Abstract
Aims
We sought to determine, using advanced echocardiography, the prevalence and type of cardiovascular sequelae after COVID19 infection with marked elevation of cardiovascular biomarkers (CVB), and their prognostic implications.
Methods
All patients admitted from March 1st to May 25th, 2020 to a tertiary referral hospital were included. Those with cardiovascular diseases or dead during admission were excluded. Patients with hs-TnI > 45 ng/L, NT-proBNP>300 pg/mL, and D-dimer >8000 ng/mL were matched with COVID controls (three biomarkers within the normal range) based on intensive care requirements and age, and separately analyzed.
Results
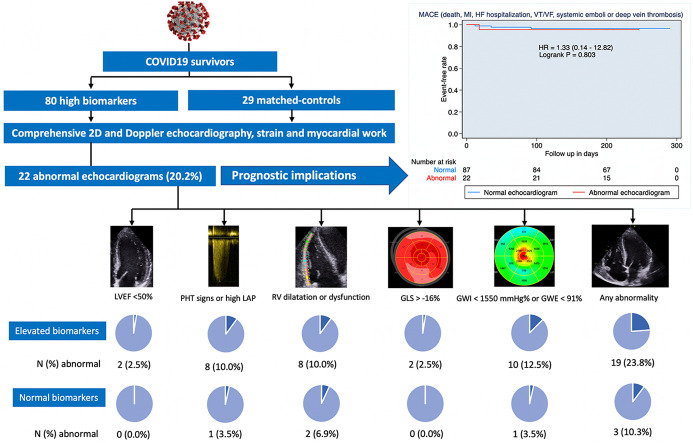
From 2025 patients, 80 patients with significantly elevated CVB and 29 controls were finally included. No differences in baseline characteristics were observed among groups, but elevated CVB patients were sicker. Follow-up echocardiograms showed no differences among groups regarding LVEF and only slight differences between groups within the normal range. Hs-TnI patients had lower myocardial work and longitudinal strain. The presence of an abnormal echocardiogram was more frequent in the elevated CVB group compared to controls (23.8 vs 10.3%, P = 0.123) but mainly associated with mild abnormalities in deformation parameters. Management did not change in any case and no major cardiovascular events except deep vein thrombosis occurred after a median follow-up of 7 months.
Conclusion
Minimal abnormalities in cardiac structure and function are observed in COVID19 survivors without previous cardiovascular diseases who presented a significant CVB rise at admission, with no impact on patient management or short-term prognosis. These results do not support a routine screening program after discharge in this population.
Keywords: COVID19, Echocardiography, Sequelae, Cardiovascular
Graphical abstract
1. Introduction
Coronavirus disease 2019 (COVID19) causes a broad spectrum of clinical manifestations, ranging from asymptomatic presentation to irreversible multiorgan damage and failure leading to death [1]. Elevated levels of high-sensitivity troponin I (hs-TnI), N-terminal proB-type natriuretic peptide (NT-proBNP), and D-dimer have been described in a high percentage of patients being associated with disease severity and worse prognosis [2,3]. Cardiac damage has been confirmed both from necropsies [4] and imaging studies [[5], [6], [7], [8], [9], [10]], but the underlying physiopathology remains unclear. Whether these cardiovascular biomarkers (CVB) elevation is associated with potentially long-lasting cardiovascular abnormalities, a baseline cardiovascular disease, or hemodynamic imbalances during hospitalization still needs to be defined.
Right (RV) and left ventricular (LV) systolic dysfunction has been associated with COVID19, CVB elevation [[5], [6], [7],10,11] and higher mortality [5,8]. Thus, an echocardiographic evaluation during hospitalization is advised to guide treatment and risk-stratification [11]. After discharge, some authors have described cardiovascular abnormalities as sequelae in patients with acute CVB rise [[12], [13], [14]]. However, there is no evidence about the real impact of these elevations of CVB over cardiac function and patient's prognosis at follow-up.
Therefore, we sought to determine, using transthoracic echocardiography and advanced myocardial deformation techniques, the prevalence and type of alterations in cardiac structure and function at discharge in patients without previously known cardiac diseases who had a significant elevation of CVB in the acute phase of a COVID19 infection, as well as the impact of these abnormalities on short-term prognosis.
2. Methods
2.1. Patient selection
From March 1st to May 25th 2020, 2025 consecutive patients admitted to Vall d'Hebron University Hospital in Barcelona, a tertiary hospital and referral center for COVID19 in Catalonia (Spain) were screened. Patients ≥18 years-old with a COVID19 diagnosis confirmed by a positive reverse-transcriptase polymerase chain reaction assay for severe acute respiratory syndrome-related coronavirus 2 in respiratory tract samples were considered for inclusion. Patients with any elevated biomarker were prospectively included as cases (hs-TnI > 45 ng/L, above the 99th percentile; NT-proBNP >300 pg/mL, the recommended cut off to rule out heart failure [15]; and D-dimer >8000 ng/mL, above the 95th percentile in our sample). Patients who died during admission or in the first 30 days after discharge were excluded. Patients with a reported history of previous heart failure, left ventricle ejection fraction (LVEF) <50%, pulmonary hypertension, coronary artery disease, inherited or congenital cardiomyopathy, valvular heart disease greater than mild, or with any type of cardiac device were also excluded.
