Abstract
A series of 4′-ethynyl (4′-E) nucleoside analogs were designed, synthesized, and identified as being active against a wide spectrum of human immunodeficiency viruses (HIV), including a variety of laboratory strains of HIV-1, HIV-2, and primary clinical HIV-1 isolates. Among such analogs examined, 4′-E-2′-deoxycytidine (4′-E-dC), 4′-E-2′-deoxyadenosine (4′-E-dA), 4′-E-2′-deoxyribofuranosyl-2,6-diaminopurine, and 4′-E-2′-deoxyguanosine were the most potent and blocked HIV-1 replication with 50% effective concentrations ranging from 0.0003 to 0.01 μM in vitro with favorable cellular toxicity profiles (selectivity indices ranging 458 to 2,600). These 4′-E analogs also suppressed replication of various drug-resistant HIV-1 clones, including HIV-1M41L/T215Y, HIV-1K65R, HIV-1L74V, HIV-1M41L/T69S-S-G/T215Y, and HIV-1A62V/V75I/F77L/F116Y/Q151M. Moreover, these analogs inhibited the replication of multidrug-resistant clinical HIV-1 strains carrying a variety of drug resistance-related amino acid substitutions isolated from HIV-1-infected individuals for whom 10 or 11 different anti-HIV-1 agents had failed. The 4′-E analogs also blocked the replication of a non-nucleoside reverse transcriptase inhibitor-resistant clone, HIV-1Y181C, and showed an HIV-1 inhibition profile similar to that of zidovudine in time-of-drug-addition assays. The antiviral activity of 4′-E-thymidine and 4′-E-dC was blocked by the addition of thymidine and 2′-deoxycytidine, respectively, while that of 4′-E-dA was not affected by 2′-deoxyadenosine, similar to the antiviral activity reversion feature of 2′,3′-dideoxynucleosides, strongly suggesting that 4′-E analogs belong to the family of nucleoside reverse transcriptase inhibitors. Further development of 4′-E analogs as potential therapeutics for infection with multidrug-resistant HIV-1 is warranted.
Combination chemotherapy or highly active antiretroviral therapy (HAART) using two or more reverse transcriptase (RT) inhibitors (RTIs) and protease inhibitors (PIs) has dramatically improved the quality of life and survival of patients infected with human immunodeficiency virus type 1 (HIV-1) (10, 23). However, the ability to provide effective long-term antiretroviral therapy for HIV-1 infection has become a complex issue, since a number of patients who initially achieved very favorable viral suppression later experience treatment failure (40). Moreover, 30 to 40% of HIV-1-infected individuals who had received no prior antiviral therapy fail to achieve viral suppression to undetectable levels (9, 17). In addition, 10 to 40% of antiviral therapy-naive individuals infected with HIV-1 have persistent viral replication (plasma HIV RNA >500 copies/ml) under HAART (11, 12, 37), possibly due to transmission of drug-resistant HIV-1 variants (40). Thus, the development of novel compounds that are active against drug-resistant HIV-1 variants and that prevent or delay the emergence of resistant HIV-1 variants is urgently needed.
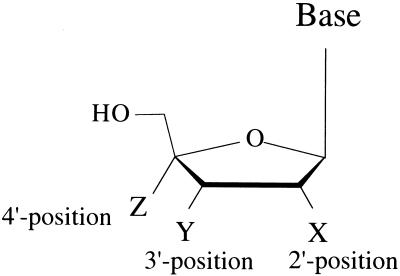
Certain 4′-substituted nucleosides have been described in the literature. Maag et al. (19) reported that 4′-azido-2′-deoxythymidine (4′-AZT), the hydrogen atom at the 4′ position of which was substituted for with an azido group, exerted potent activity against HIV-1 in vitro. Subsequently, Chen and colleagues (4) reported that 4′-AZT was active against HIV-1 through its DNA chain-terminating activity. More recently, Sugimoto et al. have reported that 4′-substituted nucleosides including 4′-ethynylthymidine exhibited potent activity against not only HIV-1, but also herpes simplex virus type 1 (38). We recently designed and synthesized a series of 4′-ethynyl (4′-E)-2′-deoxynucleosides and their analogs and identified several highly potent anti-HIV-1 compounds, including 4′-E-2′-deoxycytidine (4′-E-dC), 4′-E-2′-deoxyadenosine (4′-E-dA), 4′-E-2′-deoxyribofuranosyl-2,6-diaminopurine (4′-E-dDAP), and 4′-E-2′-deoxyguanosine (4′-E-dG). These 4′-E compounds, unlike all of the currently available nucleoside RTIs (NRTIs), lack the 2′,3′-dideoxyribose configuration but have a 2′-deoxyribose configuration (Fig. 1 and Table 1).
FIG. 1.
Structures of 4′-substituted nucleosides. All nucleoside analogs discussed here have substitutions at the 4′ position of the sugar moiety shown here except for the two compounds 4′-E-araT and 4′-E-araC, which have an arabinofuranosyl sugar moiety. See Table 1.
TABLE 1.
