Abstract
Objective
Spontaneous hemorrhagic stroke is a devastating disease with high mortality and grave neurological outcomes worldwide. This study aimed to evaluate the association between the elapsed time from emergency department (ED) visit to emergency neurosurgery and clinical outcomes in patients with spontaneous hemorrhagic stroke.
Methods
A nationwide cross-sectional study was conducted using the nationwide emergency database in Korea. Spontaneous hemorrhagic stroke patients who received neurosurgery within 12 hours of ED visit between January 2018 and December 2019 were enrolled. The main exposure was time to neurosurgery and the primary outcome was in-hospital mortality. Multivariable logistic regression was conducted.
Results
Among 2,602 study populations (incidence rate: 2.5 per 100,000 person-years, 15.8% of SAH, 78.6% of ICH, and 5.6% of mixed type), 525 (20.2%) patients received surgery in the ultra-early (0–2 hours) group, 1,093 (42.0%) in the early (2–4 hours) group, and 984 (37.8%) in the late (4–12 hours) group. The early group showed better survival outcomes than the ultra-early and late group (in-hospital mortality 22.2% vs. 26.5% and 26.1%, p = 0.06). Compared to the late group, adjusted OR (95% CI) for in-hospital mortality was 0.78 (0.63–0.96) for the early group, while there was no significant difference in the ultra-early group (0.90 (0.69–1.16)).
Conclusions
Early neurosurgery within 2–4 hours of the ED visit was associated with favorable survival outcomes in patients with spontaneous hemorrhagic stroke.
Introduction
Spontaneous hemorrhagic stroke is a devastating disease with high mortality and grave neurological outcomes worldwide [1]. As the aging population increases, the burden of the disease continues to increase due to the high incidence of the elderly and poor prognosis [2,3]. A recent study reported that the expected years of life lost for spontaneous hemorrhagic stroke was 6 to 9.7 years, and the expected lifetime cost was $86,327 [4]. Despite many efforts to improve clinical outcomes for patients with spontaneous hemorrhagic stroke, specific breakthrough treatments have not been developed [5].
The effects of time to vascular interventions on clinical outcomes have been well investigated in emergency cardio- and cerebro-vascular diseases such as acute myocardial infarction and ischemic stroke [6,7]. In terms of hemorrhagic stroke, early intervention is recommended to preserve the nervous system [8,9], reduce intracranial pressure (ICP) and improve neurological outcomes for subarachnoid hemorrhage (SAH) [10].
The elapsed time from emergency department (ED) visits to definitive care is associated with survival outcomes for patients with critical illnesses, and better outcomes are associated with treatments initiated in the hours immediately after diagnosis. Overall, early intervention is known to be associated with favorable clinical outcomes for patients with spontaneous hemorrhagic stroke who have undergone emergency surgery [8–11]. However, the gold standard of time to initiate surgery has not been well studied, and there are no emergency operation protocols to improve clinical outcomes and reduce complications for patients with spontaneous hemorrhagic stroke [12].
We hypothesized that a delay of operation reduces the chance of survival and increases complications, including reoperations, for patients with spontaneous hemorrhagic stroke. The purpose of this study was to evaluate the association between time elapsed from ED admission to surgery and clinical outcomes in patients with spontaneous hemorrhagic stroke who need emergency neurosurgery, and to determine the optimal cutoff time intervals for favorable survival outcomes.
Methods
Study design and data source
A nationwide cross-sectional study was conducted using the National Emergency Department Information System (NEDIS) database in Korea. The Ministry of Health and Welfare established the emergency medicine information network to measure emergency care qualities in 2003. The database collects data in real time from EDs nationwide, including demographics, treatments in the ED and hospital, and clinical outcomes. All information is automatically transferred from each hospital to a central government server following patients’ discharge from an ED or hospital. Inaccurate data is filtered by a data processing system. The NEDIS data is updated in real time by the National Emergency Medical Center and approved annually by National Statistics for data quality management [13,14].
Study setting
Korea has approximately 50 million residents living in 17 provinces. The Ministry of Health and Welfare designated 3 levels of ED according to emergency medical resources and capacity (including facilities, equipment, and medical staff) and operated 38 Level 1 EDs, 125 Level 2 EDs, and 239 Level 3 EDs (a total of 402 EDs) in 2020. A Level 1 ED is designed to provide care for serious and severe emergency patients. Level 1 and Level 2 EDs are mostly assigned on-call neurosurgeons in the hospital, and Level 1 EDs have an emergency operating room in the ED. Hemorrhagic stroke is mostly diagnosed by computed tomography. Most hospitals follow standard protocols for management of hemorrhagic stroke including ICP monitoring, medical management, and determination of surgery [15,16].
The emergency medical services (EMS) system is a government-based system operated by the National Fire Agency. EMS providers perform basic to intermediate levels of prehospital care at the scene and transport suspected stroke patients to nearby Level 1 or Level 2 EDs, according to standard operating protocols. Interhospital transport is mainly performed by private EMS agencies that are regulated by the EMS Act.