Patients with all three CVB below the prespecified threshold (except for D-dimer, for which a cut-off of <1000 ng/ml was used [16]), were considered as controls. Each case was matched with a control based upon age and if they required intensive care unit (ICU) admission. Matching with replacement was used, so more than one case was allowed to match the same control if no other similar control was found in the sample.
Baseline demographic variables, comorbidities, need for invasive mechanical ventilation, use of vasoactive drugs, laboratory findings, medication at discharge, and in-hospital clinical events were obtained from electronic records. Clinical events during hospitalization were recorded and categorized as ischemic events (myocardial infarction, stroke, pulmonary embolism, or deep vein thrombosis) or bleeding events (defined as a transfusion of 2 packed red blood cells, a drop in more than 2 g/dl, fatal bleeding or bleeding in a critical organ [17]). After discharge, patients were followed-up until November 25th 2020, at which point the study was considered completed. We reported the incidence of major cardiovascular adverse events (MACE), comprising all-cause death, admission for heart failure, myocardial infarction (as defined in the 4th universal definition of myocardial infarction [18]), ventricular arrhythmias, systemic emboli (stroke or peripheral emboli), or venous thrombosis (deep vein thrombosis or pulmonary embolism). The follow-up was complete for all patients. All patients enrolled provided their informed consent and the study was approved by the local ethics committee (PR(AG)437/2020). Patient were not involved in the design of the study.
2.2. Echocardiographic assessment
At least 30 days after discharge to allow for recovery from the acute infection, all patients underwent a comprehensive echocardiographic assessment performed in the Cardiovascular Imaging Department and using the same equipment (Vivid E9 or Vivid E95; GE Healthcare, Horten, Norway). Four-chambers' dimensions, LV volumes, mass, ejection fraction (LVEF), RV function, and LV diastolic function were assessed as recommended by clinical guidelines [19,20]. Systolic pulmonary artery pressure was estimated using tricuspid regurgitation peak velocity and adding the estimated right atrial pressure based on the collapsibility and diameter of the inferior vena cava.
Advanced myocardial deformation techniques were used to assess for subclinical abnormalities. LV global longitudinal strain (GLS) was measured using images in 4, 3, and 2-chamber at 50–80 frames per second [21]. Peak systolic RV free wall longitudinal strain (RVFWLS) was measured using an RV-focused 4-chamber view. Noninvasive brachial artery blood pressure using a standard blood pressure cuff was obtained at the time of the echocardiographic acquisition to enable myocardial work calculation [22], including global work index (GWI) and global work efficiency (GWE). Images were analyzed using specific software (EchoPAC v. 203, GE Healthcare) by two different cardiologists who were blinded to the patient's status.
To categorize the results, several parameters were dichotomized as normal or abnormal based on previously described reference values. Pulmonary hypertension (PHT) was considered elevated if tricuspid regurgitation velocity was >2.8 m/s or indirect signs of PHT (septal systolic shift towards the LV or pulmonary acceleration time < 80 ms with mid-systolic notch) were observed. Left atrial (LA) pressure was considered to be increased according to the current guideline-recommended algorithm [20]. RV enlargement was considered if two of the basal, mid, or longitudinal diameters exceeded their reference values (41 mm basal, 35 mm mid, and 83 mm for longitudinal RV), and with systolic dysfunction (TAPSE <17 mm, RV s' < 9.5 cm/s or RV FAC <35%) [19]. To allow for comparisons between groups, the global result of the echocardiographic study was also dichotomized as normal or abnormal. An echocardiogram was considered abnormal if any of the following was found: LVEF <50%, GLS > -16%, RVFWLS >-19% [23], GWI <1550 mmHg%, GWE <91% [24], dilated RV, RV systolic dysfunction, pulmonary hypertension or an estimated increase in LA pressure.
2.3. Statistical analysis
Baseline characteristics are shown as absolute numbers and frequencies for categorical variables and as a median and interquartile range (IQR) for continuous variables. Normality was assessed using the Shapiro-Wilk test, and according to the results t-test for matched pairs or two-sided Wilcoxon sign rank-sum test were used for continuous variables, to account for matching with replacement. For categorical variables, McNemar's test was used. When comparing patients with abnormal echocardiographic results with those with a normal echocardiogram, a t-test for independent variables or Wilcoxon rank-sum test were used, according to normality, for quantitative variables, and Chi-square test or Fisher's test, as appropriate, for categorical variables. Multivariate analysis using logistic regression was conducted and the odds ratio (OR) with a 95th confidence interval is provided. A Kaplan Meier display was elaborated to compare the occurrence of MACE between patients with normal and abnormal echocardiograms, and Hazard Ratio (HR) using unadjusted Cox regression was provided. Statistical significance was tested with the log-rank test. A two-tailed p-value <0.05 was considered to be significant. All analyses were performed using Stata 15.1.