Anti-HIV-1 activity of 4′-substituted nucleosides in MT-4 cellsa
| Compound (abbreviation) | Base | Sugar modification at X(2′-) Y(3′-) Z(4′-) | EC50 (μM)b | CC50 (μM)b | Selectivity index |
|---|---|---|---|---|---|
| 4′-Ethynylribosylthymine (4′-E-rT) | Thymine | -OH -OH -CCH | >400 | >400 | NDc |
| 4′-Ethynylthymidine (4′-E-T) | Thymine | -H -OH -CCH | 0.83 ± 0.46 | >400 | >482 |
| 4′-Ethynylarabinofuranosylthymine (4′-E-araT) | Thymine | β-OH -OH -CCH | 119 ± 22 | >400 | >4 |
| 4′-Ethylthymidine (4′-Et-T) | Thymine | -H -OH -CH2CH3 | >400 | >400 | ND |
| 4′-Vinylthymidine (4′-V-T) | Thymine | -H -OH -CHCH2 | 3.9 ± 1.0 | >400 | >103 |
| 4′-Hydroxyethylthymidine (4′-HE-T) | Thymine | -H -OH -CH2CH2OH | 7.0 ± 4.3 | >400 | >57 |
| 4′-Ethynyl-5-iodo-2′-deoxyuridine (4′-E-IdU) | 5-Iodo-uracil | -H -OH -CCH | 0.29 ± 0.063 | >400 | >1,380 |
| 4′-Ethynyl-5-ethyl-2′-deoxyuridine (4′-E-EtdU) | 5-Ethyl-uracil | -H -OH -CCH | >400 | >400 | ND |
| 4′-Ethynyl-5-bromovinyl-2′-deoxyuridine (4′-E-BVDU) | 5-Bromovinyl-uracil | -H -OH -CCH | >15 | 1.5 | ND |
| 3′-Azido-3′-deoxythymidine (AZT) | Thymine | -H -N3 -H | 0.032 ± 0.0011 | 29.4 ± 8.7 | 9,190 |
| 4′-Ethynyl-2′-deoxycytidine (4′-E-dC) | Cytosine | -H -OH -CCH | 0.0048 ± 0.001 | 2.2 ± 1.0 | 458 |
| 4′-Ethynylarabinofuranosylcytosine (4′-E-araC) | Cytosine | β-OH -OH -CCH | 0.043 ± 0.011 | 2.0 ± 0.4 | 46.5 |
| 4′-Methyl-2′-deoxycytidine (4′-Me-dC) | Cytosine | -H -OH -CH3 | 0.015 ± 0.063 | 1.0 ± 0.41 | 66.7 |
| 4′-Fluoromethyl-2′-deoxycytidine (4′-FMe-dC) | Cytosine | -H -OH -CH2F | 0.0068 ± 0.0036 | 0.12 ± 0.02 | 18 |
| 2′,3′-Dideoxy-3′-thiacytidine (3TC) | Cytosine | -H none -H | 0.10 ± 0.035 | >100 | >1,000 |
| 4′-Ethyl-N6-methyladenosine (4′-E-NM-A) | 6-Methyladenine | -OH -OH -CCH | >400 | >400 | ND |
| 4′-Ethynyladenosine (4′-E-A) | Adenine | -OH -OH -CCH | >400 | >400 | ND |
| 4′-Ethynyl-2′-deoxyadenosine (4′-E-dA) | Adenine | -H -OH -CCH | 0.0098 ± 0.0043 | 16 ± 7.9 | 1,630 |
| 4′-Ethynyl-2′-deoxyribofuranosylpurine (4′-E-dP) | Purine | -H -OH -CCH | 135 ± 25 | >400 | >3 |
| 4′-Ethynyl-2′-deoxyribofuranosyl-2,6-diaminopurine (4′-E-dDAP) | 2,6-Diaminopurine | -H -OH -CCH | 0.00034 ± 0.00003 | 0.9 ± 0.17 | 2,600 |
| 4′-Ethynyl-2′-deoxyinosine (4′-E-dI) | Hypoxanthine | -H -OH -CCH | 0.13 ± 0.035 | 137 ± 39 | 1,053 |
| 4′-Ethynyl-2′-deoxyguanosine (4′-E-dG) | Guanine | -H -OH -CCH | 0.0015 ± 0.0003 | 1.4 ± 0.16 | 933 |
| 4′-Ethynyl-6-chloro-2′-deoxyguanosine (4′-E-CldG) | 6-Chloroguanine | -OH -OH -CCH | >400 | >400 | ND |
Anti-HIV-1 activity was determined by the MTT method. AZT and 3TC served as controls.
The data shown are mean values with standard deviations derived from the results of three independent experiments.
ND, not determined.
All of these 4′-E compounds blocked the replication of a wide spectrum of laboratory and clinical HIV-1 strains in vitro with low cellular toxicities. These compounds also suppressed the replication of various drug-resistant HIV-1 clones, including HIV-1M41L/T215Y, HIV-1L74V, HIV-1K65R, HIV-1M41L/T69S-S-G/T215Y, HIV-1Y181C, and HIV-1A62V/V75I/F77L/F116Y/Q151M. These 4′-E compounds also suppressed various multidrug-resistant clinical HIV-1 variants carrying a variety of drug resistance-related amino acid substitutions, which were isolated from patients for whom virtually all of the currently available antiviral regimens had failed. Furthermore, we demonstrate in this study that these 4′-E analogs most likely block HIV-1 by functioning as NRTIs.
MATERIALS AND METHODS
Antiviral agents.
3′-Azido-3′-deoxythymidine (AZT, or zidovudine), 2′,3′-dideoxyinosine (ddI, or didanosine), and 2′,3′-dideoxycytidine (ddC, or zalcitabine) were purchased from Sigma (St. Louis, Mo.). (−)-2′,3′-Dideoxy-3′-thiacytidine (3TC, or lamivudine) was a kind gift from R. F. Schinazi (Atlanta, Ga.). A series of 4′-position-substituted nucleosides were designed and synthesized as described elsewhere (16, 27, 28). Their basic formula is shown in Fig. 1. A non-NRTI (NNRTI), MKC-442, was a gift from Mitsubishi Kasei Corporation (Yokohama, Japan) (2).
Cells.
MT-4 and H9 cells were grown in an RPMI 1640-based culture medium, and Cos-7 cells were grown in Dulbecco's modified Eagle medium (DMEM); each of these media was supplemented with 10% fetal calf serum (FCS; HyClone Laboratories, Logan, Utah), 2 mM l-glutamine, 50 U of penicillin per ml, and 50 μg of streptomycin per ml. HeLa-CD4-LTR/β-gal cells (14) were kindly provided by M. Emerman through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (Bethesda, Md.). Prior to use, HeLa-CD4-LTR/β-gal cells were propagated in DMEM supplemented with 10% FCS, 0.1 ng of hygromycin B per ml, and 200 μg of Geneticin per ml. In the anti-HIV assay, cells were cultured in the DMEM-based culture medium with addition of 50 U of penicillin per ml and 50 μg of streptomycin per ml instead of hygromycin B and Geneticin. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy HIV-1-seronegative donors by Ficoll-Hypaque gradient centrifugation and were stimulated for 3 days with phytohemagglutinin M (PHA; 10 μg/ml; Sigma) prior to use.
Viruses and construction of recombinant HIV-1 clones.
Three laboratory strains, HIV-1LAI, HIV-2ROD, and HIV-2EHO, were employed. Multidrug-resistant clinical HIV-1 strains were isolated from patients with AIDS who had been treated with 9 to 11 anti-HIV-1 drugs for 39 to 64 months, as previously described (42). These clinical HIV-1 isolates were passaged once or twice in PHA-stimulated PBMCs (PHA-PBMCs), and titers of the culture supernatants obtained were determined for infectivity and stored at −70°C until use.
