Abstract
Coronavirus disease 2019 (COVID-19) is a highly contagious respiratory illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) that began spreading globally in late 2019. While most cases of COVID-19 present with mild to moderate symptoms, COVID-19 was the third leading cause of mortality in the United States in 2020 and 2021. Though COVID-19 affects individuals of all races and ethnicities, non-Hispanic Black and Hispanic/Latinx populations are facing an inequitable burden of COVID-19 characterized by an increased risk for hospitalization and mortality. Importantly, non-Hispanic Black and Hispanic/Latinx adults have also faced a greater risk of non-COVID-19-related mortality (e.g., from cardiovascular disease/CVD) during the pandemic. Contributors to the racial disparities in morbidity and mortality during the pandemic are multi-factorial as we discuss in our companion article on social determinants of health. However, profound racial variation in the prevalence of CVD and metabolic diseases may serve as a key driver of worse COVID-19-related and non-COVID-19-related health outcomes among racial and ethnic minority groups. Within this review, we provide data emphasizing the inequitable burden of CVD and metabolic diseases among non-Hispanic Black and Hispanic/Latinx populations. We also discuss the pathophysiology of these conditions, with a focus on how aberrant physiological alterations in the context of CVD and metabolic diseases manifest to increase susceptibility to severe COVID-19.
Keywords: SARS-CoV-2, Cardiovascular disease, Metabolic disease, Health disparities, Obesity
Abbreviations: ACE2, angiotensin converting enzyme 2; BMI, body mass index; BP, blood pressure; CDC, Centers for Disease Control and Prevention; cf-PWV, carotid femoral pulse wave velocity; CHD, coronary heart disease; COVID-19, coronavirus disease 2019; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; FMD, flow-mediated dilation; HF, heart failure; HRV, heart rate variability; ICU, intensive care unit; MESA, Multi-Ethnic Study of Atherosclerosis; MSNA, muscle sympathetic nerve activity; NHANES, National Health and Nutrition Examination Survey; RR, relative risk or risk ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; T2D, type 2 diabetes; US, United States
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a novel coronavirus discovered in December 2019 in Wuhan, China.1 SARS-CoV-2 is part of the coronavirus family, which includes common viruses that cause a variety of diseases from head or chest colds to more severe, but rarer, diseases such as SARS-CoV-1 and Middle East respiratory syndrome.2 SARS-CoV-2 began spreading around the globe in 2019 and reached the United States (US) in early 2020.1 While most cases of COVID-19 present with mild to moderate symptoms, there is a heterogeneous burden of severe COVID-19 symptoms and mortality in nursing homes, older individuals, and in individuals with pre-existing conditions.3
By December 2021, global COVID-19 cases surpassed 250 million, and despite a less than 1% case-fatality rate, approximately 5 million deaths worldwide were attributed to COVID-19.4 In the US, there were more than 50 million cases and approximately 800,000 deaths attributable to COVID-19, making COVID-19 the third leading cause of mortality behind only cardiovascular (CV) disease (CVD) and cancer in 2020 and 2021.5 As the pandemic spread throughout the US, there was mounting evidence relatively early on that racial and ethnic minorities, as well as socioeconomically disadvantaged groups, were bearing a disproportionate burden of severe illness and mortality from COVID-19.6 The focus of this review is to summarize major physiological factors that may contribute to racial and ethnic-related health disparities in COVID-19 with an emphasis on pre-existing health conditions, particularly CVD and metabolic diseases. Readers may also refer to a companion review article, which focuses on social determinants of health that may play a role in racial and ethnic-related heath disparities and COVID-19 outcomes.7
Data from Spring 2020 (initial COVID-19 wave in the US) indicated non-Hispanic Black adults with confirmed COVID-19 were significantly more likely to be admitted to the hospital compared to individuals from other racial or ethnic groups.6 Data from the second COVID-19 wave in Summer through Winter 2020 indicated that COVID-19-related hospitalizations were higher in non-Hispanic Black and Hispanic/Latinx individuals compared with non-Hispanic White individuals.8 Specifically, the relative risk ((RR), 95% confidence interval (CI)) for hospitalizations was 3.1 (95% CI 2.8–3.3) for non-Hispanic Black individuals and 5.9 (95% CI 5.6–6.3) for Hispanic/Latinx individuals compared to non-Hispanic White individuals.8 Recent data also indicated that non-Hispanic Black, Hispanic/Latinx, and other racial/ethnic minority groups (i.e., American Indian or Alaska Native, and Asian or Pacific Islander) were more likely than non-Hispanic White individuals to have a COVID-19-associated hospitalization, intensive care unit (ICU) admission, or in-hospital mortality during the first year of the COVID-19 pandemic.9 For example, compared to non-Hispanic White individuals, the RR for non-Hispanic Black individuals was 2.9 (95% CI, 2.8–2.9) for hospitalization, 3.2 (95% CI, 3.1–3.3) for ICU admission, and 2.6 (95% CI, 2.5–2.7) for death.9 Meanwhile, for Hispanic/Latinx individuals, the RR was 3.1 (95% CI, 3.0–3.1) for hospitalization, 4.2 (95% CI, 4.1–4.3) for ICU admission, and 3.9 (95% CI, 3.7–4.0) for death compared to non-Hispanic White individuals.9 The greater risk for COVID-19-related hospitalization and death in racial/ethnic minorities is supported by findings from a recent systematic review of 52 studies, 71% of which were from the US.10
Although racial and ethnic disparities in COVID-19 death rates are striking, focusing on confirmed COVID-19 deaths alone may underestimate the true effect of the pandemic on perpetuating, or even amplifying, health disparities.11 However, excess death estimates capture deaths both directly and indirectly caused by COVID-19. In 2020, the age-adjusted death rate increased by 16% in the US.5 Overall death rates were highest among non-Hispanic Black and non-Hispanic American Indian or Alaska Native individuals, while COVID-19-related deaths were highest among Hispanic/Latinx and non-Hispanic American Indian or Alaska Native individuals.5 Between March and December 2020, there were nearly 500,000 excess deaths compared to the same time period in 2019, 74% of which were attributable to COVID-19.11 Age-standardized excess death rates among non-Hispanic Black, Hispanic/Latinx, and non-Hispanic American Indian or Alaska Native individuals were more than double those in non-Hispanic White and Asian individuals.11 Moreover, compared with non-Hispanic White individuals, age-standardized non-COVID-19 excess deaths (i.e., diabetes, CVD and cerebrovascular diseases, and Alzheimer's disease) also disproportionately affected non-Hispanic Black, Hispanic/Latinx, and non-Hispanic American Indian or Alaska Native individuals.11 A separate investigation specific to excess CVD-related mortality during the COVID-19 pandemic demonstrated that non-Hispanic Black, Hispanic/Latinx, and Asian populations each experienced a ~ 20% greater rise in mortality caused by CVD (e.g., myocardial infarction and cardiac arrest) and cerebrovascular disease (e.g., stroke) compared to non-Hispanic White adults.12
The reasons for these alarming health disparities during COVID-19 are multi-factorial and include pre-existing health conditions, numerous social determinants of health, and health behaviors7 (See Fig. 1 ). Below, we will discuss the racial and ethnic disparities in pre-existing conditions, specifically CVD and metabolic diseases, and how the pathophysiology underlying these disease states may be associated with worse COVID-19 outcomes (see Fig. 2 ).
Fig. 1.
