Abstract
Animal and clinical data show that high ratios of the area under the concentration-time curve and the peak concentration in blood to the MIC of fluoroquinolones for a given pathogen are associated with a favorable outcome. The present study investigated whether improvement of the therapeutic potential of ciprofloxacin could be achieved by encapsulation in polyethylene glycol (PEG)-coated long-circulating sustained-release liposomes. In a rat model of unilateral Klebsiella pneumoniae pneumonia (MIC = 0.1 μg/ml), antibiotic was administered at 12- or 24-h intervals at twofold-increasing doses. A treatment period of 3 days was started 24 h after inoculation of the left lung, when the bacterial count had increased 1,000-fold and some rats had positive blood cultures. The infection was fatal within 5 days in untreated rats. Administration of ciprofloxacin in the liposomal form resulted in delayed ciprofloxacin clearance and increased and prolonged ciprofloxacin concentrations in blood and tissues. The ED50 (dosage that results in 50% survival) of liposomal ciprofloxacin was 3.3 mg/kg of body weight/day given once daily, and that of free ciprofloxacin was 18.9 mg/kg/day once daily or 5.1 mg/kg/day twice daily. The ED90 of liposomal ciprofloxacin was 15.0 mg/kg/day once daily compared with 36.0 mg/kg/day twice daily for free ciprofloxacin; 90% survival could not be achieved with free ciprofloxacin given once daily. In summary, the therapeutic efficacy of liposomal ciprofloxacin was superior to that of ciprofloxacin in the free form. PEG-coated liposomal ciprofloxacin was well tolerated in relatively high doses, permitting once daily administration with relatively low ciprofloxacin clearance and without compromising therapeutic efficacy.
The pharmacodynamic and pharmacokinetic properties of antimicrobial agents have been intensively investigated in the recent past, years resulting in the optimization of dosage regimens. In vitro studies and dose-effect studies of experimental infections in animals have been performed with various classes of antibiotics to investigate which pharmacodynamic parameters determine therapeutic efficacy (9, 24, 26, 28, 42). Comparative studies of humans with different doses and dosage regimens have also been utilized to optimize antimicrobial treatment (9, 24).
The fluoroquinolones are concentration dependent in their rates of bacterial killing. The area under the concentration-time curve (AUC)/MIC ratio is the parameter that best correlates with the therapeutic efficacy of these agents. In addition, a sufficiently high peak concentration in serum/MIC ratio seems necessary to suppress the emergence of resistant mutants during treatment. This can be concluded from in vitro models that simulate human pharmacokinetics to study the effects of changing the concentrations of fluoroquinolones on a number of pathogens (6, 12, 13, 15, 23, 29, 32). For fluoroquinolones, a 24-h AUC/MIC ratio of >125 is needed for rapid bacterial eradication, whereas a peak serum drug concentration/MIC ratio of >8 is needed to prevent the selection of resistant organisms.
The effect of dose or dose interval on the therapeutic efficacy of fluoroquinolones has been evaluated in experimental infections such as pneumonia, sepsis, peritonitis, and thigh infection caused by gram-negative pathogens in mice, rats, guinea pigs, and rabbits (11, 19, 20, 27, 33, 41). Dosage schedules resulting in high AUC/MIC ratios and high peak serum drug concentration/MIC ratios of 10 or 20 effected higher therapeutic efficacy than did regimens in which a more fractionated schedule was used at the same daily dose (24, 28).
In clinical studies with fluoroquinolones, the AUC/MIC ratio and the peak serum drug concentration/MIC ratio are important predictors of both clinical and microbiological cure (16, 24, 28, 36). Forrest et al. investigated the pharmacodynamics of intravenous ciprofloxacin in seriously ill patients and found that a 24-h AUC/MIC ratio of ≥125 was significant for a satisfactory clinical and microbiological outcome (16). The data from this study show that most treatment failures with ciprofloxacin are consequences of high MIC, low AUC, or both. More recent clinical studies with grepafloxacin and levofloxacin showed that a peak serum drug concentration/MIC ratio of 10 or greater was associated with successful outcome; when the ratio was less than 10 the AUC/MIC ratio was most closely linked to outcome (17, 38).
Based on the pharmacodynamic properties of the fluoroquinolones, as shown in in vitro studies and in dose-response studies in animals and patients, regimens of high doses at infrequent intervals might be most efficacious in terms of eradication time, killing bacteria, and reducing the selection of drug-resistant bacteria. Relatively infrequent dosing (possibly once daily) may be superior. However, very high doses of some quinolones might prove to be toxic when given once daily.
The present study was undertaken to investigate whether improvement of the therapeutic potential of fluoroquinolones could be obtained by liposomal encapsulation of the drugs. In a rat model of left-sided Klebsiella pneumoniae pneumonia, the therapeutic efficacy of liposome-encapsulated ciprofloxacin versus free ciprofloxacin was determined. The liposome type used has shown relatively long blood circulation times, as a result of coating of the liposome surface with polyethylene glycol (PEG), and the PEG-coated liposomes are engineered to release ciprofloxacin slowly. Liposomal encapsulation results in a decrease in toxic side effects of the drug, permitting the use of relatively high doses. The aim of using liposomes as carriers of ciprofloxacin in the present study was, first, to delay ciprofloxacin clearance and achieve sustained liposomal release of ciprofloxacin in the blood over time, thus extending ciprofloxacin activity in the blood (increased AUC) and tissues. The second objective was to permit relatively high doses and decreased dose frequency and, as a result, a once-daily treatment schedule.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female RP/AEur/RijHsd albino rats (Harlan, Horst, The Netherlands) were used in all experiments. Animals were 18 to 25 weeks old and weighed 185 to 225 g.
