Abstract
Background
Hyperkalemia (HK) may be associated with poor clinical outcomes among COVID-19 patients. This study aimed to describe the prevalence of HK and evaluate the associations between HK and in-hospital mortality, intensive care unit (ICU) admission, length of hospital stay (LOS), and hospitalization cost among COVID-19 inpatients.
Methods
A retrospective cohort study was conducted using a large hospital discharge database (PINC AI Healthcare Database) for COVID-19 inpatients discharged between April 1 and August 31, 2020. HK was defined with discharge diagnosis and potassium binder use.
Results
Of 192,182 COVID-19 inpatients, 12% (n = 22,702) had HK. HK patients were more likely to be older (median age 67 vs 63 years), male (63% vs 50%), black (30% vs 22%), and have a history of chronic kidney disease (45% vs 16%) or diabetes mellitus (55% vs 35%) than non-HK patients (all p<.001). A significantly higher proportion of patients with HK had in-hospital mortality (42% vs 11%, p<.001) than those without HK; this was persistent after adjusting for confounders (adjusted odds ratio [aOR] 1.69, 95% confidence interval [CI]1.62-1.77). Patients with HK were also more likely to be admitted to ICU (aOR 1.05, 95% CI 1.01-1.09), incur higher cost of care (adjusted mean difference $5,389) and have longer LOS (adjusted mean difference 1.3 days) than non-HK patients.
Conclusions
Presence of HK was independently associated with higher in-hospital mortality, LOS, and cost of care among COVID-19 inpatients. Detecting and closely monitoring HK are recommended to improve clinical outcomes and reduce LOS and healthcare cost among COVID-19 patients.
Key Indexing Terms: COVID-19, Hyperkalemia, Mortality, Healthcare resource utilization
Introduction
Newly emerged coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is responsible for almost one million deaths in the United States (US) alone.1 Mortality rate was especially high among adults with advanced age and pre-existing conditions such as cardiovascular disease, diabetes mellitus (DM), hypertension, chronic lung disease, cancer, chronic kidney disease (CKD), and obesity.2, 3, 4, 5
Renal complications of the disease were also closely associated with the risks of severe illness and death among COVID-19 inpatients.6, 7, 8, 9 In a systemic review, acute kidney injury (AKI) was present in 17% of the hospitalized COVID-19 patients, of which 77% had a severe illness and 52% died.9 For patients with AKI, the primary indication of renal replacement therapy was hyperkalemia – high serum potassium level (>5.0 mmol/L or >5.5mmol/L).10, 11, 12, 13, 14
Not surprisingly, hyperkalemia was one of the most frequent electrolyte imbalances with an incidence of 12.5% among COVID-19 inpatients.15 In a large cohort study of US patients with COVID-19, hyperkalemia was more frequently observed among the deceased compared to recovered patients.16 , 17 In another recent study, serum potassium level of ≥5.0 mmol/L was associated with significantly increased 30-day mortality among COVID-19 patients independent of age, sex, history of CKD, pulse oxygen saturation, and serum creatinine.18
COVID-19 inpatients are already at an increased risk of developing hyperkalemia, due to the high prevalence of congestive heart failure (CHF), DM, and CKD.17 Furthermore, hypertension is the most common comorbidity among COVID-19 inpatients and use of renin–angiotensin–aldosterone system (RAAS) inhibitors in these patients could lead to disturbed potassium homeostasis and elevated serum potassium levels.9 , 17 , 19
However, data are limited on the independent risk conferred by hyperkalemia and how RAAS inhibitors are used among hospitalized COVID-19 patients. Furthermore, no study to date has assessed the impact of hyperkalemia on healthcare resource utilization (HRU) and cost among COVID-19 inpatients. Using the largest hospital discharge database in the US, the aims of this study were: 1) to describe the prevalence of hyperkalemia, 2) to evaluate the association between hyperkalemia and in-hospital mortality, and 3) to assess the impact of hyperkalemia on HRU and cost among COVID-19 patients.
Methods
Study design and data source
A retrospective cohort study was performed using PINC AI Healthcare Database (PHD, formerly known as Premier Healthcare Database). The PHD is a hospital-based, service-level, all-payer discharge database for geographically diverse inpatient and outpatient visits. Inpatient discharges in PHD represents approximately 20-25% of all inpatient admissions in the US since 2000.17 , 20 PHD has been used by the National Institute of Health and the Centers for Disease Prevention and Control for evaluating the impact of COVID-19 on patients across the US.21 , 22 The standard hospital discharge files included demographic characteristics, disease states, and time-stamped log of billed items (e.g., procedures, medications, laboratory services, and diagnostic services) of patient visits and geographic location, urbanicity of served population, teaching status, and bed capacity of the hospitals.20 All data were statistically deidentified and compliant with the Health Insurance Portability and Accountability Act. The study was exempted from institutional board review based on US Title 45 Code of Federal Regulations, Part 46. We did not pursue informed consent from the study participants because individuals could not be identified directly or through linked identifiers. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) 23 reporting guideline.
Study population
All COVID-19-related inpatient visits were identified using the principal or secondary discharge diagnosis of COVID-19 (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] diagnosis code U07.1) between April 1 and August 31, 2020.24 If the patient had multiple inpatient visits with the discharge diagnosis of COVID-19 during the study period, only the first inpatient visit (index hospitalization) was included in the analysis to represent patient-level data. Patients were only included in the study if they were 18 years old or older upon admission and did not acquire COVID-19 at the hospital (presence on admission is not equal to ‘No’).
Exposure & outcome measures
Hyperkalemia was identified using the principal or secondary discharge ICD-10-CM diagnosis code for hyperkalemia (E87.5) or hospital chargemaster descriptions of potassium binder use (sodium zirconium cyclosilicate, sodium polystyrene sulfonate, or patiromer).
