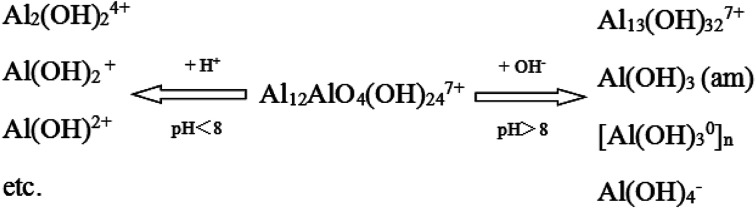Abstract
High-poly-aluminum chloride sulfate (HPACS) coagulants with different [SO42−]/[Al3+] molar ratio (S) were prepared and proved to have high coagulation efficiency for the removal of humic acid and strong stability for storage and application. The results showed that the higher the SO42− addition, the bigger the aluminum polymerization particles and the more the polymerization Alc existed in the prepared HPACS coagulants. The HPACS exhibited higher coagulation efficiency, a better aging stability and stronger resistance to the change of pH and Ca2+ concentration of raw water than the polyaluminum chloride (PAC) and poly-aluminum chloride sulfate (PACS) reported before. The Sips adsorption neutralization model was established to illustrate the relationship between coagulant dosage and zeta potential of the water system. The adsorption neutralization capacity was proved to be HPACS (S = 0) > HPACS (S = 0.02) > HPACS (S = 0.06) > HPACS (S = 0.10), which was not completely consistent with the coagulation effect of HPACS with different S values and indicated that in addition to adsorption neutralization, actions like bridge-aggregation, precipitation, and sweep-flocculation also played an important role during HPACS coagulation. Moreover, the negative Gibbs free energy indicated that the coagulant adsorption neutralization reaction was a spontaneous process.
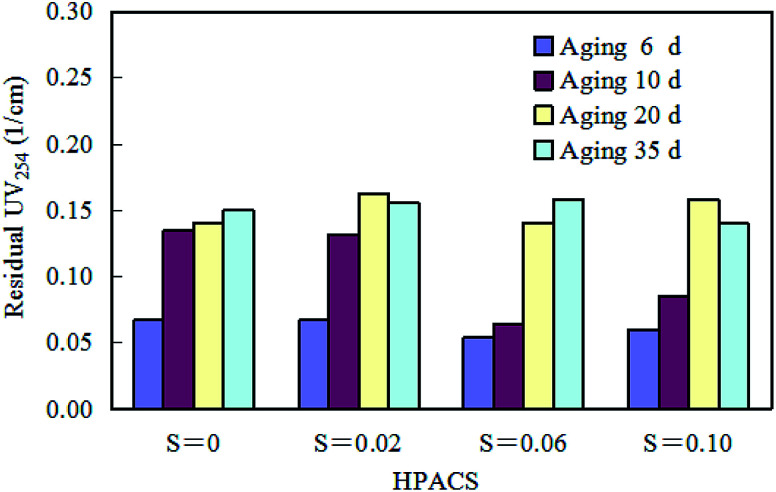
Effect of aging time of HPACS on HA removal (with a minimum absolute deviation of 5%).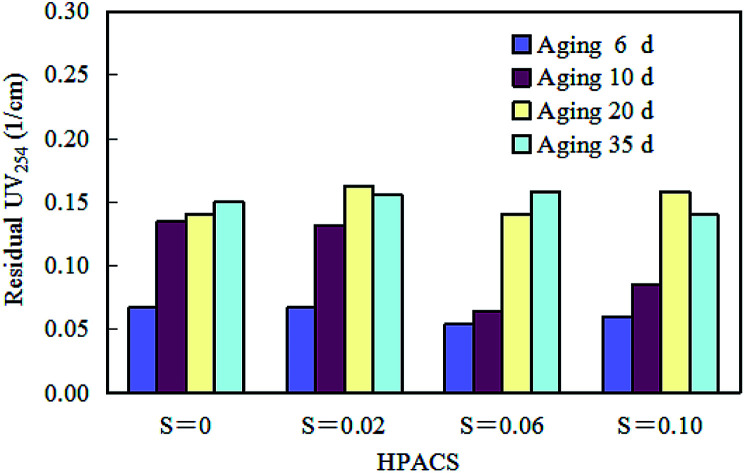
1. Introduction
Coagulation is one of the most widely used techniques in the field of water and wastewater treatment with its advantages of simplicity, economy, convenience and high efficiency. Although the development of coagulation technology is relatively mature, the mechanisms of coagulation are not completely clear, since the coagulation involves many fields, such as aqueous chemistry, colloid and micro-interface chemistry, and fluid mechanics. Moreover, with the development of society, the continuous emergence of new pollutants and the increasing demand of water quality present new challenges and opportunities for coagulation.
Coagulation is important for particle and natural organic matter (NOM) removal,1–3 in which charge neutralization, bridge-aggregation, precipitation, and sweep-flocculation were recognized as the general mechanisms. In order to improve the coagulation efficiency, many researchers have been devoted to developing more efficient and low-residue coagulant species. Compared with organic coagulants, inorganic coagulants are more widely used in the water supply and experienced the developing trend from single-molecule salts (e.g. aluminum chloride, AlCl3) to polymer salts (e.g. polyaluminum chloride, PAC), or polymer composite coagulants.
In recent decades, people have produced various types of polymer composite coagulants like poly-silicic-cation (PSiCs),4,5 polymeric aluminum ferric sulfate (PAFS),6 polyaluminum ferric silicate chloride (PAFSC)7,8 and polyaluminum chloride-chitosan (PACC)9etc. The efficiency of these new coagulants is higher than those without complex substances. An excellent removal efficiency of humic acid (HA) was achieved with a series of polyaluminum chloride sulfate (PACS) coagulants in our previous study.10 However, we found that after introducing a certain amount of SO42− into the PAC, the storage stability of the coagulants was lowered, and the coagulation capability greatly decreased in less than a month.
