Abstract
Cataplexy is the abrupt loss of muscle tone experienced by narcoleptics. It is usually precipitated by strong emotions or athletic activity. It has been hypothesized that cardiovascular variables have a role in the triggering of cataplexy. In the present study, we have utilized the narcoleptic canine model to directly investigate changes in heart rate and blood pressure in relation to cataplectic episodes. We found that heart rate increased 18% on average in the 20 s preceding cataplexy onset and then fell during cataplexy. Thus, from a cardiovascular standpoint, cataplexy can be subdivided into two very different periods, the cataplexy onset period with very high and declining heart rate, and the period ≥10 s after onset, with greatly reduced heart rate. Heart rate at cataplexy onset was significantly higher than heart rate in rapid-eye-movement (REM) sleep, non-REM sleep, and quiet waking. Blood pressure did not markedly change before the onset of spontaneous cataplexies but decreased significantly during cataplexy. Although blood pressure increases did not precede spontaneous cataplexies, sudden increases in blood pressure, induced pharmacologically or by obstruction of the descending aorta, triggered cataplexy in the most severely affected subjects. A hypothesized role for cataplexy as a homeostatic reflex, triggered by interactions between blood flow, central chemoreceptors, and atonia control mechanisms in the medial medulla, is discussed.
Keywords: muscle tone, hypertension, hypotension, narcolepsy, canine, non-rapid-eye-movement sleep, rapid-eye-movement sleep
CATAPLEXY is the pathological loss of muscle tone experienced periodically by narcoleptics. During cataplectic episodes, consciousness is preserved, while voluntary movement is blocked and tone in the skeletal muscles is greatly reduced or absent. Cataplectic episodes may last for a few seconds or as long as several minutes. They can terminate abruptly with arousal into the waking state or may progress into rapid-eye-movement (REM) sleep. Episodes are typically triggered by situations in which strong emotions are aroused, including anger, embarrassment, and sexual activity, and may also result from athletic exertion (10). The physiological changes responsible for the triggering of cataplexy are unknown.
The decerebrate animal can serve as a model for certain aspects of cataplexy. In this preparation, electrical stimulation of the medial medulla can precipitate a complete loss of muscle tone (17). Similar stimulation in the intact animal is ineffective. Furthermore, many decerebrate preparations do not show this loss of tone after stimulation (31). In our initial studies of the source of this variability, we identified transection level as one determinant of the probability of loss of muscle tone after medullary stimulation (29). In a subsequent study, we found that small reductions in blood pressure could prevent atonia after medullary stimulation (15). Conversely, increases in blood pressure facilitated the atonia response to medullary stimulation. We hypothesized that since blood pressure increases facilitate the atonia response in the decerebrate cataplexy model, they might have a role in triggering cataplexy in narcoleptics.
The narcolepsy-cataplexy syndrome has been shown to occur in several breeds of dog and in other animals (2, 8, 14, 19, 20, 32, 33). We have used the narcoleptic dog to test the hypothesis derived from our decerebrate work and to examine changes in heart rate and blood pressure linked to spontaneous cataplectic attacks. Some of these results have been reported in preliminary form (28).
METHODS
Five narcoleptic doberman-labrador crossbred dogs, born at the Stanford Narcoleptic Dog Colony, served as subjects. Dog 142 was male, and dogs 139, 141, 146, and 148 were female. The ages of the dogs at the time of the studies were 3–4 mo (dogs 139, 141, and 142) or 5 mo (dogs 146 and 148). The dogs were transported from Stanford to Los Angeles, where the studies were conducted. After being anesthetized with halothane dogs 139–146 were implanted with screw electrodes over sensorimotor and posterior lateral cortex for recording the electroencephalogram (EEG). Screw electrodes were also placed in the orbit or frontal bone for recording the electrooculogram (EOG). Stranded stainless steel wires were inserted into the dorsal neck musculature for recording the electromyogram (EMG). A catheter was inserted through the femoral artery and guided into the descending aorta. It was led subcutaneously to terminate on the headplug. The catheter was flushed with heparinized saline every 12 h, and a continuous flow of 4 ml/h of heparinized saline was maintained during recording sessions using a Sorenson valve and pressure-infusion bag. Blood pressure and heart rate data were obtained during spontaneously occurring sleep and waking states from dogs 139, 141, and 142. Dogs 146 and 148 were used only for studies of the effect of blood pressure manipulation on the triggering of cataplexy. Norepinephrine (NE) and sodium nitroprusside (SNP) were administered through the catheter followed by a flush of 12 ml of saline. NE doses ranged from 0.9 to 20 μg/kg. SNP doses ranged from 0.04 to 0.15 mg/kg. Blood pressure was recorded with a Statham pressure transducer and displayed on a polygraph along with EEG, EOG, and EMG. In dogs 141, 142, and 146, a Swan-Ganz 5-F balloon-tipped catheter was employed. Inflation of the balloon obstructed the descending aorta, causing a rapid rise in blood pressure. A cardiotachometer displayed heart interbeat intervals that were derived from the blood pressure pulse wave. Dog 148 did not receive a chronic implant. Instead, two pairs of stranded stainless steel suture wires (Ethicon) were inserted into the left and right dorsal neck musculature through 21-gauge syringe needles, EKG was recorded using transcutaneous needle electrodes, and drugs were administered through a butterfly needle implanted in the cephalic vein. In this dog, heart rate slowing was used to indicate the time course of cardiovascular changes after NE administration.
