Abstract
Background
Little is known about the relative harms of different antibiotic regimens prescribed to treat uncomplicated urinary tract infection (UTI). We sought to compare the risk of adverse events associated with commonly used oral antibiotic regimens for the outpatient treatment of uncomplicated UTI.
Methods
Using data from the IBM® MarketScan® Commercial Database, we identified 1 169 033 otherwise healthy, nonpregnant women aged 18–44 years with uncomplicated UTI who initiated an oral antibiotic with activity against common uropathogens from 1 July 2006 to 30 September 2015. We used propensity score–weighted Kaplan-Meier methods and Cox proportional hazards regression models to estimate the association between antibiotic agent and adverse events.
Results
Of 2 first-line agents, trimethoprim-sulfamethoxazole (vs nitrofurantoin) was associated with higher risk of several adverse drug events including hypersensitivity reaction (hazard ratio, 2.62; 95% confidence interval, 2.30–2.98), acute renal failure (2.56; 1.55–4.25), skin rash (2.42; 2.13–2.75), urticaria (1.37; 1.19–1.57), abdominal pain (1.14; 1.09–1.19), and nausea/vomiting (1.18; 1.10–1.28), but a similar risk of potential microbiome-related adverse events. Compared with nitrofurantoin, non–first-line agents were associated with higher risk of several adverse drug events and potential microbiome-related adverse events including non–Clostridium difficile diarrhea, C. difficile infection, vaginitis/vulvovaginal candidiasis, and pneumonia. Treatment duration modified the risk of potential microbiome-related adverse events.
Conclusions
The risks of adverse drug events and potential microbiome-related events differ widely by antibiotic agent and duration. These findings underscore the utility of using real-world data to fill evidentiary gaps related to antibiotic safety.
Keywords: administrative data, antibiotics, comparative safety, adverse events, urinary tract infection
In this cohort study of commercially insured women with uncomplicated urinary tract infection, risks of adverse drug events and potential microbiome-related adverse events were generally lower for nitrofurantoin than for other agents. Treatment duration modified the risk of potential microbiome-related adverse events.
The majority of antibiotics are prescribed in the outpatient setting [1, 2], and uncomplicated urinary tract infection (UTI) is among the most common indications for antibiotics [3, 4]. Clinical practice guidelines for the treatment of uncomplicated UTI in women recommend empirical antibiotic therapy [3]. Nitrofurantoin and trimethoprim-sulfamethoxazole (TMP/SMX) are recommended as first-line agents; fluoroquinolones and β-lactams are non–first-line agents [3]. These recommendations are based on efficacy in randomized clinical trials (RCTs), rates of in vitro resistance among urinary pathogens, ecological adverse effects (eg, the selection of drug-resistant organisms and colonization or infection with multidrug-resistant organisms), and adverse effects [3]. The first-line agents are advantageous because of high efficacy and low propensity for ecological adverse effects. Additionally, for nitrofurantoin, resistance among uropathogens is uncommon [5–7]. TMP/SMX has long been considered a “workhorse” antibiotic for UTI therapy; however, resistance rates to TMP/SMX may be rising, and tolerability remains problematic. Fluoroquinolones are highly efficacious but have a high propensity for uropathogen resistance; guidelines suggest reserving them for important uses other than uncomplicated UTI [3, 8]. β-Lactam agents, particularly amoxicillin and ampicillin (AMX/AMP), have lower efficacy and a higher prevalence of antibiotic resistance versus other UTI antibiotics [3]. However, the evidence base remains limited regarding the relative benefits and harms of various antibiotic regimens for UTI therapy; consequently, clinical equipoise exists regarding selection of agent, as demonstrated by wide variation in prescribing practices [9–12].
Estimates on the comparative safety of antibiotic agents to treat uncomplicated UTI remain limited, despite the importance for informing guideline development and antibiotic prescribing. Existing evidence is predominantly from RCTs, which are limited by small sample size, short follow-up, heterogeneous study populations, and wide variation in duration of antibiotic prescriptions. Additionally, RCTs only compare antibiotic agents in limited combinations (eg, ciprofloxacin vs amoxicillin-clavulanate) [3]. Meta-analyses of RCTs demonstrate increased risk of skin rash (TMP/SMX vs nitrofurantoin; β-lactams vs fluoroquinolones) and a similar risk of diarrhea (fluoroquinolones vs TMP/SMX); however, estimates are based on small numbers (N ≤16 cases total per comparison) and are unavailable for many adverse event types [13]. Meta-analyses demonstrate similar agent-specific rates of any adverse events (composite outcome) [13–15], but analyses lack granular information on type/severity of adverse events. Observational studies report conflicting results regarding the association between adverse events and antibiotic agents; however, many studies may be susceptible to confounding by indication because they compare antibiotic users with nonusers [16], fail to appropriately account for the role of infection type on the risk of adverse events, or are not restricted to treatment of UTI.
Using data from a large administrative claims database, we compared the risk of several adverse events associated with commonly used antibiotic agents for outpatient treatment of uncomplicated UTI among young women in the United States. We classified adverse events into 2 categories: (1) adverse drug events (ie, specific to drugs but not specific to antibiotics) and (2) potential microbiome-related adverse events (ie, specific to antibiotics due to the pathophysiology of antibiotic-induced microbiome disruption [17–19]). For microbiome-disruption–related adverse event outcomes, we evaluated the association between each outcome and treatment duration, stratified by antibiotic agent.
METHODS
Data Source
We used the IBM® MarketScan® Commercial Database (2006–2015), which contains standardized, de-identified, person-level information on enrollment and adjudicated insurance claims for inpatient and outpatient procedures, as well as pharmacy-dispensed medications, for individuals residing in all 50 US states and the District of Columbia with primarily employer-sponsored commercial insurance and their spouses and dependents [20, 21]. The Institutional Review Board at Washington University School of Medicine deemed this study exempt from human subject review.