Recombinant infectious HIV-1 clones carrying various mutations in the pol gene were generated as previously described (35, 39, 42). Briefly, the desired mutations were introduced into the XmaI-NheI region (759 bp) of pTZNX1, which encoded Gly-15 to Ala-267 of HIV-1 RT (strain BH 10), by the oligonucleotide-based mutagenesis method (42). The XmaI-NheI fragment was inserted into a pHXB2RIP7 (a kind gift from M. Reitz, Jr., National Institutes of Health, Bethesda, Md.)-based plasmid, pSUM9, generating various molecular clones with the desired mutations. Each molecular clone (10 μg/ml as DNA) was transfected into Cos-7 cells (4 × 105 cells/100-mm-diameter dish) by the calcium phosphate method (Promega, Madison, Wis.). After 24 h, MT-2 cells (106 cells/dish) were added and cocultured with Cos-7 cells for an additional 24 h. When an extensive cytopathic effect was observed, cell supernatants were harvested, and the virus was further propagated in H9 cells. The culture supernatant was harvested, the titer was determined for infectivity, and the sample was stored at −70°C until use. The presence of intended mutations and the absence of unintended mutations in infectious clones were confirmed by determination of the nucleotide sequence of proviral DNA isolated from the virus-producing H9 cells. HIV-1HXB2D was generated by using pSUM9 (35) and served as a wild-type infectious clone (HIV-1wt).
Determination of drug susceptibility of HIV-1.
The inhibitory effects of test compounds on HIV-1 replication were monitored by the inhibition of virally induced cytopathicity in MT-4 cells. Briefly, MT-4 cells were suspended at 105 cells/ml and exposed to HIV-1LAI at 100 50% tissue culture infectious doses (TCID50s). Immediately after viral exposure, the cell suspension (104 cells in 100 μl) was brought into each well of a 96-well flat microtiter culture plate (Costar, Cambridge, Mass.) containing various concentrations of test compounds. After incubation for 5 days, the number of viable cells was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method as previously described (15, 30).
The sensitivity of infectious clones to various RTIs was determined in the multinuclear activation of the galactosidase indicator (MAGI) assay (14), with some modifications using the viral preparations titrated as previously described (20). Briefly, target cells (HeLa CD4-LTR/β-gal; 104/well) were plated in 96-well flat microtiter culture plates. On the following day, the medium was aspirated, and the cells were inoculated with HIV-1 clones (70 MAGI units/well, which gave 70 blue cells after 48 h of incubation) and cultured in the presence of various concentrations of drug in fresh medium. Forty-eight hours after viral exposure, all blue cells in each well were counted. The cytotoxicity of the compound was determined by the MTT method as previously described (15). All experiments were performed in triplicate.
The drug susceptibility of HIV-1 clinical isolates was determined as previously described (42). Briefly, PHA-PBMCs (106 cells/ml) were exposed to each viral preparation at a TCID50 of 50 and cultivated in 200 μl of culture medium containing various concentrations of the drug in 10-fold serial dilutions in 96-well culture plates. All assays were performed in triplicate, and the amounts of p24 antigen produced by the cells into the culture medium were determined on day 7 in culture with a commercially available radioimmunoassay kit (Du Pont, Boston, Mass.).
The activity of test compounds is shown as the concentration that blocks HIV-1 replication by 50% (EC50), and cytotoxicity is shown as the concentration that suppresses the viability of HIV-1-unexposed cells by 50% (CC50). The window between CC50 and EC50 is shown by the CC50/EC50 ratios (selectivity indices).
RESULTS
Activity of 4′-substituted nucleosides against HIV-1 in vitro.
We designed and synthesized more than 20 novel 4′-substituted nucleoside analogs (16, 27, 28, 38) and tested them for in vitro activity against HIV-1 by using the MTT colorimetric assay employing MT-4 cells. A thymidine analog, 4′-ethynyl (E)-T, and a uracil analog, 4′-E-IdU, were found to be moderately active against HIV-1LAI, with EC50s of 0.83 and 0.29 μM, respectively (Table 1). Conversion of the 2′-deoxyribose of 4′-E-T to ribose, generating 4′-E-rT, nullified the antiviral activity of 4′-E-T. Other 4′-substituted thymidine and uridine analogs had marginal or no activity. All four 4′-substituted cytidine analogs examined were active, among which 4′-E-dC and 4′-fluoromethyl (FMe)-dC were most potent against HIV-1, although the latter was substantially cytotoxic (Table 1). It was noted that 4′-E-araC was moderately active.
Among 4′-E purine analogs, 4′-E-dA, 4′-E-dDAP, and 4′-E-dG were highly potent against HIV-1, with subnanomolar to nanomolar EC50s (Table 1). 4′-E-dI was only moderately active against the virus. It is noteworthy that both 4′-E-dDAP and 4′-E-dA had a favorable toxicity profile, with selectivity indices of 2,600 and 1,630, respectively (Table 1). All three 4′-E purine analogs with a ribose configuration (4′-E-NM-A, 4′-E-A, and 4′-E-CldG) were inactive.
Activity of 4′-substituted nucleosides against HIV-1 variants resistant to various RTIs.
We also evaluated whether 4′-substituted nucleosides were active against HIV-1 variants resistant to various nucleoside RT inhibitors (NRTIs) by using the MAGI assay. Four pyrimidine analogs (4′-E-dC, 4′-E-araC, 4′-Me-dC, and 4′-FMe-dC) that were potent against HIV-1LAI were selected and tested further against various infectious HIV-1 clones carrying resistance-conferring amino acid substitutions (Table 2). It was noteworthy that all four of these cytidine analogs examined suppressed the replication of ddI- and ddC-resistant HIV-1K65R and HIV-1L74V, AZT-resistant HIV-1M41L/T215Y, and multi-dideoxynucleoside-resistant (resistant to AZT, ddI, ddC and d4T) variant HIV-1A62V/V75I/F77L/F116Y/Q151M (35, 36). It was noted that among 4′-substituted pyrimidine analogs, only 4′-E-dC remained active against 3TC-resistant variants HIV-1M184I and HIV-1M184V, but was less active against the multi-NRTI-resistant (resistant to AZT, ddI, ddC, d4T and 3TC) variant HIV-1M41L/T69S-S-G/T215Y (41), while 4′-FMe-dC remained potent against HIV-1M41L/T69S-S-G/T215Y. However, all three 4′-E purine compounds (4′-E-dA, 4′-E-dDAP, and 4′-E-dG) were active against the infectious clones tested, including HIV-1M184V and HIV-1M41L/T69S-S-G/T215Y. These purine analogs were also active against an NNRTI-resistant infectious clone, HIV-1Y181C. When tested in HeLa-CD4-LTR/β-gal cells, the CC50s of the analogs examined were all >100 or >200 μM, except for that of 4′-E-dG.
TABLE 2.