Bacteria.
K. pneumoniae ATCC 43816, capsular serotype 2, was used to infect the rats. The minimum bactericidal concentration of ciprofloxacin for this strain was 0.1 μg/ml as determined by the tube dilution test.
Infection model.
A left-sided pneumonia was produced as previously described (2). In brief, rats were anesthetized with fluanisone and fentanyl citrate (Hypnorm) (Janssen, Animal Health, Saunderton, United Kingdom), followed by pentobarbital (Nembutal) (Sanofi Santé b.v., Maassluis, The Netherlands). The left primary bronchus was intubated, and the left lung was inoculated with 0.02 ml of a saline suspension containing 106 viable K. pneumoniae bacteria in the logarithmic phase of growth. After bacterial inoculation, the narcotic antagonist nalorphine bromide (Onderlinge Pharmaceutische Groothandel, Utrecht, The Netherlands) was injected. Inoculation of the lung resulted in an acute unilateral pneumonia. The course of the infection was assessed by measuring the number of viable bacteria in the infected left lung, the right lung, and the blood. Animals were sacrificed by CO2 inhalation, a blood sample was taken, and the left and right lungs were removed and homogenized (VirtTis, Gardiner, N.Y.) in 20 ml of phosphate-buffered saline for 30 s at 10,000 rpm. Lung homogenate suspensions and blood were serially diluted and plated on tryptone soy agar (Unipath Ltd., Basingstoke, United Kingdom). At 24 h after bacterial inoculation, the bacterial numbers in the left lung had increased 103-fold, up to 109 (range, 4 × 108 to 5 × 109; n = 10). Untreated rats developed septicemia and pleuritis; at 24 h after inoculation some rats had positive blood cultures. Untreated rats died between day 3 and day 6 after bacterial inoculation.
Liposomes.
PEG-coated liposome preparations of ciprofloxacin (PL Cipro) consisted of the PEG 2000 derivative of distearoylphosphatidylethanolamine, hydrogenated soybean phosphatidylcholine, and cholesterol in a molar ratio of 5:50:45. Ciprofloxacin-containing liposomes and placebo liposomes were kindly supplied by ALZA Corporation (Mountain View, Calif.). Mean particle size was determined by dynamic light scattering (4700 system; Malvern Instruments, Malvern, United Kingdom). The ciprofloxacin-containing liposomes had a mean particle size of 107 ± 7 nm and contained 256 ± 51 μg of ciprofloxacin/μmol of total lipid (mean ± standard deviation [SD] of 10 preparations). The mean particle size of the placebo liposomes was 105 ± 6 nm (mean ± SD of two preparations).
Radiolabeling of liposomes.
Pharmacokinetics and biodistribution of intact liposomes were determined by the use of a high-affinity 67Ga-deferoxamine-mesylate (67Ga-DF) complex as an aqueous liposomal marker (44). As shown by Gabizon et al. (18), this complex is appropriate for in vivo tracing of intact liposomes because of the advantages of minimal translocation of radioactive labels to plasma proteins and the rapid renal clearance rate when the label is released from the liposomes. 67Ga was obtained as 67Ga-citrate from Mallinckrodt Medical b.v., Petten, The Netherlands. The labeling was performed as described by Gabizon et al. (18). The radiolabeling resulted in the formation of a 67Ga-DF complex in the aqueous interior of the liposomes. Nonentrapped DF and radiolabels were removed by gel filtration on a Sephadex G-50 column eluted with HEPES buffer. The circulation times of liposomes in the blood and localization in the infected left lung, right lung, liver, spleen, and kidneys were determined using the 67Ga-DF complex as a marker for intact liposomes.
Pharmacokinetics and biodistribution.
The pharmacokinetics and biodistribution of antimicrobially active ciprofloxacin after administration in the free or liposome-encapsulated form were determined in rats at 24 h after bacterial inoculation. At different intervals after intravenous (i.v.) administration, blood samples were obtained by retro-orbital bleeding under CO2 anesthesia. Then the rats were sacrificed, and the left lung, right lung, spleen, liver, and kidneys were removed. 67Ga served as a marker for intact liposomes and was quantitated in a Minaxi Autogamma 5000 gamma counter (Packard Instrument Company, Meriden, Conn.). Correction for the blood content of the tissues was done using 111In-oxine-labeled syngeneic erythrocytes, injected i.v. 10 min before dissection. Erythrocytes were labeled as previously described (25). Ciprofloxacin concentrations in the blood and tissues were determined as previously described (5). In short, with diagnostic sensitivity test agar (Oxoid, Basingstoke, United Kingdom) and an Escherichia coli test strain susceptible to 0.025 μg of ciprofloxacin per ml, all tests were done by a standard large-plate agar diffusion procedure. Samples of 100 μl were assayed. Twofold-increasing standard concentrations ranging from 0.1 to 1.6 μg of ciprofloxacin per ml were used. The assay system was sensitive to 0.1 μg of ciprofloxacin per ml. The coefficient of variation of 15 determinations of solutions containing 0.1 to 1.6 μg of ciprofloxacin per ml was 1 to 3%.
