–2015 (Table 1). The PAPs of CMC and IC were higher in the elderly, 19.2% (16.0–22.3) and 32.2% (29.1–35.2), respectively, compared with 14.1% (10.9–17.2) and 23.2% (19.9–26.3) for those aged 5–64 years. The attribution of IC was higher for non-PCV13 and non-PCV13/non-PPV23 serotypes compared with PCV13 serotypes, 33.2% (30.3–35.9), 39.5% (34.9–43.8), and 22.5% (18.8–26.1), respectively, while the attribution of CMC was similar. This IC attribution was also observed in age-stratified analyses. The specific comorbidities that attributed to most IPD cases were lung diseases (15.9%; 13.8–17.9), immunodeficiency (11.3%; 9.3–13.2), and heart diseases (8.5%; 5.7–11.2; Supplementary Table 3A–3C).
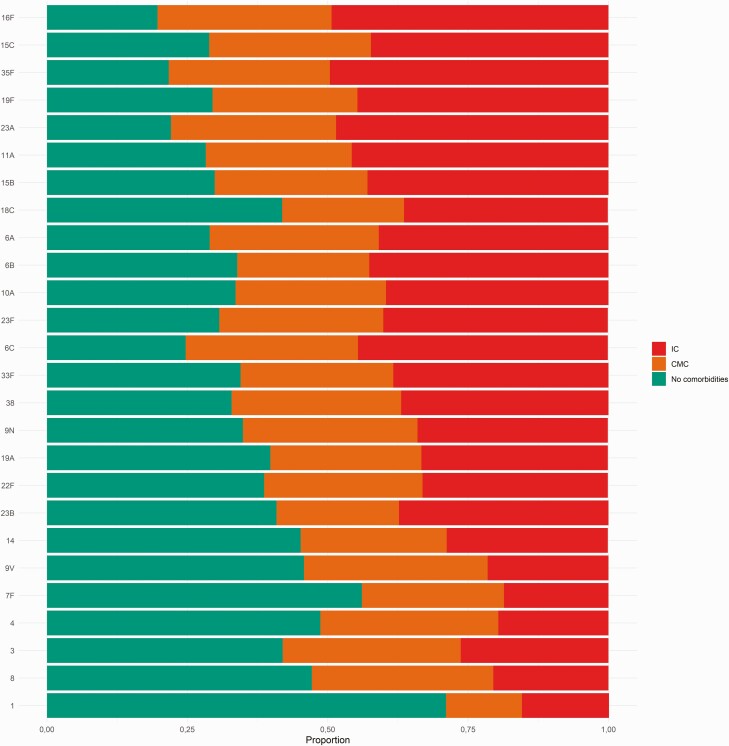
The IPD risk by specific serotypes varied for individuals with CMC and IC, with ORs ranging from 1 to 5 for CMC and from 2 to 14 for IC (Figure 1, Table 2). The serotypes that were associated with the highest risk in descending order were serotypes 16F, 15C, 35F, 19F, and 23A (ORs of 3–5 for CMC and >10 for IC). Non-PCV13 serotypes 9N, 11A, and 22F, which expanded after PCV13 introduction [15], were associated with ORs of 2–3 for individuals with CMC and 4–9 for IC.
Figure 1.
Proportion of ICs and CMCs in cases with invasive pneumococcal disease by serotype. Abbreviations: CMC, chronic medical condition; IC, immunocompromising condition.
Table 2.