Actually, a poor stability has always been a limiting factor for all polymer composite coagulants, which restricts the production and application of coagulants. It was reported that acidic chemical, such as phosphate, acetate, silicate and citric acid, could be added into polymer composite coagulants as a stabilizer to enhance the stability of coagulants.11 However, this method is not suitable for water supply because these added stabilizers may remain in water to cause secondary pollution. It has been reported that besides adding stabilizers, thermal treatment can also improve coagulation performance.12,13 In this study, high-poly-aluminum chloride sulfate (HPACS) coagulants with different [SO42−]/[Al3+] molar ratios (S) were prepared by a simple thermal treatment of PACS. The hydrolysis morphology and particle size of prepared HPACS were characterized. The coagulation behaviors of HPACS for humic acid (HA, a group of typical natural organic matters (NOM), accounting for more than 50% of whole NOM in water) removal from water were examined under different initial pH and coagulant dosages. The charge neutralization performances of HPACS were investigated and the relationship between coagulant dosage and zeta potential of water samples was simulated with Sips adsorption isotherm. In addition, the coagulant stability along with aging time and the effect of coexisting Ca2+ in raw water were investigated. The purpose of this study is to find a novel coagulant with superior coagulation performance and clarify the adsorption neutralization process.
2. Materials and methods
2.1. Preparation of HPACS
All reagents used in the research were analytical grade except for HA. The HA was a mixture and with the average molecular weight of about 3000. All solutions were prepared by deionized water except those pointed out specifically. PACS with a S value of 0, 0.02, 0.06 and 0.10 was prepared as reported.10 HPACS was prepared by thermal treatment of PACS solutions at 95 °C for 12 h under stirring and refluxing. The total aluminum concentration (Alt) of all coagulant solution was 200 mmol L−1 and the basicity (B = [OH−]/[Al3+]) value was 2.0. All coagulants were in liquid and aged at room temperature for 6 d before coagulation experiments except for that the aging time of coagulants was 20 d when the effect of coexisting Ca2+ was analyzed.
2.2. Water sample and coagulation experiments
The stock HA solution and working solution were prepared as reported.10 The pH of working solutions was adjusted to around 7.0 except for specified. For the effect of coexisting Ca2+ on the HA removal, a calculated amount of CaCl2 was added in the working solution.
Coagulation experiments were all carried out at room temperature (about 20 °C) using jar test on a six-paddle gang stirrer (MY3000-6B, Meiyu Co., China). 500 ml working solution was added into 1000 ml beaker. A measured amount of coagulant was added into the working solution under rapid stirring at 200 rpm for 2 min, followed by slow stirring at 30 rpm for 20 min, and then settled for 30 min. The coagulant dosage was 0.02 mmol L−1 as Alt (unless otherwise given).
2.3. Analytical methods
The Al-ferron time-wise complex colorimetric analysis was applied to investigate the hydrolysis forms of aluminum in HPACS using a UV-Vis spectrometer (2550, Shimadzu Co., Japan). Absorbance values at 370 nm were picked out from 1 min to 120 min after mixing the sample with ferron reagent.14,15 After reacting for 1 min, the reacted species was regarded as Ala, for 1 to 120 min as Alb, and no reaction before 120 min as Alc. Particle size of HPACS was measured with a Zetasizer Nano ZS (3000HS, Malvern Co., U.K.) after filtration with a microfiltration membrane of 0.2 μm pore size. The UV254 (absorbance at 254 nm) was measured through a UV-Vis spectrometer (2550, Shimadzu Co., Japan). The zeta potential of water sample was immediately measured after addition of coagulant and rapid mixing with a Zetasizer Nano ZS (3000HS, Malvern Co., U.K.).
3. Results and discussion
3.1. Hydrolysis morphology and particle size of HPACS
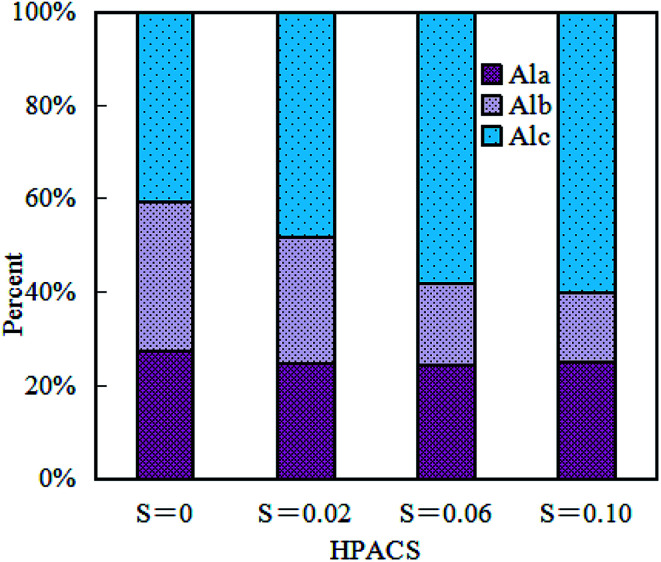
Many aluminum hydroxyl forms have been found in aluminum salts so far,16–18 and the mononuclear or primary polymer like Al3+, Al(OH)2+, Al(OH)2+, Al(OH)4−, Al2(OH)24+, Al2(OH)5+, Al3(OH)8+, Al3(OH)45+ was defined as Ala, the medium polymer like AlO4Al12(OH)24(H2O)127+ (Al13) was defined as Alb, and the high polymer or precipitate like Al15(OH)369+, [A130O8(OH)56(H2O)24]18+ (Al30), Alx(OH)y(3x−y)+ or [Al(OH)30]n was defined as Alc.19Fig. 1 shows the Al-ferron complex colorimetric morphology distribution of the prepared HPACS. With the increase of S value, the morphology of Ala in HPACS remained at around 25%, while Alb was further transferred into Alc gradually. The Alc content was 40.6%, 48.4%, 58.1% and 60.1% for HPACS with a S of 0, 0.02, 0.06 and 0.10, respectively.
Fig. 1. Hydrolysis species distribution of HPACS.
The Alc with high degree of polymerization may play a better bridge-aggregation, precipitation, and sweep-flocculation role in the coagulation process. Wang et al.20 found that when the charge neutralization ability of inorganic polymer coagulant reached a certain level, polymerization degree of the inorganic polymer coagulant would be the decisive factor affecting the coagulation efficiency. Zhao et al.21 obtained a polyaluminum chloride solution with an Alc content of 81% by membrane distillation. The importance of Alc in the hydrolysis of aluminum has attracted more and more attention.