Study Design and Population
We identified women aged 18–44 years who received a new outpatient diagnosis of uncomplicated UTI (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 595.0, 595.9, 599.0) with an accompanying antibiotic prescription between 1 July 2006 and 30 September 2015, as previously described [22]. The index date was the date of a filled oral prescription for an antibiotic with activity against common uropathogens, occurring on the day of or day after diagnosis of uncomplicated UTI. We required continuous enrollment in a payer plan during the 180-day baseline period before the index date. To restrict to healthy women with minimal antibiotic and healthcare exposures, we excluded patients hospitalized within 90 days before the index date. To restrict to patients who met the definition of uncomplicated UTI, in accordance with Infectious Diseases Society of America (IDSA) guideline criteria [3], we excluded patients who received diagnoses or prescription medications during the 180-day baseline period for UTI, pregnancy, urinary comorbidities or abnormalities, pyelonephritis, diabetes, systemic autoimmune conditions, spinal cord injuries, or hematologic or solid-organ malignancies (Supplementary Tables 1 and 2). We excluded patients who filled an antibiotic prescription or had non-UTI bacterial or viral infections in the 30 days before the index date (Supplementary Table 3).
Antibiotic Exposure Assessment
We collected information on the index antibiotic prescription, including agent, route, days’ supply, and dose. We categorized antibiotics in accordance with IDSA guidelines: nitrofurantoin, TMP/SMX, fluoroquinolones, broad-spectrum β-lactam/β-lactamase inhibitor combinations (henceforth referred to as broad-spectrum β-lactams), narrow-spectrum β-lactams, and AMX/AMP (Supplementary Table 4) [3]. We further categorized antibiotics as first-line agents (nitrofurantoin, TMP/SMX), non–first-line agents (fluoroquinolones, broad-spectrum β-lactams, narrow-spectrum β-lactams), and nonrecommended agents (AMX/AMP). We required antibiotic prescription days’ supply to be 10 days or fewer. We excluded patients with antibiotic prescriptions characterized by unusual doses, as determined by an infectious diseases physician.
Safety Outcome Definitions
We used ICD-9-CM and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM)/Procedure Coding System codes to identify individual safety outcomes including skin rash, urticaria, Stevens-Johnson syndrome; anaphylaxis, angioedema/laryngeal edema, hypersensitivity reaction; abdominal pain, nausea/vomiting, non–Clostridium difficile diarrhea, C. difficile infection; vaginitis/vulvovaginal candidiasis; aortic aneurysm/dissection; acute renal failure; tendinopathy including tendon rupture; and pneumonia (Supplementary Table 5). We further categorized outcomes as adverse drug events or potential microbiome-related adverse events. To ensure identification of new-onset outcomes, we excluded individuals diagnosed with the outcome of interest within the 180-day baseline period. Outcome-specific follow-up periods ranged from 3 to 90 days, depending on anticipated onset after antibiotic initiation [23–25].
Covariates
Potential confounders of the association between antibiotic agent and safety outcomes during the 180-day baseline period before the index antibiotic prescription were identified a priori using subject matter expertise of the research team. Information was collected on age, month/year of prescription, geographic region, provider specialty, and comorbid conditions defined using the Elixhauser classification [26, 27].
Statistical Analysis
To account for possible confounding, we estimated 5 separate propensity scores (PSs), computed as the estimated probability that a patient initiated nitrofurantoin versus each other antibiotic category of interest, conditional on all baseline covariates. We used PSs to estimate standardized mortality ratio (SMR) weights, equal to 1 for individuals in the reference cohort (i.e., nitrofurantoin initiators) and the propensity odds (PS/[1 – PS]) for individuals in other cohorts (eg, TMP/SMX initiators). Standardized mortality ratio weighting allows standardization of the covariate distribution in each comparator cohort to that in the reference cohort (ie, nitrofurantoin) [28, 29]. We plotted standardized mean differences for each baseline covariate in the unweighted and SMR-weighted populations to determine whether weighting reduced imbalances of observed covariates and made treatment groups more exchangeable; standardized mean differences less than 10% in the SMR-weighted population were considered adequate [30]. To examine the relationship between antibiotic agents and safety outcomes, we estimated SMR-weighted cumulative risk functions using Kaplan-Meier methods. We used Cox proportional hazards models to estimate unadjusted and SMR-weighted hazard ratios (HRs) comparing each antibiotic agent category with nitrofurantoin. For all outcomes, we stratified models by age. For microbiome-disruption–related adverse event outcomes, we evaluated the antibiotic agent–specific association between each outcome and appropriate versus inappropriately long treatment duration (appropriate duration defined as 3 days for fluoroquinolones and TMP/SMX, 5 days for nitrofurantoin, and 3–7 days for β-lactams [3]). To account for weighting, we used robust variance estimators to calculate 95% confidence intervals [28]. We verified the proportional hazards assumption with an interaction term between (log) time and treatment. Patients were censored at the end of the outcome-specific follow-up period, end of continuous coverage, subsequent antibiotic prescription for a different agent, hospitalization (due to lack of information on inpatient drug administration), or administratively (31 December 2015).
We conducted sensitivity analyses to examine the robustness of our findings, by excluding patients who (1) received kidney imaging to rule out pyelonephritis at UTI diagnosis (Supplementary Table 6), (2) received antibiotic agents that have indications for both UTI and community-acquired pneumonia, and (3) were diagnosed in the 30-day (rather than 180-day) period before antibiotic initiation.
RESULTS
Study Cohort
We identified a total of 1 169 033 eligible women (Supplementary Figure 1). The distribution of antibiotic agent was as follows: fluoroquinolone (43%), TMP/SMX (28%), nitrofurantoin (24%), narrow-spectrum β-lactam (3%), broad-spectrum β-lactam (1%), and AMX/AMP (1%). Patient characteristics are presented by treatment group in Table 1 and Supplementary Table 7. After weighting, all measured patient characteristics were well balanced between treatment groups (standardized mean differences <0.10) (Supplementary Figure 2).
Table 1.