Study population
Patients who were diagnosed with spontaneous hemorrhagic stroke and received neurosurgery within 12 hours of admission to an ED between January 1, 2018, and December 31, 2019 were included. Patients with unknown information on outcomes and patients who received procedures delayed for more than 12 hours were excluded.
Patients with spontaneous hemorrhagic stroke were defined as patients who visited the ED with medical illnesses rather than injury and who were diagnosed with hemorrhagic stroke based on the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) code: I60 for SAH and I61 for ICH, with or without intraventricular hemorrhage. Patients diagnosed with subdural hemorrhage or epidural hemorrhage (ICD-10, I62) and those with traumatic brain injuries (ICD-10, S06) were not included.
Emergency neurosurgery was performed for spontaneous hemorrhagic stroke, including intraventricular burr-hole trephination, ventriculostomy, intraventricular hematoma evacuation, resection of cerebral arteriovenous malformation, craniotomy, and craniectomy [17]. Other vascular interventions, such as coil embolization, were not included.
Exposure and outcome measurements
The exposure of interest was the time from ED visit to surgery. Information on the time of surgery in the NEDIS database was collected based on the time of skin incision. Restricted cubic spline analysis was performed to evaluate cutoff values for time intervals associated with survival outcomes.
The primary outcome was in-hospital mortality. The secondary outcome was reoperation defined as any neurosurgery during ED/hospital admission following the first emergency operation. Although it has been assumed that reoperation usually represents major rebleeding or complications, it is not possible to distinguish between emergency reoperation and planned definite operation after emergency procedure in the NEDIS database.
Information on age, sex, EMS use, transfer from another hospital, level of ED, date and time of ED visit (MN–8AM, 8AM–4PM, and 4PM–MN), mental status at triage (alert, verbal response, pain response, and unresponsive), diagnosis at ED and hospital discharge (ICD-10 code), time and type of surgery (burr-hole trephination and other neurosurgery), disposition of ED and hospital, and in-hospital mortality were collected and analyzed.
Statistical analysis
For the study population, age- and sex-specific incidence rates per 100,000 person-years were calculated by subtype of hemorrhagic stroke using the 2019 midyear census population. Demographic and clinical findings with outcomes according to main exposure were described. Statistical differences between study groups were analyzed using the Wilcoxon rank sum test for continuous variables and the chi-square test for categorical variables.
Multivariable logistic regression analysis was conducted to evaluate the association between study groups and outcomes. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Potential confounders were selected, including age group (<50, 50–69, and over 70 years old), sex, EMS use, transfer from another hospital, level of ED (Level 1 and others), mental status at ED triage, type of hemorrhagic stroke (SAH, ICH, and mixed), and combined intraventricular hemorrhage.
Sensitivity analyses were performed to determine the optimal cutoff point for the elapsed time from the ED visit to surgical decompression, which is associated with survival outcomes. To enhance clinical utility, the elapsed time variable was dichotomized at each cutoff value in this analysis.
All statistical analyses were performed with SAS, version 9.4 (SAS, Cary, SC, USA), Microsoft Excel software (Microsoft Corp. 2020) and R, version 3.5 (R Foundation for Statistical Computing, Vienna, Austria) with the Hmisc package.
Ethics statement
This study complies with the Declaration of Helsinki. Its protocol was approved by the Institutional Review Board (IRB No. 2012-104-1183), and the requirement for informed consent was waived due to the retrospective nature of this study.
Results
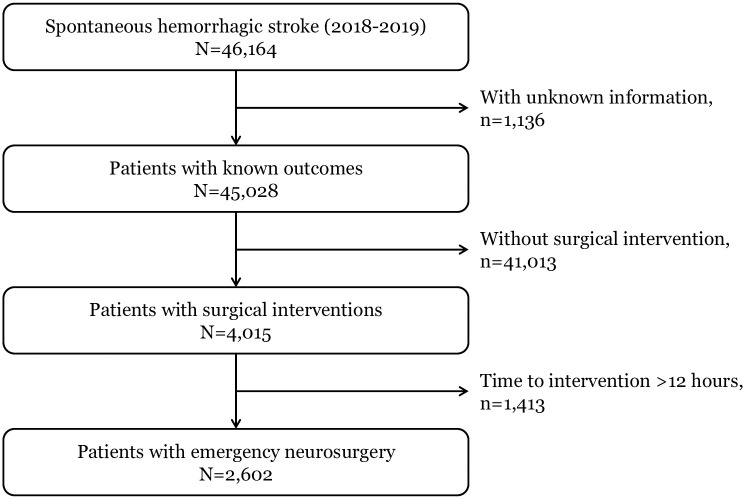
Among 46,164 patients (incidence rate: 46.2 per 100,000 person-years) who visited the ED and were diagnosed with spontaneous hemorrhagic stroke during the study period, a total of 2,602 patients (incidence rate: 2.5 per 100,000 person-years) were included in the final analysis after excluding 1,136 patients with unknown information on outcomes, 41,013 patients who did not receive procedures and 1,413 patients who received surgery delayed for more than 12 hours (Fig 1).