Blood and tissue homogenates from rats that received PL Cipro were incubated in 0.1% Triton X-100 for 30 min at 25°C to disintegrate intact liposomes before determination of the presence of antimicrobially active ciprofloxacin. After centrifugation of the samples for 5 min at 12,000 × g, ciprofloxacin concentrations in the supernatant were determined. To exclude the possibility that the presence of Triton X-100 in the unknown samples may have had an effect on the bacterial growth inhibition zone on the agar plates, the standard concentrations were also incubated with Triton X-100.
Toxicity.
The maximum tolerated dose (MTD) was assessed using various parameters. Acute toxicity was characterized in terms of seizures, irritability, and an apparent dazed state. Long-term toxicity was assessed in terms of a significant change in renal or hepatic function. Renal function abnormalities were determined by measuring blood urea nitrogen and serum creatinine; hepatic function abnormalities were detected by measuring the serum aspartate aminotransferase and alanine aminotransferase by established tests (Merck Diagnostica, Darmstadt, Germany).
Antimicrobial treatment.
Ciprofloxacin in the free form (CIP) or PL Cipro was administered at 24 h after bacterial inoculation of the left lung. The doses were escalated by twofold increases (n, 8 to 10 per dosage), ranging from 0.3 to 80 mg/kg of body weight/day. The injection frequency was 12 or 24 h for CIP and 24 h for PL Cipro. The duration of treatment was 3 days in all cases. Therapeutic efficacy was assessed by the survival of rats at day 21 after bacterial inoculation. Estimates of ED50 (dosage that effects 50% survival of rats) and ED90 were obtained using the PROBIT procedure from the SAS program (SAS user's guide, SAS Institute Inc., Cary, N.C.), assuming a logistic distribution of data. Postmortem cultures of the left lung and blood from rats were performed to check for the presence of K. pneumoniae only, as well as for susceptibility to ciprofloxacin. Cultures were also done for rats that survived at day 21 postinoculation.
RESULTS
Blood circulation time of liposomes and ciprofloxacin.
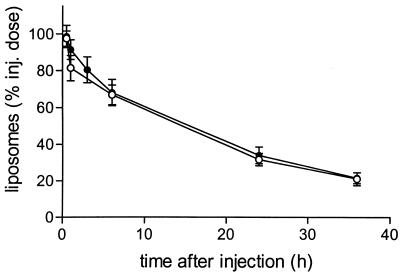
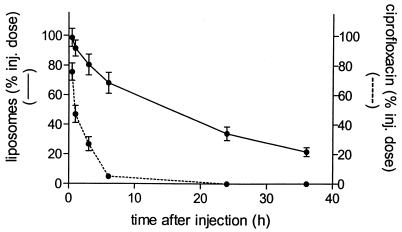
After i.v. administration, intact liposomes demonstrated a relatively long blood residence time, with a half-life of approximately 18 h (Fig. 1). This long-term circulation in blood was not altered when the liposomes contained encapsulated ciprofloxacin. Figure 2 shows that ciprofloxacin is slowly released from the intact liposomes in blood. Within 6 h after i.v. administration about 90% of the encapsulated ciprofloxacin is released, while the liposomes remain intact (Fig. 2). The concentrations of ciprofloxacin indicated in Fig. 2 represent primarily PL Cipro, since it is known that CIP rapidly diffuses from intravascular to extravascular space.
FIG. 1.
Levels of 67Ga-DF-labeled liposomes containing ciprofloxacin or placebo liposomes in blood. Liposomes were injected (inj.) i.v. as a single dose (ciprofloxacin, 20 mg/kg; total lipid, 83 μmol/kg) in rats at 24 h after inoculation of K. pneumoniae in the left lung. Levels of 67Ga-DF label in the blood after injection of ciprofloxacin-containing liposomes (●) or placebo liposomes (○) were determined. Data are expressed as mean ± SD for six rats.
FIG. 2.
Levels of 67Ga-DF-labeled liposomes containing ciprofloxacin in blood. Liposomes were injected (inj.) i.v. as a single dose (ciprofloxacin, 20 mg/kg; total lipid, 83 μmol/kg) into rats at 24 h after inoculation of K. pneumoniae in the lung. Levels of 67Ga-DF label and antimicrobially active ciprofloxacin in the blood were determined. Data are expressed as mean ± SD for six rats.
Concentration of ciprofloxacin in blood after administration in the free or liposome-encapsulated form.
Concentrations of ciprofloxacin in the blood were determined at various intervals after i.v. administration of 20 mg/kg as a single dose. Total (CIP plus PL Cipro) concentrations of ciprofloxacin are presented in Table 1. At 3 min after administration of CIP only 5% of the injected dose was present in blood, and at 30 min only 1% was present, corresponding with 5 μg of ciprofloxacin/ml. In contrast, after treatment with PL Cipro, 77% of the injected dose was still circulating in the blood 30 min after administration. Administration of ciprofloxacin in the liposome-encapsulated form resulted in a substantial increase in AUC0–6 h, 800 μg · h/ml for PL Cipro compared to 15.8 μg · h/ml for CIP.