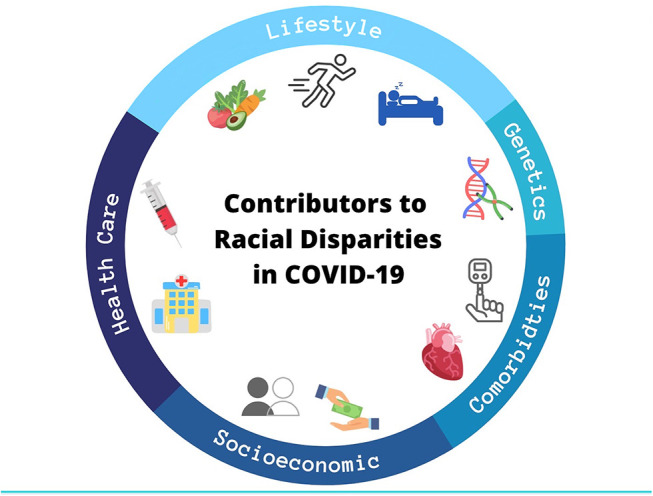
Illustration of the multi-factorial etiology of racial disparities in COVID-19 health outcomes. Though overlap among these factors exists, lower socioeconomic status and segregation/discrimination, inequities in healthcare and vaccine status, health behaviors, (epi)genetic and environmaental variation, and an increased burden of comorbidities such as cardiovascular and metabolic disease synergize to increase the prevalence and severity of COVID-19 among racial and ethnic minorities. This figure is also depicted in our companion paper.7 Resused with permission.
Fig. 2.
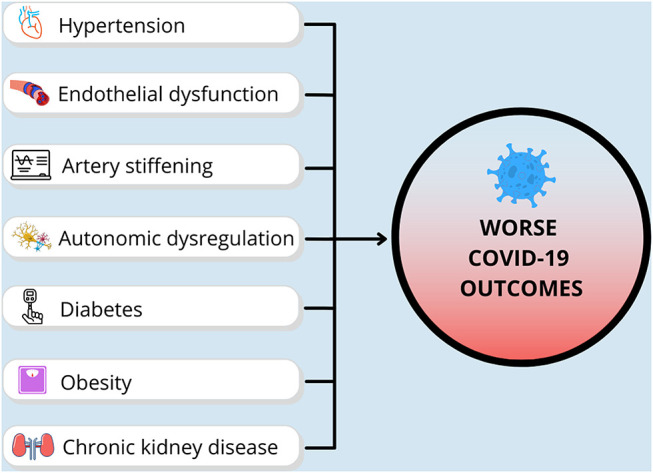
Illustration of cardiometabolic health factors that contribute to COVID-19 health outcomes. We postulate that the greater prevalence of cardiometabolic diseases, and their pathophysiological manifestations, likely play a role in increased susceptibility for severe COVID-19 and worse COVID-19-related health outcomes in non-Hispanic Black and Hispanic/Latinx populations.
Disparities in CVD and COVID-19 outcomes
Common CVDs including stroke, coronary heart disease (CHD), and heart failure (HF), are the leading cause of mortality worldwide. In the US, the prevalence of lifetime CVD in adults aged ≥20 years is nearly 50%, with annual healthcare costs reaching $364 billion.13 Although CVDs afflict individuals from all races and ethnicities, the prevalence and mortality rates appear to be exacerbated in non-Hispanic Black and Hispanic/Latinx populations compared to non-Hispanic White adults.13 For instance, the annual age-adjusted rate of first ischemic stroke per 1000 persons is greater in non-Hispanic Black (1.91) and Hispanic/Latinx (1.49) compared to non-Hispanic White adults (0.88).14 Moreover, these disparities persist in sub-analyses comparing incidence of various stroke types (i.e., intracranial and extracranial atherosclerotic, lacunar, and cardiometabolic) in both non-Hispanic Black and Hispanic/Latinx adults relative to non-Hispanic White adults (RR ranges from 1.42 to 5.85).14 Importantly, while age-adjusted stroke-related mortality has declined by ~7% in recent decades in the general population, mortality rates from stroke remain substantially higher in non-Hispanic Black adults compared with non-Hispanic White adults.13 These findings warrant an improved understanding of factors that underpin racial/ethnic disparities in stroke incidence and mortality.
In addition to stroke, the presence of racial/ethnic disparities in CHD and HF have been noted. For instance, improvements in clinical care have contributed to a decline in the incidence of CHD in the US, but this trend is less apparent in non-Hispanic Black compared to non-Hispanic White adults.15 Regarding COVID-19-related outcomes, pre-existing CHD or HF exacerbates risk for negative health outcomes with myocardial injury in patients hospitalized for COVID-19.16 To uncover factors that may contribute to racial/ethnic disparities in the incidence of CHD, a recent study evaluated temporal trends in the prevalence of risk factors using a large dataset from the multicenter Atherosclerosis Risk in Communities study.17 While the contribution of major risk factors, such as hypertension and hypercholesterolemia, to the incidence of CHD appeared to decline in recent decades in non-Hispanic White adults, similar findings were not observed in non-Hispanic Black adults.17 Specifically, the contribution from hypertension remained markedly higher in non-Hispanic Black adults despite higher rates of non-Hispanic Black individuals undergoing antihypertensive treatments.17 Likewise, the contribution from hypercholesterolemia was 3-fold greater in non-Hispanic Black adults despite higher statin use.17
Recent data indicate age-adjusted hospitalization RR for CHD in Hispanic/Latinx adults relative to non-Hispanic White adults is 1.6 (95% CI, 1.2–1.9) for females and 1.4 (95% CI, 1.2–1.7) for males. However, in 2018, the age-adjusted mortality rates for CHD per 10,000 persons females was 12.9 in non-Hispanic White males and 6.5 in White females, 14.1 in non-Hispanic Black males and 8.0 in Black females, and 9.3 in Hispanic/Latinx males and 5.0 in Hispanic/Latinx females.13 These findings suggest males seem to exhibit lower CHD survival rates relative to females. The RR for myocardial infarction-related in-hospital mortality for individuals less than 65 years of age was 1.48-fold higher in Hispanic/Latinx females (3.7%) and 1.24-fold higher in non-Hispanic Black (3.1%) compared to non-Hispanic White females (2.5%), but non-Hispanic White females had the greatest risk after 65 years of age.18 Taken together, these findings demonstrate a greater burden of CHD for non-Hispanic Black adults whereas disparities in CHD among Hispanic/Latinx populations are not as clear and may depend on age and biological sex.
Lastly, HF currently afflicts approximately six million adults in the US, and the prevalence of HF is projected to reach eight million by 2030.13 Compared to non-Hispanic White adults, longitudinal data from the Multi-Ethnic Study of Atherosclerosis (MESA) suggest a 4.6-fold greater risk in non-Hispanic Black adults and 3.5-fold greater risk in Hispanic/Latinx adults for developing congestive HF.19 However, HF-related mortality (per 10,000 persons) does not appear to be appreciably greater in non-Hispanic Black males (12.1) or Hispanic/Latinx males (7.2) compared to non-Hispanic White males (11.4), or in non-Hispanic Black females (8.7) or Hispanic/Latinx females (5.0) compared to non-Hispanic White females (8.2).13 However, males with HF seem to exhibit lower survival rates relative to females, irrespective of race or ethnicity.13
The mechanisms underlying racial disparities in CVD are not fully resolved, but involve hypertension and vascular dysfunction, which may manifest as endothelial dysfunction of both the macro- and microvasculature, central arterial stiffening, and autonomic dysregulation.20 , 21 In the next section of this review, we will discuss racial/ethnic-related disparities in hypertension, endothelial dysfunction, central arterial stiffening, and autonomic regulation of blood pressure (BP), as well as how each of these may impact COVID-19 outcomes. Given the large body of literature concerning racial/ethnic-related health disparities in non-Hispanic Black individuals, the majority of content in the following sections will feature studies comparing non-Hispanic Black and non-Hispanic White adults. However, we will incorporate findings in Hispanic/Latinx populations when data are available.
