Abstract
Background
Variations in the ACE2 activity in saliva could explain the striking differences of susceptibility to infection and risk of severe disease.
Methods
We analyze the activity of ACE2 in saliva in different population groups across a wide age range and disease status during April to June 2020, before SARS-CoV-2 vaccine implementation, and we establish differences between infected people and participants considered resistant (highly exposed healthcare workers and children who cohabited with parents with COVID-19 without isolation and remain IgG negative).
Results
We included 74 adults, of which 47 (64%) were susceptible and 27 (36%) were resistant, and 79 children, of which 41 (52%) were susceptible and 38 (48%) were resistant. Resistant adults have significantly lower ACE2 activity in saliva than susceptible adults and non-significant higher values than susceptible and resistant children. ACE2 activity is similar in the susceptible and resistant pediatric population (p = 0.527). In contrast, we observe an increase in activity as the disease's severity increases among the adult population (mild disease vs. severe disease, 39 vs. 105 FU, p = 0.039; severe disease vs. resistant, 105 vs. 31 FU, p < 0.001).
Conclusions
using an enzymatic test, we show that ACE2 activity in saliva correlates with the susceptibility to SARS-Cov-2 infection and disease severity. Children and adults with low-susceptibility to SARS-Cov-2 infection showed the lowest ACE2 activity. These findings could inform future strategies to identify at-risk individuals.
Keywords: ACE2, Saliva, SARS-CoV-2, Susceptibility, Severity
Background
Because the angiotensin-converting enzyme 2 (ACE2) is the molecular target for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cell entry, variations in the activity of ACE2 may play a significant role in determining an individual's susceptibility to COVID-19 and disease severity.1 ACE2 is widely expressed in different tissues, including the surface of alveolar epithelial cells and the epithelium of oral, nasal, and nasopharynx mucosa, explaining why direct person-to-person respiratory transmission is the primary means of SARS-CoV-2 transmission.2
Reports of pathologic findings in tissue specimens of COVID-19 patients are emerging and reinforce the role of ACE2 expression and activity in disease pathogenesis.3 ACE2 has a higher expression in the salivary gland and the oral cavity's epithelium than in the lung. Thus, saliva could be used to study the COVID-19 pathogenesis due to the ease of detecting SARS-CoV2 in these samples.4 ACE2 expression in the lungs seems to increase with age, which might explain the higher disease severity observed in the older population with COVID-19.5 However, it is still unclear whether ACE2 expression is a cause of increased susceptibility in older people.6 While children and women are assumed to have a lower incidence of infection than adult men,6 , 7 the potential role of ACE2 expression has not been fully explored.
Here, we hypothesized that the activity of ACE2 in saliva would correlate with lower susceptibility to SARS-CoV-2 infection and will explain, at least in part, the formidable clinical phenotype of individuals with repeated high-risk exposures to SARS-CoV-2 who did not become infected before SARS-CoV-2 vaccine implementation. We analyze the expression of ACE2 in saliva in different population groups across a wide age range and disease status, and we establish differences between infected people and those exposed to the virus but not infected.
Methods
Study design and setting
We analyzed data from children recruited at Hospital Universitario La Paz and adults recruited at Hospital Universitario Ramón y Cajal, between April and June 2020, before SARS-CoV-2 vaccine implementation. We classified children as "susceptible” if they were positive for SARS-CoV-2 IgG, and as "resistant" if they had repeated high-risk exposures8 to SARS-Cov-2 (specifically, cohabitation with parents with confirmed COVID-19), but remained IgG negative at least after eight weeks after the exposure. We considered adults as "susceptible" when they had positive IgG for SARS-CoV-2 or previous COVID19 confirmed by polymerase chain reaction (PCR) in nasopharyngeal exudate. Non-susceptible adults were healthy healthcare workers who had been on duty for at least three months in COVID19 wards or intensive care units and reported at least three high-risk exposures to SARS-CoV-2,8 without having experienced symptoms suggestive of SARS-CoV-2 infection, persistently negative PCR SARS-CoV-2 testing, and absence of SARS-CoV-2 IgM and IgG in plasma. The most frequent exposure was largely unprotected exposure to aerosol-generating procedures or patient secretions and close contact without face masks with other confirmed cases of COVID-19. We measured SARS-CoV-2 antibodies by indirect chemiluminescence immunoassay (Vircell, Granada, Spain). Participants with mild disease were those without a need of supplemental oxygen and asymptomatic after one week of diagnosis. Severe disease was defined as the presence of bilateral radiologic infiltrates or opacities and clinical assessment requiring supplemental oxygen.