3. Results
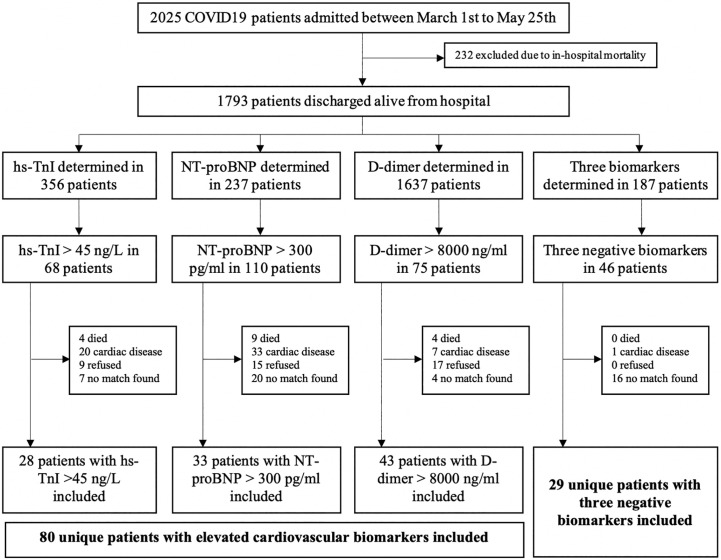
The patients' flow-chart is displayed in Fig. 1 . From March 1st to May 25th 2020, 2025 patients with COVID19 infection were admitted, and 276 exhibited elevated CVB. However, 196 patients were excluded from the analysis: 82 patients (29.7%) died during hospitalization, 16 (5.8%) died in the first 30 days after discharge, 39 (14.1%) had previous cardiovascular diseases, 29 (10.5%) refused to be included and 28 (10.9%) could not be matched with a control. A total of 80 unique patients with elevated CVB and 29 matched-controls were included, conforming 3 comparison groups (28 pairs for hs-TnI, 33 pairs for NT-proBNP, and 43 pairs for D-dimer). All of them had symptoms, mostly fever (89%) and dyspnea (71.8%), but also diarrhea (24%). The median days after admission when the peak values for hs-TnI, BNP and D-dimer were registered were 4, 4 and 5 days, respectively.
Fig. 1.
Patient flow-chart distribution. hs-TnI: high-sensitivity troponin I; NT-proBNP: N-terminal pro-B-type natriuretic peptide; COVID19: coronavirus disease 2019.
3.1. Clinical characteristics
Demographics and clinical characteristics of patients with elevated CVB and controls are displayed in Table S1. The median age was 55.7 years (IQR: 46.2–66.1 years), 62.7 (53.2–72.1), and 62.7 (55.0–66.8) in the hs-TnI, NT-proBNP, and D-dimer groups, respectively, with no difference with controls. Women were more frequent in the control group being only statistically significant in the elevated hs-TnI group (32.1% vs 52.4%, P = 0.049) and ICU stay was longer in all groups with elevated CVB (P < 0.05). Also, creatinine and C-reactive protein were higher (P < 0.05 for all comparisons), and hemoglobin and lymphocyte count were lower (P < 0.05 for all comparisons) in the group with elevated CVB. In the hs-TnI and D-dimer group, more patients required invasive mechanical ventilation (75.0% vs 19.1% and 69.8% vs 16.7%, P < 0.001, respectively), vasoactive drugs (54.6% vs 14.3%, P = 0.013, and 41.9% vs 12.5%, P = 0.002, respectively) and had more ischemic events (32.1% vs 4.8%, P = 0.004, and 30.2% vs 8.3%, P < 0.001, respectively). Differences were non-significant in the NT-proBNP group.
3.2. Echocardiographic findings
Echocardiographic follow-up was performed 4.3 months (IQR: 3.5–5.3) after discharge, and results are displayed in Table S2. There was no missing data for conventional echocardiographic measures, but GLS could not be measured in 28% patients and RVFWLS was available in 56% patients. All patients were in sinus rhythm. No differences were seen among groups regarding LVEF, LV or RV diameters, LV mass, or LA volumes. Compared to the other 2 groups, patients with elevated hs-TnI had a slightly lower TAPSE (20 vs 23 mm, P < 0.001) and RV s' (12 vs 13 cm/s, P < 0.001). Also, patients with D-dimer elevation, compared to controls, presented lower TAPSE (20 vs 23 mm, P = 0.007) with no difference in RVFWLS. Patients with elevated hs-TnI exhibited higher E/e’ ratio (8.4 vs 6.8, P = 0.003) and lower GWI (1930 mmHg% vs 2132 mmHg%, P = 0.028) compared to the rest of the groups. Patients with elevated NT-proBNP had worse GLS (−19.2 vs −21.2%, P = 0.015), similarly as was observed in those with elevated hs-TnI (−20.3 vs −21.1%, P = 0.078). Patients who presented a pulmonary embolism during the index admission (N = 9) did not have worse RV function at the follow-up echocardiogram according to any of the parameters assessed (P > 0.05 for all comparisons).
