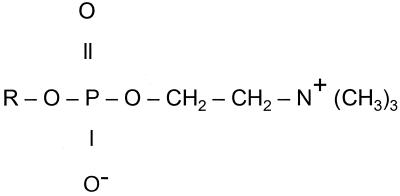Abstract
The protozoan parasite Entamoeba histolytica is the cause of amoebic dysentery and liver abscess. It is therefore responsible for significant morbidity and mortality in a number of countries. Infections with E. histolytica are treated with nitroimidazoles, primarily with metronidazole. At this time, there is a lack of useful alternative classes of substances for the treatment of invasive amoebiasis. Alkylphosphocholines (alkyl-PCs) such as hexadecyl-PC (miltefosine) were originally developed as antitumor agents, but recently they have been successfully used for the treatment of visceral leishmaniasis in humans. We examined hexadecyl-PC and several other alkyl-PCs with longer alkyl chains, with and without double bond(s), for their activity against two strains of E. histolytica. The compounds with the highest activity were oleyl-PC, octadecyl-PC, and nonadecenyl-PC, with 50% effective concentrations for 48 h of treatment between 15 and 21 μM for strain SFL-3 and between 73 and 98 μM for strain HM-1:IMSS. We also tested liposomal formulations of these alkyl-PCs and miltefosine. The alkyl-PC liposomes showed slightly lower activity, but are expected to be well tolerated. Liposomal formulations of oleyl-PC or closely related alkyl-PCs could be promising candidates for testing as broad-spectrum antiprotozoal and antitumor agents in humans.
Entamoeba histolytica is a protozoan parasite causing amoebic dysentery and liver abscess. Its life cycle is simple and includes the cysts, which are able to survive in the environment, and the trophozoites, which cause tissue damage. Infection with this protist occurs in many countries of the world, resulting in 36 to 50 million cases of invasive disease and up to 110,000 deaths per year (32).
The first-line drugs of amoebiasis chemotherapy are nitroimidazoles, with their prototype metronidazole [1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole] being the drug of choice (10, 21). It is effective, available at low cost, and the most widely used medicament for treating amoebiasis. Although treatment failures have been reported, there is at present no documented clinically relevant resistance. However, resistance against metronidazole is common in bacteria and other protozoan organisms, and in vitro trophozoites of E. histolytica are able to adapt to therapeutically relevant levels of the drug (25, 33). Because cross-resistance exists among the nitroimidazoles (29), there is no equally effective and tolerated class of drug available in case of rising resistance to metronidazole.
Alkylphosphocholines (alkyl-PCs) were originally investigated for their antineoplastic activity (1, 7, 13). The model compound hexadecyl-PC (miltefosine) is licensed for the topical treatment of breast cancer skin metastases. In addition, hexadecyl-PC was shown to be active against trypanosomatids, such as Leishmania donovani (20), Trypanosoma brucei and Trypanosoma cruzi (3, 19, 26). Recently, a phase II trial using hexadecyl-PC for the treatment of visceral leishmaniasis has resulted in a 95% overall cure rate (15). In view of these promising results, we studied several alkyl-PCs that differed in length and saturation of the alkyl chain for their effect on E. histolytica.
Oral administration of hexadecyl-PC is associated with considerable gastrointestinal side effects (15). Because liposomal formulations of alkyl-PCs might be a way of avoiding or reducing these side effects, we also tested liposomes of alkyl-PCs and cholesterol. In this study, we demonstrate that both pure substances and liposomal formulations have significant amoebicidal activity in vitro.
MATERIALS AND METHODS
Chemicals.
Metronidazole was obtained from Sigma (St. Louis, Mo.).
Synthesis of alkyl-PCs.