Fig 1. Study population flow.
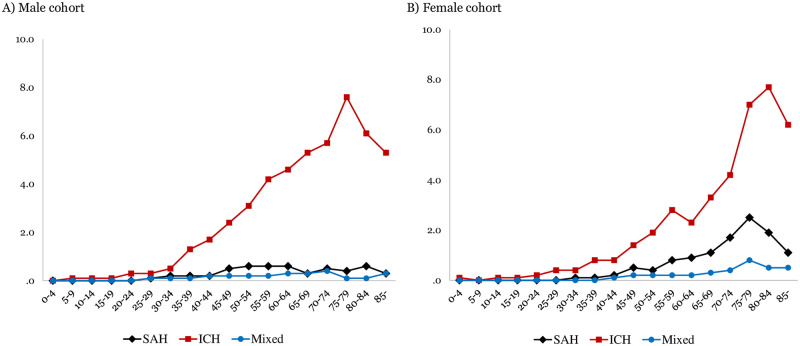
Age- and sex-specific incidence rates per 100,000 person-years are demonstrated by subtype of hemorrhagic stroke in Fig 2.
Fig 2. Age- and sex-specific incidence rates of spontaneous hemorrhagic stroke with emergency neurosurgery.
SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage.
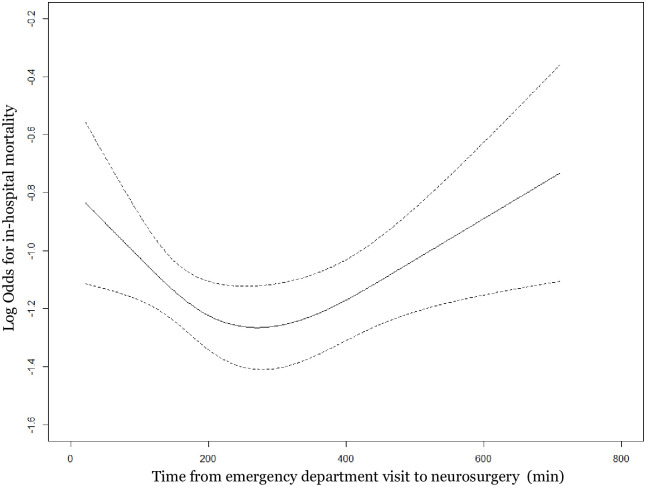
The restricted cubic spline graph between the time from ED admission to neurosurgery and in-hospital mortality for the study population is shown in Fig 3. Based on the U-shaped graph, 2 hours and 4 hours were considered cutoff values.
Fig 3. Restricted cubic spline graph of time from emergency department visit to neurosurgery and in-hospital mortality.
Demographics and clinical characteristics were compared between the ultra-early (0–2 hours), early (2–4 hours), and late (4–12 hours) groups; 525 (20.2%) patients received surgery in the ultra-early group, 1,093 (42.0%) in the early group, and 984 (37.8%) in the late group. In-hospital mortalities were 24.6% among the study population, 26.5% in the ultra-early group, 22.2% in the early group, and 26.1% in the late group (p-value, 0.06) (Table 1).
Table 1. Demographics of study population according to time from emergency department visit to emergency neurosurgery.