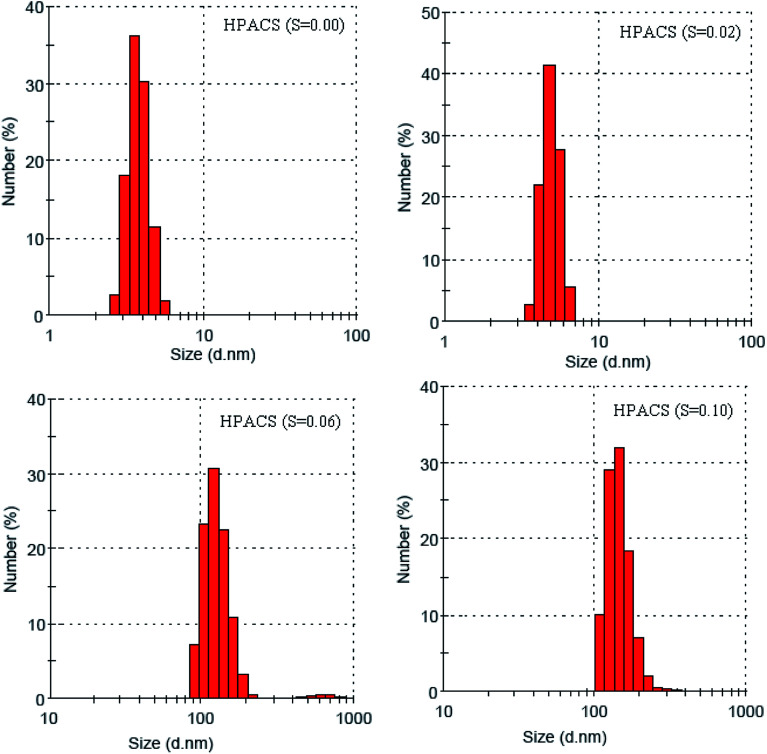
The particle size distribution of prepared HPACS is shown in Fig. 2. The HPACS (S = 0.06) and HPACS (S = 0.10) had a much higher polymerization degree than the HPACS (S = 0) and HPACS (S = 0.02). The average diameter of HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10) were 3.9, 5.0, 137.7 and 142.9 nm, respectively. Many researchers believed that the Alb had a good relationship with [Al13O4(OH)24]7+ (Al13),18,22 which with a molecular weight of 1039 and a molecular particle size of 2–3 nm. The HPACS (S = 0) and HPACS (S = 0.02) possessed quite more content of Alb, so their particle size was nearly close to Al13. The macromolecular particles appeared in HPACS (S = 0.06) and HPACS (S = 0.10) may be caused by the aggregation of Alb or Alc, in which the sulfate may act as a bridge.10
Fig. 2. Particle size distribution of HPACS.
3.2. Effect of initial pH on HA removal by HPACS
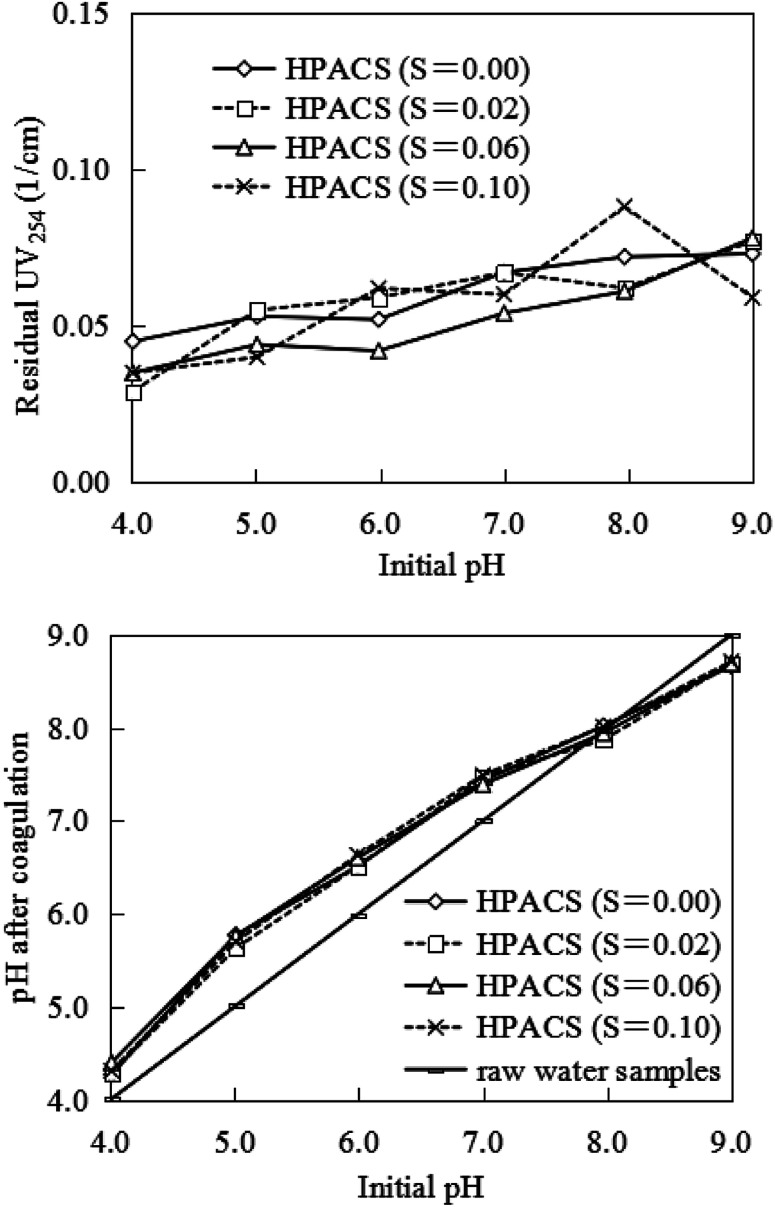
The initial pH of water sample is one of the important factors affecting the coagulation effect.23 The principle of this action is generally considered to be that different pH may change the hydrolysis species of aluminum salts and the protonation degree of HA. However, this effect became insignificant when examining the effect of initial pH on HPACS coagulation as shown in Fig. 3. Although the residual UV254 of water sample tended to increase with the increase of pH, the initial pH had no significant effect on the HPACS coagulation performances compared with the PAC and PACS as coagulant.10,15,24 For the HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10), the residual UV254 varied only 0.028, 0.048, 0.043 and 0.053 cm−1, respectively at the pH rang of 4.01 to 9.00. This behavior of HPACS breaks the conventional knowledge that aluminum coagulants usually present worse coagulation performances in alkaline condition.25,26
Fig. 3. Effect of initial pH on HA removal by HPACS (with a minimum absolute deviation of 5%).