Association Between Serotype and Comorbidity Group and Serotype and 30-Day Mortality for Serotypes With >100 Invasive Pneumococcal Disease Cases on Average From All Imputed Datasets (N) in the Study Period: 2006–2015
| Serotype | N | 2006–2015 | 2006–2007 | 2014–2015 | |||
|---|---|---|---|---|---|---|---|
| OR of Chronic Medical Condition vs No Comorbidities (95% CI)a | OR of Being Immunocompromised vs No Comorbidities (95% CI)a | 30-Day Mortality, N (%) | OR of 30-Day Mortality (95% CI)b | Incidence Rate (95% CI) | Incidence Rate (95% CI) | ||
| 16Fc | 147 | 4.50 (2.62–7.73) | 13.07 (7.68–22.26) | 29 (19.7) | 1.97 (1.21–3.20) | 0.09 (0.06–0.15) | 0.22 (0.16–0.30) |
| 15Cc | 144 | 4.67 (2.77–7.87) | 11.82 (6.92–20.20) | 20 (13.9) | 1.58 (0.91–2.74) | 0.09 (0.05–0.16) | 0.16 (0.11–0.22) |
| 35Fc | 272 | 3.74 (2.52–5.55) | 10.94 (7.48–16.02) | 37 (13.7) | 1.27 (0.82–1.97) | 0.16 (0.10–0.24) | 0.33 (0.25–0.42) |
| 19Fd | 302 | 3.78 (2.63–5.42) | 10.76 (7.58–15.29) | 58 (19.2) | 2.29 (1.57–3.34) | 0.52 (0.42–0.64) | 0.12 (0.08–0.19) |
| 23Ac | 305 | 4.30 (2.94–6.27) | 10.75 (7.46–15.50) | 55 (18.0) | 1.70 (1.16–2.50) | 0.17 (0.12–0.25) | 0.44 (0.36–0.55) |
| 11Ae | 386 | 2.89 (2.09–3.99) | 9.08 (6.66–12.39) | 94 (24.4) | 2.93 (2.08–4.13) | 0.28 (0.21–0.38) | 0.37 (0.30–0.47) |
| 15Be | 145 | 2.57 (1.54–4.28) | 7.56 (4.68–12.19) | 28 (19.3) | 2.01 (1.22–3.30) | 0.05 (0.02–0.10) | 0.17 (0.12–0.24) |
| 18Cd | 314 | 2.40 (1.68–3.41) | 7.08 (5.03–9.98) | 36 (11.5) | 1.34 (0.86–2.07) | 0.52 (0.42–0.65) | 0.08 (0.04–0.13) |
| 6Bd | 517 | 2.06 (1.57–2.70) | 6.71 (5.16–8.73) | 81 (15.7) | 1.52 (1.08–2.14) | 0.93 (0.79–1.09) | 0.17 (0.12–0.25) |
| 6Ad | 484 | 2.87 (2.20–3.74) | 6.69 (5.18–8.65) | 61 (12.6) | 1.09 (0.76–1.56) | 0.73 (0.61–0.88) | 0.13 (0.09–0.20) |
| 10Ae | 210 | 2.44 (1.63–3.64) | 6.51 (4.37–9.69) | 29 (13.8) | 1.52 (0.94–2.45) | 0.09 (0.05–0.15) | 0.41 (0.33–0.51) |
| 23Fd | 576 | 2.71 (2.11–3.47) | 6.32 (4.92–8.13) | 69 (12.0) | 1.06 (0.75–1.50) | 1.05 (0.90–1.22) | 0.10 (0.06–0.17) |
| 6Cc | 327 | 2.70 (1.94–3.76) | 6.25 (4.54–8.59) | 55 (16.8) | 1.44 (0.98–2.14) | 0.03 (0.01–0.10) | 0.65 (0.55–0.78) |
| 33Fe | 409 | 2.80 (2.09–3.75) | 6.15 (4.64–8.15) | 38 (9.3) | 0.93 (0.61–1.43) | 0.17 (0.11–0.27) | 0.58 (0.48–0.70) |
| 38c | 123 | 2.88 (1.66–5.00) | 5.64 (3.27–9.72) | 18 (14.6) | 1.41 (0.79–2.51) | 0.10 (0.06–0.16) | 0.09 (0.06–0.15) |
| 9Ne | 499 | 2.79 (2.17–3.59) | 5.30 (4.09–6.88) | 67 (13.4) | 1.45 (1.01–2.07) | 0.42 (0.33–0.55) | 0.71 (0.60–0.84) |