Antiviral activity of 4′-substituted nucleosides against drug-resistant infectious clonesa
| Compound | EC50 (μM)b
|
CC50 (μM)b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HXB2 | K65R | L74V | M41L/T215Y | M184V | M184I | M41L/T69S-S-G/T215Y | MDRc | Y181C | ||
| Pyrimidine analogs | ||||||||||
| 4′-E-T | 0.36 | 0.53 | 0.68 | 0.43 | 0.18 | 0.14 | 0.44 | 0.12 | 0.13 | >200 |
| 4′-E-IdU | 0.37 | NDd | ND | ND | 0.21 | ND | ND | 0.32 | ND | >200 |
| 4′-V-T | 8.1 | ND | ND | ND | >100 | ND | ND | 9.7 | ND | >100 |
| 4′-HE-T | 15.8 | ND | ND | ND | >100 | ND | ND | 20.7 | ND | >100 |
| 4′-Et-T | >100 | ND | ND | ND | ND | ND | ND | >100 | ND | >100 |
| 4′-E-dC | 0.0012 | 0.0008 | 0.0013 | 0.006 | 0.0024 | 0.0026 | 0.015 | 0.0012 | 0.0021 | >200 |
| 4′-E-araC | 0.0071 | 0.015 | 0.026 | 0.026 | 0.71 | 0.48 | 0.17 | 0.0079 | 0.016 | >200 |
| 4′-Me-dC | 0.0058 | 0.0071 | 0.0062 | ND | 0.2 | 0.74 | ND | 0.0033 | ND | >200 |
| 4′-FMe-dC | 0.0046 | 0.065 | 0.0019 | 0.0035 | 2.0 | ND | 0.066 | 0.0039 | ND | >200 |
| AZT | 0.022 | 0.02 | 0.02 | 0.3 | 0.01 | 0.017 | 1.6 | 15.3 | 0.014 | >100 |
| 3TC | 0.71 | ND | ND | ND | >100 | >100 | 9.9 | 1.1 | ND | >100 |
| ddC | 0.2 | 3.0 | 1.5 | ND | 2.2 | ND | 1.3 | 5.5 | ND | >100 |
| Purine analogs | ||||||||||
| 4′-E-dA | 0.008 | 0.033 | 0.004 | 0.012 | 0.047 | 0.022 | 0.065 | 0.0062 | 0.011 | >200 |
| 4′-E-dDAP | 0.0014 | 0.00035 | 0.0007 | 0.0017 | 0.0059 | 0.0027 | 0.0041 | 0.001 | 0.0008 | >200 |
| 4′-E-dI | 0.81 | 0.25 | 0.61 | 1.3 | 16.6 | 1.5 | 2.2 | 0.51 | ND | >200 |
| 4′-E-dG | 0.007 | 0.001 | 0.0012 | 0.019 | 0.008 | 0.0041 | 0.0068 | 0.0048 | 0.01 | 52 |
| ddI | 3.9 | 12.7 | 19.5 | 3.6 | 10.1 | ND | 12.2 | 25 | ND | >100 |
Anti-HIV activity was determined with the MAGI assay. The abbreviations are defined in Table 1.
The data shown are mean values derived from the results of three independent experiments.
Multidideoxynucleoside-resistant HIV-1 that contains mutations in the pol region: an Ala-62 to Val substitution (A62V), V751, F77L, F116Y, and Q151M.
ND, not determined.
4′-Ethynyl group is required for activity against HIV-1M184V and HIV-1M184I.
To investigate whether and to what extent the 4′-E configuration was crucial for activity against HIV-1, three thymidine analogs and two 2′-deoxycytidine analogs substituted at the 4′-position with different groups (vinyl, ethyl, hydroxyethyl, methyl, or fluoromethyl) were synthesized. 4′-ethylthymidine (4′-Et-T) was inert, but 4′-vinylthymidine (4′-V-T) and 4′-hydroxyethylthymidine (4′-HE-T) were active against HIV-1LAI, although they were less so than to 4′-E-T (Table 1). These two thymidine analogs were active against the control wild-type infectious clone HIV-1HXB2 and a multidideoxynucleoside-resistant infectious clone (HIV-1A62V/V75I/F77L/F116Y/Q151M), but were totally inert (>100 μM) against the 3TC-resistant clone HIV-1M184V (Table 2). Two 2′-deoxycytidine analogs, 4′-Me-dC and 4′-FMe-dC, were as potent against HIV-1LAI as 4′-E-dC. These analogs were also active against various drug-resistant infectious clones, but were less potent against HIV-1M184V and HIV-1M184I (Table 2). Taken together, although the 4′-E substitution is not essential for antiviral activity against HIV-1, the 4′-E configuration appears to be required for activity against 3TC-resistant HIV-1M184V and HIV-1M184I.
Activity of selected 4′-substituted nucleosides against HIV-2 strains.
We also examined selected 4′-E analogs against two HIV-2 strains, HIV-2ROD and HIV-2EHO, in the MAGI assay (Table 3). Although the thymidine analog 4′-E-T showed only moderate activity, three cytidine analogs, 4′-E-dC, 4′-E-araC, and 4′-Me-dC, were highly active against HIV-2, and 4′-E-dC was the most potent analog, with 50% inhibitory concentrations (IC50s) of ∼0.001 μM (Table 3). Four 4′-E purine analogs examined also suppressed the replication of both HIV-2 strains. AZT, tested as a control compound, was active against both HIV-2 strains, as previously described (21). Considering that all of the currently known NNRTIs fail to suppress the replication of HIV-2 (6), it appears that 4′-substituted nucleosides do not belong to NNRTIs.
TABLE 3.
Anti-HIV-2 activity of 4′-substituted nucleosides in MAGI cellsa
| Compound | EC50 (μM)
|
|
|---|---|---|
| HIV-2ROD | HIV-2EHO | |
| 4′-E-T | 0.34 | 0.29 |
| 4′-E-dC | 0.001 | 0.0009 |
| 4′-E-araC | 0.012 | 0.003 |
| 4′-Me-dC | 0.002 | 0.0013 |
| 4′-E-dA | 0.0019 | 0.001 |
| 4′-E-dI | 0.29 | 0.37 |
| 4′-E-dDAP | 0.001 | 0.0008 |
| 4′-E-dG | 0.026 | 0.0042 |
| AZT | 0.0061 | 0.013 |
Anti-HIV-2 activity was determined with the MAGI assay. The abbreviations are defined in Table 1. The data shown are mean values derived from the results of three independent experiments.
Activity of 4′-ethynyl-2′-deoxynucleosides against HIV-1 isolated from heavily drug-experienced patients.
4′-E analogs were then evaluated for their activity against multidrug resistant clinical HIV-1 isolates in vitro. In this study, we chose three most potent 4′-E compounds, 4′-E-dC, 4′-E-dA, and 4′-E-dDAP. Four clinical HIV-1 strains were isolated from patients who received a variety of anti-HIV-1 agents for 39 to 64 months and had failed to respond to any existing regimens of antiviral therapy (42). As shown in Table 4, all four clinical HIV-1 strains contained a variety of drug resistance-conferring amino acid substitutions in the RT- and protease-encoding regions of HIV-1 gene. All three 4′-E compounds suppressed the replication of these highly drug-resistant clinical strains as effectively as that of the wild-type clinical strain HIV-1ERS104pre (36) and two HIV-1 clinical strains (HIV-1IVR205 and HIV-1IVR207) isolated from drug-naive AIDS patients. It was noted, however, that 4′-E-dC was moderately active against HIV-1Pt6 and HIV-1Pt9. There was no apparent association of the observed reduced antiviral activity with amino acid substitutions identified in these clinical isolates (Table 4).