Main outcomes of interest were in-hospital mortality, intensive care unit (ICU) admission, hospital length of stay (LOS), and hospitalization cost during index hospitalization. Secondary outcomes included acute complications of COVID-19 during index hospitalization. In-hospital mortality was identified when the patient's discharge status was ‘expired’; ICU admission was identified when the patient incurred any room and board charges related to ICU (observations after surgery and step-down ICU were excluded). Total cost for the index hospitalization included the sum of all costs incurred by the hospital including room and board, pharmacy, laboratory, imaging, and central supply.
For sensitivity analysis, we examined the composite measure of in-hospital mortality + referral to hospice at the time of discharge. Furthermore, we examined the association between in-hospital mortality and hyperkalemia in a subgroup of patients who developed AKI during their inpatient stay.
Patient, visit, and hospital characteristics
Baseline patient characteristics including age, sex, patients’ self-reported race and ethnicity, and hospital characteristics including geographical region (i.e., Midwest, Northeast, South, or West), hospital size (i.e., number of beds), urbanicity of served population (rural vs. urban) and teaching status were provided by the participating hospitals. For baseline comorbidities, Charlson-Deyo comorbidities were identified using ICD-10-CM diagnosis codes (eTable 1 in Supplement) and Charlson Comorbidity Index (CCI) score was calculated using a previously validated method.25 , 26 In addition to Charlson-Deyo comorbidities, morbid obesity was identified using ICD-10-CM diagnosis codes (E66.01, E66.2, Z68.34-Z68.39, Z68.41-Z68.45). Comorbidities were assessed during index hospitalization and any visit to the same hospital within 180 days prior to the index hospitalization.
The severity of illness was assessed using All Patient Refined Diagnosis Related Groups (APR-DRG) Severity of Illness categories. APR-DRG Severity of Illness is a previously validated method of estimating the extent of physiologic decompensation or organ system loss of function using four subclasses (minor, moderate, major, and extreme).27 , 28 In addition, in-hospital dialysis was identified using ICD-10-CM diagnosis codes (Z49.xx, Z99.2) and procedure codes (5A1D70Z, 5A1D80Z, 5A1D90Z) during the index hospitalization.
COVID-19-related medications and supplements (including dexamethasone, remdesivir, convalescent plasma, zinc, vitamin C or D) were identified using hospital chargemaster descriptions during the index hospitalization.29, 30, 31, 32, 33 In addition to COVID-19 medications, we assessed in-hospital use of hyperkalemia-related medications including RAAS inhibitors (e.g., angiotensin-converting-enzyme [ACE] inhibitors, angiotensin receptor blocker [ARB], aldosterone receptor antagonist [ARA]) and intravenous administration of insulin, diuretics, bicarbonates, and calcium gluconates.
COVID-19-related acute complications assessed were acute ischemic heart disease, AKI, acute liver injury, acute respiratory failure, acute respiratory distress syndrome (ARDS), hypokalemia, hyponatremia, metabolic or respiratory acidosis, epileptic seizures, rhabdomyolysis, sepsis, shock, and venous thromboembolism, using ICD-10-CM diagnosis codes (eTable 2 in Supplement).
Statistical analysis
Descriptive statistics were used to compare baseline patient and hospital characteristics of COVID-19 patients with and without hyperkalemia. Continuous variables were reported as mean (standard deviation) or median (1st quartile, 3rd quartile) and categorical variables were reported as counts and percentages. For statistical difference, we used pooled t-test or Mann-Whitney test for continuous variables and χ2 test for categorical variables.
For in-hospital mortality and ICU admission, we first examined the association between hyperkalemia and mortality using a multivariable logistic regression model, only adjusting for patient demographics (i.e., sex, age group, race, and ethnicity) (data not shown, available upon request). We then adjusted for patient characteristics, treatment medications and supplements, and complications. A priori covariates were factors that varied by greater than 10% across exposure groups at baseline: sex, age group, race, ethnicity, CCI category, APR-DRG category, AKI, in-hospital dialysis, ARDS, hyponatremia, acidosis, sepsis, shock, medication use (albumin, antiarrhythmic, beta blocker, blood growth factor, bronchodilator, calcium channel blocker, corticosteroid, antibiotics other than azithromycin, statin, and intravenous administration of insulin, diuretics, bicarbonates, and calcium gluconates). Final covariates were selected using backward elimination method, with significance level of p < .05 to stay in the model (ethnicity, corticosteroids, and sepsis were eliminated). AKI and CHF did not significantly modify the association between hyperkalemia and in-hospital mortality (interaction terms). Multicollinearity between covariates in the final model was tested using variance inflation factor and was not present.
For LOS and hospitalization cost, we used multivariable generalized linear regression models with gamma (for cost) and Poisson (for LOS) variances and log-link functions using the same final covariates described above. Mean values for both variables were estimated using recycled prediction methods and bootstrapping for 95% confidence interval (95% CI) estimations.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina), and the figure was generated using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and forestplot package.
Results
Prevalence of hyperkalemia
We identified 192,182 hospitalized COVID-19 patients with a median age of 63 years, 51.6% male, 51.9% white, 23.1% black, and 23.0% Hispanic (Table 1 ). Most common comorbidity was DM (37.4%), followed by CKD (19.8%), chronic pulmonary disease (19.2%), and morbid obesity (18.0%).
TABLE 1.