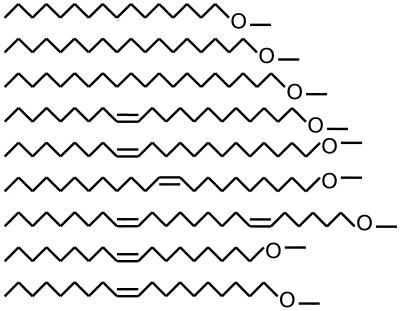
The different alkyl-PCs were prepared from the respective alcohols according to the synthetic routes described by Eibl (5). Detailed conditions have been reported for the synthesis of hexadecyl-PC (6). Briefly, the long-chain alcohols are converted to the alkyl-PCs in a two-step procedure. Step 1 includes three reactions: (i) phosphorylation of the long-chain alcohols with phosphorus oxychloride, (ii) ring closure of the respective long-chain alkylphosphorus dichloride with ethanolamine, and (iii) selective opening of the oxazaphospholane ring at the P-N bond under slightly acidic conditions to form the respective long-chain alkylphosphoethanolamines. In step 2, permethylation of the alkylphosphoethanolamines is achieved with dimethyl sulfate in the presence of potassium carbonate to result in the alkyl-PCs used in this study, as shown in Table 1. Liposomes were prepared and stored essentially as described in previous publications (8, 17, 18).
TABLE 1.
Alkyl-PCs examined in this studya
The alkyl-PCs are PC (top) esters with various long-chain saturated and unsaturated alkyl groups. The designations for the alkyl chains indicate first the number of carbon atoms of the alkyl chain followed by a colon and the number of double bonds.
Strains and growth conditions.
E. histolytica SFL-3 and HM-1:IMSS (ATCC 30459), both pathogenic strains having the zymodeme II isoenzyme pattern (27), were cultured axenically at 37°C in TYI-S-33 medium (4) containing 10% bovine serum. Strain SFL-3 was isolated in 1962 by G. L. Robinson at the Seamen's Hospital in Greenwich, United Kingdom, and later was passaged three times through hamster liver (14). Strain HM-1:IMSS was isolated in 1975 by B. Sepulveda and M. de la Torre.
Susceptibility tests.
Stock solutions (2 mM) of each alkyl-PC or metronidazole were prepared. APC1 to -6 and APC8 were dissolved in distilled water containing 5% (wt/vol) ethanol; the other compounds and the liposomal formulations were dissolved in distilled water. The compounds were made up in standard volumes of 660 μl that, when added to the culture vials, yielded final concentrations of 100, 50, 20, 10, and 5 μM. For comparison, metronidazole was tested against the same strains at the concentrations described above, and an additional sample was tested at a final concentration of 2 μM.
Experiments were performed with 48-h cultures. Trophozoites were harvested by centrifugation for 3 min at 500 × g and 4°C, resuspended in 20 ml of TYI-S-33 medium and counted in a Bürker Türk counting chamber. Between 8 × 105 and 1 × 106 trophozoites were suspended in 12 ml of prewarmed medium in 15-ml screw-cap glass test tubes (Dow Corning, Corning, N.Y.), the various substances were added, and incubation was continued at 37°C.
Cytotoxic activity of the alkyl-PCs was assessed after 24 and 48 h. Three cultures of each concentration and the control were briefly chilled on ice, transferred into 15-ml conical polypropylene tubes, and centrifuged (5 min, 500 × g, 4°C). Cells were resuspended in a final volume of 1 ml of a 1:1 mixture of 0.4% (wt/vol) trypan blue (Sigma) and phosphate-buffered saline for the determination of viability. Live and dead cells in each sample were counted in a Bürker Türk chamber.
Microscopy.
Trophozoites were treated with 100 μM metronidazole or with 100 μM oleyl-PC for 16 h at 37°C, and the effects on morphology of the amoebae were recorded by phase-contrast microscopy.
Statistical analysis.
Since the concentration-specific response of both strains of E. histolytica shows a log-normal distribution, the method of Litchfield and Wilcoxon (22) was applied to the analysis of the test data. This method is based on the log transformation of the drug concentrations and the probit transformation of the percentage of inhibition. The transformed data were used for the calculation of a linear regression according to the principle of least squares. Computer adaptations of the method were employed for analysis of single tests (12) and for processing of grouped data (34).
RESULTS
Treatment of parasites.