To prevent artifactual changes in blood pressure readings caused by movement of the dog relative to the transducer, studies were conducted while the animals were supported by a sling (Chatham Medical Arts). Recordings were performed in a quiet room with a low level of natural light. The dogs could see only the wall of the laboratory, ~3 ft away. No attempt was made to trigger cataplexy by presenting food, water, or other stimuli. Spontaneous episodes of sleep and cataplexy occurred while the dogs were in the sling.
State data were based on five episodes of each state in each dog. Each episode score for heart rate and blood pressure was based on a mean of three values of each parameter. “Active waking” values were taken from those periods of vigorous motor activity with maximal heart rate values in which cataplectic attacks did not occur. “Quiet waking,” non-REM and REM sleep values were taken at the midpoint of episodes, although heart rate and blood pressure parameters did not differ substantially between the beginning, middle, and end of these states. “Cataplexy onset” values were taken from the first 1-s interval after the complete loss of muscle tone. “Cataplexy” values were taken at the midpoint of cataplexy episodes. Time-course plots were made from data normalized for varying cataplexy durations, with samples at intervals equal to 10% of the episode duration starting 10% before onset and ending 10% after offset. Time-course data were averaged from 7 to 10 cataplexy episodes in each dog. Episodes that terminated in awakening were chosen, whereas those leading to extended sleep periods were excluded from this analysis, to have a homogeneous sample including both onset and offset values. Values at and shortly after onset were similar in both kinds of episodes.
RESULTS
Heart rate as a function of state.
Figure 1 presents the changes in heart rate across states in the three dogs examined. Heart rate varied significantly as a function of state (P < 0.01, F test with repeated measures). Post hoc comparisons with correction for multiple tests [P < 0.05, Scheffe’s test (12)] showed that heart rates in active waking were significantly greater than rates in either quiet waking, cataplexy, REM sleep, or non-REM sleep (Table 1). Heart rate at cataplexy onset was significantly greater than heart rates in quiet waking, REM sleep, and non-REM sleep and was also significantly greater than heart rate during cataplexy. Mean heart rate at cataplexy onset was greater than heart rate in active waking, although this difference was not significant (Table 1). As explained below, cataplexy onset was consistently preceded by an increase in heart rate.
FIG. 1.