Selected Baseline Characteristics of Patients With Urinary Tract Infection by Initial Antibiotic Agent
| First-Line Recommended Agents | Non–First-Line Recommended Agents | Nonrecommended Agents | ||||
|---|---|---|---|---|---|---|
| Characteristic | Nitrofurantoin (n = 279 994) | TMP/SMX (n = 329 184) | Fluoroquinolone (n = 508 062) | Broad-Spectrum β-Lactam (n = 12 889) | Narrow-Spectrum β-Lactam (n = 31 178) | AMX/AMP (n = 7726) |
| Age, median (IQR), years | 30 (23–37) | 29 (22–37) | 32 (24–39) | 28 (20–36) | 29 (21–36) | 30 (23–37) |
| Region of residence | ||||||
| Northeast | 14.0 | 13.9 | 13.7 | 14.9 | 8.9 | 19.1 |
| Midwest | 20.9 | 28.9 | 21.7 | 16.1 | 23.3 | 25.0 |
| South | 41.6 | 40.7 | 45.8 | 55.5 | 30.2 | 40.9 |
| West | 23.5 | 16.4 | 18.8 | 13.5 | 37.7 | 15.0 |
| Provider specialty | ||||||
| Emergency medicine | 6.9 | 7.2 | 7.4 | 9.1 | 19.2 | 3.0 |
| Internal medicine | 11.3 | 13.3 | 16.8 | 13.6 | 10.3 | 18.1 |
| Family medicine and pediatrics NEC | 32.2 | 40.0 | 38.2 | 40.9 | 30.3 | 41.5 |
| OBGYN | 14.2 | 4.3 | 4.2 | 4.0 | 5.1 | 5.4 |
| MD/DO/NEC | 6.1 | 7.5 | 6.2 | 5.0 | 7.0 | 6.8 |
| Non-MD | 5.5 | 6.7 | 4.7 | 3.7 | 2.9 | 3.4 |
| Other | 20.6 | 17.8 | 19.4 | 21.7 | 23.0 | 19.4 |
| Unknown | 3.2 | 3.3 | 3.0 | 2.0 | 2.2 | 2.5 |
| Comorbid conditions | ||||||
| COPD | 0.6 | 0.6 | 0.7 | 0.8 | 0.9 | 0.7 |
| Deficiency anemias | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 |
| Depression | 1.6 | 1.6 | 1.6 | 1.6 | 1.5 | 1.3 |
| Drug/alcohol abuse | 0.2 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 |
| Hypertension | 0.9 | 1.0 | 1.2 | 1.2 | 1.1 | 1.2 |
| Obesity | 0.6 | 0.7 | 0.7 | 0.8 | 0.8 | 0.5 |
| Psychoses | 2.3 | 2.1 | 2.3 | 2.5 | 2.3 | 2.0 |
N = 1 169 033. Results are expressed as percentages unless otherwise indicated. Baseline covariates were assessed on the fill date of the index antibiotic prescription. Abbreviations: AMX/AMP, amoxicillin or ampicillin; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; NEC, not elsewhere classified; OBGYN, obstetrics and gynecology; TMP/SMX, trimethoprim-sulfamethoxazole.
Crude Incidence Rates
The number of patients excluded from outcome-specific cohorts for outcomes occurring before antibiotic initiation ranged from 0.0% (acute renal failure) to 12.6% (abdominal pain) (Supplementary Table 8). Agent-specific rates of adverse events varied widely, ranging from 0.00 to 0.02 cases/10 000 person-days for Stevens-Johnson syndrome to 12.94 to 19.59 cases/10 000 person-days for abdominal pain (Table 2).
Table 2.
Comparative Risk of Adverse Events by Antibiotic Agent
| Outcome | Follow-up, days | Antibiotic Agenta | No. of Events | Person-Time, days | Rate per 10 000 Person-Days | Crude HR (95% CI) | Weighted HR (95% CI) |
|---|---|---|---|---|---|---|---|
| Adverse drug events | |||||||
| Dermatologic | |||||||
| Skin rash | 14 | Nitrofurantoin | 335 | 3 605 771 | 0.93 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 919 | 4 166 723 | 2.21 | 2.38 (2.10–2.70) | 2.42 (2.13–2.75) | ||
| Fluoroquinolone | 453 | 6 637 803 | 0.68 | .73 (.64–.85) | .76 (.64–.89) | ||
| Broad-spectrum BL | 25 | 165 296 | 1.51 | 1.63 (1.08–2.45) | 1.70 (1.49–1.95) | ||
| Narrow-spectrum BL | 42 | 400 929 | 1.05 | 1.13 (.82–1.55) | 1.13 (.97–1.31) | ||
| AMX/AMP | 11 | 96 631 | 1.14 | 1.23 (.67–2.24) | .75 (.63–.88) | ||
| Urticaria | 14 | Nitrofurantoin | 353 | 3 625 362 | 0.97 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 598 | 4 191 050 | 1.43 | 1.47 (1.29–1.68) | 1.37 (1.19–1.57) | ||
| Fluoroquinolone | 226 | 6 676 869 | 0.34 | .35 (.29–.41) | .35 (.29–.43) | ||
| Broad-spectrum BL | 15 | 166 103 | 0.90 | .93 (.55–1.56) | .76 (.65–.89) | ||
| Narrow-spectrum BL | 18 | 403 783 | 0.45 | .46 (.29–.73) | .42 (.34–.50) | ||
| AMX/AMP | 4 | 97 041 | NE | NE | |||
| Stevens-Johnson syndrome | 14 | Nitrofurantoin | 1 | 3 649 427 | 0.00 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 7 | 4 217 227 | 0.02 | NE | NE | ||
| Fluoroquinolone | 3 | 6 717 509 | 0.00 | NE | NE | ||
| Broad-spectrum BL | 0 | 167 462 | 0.00 | NE | NE | ||
| Narrow-spectrum BL | 0 | 406 219 | 0.00 | NE | NE | ||