TABLE 4.
Antiviral activity of 4′-substituted nucleosides against clinical isolates
| Strain | Amino acid substitution(s)
|
EC50 (μM)a
|
||||
|---|---|---|---|---|---|---|
| RT region | Protease region | 4′-E-dC | 4′-E-dA | 4′-E-dDAP | AZT | |
| ERS104pre | None | None | 0.0012 | 0.013 | 0.00083 | 0.0056 |
| IVR205 | None | K20M, M36I, D60E | 0.0023 | 0.0034 | 0.0001 | 0.0015 |
| IVR207 | G190Q | V77I | 0.0046 | 0.0022 | <0.0001 | 0.0036 |
| Pt 1 | T69G, K70R, L74V, A98G, K103N, V179D, M184V, T215F, K219F | L10I, L33I, M36I, M46I, L63P, A71, G73S, V82A, L90M | 0.00064 (0.5 fold) | 0.00054 (0.4 fold) | 0.0011 (1.3 fold) | 0.029 (52 fold) |
| Pt 6 | M41L, D67N, M184V, L210W, T215Y | L10I, K20R, L24I, M36I, M46L, I54V, L63P, V82A, L89M | 0.013 (11 fold) | 0.040 (3 fold) | 0.0001 (0.1 fold) | 0.28 (50 fold) |
| Pt7 | M41L, D67N, T69D, M184V, T215F | L10I, K45R, I54V, L63P, A71V, V82T, L90M | 0.0016 (1.3 fold) | 0.009 (0.7 fold) | 0.0005 (0.6 fold) | 1.9 (340 fold) |
| Pt 9 | M41L, M184V, T215Y | M46I, L63P, A71V, V77I, I84V, N88D, L90M | 0.023 (19 fold) | 0.029 (2.2 fold) | 0.0031 (3.7 fold) | 0.97 (170 fold) |
EC50 were determined with PHA-PBMC. All assays were conducted in triplicate. Numbers in parentheses represent fold changes of EC50s against each HIV-1 isolate compared to those against the wild-type clinical HIV-1 strain, ERS104pre. The abbreviations are defined in Table 1.
Testing 4′-ethynyl nucleosides in the time-of-drug-addition assay.
The triphosphate form of 4′-E nucleosides is not presently available, and how 4′-E nucleosides block the replication of HIV-1 and HIV-2 remains to be clarified. The time-of-drug-addition assays have been used to approximately determine what stage of the replication cycle of HIV-1 the compound in question blocks (29). We therefore tested two potent 4′-E compounds, 4′-E-dC and 4′-E-araC, in the assay together with three controls: a surface reactant, dextran sulfate 5000 (DS5000); NRTI AZT; and NNRTI MKC-442.
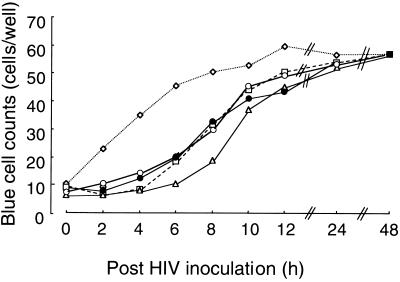
The concentrations of compounds tested were all fixed at their EC90s. At indicated time points, compounds were added to the HIV-1LAI-exposed MAGI cells. As shown in Fig. 2, DS5000, which like DS8000 (24) inhibits the absorption of HIV and formation of syncytia, showed anti-HIV-1 activity only when it was added early after viral exposure. AZT, which requires triphosphorylation before it becomes active (23), exerted its antiviral activity when its addition was delayed for up to 4 h. MKC-442, which does not require activation, was effective when its addition was delayed for up to 6 h. The profiles of anti-HIV-1 activity of 4′-E-dC and 4′-E-araC were quite similar to that of AZT (Fig. 2). These results suggest that 4′-E nucleosides suppress the replication of HIV at or around the step of reverse transcription.
FIG. 2.
Antiviral activity of 4′-ethynyl nucleosides in the time-of-drug-addition assays. At indicated time points, 4′-E-dC (open circles) and 4′-E-araC (closed circles) were added to the HIV-1LAI-exposed MAGI (HeLa CD4/LTR-β-gal) cells, and the blue cells produced were counted at the completion of the 48-h period of incubation. DS5000 (open diamonds), AZT (open squares), and MKC-442 (open triangles) served as controls.
Reversal of antiviral activity of 4′-ethynyl nucleosides by natural 2′-deoxynucleosides.
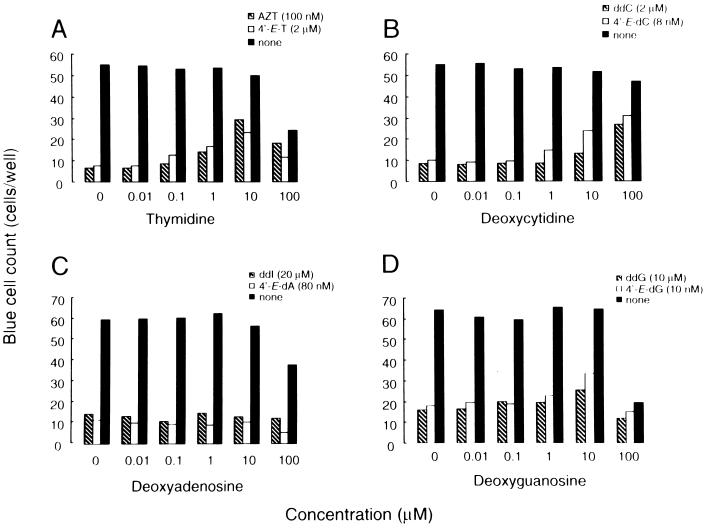
It has been shown that biological effects such as antitumor or antiviral activity of nucleoside analogs are reversed by the addition of natural nucleosides (5, 8, 26). We first tested whether thymidine and 2′-deoxycytidine reversed the antiviral activity of 4′-E-T and 4′-E-dC, respectively (Fig. 3). The concentrations of compounds tested were all fixed at their EC90s. Antiviral activity of 4′-E-T (2 μM) was suppressed by the addition of thymidine in a dose-dependent manner, and the antiviral activity of AZT (100 nM) was also reversed by the addition of thymidine, in agreement with our previous published data (26), although 100 μM thymidine caused cytotoxicity (Fig. 3A). Activity of 4′-E-dC (8 nM) and 4′-E-dG (10 nM) was similarly reversed by the addition of 2′-deoxycytidine and 2′-deoxyguanosine, respectively (Fig. 3B and D). Likewise, the activity of ddC (2 μM) and ddG (10 μM) was reversed by the addition of dC and dG, respectively, in agreement with our previous data (25). In contrast, the antiviral activity of 4′-E-dA was not reversed by the addition of dA, a similar profile of the activity of ddI versus dA (25) (Fig. 3C).