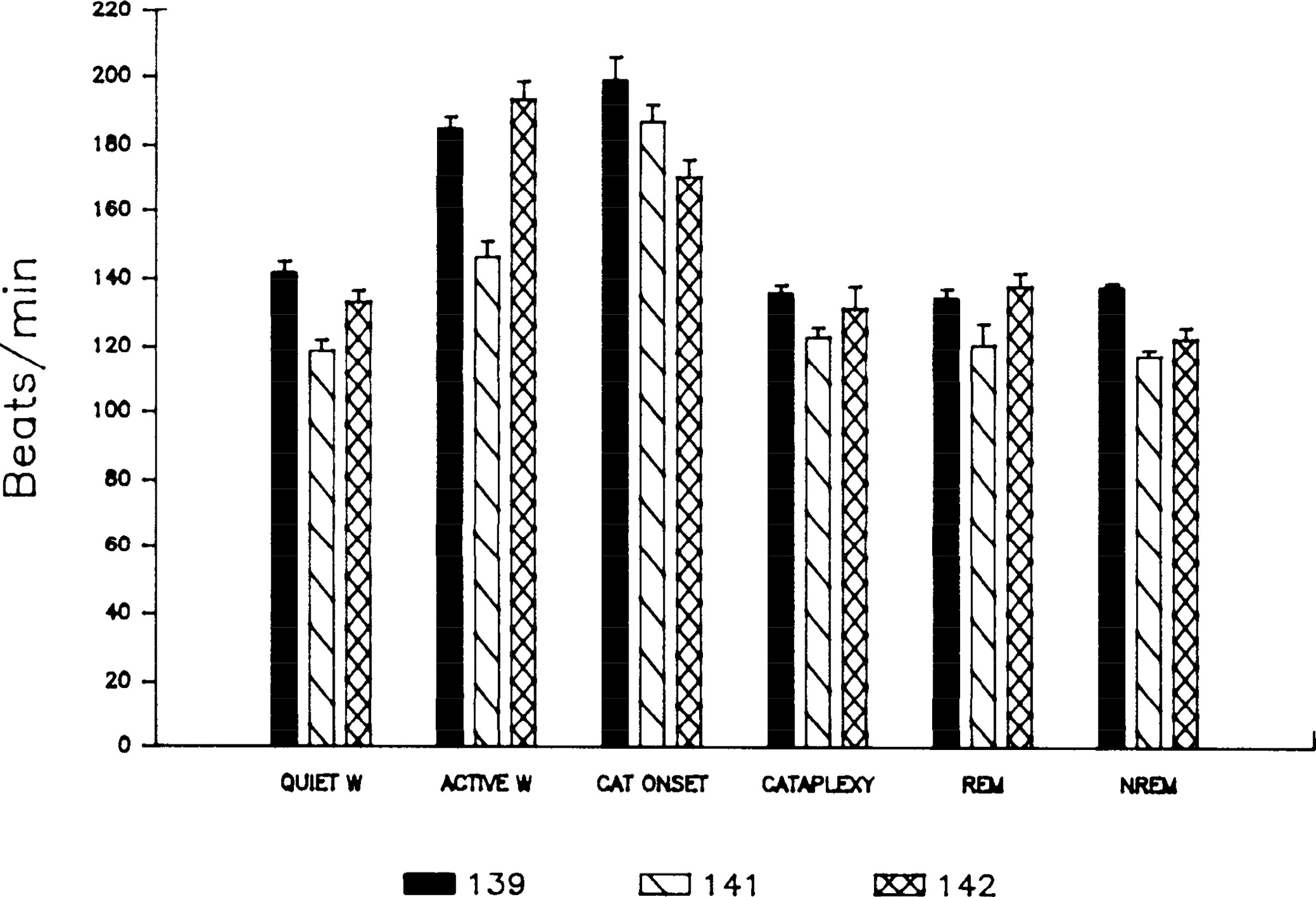
Heart rate as a function of state ± SE in dogs 139,141,and 142. W, waking; CAT, cataplexy; REM, rapid-eye movement; NREM, non-REM.
TABLE 1.
Heart rate and blood pressure differences between states
| QW | CAT-ON | CAT | REM Sleep | NREM Sleep | |
|---|---|---|---|---|---|
| AW | H S | - - | H S | H S | H S |
| QW | L - | - - | - - | - - | |
| CAT-ON | H - | H - | HS | ||
| CAT | - - | - - | |||
| REM Sleep | - - |
H, Heart rate of state listed in left column was significantly greater than heart rate of state listed above top line; L, heart rate of state listed in left column was significantly less than heart rate of state listed above top line; S, systolic blood pressure of state listed in left column was significantly greater than systolic blood pressure of state listed above top line; F test was used to verify the overall significance of changes in heart rate and blood pressure as a function of state (P < 0.01). Scheffe’s method for post hoc comparisons was employed to determine the probability of differences between groups (P < 0.05). AW, active waking; QW, quiet waking; CAT-ON, cataplexy onset; CAT, cataplexy midpoint; REM, rapid-eye movement, NREM, non-REM. -, no significant difference.
Blood pressure as a function of state.
Figures 2 and 3 present the changes in blood pressure across states. Both systolic and diastolic blood pressure varied significantly with state (P < 0.01, F test with repeated measures). Paired comparisons (P < 0.05, Scheffe’s test) showed that systolic blood pressure, like heart rate, was significantly greater in active waking than in either quiet waking, cataplexy, REM sleep, or non-REM sleep (Table 1). Systolic blood pressure was not significantly greater in active waking than at cataplexy onset. Systolic and mean blood pressure values decreased as the dogs shifted from cataplexy onset, to cataplexy, to REM sleep, the three conditions in which muscle atonia is present (P < 0.03, Friedman one-way analysis of variance by ranks). Paired comparisons of diastolic blood pressure across states were not significant. There was no significant correlation between base-line blood pressures and the frequency of cataplectic attacks between dogs.