TABLE 1.
Concentrations of total ciprofloxacin in blood at various intervals after i.v. administration of PL Cipro or CIP in rats with K. pneumoniae lung infectiona
| Time after treatment | Concn (μg/ml) (% of injected dose) of ciprofloxacin in blood after treatment with:
|
|
|---|---|---|
| PL Ciprob | CIPc | |
| 3 min | 17.6 ± 1.6 (4.7) | |
| 10 min | 8.6 ± 2.1 (2.3) | |
| 30 min | 291 ± 32 (77) | 5.0 ± 1.2 (1.3) |
| 1 h | 212 ± 30 (56) | 3.3 ± 1.1 (0.9) |
| 3 h | 97 ± 7.5 (26) | |
| 6 h | 35 ± 7.0 (9.2) | |
PL Cipro (20 mg/kg; total lipid, 86 μmol/kg) or CIP (20 mg/kg) was injected as a single dose into rats at 24 h after inoculation of K. pneumoniae in the left lung. Total (CIP plus PL Cipro) concentrations of ciprofloxacin are presented. Data are expressed as mean ± SD six rats.
AUC0–6h was 800 μg · h/ml.
AUC0–1h was 15.8 μg · h/ml.
Toxic side effects.
Toxicity was assessed using various parameters in a treatment schedule at twofold-increasing doses for a period of 3 days, with PL Cipro administered once daily and CIP administered once or twice daily. The MTD for CIP was 40 mg/kg/dose. At higher doses, acute toxicity was observed (e.g., seizures, irritability followed by an apparent dazed state) shortly after the first dose. After administration of PL Cipro, no signs of acute toxicity were observed at dosages up to 160 mg/kg/dose. Long-term toxicity was investigated at dosage schedules yielding 100% survival of rats: 40 mg of CIP/kg/dose twice daily and 20 mg of PL Cipro/kg/day once daily. At 12 or 24 h after the last dose of CIP or PL Cipro, respectively, significant abnormalities in renal or hepatic functions were not observed. Thus, PL Cipro was well tolerated at doses above the MTD of CIP.
Therapeutic efficacy of PL Cipro versus CIP.
In untreated rats, after inoculation of the left lung with 106 CFU of K. pneumoniae, the infection in the left lung developed progressively, whereas no infection developed in the right lung.
Antimicrobial treatment was started at 24 h after bacterial inoculation, when the bacterial count in the left lung had increased approximately 103-fold, to 3 × 109 CFU (range, 5 × 108 to 8 × 109; n = 10), and 7 out of 10 rats had developed positive blood cultures. All untreated rats died between day 3 and day 6 after bacterial inoculation. The parameter for therapeutic efficacy of antimicrobial treatment was the survival of rats assessed for a period of 21 days after bacterial inoculation. Treatment with CIP twice daily was effective in a dose-dependent manner and resulted in 100% survival of rats at doses that were well tolerated (Table 2). When CIP was administered once daily, 100% survival of rats could not be achieved at doses below the MTD. In contrast, the administration of PL Cipro once daily was fully effective at a dosage of 20 mg/kg/day.
TABLE 2.
Therapeutic efficacy of treatment of rats with K. pneumoniae lung infectiona
| Dosage (mg/kg/day)b | No. of surviving rats/total no. of rats receiving treatment
|
||
|---|---|---|---|
| CIP twice daily | CIP once daily | PL Cipro once daily | |
| 80 | 8/8c | ||
| 40 | 15/15 | 4/8 | 8/8 |
| 20 | 9/10 | 5/8 | 8/8 |
| 10 | 7/10 | 2/8 | 7/8 |
| 5 | 5/10 | 2/8 | 3/8 |
| 2.5 | 2/10 | 0/8 | 3/8 |
| 1.25 | 2/10 | 3/8 | |
| 0.6 | |||
| 0.3 | 0/5 | ||
| ED50 | 5.1 (2.6–8.6) | 18.9 (10.5–37.0) | 3.3 (1.7–5.7) |
| ED90 | 36.0 (18.3–164) | >MTD | 15.0 (8.0–78.9) |
Treatment was started at 24 h after inoculation of K. pneumoniae in the lung and continued for 3 days. Survival of rats was assessed for a period of 21 days. Untreated rats died before day 6 after inoculation.
ED50s and ED90s are expressed as milligrams per kilograms per day. Values in parentheses are 95% confidence intervals.
Toxic side effects (acute toxicity) were observed in four out of eight rats.
ED50s and ED90s calculated from the survival data demonstrate that the therapeutic efficacy of PL Cipro was superior to that of CIP (Table 2). PL Cipro was significantly (P < 0.05) more effective than CIP in achieving 50% survival of rats with once-daily administration and slightly, but not significantly, more active than CIP given twice daily. The difference between PL Cipro and CIP with once-daily administration is even more striking at the ED90. Survival of 90% of rats could not be achieved with once-daily treatment with CIP; twice-daily treatment was required. In contrast, PL Cipro given once daily was effective. The ED90 daily dose for CIP given twice daily was slightly higher than the ED90 daily dose for PL Cipro given once daily.