Hypertension
While several traditional and non-traditional risk factors may be involved in the pathogenesis and progression of CVD, hypertension is a major modifiable risk factor. Racial differences in BP emerge early in life,22 , 23 and non-Hispanic Black adults suffer from the highest rates of hypertension of any racial/ethnic groups in the United States.13 According to National Health and Nutrition Examination Survey (NHANES) data, the prevalence of hypertension (defined as systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or self-reported use of an antihypertensive medication) is 45.3% in non-Hispanic Black adults compared with 31.4% in non-Hispanic White adults.24 In persons 55 years of age or older, the cumulative incidence of hypertension in non-Hispanic Black adults (76%, both sexes) is also greater than non-Hispanic White males (55%) and females (40%).25 Furthermore, fewer cases of hypertension are controlled in non-Hispanic Black adults (39.2%) compared to non-Hispanic White adults (49.1%).24 Hispanic/Latinx adults (31.6%) exhibit a similar hypertension prevalence as non-Hispanic White adults, although rates of awareness, treatment, and control are lower.24
A recent study quantified the incidence of hypertension (defined as systolic BP ≥130 mmHg, diastolic BP ≥80 mmHg, or use of antihypertensive medication) among self-identified Hispanic/Latinx adults, aged 18 to 74 years, from four US communities.26 The 6-year-age-adjusted probability of incident hypertension was 21.7% (95% CI, 19.5–24.1) among males and 19.7% (95% CI, 18.1–21.5) among females.26 When stratified by different ethnic backgrounds, the probability of incident hypertension was markedly higher among adults of Cuban (male: 27.1% and female: 22.6%) and Dominican (male: 28.1% and female: 23.3%) backgrounds relative to those of Mexican American background (male: 17.6% and female: 16.0%). Collectively, these findings of racial/ethnic-related disparities in the prevalence, awareness, and control rates of hypertension underscore the need for additional targeted strategies for preventing and combating uncontrolled hypertension.
The exact mechanisms by which racial/ethnic-related differences in the prevalence and management of hypertension contribute to increased risks of CVD-related morbidity and mortality are incompletely understood. However, there is evidence that a high prevalence of end organ damage in young non-Hispanic Black adults with hypertension may contribute to worse health outcomes.27 For instance, 25%–30% of young non-Hispanic Black adults exhibit left ventricular hypertrophy,27 an abnormality that is associated with target organ damage and has been independently associated with adverse CVD events.28 In addition, evidence of left ventricular systolic dysfunction has been detected in 8–10% of young non-Hispanic Black adults with uncontrolled hypertension,27 which is clinically alarming given that non-Hispanic Black individuals are more likely than non-Hispanic White individuals to suffer from severe HF and mortality.29 Likewise, concentric and eccentric left ventricular hypertrophy are more frequently observed in Hispanic/Latinx adults compared to non-Hispanic White individuals.30 Furthermore, in a cohort of middle-aged to older Hispanic/Latinx adults, the prevalence of left systolic dysfunction was 3.6% and the prevalence of left diastolic dysfunction was an alarming 50.3%, much of which appeared to be related to hypertension.31
Observational studies repeatedly demonstrate an association between hypertension and COVID-19 infection and mortality rates. In fact, nearly a third of patients infected with COVID-19 have underlying hypertension.32 When admitted to the hospital with COVID-19, a greater percentage of patients with hypertension (31.8%) than patients with normotension (21.3%) progress to severe COVID-19. Patients with hypertension were more likely to need mechanical ventilation.33 Moreover, the risk of COVID-19 mortality was twice as great in patients with hypertension compared to patients with normotension, and those with uncontrolled hypertension were more likely to die than individuals with controlled hypertension.33
When compared to case-controls, hospitalized patients with COVID-19 present with elevations in BP and biomarkers that are related to inflammation (e.g., C-reactive protein, tumor necrosis factor-α), cardiac tissue injury (e.g., troponin, procalcitonin), and vasoconstriction (e.g., angiotensin 2).34 The elevations in biomarkers of inflammation and tissue damage may underlie the increased risk for myocardial angina, stroke, and need for percutaneous coronary intervention among patients with COVID-19.33 While the underlying mechanisms responsible for COVID-19-mediated induction of hypertension are unclear, vascular dysfunction and renal mechanisms may be implicated.35
Endothelial dysfunction
The endothelium is the innermost cell layer of the vasculature and serves as a key regulator of vascular homeostasis.36 Healthy endothelium produces several vasoactive substances, such as nitric oxide (NO), prostaglandins, and bradykinin, that help to regulate vascular tone, cellular adhesion, smooth muscle cell proliferation, vascular inflammation, and thrombosis.36 Conversely, endothelial dysfunction is characterized by reduced NO bioavailability and increased production of vasoconstrictors, such as endothelin-1 and thromboxane A2, which contribute to impaired vasodilation.36 Thus, it is not surprising that endothelial dysfunction is an early atherosclerotic event that precedes the development and progression of several pathological conditions.36 For instance, impaired endothelium-dependent vasodilation of coronary arteries independently predicts the future development of CHD.37 In addition, attenuated brachial artery flow-mediated dilation (FMD), a non-invasive technique to assess conduit artery endothelial function, independently predicts CVD-related morbidity and mortality.38, 39, 40 In fact, every 1% change in FMD confers a reciprocal ~13% change in risk for CVD events.41
The heightened risk of CVD in non-Hispanic Black and Hispanic/Latinx populations may be attributed, in part, to endothelial dysfunction. For instance, postmenopausal non-Hispanic Black females exhibit a lower brachial artery FMD compared with non-Hispanic White females.42 In contrast, multiple studies reported no group differences in brachial artery FMD in young non-Hispanic Black compared to non-Hispanic White males and females.43, 44., 45 Interestingly, one study demonstrated that Hispanic/Latinx adults of Mexican descent exhibited higher brachial artery FMD than non-Hispanic White adults.46 In the microvasculature, multiple studies have demonstrated blunted microvascular vasodilator function in non-Hispanic Black adults compared with non-Hispanic White adults.47, 48., 49
Regarding mechanisms underlying racial disparities in endothelial dysfunction, lower NO bioavailability was recently detected in human umbilical vein endothelial cells from non-Hispanic Black versus non-Hispanic White adults, which appeared to be mediated, at least in part, by greater oxidative stress in the cells from non-Hispanic Black donors.50 Further evidence for reduced NO bioavailability in non-Hispanic black adults was provided in experiments from a separate investigation demonstrating that intradermal L-arginine (key substrate for producing NO) attenuates racial disparities in the cutaneous microvasculature.47 Plasma concentrations of the vasoconstrictor endothelin-1 are also elevated in non-Hispanic Black adults with hypertension compared to non-Hispanic White adults with hypertension.51 Taken together, current findings suggest racial/ethnic-related disparities in endothelial function may manifest differentially along the arterial tree (e.g., macro- vs microvascular function). The heterogeneous findings regarding race and endothelial function may also be due to social determinants (e.g., adverse childhood experiences), lifestyle (e.g., diet, exercise, and sleep), and/or biological (age and sex) differences among the cohorts and highlight a need for future studies in this area featuring well-characterized participants. Regardless, given the prognostic ability of endothelial health to CVD,38, 39, 40 it stands to reason that endothelial dysfunction may be an important contributing factor to the greater prevalence of CVD in the non-Hispanic Black population while additional data are needed in the Hispanic/Latinx population.
Endothelial health is also important in the development and progression of COVID-19. Angiotensin-converting enzyme 2 (ACE2) plays a critical role in endothelial function and maintenance of blood vessel integrity through vasodilatory, anti-inflammatory, and anti-fibrotic pathways (via renin-angiotensin-aldosterone system signaling).52 However, ACE2 receptors ubiquitously found on the cell membranes of various organs and tissues also serve as an entry point for SARS-CoV-2.53 Once bound to ACE2 receptors, the coronavirus spike protein triggers a downregulation of intracellular ACE2 expression, while ACE levels seem to be unaffected.54 The subsequent imbalance in ACE/ACE2 levels is thought to trigger an overactivation of the renin angiotensin aldosterone system and a subsequent progression of COVID-19,53 possibly contributing to vascular thrombosis.54 Indeed, a prospective cohort study in patients with COVID-19 who were in the ICU observed elevated levels of atherothrombotic indicators compared to age- and sex-matched counterparts.55 Moreover, there is evidence for reduced endothelial function in patients who were recovering from COVID-19.56 , 57 Collectively, these findings highlight that endothelial activation and dysfunction plays an important role in the pathogenesis of COVID-19.