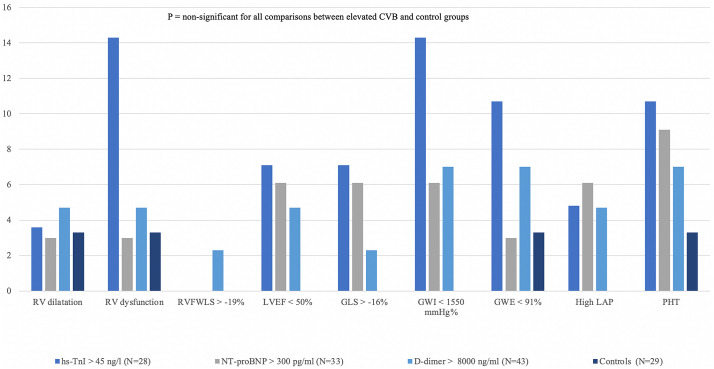
After dichotomizing quantitative variables, 22 echocardiograms (20.2% of the global cohort: 19 [23.8%] in cases and 3 [10.3%] in controls, P = 0.123) were considered as abnormal (39.3% in the hs-TnI group, 25.6% in D-dimer group, and 15.2% in NT-proBNP group, Fig. S1). Fig. 2 and Table S3 display the echocardiographic findings. Even though differences among groups were non-significant, a tendency towards more RV dysfunction (14.3% vs 0%, P = 0.125) and low GWI (14.3% vs 0%, P = 0.125) was found in patients with elevated hs-TnI when compared to controls. However, as shown in Table S4, of the 22 patients with an abnormal echocardiogram, the majority had borderline alterations in biventricular function: altered myocardial deformation mechanics (mainly GWI and GWE) in 10 patients, LVEF <50% in 2 patients, and just 1 with RV dysfunction. No pericardial effusions were found in any group.
Fig. 2.
Distribution of abnormal echocardiographic findings. Each quantitative echocardiographic parameter was dichotomized as normal or abnormal according to reference values. Although a tendency towards more RV dysfunction and low GWI in patients with hs-TnI, it did not reach statistical significance (P = 0.125). Abbreviations as in Fig. 1; LVEF: left ventricle ejection fraction; RV: right ventricle; GLS: global longitudinal strain; RVFWLS: peak systolic right ventricle free wall longitudinal strain; GWI: global work index; GWE: global work efficiency; LAP: left atrial pressure; PHT: pulmonary hypertension signs.
Table 1 displays the clinical and echocardiographic differences between those with normal and abnormal echocardiographic findings. Patients with abnormal echocardiographic findings were more frequently male (81.8% vs 54.0%, P = 0.018), required more often ICU admission (90.0% vs 68.6%, P = 0.035) and had higher hs-TnI (57 vs 10 ng/L, P = 0.008). In the multivariate analysis, male sex (OR = 3.80 [1.06–13.6], P = 0.04), ICU admission requirement (OR = 7.42 [1.13–48.88], P = 0.037) and hs-TnI >45 ng/L (OR = 4.14 [1.32–13.02], P = 0.015) were associated with an abnormal echocardiogram at follow-up.
Table 1.
Comparison of clinical characteristics between patients with an abnormal echocardiogram versus those with a normal echocardiogram.