| 19Ad | 791 | 2.06 (1.69–2.52) | 4.70 (3.84–5.76) | 100 (12.6) | 1.44 (1.04–1.99) | 0.38 (0.29–0.49) | 1.02 (0.89–1.18) |
| 22Fe | 1214 | 2.29 (1.95–2.68) | 4.65 (3.95–5.49) | 120 (9.9) | 1.07 (0.79–1.45) | 0.51 (0.39–0.67) | 1.46 (1.29–1.64) |
| 23Bc | 164 | 1.63 (1.02–2.60) | 4.19 (2.69–6.51) | 17 (10.4) | 1.20 (0.67–2.15) | 0.02 (0.00–0.12) | 0.52 (0.43–0.64) |
| 14d | 1026 | 1.95 (1.64–2.33) | 3.79 (3.17–4.53) | 98 (9.6) | 1 (Ref.) | 2.04 (1.83–2.28) | 0.15 (0.10–0.23) |
| 9Vd | 854 | 2.90 (2.42–3.49) | 3.67 (2.96–4.54) | 65 (7.6) | 0.92 (0.65–1.31) | 1.68 (1.48–1.91) | 0.15 (0.10–0.22) |
| 7Fd | 1240 | 2.22 (1.89–2.60) | 3.32 (2.76–3.98) | 63 (5.1) | 0.72 (0.50–1.03) | 1.62 (1.42–1.85) | 0.52 (0.42–0.63) |
| 4d | 825 | 2.78 (2.30–3.36) | 3.24 (2.60–4.05) | 67 (8.1) | 1.05 (0.73–1.50) | 1.32 (1.14–1.51) | 0.14 (0.09–0.21) |
| 3d | 1466 | 2.39 (2.07–2.76) | 3.24 (2.77–3.80) | 222 (15.1) | 1.80 (1.36–2.37) | 1.15 (0.99–1.34) | 1.71 (1.53–1.91) |
| 8e | 413 | 3.00 (2.29–3.93) | 3.06 (2.25–4.17) | 29 (7.0) | 0.90 (0.57–1.43) | 0.45 (0.36–0.57) | 0.59 (0.49–0.71) |
| 1d | 224 | 1.06 (0.67–1.67) | 2.06 (1.31–3.24) | 10 (4.5) | 0.77 (0.37–1.60) | 0.20 (0.14–0.29) | 0.08 (0.05–0.13) |
| PCV13 serotypes | 8627 | 2.36 (2.23–2.50) | 4.31 (4.04–4.59) | 930 (10.8) | 1 (Ref.) | 12.16(11.63–12.70) | 4.38 (4.09–4.69) |
| Non-PCV13 serotypes | 5469 | 2.78 (2.57–3.01) | 6.26 (5.78–6.78) | 741 (13.5) | 1.13 (1.01–1.27) | 3.23 (2.93–3.56) | 7.92 (7.53–8.33) |
| PPV23–non-PCV13 serotypes | 3467 | 2.60 (2.37–2.87) | 5.38 (4.88–5.94) | 425 (12.3) | 1.08 (0.95–1.23) | 2.20 (1.96–2.46) | 4.59 (4.29–4.91) |
| Non-PCV13/non-PPV23 serotypes | 2003 | 3.22 (2.80–3.69) | 8.20 (7.18–9.38) | 316 (15.8) | 1.22 (1.05–1.42) | 1.03 (0.86–1.23) | 3.33 (3.08–3.60) |
| All serotypes | 14096 | 2.50 (2.39–2.62) | 5.01 (4.78–5.25) | 1671 (11.9) | … | 15.4 (14.8–16–0) | 12.3 (11.8–12.8) |
Incidence rates (invasive pneumococcal disease cases/100000 population) presented for pre- and post-vaccine period. No significant interactions of period and comorbidity group per serotype, after adjustment for multiple comparison.
Abbreviations: CI, confidence interval; OR, odds ratio.
ORs calculated using conditional logistic regression.
ORs calculated using logistic regression adjusted for age group, sex, year, and comorbidity group. Serotype 14 used as reference.
Non-PCV13/non-PPV23 serotypes.
PCV13 serotypes.
PPV23–non-PCV13 serotypes.