FIG. 3.
Reversal of anti-HIV activity of 4′-ethynyl-2′-deoxynucleosides by 2′-deoxynucleosides. Antiviral activities of 4′-E-thymidine (4′-E-T; 2 μM [open bars]) and AZT (100 nM [hatched bars]) were reversed by the addition of their physiologic counterpart (thymidine) in a dose-dependent manner (A). In the culture without antiviral agents (solid bars), the activities of 4′-E-2′-deoxycytidine (4′-E-dC; 8 nM) and ddC (2 μM [hatched]) were similarly reversed by the addition of 2′-deoxycytidine (dC) (B). In contrast, the anti-HIV-1 activities of 4′-E-2′-deoxyadenosine (4′-E-dA; 80 nM [open bars]) and ddI (10 μM [hatched bars]) were not suppressed by the addition of 2′-deoxyadenosine (C). The activities of 4′-E-2′-deoxyguanosine (4′-E-dG; 10 nM [open bars]) and ddG (10 μM [hatched bars]) were moderately reversed by the addition of 2′-deoxyguanosine (dG) (D).
Acid stability of selected 4′-ethynyl nucleosides.
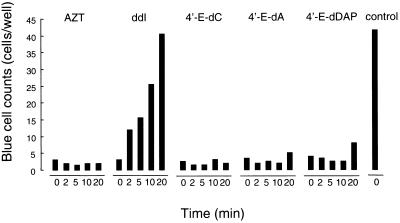
Certain nucleoside analogs such as ddI are acid labile and require antacid upon oral administration, generating adverse reactions, such as abdominal discomfort and diarrhea (33). We therefore examined the acid sensitivity of three selected 4′-E analogs (4′-E-dC, 4′-E-dA, and 4′-E-dDAP). These compounds were exposed to 1 N HCl for up to 20 min and then neutralized with 1 N NaOH and added to target cells exposed to HIV-1. As shown in Fig. 4, ddI lost all of its antiviral activity following acid exposure for 20 min; however, 4′-E-dC, 4′-E-dA, 4′-E-dDAP, and the control acid-resistant AZT did not lose their antiviral activity.
FIG. 4.
Acid stability of 4′-ethynyl nucleosides. AZT, ddI, 4′-E-dC, 4′-E-dA, and 4′-E-dDAP were exposed to 1 N HCl for the indicated periods of time, neutralized with 1 N NaOH, and added to the culture in the MAGI assay at final concentrations of 100 nM, 20 μM, 8 nM, 80 nM, and 8 nM, respectively, which approximately represent the EC90 of each compound under the conditions used.
DISCUSSION
All of the currently available NRTIs have a 2′,3′-dideoxyribofuranose configuration, and only after anabolic intracellular phosphorylation do they exert their antiviral activity against HIV by functioning as viral DNA chain terminators (22). In this study, we identified 4′-E nucleoside analogs potent against a variety of HIV strains, including laboratory strains of HIV-1 and HIV-2, infectious clones, clinical HIV-1 isolates, and multidrug-resistant clinical strains.
As of this writing, no 5′-triphosphate forms of the 4′-substituted nucleoside analogs examined in this study have been available, and enzymatic analyses with wild-type and mutant RT to elucidate the exact mechanism of antiretroviral activity of the 4′-substituted nucleoside analogs have not yet been carried out. Nevertheless, it is likely that these 4′-substituted nucleoside analogs serve as NRTIs but not as NNRTIs. First, the 4′-substituted nucleoside analogs were active against the virus with any pyrimidines or purines, although their antiviral potency varied (Table 1), a different profile from that of HEPT and its analogs, which are active only with thymine as its base component (1). Second, although all of the currently available NNRTIs are incapable of inhibiting HIV-2, and this property has been one of the salient features of NNRTIs, the selected 4′-substituted nucleoside analogs examined were active against both HIV-1 and HIV-2 (Tables 1 and 3). Moreover, 4′-E-dC, 4′-E-dA, and 4′-E-dDAP inhibited an NNRTI-resistant clone, HIV-1Y181C (Table 2). Third, in the time-of-drug-addition assays (7), the profiles of anti-HIV-1 activity of two 4′-E nucleosides (4′-E-dC and 4′-E-araC) were quite similar to that of AZT (Fig. 2), suggesting that 4′-E nucleosides suppress the replication of HIV-1 at or around the step of reverse transcription in the replication cycle. Fourth, antiviral activity of 4′-E-T, 4′-E-dC, and 4′-E-dG was reversed by the addition of their corresponding physiologic 2′-deoxynucleosides (Fig. 3A, B, and D), strongly suggesting that 4′-E nucleosides serve as substrates for RT. In contrast, the activity of 4′-E-dA was not reversed by its corresponding physiologic nucleoside 2′-deoxyadenosine (dA)(Fig. 3C). In this respect, the activity of ddA (and ddI) against HIV-1 is not reversed by dA (26), which is possibly due to there being no intracellular change in dATP levels regardless of extracellular dA levels, since the ubiquitous adenosine deaminase (ADA) in the target cells immediately converts dA to 2′-deoxyinosine (3, 34). In fact, the addition of dA in the presence of an ADA inhibitor (2′-deoxycoformycin) reversed the activity of 4′-E-dA against HIV-1 (data not shown). Taken together, like the currently available NRTIs, 4′-substituted nucleosides most likely block the replication of HIV by functioning competitive inhibitors for RT.
It is noteworthy that there are a few previously reported 4′-substituted nucleoside analogs active against HIV. Maag et al. synthesized 4′-azidothymidine (4′-AZT), 4′-azido-2′-deoxyguanosine (4′-AZG), and 4′-azido-5-chloro-2′-deoxyuridine (4′-AZU) and found that these compounds were active against HIV-1 (18, 19). With respect to their specific anti-HIV activity, Chen et al. reported that following intracellular anabolic phosphorylation of 4′-AZT, HIV RT efficiently incorporates two consecutive 4′-AZT molecules preventing RT from further elongating the DNA chain (4). Incorporation of two 4′-AZT-monophosphate (MP) molecules separated by one 2′-deoxynucleoside 5′-MP (dAMP, dCMP, or dGMP) also abolishes DNA chain elongation by HIV RT. In contrast, cellular DNA polymerases α and β incorporate a single 4′-AZT-MP molecule into nascent DNA at a very slow rate, but do not incorporate a second consecutive 4′-AZT-MP and continue to elongate cellular DNA (4).