| Time from ED visit to neurosurgery | |||||
|---|---|---|---|---|---|
| Total | Ultra-early (0–2 hours) | Early (2–4 hours) | Late (4–12 hours) | p-value | |
| N (%) | N (%) | N (%) | N (%) | ||
| Total | 2,602 | 525 (20.2) | 1,093 (42.0) | 984 (37.8) | |
| Age, year, median (IQR) | 61 (51–73) | 61 (51–71) | 61 (51–74) | 61 (52–73) | 0.71 |
| 0–49 | 562 (21.6) | 116 (22.1) | 252 (23.1) | 194 (19.7) | 0.48 |
| 50–59 | 649 (24.9) | 134 (25.5) | 260 (23.8) | 255 (25.9) | |
| 60–69 | 568 (21.8) | 120 (22.9) | 226 (20.7) | 222 (22.6) | |
| 70–79 | 538 (20.7) | 106 (20.2) | 224 (20.5) | 208 (21.1) | |
| 80–120 | 285 (11.0) | 49 (9.3) | 131 (12.0) | 105 (10.7) | |
| Sex, male | 1,341 (51.5) | 268 (51.0) | 552 (50.5) | 521 (52.9) | 0.52 |
| EMS use | 1,670 (64.2) | 330 (62.9) | 703 (64.3) | 637 (64.7) | <0.01 |
| Transferred from other hospital | 789 (30.3) | 172 (32.8) | 339 (31.0) | 278 (28.3) | 0.16 |
| Level 1 ED | 1,185 (45.5) | 251 (47.8) | 513 (46.9) | 421 (42.8) | 0.08 |
| Time of ED visit | <0.01 | ||||
| MN–8AM | 482 (18.5) | 84 (16.0) | 182 (16.7) | 216 (22.0) | |
| 8AM–4PM | 1,138 (43.7) | 220 (41.9) | 462 (42.3) | 456 (46.3) | |
| 4PM–MN | 982 (37.7) | 221 (42.1) | 449 (41.1) | 312 (31.7) | |
| Mental status at triage | <0.01 | ||||
| Alert | 627 (24.1) | 82 (15.6) | 256 (23.4) | 289 (29.4) | |
| Verbal response | 500 (19.2) | 85 (16.2) | 216 (19.8) | 199 (20.2) | |
| Pain response | 1,114 (42.8) | 257 (49.0) | 483 (44.2) | 374 (38.0) | |
| Unresponsive | 361 (13.9) | 101 (19.2) | 138 (12.6) | 122 (12.4) | |
| Hemorrhagic stroke | <0.01 | ||||
| SAH | 411 (15.8) | 45 (8.6) | 155 (14.2) | 211 (21.4) | |
| ICH | 2,044 (78.6) | 460 (87.6) | 879 (80.4) | 705 (71.6) | |
| Mixed | 147 (5.6) | 20 (3.8) | 59 (5.4) | 68 (6.9) | |
| Combined IVH | 701 (26.9) | 187 (35.6) | 309 (28.3) | 205 (20.8) | <0.01 |
| Type of neurosurgery | <0.01 | ||||
| Burr-hole, in ED | 226 (8.7) | 83 (15.8) | 111 (10.2) | 32 (3.3) | |
| Burr-hole, in operation room | 1,250 (48.0) | 248 (47.2) | 556 (50.9) | 446 (45.3) | |
| Other neurosurgery | 1,126 (43.3) | 194 (37.0) | 426 (39.0) | 506 (51.4) | |
| ED disposition | <0.01 | ||||
| ICU admission | 500 (19.2) | 94 (17.9) | 141 (12.9) | 265 (26.9) | |
| Operation room | 2,048 (78.7) | 423 (80.6) | 923 (84.4) | 702 (71.3) | |
| Reoperation | 332 (12.8) | 61 (11.6) | 136 (12.4) | 135 (13.7) | 0.47 |
| In-hospital mortality | 639 (24.6) | 139 (26.5) | 243 (22.2) | 257 (26.1) | 0.06 |
ED, emergency department; IQR, interquartile range; EMS, emergency medical services; SAH, subarachnoid hemorrhage; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; ICU, intensive care unit.
By subtype of spontaneous hemorrhagic stroke, 10.9% of SAH, 22.5% of ICH, and 13.6% of mixed-type hemorrhage patients received neurosurgery in the ultra-early interval group. The in-hospital mortality rates were 128 (31.1%) in the SAH, 457 (22.4%) in the ICH, and 54 (36.7%) in the mixed hemorrhage groups (Table 2).
Table 2. Demographics of study population by subtype of spontaneous hemorrhagic stroke.
| Hemorrhagic stroke | |||
|---|---|---|---|
| Subarachnoid hemorrhage | Intracerebral hemorrhage | Mixed | |
| N (%) | N (%) | N (%) | |
| Total | 411 | 2,044 | 147 |
| Time to neurosurgery | |||
| Ultra-early (0–2 hours) | 45 (10.9) | 460 (22.5) | 20 (13.6) |
| Early (2–4 hours) | 155 (37.7) | 879 (43.0) | 59 (40.1) |
| Late (4–12 hours) | 211 (51.3) | 705 (34.5) | 68 (46.3) |
| Age, year, median (IQR) | 60 (51–73) | 61 (52–73) | 60 (49–72) |
| 0–49 | 86 (20.9) | 437 (21.4) | 39 (26.5) |
| 50–59 | 105 (25.5) | 512 (25.0) | 32 (21.8) |
| 60–69 | 87 (21.2) | 450 (22.0) | 31 (21.1) |
| 70–79 | 92 (22.4) | 415 (20.3) | 31 (21.1) |
| 80–120 | 41 (10.0) | 230 (11.3) | 14 (9.5) |
| Sex, male | 141 (34.3) | 1,134 (55.5) | 66 (44.9) |