Large artery stiffening
Large elastic arteries (i.e., aortic and carotid) play a pivotal role in cardiovascular health by protecting the microvasculature from exposure to excessive pulsatility due to fluctuations in BP and flow with intermittent left ventricular ejection.58 Conversely, when large elastic arteries become stiff, the overall buffering capacity of these arteries becomes impaired, enabling pulsatile energy to penetrate microvessels yielding microvascular and end-organ (e.g., heart, kidneys, brain, eyes) damage.58 In addition, central (aortic) BP, which is more reflective of the arterial pressures that the heart, brain, and kidneys are exposed to, becomes elevated due to central (large) arterial stiffening.59 Importantly, elevations in central arterial stiffness (assessed via carotid-femoral pulse wave velocity; cf-PWV), which is the gold-standard for the non-invasive assessment of large artery stiffness, and central BP are independently associated with increased risk of incident CVD.60 , 61 Therefore, identification of factors implicated in central arterial stiffening may serve to provide insight into potential therapeutic targets for improving CV health.
While multiple factors, including aging and pathological conditions (i.e., hypertension) contribute to central arterial stiffening,58 accumulating evidence indicates that race/ethnicity may play a role.20 For example, there are data indicating that non-Hispanic Black children exhibit significantly higher cf-PWV compared with non-Hispanic White children even after adjustment for confounders.62 Likewise, young non-Hispanic Black males without hypertension also present with elevations in cf-PWV compared with non-Hispanic White males.63 Additional data suggest non-Hispanic Black adults (inclusive of males and females) had higher cf-PWV values than non-Hispanic White adults, though these group differences disappeared after controlling for CVD risk factors, including BP.64 However, in a large cohort of 2500 apparently healthy middle-aged adults, greater central artery stiffness, assessed via carotid ꞵ-stiffness, was observed in non-Hispanic Black compared to non-Hispanic White individuals after covariate adjustment.65 These findings are consistent with data indicating middle-aged non-Hispanic Black and Hispanic/Latinx adults exhibit greater proximal aortic stiffness, assessed via aortic arch PWV, compared with non-Hispanic White adults.66
The higher central arterial stiffness in non-Hispanic Black and Hispanic/Latinx populations likely involves several pathways implicated in vascular inflammation and oxidative stress. Underlying factors likely include a combination of social determinants (e.g., adverse childhood experiences), lifestyle (e.g., diet, exercise, and sleep) and environmental and (epi)genetic interactions that predispose individuals to premature vascular aging.7 , 67 , 68 Greater central arterial stiffening in non-Hispanic Black and Hispanic/Latinx populations likely contributes to the increased risk of CVD and disparities in COVID-19 health outcomes. For example, a case-control study demonstrated significantly higher cf-PWV and brachial-ankle PWV (peripheral arterial stiffness) in patients with COVID-19 versus healthy controls.69 Additionally, COVID-19 was independently associated with higher cf-PWV and brachial-ankle PWV; PWV values were higher (i.e., worse) in non-survivors. In survivors, PWV values correlated with length of hospital stay.69 Additional studies have demonstrated elevated arterial stiffness in young healthy patients recovering from COVID-19 relative to healthy controls.57 , 70 In a large cohort of hospitalized patients with COVID-19 in Spain, estimated arterial stiffness (using pulse pressure) was associated with all-cause in-hospital mortality.71 Collectively, these data illustrate a concerning bi-directional relationship between arterial stiffness and COVID-19 outcomes whereby underlying arterial stiffness is associated with COVID-19 severity, and COVID-19 exacerbates arterial stiffening, likely through heightened inflammation and oxidative stress.72
Autonomic control of BP
The autonomic nervous system plays a fundamental role in the control of BP.73 Adjustments in parasympathetic and sympathetic neural outflow to the heart result in changes in heart rate and cardiac contractility, while adjustments in sympathetic neural outflow to the peripheral vasculature modulate vessel diameter and thus peripheral resistance.73 Autonomic imbalance is demonstrated to contribute to the development of CVD-associated pathologies such as hypertension and HF.73 , 74 Importantly, both cardiac autonomic dysfunction (assessed indirectly via heart rate variability, HRV)75 and sympathetic hyperactivity (assessed directly via muscle sympathetic nerve activity, MSNA)76 have been independently associated with an increased mortality risk. Therefore, it is possible that racial/ethnic-related disparities in the prevalence and severity of CVDs may be mediated, in part, by dysfunction of the autonomic nervous system.
To our knowledge relatively few studies have sought to investigate cardiac autonomic function in non-Hispanic Black adults, and mixed findings have been observed.77, 78, 79. For instance, one study found middle-aged non-Hispanic Black adults were 3.45 fold (95% CI, 1.74–6.98) more likely than non-Hispanic White adults to have depressed HRV, a maladaptation that is predictive of incident hypertension.80 In contrast, there are data demonstrating higher cardiac parasympathetic modulation in non-Hispanic Black teenagers compared to non-Hispanic White teenagers.78 Additionally, there are data demonstrating no racial difference in cardiac parasympathetic modulation in non-Hispanic Black and non-Hispanic White young adults.79 Together, these findings suggest that cardiac autonomic function may be altered in non-Hispanic Black individuals in an age-dependent manner.
Sympathetic neural control of BP in non-Hispanic Black adults has been a topic of great interest, though mixed findings have also been observed.21 , 81 For instance, no race-related differences in sympathetic neural outflow, as measured via resting MSNA, have been detected between non-Hispanic Black adults compared with non-Hispanic White adults in younger or older cohorts.82, 83, 84., 85 However, young non-Hispanic Black males exhibited greater sympathetic vascular transduction compared with young non-Hispanic White males, as evidenced by greater reductions in leg vascular conductance (i.e., leg blood flow normalized to mean arterial BP) and greater increases in mean arterial BP following spontaneous bursts of MSNA.83 Likewise, direct stimulation of α1-adrenergic receptors via intra-arterial infusion of phenylephrine yields significant reductions in forearm blood flow in young non-Hispanic Black males compared with non-Hispanic White males, indicating augmented sympathetic vasoconstrictor responsiveness in young non-Hispanic Black males.86 In response to an orthostatic challenge induced by lower body negative pressure, young non-Hispanic Black adults had smaller increases in MSNA than did non-Hispanic White adults despite similar increases in forearm vascular resistance.82 Similar observations have been observed in older non-Hispanic Black adults during upright tilt,85 alluding to elevated sympathetic vascular transduction in non-Hispanic Black adults when faced with an orthostatic perturbation. Furthermore, findings of heightened MSNA and BP responses to a cold pressor test in young non-Hispanic Black adults compared with non-Hispanic White adults provide evidence for augmented sympathetic and BP reactivity in non-Hispanic Black adults.87 , 88 Collectively, these findings suggest that increased sympathetic vascular transduction and sympathetic reactivity are more likely in non-Hispanic Black individuals. It is also important to note that while studies concerning autonomic neural control of BP in non-Hispanic Black populations have increased in number, information regarding this aspect of physiology is extremely limited in Hispanic/Latinx individuals, warranting additional studies in this population.