| Abnormal echocardiogram(N = 22) | Normal echocardiogram (N = 87) | P value | |
|---|---|---|---|
| Age (years) | 62.1 (54.9–66.8) | 60.8 (51.3–67.7) | 0.695 |
| Female sex | 4 (18.2%) | 40 (46.0%) | 0.018 |
| BMI (kg/m2) | 27.4 (24.9–31.8) | 28.6 (25.8–32.9) | 0.269 |
| Tobacco use | 10 (45.5%) | 24 (27.6%) | 0.106 |
| Hypertension | 11 (50.0%) | 38 (43.7%) | 0.594 |
| Dyslipemia | 8 (36.4%) | 34 (39.1%) | 0.815 |
| Diabetes | 6 (27.3%) | 18 (20.7%) | 0.506 |
| eGFR <60 mL/min/1.73m2 | 3 (13.6%) | 8 (9.2%) | 0.537 |
| COPD | 2 (9.1%) | 13 (15.9%) | 0.477 |
| Cancer | 1 (4.6%) | 7 (8.1%) | 0.574 |
| ICU admission | 20 (90,9%) | 59 (68.6%) | 0.035 |
| Days before ICU admission | 0 (0–1) | 1 (0–3) | 0.023 |
| Days in ICU | 15 (9–31) | 15 (8–27) | 0.811 |
| Invasive mechanical ventilation | 14 (63.6%) | 40 (46.0%) | 0.139 |
| Vasoactive drugs* | 9 (40.9%) | 28 (32.2%) | 0.440 |
| Ischemic event | 5 (22.7%) | 15 (17.2%) | 0.553 |
| Bleeding event | 6 (27.3%) | 11 (12.6%) | 0.091 |
| TnI peak (ng/L) | 57 (10–184) | 10 (4–39) | 0.008 |
| NT-proBNP peak (pg/mL) | 152 (121–2430) | 240 (99–633) | 0.727 |
| D dimer peak (ng/mL) | 6504 (843–14,247) | 2706 (589–12,973) | 0.334 |
| CRP peak (mg/dL) | 21.2 (16.5–33) | 17.5 (10.2–27.3) | 0.055 |
| Creatinine peak (mg/dL) | 1.1 (0.9–2.3) | 0.9 (0.8–1.2) | 0.033 |
| Lowest hemoglobin (g/dL) | 9.0 (7.6–11.0) | 10.4 (8.8–12.6) | 0.033 |
| Lowest lymphocyte count (per mcL) | 0.6 (0.3–0.8) | 0.7 (0.5–1) | 0.010 |
| Anticoagulant | 9 (40.9%) | 22 (25.6%) | 0.156 |
| Aspirin | 4 (18.2%) | 6 (7.0%) | 0.106 |
| Statins | 6 (27.3%) | 18 (20.9%) | 0.523 |
| ACEI/ARB/ARNI | 8 (36.4%) | 26 (29.9%) | 0.558 |
| Beta-blockers | 4 (18.2%) | 10 (11.6%) | 0.414 |
| Calcium-channel blockers | 4 (18.2%) | 12 (14.0%) | 0.618 |
| Loop diuretics | 1 (4.6%) | 2 (2.3%) | 0.572 |
| LVEF (%) | 59 (54–63) | 60 (57–64) | 0.092 |
| TAPSE (mm) | 21 (18–22) | 22 (19–24) | 0.075 |
| RV s' (cm/s) | 12 (10–14) | 13 (12–15) | 0.020 |
| Mean E/e’ | 6.8 (6.1–9.4) | 7.7 (6.3–8.7) | 0.973 |
| SPAP (mmHg) | 23 (22–27) | 27 (24–31) | 0.211 |
| GLS (%) | −19.2 (−16.7 to −21.2) | −20.5 (−18.9 to −22.1) | 0.028 |
| GWI (mmmHg%) | 1792 (1516–2287) | 2157 (1839–2418) | 0.018 |
| GWE (%) | 95 (90–96) | 96 (95–97) | 0.065 |
| RVFWLS (%) | −25 (−22 to −27) | −25 (−23 to −27) | 0.564 |
hs-TnI: high-sensitivity troponin I; NT-proBNP: N-terminal pro B-type natriuretic peptide; BMI: body mass index; eGFR: estimated glomerular filtration rate; COPD: chronic obstructive pulmonary disease; ICU: intensive care unit; CRP: C-reactive protein; ACEI: angiotensin-converting-enzyme inhibitors; ARB: angiotensin II receptor blockers; ARNI: angiotensin receptor-neprilysin inhibitors.
From the 109 patients included in the study, 17 patients (15.6%) were studied by echocardiography during hospitalization, 6 of which (35.3%) presented an abnormal echocardiogram: 1 patient had an LVEF of 41%, 4 patients had a GLS > -16% (range − 6%–-13%), 1 had a TAPSE <17 mm and 1 had a FAC <35%. All of these parameters normalized in the follow-up echocardiogram, except for a patient with LVEF 41%, also studied by cardiovascular magnetic resonance (CMR), who was suspected to have a preexistant non-ischemic cardiomyopathy.
3.3. Follow-up
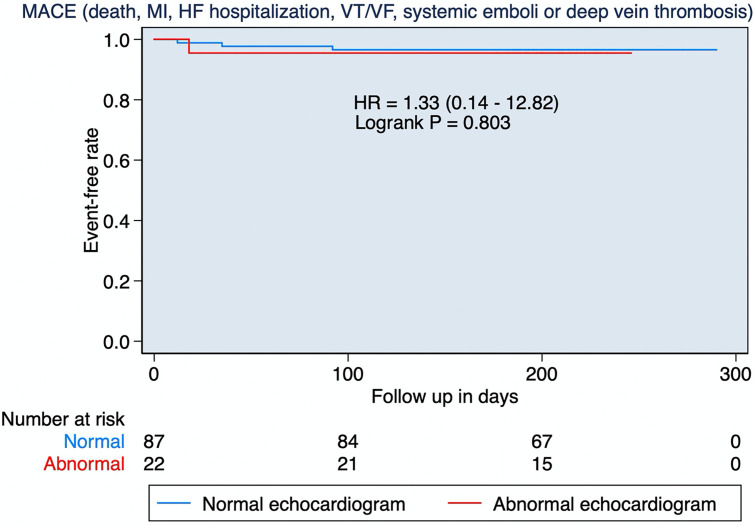
After a median follow-up of 7 months (IQR: 6.6–7.5), no patients died or were admitted due to heart failure, myocardial infarction, systemic emboli, or ventricular arrhythmias. Deep vein thrombosis was diagnosed in 4 patients (3 cases and 1 control) after a median of 27 days (IQR: 15–64, Fig. 3 ). No change in clinical management or treatment was based on echocardiographic findings.