Changes in IPD Incidence Post-Vaccine Introduction According to Presence of Underlying Diseases
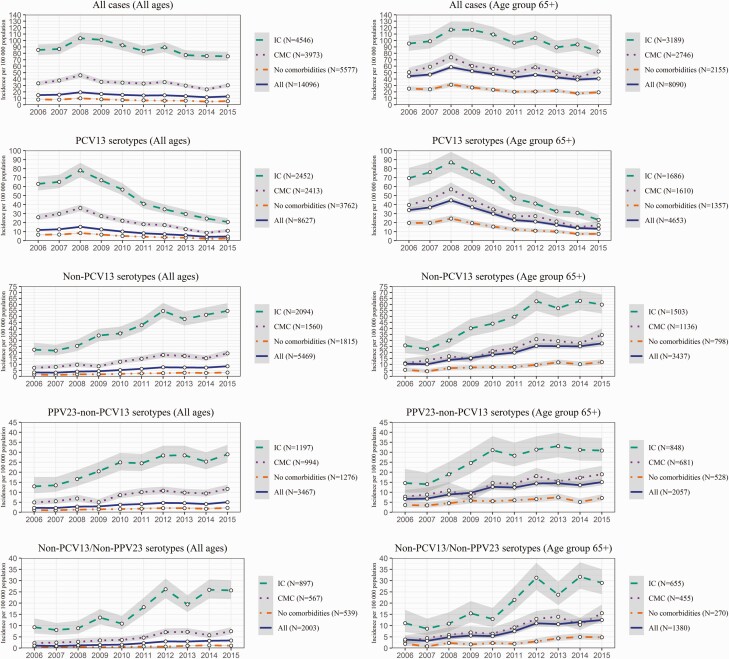
When years 2006–2007 were compared with years 2014–2015, the IPD incidence per 100000 decreased in individuals with IC from 86.1 to 75.6 (RR, 0.88; 0.80,0.97 and RD, –10.5; –18.2,–2.7), in persons with CMC from 35.5 to 27.1 (RR, 0.76; 0.69,0.85 and RD, –8.4; –11.6,–5.2), and in those without comorbidities from 8.1 to 5.3 (RR, 0.65; 0.60,0.71 and RD, –2.8; –3.4,–2.2; Figure 2, Supplementary Figure 1). In young children with comorbidities, the incidence decreased from 34.2 to 7.3 (RR, 0.21; 0.10,0.46 and RD, –26.9; –39.6,–14.2), and in children without comorbidities from 11.4 to 3.2 (RR, 0.28; 0.19,0.41 and RD, –8.2; –10.6,–5.8; Supplementary Figure 2). In the elderly, the incidence reduction was modest: from 97.1 to 88.3 (RR, 0.91 [95% CI 0.81 to 1.02] and RD, –8.8 [95% CI –19.4 to 1.9]) in individuals with IC, and from 55.0 to 47.2 (RR, 0.86 [95% CI 0.76 to 0.97] and RD, –7.8 [95% CI –14.1 to –1.4] in individuals with CMC. (Figure 2)
Figure 2.
Incidence per 100000 population per year for all serotypes and by serotype group, presented separately for individuals without comorbidities and for those with IC and CMC. Left column shows results for all ages, right column for the those aged ≥65 years. The gray areas indicate 95% confidence intervals. Abbreviations: CMC, chronic medical condition; IC, immunocompromising condition; PCV, pneumococcal conjugated vaccine.
PCV13 serotypes decreased in individuals aged 5–64 years and ≥65 years with CMC and IC and rarely caused IPD in children in 2015 (Supplementary Figure 2, Figure 2).The absolute increases of IPD caused by non-PCV13, PPV23–non-PCV13, and non-PCV13/non-PPV23 serotypes was higher in individuals with IC: RD, 31.1 (95% CI 25.7 to 36.5), 14.0 (95% CI 10.0 to 18.0), and 17.1 (95% CI 13.5 to 20.8), respectively, and with CMC: RD, 9.6 (95% CI 7.5 to 11.7), 5.3 (95% CI 3.6 to 7.0), and 4.3 (95% CI 3.0 to 5.5), respectively, compared with those without comorbidities: RD, 1.7 (95% CI 1.3 to 2.1), 0.90 (95% CI 0.61 to 1.19), and 0.79 (95% CI 0.58 to 1.01), respectively (Figure 2).
The incidence of PCV13 serotypes 3 and 19A increased post-vaccination from 1.2 to 1.7 and from 0.4 to 1.0 per 100000, respectively. The absolute increase was more pronounced in individuals with CMC (from 2.9 to 4.4 and from 0.6 to 2.1, respectively) or IC (from 6.2 to 7.9 and from 2.2 to 5.8, respectively) compared with individuals without comorbidities (from 0.6 to 0.8 and 0.2 to 0.5, respectively), although the risk ratios were similar (Supplementary Table 4, Supplementary Figure 3). Despite some incidence fluctuations during the study period, the trend was similar for non-PCV13 serotypes 6C, 9N, 11A, 22F, 23A, and 35F, with the most pronounced increase for 22F in individuals with IC (from 3.0 to 7.9). Furthermore, we observed an increase in the incidence post-vaccination of several non-PCV13/non-PPV23 serotypes that were associated with high risk for IPD in individuals with comorbidities, such as serotypes 16F, 15C, 35F, and 23B (Table 2). Yet, the relative changes in the serotype-specific incidences between 2006–2007 and 2014–2015 among individuals with or without comorbidities were not significantly different (all P values > .05).