FIG. 2.
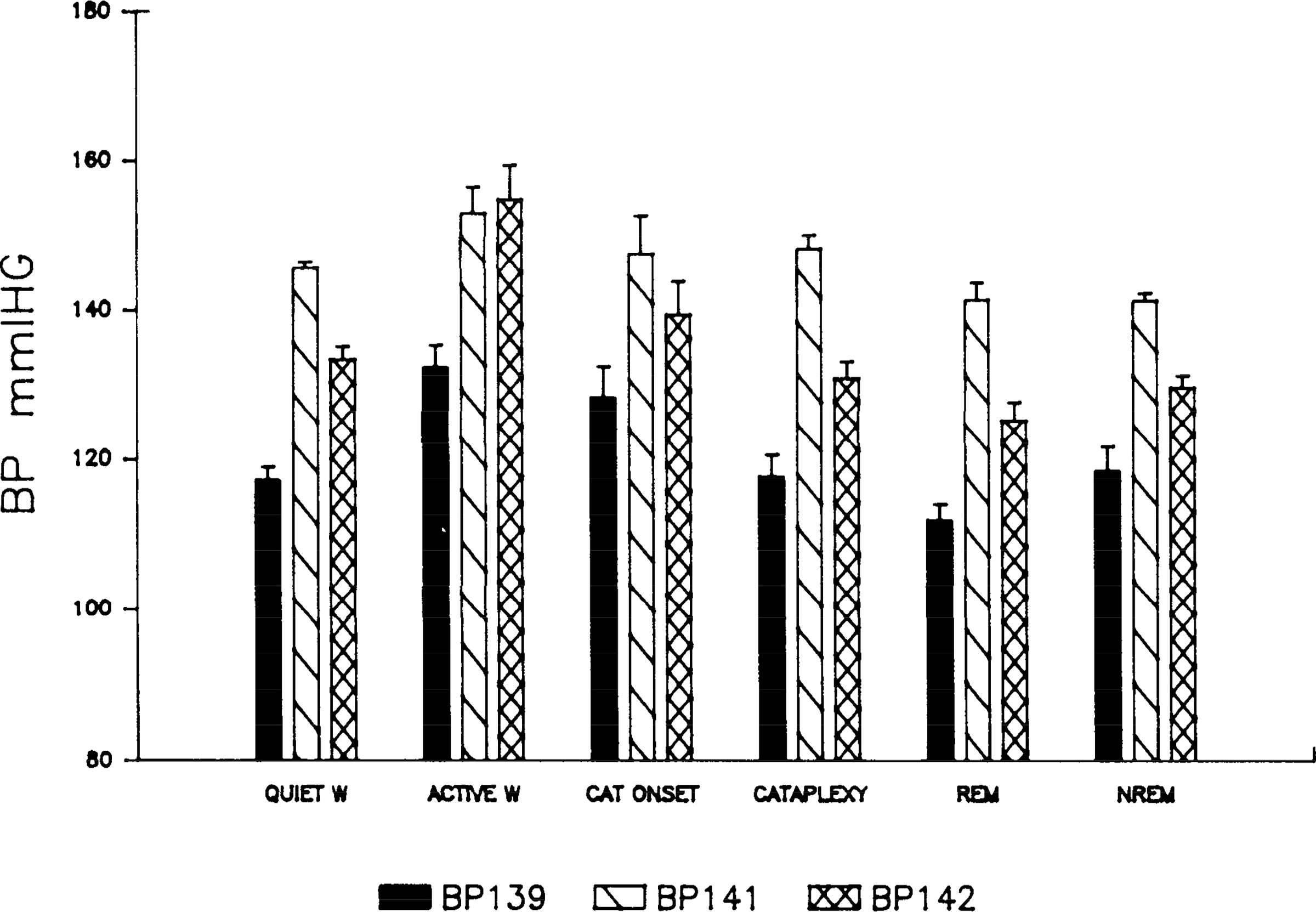
Systolic blood pressure (BP) as a function of state. See Fig. 1 for abbreviations.
FIG. 3.