It should be noted that 4′-E-dC, 4′-E-dA, and 4′-E-dDAP suppressed all infectious HIV-1 clones examined which were resistant to all the currently available NRTIs, including two multidrug-resistant HIV-1 variants, HIV-1M41L/T69S-S-G/T215Y (41) and HIV-1A62V/V75I/F77L/F116Y/Q151M (35, 36) (Table 2). It is also of note that 4′-E-araC, 4′-Me-dC, and 4′-FMe-dC were less potent against two 3TC-resistant HIV-1 variants, HIV-1M184I and HIV-1M184V (Table 2). This difference in drug resistance profiles between the former three 4′-E nucleosides and the latter two analogs should be intriguing from a structure-activity relationship point of view. Recently, the extensive structural analysis of RT has shed light on the mechanism of viral resistance to NRTIs (13, 31, 32). Substitutions of amino acids by mutations, which confer drug resistance, are accumulated around the deoxynucleoside triphosphate (dNTP) binding sites. Considering that all of the currently available NRTIs contain 3′-position modifications, HIV-1 is thought to alter the putative dNTP binding site to develop drug resistance. Indeed, codons K65, L74, Q151, and M184, which are associated with resistance to NRTIs, are located in the neighborhood of the enzyme sites. These sites interact with incoming nucleotides and are thought to be critical for the binding of the active site of RT to NRTIs, which prevents the NRTIs from being incorporated into the growing proviral DNA chain (32). However, 4′-E nucleosides examined in this study retain 3′-OH moiety like natural substrates, which may enable 4′-E nucleosides to interact with the mutated 3′-OH binding site of various types of drug-resistant HIV. Isolation and characterization of resistant mutants against 4′-E nucleosides should provide more insights into nucleoside-enzyme interactions. Further development of 4′-E analogs as potential therapeutics for infection with multidrug-resistant HIV-1 is warranted.
ACKNOWLEDGMENTS
We thank Shin-ichi Oka and Setsuko Ida for providing HIV-1 clinical strains and Miyuki Itoh and Takehiro Suzuki for excellent technical support.
This work was supported in part by a grant from a Research for the Future Program of Japan Society for the Promotion of Science (JSPS-RFTF 97L00705; H.M.), a Grant-in-Aid for Scientific Research (Priority Areas) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan ( E.K. and H.M.), and a Grant for Promotion of AIDS Research from the Ministry of Health, Welfare, and Labor of Japan (E.K., M.M., and H.M.).
REFERENCES
- 1.Baba M, De Clercq E, Tanaka H, Ubasawa M, Takashima H, Sekiya K, Nitta I, Umezu K, Nakashima H, Mori S, Shigeta S, Walker R T, Miyasaka T. Potent and selective inhibition of human immunodeficiency virus type 1 (HIV-1) by 5-ethyl-6-phenylthiouracil derivatives through their interaction with the HIV-1 reverse transcriptase. Proc Natl Acad Sci USA. 1991;88:2356–2360. doi: 10.1073/pnas.88.6.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baba M, Shigeta S, Yuasa S, Takashima H, Sekiya K, Ubasawa M, Tanaka H, Miyasaka T, Walker R T, De Clercq E. Preclinical evaluation of MKC-442, a highly potent and specific inhibitor of human immunodeficiency virus type 1 in vitro. Antimicrob Agents Chemother. 1994;38:688–692. doi: 10.1128/aac.38.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begleiter A, Verburg L, Israels L G, Johnston J B. Factors influencing the inhibition of repair of irradiation-induced DNA damage by 2′-deoxycoformycin and deoxyadenosine. Cancer Chemother Pharmacol. 1992;30:65–69. doi: 10.1007/BF00686487. [DOI] [PubMed] [Google Scholar]
- 4.Chen M S, Suttmann R T, Papp E, Cannon P D, McRoberts M J, Bach C, Copeland W C, Wang T S. Selective action of 4′-azidothymidine triphosphate on reverse transcriptase of human immunodeficiency virus type 1 and human DNA polymerases alpha and beta. Biochemistry. 1993;32:6002–6010. doi: 10.1021/bi00074a011. [DOI] [PubMed] [Google Scholar]
- 5.Chu M Y, Fisher G A. A proposed mechanism of action of 1-β-D-arabiofuranosylcytosine as an inhibitor of the growth of leukemic cells. Biochem Pharm. 1962;11:423–430. doi: 10.1016/0006-2952(62)90225-3. [DOI] [PubMed] [Google Scholar]
- 6.De Clercq E. Perspectives of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Farmaco. 1999;54:26–45. doi: 10.1016/s0014-827x(98)00103-7. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E, Yamamoto N, Pauwels R, Baba M, Schols D, Nakashima H, Balzarini J, Debyser Z, Murrer B A, Schwartz D, Thornton D, Bridger G, Fricker S, Henson G, Abrams M, Picker D. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc Natl Acad Sci USA. 1992;89:5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans J S, Mengel G D. The reversal of cytosine arabionoside activity in vivo by deoxycytidine. Biochem Pharmacol. 1964;13:984–994. doi: 10.1016/0006-2952(64)90095-4. [DOI] [PubMed] [Google Scholar]
- 9.Fatkenheuer G, Theisen A, Rockstroh J, Grabow T, Wicke C, Becker K, Wieland U, Pfister H, Reiser M, Hegener P, Franzen C, Schwenk A, Salzberger B. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. AIDS. 1997;11:F113–F116. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fischl M A. Antiretroviral therapy in 1999 for antiretroviral-naive individuals with HIV infection. AIDS. 1999;13(Suppl. 1):S49–S59. [PubMed] [Google Scholar]
- 11.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 12.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Jr, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Chopra R, Verdine G, Harrison S. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 14.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama E, Shigeta S, Suzuki T, De Clercq E. Application of a gastric cancer cell line (MKN-28) for anti-adenovirus screening using the MTT method. Antiviral Res. 1996;31:159–164. doi: 10.1016/0166-3542(96)06966-5. [DOI] [PubMed] [Google Scholar]
- 16.Kohgo S, Horie H, Ohrui H. Synthesis of 4′-C-ethynyl-beta-D-arabino- and 4′-C-ethynyl-2′-deoxy-beta-D-ribo-pentofuranosyl pyrimidines, and their biological evaluation. Biosci Biotechnol Biochem. 1999;63:1146–1149. doi: 10.1271/bbb.63.1146. [DOI] [PubMed] [Google Scholar]