| AMX/AMP | 0 | 97 686 | 0.00 | NE | NE | ||
| Hypersensitivity | |||||||
| Anaphylaxis | 3 (index + 2) | Nitrofurantoin | 10 | 837 830 | 0.12 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 12 | 985 021 | 0.12 | 1.02 (.44–2.36) | .92 (.38–2.26) | ||
| Fluoroquinolone | 11 | 1 520 232 | 0.07 | .61 (.26–1.43) | .55 (.20–1.56) | ||
| Broad-spectrum BL | 2 | 38 549 | 0.52 | NE | NE | ||
| Narrow-spectrum BL | 2 | 93 249 | 0.21 | NE | NE | ||
| AMP/AMX | 1 | 23 099 | 0.43 | NE | NE | ||
| Angioedema/laryngeal edema | 3 (index + 2) | Nitrofurantoin | 20 | 837 229 | 0.24 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 19 | 984 329 | 0.19 | .81 (.43–1.51) | .69 (.35–1.37) | ||
| Fluoroquinolone | 37 | 1 519 165 | 0.24 | 1.02 (.59–1.76) | .93 (.50–1.75) | ||
| Broad-spectrum BL | 1 | 38 516 | 0.26 | NE | NE | ||
| Narrow-spectrum BL | 3 | 93 163 | 0.32 | NE | NE | ||
| AMP/AMX | 1 | 23 067 | 0.43 | NE | NE | ||
| Hypersensitivity reaction | 14 | Nitrofurantoin | 316 | 3 623 701 | .87 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 1012 | 4 187 077 | 2.42 | 2.78 (2.45–3.15) | 2.62 (2.30–2.98) | ||
| Fluoroquinolone | 377 | 6 670 039 | .57 | .65 (.56–.75) | .68 (.57–.80) | ||
| Broad-spectrum BL | 11 | 165 933 | .66 | .76 (.42–1.39) | .53 (.44–.64) | ||
| Narrow-spectrum BL | 32 | 403 004 | .79 | .91 (.63–1.31) | .64 (.53–.76) | ||
| AMP/AMX | 5 | 96 897 | .52 | .59 (.24–1.43) | .60 (.50–.72) | ||
| Gastrointestinal | |||||||
| Abdominal pain | 14 | Nitrofurantoin | 4199 | 3 244 168 | 12.94 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 5589 | 3 698 287 | 15.11 | 1.16 (1.12–1.21) | 1.14 (1.09–1.19) | ||
| Fluoroquinolone | 10 532 | 5 728 628 | 18.38 | 1.42 (1.37–1.48) | 1.36 (1.31–1.42) | ||
| Broad-spectrum BL | 269 | 137 346 | 19.59 | 1.51 (1.34–1.71) | 1.52 (1.46–1.58) | ||
| Narrow-spectrum BL | 547 | 321 443 | 17.02 | 1.32 (1.20–1.44) | 1.30 (1.25–1.35) | ||
| AMX/AMP | 157 | 83 715 | 18.75 | 1.44 (1.23–1.69) | 1.45 (1.39–1.50) | ||
| Nausea/vomiting | 14 | Nitrofurantoin | 1299 | 3 510 085 | 3.70 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 1817 | 4 042 451 | 4.49 | 1.21 (1.13–1.30) | 1.18 (1.10–1.28) | ||
| Fluoroquinolone | 2781 | 6 409 201 | 4.34 | 1.18 (1.10–1.26) | 1.17 (1.08–1.26) | ||
| Broad-spectrum BL | 97 | 157 715 | 6.15 | 1.66 (1.35–2.04) | 1.52 (1.42–1.63) | ||
| Narrow-spectrum BL | 200 | 374 650 | 5.34 | 1.44 (1.24–1.67) | 1.11 (1.03–1.20) | ||
| AMX/AMP | 34 | 93 028 | 3.65 | .98 (.70–1.38) | .95 (.88–1.03) | ||
| Cardiovascular | |||||||
| Aortic aneurysm and dissection | 90 | Nitrofurantoin | 16 | 18 585 791 | 0.01 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 16 | 21 688 918 | 0.01 | .86 (.43–1.72) | 1.10 (.56–2.17) | ||
| Fluoroquinolone | 32 | 35 015 856 | 0.01 | 1.06 (.58–1.94) | 1.02 (.51–2.02) | ||
| Broad-spectrum BL | 4 | 835 791 | 0.05 | NE | NE | ||
| Narrow-spectrum BL | 0 | 2 066 881 | 0.00 | NE | NE | ||
| AMX/AMP | 0 | 483 202 | 0.00 | NE | NE | ||
| Renal | |||||||
| Acute renal failure | 14 | Nitrofurantoin | 21 | 3 649 482 | 0.06 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 57 | 4 217 219 | 0.14 | 2.33 (1.41–3.84) | 2.56 (1.55–4.25) | ||
| Fluoroquinolone | 93 | 6 717 353 | 0.14 | 2.42 (1.51–3.89) | 2.39 (1.43–3.97) | ||
| Broad-spectrum BL | 1 | 167 468 | 0.06 | NE | NE | ||
| Narrow-spectrum BL | 5 | 406 236 | 0.12 | 2.14 (.81–5.68) | 1.20 (.67–2.14) | ||
| AMX/AMP | 1 | 97 675 | 0.10 | NE | NE | ||
| Neuromuscular and skeletal | |||||||
| Tendinopathy (including tendon rupture) |
90 | Nitrofurantoin | 433 | 18 475 398 | 0.23 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 454 | 21 566 921 | 0.21 | .90 (.79–1.02) | .92 (.80–1.06) | ||
| Fluoroquinolone | 1019 | 34 787 372 | 0.29 | 1.25 (1.12–1.40) | 1.19 (1.04–1.35) | ||
| Broad-spectrum BL | 24 | 830 851 | 0.29 | 1.23 (.82–1.86) | 1.48 (1.31–1.67) | ||
| Narrow-spectrum BL | 52 | 2 054 294 | 0.25 | 1.08 (.81–1.44) | 1.09 (.96–1.25) | ||
| AMX/AMP | 10 | 481 107 | 0.21 | .89 (.47–1.66) | .86 (.75–.99) | ||
| Potential microbiome-related adverse events | |||||||
