Abstract
Non-small-cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma (SCLC), which accounts for about 85% of all lung cancers. Bone morphogenetic protein (BMP)-9 in humans is encoded by the growth differentiation factor 2 gene, which belongs to the transforming growth factor-beta superfamily. In the present study, we explored the role of BMP-9 in A549 and NCI-H1650 cell proliferation and its possible molecular mechanisms. 25 NSCLC patients were recruited to evaluate mRNA expression of BMP-9 to determine its clinicopathologic significance. We found that recombinant protein BMP-9 and overexpression of BMP-9 promoted A549 and NCI-H1650 cell proliferation in vitro, which was abolished by phosphatidylinositol 3-kinase (PI3K) inhibitor (LY294002). Western blot results revealed that BMP-9 significantly activated the PI3K/Akt and Smad1/5 pathway signaling. In vivo, BMP-9 promoted tumor growth and PI3K/Akt and Smad1/5 signaling pathways in an A549 or NCI-H1650 cell line-derived xenograft model. Knockdown BMP-9 or BMP-9 receptor ALK1 inhibited A549 cell growth in vitro and in vivo, which was associated with regulating the PI3K/Akt and Smad1/5 signaling pathways. These results demonstrated that BMP-9 promoted A549 and NCI-H1650 cell proliferation via PI3K/Akt and Smad1/5 signaling pathways.
Non-small-cell lung carcinoma (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma (SCLC), which accounts for about 85% of all lung cancers.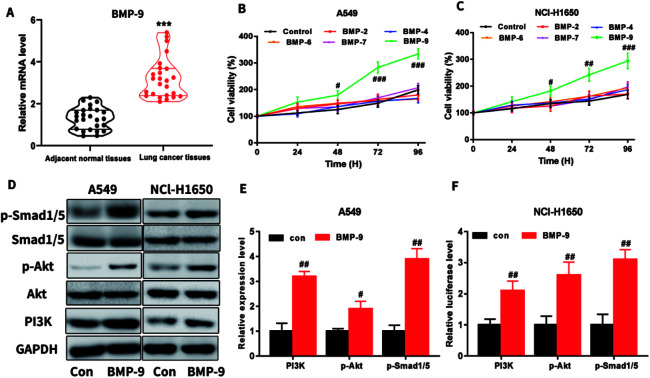
1. Introduction
Lung cancer is one of the most common malignant tumors and its five-year survival rate is significantly lower than for many clinical tumors in clinical practice.1 Non-small cell lung cancer accounts for about 85% of the total number of lung cancers. Unfortunately, only less than 15% of non-small cell lung cancer patients can survive over 5 years.2–4 So far, the pathogenesis of non-small cell lung cancer has not been well elucidated, so it is of great significance to identify key genes for non-small cell lung cancer.
Bone morphogenetic protein 9 (BMP-9), which is also known as growth differentiation factor 2 (GDF2), belongs to the TGF-β superfamily.5 It has been reported that BMPs is related to the regulation of tumorigenesis, development and bone metastases.6 In higher pathological grade prostate cancer, BMP-9 expression evidently decreased, whereas overexpression of BMP-9 and BMP-10 inhibits in vitro growth, cell matrix adhesion, invasion, and migration of prostate cancer cells.7 BMP-9 induces apoptosis in prostate cancer by upregulating of prostate apoptosis response-4,7 which may indicate that BMP-9 is a tumor suppressor. Conversely, BMP-9 promotes the proliferation and migration of bladder cancer cells through up-regulating lncRNA UCA1 and promotes ovarian cancer cell proliferation through activating receptor-like kinase-2/Smad1/Smad4.8,9
IL-6, a cytokine, is produced by a variety of cells, which plays an extensive biological role and is closely related to the occurrence and development of malignant tumors. Current studies have shown that IL-6 is mainly related to cell proliferation, apoptosis and metastasis and IL-6 plays an important regulatory role in most tumors, such as prostate cancer, breast and ovarian cancer.10,11In vitro and in vivo studies have shown that IL-6 can promote the proliferation of lung cancer cells. After blockade of the IL-6 receptor, the proliferation of H460 lung cancer stem cells was inhibited.12 IL-6 is an endocrine factor, which can promote the development of tumors through a variety of mechanisms, such as the PI3K/Akt signaling pathway.