Baseline characteristics of hospitalized COVID-19 patients with and without hyperkalemia.
| All | Hyperkalemia | No Hyperkalemia | P-Value | |
|---|---|---|---|---|
| n = 192,182 | n = 22,702 | n = 169,480 | ||
| Patient Characteristics | ||||
| Age, median (q1, q3) | 63.0 (50.0, 75.0) | 67.0 (57.0, 76.0) | 63.0 (49.0, 75.0) | < .001 |
| Age Category, years, n(%) | < .001 | |||
| 18-39 | 25694 (13.4) | 1007 (4.4) | 24687 (14.6) | |
| 40-59 | 54833 (28.5) | 5710 (25.2) | 49123 (29.0) | |
| 60-74 | 60288 (31.4) | 9256 (40.8) | 51032 (30.1) | |
| 75 + | 51367 (26.7) | 6729 (29.6) | 44638 (26.3) | |
| Sex, n(%) | < .001 | |||
| Female | 92846 (48.3) | 8428 (37.1) | 84418 (49.8) | |
| Male | 99073 (51.6) | 14258 (62.8) | 84815 (50.0) | |
| Unknown | 263 (0.1) | 16 (0.1) | 247 (0.1) | |
| Race, n(%) | < .001 | |||
| White | 99719 (51.9) | 9533 (42.0) | 90186 (53.2) | |
| Black | 44354 (23.1) | 6801 (30.0) | 37553 (22.2) | |
| Other | 48109 (25.0) | 6368 (28.1) | 41741 (24.6) | |
| Ethnicity, n(%) | < .001 | |||
| Hispanic or Latino | 44276 (23.0) | 4879 (21.5) | 39397 (23.2) | |
| Not Hispanic or Latino | 111610 (58.1) | 12938 (57.0) | 98672 (58.2) | |
| Other | 36296 (18.9) | 4885 (21.5) | 31411 (18.5) | |
| Health Insurance Status, n(%) | < .001 | |||
| Medicaid | 33345 (17.4) | 3517 (15.5) | 29828 (17.6) | |
| Medicare | 93409 (48.6) | 13687 (60.3) | 79722 (47.0) | |
| Private Insurance | 47844 (24.9) | 4176 (18.4) | 43668 (25.8) | |
| Uninsured/Other/Unknown | 17584 (9.1) | 1322 (5.8) | 16262 (9.6) | |
| Charlson-Deyo Comorbidities, n(%) | ||||
| Myocardial infarction | 15112 (7.9) | 3146 (13.9) | 11966 (7.1) | < .001 |
| Congestive heart failure | 27449 (14.3) | 5498 (24.2) | 21951 (13.0) | < .001 |
| Peripheral vascular disease | 6938 (3.6) | 1155 (5.1) | 5783 (3.4) | < .001 |
| Cerebrovascular disease | 6769 (3.5) | 1285 (5.7) | 5484 (3.2) | < .001 |
| Dementia | 24277 (12.6) | 2984 (13.1) | 21293 (12.6) | .013 |
| Chronic pulmonary disease | 36822 (19.2) | 5043 (22.2) | 31779 (18.8) | < .001 |
| Rheumatic disease | 3568 (1.9) | 407 (1.8) | 3161 (1.9) | .448 |
| Peptic ulcer disease | 1773 (0.9) | 389 (1.7) | 1384 (0.8) | < .001 |
| Mild liver disease | 1729 (0.9) | 327 (1.4) | 1402 (0.8) | < .001 |
| Diabetes mellitus | 71797 (37.4) | 12362 (54.5) | 59435 (35.1) | < .001 |
| Hemiplegia or paraplegia | 2313 (1.2) | 367 (1.6) | 1946 (1.1) | < .001 |
| Chronic kidney disease | 37965 (19.8) | 10193 (44.9) | 27772 (16.4) | < .001 |
| Moderate or severe liver disease | 1811 (0.9) | 489 (2.2) | 1322 (0.8) | < .001 |
| Any malignancy, including leukemia and lymphoma | 6672 (3.5) | 1004 (4.4) | 5668 (3.3) | < .001 |
| Metastatic solid tumor | 2019 (1.1) | 251 (1.1) | 1768 (1.0) | .386 |
| HIV disease | 669 (0.3) | 152 (0.7) | 517 (0.3) | < .001 |
| Charlson Comorbidity Index (CCI) Score Category, n(%) | < .001 | |||
| 0 | 71920 (37.4) | 4575 (20.2) | 67345 (39.7) | |
| 1-4 | 95882 (49.9) | 11909 (52.5) | 83973 (49.5) | |
| 5+ | 24380 (12.7) | 6218 (27.4) | 18162 (10.7) | |
| Morbid Obesity | 34577 (18.0) | 4898 (21.6) | 29679 (17.5) | < .001 |
| Hospital Characteristics | ||||
| Hospital Size, n(%) | < .001 | |||
| 1-299 | 68438 (35.6) | 7015 (30.9) | 61423 (36.2) | |
| 300-499 | 57373 (29.9) | 7082 (31.2) | 50291 (29.7) | |
| 500+ | 66224 (34.5) | 8593 (37.9) | 57631 (34.0) | |
| Unknown | 147 (0.1) | 12 (0.1) | 135 (0.1) | |
| Teaching Status, n(%) | < .001 | |||
| Non-Teaching | 96910 (50.4) | 9719 (42.8) | 87191 (51.4) | |
| Teaching | 95272 (49.6) | 12983 (57.2) | 82289 (48.6) | |
| Population Served, n(%) | < .001 | |||
| Rural | 17966 (9.3) | 1822 (8.0) | 16144 (9.5) | |
| Urban | 174216 (90.7) | 20880 (92.0) | 153336 (90.5) | |
| Geographic Location, n(%) | < .001 | |||
| Midwest | 33511 (17.4) | 3545 (15.6) | 29966 (17.7) | |
| Northeast | 47368 (24.6) | 7450 (32.8) | 39918 (23.6) | |
| South | 81982 (42.7) | 8756 (38.6) | 73226 (43.2) | |
| West | 29321 (15.3) | 2951 (13.0) | 26370 (15.6) | |
HIV: human immunodeficiency virus.