| Gastrointestinal | |||||||
| Non–Clostridium difficile diarrhea | 30 | Nitrofurantoin | 1046 | 7 146 911 | 1.46 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 1268 | 8 259 018 | 1.54 | 1.05 (.97–1.14) | 1.02 (.93–1.11) | ||
| Fluoroquinolone | 2255 | 13 193 422 | 1.71 | 1.17 (1.09–1.26) | 1.14 (1.05–1.24) | ||
| Broad-spectrum BL | 86 | 323 185 | 2.66 | 1.82 (1.46–2.26) | 1.78 (1.65–1.92) | ||
| Narrow-spectrum BL | 189 | 783 229 | 2.41 | 1.65 (1.41–1.92) | 1.50 (1.38–1.62) | ||
| AMX/AMP | 45 | 187 533 | 2.40 | 1.63 (1.21–2.20) | 1.54 (1.43–1.67) | ||
| C. difficile infection | 90 | Nitrofurantoin | 8 | 18 585 361 | 0.00 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 5 | 21 689 566 | 0.00 | .54 (.18–1.64) | .50 (.15–1.67) | ||
| Fluoroquinolone | 61 | 35 018 524 | 0.02 | 4.07 (1.95–8.50) | 4.22 (1.96–9.10) | ||
| Broad-spectrum BL | 3 | 835 944 | 0.04 | NE | NE | ||
| Narrow-spectrum BL | 3 | 2 066 940 | 0.01 | NE | NE | ||
| AMX/AMP | 1 | 483 186 | 0.02 | NE | NE | ||
| Genitourinary | |||||||
| Vaginitis/vulvovaginal candidiasis | 30 | Nitrofurantoin | 3978 | 6 639 901 | 5.99 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 4335 | 7 711 026 | 5.62 | .94 (.90–.98) | .98 (.94–1.03) | ||
| Fluoroquinolone | 6839 | 12 265 099 | 5.58 | .93 (.90–.97) | .98 (.94–1.03) | ||
| Broad-spectrum BL | 224 | 300 178 | 7.46 | 1.24 (1.09–1.42) | 1.32 (1.26–1.37) | ||
| Narrow-spectrum BL | 550 | 743 141 | 7.40 | 1.24 (1.13–1.35) | 1.30 (1.25–1.35) | ||
| AMX/AMP | 144 | 172 293 | 8.36 | 1.39 (1.18–1.64) | 1.59 (1.53–1.65) | ||
| Respiratory/pulmonary | |||||||
| Pneumonia | 90 | Nitrofurantoin | 353 | 18 540 894 | 0.19 | 1 [Reference] | 1 [Reference] |
| TMP/SMX | 431 | 21 628 868 | 0.20 | 1.05 (.91–1.21) | 1.04 (.90–1.21) | ||
| Fluoroquinolone | 774 | 34 917 175 | 0.22 | 1.17 (1.03–1.33) | 1.12 (.97–1.29) | ||
| Broad-spectrum BL | 35 | 832 813 | 0.42 | 2.20 (1.56–3.11) | 2.21 (1.94–2.50) | ||
| Narrow-spectrum BL | 50 | 2 062 231 | 0.24 | 1.27 (.95–1.71) | 1.49 (1.30–1.71) | ||
| AMX/AMP | 11 | 481 486 | 0.23 | 1.19 (.66–2.18) | 1.02 (.88–1.18) |
Abbreviations: AMX/AMP, amoxicillin or ampicillin; BL, β-lactam; CI, confidence interval; HR, hazard ratio; NE, not estimable due to small case counts; TMP/SMX, trimethoprim-sulfamethoxazole.
aFor HR estimation, we required ≥5 adverse event cases in both the reference antibiotic treatment group (ie, nitrofurantoin) and the comparator treatment group to ensure stability of the effect estimate.
Adverse Drug Events
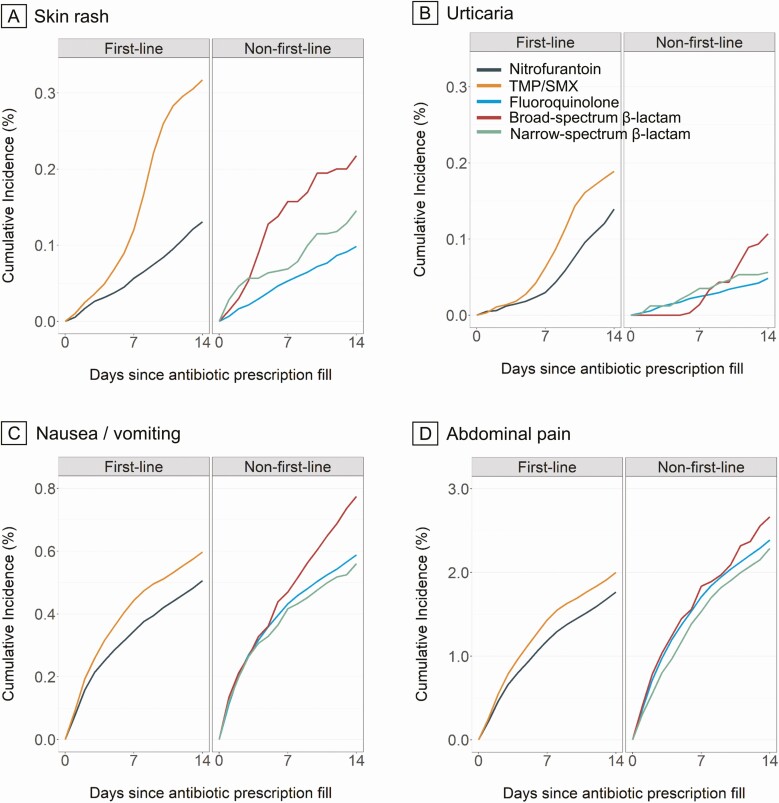
Figure 1, Supplementary Figure 3, and Supplementary Table 9 present weighted cumulative incidence estimates for each adverse drug event outcome over the follow-up duration, by treatment group. Table 2 presents case counts, person-days of follow-up, rates, and HR estimates for each adverse event analysis by antibiotic agent. Of the 2 first-line agents, we observed higher risks of adverse drug event outcomes among TMP/SMX versus nitrofurantoin initiators for hypersensitivity reaction, acute renal failure, skin rash, urticaria, abdominal pain, and nausea/vomiting. Compared with nitrofurantoin, non–first-line agents were also associated with higher risk of several adverse drug events. Specifically, fluoroquinolones were associated with higher risk of acute renal failure, abdominal pain, tendinopathy, and nausea/vomiting; broad-spectrum β-lactams were associated with higher risk of skin rash, nausea/vomiting, abdominal pain, and tendinopathy; and narrow-spectrum β-lactams were associated with higher risk of abdominal pain and nausea/vomiting. Nonrecommended AMP/AMX was associated with higher risk of abdominal pain. Compared with nitrofurantoin, we observed a lower risk of skin rash, urticaria, and hypersensitivity reaction among initiators of some non–first-line or nonrecommended antibiotics. Case counts were too rare to estimate HRs for any antibiotic agent comparisons for Stevens-Johnson syndrome and for some comparisons for urticaria, anaphylaxis, angioedema/laryngeal edema, aortic aneurysm and dissection, and acute renal failure.
Figure 1.