Trophozoites of E. histolytica were treated with hexadecyl-PC (miltefosine) and related alkyl-PCs (Table 1) or with metronidazole in regular test tube cultures. Two separate experiments were performed for each of the two strains, cytotoxicity was assayed after 24 and 48 h by trypan-blue exclusion, and at each time point and concentration, cells were counted from triplicate cultures. During the course of the experiments, there was some loss of viable trophozoites, which was observed in the untreated control cultures (SFL-3, after 24 h, 1.3%, and after 48 h, 5.6%; HM-1:IMSS, after 24 h, 3.3%, and after 48 h, 18.7%). This was taken into account for further analysis. Fifty percent effective concentrations (EC50s) and EC90s were calculated based on log probit statistics. The results are summarized in Table 2.
TABLE 2.
Effect of alkyl-PCs on E. histolytica SFL-3 and HM-1:IMSS trophozoites
| Substance | Alkyl chain | Time (h) | EC (μM) for straina:
|
|||
|---|---|---|---|---|---|---|
| SFL-3
|
HM-1:IMSS
|
|||||
| 50% | 90% | 50% | 90% | |||
| APC1 | C16:0 | 24 | 113 (57–225) | 441 (123–1,583) | 124 (98–157) | 347 (221–547) |
| 48 | 53 (28–99) | 260 (70–957) | 83 (67–104) | 187 (148–236) | ||
| APC2 | C18:0 | 24 | 35 (21–57) | 107 (48–239) | 94 (81–109)* | 301 (184–489) |
| 48 | 21 (13–34) | 46 (24–87) | 73 (63–85)* | 217 (146–325)* | ||
| APC3 | C20:0 | 24 | 96 (76–123) | 326 (164–649) | 137 (119–157)* | 496 (385–640) |
| 48 | 48 (43–55) | 86 (70–105) | 87 (83–91)* | 160 (124–204)* | ||
| APC4 | C21:1 | 24 | 39 (35–42) | 79 (69–91) | 206 (128–331)* | 790 (357–1,749)* |
| 48 | 35 (33–37) | 65 (57–73) | 109 (85–139)* | 257 (139–509)* | ||
| APC5 | C22:1 | 24 | 69 (44–108) | 191 (97–379) | 1,117 (220–5,672)* | 8,280 (531–129,111)* |
| 48 | 46 (28–75) | 120 (59–244) | 181 (125–262)* | 457 (243–858)* | ||
| APC6 | C22:1 | 24 | 62 (38–101) | 190 (85–424) | 351 (190–647)* | 1,257 (363–4,351)* |
| 48 | 33 (18–59) | 128 (42–388) | 204 (145–286)* | 1,316 (477–3,632)* | ||
| APC8 | C24:2 | 24 | 99 (52–189) | 354 (114–1,101) | 128 (62–268) | 288 (120–689) |
| 48 | 63 (36–110) | 225 (83–610) | 82 (58–116) | 169 (136–211) | ||
| APC9 | C18:1 | 24 | 29 (18–46) | 72 (36–142) | 260 (140–484)* | 1,152 (413–3,213)* |
| 48 | 15 (9–26) | 44 (19–100) | 88 (78–99)* | 159 (142–178)* | ||
| APC10 | C19:1 | 24 | 29 (19–45) | 68 (37–125) | 190 (121–297)* | 712 (307–1,653)* |
| 48 | 19 (12–30) | 48 (24–98) | 98 (68–140)* | 189 (131–272)* | ||
| Metronidazole | 24 | 89 (76–104) | 205 (155–272) | 106 (94–118) | 358 (295–434)* | |
| 48 | 53 (42–66) | 114 (86–149) | 65 (53–81) | 173 (139–215)* | ||
50% and 90%, EC50 and EC90, respectively. The EC represents the drug concentration at which 50 or 90% of the trophozoites were nonviable based on trypan blue staining. In parentheses are shown 95% confidence intervals. Values significantly higher (P < 0.05) for HM-1:IMSS than for SFL-3 are marked with asterisks.
Activity of hexadecyl-PC (miltefosine) and other alkyl-PCs against E. histolytica strain SFL-3.