Fig. 3.
Events occurring during follow-up. Kaplan Meier display comparing the occurrence of MACE during follow-up between patients with abnormal versus a normal echocardiogram. MACE: major cardiovascular adverse events; MI: myocardial infarction; HF: heart failure hospitalization; VT: ventricular tachycardia; VF: ventricular fibrillation.
4. Discussion
To the best of our knowledge, this is the first prospective study to report the results of the systematic advanced echocardiographic assessment in COVID19 survivors. Our study (Graphical abstract) reveals that in patients without previous structural heart disease who survive a COVID-19 infection, there is a minimal impact of the infection on myocardial structure and function despite having marked elevation of CVB during index admission. This is demonstrated by a low prevalence of mild echocardiographic alterations (23.8%) with no impact on the patient's management or short-term prognosis. Therefore, an indiscriminate routine echocardiographic study may not be justified in this population.
The existence of image-proven myocardial damage during the acute phase of COVID19 is well established and is associated with the elevation of CVB [11], more severe illness and higher mortality [5,8]. Although it may change clinical management in the acute setting [11], echocardiographic screening during hospitalization is challenging due to personal safety concerns and overwork. In the acute infection, several cardiac abnormalities have been described, especially RV systolic dysfunction but also low LVEF or impaired LV GLS [[5], [6], [7], [8],10,25]. The cause for CVB elevation is not clear, and may be related to the acute inflammatory process, volume overload during the ICU admission, increased RV afterload related to pulmonary hypertension in the setting of acute respiratory distress syndrome with or without pulmonary emboli or microvascular disease. These findings have raised the interest in echocardiographic screening after discharge. In our cohort, 35.3% of those who had an echocardiogram performed during admission showed major abnormalities (LV or RV dysfunction), all of which had resolved at the time of echocardiographic follow-up except for one. Altogether, our study supports the idea that the rise in CVB and some structural abnormalities observed during the acute phase in previously healthy subjects reflect hemodynamic imbalances, multiorgan failure severity, or transient cardiac damage with no lasting sequelae, in concordance with other studies [26]. Nonetheless, if any specific cause for the elevation in biomarkers is found, then appropriate follow-up and management should be performed.
Four studies have reported so far echocardiographic findings after recovering from COVID19. Churchill et al. included 125 patients with an echocardiogram performed during in-hospital admission and found that 22 of them had an LVEF <50%, many of them with hs-TnI > 50 ng/L. Among patients with LVEF <50%, only 9 underwent a second echocardiogram after discharge in a non-protocolized manner and 8 had normalized their LVEF [9]. Similarly, Daher et al. found no abnormalities in an echocardiogram performed 6 weeks after discharge in 33 patients. However, these patients had presented a low CVB elevation during admission [27]. In an all-comer population of 145 hospitalized and non-hospitalized patients, with no data regarding CVB, studied 60 days after the COVID19 diagnosis, Sonnweber et al. described a 3% prevalence of LV dysfunction and 10% of pulmonary hypertension [28]. Catena et al. described no differences in any conventional echocardiographic parameter between 18 patients with TnI >0.015 and controls, but the CVB elevation was only mild, no other CVB were assessed and abnormalities in myocardial deformation were not explored [29]. All of the above results would be in line with our observations; however, our study covers an unexplored area regarding patients with marked elevation of biomarkers during admission and also implements strain and myocardial work analysis in the evaluation of COVID patients, but data regarding reversibility should be interpreted with caution due to the small number of abnormal echocardiograms during the acute phase.