ACE-2 activity measurement and statistical analysis
Non-induced saliva samples were collected in sterile tubes and cryopreserved at −80 °C. After thawing, ACE2 activity was measured in batch through a fluorometric assay, using a synthetic ACE2-specific substrate (Mca-APK(Dnp); Reactomix S.L., Granada, Spain) that is metabolized to a fluorescent compound in the presence of a functional enzyme. Saliva samples were incubated with the buffer solution (150 nM Tris-HCl pH 7.5, 200 nM NaCl, 10 uM ZnCl2, protease inhibitor) and the substrate (portions 10-99-1, respectively). After incubation at room temperature for 16 h, we quantified each sample's fluorescence using Varioskan Lux (Thermo), a fluorescence reader with an excitation of 320 nm and an emission of 420 nm. Measurements in duplicate showed a coefficient of variation of 5.1%. According to this technique, increased fluorescence indicates increased ACE2 activity. The protein concentration, measured colorimetrically by the PierceTM 660 nm Protein Assay reagent (Thermo Scientific, USA), for all samples was on average 1.58 ± 0.07 µg protein/µL saliva, with no significant differences across samples. Therefore, the differences in ACE2 activity have a biological significance and not due to a bias in the amount of protein in each simple.
We used the one-way ANOVA followed by the Tukey test to correct for multiple comparisons and logistic regression to establish the association between susceptibility to SARS-CoV-2 and ACE2 activity after controlling for the potential confounding effect of sex. We used Stata version 16.0 (StataCorp LP, College Station, Texas) for the statistical analysis and Prism version 9.0 (GraphPad, La Jolla, California) for figure generation.
Results
We included a total of 153 unvaccinated participants; 74 adults, of which 47 (64%) were considered susceptible and 27 (36%) resistant, and 79 children, of which 41 (52%) were considered susceptible and 38 (48%) resistant. The age range was 5–92 years. Among the 47 susceptible adults, 20 (43%) had severe disease, 16 (34%) mild disease, and 11 (23%) were people who had asymptomatic COVID-19 and had positive IgG with negative PCR test at the time of inclusion in the study. Supplemental Table 1 shows the baseline characteristics of the sample population.