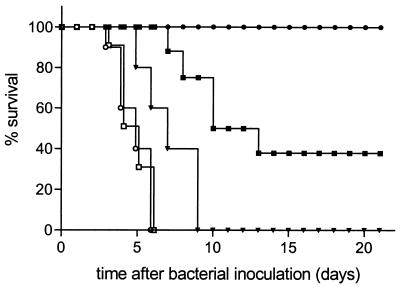
As shown in Fig. 3, the administration of placebo liposomes had no effect on the mortality rate. In this figure, the mortality rates of rats treated with PL Cipro at doses of 20 mg/kg, 2.5 mg/kg, or 0.3 mg/kg for 3 days are also represented.
FIG. 3.
Survival of rats with K. pneumoniae lung infection after administration of PL Cipro at 20 mg/kg (●) (n = 8), 2.5 mg/kg (▪) (n = 8), or 0.3 mg/kg (▾) (n = 5); placebo liposomes (□) (n = 10); or buffer (○) (n = 10) at 24, 48, and 72 h after bacterial inoculation of the lung. Total lipid doses were 81 μmol/kg/dose.
K. pneumoniae was present only in the infected left lung of rats that had died. In addition, the susceptibility to ciprofloxacin of the bacteria that were recovered from the infected left lung tissue of treated rats appeared to be unchanged.
Biodistribution of PL Cipro versus CIP.
Concentrations of ciprofloxacin (CIP plus PL Cipro) in different organs and the blood of infected rats were determined at 1- and 6-h intervals after administration of a single dose of CIP or PL Cipro at 20 mg/kg (Table 3). When rats were sacrificed 1 h after injection of CIP, about 1% of the injected dose was still present in the blood. Rats treated with PL Cipro were sacrificed at 1 or 6 h after administration. One hour after the administration of PL Cipro, the total recovery of ciprofloxacin based on its levels in the blood, liver, spleen, kidney, and lung was 75% of the injected dose. In contrast, when CIP was administered, recovery was only 3% of the injected dose at the same time point. Concentrations of ciprofloxacin in tissue were substantially increased following administration in the liposomal form compared with injected CIP. The degrees of localization of ciprofloxacin in the infected left lung and in the uninfected right lung were not different and appeared to decrease with time.
TABLE 3.
Biodistribution of total ciprofloxacin at various intervals after i.v. administration of PL Cipro or CIP in rats with K. pneumoniae lung infectiona
| Location | Concnb (% of injected dose) of ciprofloxacin at time
|
||
|---|---|---|---|
| 1 h
|
6 h, PL Cipro | ||
| CIP | PL Cipro | ||
| Blood | 3.3 ± 1.1 (0.9) | 212 ± 32 (56) | 35 ± 7.0 (9.2) |
| Liver | 33 ± 4.2 (0.8) | 552 ± 50 (14) | 83 ± 27 (2.0) |
| Spleen | 7.3 ± 0.5 (0.2) | 68 ± 4.1 (1.7) | 134 ± 22 (3.2) |
| Kidney | 42 ± 17 (1.0) | 44 ± 19 (1.1) | 9.5 ± 3.3 (0.2) |
| Left lung | 3.7 ± 0.4 (0.1) | 54 ± 20 (1.4) | 12 ± 2.9 (0.3) |
| Right lung | 4.6 ± 0.9 (0.1) | 47 ± 21 (1.2) | 7.5 ± 3.4 (0.2) |
PL Cipro (20 mg/kg; total lipid, 86 μmol/kg) or CIP (20 mg/kg) was injected as a single dose into rats at 24 h after inoculation of K. pneumoniae in the left lung. Total (CIP plus PL Cipro) concentrations of ciprofloxacin are presented.
Concentrations of ciprofloxacin (mean ± SD for six rats) are presented as micrograms per milliliter of blood or per organ.
DISCUSSION
A possible approach for intensifying antibiotic treatment may be the use of an appropriate delivery system, such as liposomes, which may enhance antibiotic pharmacokinetics or penetration to infected sites. Liposomes are considered to be versatile delivery systems. Depending on the liposomal size and physicochemical characteristics, which can be manipulated by changing the lipid composition (surface charge, surface coating, bilayer rigidity), liposomes can be used in various ways. One rationale for using liposomes as carriers of antibiotics is to reduce the toxicity of potentially toxic antibiotics such as amphotericin B (21, 22). Liposomes can also be exploited to achieve high and prolonged intracellular antibiotic concentrations in infected cells (3, 37). Another application of liposomes targets the delivery of antibiotics to infected tissues. In this respect, previous studies in this experimental model of K. pneumoniae pneumonia in rats have demonstrated a substantial increase in therapeutic efficacy for gentamicin or ceftazidime resulting from administration in long-circulating liposomes (4, 40). Finally, liposomes carrying antibiotics may also be used as microreservoirs of antibiotics during circulation. Again, long-circulating liposomes are needed for this purpose. Experimental evidence to support the application of liposomes in this way is provided in the present study.