Increased Mortality Observed in Individuals With Underlying Diseases Infected With Nonvaccine Types Post-Vaccine Introduction
The overall mortality incidence due to IPD was largely unchanged between 2006 and 2015 (range, 1.4–2.1 per 100000; Supplementary Figure 4). In individuals with IC, there was an increase during 2006–2010, from 12.2 to 18.4 per 100000, thereafter, the mortality decreased to 10.4 in 2015. The mortality due to PCV13 serotypes decreased from 10.1 in 2006–2007 to 3.9 per 100000 in 2014–2015 in individuals with IC (RR, 0.38; [95% CI 0.27 to 0.55] and RD, –6.2; [95% CI –8.6 to –3.9]), and from 0.4 to 0.2 in individuals without comorbidities (RR, 0.38; [95% CI 0.24 to 0.59] and RD, –0.3; [95% CI –0.4 to –0.2]). In contrast, mortality due to non-PCV13 serotypes increased from 3.0 to 7.7 in individuals with IC (RR, 2.56; [95% CI 1.63 to 4.00] and RD, 4.7; [95% CI 2.7 to 6.7]), and from 1.1 to 2.1 in individuals with CMC (RR, 1.94; [95% CI 1.18 to 3.20] and RD, 1.0; [95% CI 0.3 to 1.8]), while the increase was less pronounced for individuals without underlying diseases, from 0.2 to 0.3 (RR, 1.64; [95% CI 0.98 to 2.76] and RD, 0.1; [95% CI –0.003 to 0.2]). The increased mortality was due to both PPV23–non-PCV13 and non-PCV13/non-PPV23 serotypes. After adjustment for age, sex, comorbidity, and year, serotypes associated with the highest mortality were 11A followed by 19F, 15B, 16F, 3, 23A, 15C, 10A, 6B, 9N, 19A, and 6C, that is, many nonvaccine types that increased post-vaccine introduction (Table 2).
Reduction of PCV13 Serotypes and Expansion of Nonvaccine Serotypes in the Elderly Have Implications for Vaccination in Adults
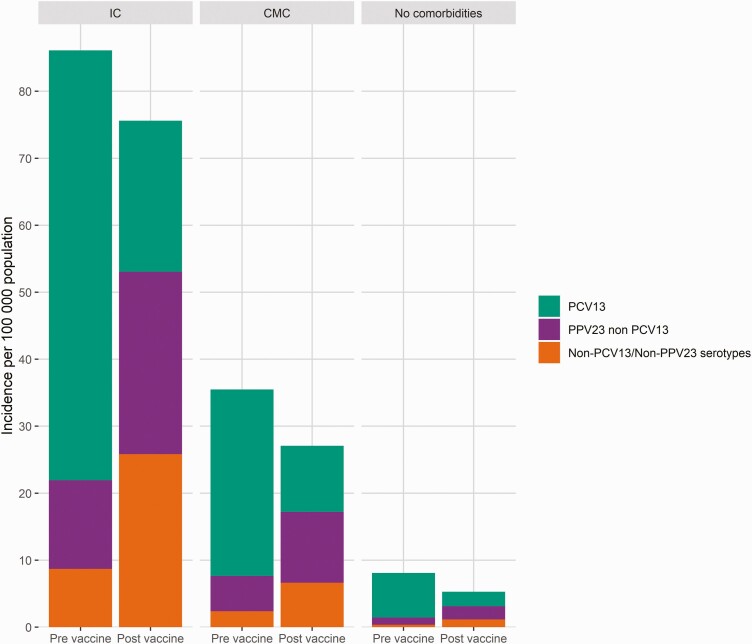
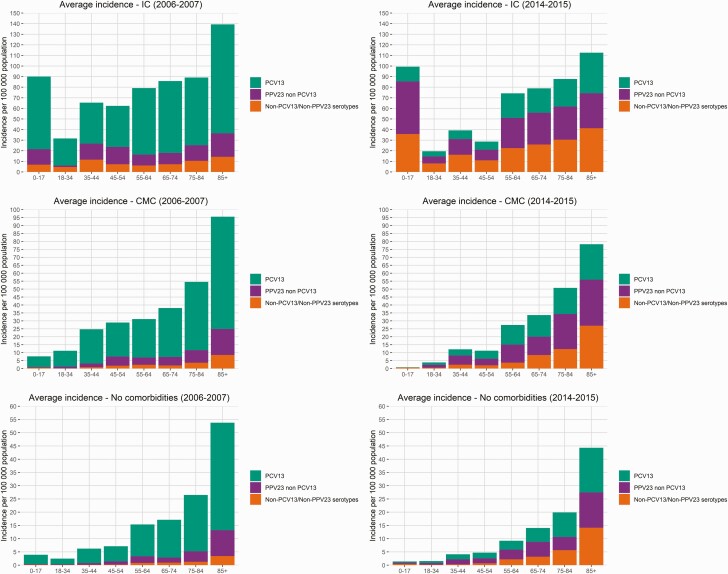
In analyses of finely stratified age groups, the PCV13 serotype incidence decreased regardless of age or underlying diseases post-vaccine introduction, and PPV23–non-PCV13 and non-PCV13/non-PPV23 serotypes increased (Figures 3 and 4). The incidence of non-PCV13/non-PPV23 serotypes was highest in individuals with IC post-vaccination, especially in children and those aged >55 years. Among individuals with CMC or IC, there were substantial IPD decreases in those aged 18–54 years post-vaccination. Among individuals without comorbidities, the IPD incidence was ≥17 per 100000 in persons aged ≥65 years in 2006–2007, while in 2014–2015 an equivalent incidence was observed in those aged ≥75 years.