Of these patients, 11.8% (n = 22,702) patients experienced hyperkalemia. Hyperkalemia patients were more likely to be older (median age 67 vs. 63 years old), black (30.0% vs. 22.2%), and have Medicare coverage (60.3% vs. 47.0%) than patients without hyperkalemia (all p < .001). Baseline comorbidities differed as well, and hyperkalemia patients were significantly more likely to have a history of DM (54.5% vs. 35.1%), CKD (44.9% vs. 16.4%), CHF (24.2% vs. 13.0%), myocardial infarction (13.9% vs. 7.1%), chronic pulmonary disease (22.2% vs. 18.8%), and morbid obesity (21.6% vs. 17.5%) than non-hyperkalemia patients (all p < .001). Relatively lower proportion of patients in the hyperkalemia group were in smaller (1−299 beds: 30.9% vs. 36.2%), non-teaching (42.8% and 51.4%), and rural hospitals (8.0% to 9.5%), and hospitals in the South (38.6% to 43.2%) (all p < .001).
Acute complications and medication use
A significantly higher proportion of patients in the hyperkalemia group (70.9%) had extreme severity of illness compared to patients in the non-hyperkalemia group (34.7%, p < .001) (Table 2 ). More patients in the hyperkalemia group experienced acute complications including AKI (70.1% vs. 25.9%), ARDS (22.2% vs. 5.9%), hyponatremia (32.2% vs. 17.3%), metabolic or respiratory acidosis (37.4% vs. 11.2%), sepsis (57.0% vs. 27.5), and shock (38.4% vs. 7.8%) than patients in the non-hyperkalemia group (all p < .001). Hyperkalemia patients were more likely to undergo dialysis during hospitalization (31.4% vs. 4.0%) than patients without hyperkalemia (p < .001).
TABLE 2.
Acute complications and treatments of hospitalized COVID-19 patients with and without hyperkalemia.
| Total | Hyperkalemia | No Hyperkalemia | P-Value | |
|---|---|---|---|---|
| n = 192182 | n = 22702 | n = 169480 | ||
| Discharge Status, n(%) | < .001 | |||
| Expired | 28369 (14.8) | 9592 (42.3) | 18777 (11.1) | |
| Home | 94233 (49.0) | 4459 (19.6) | 89774 (53.0) | |
| Home health | 22134 (11.5) | 2203 (9.7) | 19931 (11.8) | |
| Hospice | 5940 (3.1) | 744 (3.3) | 5196 (3.1) | |
| Nursing or rehabilitation facility | 32388 (16.9) | 4838 (21.3) | 27550 (16.3) | |
| Transferred | 793 (0.4) | 56 (0.3) | 737 (0.4) | |
| Other/unknown | 8324 (4.3) | 809 (3.6) | 7515 (4.4) | |
| APR-DRG Severity of Illness, n(%) | < .001 | |||
| Minor or Moderate | 9662 (5.0) | 141 (0.6) | 9521 (5.6) | |
| Major | 107648 (56.0) | 6465 (28.5) | 101183 (59.7) | |
| Extreme | 74872 (39.0) | 16096 (70.9) | 58776 (34.7) | |
| Acute Complications, n(%) | ||||
| Acute ischemic heart disease | 8175 (4.3) | 2049 (9.0) | 6126 (3.6) | < .001 |
| Acute kidney injury | 59895 (31.2) | 15921 (70.1) | 43974 (25.9) | < .001 |
| Acute liver injury | 4428 (2.3) | 1683 (7.4) | 2745 (1.6) | < .001 |
| Acute respiratory failure | 101602 (52.9) | 13593 (59.9) | 88009 (51.9) | < .001 |
| Acute respiratory distress syndrome | 14981 (7.8) | 5040 (22.2) | 9941 (5.9) | < .001 |
| Cerebrovascular disease | 4899 (2.5) | 1036 (4.6) | 3863 (2.3) | < .001 |
| Hypokalemia | 37828 (19.7) | 3650 (16.1) | 34178 (20.2) | < .001 |
| Hyponatremia | 36617 (19.1) | 7310 (32.2) | 29307 (17.3) | < .001 |
| Metabolic or respiratory acidosis | 27460 (14.3) | 8490 (37.4) | 18970 (11.2) | < .001 |
| Epileptic seizures | 9735 (5.1) | 1464 (6.4) | 8271 (4.9) | < .001 |
| Rhabdomyolysis | 3496 (1.8) | 1031 (4.5) | 2465 (1.5) | < .001 |
| Sepsis | 59842 (31.1) | 12942 (57.0) | 46900 (27.7) | < .001 |
| Shock | 21987 (11.4) | 8724 (38.4) | 13263 (7.8) | < .001 |
| Venous thromboembolism | 9676 (5.0) | 2232 (9.8) | 7444 (4.4) | < .001 |
| Dialysis during Hospitalization, n(%) | 13924 (7.2) | 7137 (31.4) | 6787 (4.0) | < .001 |
| Medication and Supplement Use, n(%) | ||||
| Albumin | 15134 (7.9) | 6051 (26.7) | 9083 (5.4) | < .001 |
| Antiarrhythmic | 11006 (5.7) | 3609 (15.9) | 7397 (4.4) | < .001 |
| Antibiotics other than azithromycin | 135641 (70.6) | 18846 (83.0) | 116795 (68.9) | < .001 |
| Anticoagulant | 136276 (70.9) | 15590 (68.7) | 120686 (71.2) | < .001 |