| EMS use | 247 (60.1) | 1,317 (64.4) | 106 (72.1) |
| Transferred from other hospital | 144 (35.0) | 606 (29.6) | 39 (26.5) |
| Level 1 ED | 222 (54.0) | 892 (43.6) | 71 (48.3) |
| Time of ED visit | |||
| MN–8AM | 92 (22.4) | 366 (17.9) | 24 (16.3) |
| 8AM–4PM | 171 (41.6) | 908 (44.4) | 59 (40.1) |
| 4PM–MN | 148 (36.0) | 770 (37.7) | 64 (43.5) |
| Mental status at triage | |||
| Alert | 107 (26.0) | 503 (24.6) | 17 (11.6) |
| Verbal response | 67 (16.3) | 419 (20.5) | 14 (9.5) |
| Pain response | 153 (37.2) | 879 (43.0) | 82 (55.8) |
| Unresponsive | 84 (20.4) | 243 (11.9) | 34 (23.1) |
| Combined IVH | 0 (0.0) | 610 (29.8) | 91 (61.9) |
| Type of neurosurgery | |||
| Burr-hole, in ED | 26 (6.3) | 190 (9.3) | 10 (6.8) |
| Burr-hole, in operation room | 240 (58.4) | 941 (46.0) | 69 (46.9) |
| Other neurosurgery | 145 (35.3) | 913 (44.7) | 68 (46.3) |
| ED disposition | |||
| ICU admission | 67 (16.3) | 417 (20.4) | 16 (10.9) |
| Operation room | 335 (81.5) | 1,583 (77.4) | 130 (88.4) |
| Reoperation | 73 (17.8) | 237 (11.6) | 22 (15.0) |
| In-hospital mortality | 128 (31.1) | 457 (22.4) | 54 (36.7) |
IQR, interquartile range; ED, emergency department; IVH, intraventricular hemorrhage; EMS, emergency medical services; ICU, intensive care unit.
Among emergency neurosurgery, intraventricular burr-hole trephination was the most common type of surgery (57.9% for SAH and 52.4% for ICH), followed by intraventricular hematoma evacuation (35.0% for ICH) and craniotomy (26.3% for SAH) (Table 3).
Table 3. Emergency neurosurgery by subtype of spontaneous hemorrhagic stroke.
| Subarachnoid hemorrhage | Intracerebral hemorrhage | Mixed | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Reoperation | In-hospital mortality | Total | Reoperation | In-hospital mortality | Total | Reoperation | In-hospital mortality | |
| N | N (%) | N (%) | N | N (%) | N (%) | N | N (%) | N (%) | |
| Total | 411 | 73 (17.8) | 128 (31.1) | 2044 | 237 (11.6) | 457 (22.4) | 147 | 22 (15.0) | 54 (36.7) |
| Intraventricular burr-hole trephination | 238 | 34 (14.3) | 64 (26.9) | 1071 | 85 (7.9) | 232 (21.7) | 16 | 3 (18.8) | 6 (37.5) |
| Ventriculostomy | 6 | 2 (33.3) | 2 (33.3) | 4 | 1 (25.0) | 1 (25.0) | 76 | 10 (13.2) | 30 (39.5) |
| Intraventricular hematoma evacuation | 52 | 15 (28.8) | 11 (21.2) | 715 | 82 (11.5) | 159 (22.2) | 51 | 8 (15.7) | 18 (35.3) |
| Resection of arteriovenous malformation | 1 | 0 (0.0) | 1 (100.0) | 28 | 5 (17.9) | 2 (7.1) | 4 | 1 (25.0) | 0 (0.0) |
| Craniotomy | 108 | 21 (19.4) | 47 (43.5) | 215 | 63 (29.3) | 59 (27.4) | 0 | - | - |
| Craniectomy | 6 | 1 (16.7) | 3 (50.0) | 11 | 1 (9.1) | 4 (36.4) | 0 | - | - |
In multivariable logistic regression analysis, the early group showed better survival outcomes than the late group (adjusted OR (95% CI) for in-hospital mortality, 0.78 (0.63–0.96)), while there was no significant difference in the ultra-early group (adjusted OR (95% CI), 0.90 (0.69–1.16)) in Model 3. For reoperation, the adjusted ORs (95% CIs) were 0.92 (0.66–1.29) in the ultra-early group and 0.96 (0.74–1.24) in the early group (Table 4).
Table 4. Multivariable logistic regression analysis by time from emergency department visit to emergency neurosurgery.
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| In-hospital mortality | |||
| Ultra-early (0–2 hours) | 1.03 (0.81–1.31) | 0.89 (0.69–1.14) | 0.90 (0.69–1.16) |
| Early (2–4 hours) | 0.81 (0.67–1.00) | 0.77 (0.62–0.95) | 0.78 (0.63–0.96) |
| Late (4–12 hours) | 1.00 | 1.00 | 1.00 |
| Reoperation | |||
| Ultra-early (0–2 hours) | 0.83 (0.60–1.14) | 0.92 (0.66–1.28) | 0.92 (0.66–1.29) |
| Early (2–4 hours) | 0.90 (0.70–1.17) | 0.96 (0.74–1.24) | 0.96 (0.74–1.24) |
| Late (4–12 hours) | 1.00 | 1.00 | 1.00 |
OR, odds ratio; CI, confidence interval.