All compounds showed an amoebicidal effect. Comparison of the EC50 after 24 and 48 h shows that the cytotoxic effect is not an immediate one, because in all cases, the EC50s after 2 days were significantly lower than after 1 day. APC1 (hexadecyl-PC) was surpassed in activity by all but one of the other alkyl-PCs, APC8, with the longest alkyl chain. The compound with the highest activity was APC9 (oleyl-PC), with an EC50 after 2 days of 15 μM, followed by APC10 and APC2. Unexpectedly, in our system, metronidazole had a much higher EC50 of 53 μM after 2 days. Even prolonged incubation for 72 h did not significantly lower the ECs.
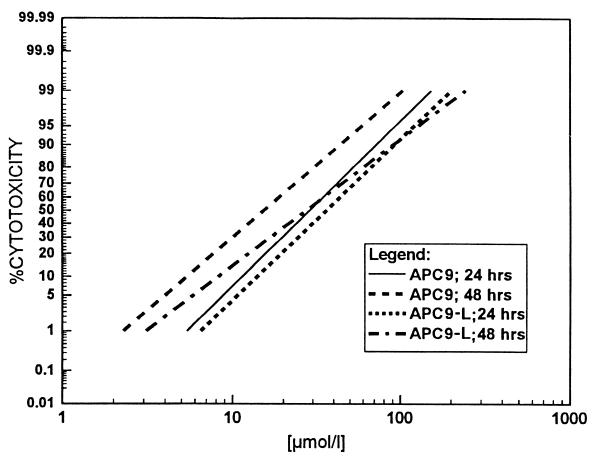
As an example, the activity regression for APC9 is shown in Fig. 1. The slope is relatively steep, so that the therapeutically important EC99 could be in the attainable range.
FIG. 1.
Comparison of the log-probit regressions obtained with APC9 and APC9-L in E. histolytica strain SFL-3 after 24 and 48 h of exposure. The percentage of cytotoxicity represents the proportion of trypan blue-positive trophozoites in the treated culture corrected by the proportion of nonviable cells in the untreated control culture.
Differences in susceptibility between strains SFL-3 and HM-1:IMSS.
In addition to strain SFL-3, we measured the effect of alkyl-PCs on strain HM-1:IMSS. Although both strains are pathogenic isolates belonging to zymodeme II, there were considerable differences; HM-1:IMSS was much less susceptible. This difference was statistically significant (P < 0.05) for many of the measurements. Those ECs that were significantly different between the two strains are marked with asterisks in Table 2. The highest activities were found for APC2 when EC50s were compared and for APC9 when EC90s were ranked. The compounds with the lowest activity, reflected in both EC50s and EC90s, were APC4, APC5, and APC6. The other compounds (APC1, APC3, APC8, and APC10) were ranked differently depending on whether EC50s or EC90s were compared. Although the ranking of the compounds was different between the two strains of amoebae, APC9, APC2 and APC10 could be considered the most promising candidates.
Activity of liposomal formulations of selected alkyl-PCs against the two E. histolytica strains.
For this part of the study, we selected compounds APC9, APC2, and APC10 for comparison with APC1 in liposomal formulations. The liposomal formulations were tested according to the same protocol as the pure substances. The results are summarized in Table 3. On average, there was a modest decrease in activity. This decrease was less pronounced with compounds having high activity, such as APC9 (see Fig. 1).
TABLE 3.