TGF-β family type I receptor ALK1 (activin receptor-like kinase 1) has been identified as a potential target for anti-angiogenic cancer treatment. It has been reported that the high affinity binding of BMP-9 to ALK1 indicated the key role of the ligand in vascular development.13 Several published preclinical studies have been reported ALK1 blockade revealed a decrease in tumor volume, tumor angiogenesis and metastasis.14,15
The previous studies have found that the PI3K/Akt signaling pathway is involved in tumor growth, invasion and metastasis. One of the important factors for the recurrence and metastasis of tumors is Akt abnormal activation.16,17 In prostate cancer, BMP-2 promotes prostate cancer metastasis associated with the PI3K/Akt signaling pathway.18 It has been reported that BMP-9 inhibits the migration and growth of human osteosarcoma cells by down-regulating the PI3K/AKT signaling pathway.19
With the development of research, it has been found that BMP-9 also plays an important role in tumors. For example, BMP-9 can inhibit the invasion and migration of prostate cancer PC-3 cells and promote the proliferation of ovarian cancer cells.9,20 However, the role of BMP-9 in lung cancer remains unknown, especially in non-small cell lung cancer. Therefore, the present study aimed to investigate the effect of BMP-9 on the proliferation of A549 and NCl-H1650 cells and to explore the possible mechanism to potentially provide a basis for targeted therapy of non-small cell lung cancer.
2. Materials and methods
2.1. Human lung samples
A total of 25 pairs of non-small cell lung cancer and adjacent normal tissues were obtained from The First Affiliated Hospital of China Medical University. All the experiments concerning the tumor specimens were approved by The First Affiliated Hospital of China Medical University, and all the patients signed the informed consent form. The procedures were performed in accordance with the guidelines of the ethics committee of China Medical University.
2.2. Cell lines and cell culture
Human lung adenocarcinoma A549 and NCI-H1650 cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI growth medium supplemented with 10% FBS, 100 U mL−1 penicillin and 100 pg mL−1 streptomycin, in a humidified atmosphere with 5% CO2 at 37 °C.
2.3. Adenovirus infection
The cells were infected with AdBMP-9 or AdGFP. The adenovirus-infected cells were used for subsequent experiments.
2.4. RNA interference
A549 and NCI-H1650 cells were transfected with shRNA using Lipofectamine™ 3000 Transfection Reagent (Invitrogen™) according to the manufacturer's instructions. Sense sequences for the Bmp9: 5′-CCGGCAACAGGTACACGTCCGATAACTCGAGTTATCGGACGTGTACCTGTTGTTTTTG-3′. Sense sequences for the ALK1: 5′-CCGGCTGCGGATCAAGAAGACACTACTCGAGTAGTGTCTTCTTGATCCGCAGTTTTT-3′.
2.5. CCK-8 assay
The effect of BMP-9 on cell viability and proliferation was determined by using the cell counting kit-8 assay. In brief, the cells were seeded onto 96-well plates (3000/well) and incubated for the indicated time points (0, 24, 48, 72, 96 h). At the indicated time, CCK-8 solution was added to each well (200 μL per well) and incubated at 37 °C for 3 h. The absorbance was detected at 490 nm using a microplate reader.
2.6. Colony formation assay
The effect of BMP-9 on long-term cell viability was evaluated by the colony formation assay. The cells (3000/well) were seeded in six-well plates and treated with LY294002 (20 μM). The cells were incubated in 5% CO2 at 37 °C for 7 days.
2.7. Xenografted tumor model
Male BALB/c-nu mice (16–18 g) were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All experimental procedures and protocols were approved by the Experimental Animal Ethics Committee of Academy of Military Medical Science of China (approval no. IACUC-2017246). Mice were randomly divided into two groups (n = 7), and AdBMP-9 or AdGFP cells were injected subcutaneously into the dorsal fank of the nude mice. The tumor volume and bodyweight were measured on alternate days. All mice were sacrificed on the 30th day.