A–D, Propensity score–weighted cumulative incidence curves of selected adverse drug event outcomes among patients with uncomplicated UTI, by antibiotic agent. For each outcome, the number of events by antibiotic agent is presented in Table 2. Estimates were adjusted for age, month of prescription, year of prescription, geographic region, provider specialty, alcohol or drug abuse, deficiency anemias, chronic pulmonary disease, depression, hypertension, obesity, and psychoses. Abbreviations: TMP/SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
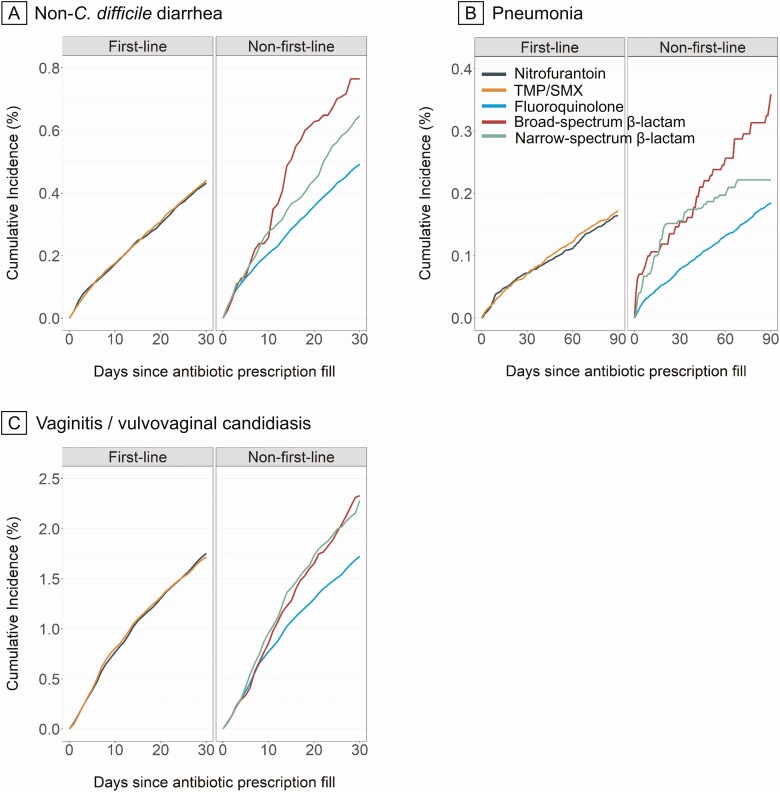
Potential Microbiome-Related Adverse Events
Figure 2 and Supplementary Table 9 present weighted cumulative incidence estimates for potential microbiome-related adverse event outcomes over the follow-up duration, by treatment group. Compared with nitrofurantoin, we observed similar risks of outcomes for TMP/SMX but a higher risk for non–first-line and nonrecommended antibiotics (Table 2). For non–C. difficile diarrhea and/or C. difficile infection, we observed higher risk for each non–first-line and nonrecommended antibiotics compared with nitrofurantoin. The magnitude of the HR estimate was particularly large for C. difficile infection and fluoroquinolones. Case counts were too rare to estimate HRs for some antibiotic agent comparisons for C. difficile infection. For vaginitis/vulvovaginal candidiasis, the risk was higher for broad-spectrum β-lactam, narrow-spectrum β-lactam, and especially AMX/AMP compared with nitrofurantoin. For pneumonia, the risk was higher for broad-spectrum β-lactam and narrow-spectrum β-lactam compared with nitrofurantoin.
Figure 2.
A–C, Propensity score–weighted cumulative incidence curves of selected potential microbiome-related adverse event outcomes among patients with uncomplicated UTI, by antibiotic agent. For each outcome, the number of events by antibiotic agent is presented in Table 2. Estimates were adjusted for age, month of prescription, year of prescription, geographic region, provider specialty, alcohol or drug abuse, deficiency anemias, chronic pulmonary disease, depression, hypertension, obesity, and psychoses. Abbreviations: TMP/SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
Subgroup and Sensitivity Analyses
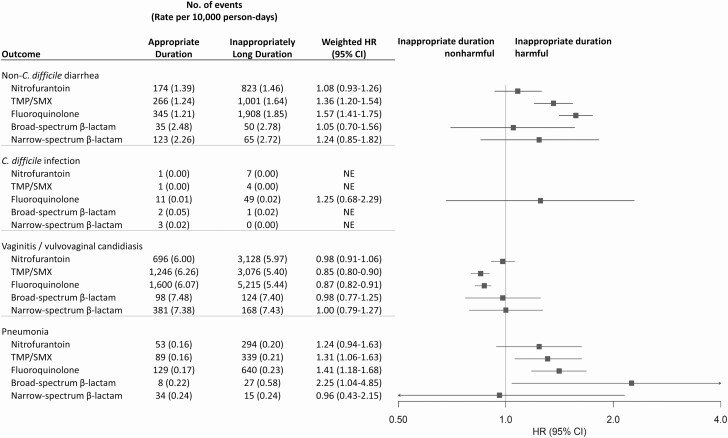
Results were generally similar by age group (Supplementary Table 10), with some exceptions (eg, TMP/SMX and urticaria: 18–29 vs 30–44 years; weighted HRs, 2.21 vs 0.98, respectively). Results varied by duration (Figure 3, Supplementary Table 11). Compared with patients who received guideline-recommended durations, we observed higher 30-day risk of non–C. difficile diarrhea among recipients of inappropriately long durations of TMP/SMX and fluoroquinolones and a higher 90-day risk of pneumonia among recipients of inappropriately long durations of TMP/SMX, fluoroquinolones, and broad-spectrum β-lactams. We also observed a lower 30-day risk of vaginitis/vulvovaginal candidiasis among recipients of inappropriately long durations of TMP/SMX and fluoroquinolones.
Figure 3.
Propensity score–weighted HR estimates of potential microbiome-related adverse events associated with antibiotic agents among patients prescribed guideline-recommended duration versus inappropriately long duration. Antibiotic duration was categorized as appropriate guideline-recommended duration (3 days for fluoroquinolones and TMP/SMX, 5 days for nitrofurantoin, 3–7 days for β-lactams) or inappropriately long duration; patients with inappropriately short duration were excluded. Propensity score weighting was implemented using standardized mortality ratio weighting wherein patients were weighted to reflect the covariate distribution in the patients who received appropriate guideline-recommended duration. Estimates were adjusted for age, month of prescription, year of prescription, geographic region, provider specialty, alcohol or drug abuse, deficiency anemias, chronic pulmonary disease, depression, hypertension, obesity, and psychoses. For HR estimation, we required 5 or more adverse event cases in both the reference antibiotic treatment group (ie, nitrofurantoin) and the comparator treatment group to ensure stability of the effect estimate. Abbreviations: CI, confidence interval; HR, hazard ratio; TMP/SMX, trimethoprim-sulfamethoxazole.