Effect on E. histolytica SFL-3 and HM-1:IMSS trophozoites of liposomal formulations of alkyl-PCs mixed with cholesterol
| Substance | Alkyl chain | Time (h) | EC (μM) for straina:
|
|||
|---|---|---|---|---|---|---|
| SFL-3
|
HM-1:IMSS
|
|||||
| 50% | 90% | 50% | 90% | |||
| APC1-L | C16:0 | 24 | 94 (77–116) | 346 (240–499) | 388 (292–515)* | 1,552 (1,069–2,252)* |
| 48 | 42 (37–47) | 112 (77–161) | 228 (87–600)* | 862 (192–3,874)* | ||
| APC2-L | C18:0 | 24 | 111 (72–170) | 454 (200–1,032) | 284 (158–512)* | 1,143 (276–4,731) |
| 48 | 39 (36–42) | 84 (72–99) | 141 (108–184)* | 371 (165–835)* | ||
| APC9-L | C18:1 | 24 | 36 (24–54) | 92 (50–170) | 507 (284–904)* | 3,257 (881–12,038)* |
| 48 | 27 (15–50) | 91 (32–256) | 192 (55–674)* | 1,044 (112–9,725) | ||
| APC10-L | C19:1 | 24 | 90 (48–170) | 317 (103–974) | 373 (158–882)* | 721 (121–4,316) |
| 48 | 51 (32–82) | 171 (76–383) | 170 (87–333)* | 689 (182–2,611) | ||
50% and 90%, EC50 and EC90, respectively. In parentheses are shown 95% confidence intervals. Values significantly higher (P < 0.05) for HM-1:IMSS than for SFL-3 are marked with asterisks.
In the case of strain SFL-3, the effects of the same alkyl-PC concentrations in liposomal and pure form were comparable, whereas for the HM-1:IMSS strain, EC50s of the liposomal formulations were much higher than those of the pure substances. The difference in susceptibility between the two strains was therefore even more pronounced with the liposomal formulations. Again, statistically significant differences (P < 0.05) are marked with an asterisk in Table 3.
Morphology of cell damage.
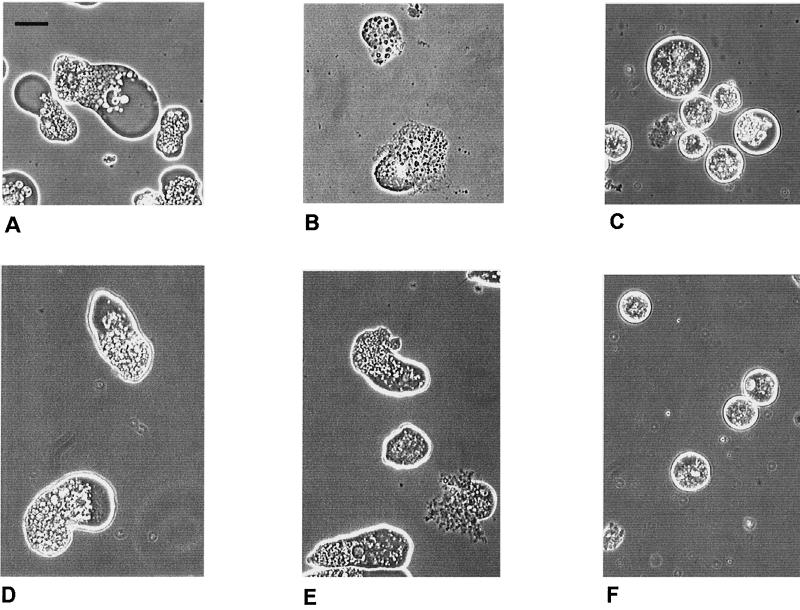
So far nothing is known about the mechanism of antiamoebic activity of alkyl-PCs. There was, however, a marked difference between the early morphological effects of oleyl-PC and metronidazole on amoebae, as shown in Fig. 2. Metronidazole typically produced rounding of cells. In contrast, some of the oleyl-PC-treated amoebae had a surface from which cytoplasmic components appeared to be bursting. In the long run, both drugs led to a complete disintegration of cells (not shown).
FIG. 2.
Phase-contrast microscopy showing the effect of treatment for 16 h with 100 μM oleyl-PC (APC9) or metronidazole on trophozoites of E. histolytica SFL-3 (A to C) and HM-1:IMSS (D to F). (A and D) Controls without added drug; (B and E) trophozoites treated with oleyl-PC; (C and F) trophozoites treated with metronidazole. The bar in panel A represents 20 μm.