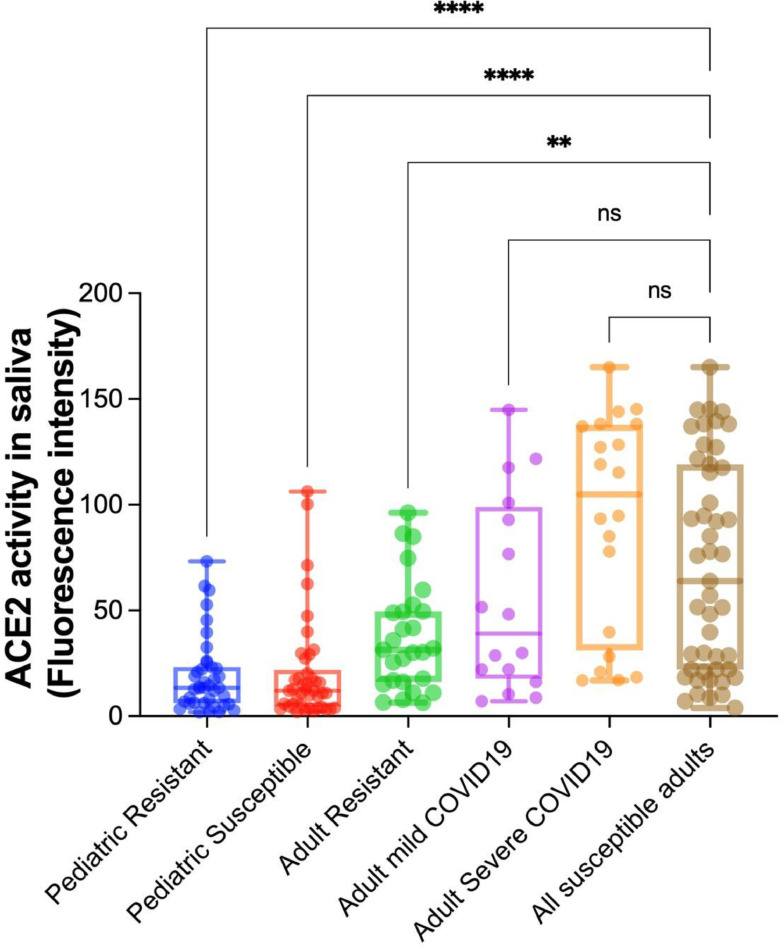
Fig. 1 shows the difference in ACE2 activity in saliva between susceptible and resistant individuals, with a much smaller difference among the pediatric population median fluorescence (12 vs. 14 FU units, p = 0.561) than in adults (64 vs. 31 FU units, p = 0.012). Resistant adults have significantly lower ACE2 activity in saliva than susceptible adults and non-significant higher values than susceptible and resistant children. ACE2 activity is similar in the susceptible and resistant pediatric population (p = 0.527). In contrast, we observe an increase in activity as the disease's severity increases among the adult population (mild disease vs. severe disease, 39 vs. 105 FU, p = 0.039; severe disease vs. resistant, 105 vs. 31 FU, p < 0.001).
Fig. 1.
Comparison of ACE2 activity in saliva according to susceptibility and disease severity in adult and pediatric population". The graph is a box and whiskers plot showing all values as points. The groups were compared by one-way ANOVA followed by Tukey test. The adult resistant group was the reference category for the multiple comparisons.
** denotes a p value < 0.01 and > 0.001
**** denotes a p value < 0.0001.
The logistic regression model yielded similar estimates in the unadjusted model and after controlling by sex (Supplemental Table 2). We observed significant differences between the susceptible and resistant adult population in the adjusted model (p = 0.008) but not in the pediatric population (p = 0.790). Supplemental Table 3 shows the results of the adjusted multinomial model by disease severity in adults.
Discussion
In this work, we observe that there is a significant correlation between ACE2 activity in saliva and susceptibility to SARS-Cov-2 and COVID19 severity in unvaccinated individuals, early in the COVID19 pandemic. First, adults susceptible to SARS-CoV-2 show higher levels of ACE2 in saliva than the resistant adults. These differences are not observed in the pediatric population, in which the saliva ACE2 activity is lower, and no differences exist between susceptible and resistant individuals. Second, an increase in ACE2 expression is found as the disease's severity increases among the adult population.
Previous work has linked ACE2 expression in different organs to the potential risk of SARS-CoV-2 infection.9 , 10 Liu et al. showed that the salivary gland's epithelial cells having elevated ACE-2 expression were infected by SARS-Cov-2,10 which raises the question of whether inter-individual variations of ACE-2 expression may contribute to the formidable spectrum of diseases susceptibility and severity. Since early in the COVID-19 pandemic, it became clear that older age was a risk factor for adverse outcomes. Recently, Baker Steven et al. have reported that ACE2 expression increases with age using data generated by RNA sequencing.11 Whereas no gender difference in ACE2 expression has been appreciated in salivary glands,12 whether a differential expression of ACE2 across age explains the more benign disease observed in children remains unclear.13
In keeping with the study hypothesis, children showed a significantly lower ACE2 activity than adults. Despite that we did not appreciate differences in resistant vs. susceptible children, the fact that all cases were either asymptomatic/mild infections or resistant could support the idea that ACE2 activity correlates with the susceptibility to SARS-CoV-2 infection. Future directions include a thorough evaluation of ACE2 activity in different anatomic compartments, and subsequent studies should further characterize the origin of this enzymatic activity by using RNA sequencing or proteomic strategies.