Effluent pH after by HPACS coagulation was also tested. As shown in Fig. 3, when the initial pH was less than 8.0, the effluent pH was higher than that of raw water, and the HPACS exhibited properties of Lewis base. When the initial pH was higher than 8.0, the effluent pH was lower than that of raw water, and the HPACS exhibited properties of Lewis acid. Tang et al.27,28 pointed out that the hydrolysis and polymerization of aluminum hydroxyls presented two parallel processes in water, and one of which would play a major role under different pH condition. At a lower pH condition, the free H+ in the system reduced the degree of polymerization by impacting the hydroxyl bridge (Al–OH–Al) and oxygen bridge (Al–O–Al) of polymerized aluminum, and the pH of water system would increase due to the consumption of H+. In a higher pH environment, the hydroxylation of aluminum would be enhanced to form Al(OH)3 (am) or even Al(OH)4−, which may cause a slight decrease in water pH, and the neutralization ability of aluminum hydroxyls became weakened simultaneously. Fig. 4 showed the possible reaction pathway of Al13 (a typical medium polyaluminum hydroxyls form in HPACS) under different pH conditions. Overall, although the HPACS dosing caused the pH change, the increased or decreased pH were all less than 0.5, which was smaller than that with other aluminum salts previously reported,15 indicating that HPACS have a stronger stability under different pH conditions.
Fig. 4. Possible reaction pathways of HPACS under different pH conditions (taking Al13 as an example).
3.3. Effect of HPACS dosage on HA removal
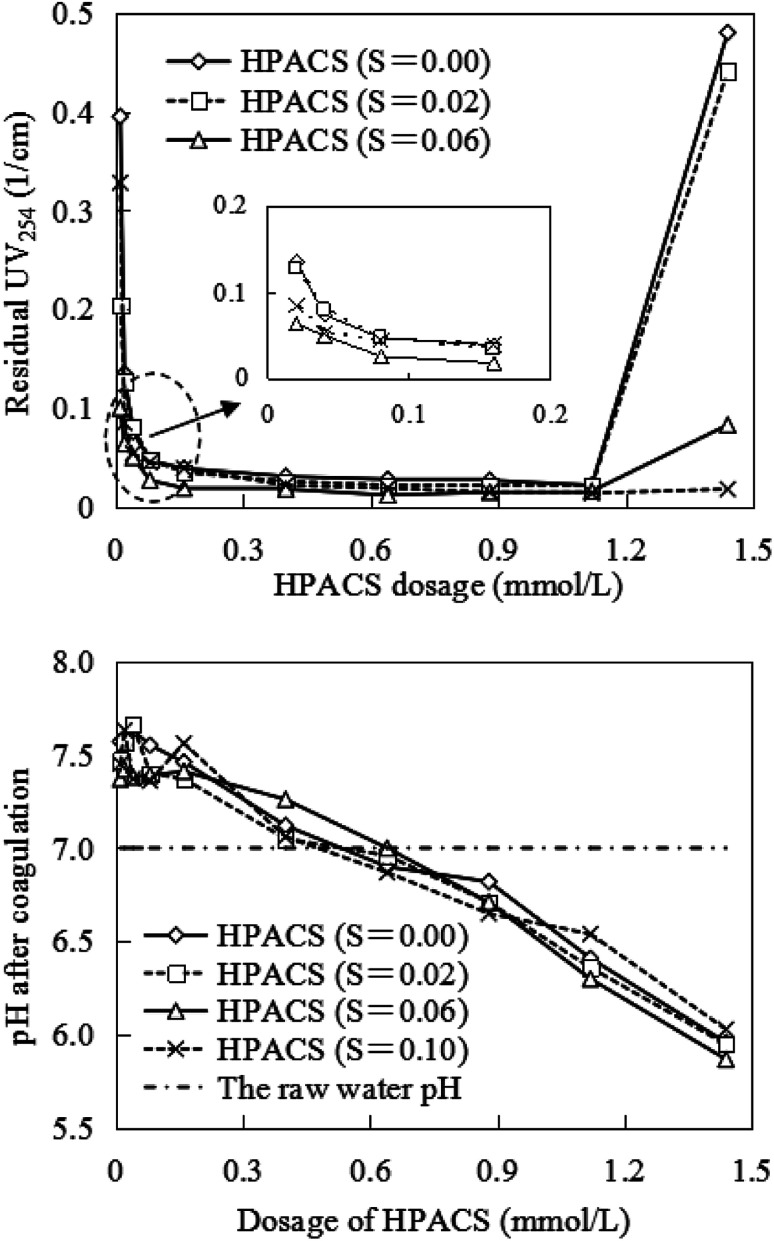
The effect of HPACS dosage on HA removal is shown in Fig. 5. The residual UV254 decreased rapidly at first and then decreased slowly along with the increase of HPACS dosage. The best HA removal was achieved at a HPACS (S = 0.06) dosage of 0.64 mmol L−1, and the HA removal was 97.3% with a UV254 value of 0.012 cm−1. Compared to the PACS studied previously,10 the HA removal was only 75.6% for PACS (S = 0.06) at the same condition, which illustrated that thermal treatment may improve the performances of PACS coagulants. Moreover, it was found that HPACS with a S value of 0.06 showed a slightly better coagulation effect at a dosage range of 0.01–1.18 mmol L−1 than the other coagulants investigated.
Fig. 5. Effect of HPACS dosage on HA removal (with a minimum absolute deviation of 5%).