| Antiemetic | 55504 (28.9) | 6565 (28.9) | 48939 (28.9) | .895 |
| ß-blocker | 62957 (32.8) | 11613 (51.2) | 51344 (30.3) | < .001 |
| Blood growth factor | 16984 (8.8) | 3948 (17.4) | 13036 (7.7) | < .001 |
| Bronchodilator | 70734 (36.8) | 12010 (52.9) | 58724 (34.6) | < .001 |
| Calcium channel blocker | 47543 (24.7) | 8415 (37.1) | 39128 (23.1) | < .001 |
| Convalescent plasma | 4051 (2.1) | 843 (3.7) | 3208 (1.9) | < .001 |
| Corticosteroid (any) | 104468 (54.4) | 15633 (68.9) | 88835 (52.4) | < .001 |
| Dexamethasone | 63070 (32.8) | 7905 (34.8) | 55165 (32.5) | < .001 |
| HIV medication | 2127 (1.1) | 461 (2.0) | 1666 (1.0) | < .001 |
| Hydroxychloroquine and azithromycin | ||||
| Both | 24802 (12.9) | 4531 (20.0) | 20271 (12.0) | < .001 |
| Hydroxychloroquine only | 14106 (7.3) | 2506 (11.0) | 11600 (6.8) | < .001 |
| Azithromycin only | 61773 (32.1) | 6601 (29.1) | 55172 (32.6) | < .001 |
| Neither | 91501 (47.6) | 9064 (39.9) | 82437 (48.6) | < .001 |
| Immunoglobulin | 668 (0.3) | 177 (0.8) | 491 (0.3) | < .001 |
| Immunomodulator | 12136 (6.3) | 3039 (13.4) | 9097 (5.4) | < .001 |
| Narcotic analgesic | 149427 (77.8) | 19296 (85.0) | 130131 (76.8) | < .001 |
| Smoking deterrent | 2518 (1.3) | 255 (1.1) | 2263 (1.3) | .008 |
| Statin | 66120 (34.4) | 9946 (43.8) | 56174 (33.1) | < .001 |
| Vitamin C or D | 57470 (29.9) | 7843 (34.5) | 49627 (29.3) | < .001 |
| Zinc | 46345 (24.1) | 6154 (27.1) | 40191 (23.7) | < .001 |
| Remdesivir | 20237 (10.5) | 2581 (11.4) | 17656 (10.4) | < .001 |
| In-Hospital RAAS Inhibitor Use, n(%) | ||||
| ACE inhibitors | 21301 (11.1) | 2184 (9.6) | 19117 (11.3) | < .001 |
| ARBs | 17211 (9.0) | 1914 (8.4) | 15297 (9.0) | .003 |
| ARAs | 3776 (2.0) | 565 (2.5) | 3211 (1.9) | < .001 |
| Any RAAS inhibitor use | 40262 (21.0) | 4379 (19.3) | 35883 (21.2) | < .001 |
| Intravenous (IV) Administration, n(%) | ||||
| IV formulation of insulin | 73083 (38.0%) | 15791 (69.6%) | 57292 (33.8%) | < .001 |
| IV formulation of diuretics | 63460 (33.0%) | 12625 (55.6%) | 50835 (30.0%) | < .001 |
| IV formulation of bicarbonates | 20627 (10.7%) | 10227 (45.0%) | 10400 (6.1%) | < .001 |
| IV formulation of calcium gluconates | 15305 (8.0%) | 8657 (38.1%) | 6648 (3.9%) | < .001 |
| Any IV administration of the above | 104464 (54.4%) | 19311 (85.1%) | 85153 (50.2%) | < .001 |
APR-DRG: All Patient Refined Diagnosis Related Groups.
HIV: human immunodeficiency virus.
RAAS: renin-angiotensin-aldosterone system.
ACE: angiotensin-converting-enzyme.
ARB: angiotensin receptor blocker.
ARA: aldosterone receptor antagonist.
Patients experiencing hyperkalemia were more likely to receive albumin (26.7% vs. 5.4%), antiarrhythmics (15.9% vs. 4.4%), beta blockers (51.2% vs. 30.3%), blood growth factors (17.4% vs. 7.7%), bronchodilators (52.9% vs. 34.6%), corticosteroids (68.9% vs. 52.4%), immunomodulators (13.4% vs. 5.4%), and statins (43.8% vs. 33.1%) than patients without hyperkalemia (all p < .001). ACE inhibitors use (9.6% vs. 11.3%) and ARBs use (8.4% vs. 9.0%) were lower in the hyperkalemia group compared to non-hyperkalemia group (all p < .01), but the difference was small. Intravenous administration of insulin, diuretics, bicarbonates, and calcium gluconates were significantly higher among hyperkalemia COVID-19 patients compared to patients without hyperkalemia (85.1% vs. 50.2%, p < .001).
In-hospital mortality
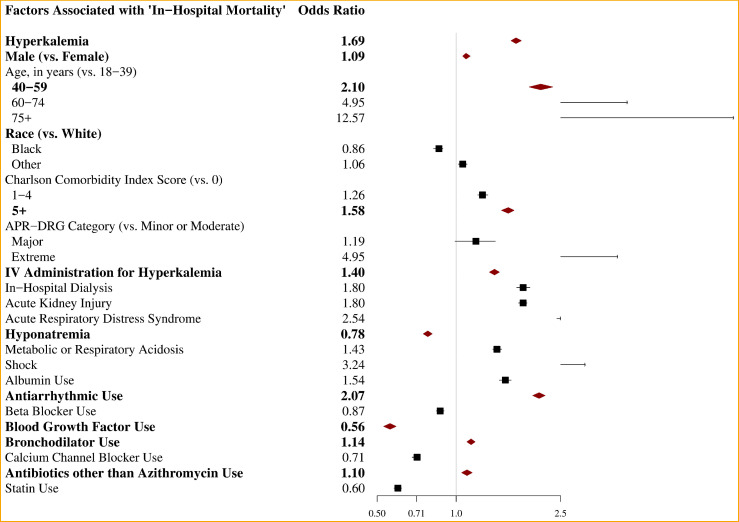
In-hospital mortality was significantly higher among COVID-19 patients experiencing hyperkalemia (42.3%) compared to patients without hyperkalemia (11.1%, p < .001) (Table 3 ). The association between hyperkalemia and in-hospital mortality attenuated after adjusting for significant predictors of mortality including age, sex, race, ethnicity, CCI score, APR-DRG severity of illness, treatment medications and complications. However, hyperkalemia was still associated with significantly increased odds of in-hospital mortality by 69% among COVID-19 patients (aOR 1.69, 95% CI 1.62−1.77) (Table 3, Fig 1 ).