In the current study, liposomes were used first to influence the pharmacokinetics of ciprofloxacin, thus extending ciprofloxacin activity in the blood and tissues, and secondly to protect encapsulated ciprofloxacin, which facilitates the use of relatively high doses and possibly once-daily dosing. The liposomes exhibited sustained liposomal release of ciprofloxacin in the blood over time while remaining intact. In earlier studies with PEG-coated liposomes containing gentamicin or ceftazidime, the liposomes retained their content during circulation (4), and as a consequence, substantial targeting of liposomal antibiotic to the infected left lung tissue was observed (40). In the present study, as expected, targeting of liposomal ciprofloxacin to the infected tissue was not achieved, because of the relatively rapid release of the antibiotic from the liposomes. Ciprofloxacin concentrations in the infected left lung and uninfected right lung were similar. However, the administration of PL Cipro resulted in relatively low ciprofloxacin clearance, prolonged ciprofloxacin concentrations in the blood, and increased ciprofloxacin concentrations in both infected and uninfected tissues. Probably as a result of this, the therapeutic efficacy of PL Cipro was superior to that of CIP. It was also observed that liposomal ciprofloxacin was well tolerated in relatively high doses and could be administered once daily without compromising its therapeutic efficacy.
Our observation that prolonged residence of ciprofloxacin in the blood is important for therapeutic efficacy agrees with the findings of others investigating the therapeutic efficacy of fluoroquinolones in the free form at various dose schedules in animal infection models. In models of pneumonitis caused by K. pneumoniae and thigh infection caused by K. pneumoniae or Pseudomonas aeruginosa in mice, Leggett et al. investigated dose-effect relations for ciprofloxacin (27). It was shown that the AUC/MIC ratio was the variable most closely linked to outcome, even though the peak serum drug concentration/MIC ratio was >10. The dosing interval had little impact. In contrast, Drusano et al. emphasized the role of the dosing interval for therapeutic efficacy. They examined the impact of dose fractionation and altered MICs in a neutropenic rat model of P. aeruginosa sepsis using lomefloxacin (11). Once-daily administration of the drug, produced a peak serum drug concentration/MIC ratio of 20 and resulted in better efficacy than a more fractionated treatment schedule at the same daily dose. At lower doses producing peak serum drug concentration/MIC ratios of <10, the AUC/MIC ratio appeared to be most closely linked to outcome. The relative importance of the peak serum drug concentration/MIC ratio for therapeutic efficacy has also been demonstrated in a model of P. aeruginosa pneumonia in neutropenic guinea pigs. Gordin et al. showed that ciprofloxacin and pefloxacin had the same rate of bacterial killing when the peak serum drug concentration/MIC ratio was the same for each agent (19). Similar conclusions can be drawn from studies by Hackbarth et al. and Shibl et al. that examined the efficacy of ciprofloxacin and pefloxacin in experimental meningitis caused by P. aeruginosa or E. coli, respectively, in rabbits (20, 41). Both studies demonstrated that the peak serum drug concentration/MIC ratio was predictive for bacterial killing in cerebrospinal fluid.
The PEG-coated liposomes used in the present study show a relatively long blood residence time due to decreased uptake by the mononuclear phagocyte system (1). Long-term circulation of liposomes is needed to achieve prolonged ciprofloxacin activity in blood. Other investigators using liposomes containing fluoroquinolones focused on the treatment of intracellular infections, which are difficult to treat due to poor penetration of antibiotics into the infected cells or decreased intracellular activity. In these studies, classical non-PEG-coated liposomes were used, which rapidly accumulate in cells of the mononuclear phagocyte system after i.v. administration, resulting in increased intracellular concentrations. Enhanced efficacy of ciprofloxacin in the protection and treatment of mice with intracellular Francisella tularensis infection was demonstrated when it was administered in the liposomal form i.v. intranasally (10), or by aerosol delivery (8). Liposomal ciprofloxacin also appeared effective in the i.v. treatment of intracellular infections caused by Salmonella enterica serovar Dublin (30) or S. enterica serovar Typhimurium (43) in mice. In vitro studies in which monocytes or macrophages in culture were infected with Mycobacterium avium-M. intracellulare complex and exposed to ciprofloxacin (31, 34) or ofloxacin (35) in the free or liposome-encapsulated form also showed the superiority of the liposome-encapsulated drugs. In contrast, free and liposomal sparfloxacin had similar effects on the growth of intracellular M. avium-M. intracellulare complex (14).
Sustained drug release of fluoroquinolones from liposomes has also been demonstrated for enrofloxacin encapsulated in non-PEG-coated liposomes composed of phosphatidylcholine and cholesterol. This formulation was administered intramuscularly in rabbits and provided therapeutic and prolonged plasma concentrations (7). In addition, ciprofloxacin liposomes have been used for external coating of catheters to prevent catheter-associated urinary tract infections in a rabbit model (39).
For the fluoroquinolones, animal data showing that high ratios of the AUC and the peak concentration in blood to the MIC for the pathogen are associated with favorable outcomes are in agreement with the clinical data. For infections caused by highly susceptible bacteria, the optimal AUC/MIC ratio and peak serum drug concentration/MIC ratio criteria can easily be reached. The difficulty lies in infections caused by bacteria such as Staphylococcus and Pseudomonas species that are only marginally susceptible to fluoroquinolones (MICs ≥ 0.5 μg/ml). In future studies the efficacy of PL Cipro will be investigated in a model of P. aeruginosa pneumonia and septicemia in rats.
ACKNOWLEDGMENT
The financial support of ALZA Corporation (Mountain View, Calif.) is gratefully acknowledged.