While the HPACS dosage was over than 1.18 mmol L−1, the water system showed significant re-stabilization except for the HPACS (S = 0.10). The HA removal reduced to 79.8% for HPACS (S = 0.06), and the residual UV254 even exceeded the raw water for HPACS (S = 0.02) and HPACS (S = 0), which may be caused by the flocs in water, preventing UV254 light transmission. Furthermore, by comparison with Fig. 1, it was surprisingly found that the re-stabilization degree of four HPACS at high dosage was inversely proportional to the Alc amount in HPACS. The Alc with high polymerization degree may play a better bridge-aggregation, precipitation, and sweep-flocculation role in the coagulation process.
The effluent pH after coagulation is shown in Fig. 5. At the low coagulant dosage, the effluent pH was slightly higher than that of the raw water due to the hydrolysis of aluminum hydroxyl compounds. As the coagulant dosage increased, the effluent pH decreased, and dropped to about 6.0 when the HPACS dosage was 1.44 mmol L−1. The hydrolysis of polymerized aluminum was maybe weakened due to mutual inhibition between the hydrolyzed products. Moreover, the prepared polymeric aluminum coagulant solution showed a pH of about 4.0, which was lower than that of the raw water (7.0), and the HPACS dosing may also cause acidification of water samples to some extent.
3.4. Zeta potential and adsorption neutralization model of HPACS
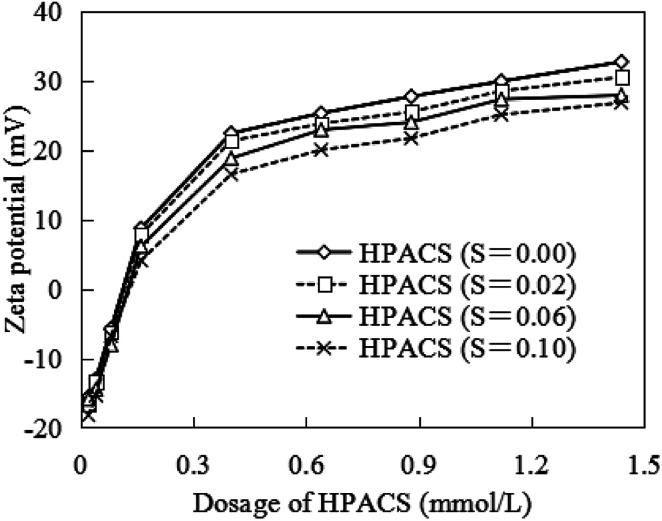
As shown in Fig. 6, when the HPACS dosage was less than 0.16 mmol L−1, the zeta potential value increased rapidly and almost reached the isoelectric points at a HPACS dosage of about 0.10 mmol L−1. Then, with the increase of HPACS dosage the increase rate of zeta potential decreased, and the difference among the four HPACS with different S value appeared. In the whole dosage range of 0.16–1.44 mmol L−1, although the disparity of zeta potential among HPACS were not very large, the zeta potential of water samples always maintained higher with a higher S value.
Fig. 6. Effect of HPACS dosage on the zeta potential of water samples (with a minimum absolute deviation of 5%).
Zeta potential change of colloidal system after coagulant dosing is usually caused by the adsorption of coagulant species onto colloidal particles so that the adsorption isotherm may be used to quantitatively describe the adsorption neutralization of aluminum species onto HA particles.19 The mathematical function of Sips isotherm is expressed as eqn (1):
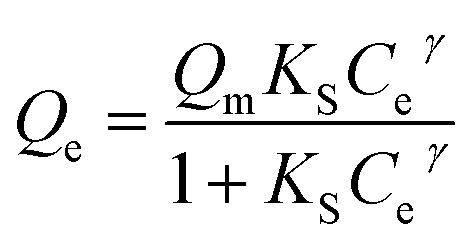 |
1 |
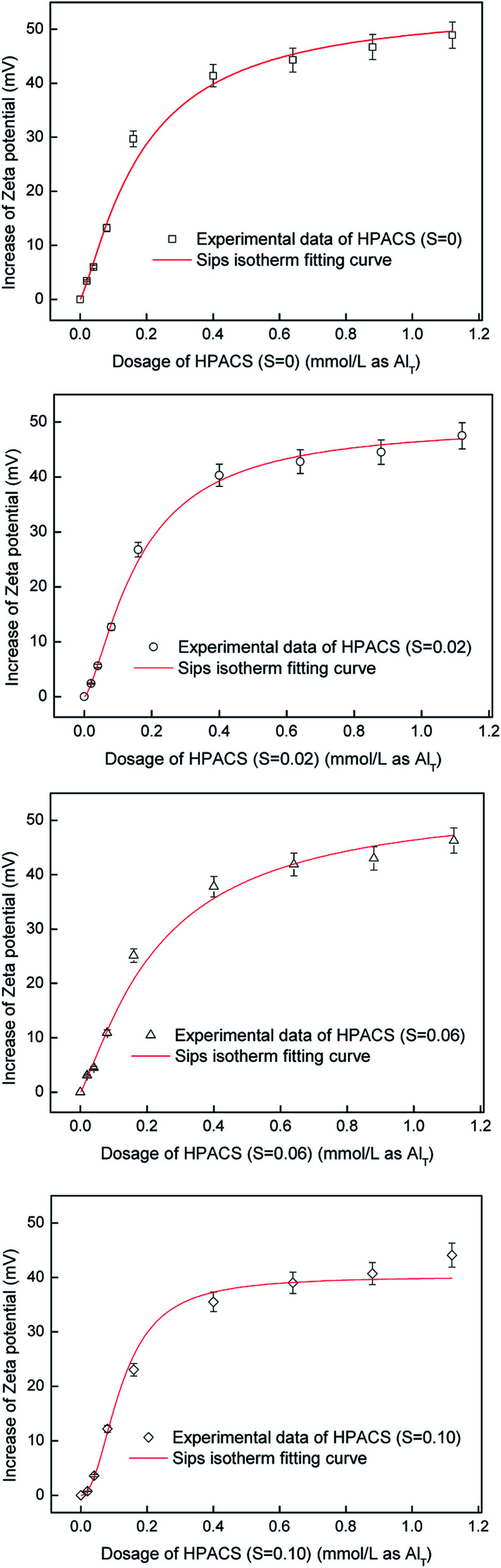
where the Ce is defined as the dosage of coagulants, Qe is the increased value of zeta potential, Qm is the maximum increased value of zeta potential when equilibrium reaches. The KS is the Sips constant, and the γ is a parameter representing the surface heterogeneity of adsorbent. The fitting results are shown in Fig. 7 and the fitted parameters are shown in Table 1. The experimental data fitting well with the Sips isotherm, and the correlation coefficients (R2) were 0.994, 0.997, 0.995 and 0.995 for HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10), respectively. With the increase of coagulant dosage, the zeta potential of water system reached a limited value. The equilibrium value of zeta potential can be obtained by adding the Qm value together with −19.0 mV (the zeta potential value of raw water), and they were 30.05, 28.75, 27.88 and 25.28 mV for HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10), respectively. The lower the zeta potential value of water system at reaction equilibrium, the less the system likely became re-stabilization at a high HPACS dosage, and the simulation result was in good agreement with the experimental data in Fig. 5 and 6.