The results did not change meaningfully after excluding 25 697 (2.2%) patients who received kidney imaging at UTI diagnosis (Supplementary Table 12). After excluding index antibiotic agents indicated to treat both UTI and pneumonia, we observed the following: (1) the estimated effects of antibiotics on risk of adverse events did not change appreciably, although the effect estimate for pneumonia among broad-spectrum β-lactams moved closer to the null (weighted HRs, 2.21 to 1.57) (Supplementary Table 13), and (2) the estimated effects of inappropriately long duration did not change appreciably, although the effect estimates generally moved closer to the null (Supplementary Table 14). Varying the length of the exclusion period from 180 to 30 days for outcome events that occurred before the index date yielded similar results (Supplementary Tables 15 and 16).
DISCUSSION
In this large, claims-based cohort study of the safety of antibiotic therapy for uncomplicated UTI among US women aged 18–44 years, we observed evidence of differential risk of adverse drug events and potential microbiome-related adverse events by both antibiotic agent and treatment duration. Of 2 first-line agents, TMP/SMX was associated with a higher risk than nitrofurantoin for several adverse drug event outcomes but similar risk of potential microbiome-related adverse events, after accounting for potential confounders. Compared with nitrofurantoin, non–first-line agents were associated with a higher risk of several adverse drug events, as well as potential microbiome-related adverse events; these results have important implications given that almost half of prescriptions were non–first-line agents. The findings did not change materially in subgroup or sensitivity analyses, with the exception that inappropriately long antibiotic prescriptions were associated with increased risk of potential microbiome-related adverse events (ie, non–C. difficile diarrhea, pneumonia).
Our findings of differential risk of adverse events by agent are consistent with previous results (ie, meta-analyses of RCTs on agent-related risks of skin rash and diarrhea) [13] and extend the knowledge on the comparative safety of antibiotic agents for additional adverse event outcomes. For example, we demonstrate differences in the safety of first-line agents, wherein TMP/SMX is associated with higher risks of additional adverse events (acute renal failure, nausea/vomiting, abdominal pain) versus nitrofurantoin. Our findings confirm previous reports on fluoroquinolone safety, demonstrating increased risk of certain adverse events (eg, C. difficile infection, tendinopathy) [31–33]. We observed that broad-spectrum β-lactams are associated with higher risks of adverse events than narrow-spectrum agents, which validates antimicrobial stewardship principles and guidance from the Centers for Disease Control and Prevention to use narrow-spectrum agents when possible to treat bacterial infections [34, 35].
The present study demonstrates that antibiotic duration modifies the risk of potential microbiome-related adverse events, likely due to antibiotic-induced disruption of the microbiota [17–19]. These findings are consistent with the well-established association between longer treatment duration and increased risk of C. difficile infection [36], as well as a meta-analysis of RCTs reporting the association between longer antibiotic duration (3 vs 5–10 days) and higher risk of any adverse event, but without accounting for type of antibiotic agent or adverse event [37]. Our results extend the knowledge on harmful effects of treatment duration on other adverse events. Specifically, we observed increased risks of non–C. difficile diarrhea and/or pneumonia among patients prescribed inappropriately long versus guideline-recommended durations of TMP/SMX, fluoroquinolones, and broad-spectrum β-lactams. Inappropriately long durations of TMP/SMX and fluoroquinolones were associated with reduced risk for vaginitis/vulvovaginal candidiasis, which requires further exploration. Among nitrofurantoin initiators, we did not observe effects of duration on potential microbiome-related events, probably since nitrofurantoin only achieves therapeutically active concentrations in urine [38].
Our results are subject to limitations. First, in our observational study, the exposure was not randomized; therefore, effect estimates are potentially subject to confounding by unobserved differences between exposure groups. However, we attempted to control confounding by performing an active comparator study that restricted the cohort to a homogeneous population with indication for treatment (ie, otherwise healthy, nonpregnant, younger women coded for uncomplicated UTI in outpatient settings; newly treated with commonly used oral antibiotic agents and durations); the use of an active comparator, new user study design reduces measured and unmeasured confounding in observational studies [39–41]. We also used propensity score methods to account for demographic and clinical covariates, but residual confounding between antibiotic exposure and each outcome may exist in the presence of unmeasured/poorly measured confounders. Second, the exposure was based on dispensed antibiotic prescriptions but adherence information was not available and likely imperfect. However, by using pharmacy-dispensing billing claims—the gold standard of prescription drug ascertainment—we were able to censor follow-up on the date of a subsequent antibiotic prescription. Third, we were not able to analyze the effects of fosfomycin therapy due to rare use in the United States. Fourth, administrative billing claims data are advantageous for studying rare events because of the large sample size, but they lack important clinical information such as laboratory results, which may result in missing or misclassified adverse event outcomes. Although patients may be less likely to report milder safety events to their provider, these databases typically have excellent ascertainment of serious events, and outcome misclassification is unlikely to be differential by exposure. Fifth, our study inclusion criteria did not require confirmation of true bacterial UTI due to the absence of data on urine testing results or signs and symptoms of infection. However, our results are useful because they generalize to women diagnosed and treated for UTIs—regardless of true UTI status—all of whom are at risk of antibiotic-related adverse events. Last, the study population was limited to commercially insured women; therefore, results may not be generalizable to uninsured or Medicaid-insured populations.
Our large comparative safety study of antibiotic therapy for uncomplicated UTI demonstrates that the risk of adverse drug events and potential microbiome-related adverse events varies widely by antibiotic agent and duration. Addressing the threat of antimicrobial resistance requires a better understanding of the consequences of antibiotic prescribing, including adverse effects, which are commonly managed by drug discontinuation and subsequent prescriptions with alternative agents [42]. These findings underscore the utility of using real-world data to fill evidentiary gaps related to antibiotic safety, which can guide clinical decision making and inform future clinical practice guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Caroline A. O’Neil, MA, MPH, for editorial assistance.
Financial support. This work was supported by a grant from the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health under award number KL2 TR002346. Data programming for this study was conducted by the Center for Administrative Data Research, which is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the NCATS of the National Institutes of Health and grant number R24 HS19455 through the Agency for Healthcare Research and Quality. M. J. D. reports a grant from the National Institutes for Dental and Craniofacial Research (grant number K23DE029514).