TABLE 3.
In-Hospital mortality, healthcare resource utilization and cost of hospitalized COVID-19 patients with and without hyperkalemia.
| Hyperkalemia | No Hyperkalemia | ||
|---|---|---|---|
| n = 22702 | n = 169480 | ||
| Unadjusted Results | p-value | ||
| In-Hospital Mortality, n(%) | 9592 (42.3) | 18777 (11.1) | < .001 |
| In-Hospital Mortality + Discharged to Hospice, n(%) | 10336 (45.5) | 23973 (14.2) | < .001 |
| Intensive Care Unit Admission, n(%) | 11381 (50.1) | 33088 (19.5) | < .001 |
| Total Hospitalization Length of Stay, in days | |||
| Mean (std) | 12.9 (11.9) | 8.0 (8.9) | < .001 |
| Median (q1, q3) | 10.0 (4.0, 18.0) | 5.0 (2.0, 11.0) | < .001 |
| Total Hospitalization Cost, in 2020 US dollars | |||
| Mean (std) | $60,193 ($80,493) | $21,628 ($36,098) | < .001 |
| Median (q1, q3) | $34,233 ($15,386, $71,888) | $11,168 ($5,944, $22,530) | < .001 |
| Adjusted Results | Difference | ||
| In-Hospital Mortality | |||
| Adjusted Odds Ratio | 1.69 | 1.00 | - |
| 95% Confidence Interval | 1.62-1.77 | [Ref] | - |
| In-Hospital Mortality + Discharged to Hospice | |||
| Adjusted Odds Ratio | 1.61 | 1.00 | - |
| 95% Confidence Interval | 1.54-1.68 | [Ref] | - |
| Intensive Care Unit Admission | |||
| Adjusted Odds Ratio | 1.05 | 1.00 | - |
| 95% Confidence Interval | 1.01-1.09 | [Ref] | - |
| Total Hospitalization Length of Stay, in days | |||
| Mean | 10.00 | 8.74 | 1.26 |
| 95% Confidence Interval | 9.98-10.02 | 8.72-8.76 | 1.26-1.26 |
| Total Hospitalization Cost, in 2020 US dollars | |||
| Mean | $29,861 | $24,472 | $5,389 |
| 95% Confidence Interval | $29,738-$29,977 | $24,371-$24,567 | $5,367-$5,410 |
*All adjusted models were adjusted for sex, age category, race, Charlson Comorbidity Index score category, All Patient Refined Diagnosis Related Groups category, dialysis, acute kidney injury, acute respiratory distress syndrome, hyponatremia, acidosis, shock, and medication use (albumin, antiarrhythmics, beta blocker, blood growth factor, bronchodilator, calcium channel blocker, antibiotics other than azithromycin, statin, and intravenous non-binder treatment for hyperkalemia).
*Cost model was adjusted using generalized linear model regression with gamma variance and log-link function.
*Length of stay model was adjusted using generalized linear model regression with Poisson variance and log-link function.
*Both cost and length of stay models were estimated using recycled prediction method and bootstrapping (n=1,000 simulation) for the 95% confidence interval.
Fig 1.
Association between in-hospital mortality and hyperkalemia (and other factors) among hospitalized COVID-19 patients.
Other factors associated with increased in-hospital mortality among COVID-19 patients were male sex, older age, 1 or greater CCI score, extreme APR-DRG severity of illness, intravenous administration of insulin, diuretics, bicarbonates, or calcium gluconates, dialysis, AKI, ARDS, hyponatremia, metabolic or respiratory acidosis, shock, albumin use, antiarrhythmic use, bronchodilator use, and antibiotics other than azithromycin use (eTable 3 in Supplement).
Using the composite measure of ‘in-hospital mortality + discharged to hospice’ as the outcome variable did not change the association between hyperkalemia and outcome. Hyperkalemia was still associated with significantly increased odds of in-hospital mortality + discharged to hospice by 61% among COVID-19 patients (aOR 1.61, 95% CI 1.54-1.68) (Table 3).
In a subgroup of COVID-19 patients with AKI, those who developed hyperkalemia were 84% more likely to die during hospitalization (aOR 1.84, 95% CI 1.75-1.93) (eTable 4 in Supplement).
Intensive care unit admission, LOS, and cost
Significantly higher proportion of patients in the hyperkalemia group were admitted to the ICU (50.1% vs. 19.5%) than patients without hyperkalemia (Table 3). Median hospital LOS was significantly longer among patients who developed hyperkalemia compared to those who didn't (10 days vs. 5 days). Total median cost of hospitalization was also significantly higher for hyperkalemic patients ($34,233 vs. $11,168) than non-hyperkalemia patients. The differences attenuated after adjusting for age, sex, race, CCI score, APR-DRG severity of illness, and treatment medications and acute complications, but patients with hyperkalemia were still more likely to be admitted to the ICU (aOR 1.05, 95% CI 1.01-1.07), incur higher hospitalization cost (adjusted mean difference $5,389, 95% CI $5,367-$5,410), and have longer LOS (adjusted mean difference 1.26 days, 95% CI 1.26-1.26).
Discussions
This study shows that hyperkalemia was relatively common among hospitalized COVID-19 patients (∼12%). More importantly, hyperkalemia was associated with significantly increased likelihood of death as well as increased cost and LOS during hospitalization. While hyperkalemia was more common among patients experiencing acute complications of COVID-19 such as AKI, ARDS, and shock, this study showed that increased odds of in-hospital mortality was independent of these complications.