Figure 3.
Stacked invasive pneumococcal disease incidence of vaccine and nonvaccine serotypes in individuals without comorbidities and in those with IC and CMC in the pre-vaccine (2006–2007) and post-vaccine (2014–2015) period for all cases. Abbreviations: CMC, chronic medical condition; IC, immunocompromising condition; PCV, pneumococcal conjugated vaccine.
Figure 4.
Stacked invasive pneumococcal disease incidence of vaccine and nonvaccine serotypes in individuals without comorbidities and in those with IC and CMC in the pre-vaccine (2006–2007) and post-vaccine (2014–2015) period in different age groups. Abbreviations: CMC, chronic medical condition; IC, immunocompromising condition; PCV, pneumococcal conjugated vaccine.
DISCUSSION
The results from this nationwide, population-based register study are important to guide pneumococcal vaccination strategies. Previous studies have shown that certain serotypes act as opportunistic pathogens, preferentially infecting individuals with comorbidities [17]. Correlations between specific serotypes and comorbidities have been confined to case series of IPD, and hence the risk of serotype-specific IPD in individuals with CMC or IC has not been assessed [17, 18]. In our study, individuals with CMC or IC showed a higher relative risk of IPD caused by non-PCV13 serotypes, especially non-PCV13/non-PPV23 serotypes, compared with PCV13 serotypes. Thus, the PAP of IC was 9.7% higher for non-PCV13 serotypes and 17.0% higher for non-PCV13/non-PPV23 serotypes compared with PCV13 serotypes post-vaccination. Serotypes 16F, 15C, 19F, 35F, 23A, and 11A, of which 4 are not included in PCV13 or PPV23, conferred the highest risk among individuals with IC and CMC. All of these serotypes, except the vaccine type 19F, increased post-vaccination, and we observed a higher absolute increase in the PPV23–non-PCV13 serotypes 9N,11A, and 22F and of serotypes 3, 19A, 6C, 3A, and 35F in those with underlying diseases. It is worrisome that many of the serotypes that increased post-vaccination preferentially infect persons with underlying diseases and were associated with high mortality. This suggests that several serotypes currently not included in licensed pneumococcal vaccines should be targeted in future vaccine development for individuals with immunocompromising conditions.