DISCUSSION
In this study, the effect of miltefosine (hexadecyl-PC) and eight other alkyl-PCs on E. histolytica was demonstrated for the first time, extending the range of protozoal species that may potentially be targeted by alkyl-PC drugs. When comparing the activities of the alkyl-PCs, the results pointed to an enhanced activity of the compounds with alkyl chains with 18 or 19 carbon atoms. Exact ranking of the compounds depended on the strain tested or whether EC50s or EC90s were compared. Taken together, oleyl-PC (APC9) was the compound with the highest activity, followed directly by octadecyl-PC (APC2) and nonadecenyl-PC (APC10). Recent experiences with oleyl-PC for the treatment of canine visceral leishmaniasis (H. Eibl, unpublished data) would support the choice of oleyl-PC as a candidate drug. When methylnitrosourea-induced mammary tumors in rats were treated with alkyl-PCs, oleyl-PC showed higher antitumor efficacy and lower toxicity than the saturated octadecyl-PC (2). In a later study of the anticancer activity of four alkyl-PCs in the same rat model (28), oleyl-PC was rated again as the substance with the broadest therapeutic ratio. The combination of high efficacy and low toxicity might make oleyl-PC a broad-spectrum antitumor and antiparasite agent. However, APC2 and APC10 showed excellent activity in the present study and should also be included in future experiments.
Both E. histolytica strains tested were pathogenic amoebae of the same isoenzyme pattern, but HM-1:IMSS was less sensitive than SFL-3. So far there are no known differences between the two strains that might explain the significant differences in susceptibility. It will require more studies to elucidate eventual differences in membrane biochemistry and whether the E. histolytica strains common in areas of endemicity are closer to SFL-3 or to HM-1:IMSS in their sensitivity to alkyl-PCs.
The lipophilic alkyl-PCs influence membrane physiology in a pleiotropic way. In tumor cells, inhibition of phospholipid biosynthesis (11) and inhibition of signal transduction (30) have been discussed as possible mechanisms of action. In leishmania, ether lipid metabolism, glycosylphosphatidylinositol (GPI) anchor biosynthesis, and signal transduction appear to be the targets (23). At this point, we know very little about the mechanism of action in E. histolytica, with the exception of the observation that a high concentration of oleyl-PC caused a visible local area of destruction of the amoeba surface. The effects are reminiscent of the morphological changes observed in scanning electron microscopy of tumor cells treated with hexadecyl-PC (16).
Although metronidazole is likely to remain the first-line drug against E. histolytica, several arguments support the use of alkyl-PCs. In rats, hexadecyl-PC is rapidly taken up and accumulates in several internal organs, such as the kidneys, lung, spleen, and adrenal gland, as well as the liver, in which concentrations of 155 to 189 nmol/g of tissue were measured after oral treatment with 30 mg/kg of body weight twice per day (24). The accumulation of alkyl-PCs in the liver might turn out to be particularly useful in the treatment of amoebic liver abscess. Hexadecyl-PC is degraded rather slowly in vivo, with a half-life of 96 h (31). This may allow treatment with a few doses. Alkyl-PCs are slowly metabolized by phospholipases to form products such as choline and long-chain alcohols that are physiological metabolites and can be recycled into phospholipids (9).
Although alkyl-PCs as pure substances are effective and may accumulate in the affected organs, they cause some side effects. Oral administration of high doses of hexadecyl-PC produces serious weight loss in rats (13). Vomiting and diarrhea were frequently observed in the patients treated for visceral leishmaniasis (15). In order to reduce these side effects, a liposomal formulation of hexadecyl-PC was tested in a tumor model in rats. It showed a superior effect compared to the free drug and had reduced gastrointestinal toxicity (17). In the present study, new liposomal formulations of alkyl-PCs and cholesterol were cytotoxic against E. histolytica in vitro; however, they had higher EC50s and EC90s than those of the pure substances. (For comparison between APC9 and APC9-L in strain SFL-3, see Fig. 1.) The gain in tolerability may well be worth the modest reduction of activity. In vivo rodent studies will be performed to test the benefits of liposomal alkyl-PCs.