* Model 1: Adjusted for age, and sex.
* Model 2: Adjusted for variables in Model 1, mental status at triage, combined intraventricular hemorrhage, and type of hemorrhagic stroke.
* Model 3: Adjusted for variables in Model 2, emergency medical services use, transferred from other hospital, and level of emergency department.
To determine the optimal binary cutoff point for elapsed time from ED visit to emergency neurosurgery, four cutoffs at 30-minute intervals were selected considering the distribution of the study population (4, 4.5, 5, and 5.5 hours). For each cutoff, a multivariable logistic regression model was conducted. The lowest estimate in adjusted OR was observed in the 4.5-hour cutoff model (adjusted OR (95% CI), 0.80 (0.66–0.98)), and no significant estimate was found for reoperation in any time cutoffs (Table 5).
Table 5. Sensitivity analysis for cutoffs of time from emergency department visit to emergency neurosurgery.
| In-hospital mortality | Reoperation | |||
|---|---|---|---|---|
| n/N (%) | AOR (95% CI) | n/N (%) | AOR (95% CI) | |
| Cut-off, 4 hours | ||||
| Early (0–4 hours) | 382/1618 (23.6) | 0.82 (0.67–0.99) | 197/1618 (12.2) | 0.95 (0.75–1.21) |
| Late (4–12 hours) | 257/984 (26.1) | 1.00 | 135/984 (13.7) | 1.00 |
| Cut-off, 4.5 hours | ||||
| Early (0–4.5 hours) | 414/1748 (23.7) | 0.80 (0.66–0.98) | 214/1748 (12.2) | 0.94 (0.74–1.21) |
| Late (4.5–12 hours) | 225/854 (26.3) | 1.00 | 118/854 (13.8) | 1.00 |
| Cut-off, 5 hours | ||||
| Early (0–5 hours) | 453/1884 (24.0) | 0.84 (0.68–1.04) | 231/1884 (12.3) | 0.92 (0.71–1.19) |
| Late (5–12 hours) | 186/718 (25.9) | 1.00 | 101/718 (14.1) | 1.00 |
| Cut-off, 5.5 hours | ||||
| Early (0–5.5 hours) | 486/2020 (24.1) | 0.80 (0.64–1.00) | 248/2020 (12.3) | 0.90 (0.68–1.18) |
| Late (5.5–12 hours) | 153/582 (26.3) | 1.00 | 84/582 (14.4) | 1.00 |
ED, emergency department; AOR, adjusted odds ratio; CI, confidence interval.
Adjusted odd ratios were calculated adjusting for age, sex, emergency medical services use, transferred from other hospital, level of emergency department, mental status at triage, combined intraventricular hemorrhage, and type of hemorrhagic stroke.
Discussion
Using a nationwide emergency patient database, this study demonstrated the effects of the elapsed time interval from ED admission to neurosurgery on clinical outcomes in patients with spontaneous nontraumatic hemorrhagic stroke who need emergency neurosurgery. A significant association was found between early surgery within 2–4 hours of the ED visit and favorable survival outcomes in patients with spontaneous hemorrhagic stroke (adjusted OR (95% CI), 0.78 (0.63–0.96) for in-hospital mortality). However, there was no association between the elapsed time intervals and reoperations after emergency neurosurgery. In sensitivity analyses, 4.5 hours from ED admission to surgery has been demonstrated as the optimal binary cutoff time interval to enhance survival outcomes. These results suggest that it is important to establish the gold standard of emergency neurosurgery and to develop standard protocols for EDs, such as critical pathways for patients with spontaneous hemorrhagic stroke, to maximize the survival gain and minimize complications.
Emergency surgical decompression of elevated ICP is important to prevent the acceleration of brain edema, reduce mortality and length of stay in the ICU, and improve functional recovery [18–21]. It has been suggested that susceptible patients should be treated as early as possible, as cerebral edema compresses adjacent tissues, causing secondary damage. Early interventions affect clinical outcomes for patients with time-sensitive emergency conditions. In patients with ST-segment elevation acute myocardial infarction, the elapsed time interval from the ED visit to coronary intervention is known to be associated with postevent ventricular function and short-term and long-term mortalities [22,23]. Therefore, providing coronary intervention to those patients within 90 minutes of ED visits is recommended as a standard worldwide protocol [24]. Similarly, for ischemic stroke, vascular interventions within the golden time period—defined as 4.5 hours from symptom onset—are associated with favorable neurologic recovery [25]. In this study, patients with spontaneous hemorrhagic stroke who needed emergency neurosurgery experienced better outcomes when early interventions began within 4.5 hours of their arrival at the ED. These results suggest that multistrategic improvements in the diagnosis and treatment process for patients with hemorrhagic stroke are needed to improve survival outcomes.