Potential conflicts of interest. M. A. O. reported receiving investigator-initiated research funds from Sanofi Pasteur, Pfizer, and Merck and serving as a consultant for Pfizer. W. G. P. reported receiving investigator-initiated research funds from Merck and serving as an advisor for Merck and Gilead. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Suda KJ, Hicks LA, Roberts RM, Hunkler RJ, Matusiak LM, Schumock GT. Antibiotic expenditures by medication, class, and healthcare setting in the United States, 2010–2015. Clin Infect Dis 2018; 66:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hicks LA, Bartoces MG, Roberts RM, et al. . US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis 2015; 60:1308–16. [DOI] [PubMed] [Google Scholar]
- 3. Gupta K, Hooton TM, Naber KG, et al. . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103–20. [DOI] [PubMed] [Google Scholar]
- 4. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. . Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315:1864–73. [DOI] [PubMed] [Google Scholar]
- 5. Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA 2014; 312: 1677–84. [DOI] [PubMed] [Google Scholar]
- 6. Miller LG, Tang AW. Treatment of uncomplicated urinary tract infections in an era of increasing antimicrobial resistance. Mayo Clin Proc 2004; 79:1048–53; quiz: 1053–4. [DOI] [PubMed] [Google Scholar]
- 7. Critchley IA, Cotroneo N, Pucci MJ, Mendes R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PloS One 2019; 14:e0220265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. FDA drug safety communication: fluoroquinolone antibiotics. Available at: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM513019.pdf. Accessed 5 February 2018.
- 9. Kobayashi M, Shapiro DJ, Hersh AL, Sanchez GV, Hicks LA. Outpatient antibiotic prescribing practices for uncomplicated urinary tract infection in women in the United States, 2002–2011. Open Forum Infect Dis 2016; 3:ofw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taur Y, Smith MA. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin Infect Dis 2007; 44:769–74. [DOI] [PubMed] [Google Scholar]
- 11. Durkin MJ, Keller M, Butler AM, et al. . An assessment of inappropriate antibiotic use and guideline adherence for uncomplicated urinary tract infections. Open Forum Infect Dis 2018; 5:ofy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark AW, Durkin MJ, Olsen MA, et al. . Rural-urban differences in antibiotic prescribing for uncomplicated urinary tract infectio n. medRxiv [Preprint]. February 24, 2021 [cited 2021 May 26]. Available at: 10.1017/ice.2021.21. [DOI] [PMC free article] [PubMed]
- 13. Zalmanovici Trestioreanu A, Green H, Paul M, Yaphe J, Leibovici L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Sys t Rev 2010: CD007182. [DOI] [PubMed] [Google Scholar]
- 14. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70:2456–64. [DOI] [PubMed] [Google Scholar]
- 15. Knottnerus BJ, Grigoryan L, Geerlings SE, et al. . Comparative effectiveness of antibiotics for uncomplicated urinary tract infections: network meta-analysis of randomized trials. Fam Pract 2012; 29:659–70. [DOI] [PubMed] [Google Scholar]
- 16. Stürmer T, Wang T, Golightly YM, Keil A, Lund JL, Jonsson Funk M. Methodological considerations when analysing and interpreting real-world data. Rheumatology (Oxford) 2020; 59:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi Y, Kellingray L, Zhai Q, et al. . Structural and functional alterations in the microbial community and immunological consequences in a mouse model of antibiotic-induced dysbiosis. Front Microbiol 2018; 9:1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elvers KT, Wilson VJ, Hammond A, et al. . Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open 2020; 10:e035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science 2016; 352:544–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butler AM, Nickel KB, Overman RA, Brookhart MA. IBM MarketScan research databases. In: Sturkenboom MC, Schink T, eds. Databases for pharmacoepidemiological research. Cham, Switzerland: Springer, 2021: 243–51. [Google Scholar]
- 21. IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers. 2020. Available at: https://www.ibm.com/downloads/cas/0NKLE57Y.
- 22. Butler AM, Durkin MJ, Keller MR, et al. . Risk of antibiotic treatment failure in premenopausal women with uncomplicated urinary tract infection. [Preprint]. March 30, 2021 [cited 2021 May 26]. Available at: 10.1002/pds.5237. [DOI] [PMC free article] [PubMed]
- 23. Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ 2008; 179:767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother 2012; 67:742–8. [DOI] [PubMed] [Google Scholar]
- 26. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36:8–27. [DOI] [PubMed] [Google Scholar]
- 27. Agency for Healthcare Research and Quality. HCUP Elixhauser comorbidity software. Healthcare Cost and Utilization Project (HCUP). Available at: www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed 21 December 2020. [Google Scholar]
- 28. Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf 2006; 15:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009; 28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HG, Stricker BH. Fluoroquinolones and risk of Achilles tendon disorders: case-control study. BMJ 2002; 324:1306–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Linden PD, Sturkenboom MC, Herings RM, Leufkens HM, Rowlands S, Stricker BH. Increased risk of Achilles tendon rupture with quinolone antibacterial use, especially in elderly patients taking oral corticosteroids. Arch Intern Med 2003; 163:1801–7. [DOI] [PubMed] [Google Scholar]
- 33. McCusker ME, Harris AD, Perencevich E, Roghmann MC. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg Infect Dis 2003; 9:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Healthcare Infection Control Practices Advisory Committee. Antibiotic stewardship statement for antibiotic guidelines—the recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC). 2016. Available at: https://www.cdc.gov/hicpac/recommendations/antibiotic-stewardship-statement.html.
- 35. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep 2016; 65: 1–12. [DOI] [PubMed] [Google Scholar]
- 36. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53:42–8. [DOI] [PubMed] [Google Scholar]
- 37. Milo G, Katchman EA, Paul M, Christiaens T, Baerheim A, Leibovici L. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database Syst Rev 2005: CD004682. [DOI] [PubMed] [Google Scholar]
- 38. Cunha BA. Nitrofurantoin—current concepts. Urology 1988; 32:67–71. [DOI] [PubMed] [Google Scholar]
- 39. Schneeweiss S, Patrick AR, Stürmer T, et al. . Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care 2007; 45:S131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015; 2:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. D’Arcy M, Stürmer T, Lund JL. The importance and implications of comparator selection in pharmacoepidemiologic research. Curr Epidemiol Rep 2018; 5:272–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hooton TM, Gupta K. Acute simplecystitis in women. Available at: https://www.uptodate.com/contents/acute-simple-cystitis-in-women#H899949213. Accessed 1 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.