2.8. Real-time quantitative PCR
Total RNA was extracted from human lung tissue or cells using TRIzol reagent according to the manufacturer's instructions, then reverse-transcribed for real-time quantitative PCR. The mRNA expressions were normalized to GAPDH and data were analyzed by using 2−ΔΔCt method.
2.9. Western blot analysis
Equal amounts of protein were separated by 8–12% SDS-PAGE and transferred onto a PVDF membrane. The membrane was sequentially blocked in 5% nonfat milk for 1 h and incubated with primary antibodies overnight at 4 °C, including GAPDH (catalog #5174S), PI3K (p85) (catalog #4292), Akt (catalog #9272), p-Akt (Ser473) (catalog #4060) p-Smad1/5 (catalog #9516S) (Cell Signaling Technology, Inc.) and Smad1/5 (Santa Cruz Biotechnology). After washed with PBST, the membranes were probed with secondary antibody. The membranes were treated with a chemiluminescent reagent visualized using a ChemicDoc XRS system.
2.10. Statistical analysis
All values were expressed as the mean ± SEM. Statistical significance was determined by using one-way ANOVA analysis. P-value of <0.05 was considered to be significant.
3. Results
3.1. Expression level of BMP-9 was upregulated in non-small cell lung cancer tissues
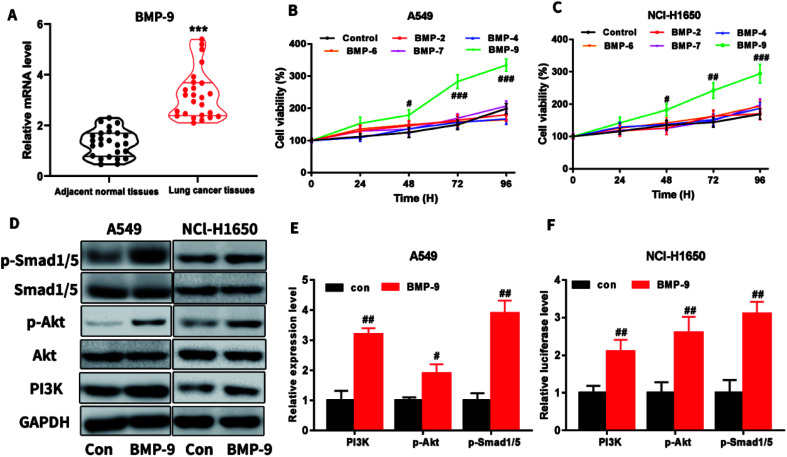
In the clinical lung cancer sample, real-time quantitative PCR results showed that the BMP-9 level in lung cancer tissues was significantly higher than the level in the adjacent normal tissues, indicating that BMP-9 played a potential role in the non-small cell lung cancer (Table 1 and Fig. 1A).
Clinical characteristics of the non-small cell lung cancer patients recruited to the study.
| Characteristics | Cases | Characteristics | Cases | ||
|---|---|---|---|---|---|
| Gender | Male | 13 | Tumor size (cm) | <5 | 11 |
| Female | 12 | ≥5 | 14 | ||
| Age (year) | <60 | 15 | Grade | I + II | 15 |
| ≥60 | 10 | III + IV | 10 | ||
| Smoking | Yes | 16 | Stage | I + II | 17 |
| No | 9 | III + IV | 8 | ||
Fig. 1. (A) The mRNA expression levels of BMP-9 in lung cancer tissues and adjacent normal tissues; (B and C) BMP-9 recombinant protein promoted the A549 (B) and NCl-H1650 (C) cells proliferation in CCK-8 assay; (D–F) BMP-9 recombinant protein up-regulated p-Smad1/5 level and PI3K/Akt pathway. Data were represented as the mean ± SEM, ***p < 0.001 versus adjacent normal tissues, #p < 0.05 versus control group, ##p < 0.01 versus control group; ##p < 0.001 versus control group.