In this study, there was a U-shaped relationship between the time of ED arrival to neurosurgery and in-hospital mortality in the restricted cubic spline graph. The ultra-early group was not associated with survival benefits from neurosurgery compared to the late group. This phenomenon is commonly found in critically ill patients who need emergency interventions [26–28]. Healthcare professionals may tend to perform early life-saving procedures for severely ill patients. In this study, the proportion of emergency burr-hole trephination in the ED was higher in the ultra-early group than in other groups, and the proportions of patients with altered mental status and cases who were transferred from other hospitals were also larger in that group. These factors may contribute to the relatively poor survival outcomes of the ultra-early group, and the survival benefits found in the early group were absent in the ultra-early group.
Determining the indications for emergency neurosurgery in patients with spontaneous hemorrhagic stroke and establishing the gold standard for timely surgery are reasonable for improving the clinical outcomes of patients facing a disease with such high mortality. There is a strong need to develop standard protocols for EDs in detail, such as critical pathways for patients with spontaneous hemorrhagic stroke who need surgical decompression for elevated ICP, to maximize the survival gain and minimize complications that may worsen rapidly in the early phase of disease progression.
Limitations
This study has several limitations. First, this study is a nationwide retrospective observational study. Potential biases could have affected the study results, and the generalizability of these data is limited. This is the inherent methodological limitation of observational studies. Second, information on the symptom onset, comorbidities, medical treatment, and disease severity of hemorrhagic stroke, including anatomical location of hemorrhage, amount of bleeding, estimated ICP, and main reason of neurosurgery are not collected in the NEDIS database. Although we limited the study population to patients who received neurosurgery within 12 hours of arrival in an ED, outcomes are limited to the in-depth interpretation of our results. Neurological or functional outcomes, such as modified Rankin Scale score, were also not be collected. In addition, since it is difficult to distinguish between emergency reoperation and planned definitive neurosurgery after emergency procedure, caution should be taken when interpreting these results given this significant limitation. Third, the time of neurosurgery is not the definite time of ICP reduction but the time of incision on the skin. Time to ICP reduction could affect the result but was unavailable in analysis.
Conclusions
Early surgery was associated with favorable survival outcomes in patients with spontaneous hemorrhagic stroke who needed emergency neurosurgery; however, there was no association between the elapsed time intervals and reoperations after emergency neurosurgery. Determining surgical indications and developing standard protocols for those patients are needed to improve survival outcomes for patients facing this fatal disease.
Data Availability
The data of this study were obtained from the National Emergency Medical Center under the Ministry of Health and Welfare in Korea but restrictions apply to the availability of these data and so are not publicly available. Data are available with the permission of the National Emergency Medical Center (contact point: bigdata@nmc.or.kr).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38(2):208–11. doi: 10.1055/s-0038-1649503 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. doi: 10.1016/s1474-4422(03)00266-7 [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: The GBD 2013 Study. Neuroepidemiology. 2015;45(3):161–76. doi: 10.1159/000441085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cha YJ. The Economic Burden of Stroke Based on South Korea’s National Health Insurance Claims Database. Int J Health Policy Manag. 2018;7(10):904–9. doi: 10.15171/ijhpm.2018.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit Care Med. 2009;37(7 Suppl):S243–9. doi: 10.1097/CCM.0b013e3181aa5de1 [DOI] [PubMed] [Google Scholar]
- 6.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703. doi: 10.1016/S0140-6736(10)60491-6 [DOI] [PubMed] [Google Scholar]
- 7.Goel K, Pinto DS, Gibson CM. Association of time to reperfusion with left ventricular function and heart failure in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: a systematic review. Am Heart J. 2013;165(4):451–67. doi: 10.1016/j.ahj.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 8.Zhang X-q, Zhang Z-m, Yin X-l, Zhang K, Cai H, Ling F. Exploring the optimal operation time for patients with hypertensive intracerebral hemorrhage: tracking the expression and progress of cell apoptosis of prehematomal brain tissues. Chinese Medical Journal. 2010;123(10). [PubMed] [Google Scholar]