Racial/ethnic-related disparities in autonomic neural control may have implications in the setting of COVID-19-related mortality. Elevations in MSNA at rest and in response to orthostatic challenge via head-up tilt in young adults recovering from COVID-19 have been observed.89 Furthermore, in patients infected with COVID-19 who were admitted to a hospital, reduced HRV was found to be related to disease severity and outcomes.90 An alarming finding warranting additional investigation is that alterations in cardiac autonomic modulation appear to persist for at least several months in patients with long COVID-19.91 This autonomic dysregulation is thought to be related, in part, to systemic inflammation induced by COVID-19 infection, which contributes to morbidity and mortality.92 , 93 In the next section of the review, we will highlight how increased risk of severe COVID-19 infection in non-Hispanic Black and Hispanic/Latinx populations, with or without CVD, may be amplified by a greater burden of metabolic diseases.
Disparities in metabolic disease and COVID-19 outcomes
Metabolic syndrome refers to a cluster of risk factors (i.e., abdominal obesity, insulin resistance, dyslipidemia, and elevated BP) that are associated with increased risk of CVD, as well as type 2 diabetes (T2D), chronic kidney disease (CKD), and cancer. Using data from NHANES, a recent study reported a metabolic syndrome prevalence of 34.7% in US adults.94 While the prevalence of metabolic syndrome increases rapidly with age, data from 2011 to 2016 indicate that the incidence of metabolic syndrome is growing most rapidly in younger (20–39 years) individuals (from 16.2% in 2011 to 21.3% in 2016).94 Regarding COVID-19, recent meta-analyses demonstrate that metabolic syndrome and associated comorbidities are associated with the development of severe COVID-19.95 Herein, we will discuss the current state of metabolic disease, with a focus on obesity, T2D, and CKD, as they relate to racial disparities in COVID-19 outcomes in the US.
Obesity & T2D
Obesity, defined as a body mass index (BMI) ≥30 kg/m2, is the most prevalent chronic disease in the US, affecting more than 40% of Americans.96 By 2030, projections indicate that nearly one-in-two adults will have obesity and one-in-four will have severe obesity.97 The prevalence of obesity is increasing among all age and racial/ethnic groups, but the greatest burden is among non-Hispanic Black individuals and Hispanic/Latinx populations.98 For example, data for 2017 to 2018 from the National Health and Nutritional Examination Survey (NHANES) documented a substantially greater burden of obesity in non-Hispanic Black (49.6%) and Hispanic/Latinx (44.8%) adults compared to non-Hispanic White (42.2%) adults.96 , 99 Interestingly, there is a greater disparity in obesity prevalence between females (56.9% Black vs. 39.8% White) than males (49.6% Black vs. 42.2% White),96 , 100 an observation that has been attributed to racial differences in social contexts (e.g., income, urbanicity).101 , 102 The public health burden of obesity is highlighted by the multitude of comorbid conditions (e.g., CVD and metabolic diseases) and healthcare costs associated with treating these conditions, which were estimated at $260 billion in 2016.103 Racial and ethnic disparities in obesity are alarming as obesity is an independent predictor of CVD and has been associated with poor COVID-19 health outcomes.104 However, obesity is also a strong risk factor for T2D, CKD, and other metabolic disturbances that increase risk for premature morbidity and mortality.105
More than 90% of patients with T2D have overweightobesity.106 In 2020, CDC estimates indicate that more than one-in-ten Americans had T2D and one-in-three had prediabetes.107 Data from the CDC and NHANES in 2017 demonstrates that the prevalence of diagnosed T2D in non-Hispanic Black (13.2%) and Hispanic/Latinx (12.8%) populations exceeds that of non-Hispanic White adults (7.6%).108 In parallel with obesity, the prevalence of T2D is rising globally, and this trend is particularly pronounced in post-industrial nations, such as the US. The rising prevalence of T2D in the US may be explained, at least in part, by the dramatic increase in young-onset T2D over the past 20 years,109 which is increasing by ~5% annually.110 Concerningly, this seems to be driven by significantly greater increases in newly diagnosed Hispanic/Latinx (+6.5% per year) and non-Hispanic Black (+6.0% per year) children compared to non-Hispanic White (+0.8% per year) children, suggesting a widening in the disparities gap.110
Though racial differences in obesity and body composition are a fundamental contributor to disparities in the prevalence of T2D, this inequity persists after adjusting for differences in BMI.111 Racial differences in socioeconomic status are demonstrated to account for much of the disparity in T2D.112 Regarding underlying physiological factors that may play a role, there are multiple studies demonstrating lower insulin sensitivity in non-Hispanic Black and Hispanic/Latinx adolescents113 and adults114 compared to non-Hispanic White adults.115 Relevant to COVID-19, poor glycemic control may contribute to the increased likelihood of severe COVID-19 infection in racial/ethnic minority groups, as diabetes (inclusive of type 1 and T2D) is estimated to account for ~30–40% of COVID-19-related hospitalization, ICU admission, and/or death.116 Moreover, in individuals without T2D, even a small incremental increase within the normal range of fasting blood glucose is associated with a substantial increase in risk of ICU admission for patients with COVID-19.117 Machine learning models have elucidated multiple pathways (e.g., dysregulated immune response, cytokine storm, inactivation of ACE2) through which elevations in blood glucose can facilitate the progression of COVID-19 throughout the viral lifecycle.118 Regardless of the underlying mechanisms, collectively, these findings highlight the significance of glycemic control as a key mediator of the progression of COVID-19 and as a plausible contributor to disparities in COVID-19-related health outcomes.
Obesity and T2D are also associated with increased inflammation and impaired immune function, which may exacerbate the risk for severe COVID-19. Derangements in the innate immune responses in patients with obesity and T2D are well-documented; these immune modifications precipitate an altered first line of defense and immune hyperactivation, as indicated by elevated inflammatory biomarkers.119 Subsequent alterations in lymphoid tissue integrity and alterations in leukocyte subsets characterized by pro-inflammatory phenotypes ultimately impair immune function and increase risk for chronic disease and COVID-19.119 A diminished capacity to produce antibodies following vaccination is another possible consequence of obesity-related impairments in immune function, though initial data suggest body mass does not impact immune response to the COVID-19 vaccine.120
Cytokine storm in patients with COVID-19 is associated with lung injury, multi-organ failure, and unfavorable prognosis of severe COVID-19.121 A greater likelihood of developing rapid and more aggressive cytokine storms in non-Hispanic Black adults compared to non-Hispanic White adults has been provided as a plausible explanation for racial disparities in COVID-19 mortality rates,122 but to our knowledge, there is scant published data on this topic. What is known is that individuals with heightened resting inflammation may be particularly at-risk for a cytokine storm, characterized by an aggressive inflammatory response that directly influences the progression of COVID-19.123 To inform therapeutic strategies attempting to mitigate cytokine-mediated COVID-19 progression and racial disparities in COVID-19 outcomes, ongoing studies are attempting to elucidate the specific cytokines responsible for aberrant physiological responses to COVID-19 infection.