The high prevalence of hyperkalemia among COVID-19 inpatients may be explained by two main reasons. First, patient's pre-existing conditions that were risk factors for developing severe illness of COVID-19 were also closely related to risk factors for experiencing hyperkalemia. Using the same database (but a different cohort), Rosenthal et al17. reported that among COVID-19 inpatients, 15.0% had CHF, 40.5% had DM, and 22.9% had CKD while only 2.5% had CHF, 12.9% had DM, and 3.3% had CKD among COVID-19 outpatients during early months of the pandemic. We observed a similar distribution of comorbidities among COVID-19 inpatients. Patients with CHF and CKD were already at a higher risk of impaired urinary potassium excretion and were more likely to develop hyperkalemia,34 , 35 and patients with DM were at risk of redistributive hyperkalemia due to uncontrolled hyperglycemia.35
Second, hyperkalemia was closely associated with AKI, one of the most prevalent and serious renal manifestations of COVID-19. Fisher et al.36 reported that AKI incidence was higher among COVID-19 inpatients (56.9%) compared to non-COVID-19 inpatients (25.1%). Furthermore, compared with non-COVID-19 ICU patients, critically ill COVID-19 patients had a higher incidence of AKI.37 , 38 Chan et al.39 reported that AKI occurred in 46% of 3,993 COVID-19 inpatients and in 75% of those admitted to ICU. In our study cohort, 31.2% of COVID-19 inpatients developed AKI and the proportion was much higher (70.1%) among those with hyperkalemia. In addition, hyperkalemia was also associated with higher likelihoods of other acute complications of COVID-19 (i.e., sepsis, shock, and ARDS).
After accounting for the severity of COVID-19 illness, hyperkalemia-related pre-existing conditions and acute complications, hyperkalemia was still associated with 69% increased odds of in-hospital mortality (aOR 1.69). Liu et al.18 also showed that increased serum potassium level was a predictor of mortality among COVID-19 patients independent of other risk factors. Furthermore, we showed that among hospitalized patients with COVID-19 and AKI, patients with hyperkalemia were 71% more likely to die than those without hyperkalemia. Our findings highlight the importance of monitoring potassium level among COVID-19 inpatients with or without the presence of previously reported conditions and acute complications.
We also presented the negative impact of hyperkalemia on HRU and cost. Betts et al.40 reported that hyperkalemia inpatients had 1.51 days longer hospital stay than patients without hyperkalemia in the pre-COVID-19 era. Our results showed a similar difference among COVID-19 inpatients, as hyperkalemia patients had an adjusted mean of 1.26 days longer LOS than those without. Betts et al.40 also showed that hyperkalemia patients incurred $4,128 higher 30-day total healthcare costs than non-hyperkalemia patients. In this study, we showed that a single hospitalization for COVID-19 patients with hyperkalemia can incur $5,389 additional hospitalization cost compared to those without hyperkalemia.
Limitations
This study has several limitations. First, this was a secondary data analysis using hospital administrative database. Hyperkalemia status was captured by ICD-10-CM diagnosis code and potassium binder use instead of by laboratory values, and were more likely to capture more prominent hyperkalemia cases. Mild hyperkalemia patients might have been misclassified as non-hyperkalemia patients and bias the results towards the null. We did not differentiate CKD and AKI stages nor specify the dosages for RAAS inhibitors. In addition, other clinical conditions and medications were also identified using hospital-reported diagnosis and procedure codes and chargemaster descriptions; misclassification bias is possible. However, the potential misclassification is expected to be non-differential between hyperkalemia and non-hyperkalemia patients and is likely to bias the association towards the null. Second, due to the nature of observational studies, we were not able to draw any causal association between hyperkalemia and in-hospital mortality due to unmeasured confounding variables. Although we know that all other conditions including acute complications preceded death, we were not able to identify the temporal association between hyperkalemia, ICU admission, and acute complications. Third, the race and ethnicity variables were self-reported by patients at the time of hospitalization and 19% of patients had unknown ethnicity and 25% of patients had other or unknown race. This could have resulted in underestimating the actual percentage of Hispanic or black patients with COVID-19.
Conclusions
Despite these limitations, this study described the association between hyperkalemia and in-hospital mortality, ICU admission, LOS, and hospitalization cost using a large COVID-19 cohort in the US. Hyperkalemia was a relatively common electrolyte imbalance among hospitalized COVID-19 patients; patients were more likely to die and get admitted to the ICU during hospitalization, be hospitalized longer, and incur higher hospitalization cost when they experienced hyperkalemia. The associations were significant after accounting for the severity of illness and hyperkalemia-related conditions and acute complications such as AKI. Our findings warrant a close monitoring of potassium levels among hospitalized COVID-19 patients. Future randomized trials are needed to confirm and better understand the impact of hyperkalemia on mortality and HRU among COVID-19 patients and to identify effective methods to predict and control hyperkalemia.
Authors Contributions
Conceptualization - R.M., N.R., A. Agiro, R.L., W.P., and A. Amin; Data curation - H.B.; Formal analysis - H.B., and R.M.; Funding acquisition - A. Agiro, N.R., R.L., and W.P.; Investigation - R.M., A. Agiro, N.R., and A. Amin; Methodology - R.M., A. Agiro, and N.R.; Project administration - A. Agiro, R.L., W.P., and N.R.; Resources - A. Agiro, R.L., W.P., and N.R.; Software - R.M., H.B., and N.R.; Supervision - A. Agiro, N.R., and A. Amin; Validation - R.M., and N.R.; Visualization - R.M.; Writing - Original draft - R.M., N.R., and A. Agiro; Writing - Review and editing - R.M., N.R., A. Agiro, A. Amin, R.L., and W.P.
Declaration of Competing Interest
A. Amin served as a principal investigator or co-investigator of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therpeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion. He served as a speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Millenium, PeraHealth, HeartRite, Aseptiscope, Sprightly.
R.M. worked on the study as a full-time employee of Premier, Inc. N.R. and H.B. worked on the study as full-time employees and stockholders of Premier, Inc.
A. Agiro, R.L., and W.P. worked on the study as full-time employees and stockholders of AstraZeneca.
Acknowledgments
Acknowledgement
Funding/Support
This study was funded by AstraZeneca.