In conclusion, using an enzymatic test, we show that ACE2 activity in saliva correlates with the susceptibility to SARS-Cov-2 infection and disease severity. Children and adults with low susceptibility to SARS-Cov-2 infection showed the lowest ACE2 activity. These findings could inform future strategies to identify at-risk individuals, as well as for the development of therapeutic strategies to reduce both susceptibility and severity of COVID-19.
Declaration of Competing Interest
Outside the submitted work, S. S.-V. reports personal fees from ViiV Healthcare, Janssen Cilag, Gilead Sciences, and MSD as well as non-financial support from ViiV Healthcare and Gilead Sciences and research grants from MSD and Gilead Sciences. J.M.-S. reports non-financial support from ViiV Healthcare, Gilead Sciences, and Jannsen Cilag. S.M. reports personal fees and non-financial from ViiV Healthcare, Janssen, Gilead Sciences, and MSD, as well as grants from MSD, ViiV Healthcare, and Gilead Sciences.
Acknowledgments
We thank to all patients and healthcare workers who participated in the study. This work was supported by Instituto de Salud Carlos III (AC17/00019, PI18/00154, COV20/00349, ICI20/00058), CRUE-Supera COVID, cofinanced by the European Development Regional Fund ‘‘A way to achieve Europe’’ (ERDF), Merck, Sharp & Dohme Investigator Studies Program (code MISP# IIS 60257), Fondo Supera COVID-19 (2020-001), and SEIMC (becas SEIMC).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.04.041.
Appendix. Supplementary materials
References
- 1.Choudhary S., Sreenivasulu K., Mitra P., Misra S., Sharma P. Role of genetic variants and gene expression in the susceptibility and severity of COVID-19. Ann Lab Med. 2021;41(2):129–138. doi: 10.3343/alm.2021.41.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174(1):69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baghizadeh Fini M. Oral saliva and COVID-19. Oral Oncol. 2020;108 doi: 10.1016/j.oraloncology.2020.104821. ISSN 1368-8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect Dis. 2020;20(5):515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciaglia E., Vecchione C., Puca annibale A. COVID-19 infection and circulating ACE2 levels: protective role in women and children. Front Pediatr. 2020;8:206. doi: 10.3389/fped.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully E.P., Haverfield J., Ursin L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Centers for Disease Control and Prevention (CDC). Interim operational considerations for public health management of healthcare workers exposed to or with suspected or confirmed COVID-19: non-U.S. healthcare settings. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/public-health-management-hcw-exposed.html
- 9.Beyerstedt S., Casaro E.B., Rangel É.B. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40(5):905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H., et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome Coronavirus infection in the upper respiratory tracts of rhesus Macaques. J Virol. 2011;85(8):4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker S.A., Kwok S., Berry G.J., Montine T.J. Angiotensin-converting enzyme 2 (ACE2) expression increases with age in patients requiring mechanical ventilation. PLoS ONE. 2021;16(2):e0247060. doi: 10.1371/journal.pone.0247060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J., Li Y., Huang X., Chen Z., Li Y., Liu C., et al. Systematic analysis of ACE2 and TMPRSS2 expression in salivary glands reveals underlying transmission mechanism caused by SARS-CoV-2. J Med Virol. 2020;92(11):2556–2566. doi: 10.1002/jmv.26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Chew KY, Karawita AC, Yamamoto A, Labzin LL, Yarlagadda T, et al. Pediatric nasal epithelial cells are less permissive to SARS-CoV-2 replication compared to adult cells. BioRxiv2021:2021.03.08.434300.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.