Fig. 7. Adsorption neutralization model of HPACS during HA coagulation (with a minimum absolute deviation of 5%).
Fitted parameters of Sips equation.
| Coagulants | Sips isotherm 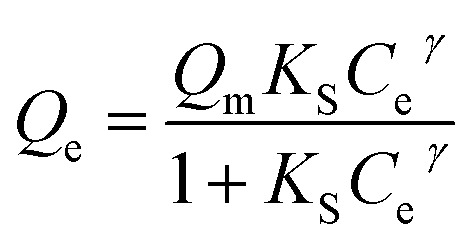
|
|||
|---|---|---|---|---|
| Q m | K S | γ | R 2 | |
| HPACS (S = 0) | 49.05 | 26.62 | 1.63 | 0.994 |
| HPACS (S = 0.02) | 47.75 | 22.90 | 1.61 | 0.997 |
| HPACS (S = 0.06) | 46.88 | 18.84 | 1.58 | 0.995 |
| HPACS (S = 0.10) | 44.28 | 18.28 | 1.57 | 0.995 |
The KL in Langmuir adsorption isotherm usually can be used to indicate the adsorption capacity of the adsorbent,29 so the KS may be used to express the adsorption neutralization capacity of coagulants. The larger the KS value, the stronger the adsorption neutralization capacity of coagulants. So it can be seen from Table 1 that the adsorption neutralization capacity was HPACS (S = 0) > HPACS (S = 0.02) > HPACS (S = 0.06) > HPACS (S = 0.10). The adsorption neutralization capacity of HPACS was not completely consistent with the coagulation effect of HPACS in Fig. 5, which indicated that in addition to adsorption neutralization, actions like bridge-aggregation, precipitation, and sweep-flocculation also played an important role during HPACS coagulation.
The γ in the Sips isotherm represents the heterogeneity of adsorbent surface, and the γ value being closer to 1 indicates that the adsorbent surface is more homogeneous.30 The different hydrolysis species of HPACS may result in different γ values. It can be seen from Table 1 that the surface heterogeneity of these coagulants was HPACS (S = 0) > HPACS (S = 0.02) > HPACS (S = 0.06) > HPACS (S = 0.10), which showed an inverse change related to the Alc content in HPACS. The relationship between the hydrolyzed species of aluminum and surface heterogeneity of adsorbent is still not very clear and needs further verification.
According to the relationship between the Gibbs free energy and the reaction equilibrium constant KS expressed in Table 1, the Gibbs free energy variation of adsorption neutralization can be calculated as eqn (2):
| ΔG = −RT ln KS | 2 |
where ΔG is the Gibbs free energy variation of reaction, KS is the Sips reaction constant, T is the thermodynamic temperature of reaction (about 293.15 K in these tests) and R is the molar gas constant. The ΔG was calculated to be −7990.33, −7624.92, −7162.06 and −7088.98 J mol−1 for HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10), respectively. The negative Gibbs free energy indicated that the coagulant adsorption neutralization reaction was a spontaneous process. The adsorption neutralization model established a link between the macroscopic operation parameters (like coagulant dosage and zeta potential of water system) and the microscopic process of adsorption neutralization. It has theoretical and practical significance for coagulation theory.
3.5. Effect of aging time on HA removal by HPACS
Aging is a process of static placement to make the solution react gradually to reach stable. The effect of aging time on HPACS coagulation to remove HA is shown in Fig. 8. The four HPACS coagulants all have the best coagulation effect after 6 day aging. The freshly prepared HPACS showed better coagulation effect, while aging would make the coagulation effect of HPACS slightly worse, but the HPACS eventually reached the stability in about 20 days. It was also observed that the four HPACS coagulant solutions remained clear throughout the coagulation test and no white flocculent precipitates were formed for one year thereafter. Compared with the PACS coagulant studied before,10 the HPACS after thermal treatment showed stronger stability and was not easily deteriorated during storage. This property of HPACS was very positive in the industrial production and storage of coagulants.
Fig. 8. Effect of aging time of HPACS on HA removal (with a minimum absolute deviation of 5%).
3.6. Effect of Ca2+ on HA removal by HPACS
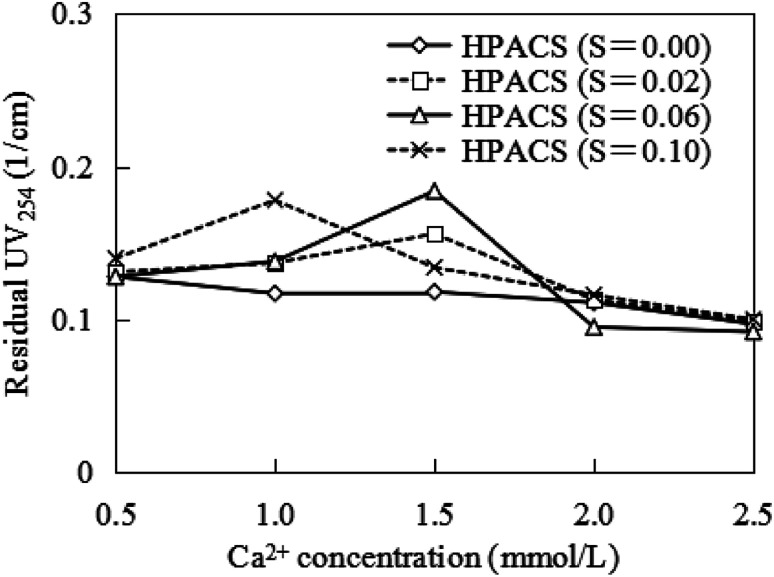
Previous researches indicated that the Ca2+ could contribute to the HA removal when the PAC dosage was deficient, because the Ca2+ in water could neutralize the negative charges on the surface of HA. In this study, the effect of Ca2+ on the removal of HA by HPACS is shown in Fig. 9. The promotion effect of Ca2+ on HPACS coagulation for HA removal was not obvious. There was no obvious rule for the residual UV254 with the change of Ca2+ concentration. Compared with PAC in previous study,15 therefore, the charge neutralization ability of HPACS was weakened due to the introduction of sulfate. But the positively charged Ca2+ did not play a significant role in the HPACS coagulation, indicated that the bridge-aggregation, precipitation, and sweep-flocculation may be the main mechanisms for the high polymerized HPACS.