3.2. BMP-9 recombinant protein promoted proliferation in A549 and NCI-H1650 cells
To determine which BMPs exhibited promote proliferation on A549 and NCI-H1650 cells, BMP2, BMP4, BMP6, BMP7, or BMP9 were used to incubate with A549 or NCI-H1650 cells for 0–96 h, then CCK-8 assay was used to determine the cell viability. As shown in Fig. 1B and C, BMP9 could stimulate proliferation in A549 and NCI-H1650 cells, whereas BMP2, BMP4, BMP6 and BMP7 were unable to promote proliferation.
To study the underlying mechanism of BMP-9 on proliferation, we determined the PI3K/Akt and Smad1/5 signaling pathway after BMP9 recombinant protein treatment. As shown in Fig. 1D–F, after BMP9 recombinant protein treatment, the expression levels of PI3K, p-Akt and p-Smad1/5 significantly increased in A549 and NCI-H1650 cells.
3.3. BMP-9 overexpression promoted proliferation and colony formation in A549 and NCI-H1650 cells
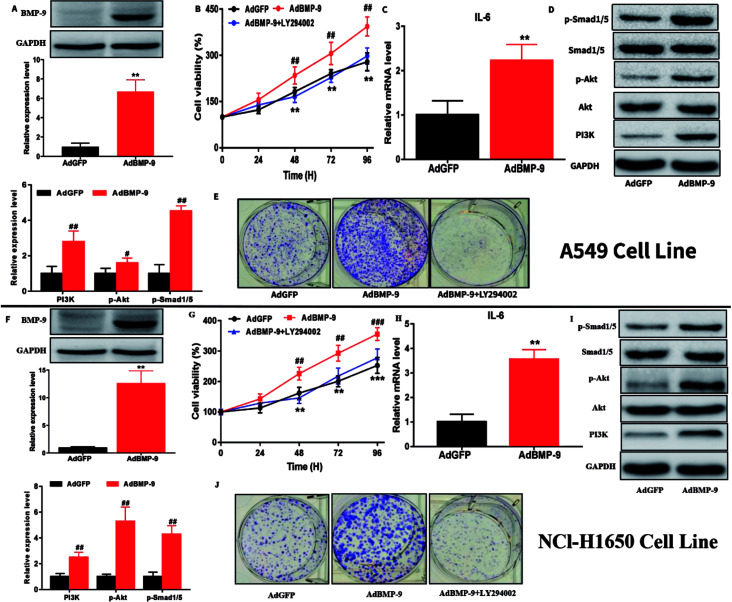
Next, A549 and NCI-H1650 cells with BMP-9 overexpression were chosen to investigate the effects of BMP-9. Western blotting showed that the BMP-9 expression in AdBMP-9 cells was significantly higher than that in AdGFP cells (Fig. 2A and F). CCK-8 results showed that BMP-9 overexpression significantly promoted A549 and NCI-H1650 cells proliferation, however, it was worth noting that the stimulation of BMP-9 was abolished by PI3K inhibitor LY294002 (20 μM) (Fig. 2B and G). Colony formation assay was used to directly evaluate the proliferative ability of a single cancer cell to form a cell colony in vitro. Compared to AdGFP cells, BMP-9 overexpression effectively promoted the colony-forming ability of AdBMP-9 cells, and PI3K inhibitor LY294002 significantly abolished the effect of BMP-9 overexpression both in A549 and NCI-H1650 cells (Fig. 2E and J).
Fig. 2. BMP-9 overexpression promoted the proliferation and up-regulated the level of IL-6 and p-Smad1/5 and PI3K/Akt pathway in A549 and NCl-H1650 cells. (A and F) BMP-9 overexpression was confirmed by western blotting in A549 and NCl-H1650 cells. (B, E, G and J) BMP-9 overexpression promoted A549 and NCl-H1650 cells proliferation in CCK8 and colony formation assays. (C, D, H and I) BMP-9 overexpression up-regulated the level of IL-6 and p-Smad1/5 and PI3K/Akt pathway in A549 and NCl-H1650 cells. Data were represented as the mean ± SEM, *p < 0.05 versus AdGFP group, **p < 0.01 versus AdGFP group, ***p < 0.01 versus AdGFP group.