Chronic kidney disease
Obesity and T2D, along with hypertension and CVD, are major risk factors for CKD.124 A total of 37 million US adults are estimated to have CKD, ~90% of whom have not been diagnosed.125 Though rates of CKD in 2014 were reported to be similar in non-Hispanic Black and non-Hispanic White individuals,126 2021 data from the CDC indicate a slightly greater prevalence of CKD in non-Hispanic Black (16%) and Hispanic/Latinx (13.6%) than non-Hispanic White (12.7%) adults.125 Irrespective of T2D status, non-Hispanic Black adults have greater odds of albuminuria than non-Hispanic White adults, indicating greater kidney disease burden.127 Additionally, compared to non-Hispanic White adults, non-Hispanic Black adults with early-stage CKD are 3.5 times more likely to progress to end stage renal disease.128 Likewise, the risk of developing kidney failure requiring dialysis or kidney transplantation is 2.6-fold higher in non-Hispanic Black populations and 1.5-fold higher in Hispanic/Latinx individuals compared to non-Hispanic White populations.129
Of relevance to COVID-19, impaired renal function during hospital admission is an independent predictor of poor prognosis among patients with COVID-19.130 Additionally, dialysis, organ transplantation, and CKD represent three of the four comorbidities associated with the highest mortality risk from COVID-19.131 This is particularly concerning as CKD was recently identified as the most prevalent risk factor for severe COVID-19 worldwide.131 Furthermore, kidney damage is common among patients with COVID-19; a prospective cohort study reported that 44% of patients with COVID-19 had proteinuria and 27% had hematuria at hospital admission, while 5% of patients experienced acute kidney injury in-hospital.132 Though the underlying pathophysiology of COVID-19-associated acute kidney injury is uncertain, hemodynamic instability (e.g., pulsatile blood flow due to central arterial stiffening) and/or tissue inflammation and local immune cell infiltration have been identified as leading candidate mechanisms.133 Elevated levels of subclinical inflammation and kidney injury in patients with COVID-19 may persist for months after infection, resulting in a progressive decline in kidney function that contributes to CKD.131 Like many other health disparities, racial disparities in social, cultural, and economic factors play a fundamental role in mediating inequities in CKD and COVID-19 related health outcomes. For example, perceived racial discrimination is longitudinally associated with lower kidney function, assessed by estimated glomerular filtration rate.134
Conclusion
CVD and metabolic diseases are the number one cause of death, both in the US and worldwide. A commonality among nearly all of these disease states is their inequitable burden on racial and ethnic minorities, specifically in non-Hispanic Black and Hispanic/Latinx individuals in the US. As mentioned above, the greater prevalence of cardiometabolic diseases, and their pathophysiological manifestations, likely play a role in increased susceptibility for severe COVID-19 and worse COVID-19-related health outcomes in non-Hispanic Black and Hispanic/Latinx populations (summarized in Fig. 2).
CV pathologies, such as hypertension and BP dysregulation, endothelial dysfunction, and large artery stiffening greatly increase risk for poor health outcomes in patients with CVD risk factors who become afflicated with COVID-19. Moreover, long-lasting cardiac, vascular, and autonomic complications associated with COVID-19 have all been observed, and further insults on these already compromised physiological systems in patients with CVD will detract from health-related quality of life and increase risk for premature mortality. Amplifying the deleterious impact of COVID-19 among non-Hispanic Black and Hispanic/Latinx individuals is a greater burden of metabolic diseases, specifically obesity, T2D, and advanced CKD. Disparities in obesity are particularly concerning, as obesity is a predictor of COVID-19 severity, and obesity increases risk for other morbidities (e.g., T2D) that are associated with worse COVID-19-related health outcomes. Poor glycemic control, immune dysfunction, and excess inflammation are hallmarks of obesity and T2D and exacerbate risk for severe COVID-19. Though less discussed, CKD is the most prevalent risk factor for severe COVID-19 worldwide, and CKD and CKD-associated complications (e.g., dialysis, organ transplant, etc.) are among the leading risk factors for COVID-19-related mortality.
Of note, the majority of applied physiology studies pertaining to racial disparities in health feature non-Hispanic Black adults but fewer include Hispanic/Latinx adults, in whom disparities in CVD and metabolic disease and COVID-related health outcomes are also present. More research in this population, as well as other racial and ethnic minorities, is urgently needed. Inspection of existing literature on the current state of chronic disease in the US, and associated risk factors, also revealed some alarming sex-differences, such as the far greater disparity in the prevalence of obesity in females compared to males. Consideration of biological sex as a potential moderator of health disparities, as well as the physiological underpinnings and downstream consequences are warranted.
There is a long history of racial and ethnic health disparities in the US. Over the past two years, the greater burden of CVD and metabolic diseases in non-Hispanic Black and Hispanic/Latinx individuals has been exacerbated by the COVID-19 pandemic, which is reflected by disease severity and mortality statistics. An improved understanding of the mechanisms (biological, social, cultural, economic, etc.) underlying these racial and ethnic disparities in the current state of chronic disease in the US as a means to guide preventive and therapeutic strategies is essential.
Funding
ATR: National Institutes of Health grant K01 HL147998; KB: National Institutes of Health grant T32 HL139451.
Disclosures
None.
References
- 1.Jernigan D.B. Update: Public Health Response to the Coronavirus Disease 2019 Outbreak — United States, February 24, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(8):216–219. doi: 10.15585/mmwr.mm6908e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Z., et al. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21(1):224. doi: 10.1186/s12931-020-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Driscoll M., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 4.Mahase E. Covid-19: death rate is 0.66% and increases with age, study estimates. Bmj. 2020;369 doi: 10.1136/bmj.m1327. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad F.B., et al. Provisional mortality data — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(14):519–522. doi: 10.15585/mmwr.mm7014e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azar K.M.J., et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff. 2020;39(7):1253–1262. doi: 10.1377/hlthaff.2020.00598. [DOI] [PubMed] [Google Scholar]
- 7.Grosicki G.J., et al. Racial and ethnic disparities in Cardiometabolic disease and COVID-19 outcomes in White, black/African American, and Latinx populations: social determinants of health. Prog Cardiovasc Dis. 2022 doi: 10.1016/j.pcad.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiumento G., et al. Persistence of racial/ethnic and socioeconomic status disparities among non-institutionalized patients hospitalized with COVID-19 in Connecticut, July to December 2020. Influenza Other Respi Viruses. 2021;16(3):532–541. doi: 10.1111/irv.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta A.M., et al. Racial and ethnic disparities in rates of COVID-19–associated hospitalization, intensive care unit admission, and in-hospital death in the United States from march 2020 to February 2021. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanijahani A., et al. A systematic review of racial/ethnic and socioeconomic disparities in COVID-19. Int J Equity Health. 2021;20(1) doi: 10.1186/s12939-021-01582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiels M.S., et al. Racial and ethnic disparities in excess deaths during the COVID-19 pandemic, march to December 2020. Ann Intern Med. 2021;174(12):1693–1699. doi: 10.7326/M21-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wadhera R.K., et al. Racial and ethnic disparities in heart and cerebrovascular disease deaths during the COVID-19 pandemic in the United States. Circulation. 2021;143(24):2346–2354. doi: 10.1161/CIRCULATIONAHA.121.054378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virani S.S., et al. Heart disease and stroke Statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 14.White H., et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2005;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 15.Chen J., et al. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: progress and continuing challenges. Circulation. 2010;121(11):1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi J. Researchers investigate what COVID-19 does to the heart. JAMA. 2021;325(9):808. doi: 10.1001/jama.2021.0107. [DOI] [PubMed] [Google Scholar]
- 17.Nadruz W., Jr., et al. Widening racial differences in risks for coronary heart disease. Circulation. 2018;137(11):1195–1197. doi: 10.1161/CIRCULATIONAHA.117.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez F., et al. Young Hispanic women experience higher in-hospital mortality following an acute myocardial infarction. J Am Heart Assoc. 2015;4(9) doi: 10.1161/JAHA.115.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bahrami H., et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schutte A.E., et al. Ethnicity and arterial stiffness. Arterioscler Thromb Vasc Biol. 2020;40(5):1044–1054. doi: 10.1161/ATVBAHA.120.313133. [DOI] [PubMed] [Google Scholar]