Using CMR, Huang et al. found in 26 recovered patients, with residual cardiovascular symptoms, a high burden of edema, fibrosis, and RV dysfunction [13]. Likewise, another study recruited 29 recovered patients with unjustified troponin T elevation during admission and found that 30% of patients had inducible ischemia, 69% showed late gadolinium enhancement (LGE) and edema, with preserved LV and RV function in all cases [14]. The largest CMR study yet published included 100 patients in the early convalescent phase after COVID19, but only 5% had hs-TnT above the 99th percentile. When compared with healthy controls from a historical cohort with normal CMR, COVID19 patients exhibited prolonged T1 and T2 times reflecting more diffuse fibrosis and edema, and LGE (also in 30% of cases) [30]. These findings showing mild cardiac involvement with little impact on the right and left systolic ventricular function in the acute phase of COVID19 are concordant with our results, with a low prevalence of LV or RV dysfunction and mildly abnormal subclinical parameters such as myocardial work or deformation, especially in the group with elevated hs-TnI. This is remarkable in our population, with high CVB, given the small percentage of patient with elevated CVB in CMR studies. We also suggest that performing a systematic echocardiogram does not change the management of these patients nor does it predict the presence of cardiovascular events at follow-up.
Considering the increasing prevalence of COVID19, the costs of assuming a screening program searching for cardiac sequelae of COVID19 would be extremely high, as well as an overwhelming workload for cardiac imaging laboratories and outpatients' evaluations. Our study demonstrates that a systematic echocardiographic follow-up study is not necessary after a COVID infection, not even in those patients who presented a significant elevation of CVB. Therefore, serial echocardiography should focus on those patients with previous structural heart disease or with persistent symptoms after discharge in whom an echocardiogram could provide relevant information for their management and follow-up.
4.1. Limitations
Although patients were prospectively included after hospital discharge, no protocol was established at the first wave of the pandemic period regarding CVB determination, and, thus, hs-TnI, NT-proBNP, and D-dimer were only ordered according to the clinical judgment, presumably in patients with a higher pre-test probability of having a cardiac condition. Though a limitation, this fact may have increased the chance of finding cardiac abnormalities during follow-up and make our results more consistent. Missing data for some echocardiographic parameters (GLS, GMW, RVFWLS) may have reduced the statistical power to show significant differences. Also, we present data from a single-center study, and the sample size may be small, however, our hospital was one of the centers throughout the country that treated the most cases of COVID-19 during the pandemic, so we believe that our results could be extrapolated to the rest of the populations. Despite our sample size may not have the statistical power to identify small differences in echocardiographic parameters, it is reassuring that only a minority of patients had echocardiographic abnormalities and, when present, these were minor and did not impact mid-term outcomes.
Finally, our results do not apply to patients with previously known cardiovascular diseases or to the acute phase of COVID-19 infection. However, this was not the aim of the study, since we intended to describe the echocardiographic findings and their prognostic value in the short-term follow-up in a high-risk population. Because of this short-term follow-up, it may not be inferred from our results that late deterioration cannot occur, especially in those patients with subclinical abnormalities.
5. Conclusions
In COVID19 survivors with no previous cardiovascular diseases, the impact on myocardial structure and function evaluated by echocardiography and advanced deformation techniques is minimal even in those with elevated CVB during the acute phase. These abnormalities are mostly mild and related to myocardial deformation parameters, with no impact on patient management or short-term prognosis. These results do not support either routine or systematic screening programs after discharge in this population.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributorship statement
Conceptualization and methodologic design: ERA, JRP, JB. Data acquisition: ERA, MBO, PJ, CB, LH, MGdA, FCT, MGdH, RFG, LS, GC. Statistical analysis and data interpretation: ERA, JRP, JB, IGF. Writing of the original draft: ERA, JRP, JB. Critically revision of the manuscript: JRP, MBO, PJ, CB, LH, MGdA, FCT, MGdH, RFG, LS, GC, JB, IFG. Final approval: all authors. JB is responsible for the overall content.
Disclosures
No conflicts of interests are declared in relation with this study.
Declaration of Competing Interest
No conflicts of interest are declared by any author regarding this topic.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcard.2022.04.070.