While alkyl-PCs were originally developed as anticancer drugs, more and more data have become available on their activity against parasites, such as those causing visceral and cutaneous leishmaniasis. This study has added E. histolytica as a possible new target. Important parameters of the pharmacology and toxicology of alkyl-PCs are studied for their application in anticancer chemotherapy and thus are also available for their application in tropical medicine. This is expected to considerably lower the costs for development of these drugs. Oleyl-PC, octadecyl-PC, and nonadecenyl-PC should be considered prime candidates for further research along these lines.
ACKNOWLEDGMENTS
We thank Hertha Erben for excellent work with the culture of the amoebae.
This work was supported by grant P12295-MED from the Austrian Science Fund and, in part, by a grant from the Hygiene Fund of the Clinical Institute for Hygiene of the University of Vienna.
REFERENCES
- 1.Berger R M, Muschiol C, Schmähl D, Eibl H J. New cytostatics with experimentally different toxic profiles. Cancer Treat Rev. 1987;14:307–317. doi: 10.1016/0305-7372(87)90023-5. [DOI] [PubMed] [Google Scholar]
- 2.Berger R M, Richter H, Seelig M H, Eibl H, Schmähl D. New cytostatics—more activity and less toxicity. Cancer Treat Rev. 1990;17:143–154. doi: 10.1016/0305-7372(90)90039-i. [DOI] [PubMed] [Google Scholar]
- 3.Croft S L, Snowdon D, Yardley V. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J Antimicrob Chemother. 1996;38:1041–1047. doi: 10.1093/jac/38.6.1041. [DOI] [PubMed] [Google Scholar]
- 4.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 5.Eibl H. Phospholipid synthesis: oxazaphospholanes and dioxaphospholanes as intermediates. Proc Natl Acad Sci USA. 1978;75:4074–4077. doi: 10.1073/pnas.75.9.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eibl H, Engel J. Synthesis of hexadecylphosphocholine (miltefosine) In: Eibl H, Hilgard P, Unger C, editors. New drugs in cancer therapy. Basel, Switzerland: Karger; 1992. pp. 1–5. [Google Scholar]
- 7.Eibl H, Unger C. Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat Rev. 1990;17:233–242. doi: 10.1016/0305-7372(90)90053-i. [DOI] [PubMed] [Google Scholar]
- 8.Eibl H, Kaufmann-Kolle P. Medical application of synthetic phospholipids as liposomes and drugs. J Liposome Res. 1995;5:131–148. [Google Scholar]
- 9.Fleer E A M, Unger C, Kim D-J, Eibl H. Metabolism of ether phospholipids and analogs in neoplastic cells. Lipids. 1987;22:856–861. doi: 10.1007/BF02535544. [DOI] [PubMed] [Google Scholar]
- 10.Freeman C D, Klutman N E, Lamp K C. Metronidazole. A therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Geilen C C, Wieder T, Reutter W. Hexadecylphosphocholine inhibits translocation of CTP:choline-phosphate cytidylyltransferase in Madin-Darby canine kidney cells. J Biol Chem. 1992;267:6719–6724. [PubMed] [Google Scholar]
- 12.Grab B, Wernsdorfer W H. Evaluation of in vitro tests for drug sensitivity in Plasmodium falciparum: probit analysis of logdose/response test from 3–8 points assay. W.H.O. document W.H.O./MAL/83.990. Geneva, Switzerland: World Health Organization; 1983. [Google Scholar]
- 13.Hilgard P, Stekar J, Voegeli R, Engel J, Schumacher W, Eibl H, Unger C, Berger M R. Characterization of the antitumor activity of hexadecylphosphocholine (D 18506) Eur J Cancer Clin Oncol. 1988;24:1457–1461. doi: 10.1016/0277-5379(88)90336-7. [DOI] [PubMed] [Google Scholar]
- 14.Jarumilinta R. A simple method of inducing amoebic liver abscess in hamsters. Ann Trop Med Parasitol. 1966;60:139–145. doi: 10.1080/00034983.1966.11686397. [DOI] [PubMed] [Google Scholar]