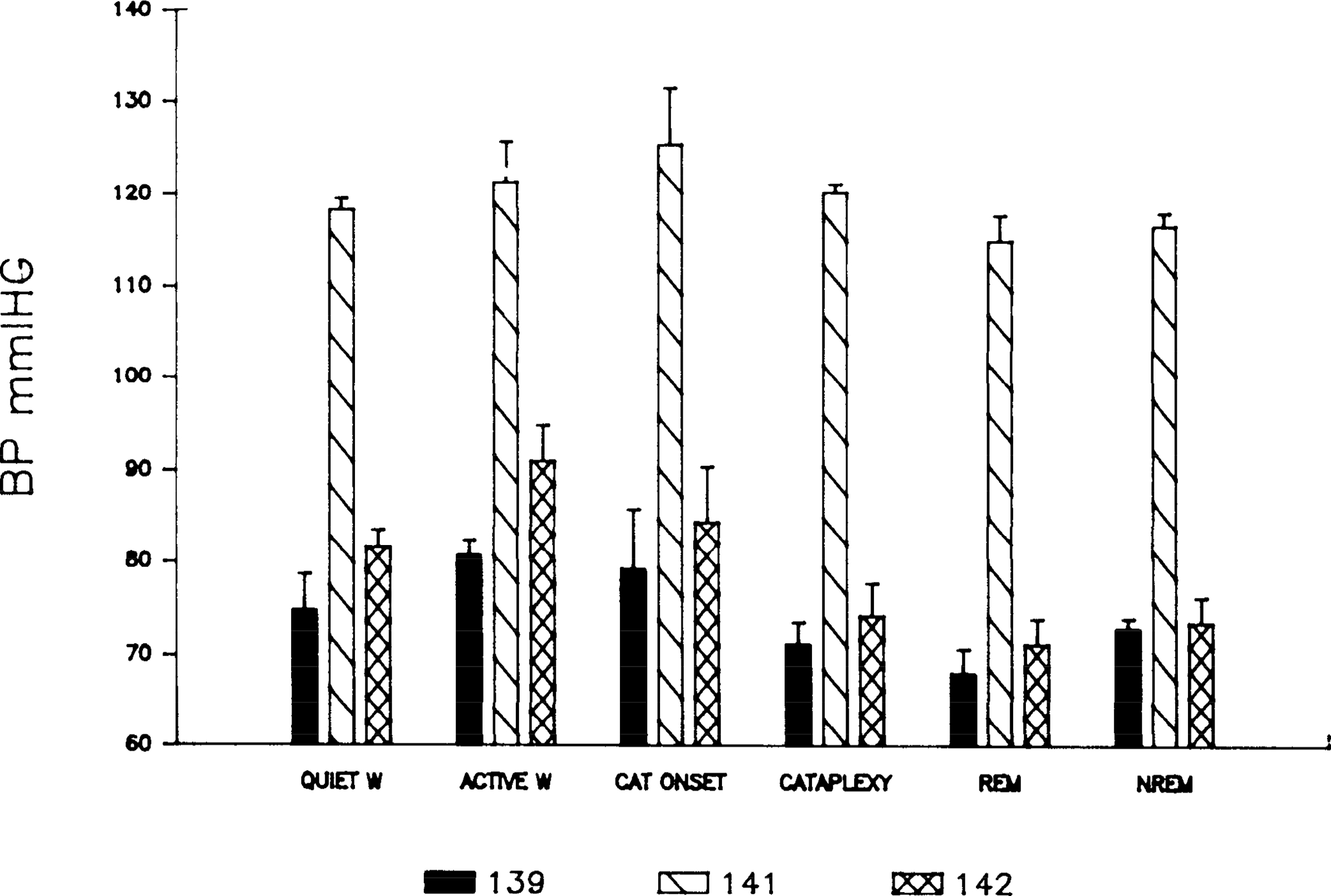
Diastolic blood pressure (BP) as a function of state. See Fig. 1 for abbreviations.
Time course of heart rate changes.
Figure 4 displays the time course of changes in recorded variables as a function of the percent of the cataplexy interval that has elapsed. In this figure, one can see a consistent increase in heart rate just before cataplexy onset followed by a rapid drop in heart rate, which stabilized ~10 s after onset. Heart rate remained at this lower level throughout the episode. After termination of the cataplexy episode, heart rate increased. Diastolic blood pressure did not markedly increase at cataplexy onset but showed a small decrease during the initial portion of the cataplexy episode. Systolic blood pressure did not change consistently before cataplexy onset. Respiration was polygraphically recorded via a thoracic strain gauge in dog 142 and was found to increase in parallel with heart rate preceding cataplexy onset. Respiration dropped dramatically at cataplexy onset, with cataplexy onset linked to a short apnea (28), followed by respiratory rates averaging less than half of those in the precataplexy period.