Appendix A. Supplementary data
Supplementary material
References
- 1.Pinney S.P., Giustino G., Halperin J.L., Mechanick J.I., Neibart E., Olin J.W., et al. Coronavirus historical perspective, disease mechanisms, and clinical outcomes: JACC focus seminar. J. Am. Coll. Cardiol. 2020 Oct;76(17):1999–2010. doi: 10.1016/j.jacc.2020.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni W., Yang X., Liu J., Bao J., Li R., Xu Y., et al. Acute myocardial injury at hospital admission is associated with all-cause mortality in COVID-19. J. Am. Coll. Cardiol. United States. 2020;76:124–125. doi: 10.1016/j.jacc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandoval Y., Januzzi J.L.J., Jaffe A.S. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J. Am. Coll. Cardiol. 2020 Sep;76(10):1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. Off. J. Soc. Cardiovasc. Pathol. 2020 Oct;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giustino G., Croft L.B., Stefanini G.G., Bragato R., Silbiger J.J., Vicenzi M., et al. Characterization of myocardial injury in patients with COVID-19. J. Am. Coll. Cardiol. 2020 Nov;76(18):2043–2055. doi: 10.1016/j.jacc.2020.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J., Volodarskiy A., Sultana R., Pollie M.P., Yum B., Nambiar L., et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J. Am. Coll. Cardiol. 2020 Oct;76(17):1965–1977. doi: 10.1016/j.jacc.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassen M.C.H., Skaarup K.G., Lind J.N., Alhakak A.S., Sengeløv M., Nielsen A.B., et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Hear Fail. 2020 Oct;7(6):4189–4197. doi: 10.1002/ehf2.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Li H., Zhu S., Xie Y., Wang B., He L., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc. Imaging. 2020 Apr;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D., et al. Echocardiographic Features of COVID-19 Illness and Association with Cardiac Biomarkers. Vol. 33. J. Am. Soc. Echocardiogr.: Off. Publ. Am. Soc. Echocardiogr. 2020:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020 Jul;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dweck M.R., Bularga A., Hahn R.T., Bing R., Lee K.K., Chapman A.R., et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Heart J. Cardiovasc. Imaging. 2020 Sep;21(9):949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudski L., Januzzi J.L., Rigolin V.H., Bohula E.A., Blankstein R., Patel A.R., et al. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19: JACC scientific expert panel. J. Am. Coll. Cardiol. 2020 Sep;76(11):1345–1357. doi: 10.1016/j.jacc.2020.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L., Zhao P., Tang D., Zhu T., Han R., Zhan C., et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc. Imaging. 2020 May;13(11):2330–2339. doi: 10.1016/j.jcmg.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Jul;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller C., McDonald K., de Boer R.A., Maisel A., Cleland J.G.F., Kozhuharov N., et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019 Jun;21(6):715–731. doi: 10.1002/ejhf.1494. [DOI] [PubMed] [Google Scholar]
- 16.Chilimuri S., Sun H., Alemam A., Mantri N., Shehi E., Tejada J., et al. Predictors of mortality in adults admitted with COVID-19: retrospective cohort study from New York City. West. J. Emerg. Med. 2020 Jul;21(4):779–784. doi: 10.5811/westjem.2020.6.47919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman S., Angerås U., Bergqvist D., Eriksson B., Lassen M.R., Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010 Jan;8(1):202–204. doi: 10.1111/j.1538-7836.2009.03678.x. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe A.S., Chaitman B.R., Morrow D.A., Bax J.J., White H.D., Alpert J.S., et al. Fourth universal definition of myocardial infarction (2018) Eur. Heart J. [Internet]. 2018 Aug 25;40(3):237–269. doi: 10.1093/eurheartj/ehy462. Available from: [DOI] [PubMed] [Google Scholar]
- 19.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015 Jan;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., 3rd, Dokainish H., Edvardsen T., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2016 Dec;17(12):1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 21.Voigt J.-U., Pedrizzetti G., Lysyansky P., Marwick T.H., Houle H., Baumann R., et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015 Feb;28(2):183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Russell K., Eriksen M., Aaberge L., Wilhelmsen N., Skulstad H., Remme E.W., et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur. Heart J. 2012 Mar;33(6):724–733. doi: 10.1093/eurheartj/ehs016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris D.A., Krisper M., Nakatani S., Kohncke C., Otsuji Y., Belyavskiy E., et al. Normal range and usefulness of right ventricular systolic strain to detect subtle right ventricular systolic abnormalities in patients with heart failure: a multicentre study. Eur. Heart J. Cardiovasc. Imaging. 2017 Feb;18(2):212–223. doi: 10.1093/ehjci/jew011. [DOI] [PubMed] [Google Scholar]
- 24.Morbach C., Sahiti F., Tiffe T., Cejka V., Eichner F.A., Gelbrich G., et al. Myocardial work - correlation patterns and reference values from the population-based STAAB cohort study. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnesi M., Baldetti L., Beneduce A., Calvo F., Gramegna M., Pazzanese V., et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020 Sep;106(17):1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metkus T.S., Sokoll L.J., Barth A.S., Czarny M.J., Hays A.G., Lowenstein C.J., et al. Myocardial injury in severe COVID-19 compared to non-COVID acute respiratory distress syndrome. Circulation. 2020 Nov;143(6):553–565. doi: 10.1161/CIRCULATIONAHA.120.050543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daher A., Balfanz P., Cornelissen C., Müller A., Bergs I., Marx N., et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020 Oct;174 doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19 - an observational prospective multi-center trial. Eur. Respir. J. 2020 Dec;57(4):2003481. doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catena C., Colussi G., Bulfone L., Da Porto A., Tascini C., Sechi L.A. Echocardiographic comparison of COVID-19 patients with or without prior biochemical evidence of cardiac injury after recovery. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2021 Feb;34(2):193–195. doi: 10.1016/j.echo.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight D.S., Kotecha T., Razvi Y., Chacko L., Brown J.T., Jeetley P.S., et al. COVID-19: myocardial injury in survivors. Circulation. 2020;142:1120–1122. doi: 10.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material