- 15.Jha T K, Sundar S, Thakur C P, Bachmann P, Karbwang J, Fischer C, Voss A, Berman J. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N Engl J Med. 1999;341:1795–1800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann-Kolle P, Fleer E A. Morphological changes of adherent and nonadherent cells by treatment with hexadecylphosphocholine and 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine observed by scanning electron microscopy. Prog Exp Tumor Res. 1992;34:47–58. doi: 10.1159/000420831. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann-Kolle P, Drevs J, Berger M R, Kötting J, Marschner N, Unger C, Eibl H. Pharmacokinetic behaviour and antineoplastic activity of liposomal hexadecylphosphocholine. Cancer Chemother Pharmacol. 1994;34:393–398. doi: 10.1007/BF00685563. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann-Kolle P, Kuhlencord A, Eibl H. Intravenous treatment of Leishmania donovani infected mice with liposomal hexadecylphosphocholine. Cell Mol Biol Lett. 1996;1:429–437. [Google Scholar]
- 19.Konstantinov S M, Kaminsky R, Brun R, Berger M R, Zillmann U. Efficacy of anticancer alkylphosphocholines in Trypanosoma brucei subspecies. Acta Trop. 1997;64:145–154. doi: 10.1016/s0001-706x(96)00628-6. [DOI] [PubMed] [Google Scholar]
- 20.Kuhlencord A, Maniera T, Eibl H, Unger C. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob Agents Chemother. 1992;36:1630–1634. doi: 10.1128/aac.36.8.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamp K C, Freeman C D, Klutman N E, Lacy M K. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36:353–373. doi: 10.2165/00003088-199936050-00004. [DOI] [PubMed] [Google Scholar]
- 22.Litchfield J T, Wilcoxon F. A simplified method for evaluating dose-effect experiments. J Pharm Exp Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- 23.Lux H, Hart D T, Parker P J, Klenner T. Ether lipid metabolism, GPI anchor biosynthesis, and signal transduction are putative targets for anti-leishmanial alkylphospholipid analogues. Adv Exp Med Biol. 1996;416:201–211. doi: 10.1007/978-1-4899-0179-8_33. [DOI] [PubMed] [Google Scholar]
- 24.Marschner M, Kötting J, Eibl H, Unger C. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother Pharmacol. 1992;31:18–22. doi: 10.1007/BF00695989. [DOI] [PubMed] [Google Scholar]
- 25.Samarawickrema N A, Brown D M, Upcroft J A, Thammapalerd N, Upcroft P. Involvement of superoxide dismutase and pyruvate:ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J Antimicrob Chemother. 1997;40:833–840. doi: 10.1093/jac/40.6.833. [DOI] [PubMed] [Google Scholar]
- 26.Santa-Rita R M, Santos Barbosa H, Meirelles M N, de Castro S L. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 2000;75:219–228. doi: 10.1016/s0001-706x(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 27.Sargeaunt P G. The reliability of Entamoeba histolytica zymodemes in clinical diagnosis. Parasitol Today. 1987;3:40–43. doi: 10.1016/0169-4758(87)90211-0. [DOI] [PubMed] [Google Scholar]
- 28.Sobottka S B, Berger M R, Eibl H. Structure-activity relationships of four anti-cancer alkylphosphocholine derivatives in vitro and in vivo. Int J Cancer. 1993;53:418–425. doi: 10.1002/ijc.2910530312. [DOI] [PubMed] [Google Scholar]
- 29.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 30.Überall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke H H. Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res. 1991;51:807–812. [PubMed] [Google Scholar]
- 31.Unger C, Fleer E, Damenz W, Hilgard P, Nagel G, Eibl H. Hexadecylphosphocholine: determination of serum concentrations in rats. J Lipid Mediat. 1991;3:71–78. [PubMed] [Google Scholar]
- 32.Walsh J A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 33.Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 1999;37:26051–26056. doi: 10.1074/jbc.274.37.26051. [DOI] [PubMed] [Google Scholar]
- 34.Wernsdorfer W H, Wernsdorfer M G. The evaluation of in vitro tests for the assessment of drug response in Plasmodium falciparum. Mitt Oesterr Ges Tropenmed Parasitol. 1995;17:221–228. [Google Scholar]