3.4. BMP-9 promoted A549 and NCI-H1650 cells proliferation by regulating PI3K/Akt and Smad1/5 signaling pathway
To further study the underlying mechanism of BMP-9 on proliferation in A549 and NCI-H1650 cells, western blotting was conducted. Western blotting results showed that the expression levels of PI3K and p-Akt in the AdBMP-9 cells were significantly increased compared to the AdGFP cells both in A549 and NCI-H1650 cells (Fig. 2D and I). In addition, the phosphorylation of Smad1/5 also significantly increased in AdBMP-9 cells (Fig. 2D and I). These results demonstrated that BMP-9 promoted A549 and NCI-H1650 cells proliferation by regulating PI3K/Akt and Smad1/5 signaling pathway.
3.5. BMP-9 promoted the tumor growth in A549 and NCI-H1650 cells line xenograft model
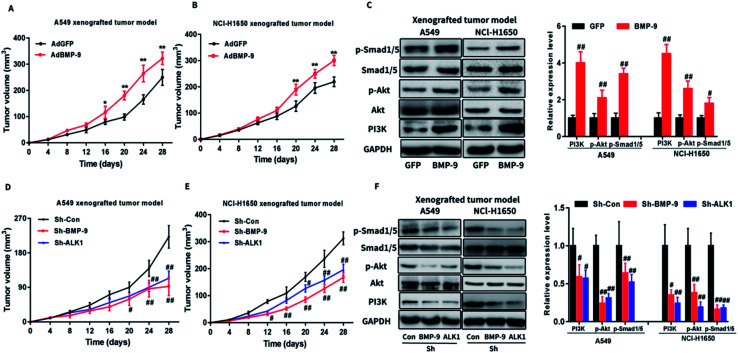
To verify the role of BMP-9 in non-small cell lung cancer, xenograft tumor assay was performed by using AdBMP-9 or AdGFP cells (A549 or NCI-H1650). As shown in Fig. 4A and B, the tumors of AdBMP-9 group were obviously larger than that of the AdGFP group. In addition, western blotting results showed that PI3K and p-Akt in tumors tissues were significantly upregulated in AdBMP-9 group compared with tissues in AdGFP group (Fig. 4C). Furthermore, Smad1/5 signaling pathway also activated in the AdBMP-9 (Fig. 4C).
Fig. 4. (A andB) BMP-9 overexpression promoted the tumor growth of A549 (A) and NCl-H1650 (B) xenograft in nude mice. (C) BMP-9 overexpression up-regulated p-Smad1/5 level and PI3K/Akt pathway of A549 and NCl-H1650 xenograft in nude mice. (D and E) Knockdown BMP-9 or ALK1 suppressed the tumor growth of A549 (D) and NCl-H1650 (E) xenograft in nude mice. (F) Knockdown BMP-9 or ALK1 suppressed p-Smad1/5 level and PI3K/Akt pathway of A549 and NCl-H1650 xenograft in nude mice. Data were represented as the mean ± SEM, #p < 0.05 versus AdGFP or Sh-Con group, ##p < 0.01 versus AdGFP or Sh-Con group.
3.6. Effect of knockdown BMP-9 or ALK1 on proliferation A549 and NCI-H1650 cells
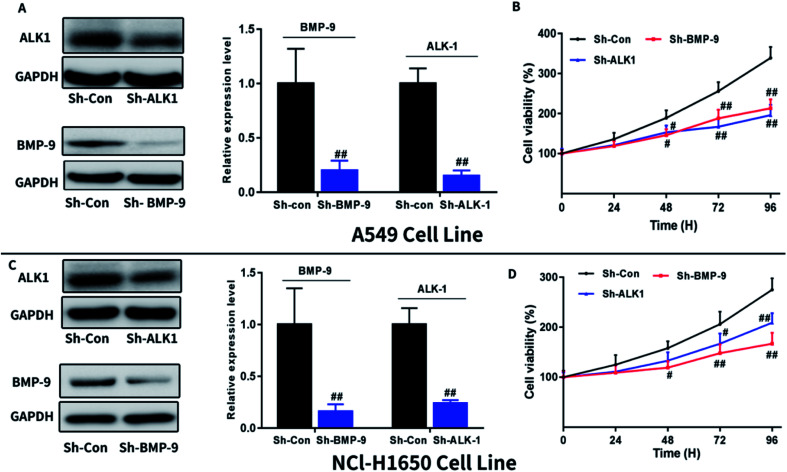
To further confirmed the effect of BMP-9 on A549 and NCI-H1650 cells proliferation, we next investigate whether knockdown BMP-9 or BMP-9 receptor ALK1 could affect A549 and NCI-H1650 proliferation. As shown in Fig. 3, knockdown BMP-9 or ALK1 significantly inhibited A549 (Fig. 3B) and NCI-H1650 cells proliferation (Fig. 3D).