- 9.Raafat M, Ragab OA, Abdelwahab OM, Salama MM, Hafez MA. Early versus delayed surgical evacuation of spontaneous supratentorial intracerebral hematoma: A prospective cohort study. Surg Neurol Int. 2020;11:145. doi: 10.25259/SNI_103_2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirmer CM, Hoit DA, Malek AM. Decompressive hemicraniectomy for the treatment of intractable intracranial hypertension after aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(3):987–92. doi: 10.1161/01.STR.0000257962.58269.e2 [DOI] [PubMed] [Google Scholar]
- 11.Wong GK, Boet R, Ng SC, Chan M, Gin T, Zee B, et al. Ultra-early (within 24 hours) aneurysm treatment after subarachnoid hemorrhage. World Neurosurg. 2012;77(2):311–5. doi: 10.1016/j.wneu.2011.09.025 [DOI] [PubMed] [Google Scholar]
- 12.Gregson BA, Broderick JP, Auer LM, Batjer H, Chen XC, Juvela S, et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43(6):1496–504. doi: 10.1161/STROKEAHA.111.640284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung E, Ro YS, Ryu HH, Shin SD, Moon S. Interaction Effects between COVID-19 Outbreak and Community Income Levels on Excess Mortality among Patients Visiting Emergency Departments. J Korean Med Sci. 2021;36(13):e100. doi: 10.3346/jkms.2021.36.e100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pak YS, Ro YS, Kim SH, Han SH, Ko SK, Kim T, et al. Effects of Emergency Care-related Health Policies during the COVID-19 Pandemic in Korea: a Quasi-Experimental Study. J Korean Med Sci. 2021;36(16):e121. doi: 10.3346/jkms.2021.36.e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho WS, Kim JE, Park SQ, Ko JK, Kim DW, Park JC, et al. Korean Clinical Practice Guidelines for Aneurysmal Subarachnoid Hemorrhage. J Korean Neurosurg Soc. 2018;61(2):127–66. doi: 10.3340/jkns.2017.0404.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JE, Ko SB, Kang HS, Seo DH, Park SQ, Sheen SH, et al. Clinical practice guidelines for the medical and surgical management of primary intracerebral hemorrhage in Korea. J Korean Neurosurg Soc. 2014;56(3):175–87. doi: 10.3340/jkns.2014.56.3.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T. Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir (Tokyo). 2014;54(11):887–94. doi: 10.2176/nmc.cr.2014-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Qiu Y, Zhang J, Zhang Q, Chen L, Chen L, et al. Emergent Single Burr Hole Evacuation for Traumatic Acute Subdural Hematoma with Cerebral Herniation: A Retrospective Cohort Comparison Analysis. World Neurosurg. 2018;120:e1024–e30. doi: 10.1016/j.wneu.2018.08.219 [DOI] [PubMed] [Google Scholar]
- 19.Kim KT, Park JK, Kang SG, Cho KS, Yoo DS, Jang DK, et al. Comparison of the effect of decompressive craniectomy on different neurosurgical diseases. Acta Neurochir (Wien). 2009;151(1):21–30. doi: 10.1007/s00701-008-0164-6 [DOI] [PubMed] [Google Scholar]
- 20.Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998;29(9):1888–93. doi: 10.1161/01.str.29.9.1888 [DOI] [PubMed] [Google Scholar]
- 21.Jabbarli R, Oppong MD, Dammann P, Wrede KH, El Hindy N, Ozkan N, et al. Time Is Brain! Analysis of 245 Cases with Decompressive Craniectomy due to Subarachnoid Hemorrhage. World Neurosurg. 2017;98:689–94.e2. doi: 10.1016/j.wneu.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 22.Yudi MB, Ramchand J, Farouque O, Andrianopoulos N, Chan W, Duffy SJ, et al. Impact of door-to-balloon time on long-term mortality in high- and low-risk patients with ST-elevation myocardial infarction. Int J Cardiol. 2016;224:72–8. doi: 10.1016/j.ijcard.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Lundergan CF, Reiner JS, Ross AM. How long is too long? Association of time delay to successful reperfusion and ventricular function outcome in acute myocardial infarction: the case for thrombolytic therapy before planned angioplasty for acute myocardial infarction. Am Heart J. 2002;144(3):456–62. doi: 10.1067/mhj.2002.124868 [DOI] [PubMed] [Google Scholar]
- 24.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 25.Kim JT, Fonarow GC, Smith EE, Reeves MJ, Navalkele DD, Grotta JC, et al. Treatment With Tissue Plasminogen Activator in the Golden Hour and the Shape of the 4.5-Hour Time-Benefit Curve in the National United States Get With The Guidelines-Stroke Population. Circulation. 2017;135(2):128–39. doi: 10.1161/CIRCULATIONAHA.116.023336 [DOI] [PubMed] [Google Scholar]
- 26.Delaveau A, Saint-Genez F, Gayet LE, Paccalin M, Ounajim A, Vendeuvre T. Impact of time to surgery in upper femoral fracture in orthogeriatrics. Orthop Traumatol Surg Res. 2019;105(5):975–8. doi: 10.1016/j.otsr.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 27.de Groot B, Ansems A, Gerling DH, Rijpsma D, van Amstel P, Linzel D, et al. The association between time to antibiotics and relevant clinical outcomes in emergency department patients with various stages of sepsis: a prospective multi-center study. Critical Care. 2015;19(1):194. doi: 10.1186/s13054-015-0936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarin N, Monga N, Adams PC. Time to endoscopy and outcomes in upper gastrointestinal bleeding. Can J Gastroenterol. 2009;23(7):489–93. doi: 10.1155/2009/604639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study were obtained from the National Emergency Medical Center under the Ministry of Health and Welfare in Korea but restrictions apply to the availability of these data and so are not publicly available. Data are available with the permission of the National Emergency Medical Center (contact point: bigdata@nmc.or.kr).