REFERENCES
- 1.Allen T M, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethyleneglycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 2.Bakker-Woudenberg I A J M, van den Berg J C, Michel M F. Therapeutic activities of cefazolin, cefotaxime, and ceftazidime against experimentally induced Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 1982;22:1042–1050. doi: 10.1128/aac.22.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakker-Woudenberg I A J M. Delivery of antimicrobials to infected tissue macrophages. Adv Drug Delivery Rev. 1995;17:5–20. [Google Scholar]
- 4.Bakker-Woudenberg I A J M, ten Kate M T, Stearne-Cullen L E T, Woodle M C. Efficacy of gentamicin or ceftazidime entrapped in liposomes with prolonged blood circulation and enhanced localization in Klebsiella pneumoniae-infected lung tissue. J Infect Dis. 1995;171:938–947. doi: 10.1093/infdis/171.4.938. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J V, Brodie J L, Benner E J, Kirby W M M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966;14:170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser J, Stone B B, Groner M C, Zinner S H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987;31:1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabanes A, Reig F, Garcia Antón J M, Arboix M. Sustained release of liposome-encapsulated enrofloxacin after intramuscular administration in rabbits. Am J Vet Res. 1995;56:1498–1501. [PubMed] [Google Scholar]
- 8.Conley J, Yang H, Wilson T, Blasetti K, Di Ninno V, Schnell G, Wong J P. Aerosol delivery of liposome-encapsulated ciprofloxacin: aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob Agents Chemother. 1997;41:1288–1292. doi: 10.1128/aac.41.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig W A. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 10.DiNinno V L, Cherwonogrodzky J W, Wong J P. Liposome-encapsulated ciprofloxacin is effective in the protection and treatment of BALB/c mice against Francisella tularensis. J Infect Dis. 1993;168:793–794. doi: 10.1093/infdis/168.3.793. [DOI] [PubMed] [Google Scholar]
- 11.Drusano G L, Johnson D E, Rosen M, Standiford H C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993;37:483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley M N, Mandler H D, Gilbert D, Ericson J, Mayer K H, Zinner S H. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin. Studies in vivo and in an in vitro dynamic model. Am J Med. 1987;82(Suppl. 4A):363–368. [PubMed] [Google Scholar]
- 13.Dudley M N. Pharmacodynamics and pharmacokinetics of antibiotics with special reference to the fluoroquinolones. Am J Med. 1991;91(Suppl. 6A):45–50. doi: 10.1016/0002-9343(91)90311-k. [DOI] [PubMed] [Google Scholar]
- 14.Düzgüneş N, Flasher D, Reddy M V, Luna-Herrera J, Gangadharam P R J. Treatment of intracellular Mycobacterium avium complex infection by free and liposome-encapsulated sparfloxacin. Antimicrob Agents Chemother. 1996;40:2618–2621. doi: 10.1128/aac.40.11.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firsov A A, Vostrov S N, Shevchenko A A, Portnoy Y A, Zinner S H. A new approach to in vitro comparisons of antibiotics in dynamic models: equivalent area under the curve/MIC breakpoints and equiefficient doses of trovafloxacin and ciprofloxacin against bacteria of similar susceptibilities. Antimicrob Agents Chemother. 1998;42:2841–2847. doi: 10.1128/aac.42.11.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forrest A, Chodosh S, Amantea M A, Collins D A, Schentag J J. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J Antimicrob Chemother. 1997;40(Suppl. A):45–57. doi: 10.1093/jac/40.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- 18.Gabizon A, Huberty J, Straubinger R M, Price D C, Papahadjopoulos D. An improved method for in vivo tracing and imaging of liposomes using a gallium 67-deferoxamine complex. J Liposome Res. 1988;1:123–135. [Google Scholar]
- 19.Gordin F M, Hackbarth C J, Scott K G, Sande M A. Activities of pefloxacin and ciprofloxacin in experimentally induced Pseudomonas pneumonia in neutropenic guinea pigs. Antimicrob Agents Chemother. 1985;27:452–454. doi: 10.1128/aac.27.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackbarth C J, Chambers H F, Stella F, et al. Ciprofloxacin in experimental Pseudomonas aeruginosa meningitis in rabbits. J Antimicrob Chemother. 1986;18(Suppl. D):65–69. doi: 10.1093/jac/18.supplement_d.65. [DOI] [PubMed] [Google Scholar]
- 21.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B. J Liposome Res. 1998;8:443–467. [Google Scholar]
- 22.Hillery A M. Supramolecular lipidic drug delivery systems: from laboratory to clinic. A review of the recently introduced commercial liposomal and lipid-based formulations of amphotericin B. Adv Drug Delivery Rev. 1997;24:345–363. [Google Scholar]
- 23.Hyatt J M, Nix D E, Schentag J J. Pharmacokinetic and pharmacodynamic activities of ciprofloxacin against strains of Streptococcus pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa for which MICs are similar. Antimicrob Agents Chemother. 1994;38:2730–2737. doi: 10.1128/aac.38.12.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kurantsin-Mills J, Jacobs H M, Siegel R, Cassidy M M, Lessin L S. Indium-111 oxine labeled erythrocytes: cellular distribution and efflux kinetics of the label. Int J Radiat Appl Instrum Part B. 1989;16:821–827. doi: 10.1016/0883-2897(89)90167-0. [DOI] [PubMed] [Google Scholar]