Fig. 3. Knockdown BMP-9 or ALK1 inhibited A549 and NCl-H1650 cells proliferation. Knockdown BMP-9 or ALK1 was confirmed by western blotting in A549 (A) and NCl-H1650 (C) cells. Knockdown BMP-9 or ALK1 inhibited A549 (B) and NCl-H1650 (D) cells proliferation in CCK8 assay. Data were represented as the mean ± SEM. #p < 0.05 versus Sh-Con group, ##p < 0.01 versus Sh-Con group, ##p < 0.001 versus Sh-Con group.
3.7. Effect of knockdown BMP-9 or ALK1 on tumor development in xenograft tumor mice model
Next, we investigated whether knockdown BMP-9 or ALK1 could affect tumor development. BALB/c-nu mice were injected A549 or NCI-H1650 cells with knockdown BMP-9 or ALK1 subcutaneously into the dorsal fank. As shown in Fig. 4D and E, knockdown BMP-9 or ALK1 significantly inhibited the tumor development. Western blotting results indicated that BMP-9 or ALK1 inhibited the PI3K/Akt and Smad1/5 signaling pathway (Fig. 4F), which demonstrated that the effect of BMP-9 on tumor development was related to the PI3K/Akt and Smad1/5 signaling pathway.
4. Discussion
The role of BMP-9 in cancer seems to be still under debate. BMP-9 expression has been found to increase in the ovarian cancer cells and RIP-Tag2 mouse model.9,14 However, a survey of 23 breast cancer samples indicated that BMP-9 expression in breast cancer tissues significantly decreased compared to adjacent tissues.21 Thus, the effect of BMP-9 in cancer may be context dependent, however, the role of BMP-9 in non-small cell lung cancer still to be explained. In the present study, we attempted to explore the role of BMP-9 in A549 and NCl-H1650 cells proliferation. To our best knowledge, our study first time uncovered that BMP-9 exerted the stimulation effect on A549 and NCl-H1650 cells proliferation, which might be associated with activating the PI3K/Akt and Smad1/5 signaling pathway.
BMP-9 belongs to the transforming growth factor-beta superfamily and mainly expressed in the liver, but also in the lung.22,23 It has been reported that BMP-9 inhibits the proliferation, adhesion, invasion, and migration in prostate cancer cells. In our study, we have demonstrated that BMP-9 expression in lung cancer tissues was significantly higher than adjacent normal tissues, demonstrating BMP-9 may be a vital molecule involved in the progression of lung cancer.
To further investigate the role of BMP-9 on lung cancer cells, overexpression of BMP-9 was performed in A549 and NCl-H1650 cells. Our results indicated that overexpression of BMP-9 effectively promoted A549 and NCl-H1650 cells growth and enhanced the proliferative ability of a single cancer cell to form a cell colony. Interestingly, the proliferation effect of BMP-9 in A549 cells was significantly abolished by PI3K inhibitor LY294002, which demonstrated PI3K played a key role in the proliferation effect of BMP-9. To evaluate the proliferation effect of BMP-9 on non-small cell lung cancer in vivo, a nude mice xenograft model was established by subcutaneous A549 or NCl-H1650 cells injection. Compared to AdGFP cells, the neoplasm volume induced by AdBMP-9 cells was significantly larger, which indicating BMP-9 promoted tumor growth.