- 17.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, Vernazza P, Sudre P, Flepp M, Furrer H, Francioli P, Weber R. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 18.Maag H, Nelson J T, Steiner J L, Prisbe E J. Solid-state and solution conformations of the potent HIV inhibitor, 4′-azidothymidine. J Med Chem. 1994;37:431–438. doi: 10.1021/jm00030a001. [DOI] [PubMed] [Google Scholar]
- 19.Maag H, Rydzewski R M, McRoberts M J, Crawford-Ruth D, Verheyden J P, Prisbe E J. Synthesis and anti-HIV activity of 4′-azido- and 4′-methoxynucleosides. J Med Chem. 1992;35:1440–1451. doi: 10.1021/jm00086a013. [DOI] [PubMed] [Google Scholar]
- 20.Maeda Y, Venzon D, Mitsuya H. Evolution and fitness of HIV-1 with the pol gene mutations conferring multi-dideoxynucleoside resistance. J Infect Dis. 1998;177:1207–1213. doi: 10.1086/515282. [DOI] [PubMed] [Google Scholar]
- 21.Mitsuya H, Broder S. Inhibition of infectivity and replication of HIV-2 and SIV in helper T-cells by 2′,3′-dideoxynucleosides in vitro. AIDS Res Hum Retrovir. 1988;4:107–113. doi: 10.1089/aid.1988.4.107. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuya H, Broder S. Strategies for antiviral therapy in AIDS. Nature. 1987;325:773–778. doi: 10.1038/325773a0. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuya H, Erickson J. Discovery and development of antiretroviral therapeutics for HIV infection. In: Merigan T C, Bartlet J G, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore, Md: Williams & Wilkins; 1999. pp. 751–780. [Google Scholar]
- 24.Mitsuya H, Looney D J, Kuno S, Ueno R, Wong-Staal F, Broder S. Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science. 1988;240:646–649. doi: 10.1126/science.2452480. [DOI] [PubMed] [Google Scholar]
- 25.Mitsuya H, Matsukura M, Broder S. Rapid in vitro systems for assessing activity of agents against HTLV-III/LAV. In: Broder S, editor. AIDS: modern concepts and therapeutic challenges. New York, N.Y: Marcel Dekker; 1987. pp. 303–333. [Google Scholar]
- 26.Mitsuya H, Weinhold K J, Furman P A, St. Clair M H, Nusinoff Lehrman S, Gallo R C, Bolognesi D, Barry D W, Broder S. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura M, Shuto S, Tanaka M, Sasaki T, Mori S, Shigeta S, Matsuda A. Nucleosides and nucleotides. 185. Synthesis and biological activities of 4′alpha-C-branched-chain sugar pyrimidine nucleosides. J Med Chem. 1999;42:2901–2908. doi: 10.1021/jm990050i. [DOI] [PubMed] [Google Scholar]
- 28.Ohrui H, Kohgo S, Kitano K, Sakata S, Kodama E, Yoshimura K, Matsuoka M, Shigeta S, Mitsuya H. Synthesis of 4′-C-ethynyl-β-D-arabino- and 4′-C-ethynyl-2′-deoxy-β-D-ribo-pentofuranosyl pyrimidines and purines and evaluation of their anti-HIV-1 activity. J Med Chem. 2000;43:4516–4525. doi: 10.1021/jm000209n. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto M, Fujiwara M, Kodama E, Yamamoto O, Shigeta S, Mitsuya H, Konno K, Yokota T, Baba M. Inhibition of human immunodeficiency virus replication by RD6–Y664, a novel benzylhydroxylamine derivative. Antivir Chem Chemother. 1999;10:71–77. doi: 10.1177/095632029901000203. [DOI] [PubMed] [Google Scholar]
- 30.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 31.Sarafianos S, Das K, Clark A J, Ding J, Boyer P, Hughes S, Arnold E. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with beta-branched amino acids. Proc Natl Acad Sci USA. 1999;96:10027–10032. doi: 10.1073/pnas.96.18.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarafianos S, Das K, Ding J, Boyer P, Hughes S, Arnold E. Touching the heart of HIV-1 drug resistance: the fingers close down on the dNTP at the polymerase active site. Chem Biol. 1999;6:137–146. doi: 10.1016/s1074-5521(99)80071-4. [DOI] [PubMed] [Google Scholar]
- 33.Serchuck L K, Wells L, Yarchoan R. Antiretroviral treatment for HIV infection. In: Merigan T C, Bartlet J G, Bolognesi D, editors. Textbook of AIDS medicine. Baltimore, Md: Williams & Wilkins; 1999. pp. 780–806. [Google Scholar]
- 34.Shimoyama R K, Seto S, Mori C. Protection by various deoxynucleosides against deoxyadenosine-induced DNA damage in adenosine deaminase-inactivated lymphocytes. Mol Genet Metab. 1999;68:455–460. doi: 10.1006/mgme.1999.2937. [DOI] [PubMed] [Google Scholar]
- 35.Shirasaka T, Kavlick M F, Ueno T, Gao W-Y, Kojima E, Alcaide M L, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, Mitsuya H. Emergence of human immunodeficiency virus type-1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirasaka T, Yarchoan R, O'Brien M C, Husson R N, Anderson B D, Kojima E, Shimada T, Broder S, Mitsuya H. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc Natl Acad Sci USA. 1993;90:562–566. doi: 10.1073/pnas.90.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staszewski S, Morales-Ramirez J, Tashima K T, Rachlis A, Skiest D, Stanford J, Stryker R, Johnson P, Labriola D F, Farina D, Manion D J, Ruiz N M. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341:1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto I, Shuto S, Mori S, Shigeta S, Matsuda A. Nucleosides and nucleotides. 183. Synthesis of 4′alpha-branched thymidines as a new type of antiviral agent. Bioorg Med Chem Lett. 1999;9:385–388. doi: 10.1016/s0960-894x(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Srinivas R V, Ueno T, Kavlick M F, Hui F K, Fridland A, Driscoll J S, Mitsuya H. In vitro induction of human immunodeficiency virus type 1 variants resistant to 2′-beta-fluoro-2′,3′-dideoxyadenosine. Antimicrob Agents Chemother. 1997;41:1313–1318. doi: 10.1128/aac.41.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wainberg M A, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 41.Winters M, Coolley K, Girard Y, Levee D, Hamdan H, Shafer R, Katzenstein D, Merigan T. A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J Clin Investig. 1998;102:1769–1775. doi: 10.1172/JCI4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshimura K, Kato R, Yusa K, Kavlick M F, Maroun V, Nguyen A, Mimoto T, Ueno T, Shintani M, Falloon J, Masur H, Hayashi H, Erickson J, Mitsuya H. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc Natl Acad Sci USA. 1999;96:8675–8680. doi: 10.1073/pnas.96.15.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]