Fig. 9. Effect of Ca2+ on HA removal by HPACS (with a minimum absolute deviation of 5%).
4. Conclusions
• The HPACS (S = 0.06) and HPACS (S = 0.10) had much more Alc than the HPACS (S = 0) and HPACS (S = 0.02), and the average diameter of HPACS (S = 0), HPACS (S = 0.02), HPACS (S = 0.06) and HPACS (S = 0.10) was 3.9, 5.0, 137.7 and 142.9 nm, respectively.
• The initial pH had no significant effect on the HPACS coagulation. This behavior breaks the conventional knowledge that aluminum coagulants usually present worse coagulation performances in alkaline condition.
• The best coagulation effect occurred when the HPACS (S = 0.06) dosage was 0.64 mmol L−1, and the HA removal reached 97.3%. When the HPACS dosage was 1.44 mmol L−1, the re-stabilization degree of water system with different S value was inversely proportional to the Alc content and charge neutralization ability.
• The order of adsorption neutralization capacity was HPACS (S = 0) > HPACS (S = 0.02) > HPACS (S = 0.06) > HPACS (S = 0.10), and was not completely consistent with the coagulation effect of HPACS, indicating that in addition to adsorption neutralization, actions like bridge-aggregation, precipitation, and sweep-flocculation also played an important role during HPACS coagulation.
• The HPACS showed stronger stability, which is very positive in the industrial production and storage of coagulants; and the coexisted Ca2+ had no significant role during HPACS coagulation.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The authors thank the financial supports from National Natural Science Foundation of China (21736001, 21776153), the “Youth Technology Talent Support Program” of Inner Mongolia for Colleges and Universities (NKYT-17-B14, NKYT-18-B23) special, the Scientific Research Project of Inner Mongolia for Colleges and Universities (NJZY19259) and the Postdoctoral Research Funding Project of Ordos.
References
- Usefi S. Asadi-Ghalhari M. Optimization of turbidity removal by coagulation and flocculation process from synthetic stone cutting wastewater. International Journal of Energy and Water Resources. 2019;3(1):33–41. doi: 10.1007/s42108-019-00010-2. [DOI] [Google Scholar]
- De S. Hazra T. Amit D. Treatment of landfill leachate by integrated sequence of air stripping, coagulation–flocculation and adsorption. Environ. Dev. Sustain. 2017;21(2):1–21. [Google Scholar]
- Liu Z. Q. You L. H. Xiong X. J. Wang Q. Yan Y. H. Tu J. L. Cui Y. H. Li X. Y. Wen G. Wu X. H. Potential of the integration of coagulation and ozonation as a pretreatment of reverse osmosis concentrate from coal gasification wastewater reclamation. Chemosphere. 2019;222:696–704. doi: 10.1016/j.chemosphere.2019.01.187. [DOI] [PubMed] [Google Scholar]
- Li R. He C. He Y. L. Preparation and characterization of poly-silicic-cation coagulants by synchronous-polymerization and co-polymerization. Chem. Eng. J. 2013;223(3):869–874. doi: 10.1016/j.cej.2013.03.010. [DOI] [Google Scholar]
- He C. Li R. Liu Z. K. Gu Z. L. Characterization and performance of poly-silicic-cation coagulant in treatment of recycled paper wastewater. Desalin. Water Treat. 2015;57(14):1–8. doi: 10.1016/j.cej.2014.12.105. [DOI] [Google Scholar]
- Zhang Y. D. Li M. Liu D. Hou X. L. Zou J. L. Ma X. T. Shang F. Y. Wang Z. W. Aluminum and iron leaching from power plant coal fly ash for preparation of polymeric aluminum ferric chloride. Environ. Technol. 2018:1–34. doi: 10.1080/09593330.2018.1426639. [DOI] [PubMed] [Google Scholar]
- Tolkou A. K. Zouboulis A. Synthesis and coagulation performance of composite poly-aluminum-ferric-silicate-chloride coagulants in water and wastewater. Desalin. Water Treat. 2015;53(12):3309–3318. doi: 10.1080/19443994.2014.933614. [DOI] [Google Scholar]
- Niu X. X. Li X. L. Zhao J. H. Ren Y. G. Yang Y. Q. Preparation and coagulation efficiency of polyaluminium ferric silicate chloride composite coagulant from wastewater of high-purity graphite production. J. Environ. Sci. 2011;23(7):1122–1128. doi: 10.1016/S1001-0742(10)60537-2. [DOI] [PubMed] [Google Scholar]
- Ng M. Liana A. E. Liu S. Lim M. Chow C. W. K. Wang D. S. Drikas M. Amal R. Preparation and characterisation of new-polyaluminum chloride-chitosan composite coagulant. Water Res. 2012;46(15):4614–4620. doi: 10.1016/j.watres.2012.06.021. [DOI] [PubMed] [Google Scholar]
- Wu Z. Zhang P. Y. Zeng G. M. Zhang M. Jiang J. H. Humic acid removal from water with polyaluminum coagulants: effect of sulfate on aluminum polymerization. J. Environ. Eng. 2012;3:293–298. doi: 10.1061/(ASCE)EE.1943-7870.0000412. [DOI] [Google Scholar]
- Chen W. Zheng H. L. Zhai J. Sun Y. J. Xue W. W. Cai N. Yang Q. Q. Feng L. Study on the preparation of a new composite coagulant: poly-ferric-titanium-sulfate and analysis of FTIR spectrum and UV-Vis spectrum. Spectrosc. Spectr. Anal. 2016;36(4):1038–1043. [PubMed] [Google Scholar]