IL-6 is a multifunctional cytokine which plays a role in different cell types. The IL-6 expression level in serum of lung cancer patients is related to the progression and the overall survival rate of lung cancer patients. IL-6 can inhibit P27/KIP1 pathway cell regulation points by activating PI3K/Akt pathway in prostate cancer cells.24 IL-6 is also reported to promote prostate cancer cell survival by activating the PI3K/Akt pathway and regulating cyclin A1.25 In our study, overexpression of BMP-9 can significantly increase IL-6 in A549 and NCl-H1650 cells. The PI3K/Akt is a major pathway in the malignant progression of various tumors, and deregulation of PI3K/Akt signaling pathway will lead to the activation of downstream signal transduction and tumorigenesis.26 In our study, overexpression of BMP-9 and recombinant BMP-9 protein facilitated the PI3K/Akt signaling pathway, indicating that the effect of BMP-9 might be associated with the activation of the PI3K/Akt signaling pathway. In addition, knockdown BMP-9 or ALK1 also inhibited cell proliferation and tumor development, which indicated the enhancement of BMP-9 in non-small cell lung cancer.
In conclusion, our study demonstrated that BMP-9 had a crucial role in non-small cell lung cancer progression and BMP-9 was an important activator of the PI3K/Akt signaling pathway. Understanding the function of BMP-9 in the pathogenesis of non-small cell lung cancer and targeting BMP-9 might be a new therapeutic strategy against non-small cell lung cancer.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
This work was funded by the National Natural Science Foundation of Youth Science Fund China (Grant No. 81601368).
References
- Juergens R. A. Brahmer J. R. J. Natl. Compr. Cancer Network. 2006;4:595–600. doi: 10.6004/jnccn.2006.0049. [DOI] [PubMed] [Google Scholar]
- Siddiqui S. Ali M. U. Ali M. A. Shah N. Nasreen S. J. Ayub Med. Coll. Abbottabad. 2010;22:116–119. [PubMed] [Google Scholar]
- Ettinger D. S. Wood D. E. Aisner D. L. Akerley W. Bauman J. Chirieac L. R. D'Amico T. A. DeCamp M. M. Dilling T. J. Dobelbower M. Doebele R. C. Govindan R. Gubens M. A. Hennon M. Horn L. Komaki R. Lackner R. P. Lanuti M. Leal T. A. Leisch L. J. Lilenbaum R. Lin J. Loo Jr B. W. Martins R. Otterson G. A. Reckamp K. Riely G. J. Schild S. E. Shapiro T. A. Stevenson J. Swanson S. J. Tauer K. Yang S. C. Gregory K. Hughes M. J. Natl. Compr. Cancer Network. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- Torre L. A. Bray F. Siegel R. L. Ferlay J. Lortet-Tieulent J. Jemal A. Ca-Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Lamplot J. D. Qin J. Nan G. Wang J. Liu X. Yin L. Tomal J. Li R. Shui W. Zhang H. Kim S. H. Zhang W. Zhang J. Kong Y. Denduluri S. Rogers M. R. Pratt A. Haydon R. C. Luu H. H. Angeles J. Shi L. L. He T. C. Am. J. Stem Cells. 2013;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- Ye L. Bokobza S. M. Jiang W. G. Int. J. Mol. Med. 2009;24:591–597. doi: 10.3892/ijmm_00000269. [DOI] [PubMed] [Google Scholar]
- Ye L. Kynaston H. Jiang W. G. J. Urol. 2009;181:2749–2759. doi: 10.1016/j.juro.2009.01.098. [DOI] [PubMed] [Google Scholar]