Role of Funder/Sponsor
The funder had a role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript as well as decision to submit the manuscript for publication; however, the funder had no role in collection of the data.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjms.2022.04.029.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.JohnsHopkinsUniversity . 2022. COVID-19 Dashboard by the Center for Systems Science and Engineering at Johns Hopkins University. https://coronavirus.jhu.edu/map.html. Accessed February 24. [Google Scholar]
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Petrilli C.M., Jones S.A., Yang J., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Team C.C.-R. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowe B., Cai M., Xie Y., et al. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y., Luo R., Wang K., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Y., Luo R., Wang X., et al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394–1402. doi: 10.2215/CJN.04650420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins-Juarez S.Y., Qian L., King K.L., et al. Outcomes for patients With COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. 2020;5(8):1149–1160. doi: 10.1016/j.ekir.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraft M.D., Btaiche I.F., Sacks G.S., et al. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm. 2005;62(16):1663–1682. doi: 10.2146/ajhp040300. [DOI] [PubMed] [Google Scholar]
- 11.Hollander-Rodriguez J.C., Calvert J.F., Jr. Hyperkalemia. Am Fam Physician. 2006;73(2):283–290. [PubMed] [Google Scholar]
- 12.Crawford A.H. Hyperkalemia: recognition and management of a critical electrolyte disturbance. J Infus Nurs. 2014;37(3):167–175. doi: 10.1097/NAN.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 13.Luo J., Brunelli S.M., Jensen D.E., et al. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11(1):90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dattani R., Hill P., Medjeral-Thomas N., et al. Oral potassium binders: increasing flexibility in times of crisis. Nephrol Dial Transplant. 2020;35(8):1446–1448. doi: 10.1093/ndt/gfaa202. [DOI] [PubMed] [Google Scholar]
- 15.Kunutsor S.K., Laukkanen J.A. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. 2020;52(7):345–353. doi: 10.1080/07853890.2020.1790643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T., Wu D., Chen H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal N., Cao Z., Gundrum J., et al. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S., Zhang L., Weng H., et al. Association between average plasma potassium levels and 30-day mortality during hospitalization in patients with COVID-19 in Wuhan, China. Int J Med Sci. 2021;18(3):736–743. doi: 10.7150/ijms.50965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weir M.R., Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clinic J Amer Soc Nephrol. 2010;5(3):531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- 20.Premier . 2021. Premier Healthcare Database (COVID-19): Data that Informs and Performs. https://offers.premierinc.com/WCFY20PASCOVIDWhitepaper_LandingPage.html. Published 2020. Accessed Aug 20. [Google Scholar]
- 21.Lavery A.M., Preston L.E., Ko J.Y., et al. Characteristics of Hospitalized COVID-19 Patients Discharged and Experiencing Same-Hospital Readmission - United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(45):1695–1699. doi: 10.15585/mmwr.mm6945e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premier . 2021. Premier Healthcare Database Being Used by National Institutes of Health to Evaluate Impact of COVID-19 on Patients Across the U.S. https://www.premierinc.com/newsroom/press-releases/premier-healthcare-database-being-used-by-national-institutes-of-health-to-evaluate-impact-of-covid-19-on-patients-across-the-u-s. Published 2020. Accessed Mar 26. [Google Scholar]
- 23.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 24.CDC . 2021. ICD-10-CM official coding and reporting guidelines April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/data/icd/COVID-19-guidelines-final.pdf. Published 2020. Accessed Mar 26. [Google Scholar]
- 25.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Rosenthal N., Cao Z., Chung J., et al. ISPOR 22nd Annual International Meeting; May 21, 2017; Boston, MA. 2017. Updated coding algorithm for assessing Charlson comorbidity index using large hospital administrative data. [Google Scholar]
- 27.Averill R.F., Goldfield N.I., Muldoon J., et al. A closer look at all-patient refined DRGs. J AHIMA. 2002;73(1):46–50. [PubMed] [Google Scholar]
- 28.Romano P.S., Chan B.K. Risk-adjusting acute myocardial infarction mortality: are APR-DRGs the right tool? Health Serv Res. 2000;34(7):1469–1489. [PMC free article] [PubMed] [Google Scholar]
- 29.FDA . 2021. FDA's Approval of Veklury (remdesivir) for the Treatment of COVID-19 - The Science of Safety and Effectiveness. https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness. Accessed Jan 27. [Google Scholar]
- 30.FDA . U.S. Food & Drug Administration; 2021. FDA Cautions Against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. Accessed Jan 27. [Google Scholar]
- 31.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 [Google Scholar]
- 32.Thomas S., Patel D., Bittel B., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 Infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tworek A., Jaron K., Uszynska-Kaluza B., et al. Convalescent plasma treatment is associated with lower mortality and better outcomes in high risk COVID-19 patients - propensity score matched case-control study. Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long B., Warix J.R., Koyfman A. Controversies in management of hyperkalemia. J Emerg Med. 2018;55(2):192–205. doi: 10.1016/j.jemermed.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Gorriz J.L., D'Marco L., Pastor-Gonzalez A., et al. Long-term mortality and trajectory of potassium measurements following an episode of acute severe hyperkalemia. Nephrol Dial Transplant. 2021 doi: 10.1093/ndt/gfab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fisher M., Neugarten J., Bellin E., et al. AKI in Hospitalized Patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang L., Zhu Y., Luo X., et al. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol. 2019;20(1):468. doi: 10.1186/s12882-019-1660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaibi K., Dao M., Pham T., et al. Severe acute kidney injury in patients with COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;202(9):1299–1301. doi: 10.1164/rccm.202005-1524LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan L., Chaudhary K., Saha A., et al. AKI in Hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betts K.A., Woolley J.M., Mu F., et al. The cost of Hyperkalemia in the United States. Kidney Int Rep. 2018;3(2):385–393. doi: 10.1016/j.ekir.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.