- Rowsell J. Nazar L. F. Speciation and thermal transformation in alumina sols: structures of the polyhydroxyoxo aluminum cluster [A130O8(OH)56(H2O)26] and its 8-Keggin moiet. J. Am. Chem. Soc. 2000;122(15):3777–3778. doi: 10.1021/ja993711+. [DOI] [Google Scholar]
- Chen Z. Y. Liu C. J. Luan Z. K. Zhang Z. G. Li Y. Z. Jia Z. P. Effect of total aluminium concentration on the formation and transformation of nanosized Al13 and Al30 in hydrolytic polymeric aluminium aqueous solutions. Chin. Sci. Bull. 2005;50(18):2010–2015. doi: 10.1360/982005-67. [DOI] [Google Scholar]
- Feng C. G. Shi B. Y. Wang D. S. Li G. H. Tang H. X. Characteristics of simplified ferron colorimetric solution and its application in hydroxy-aluminum speciation. Colloids Surf., A. 2006;287(1–3):203–211. doi: 10.1016/j.colsurfa.2006.03.053. [DOI] [Google Scholar]
- Wu Z. Zhang X. Zhou C. J. Pang J. L. Zhang P. Y. A comparative study on the characteristics and coagulation mechanism of PAC-Al13 and PAC-Al30. RSC Adv. 2016;6:108369–108374. doi: 10.1039/C6RA21147J. [DOI] [Google Scholar]
- Masion A. Vilgé-Ritter A. Rose J. Stone W. E. E. Teppen B. J. Rybacki D. Bottero J. Y. Coagulation-flocculation of natural organic matter with Al salts: speciation and structure of the aggregates. Environ. Sci. Technol. 2000;34(15):3242–3246. doi: 10.1021/es9911418. [DOI] [Google Scholar]
- Allouche L. Gerardin C. Loiseau T. Férey G. Taulelle F. A130: a giant aluminum polycation. Angew. Chem., Int. Ed. 2000;39(3):511–514. doi: 10.1002/(SICI)1521-3773(20000204)39:3<511::AID-ANIE511>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Hu C. Z. Liu H. J. Qu J. H. Wang D. S. Rut J. Coagulation behavior of aluminum salts in eutrophic water: significance of Al13 species and pH control. Environ. Sci. Technol. 2006;40(1):325–331. doi: 10.1021/es051423+. [DOI] [PubMed] [Google Scholar]
- Wu Z. Zhang X. Zhou C. J. Pang J. L. Zhang P. Y. Adsorption neutralization model and floc growth kinetics properties of aluminum coagulants based on sips and boltzmann equations. ACS Appl. Mater. Interfaces. 2017;9(7):5992–5999. doi: 10.1021/acsami.6b14273. [DOI] [PubMed] [Google Scholar]
- Wang D. S. Tang H. X. John G. Relative importance of charge neutralization and precipitation on coagulation of kaolin with PACl: effect of sulfate ion. Environ. Sci. Technol. 2002;36(8):1815–1820. doi: 10.1021/es001936a. [DOI] [PubMed] [Google Scholar]
- Zhao C. W. Wang J. Luan Z. K. Preparation of high concentration polyaluminum chloride with high Alc content by membrane distillation. Chin. J. Chem. Eng. 2011;19(1):173–176. doi: 10.1016/S1004-9541(09)60195-6. [DOI] [Google Scholar]
- Parker D. R. Bertsch P. M. Identification and quantification of the “Al13” tridecameric polycation using ferron. Environ. Sci. Technol. 1992;26:908–914. doi: 10.1021/es00029a006. [DOI] [Google Scholar]
- Wang X. L. Jiang S. J. Tan S. Y. Wang X. Wang H. W. Preparation and coagulation performance of hybrid coagulant polyacrylamide–polymeric aluminum ferric chloride. J. Appl. Polym. Sci. 2018;135(23):46355. doi: 10.1002/app.46355. [DOI] [Google Scholar]
- Zhang P. Y. Wu Z. Zhang G. M. Zeng G. M. Zhang H. Y. Li J. Song X. G. Dong J. H. Coagulation characteristics of polyaluminum chlorides PAC-Al30 on humic acid removal from water. Sep. Purif. Technol. 2008;63:642–647. doi: 10.1016/j.seppur.2008.07.008. [DOI] [Google Scholar]
- Hussain S. Van Leeuwen J. Chow C. Beecham S. Kamruzzaman M. Wang D. S. Drikas M. Aryal R. Removal of organic contaminants from river and reservoir waters by three different aluminum-based metal salts: coagulation adsorption and kinetics studies. Chem. Eng. J. 2013;225:394–405. doi: 10.1016/j.cej.2013.03.119. [DOI] [Google Scholar]
- Wu X. H. Ge X. P. Wang D. S. Tang H. X. Distinct coagulation mechanism and model between alum and high Al13-PACl. Colloids Surf., A. 2007;305(1–3):89–96. doi: 10.1016/j.colsurfa.2007.04.046. [DOI] [Google Scholar]
- Tang H. X., Inorganic Polymer Flocculation Theory and Flocculant [M], China Building Industry Press, 2006, pp. 35–52 [Google Scholar]
- Tang H. X., Wang D. S. and Ge X. P., Optimation of the Concepts for Polyaluminum Species, Chemical Water and Waste Water Treatment VIII, ed. Hahn H. H., Hoffman E. and Odeggard H., 2004, pp. 139–149 [Google Scholar]
- Chung H. K. Kim W. H. Park J. Cho J. Jeong T. Y. Park P. K. Application of Langmuir and Freundlich isotherms to predict adsorbate removal efficiency or required amount of adsorbent. J. Ind. Eng. Chem. 2015;28:241–246. doi: 10.1016/j.jiec.2015.02.021. [DOI] [Google Scholar]
- Kurniawan A. Sutiono H. Indraswati N. Ismadji S. Removal of basic dyes in binary system by adsorption using Rarasaponin−Bentonite: revisited of extended Langmuir model. Chem. Eng. J. 2012;189–190:264–274. doi: 10.1016/j.cej.2012.02.070. [DOI] [Google Scholar]