- Gou L. Liu M. Xia J. Wan Q. Jiang Y. Sun S. Tang M. Zhou L. He T. Zhang Y. Int. J. Mol. Sci. 2018;19:1116–1127. doi: 10.3390/ijms19041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera B. van Dinther M. Ten Dijke P. Inman G. J. Cancer Res. 2009;69:9254–9262. doi: 10.1158/0008-5472.CAN-09-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tunon I. Ricote M. Ruiz A. Fraile B. Paniagua R. Royuela M. Histopathology. 2005;47:82–89. doi: 10.1111/j.1365-2559.2005.02178.x. [DOI] [PubMed] [Google Scholar]
- Zakrzewska I. Poznanski J. Pol. Merkuriusz Lek. 2001;11:210–213. [PubMed] [Google Scholar]
- Yi H. Cho H. J. Cho S. M. Jo K. Park J. A. Kim N. H. Amidon G. L. Kim J. S. Shin H. C. Internet J. Oncol. 2012;41:310–316. doi: 10.3892/ijo.2012.1447. [DOI] [PubMed] [Google Scholar]
- Brown M. A. Zhao Q. Baker K. A. Naik C. Chen C. Pukac L. Singh M. Tsareva T. Parice Y. Mahoney A. Roschke V. Sanyal I. Choe S. J. Biol. Chem. 2005;280:25111–25118. doi: 10.1074/jbc.M503328200. [DOI] [PubMed] [Google Scholar]
- Cunha S. I. Pardali E. Thorikay M. Anderberg C. Hawinkels L. Goumans M. J. Seehra J. Heldin C. H. ten Dijke P. Pietras K. J. Exp. Med. 2010;207:85–100. doi: 10.1084/jem.20091309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Lowe D. D. Chen E. Zhang L. Watson K. D. Mancuso P. Lappin P. Wickman G. Chen J. H. Wang J. Jiang X. Amundson K. Simon R. Erbersdobler A. Bergqvist S. Feng Z. Swanson T. A. Simmons B. H. Lippincott J. Casperson G. F. Levin W. J. Stampino C. G. Shalinsky D. R. Ferrara K. W. Fiedler W. Bertolini F. Cancer Res. 2011;71:1362–1373. doi: 10.1158/0008-5472.CAN-10-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardavas D. Fumagalli D. Loi S. Curr. Opin. Oncol. 2012;24:623–634. doi: 10.1097/CCO.0b013e328358a2b5. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Wang X. Wang H. Yan W. Zhang Q. Chang X. Tumour Immunobiol. 2014;35:5189–5198. doi: 10.1007/s13277-014-1673-y. [DOI] [PubMed] [Google Scholar]
- Graham T. R. Odero-Marah V. A. Chung L. W. Agrawal K. C. Davis R. Abdel-Mageed A. B. Prostate. 2009;69:168–180. doi: 10.1002/pros.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. Yang Y. Jiang S. Ni B. Chen K. Jiang L. Internet J. Oncol. 2012;41:1809–1819. doi: 10.3892/ijo.2012.1617. [DOI] [PubMed] [Google Scholar]
- Ye L. Kynaston H. Jiang W. G. Mol. Cancer Res. 2008;6:1594–1606. doi: 10.1158/1541-7786.MCR-08-0171. [DOI] [PubMed] [Google Scholar]
- Wang K. Feng H. Ren W. Sun X. Luo J. Tang M. Zhou L. Weng Y. He T. C. Zhang Y. J. Cancer Res. Clin. Oncol. 2011;137:1687–1696. doi: 10.1007/s00432-011-1047-4. [DOI] [PubMed] [Google Scholar]
- Chen H. Brady Ridgway J. Sai T. Lai J. Warming S. Chen H. Roose-Girma M. Zhang G. Shou W. Yan M. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11887–11892. doi: 10.1073/pnas.1306074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. F. Harvey S. A. Thies R. S. Olson M. S. J. Biol. Chem. 2000;275:17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- Smith D. A. Kiba A. Zong Y. Witte O. N. Mol. Cancer Res. 2013;11:1159–1165. doi: 10.1158/1541-7786.MCR-13-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel B. Bjartell A. Culig Z. Persson J. L. Int. J. Cancer. 2008;122:1521–1529. doi: 10.1002/ijc.23261. [DOI] [PubMed] [Google Scholar]
- Eda H. Burnette B. L. Shimada H. Hope H. R. Monahan J. B. Cell Biol. Int. 2011;35:355–358. doi: 10.1042/CBI20100247. [DOI] [PubMed] [Google Scholar]