The absolute increase, which is an important public health measure of IPD, caused by non-PCV13, PPV23–non-PCV13, and non-PCV13/non-PPV23 serotypes post-vaccination was higher in individuals with IC or CMC compared with those without underlying diseases. Concomitantly, there was an increased mortality due to non-PCV13 serotypes in individuals with IC and CMC that was not observed in those without comorbidities. The IPD incidence caused by PCV13 serotypes post-vaccination was substantially reduced compared with pre-vaccination, regardless of age group or presence of comorbidities. The reduction in PCV13 serotypes and concomitant increase in PPV23–non-PCV13 serotypes favors vaccinations that include PPV23 for individuals with CMC and IC, although the protective effect of PPV23 in individuals with IC is debated [6-8]. Interestingly, the IPD incidence caused by serotypes included in PPV23 was similar for persons aged 75–84 years in 2014–2015 as for those aged 65–74 years in 2006–2007. This suggests that vaccinations that target the healthy elderly could be started at an older age than currently recommended.
A study from the United States observed a decreased incidence of PCV13 serotypes in individuals with CMC and IC regardless of age following PCV13 introduction in children [9]. However, they did not observe a consistent increase in nonvaccine serotypes. A case-cohort study from Norway reported a decrease of PCV13 serotypes and an increase in nonvaccine serotypes in individuals with and without immunosuppressive therapy [20]. However, individual serotypes were not assessed, and they did not account for calendar time in the selection of controls. We observed that many underlying diseases increased from pre- to post-vaccination among the controls [28], thus this should be taken into account. A Danish study included serotype data for a long pre-vaccine period, which enabled modeling of epidemic cycles of serotype-specific IPD trends [19]. However, due to limited sample size, all comorbidities were grouped together, which prevented analyses of differential effects according to IC and CMC.
Our study has several strengths. The sample size was large using national Swedish registers and the controls were sampled from the same population as the cases. Comorbidities were measured in an identical way for cases and controls, and by using incidence density sampling, we could account for calendar time in a similar way for cases and controls. Limitations are potential misclassification of underlying diseases since we relied on ICD-10 and Anatomical Therapeutic Chemical codes from Swedish national registers, although these registers have high validity [23]. Due to a limited pre-vaccine period, we did not perform interrupted time-series analyses, and hence we cannot rule out that some of the observed findings are due to underlying trends in serotype incidences [19]. Yet, this is unlikely to affect analyses where serotypes are grouped together. Since we did not assess counties using PCV10 and PCV13 separately, the results of serotypes 3 and 19A need to be interpreted cautiously [15]. Finally, we did not have individual data on vaccination status. However, the uptake in Sweden has been low for PPV23 (at most, around 30% among persons aged ≥65 years during a vaccination campaign in 1998-2000 in Region Stockholm, whereafter the coverage declined) and did not substantially change during the study period. Also, PCV13 was not recommended nationally for adult risk groups until 2016, a reason for ending the study period in 2015 [22]. Therefore, it is likely that the observed changes in incidence are due to indirect effects of the child immunization program. Yet, it is possible that PPV23 and PCV13 vaccination among persons with IC explains some of the observed shifts toward infections and mortality due to nonvaccine serotypes.
In conclusion, our data show that the indirect effects of PCV introduction have resulted in a shift from PCV13 to nonvaccine serotypes, causing IPD among individuals with CMC and IC, where the absolute increase in nonvaccine serotypes is substantial, especially in individuals with IC compared with healthy people. Infections caused by these nonvaccine serotypes are associated with high mortality. Identification of these specific serotypes will help in finding optimal vaccine strategies for the elderly and for those with underlying diseases. This is of particular importance due to changes in health status and underlying disease severity in aging populations [29].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding sources had no input on the design of the study, collection of data, analyses, or manuscript writing.