- 21.Brothers R.M., Fadel P.J., Keller D.M. Racial disparities in cardiovascular disease risk: mechanisms of vascular dysfunction. Am J Physiol Heart Circ Physiol. 2019;317(4):H777–h789. doi: 10.1152/ajpheart.00126.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelton P.K., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):e426–e483. doi: 10.1161/CIR.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 23.Voors A.W., et al. Studies of blood pressures in children, ages 5-14 years, in a total biracial community: the Bogalusa heart study. Circulation. 1976;54(2):319–327. doi: 10.1161/01.cir.54.2.319. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal R., et al. Racial/ethnic disparities in hypertension prevalence, awareness, treatment, and control in the United States, 2013 to 2018. Hypertension. 2021;78(6):1719–1726. doi: 10.1161/HYPERTENSIONAHA.121.17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas S.J., et al. Cumulative incidence of hypertension by 55 years of age in blacks and whites: the CARDIA study. J Am Heart Assoc. 2018;7(14) doi: 10.1161/JAHA.117.007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elfassy T., et al. Incidence of hypertension among US Hispanics/Latinos: the Hispanic community health study/study of Latinos, 2008 to 2017. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.119.015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post W.S., et al. High prevalence of target organ damage in young, African American inner-city men with hypertension. J Clin Hypertens (Greenwich) 2003;5(1):24–30. doi: 10.1111/j.1524-6175.2003.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manyari D.E. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;323(24):1706–1707. doi: 10.1056/NEJM199012133232413. [DOI] [PubMed] [Google Scholar]
- 29.Dries D.L., et al. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med. 1999;340(8):609–616. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez C.J., et al. Left ventricular mass and ventricular remodeling among Hispanic subgroups compared with non-Hispanic blacks and whites: MESA (multi-ethnic study of atherosclerosis) J Am Coll Cardiol. 2010;55(3):234–242. doi: 10.1016/j.jacc.2009.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta H., et al. Burden of systolic and diastolic left ventricular dysfunction among Hispanics in the United States: insights from the echocardiographic study of Latinos. Circ Heart Fail. 2016;9(4) doi: 10.1161/CIRCHEARTFAILURE.115.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao C., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen G., et al. Hypertension as a sequela in patients of SARS-CoV-2 infection. PLoS One. 2021;16(4) doi: 10.1371/journal.pone.0250815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muhamad S.-A., et al. COVID-19 and hypertension: the what, the why, and the how. Front Physiol. 2021;12(589) doi: 10.3389/fphys.2021.665064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deanfield J.E., Halcox J.P., Rabelink T.J. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 37.Bugiardini R., et al. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109(21):2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- 38.Shechter M., et al. Usefulness of brachial artery flow-mediated dilation to predict long-term cardiovascular events in subjects without heart disease. Am J Cardiol. 2014;113(1):162–167. doi: 10.1016/j.amjcard.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 39.Matsuzawa Y., et al. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeboah J., et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inaba Y., Chen J.A., Bergmann S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 42.Loehr L.R., et al. Racial differences in endothelial function in postmenopausal women. Am Heart J. 2004;148(4):606–611. doi: 10.1016/j.ahj.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 43.D’Agata M.N., et al. Young black women demonstrate impaired microvascular but preserved macrovascular function compared to white women. Exp Physiol. 2021;106(10):2031–2037. doi: 10.1113/EP089702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbosa T.C., et al. Attenuated forearm vascular conductance responses to rhythmic handgrip in young African-American compared with Caucasian-American men. Am J Physiol Heart Circ Physiol. 2018;315(5):H1316–h1321. doi: 10.1152/ajpheart.00387.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kappus R.M., et al. No evidence of racial differences in endothelial function and exercise blood flow in young, healthy males following acute antioxidant supplementation. Int J Sports Med. 2017;38(3):193–200. doi: 10.1055/s-0042-119203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardin J.M., et al. Endothelial function and urine albumin levels among asymptomatic Mexican-Americans and non-Hispanic whites. Cardiovasc Ultrasound. 2008;6:43. doi: 10.1186/1476-7120-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim K., et al. Attenuated cutaneous microvascular function in healthy young African Americans: role of intradermal l-arginine supplementation. Microvasc Res. 2018;118:1–6. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Hurr C., et al. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol. 2018;103(3):343–349. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 49.D’Agata M.N., et al. Evidence of reduced peripheral microvascular function in young black women across the menstrual cycle. J Appl Physiol. 1985;131(6):1783–1791. doi: 10.1152/japplphysiol.00452.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalinowski L., Dobrucki I.T., Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004;109(21):2511–2517. doi: 10.1161/01.CIR.0000129087.81352.7A. [DOI] [PubMed] [Google Scholar]
- 51.Ergul S., et al. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension. 1996;28(4):652–655. doi: 10.1161/01.hyp.28.4.652. [DOI] [PubMed] [Google Scholar]
- 52.Murakami M., Simons M. Regulation of vascular integrity. J Mol Med (Berl) 2009;87(6):571–582. doi: 10.1007/s00109-009-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beyerstedt S., Casaro E.B., Rangel B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar A., et al. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Juneja G.K., et al. Biomarkers of coagulation, endothelial function, and fibrinolysis in critically ill patients with COVID-19: a single-center prospective longitudinal study. J Thromb Haemost. 2021;19(6):1546–1557. doi: 10.1111/jth.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mejia-Renteria H., et al. Assessment of vascular endothelial function in COVID-19 patients. Eur Heart J. 2021;42(Supplement_1) [Google Scholar]
- 57.Ratchford S.M., et al. Vascular alterations among young adults with SARS-CoV-2. American Journal of Physiology-Heart and Circulatory Physiology. 2021;320(1):H404–H410. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chirinos J.A., et al. Large-artery stiffness in health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(9):1237–1263. doi: 10.1016/j.jacc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McEniery C.M., et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35(26):1719–1725. doi: 10.1093/eurheartj/eht565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pini R., et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano study. J Am Coll Cardiol. 2008;51(25):2432–2439. doi: 10.1016/j.jacc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 61.Mitchell G.F., et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lefferts W.K., et al. Racial differences in aortic stiffness in children. J Pediatr. 2017;180:62–67. doi: 10.1016/j.jpeds.2016.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heffernan K.S., Jae S.Y., Fernhall B. Racial differences in arterial stiffness after exercise in young men. Am J Hypertens. 2007;20(8):840–845. doi: 10.1016/j.amjhyper.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 64.Baldo M.P., et al. Racial differences in arterial stiffness are mainly determined by blood pressure levels: results from the ELSA-Brasil study. J Am Heart Assoc. 2017;6(6) doi: 10.1161/JAHA.117.005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Din-Dzietham R., et al. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004;17(4):304–313. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Goel A., et al. Ethnic difference in proximal aortic stiffness: an observation from the Dallas heart study. JACC Cardiovasc Imaging. 2017;10(1):54–61. doi: 10.1016/j.jcmg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 67.Lacolley P., Regnault V., Laurent S. Mechanisms of arterial stiffening: from Mechanotransduction to epigenetics. Arterioscler Thromb Vasc Biol. 2020;40(5):1055–1062. doi: 10.1161/ATVBAHA.119.313129. [DOI] [PubMed] [Google Scholar]
- 68.Zieman S.J., Melenovsky V., Kass D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 69.Schnaubelt S., et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J Intern Med. 2021;290(2):437–443. doi: 10.1111/joim.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szeghy R.E., et al. Carotid stiffness, intima–media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol. 2021 doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodilla E., et al. Impact of arterial stiffness on all-cause mortality in patients hospitalized with COVID-19 in Spain. Hypertension. 2021;77(3):856–867. doi: 10.1161/HYPERTENSIONAHA.120.16563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saeed S., Mancia G. Arterial stiffness and COVID-19: a bidirectional cause-effect relationship. The Journal of Clinical Hypertension. 2021;23(6):1099–1103. doi: 10.1111/jch.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mancia G., Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114(11):1804–1814. doi: 10.1161/CIRCRESAHA.114.302524. [DOI] [PubMed] [Google Scholar]
- 74.Malpas S.C. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90(2):513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 75.Tsuji H., et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 76.Barretto A.C., et al. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol. 2009;135(3):302–307. doi: 10.1016/j.ijcard.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 77.Lampert R., et al. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150(1):153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., et al. Ethnic differences and heritability of heart rate variability in African- and European American youth. Am J Cardiol. 2005;96(8):1166–1172. doi: 10.1016/j.amjcard.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 79.Hill L.K., et al. Racial differences in the association between heart rate variability and left ventricular mass. Exp Physiol. 2017;102(7):764–772. doi: 10.1113/EP086228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schroeder E.B., et al. Hypertension, blood pressure, and heart rate variability: the atherosclerosis risk in communities (ARIC) study. Hypertension. 2003;42(6):1106–1111. doi: 10.1161/01.HYP.0000100444.71069.73. [DOI] [PubMed] [Google Scholar]