- 26.Leggett J E, Fantin B, Ebert S, Totsuka K, Vogelman B, Calame W, Mattie H, Craig W A. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J Infect Dis. 1989;159:281–292. doi: 10.1093/infdis/159.2.281. [DOI] [PubMed] [Google Scholar]
- 27.Leggett J E, Ebert S, Fantin B, Craig W A. Comparative dose-effect relations at several dosing intervals for beta-lactam, aminoglycoside and quinolone antibiotics against gram-negative bacilli in murine thigh-infection and pneumonitis models. Scand J Infect Dis Suppl. 1991;74:179–184. [PubMed] [Google Scholar]
- 28.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–39. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 29.Madaras-Kelly K J, Ostergaard B E, Baeker Hovde L, Rotschafer J C. Twenty-four-hour area under the concentration-time curve/MIC ratio as a generic predictor of fluoroquinolone antimicrobial effect by using three strains of Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1996;40:627–632. doi: 10.1128/aac.40.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magallanes M, Dijkstra J, Fierer J. Liposome-incorporated ciprofloxacin in treatment of murine salmonellosis. Antimicrob Agents Chemother. 1993;37:2293–2297. doi: 10.1128/aac.37.11.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majumdar S, Flasher D, Friend D S, Nassos P, Yajko D, Hadley W K, Düzgüneş N. Efficacies of liposome-encapsulated streptomycin and ciprofloxacin against Mycobacterium avium-M. intracellulare complex infections in human peripheral blood monocyte/macrophages. Antimicrob Agents Chemother. 1992;36:2808–2815. doi: 10.1128/aac.36.12.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchbanks C R, McKiel J R, Gilbert D H, Robillard N J, Painter B, Zinner S H, Dudley M N. Dose ranging and fractionation of intravenous ciprofloxacin against Pseudomonas aeruginosa and Staphylococcus aureus in an in vitro model of infection. Antimicrob Agents Chemother. 1993;37:1756–1763. doi: 10.1128/aac.37.9.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michéa-Hamzehpour M, Auckenthaler R, Regamey P, Pechère J-C. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother. 1987;31:1803–1808. doi: 10.1128/aac.31.11.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh Y-K, Nix D E, Straubinger R M. Formulation and efficacy of liposome-encapsulated antibiotics for therapy of intracellular Mycobacterium avium infection [sic] Antimicrob Agents Chemother. 1995;39:2104–2111. doi: 10.1128/aac.39.9.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onyeji C O, Nightingale C H, Nicolau D P, Quintiliani R. Efficacies of liposome-encapsulated clarithromycin and ofloxacin against Mycobacterium avium-M. intracellulare complex in human macrophages. Antimicrob Agents Chemother. 1994;38:523–527. doi: 10.1128/aac.38.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peloquin C A, Cumbo T J, Nix D E, Sands M F, Schentag J J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration and clinical condition on bacterial eradication. Arch Intern Med. 1989;149:2269–2273. [PubMed] [Google Scholar]
- 37.Petersen E A, Grayson J B, Hersh E M, Dorr R T, Chiang S-M, Oka M, Proffitt R T. Liposomal amikacin: improved treatment of Mycobacterium avium complex infection in the beige mouse model. J Antimicrob Chemother. 1996;38:819–828. doi: 10.1093/jac/38.5.819. [DOI] [PubMed] [Google Scholar]
- 38.Preston S L, Drusano G L, Berman A L, Fowler C L, Chow A T, Dornseif B, Reichl V, Natarajan J, Corrado M. Pharmacodynamics of levofloxacin. A new paradigm for early clinical trials. JAMA. 1998;279:125–129. doi: 10.1001/jama.279.2.125. [DOI] [PubMed] [Google Scholar]
- 39.Pugach J L, Ditizio V, Mittelman M W, Bruce A W, Dicosmo F, Khoury A E. Antibiotic hydrogel coated Foley catheters for prevention of urinary tract infection in a rabbit model. J Urol. 1998;162:883–887. doi: 10.1097/00005392-199909010-00084. [DOI] [PubMed] [Google Scholar]
- 40.Schiffelers R M, Storm G, ten Kate M T, Bakker-Woudenberg I A J M. Therapeutic efficacy of liposome-encapsulated gentamicin in rat Klebsiella pneumoniae pneumonia in relation to impaired host defense and low bacterial susceptibility to gentamicin. Antimicrob Agents Chemother. 2001;45:464–470. doi: 10.1128/AAC.45.2.464-470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibl A M, Hackbarth C J, Sande M A. Evaluation of pefloxacin in experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1986;29:409–411. doi: 10.1128/aac.29.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- 43.Webb M S, Boman N L, Wiseman D J, Saxon D, Sutton K, Wong K F, Logan P, Hope M J. Antibacterial efficacy against an in vivo Salmonella typhimurium infection model and pharmacokinetics of a liposomal ciprofloxacin formulation. Antimicrob Agents Chemother. 1998;42:45–52. doi: 10.1128/aac.42.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodle M C. 67Gallium-labeled liposomes with prolonged circulation: preparation and potential as nuclear imaging agents. Nucl Med Biol. 1993;20:149–155. doi: 10.1016/0969-8051(93)90107-6. [DOI] [PubMed] [Google Scholar]