Financial support. The work was supported by grants from the Swedish Foundation for Strategic Research, the Swedish Research Council, the Stockholm County Council, and the Knut and Alice Wallenberg Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018; 18:1191-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klemets P, Lyytikäinen O, Ruutu P, Ollgren J, Nuorti JP.. Invasive pneumococcal infections among persons with and without underlying medical conditions: implications for prevention strategies. BMC Infect Dis 2008; 8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kyaw MH, Rose CE Jr, Fry AM, et al. ; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377-86. [DOI] [PubMed] [Google Scholar]
- 4. van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect 2012; 65:17-24. [DOI] [PubMed] [Google Scholar]
- 5. Shigayeva A, Rudnick W, Green K, et al. ; Toronto Invasive Bacterial Diseases Network. Invasive pneumococcal disease among immunocompromised persons: implications for vaccination programs. Clin Infect Dis 2016; 62:139-47. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453-60. [DOI] [PubMed] [Google Scholar]
- 7. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR.. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA 1993; 270:1826-31. [PubMed] [Google Scholar]
- 8. Djennad A, Ramsay ME, Pebody R, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine 2018; 6:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed SS, Pondo T, Xing W, et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions—United States. Clin Infect Dis 2020; 70:2484-92. [DOI] [PubMed] [Google Scholar]
- 10. Savulescu C, Krizova P, Lepoutre A, et al. ; SpIDnet Group. Effect of high-valency pneumococcal conjugate vaccines on invasive pneumococcal disease in children in SpIDnet countries: an observational multicentre study. Lancet Respir Med 2017; 5:648-56. [DOI] [PubMed] [Google Scholar]
- 11. Hanquet G, Krizova P, Valentiner-Branth P, et al. ; SpIDnet/I-MOVE+ Pneumo Group. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax 2019; 74:473-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galanis I, Lindstrand A, Darenberg J, et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J 2016; 47:1208-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lindstrand A, Galanis I, Darenberg J, et al. Unaltered pneumococcal carriage prevalence due to expansion of non-vaccine types of low invasive potential 8 years after vaccine introduction in Stockholm, Sweden. Vaccine 2016; 34:4565-71. [DOI] [PubMed] [Google Scholar]
- 14. Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naucler P, Galanis I, Morfeldt E, Darenberg J, Örtqvist Å, Henriques-Normark B.. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis 2017; 65:1780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meyer AC, Drefahl S, Ahlbom A, Lambe M, Modig K.. Trends in life expectancy: did the gap between the healthy and the ill widen or close? BMC Med 2020; 18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sjöström K, Spindler C, Ortqvist A, et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis 2006; 42:451-9. [DOI] [PubMed] [Google Scholar]
- 18. Luján M, Burgos J, Gallego M, et al. Effects of immunocompromise and comorbidities on pneumococcal serotypes causing invasive respiratory infection in adults: implications for vaccine strategies. Clin Infect Dis 2013; 57:1722-30. [DOI] [PubMed] [Google Scholar]
- 19. Weinberger DM, Warren JL, Dalby T, et al. Differences in the impact of pneumococcal serotype replacement in individuals with and without underlying medical conditions. Clin Infect Dis 2019; 69:100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steens A, Winje BA, White RA, Odsbu I, Brantsæter AB, Vestrheim DF.. Indirect effects of pneumococcal childhood vaccination in individuals treated with immunosuppressive drugs in ambulatory care: a case-cohort study. Clin Infect Dis 2019; 68:1367-73. [DOI] [PubMed] [Google Scholar]
- 21. Cabaj JL, Nettel-Aguirre A, MacDonald J, Vanderkooi OG, Kellner JD.. Influence of childhood pneumococcal conjugate vaccines on invasive pneumococcal disease in adults with underlying comorbidities in Calgary, Alberta (2000-2013). Clin Infect Dis 2016; 62:1521-6. [DOI] [PubMed] [Google Scholar]
- 22. Naucler P, Henriques-Normark B, Hedlund J, Galanis I, Granath F, Örtqvist Å.. The changing epidemiology of community-acquired pneumonia: nationwide register-based study in Sweden. J Intern Med 2019; 286:689-701. [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2012; 61:816-9. [PubMed] [Google Scholar]
- 25. Henriqus Normark B, Christensson B, Sandgren A, et al. Clonal analysis of Streptococcus pneumoniae nonsusceptible to penicillin at day-care centers with index cases, in a region with low incidence of resistance: emergence of an invasive type 35B clone among carriers. Microb Drug Resist 2003; 9:337-44. [DOI] [PubMed] [Google Scholar]
- 26. Rothman KJ, Sander G, Lash TL.. Modern epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 27. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27-38. [Google Scholar]
- 28. Andersson T, Ahlbom A, Carlsson S.. Diabetes prevalence in Sweden at present and projections for year 2050. PloS One 2015; 10:e0143084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL.. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019; 4:e159-e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.