- 81.Drew R.C., Charkoudian N., Park J. Neural control of cardiovascular function in black adults: implications for racial differences in autonomic regulation. Am J Physiol Regul Integr Comp Physiol. 2020;318(2):R234–r244. doi: 10.1152/ajpregu.00091.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ray C.A., Monahan K.D. Sympathetic vascular transduction is augmented in young normotensive blacks. J Appl Physiol. 1985;92(2):651–656. doi: 10.1152/japplphysiol.00788.2001. [DOI] [PubMed] [Google Scholar]
- 83.Vranish J.R., et al. Exaggerated vasoconstriction to spontaneous bursts of muscle sympathetic nerve activity in healthy young black men. Hypertension. 2018;71(1):192–198. doi: 10.1161/HYPERTENSIONAHA.117.10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fonkoue I.T., et al. Sympathetic neural reactivity to mental stress differs in black and non-Hispanic white adults. J Appl Physiol. 1985;124(1):201–207. doi: 10.1152/japplphysiol.00134.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okada Y., et al. Elderly blacks have a blunted sympathetic neural responsiveness but greater pressor response to orthostasis than elderly whites. Hypertension. 2012;60(3):842–848. doi: 10.1161/HYPERTENSIONAHA.112.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stein C.M., et al. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36(6):945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 87.Calhoun D.A., et al. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22(6):801–805. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 88.Calhoun D.A., Mutinga M.L. Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press. 1997;6(4):209–213. doi: 10.3109/08037059709062071. [DOI] [PubMed] [Google Scholar]
- 89.Stute N.L., et al. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol. 2021;599(18):4269–4285. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Y., et al. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.630038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barizien N., et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11(1):14042. doi: 10.1038/s41598-021-93546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Del Rio R., Marcus N.J., Inestrosa N.C. Potential role of autonomic dysfunction in Covid-19 morbidity and mortality. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.561749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larsen N.W., Stiles L.E., Miglis M.G. Preparing for the long-haul: autonomic complications of COVID-19. Auton Neurosci. 2021;235 doi: 10.1016/j.autneu.2021.102841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirode G., Wong R.J. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020;323(24):2526–2528. doi: 10.1001/jama.2020.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yanai H. Metabolic syndrome and COVID-19. Cardiol Res. 2020;11(6):360–365. doi: 10.14740/cr1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hales C., et al. National Center for Health Statistics; Hyattsville, MD: 2020. Prevalence of obesity and severe obesity among adults: United States, 2018-2018. NCHS Data Brief, no 360. [PubMed] [Google Scholar]
- 97.Ward Z.J., et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. New England Journal of Medicine. 2019;381(25):2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 98.Cossrow N., Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metabol. 2004;89(6):2590–2594. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 99.Petersen R., Pan L., Blanck H.M. Racial and ethnic disparities in adult obesity in the United States: CDC’s tracking to inform state and local action. Prev Chronic Dis. 2019;16:E46. doi: 10.5888/pcd16.180579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ogden C.L., et al. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- 101.Bleich S.N., et al. Social context explains race disparities in obesity among women. J Epidemiol Community Health. 2010;64(5):465–469. doi: 10.1136/jech.2009.096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seamans M.J., et al. Exploring racial differences in the obesity gender gap. Ann Epidemiol. 2015;25(6):420–425. doi: 10.1016/j.annepidem.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cawley J., et al. Direct medical costs of obesity in the United States and the most populous states. J Manag Care Spec Pharm. 2021;27(3):354–366. doi: 10.18553/jmcp.2021.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hajifathalian K., et al. Obesity is associated with worse outcomes in COVID-19: analysis of early Data from new York City. Obesity (Silver Spring) 2020;28(9):1606–1612. doi: 10.1002/oby.22923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121(6):21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bramante C.T., Lee C.J., Gudzune K.A. Treatment of obesity in patients with diabetes. Diabetes Spectr. 2017;30(4):237–243. doi: 10.2337/ds17-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, USdoHaH Services, Editor. 2020. [Google Scholar]
- 108.Rodríguez J.E., Campbell K.M. Racial and ethnic disparities in prevalence and Care of Patients with Type 2 diabetes. Clin Diabetes. 2017;35(1):66–70. doi: 10.2337/cd15-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nadeau K.J., et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care. 2016;39(9):1635–1642. doi: 10.2337/dc16-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Divers J., et al. Trends in incidence of type 1 and type 2 diabetes among youths — selected counties and Indian reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69:161–165. doi: 10.15585/mmwr.mm6906a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shai I., et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29(7):1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 112.Signorello L.B., et al. Comparing diabetes prevalence between African Americans and whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–2267. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasson R.E., et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95(8):4048–4051. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Osei K., Cottrell D.A., Harris B. Differences in basal and poststimulation glucose homeostasis in nondiabetic first degree relatives of black and white patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 1992;75(1):82–86. doi: 10.1210/jcem.75.1.1619033. [DOI] [PubMed] [Google Scholar]
- 115.Haffner S.M., et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study Diabetes. 1996;45(6):742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 116.Gregg E.W., Sophiea M.K., Weldegiorgis M. Diabetes and COVID-19: population impact 18 months into the pandemic. Diabetes Care. 2021:dci210001. doi: 10.2337/dci21-0001. [DOI] [PubMed] [Google Scholar]
- 117.Alahmad B., et al. Fasting blood glucose and COVID-19 severity: nonlinearity matters. Diabetes Care. 2020;43(12):3113–3116. doi: 10.2337/dc20-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Logette E., et al. A machine-generated view of the role of blood glucose levels in the severity of COVID-19. Frontiers. Public Health. 2021;9(1068) doi: 10.3389/fpubh.2021.695139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Advances in Nutrition. 2016;7(1):66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pellini R., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36 doi: 10.1016/j.eclinm.2021.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ragab D., et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11(1446) doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tal Y., et al. Racial disparity in Covid-19 mortality rates - a plausible explanation. Clin Immunol. 2020;217 doi: 10.1016/j.clim.2020.108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Demeulemeester F., et al. Obesity as a risk factor for severe COVID-19 and complications. A Review Cells. 2021;10(4) doi: 10.3390/cells10040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maric-Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am. 2013;97(1):59–74. doi: 10.1016/j.mcna.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Center for Disease Control and Prevention. Chronic Kidney Disease in the United States . Center for Disease Control and Prevention; 2021. U.S.D.o.H.a.H. Services, Editor. 2021. [Google Scholar]
- 126.Saran R., et al. US renal data system 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66(1 Suppl 1) doi: 10.1053/j.ajkd.2015.05.001. (p. Svii, S1-305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bryson C.L., et al. Racial and ethnic variations in albuminuria in the US third National Health and nutrition examination survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48(5):720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 128.Norton J.M., et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saran R., et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–a7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 130.Chen K., et al. Clinical outcomes of hospitalized COVID-19 patients with renal injury: a multi-hospital observational study from Wuhan. Sci Rep. 2021;11(1):15205. doi: 10.1038/s41598-021-94570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yende S., Parikh C.R. Long COVID and kidney disease. Nat Rev Nephrol. 2021;17(12):792–793. doi: 10.1038/s41581-021-00487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng Y., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Legrand M., et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beydoun M.A., et al. Perceived discrimination and longitudinal change in kidney function among urban adults. Psychosom Med. 2017;79(7):824–834. doi: 10.1097/PSY.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]