FIG. 4.
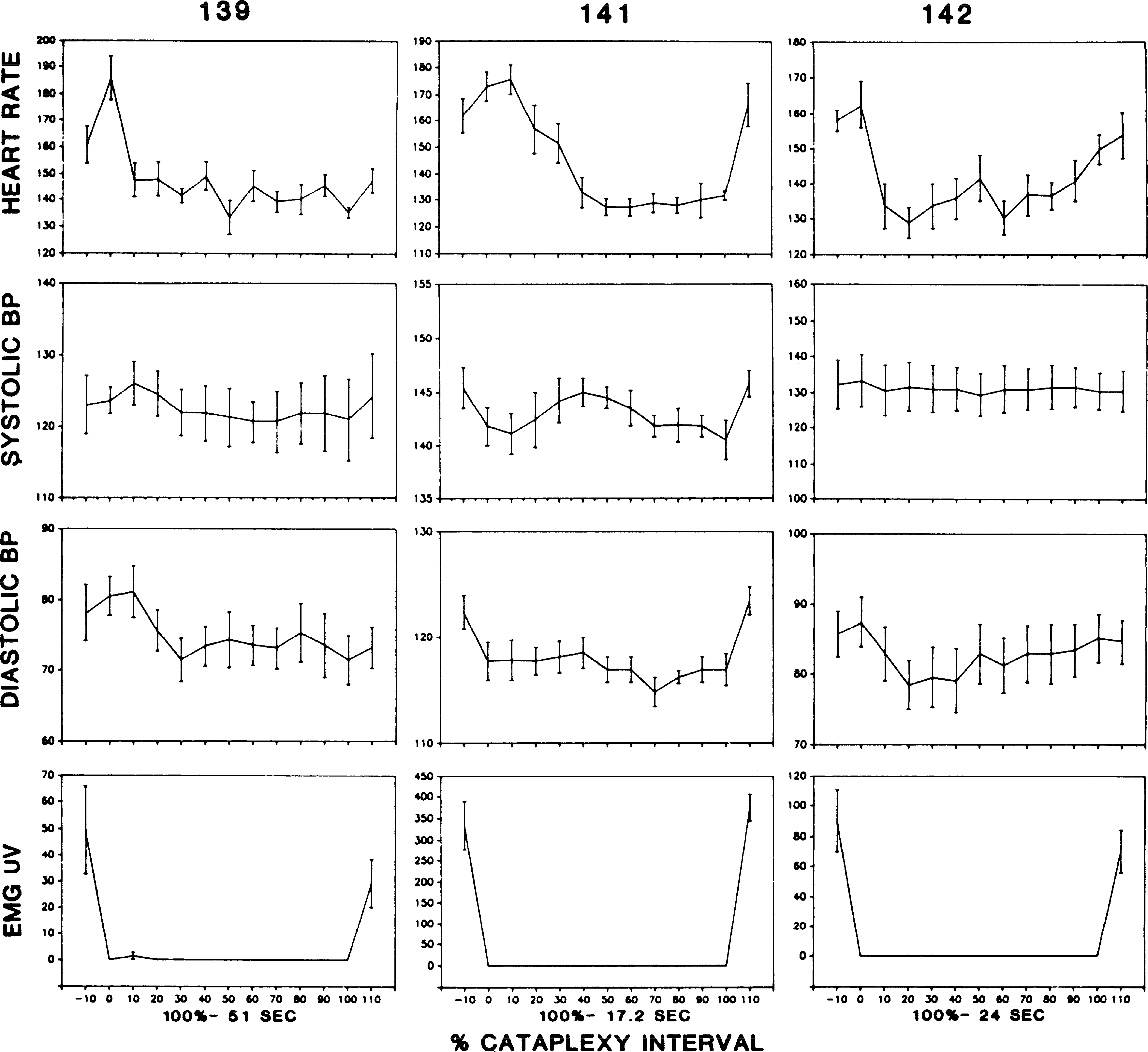
Time course of changes in heart rate, blood pressure (BP), and nuchal muscle tone as a function of percentage of cataplexy interval completed in dogs 139, 141, and 142. Mean duration of entire cataplexy interval is indicated for each dog. EMG, electromyogram.
To better examine the time course of events at cataplexy onset, we plotted out recorded variables for the 10 s preceding and following onset (Fig. 5). The change in heart rate at onset was quite marked and of enormous magnitude. On average, heart rate increased 18% in the 10 s preceding cataplexy onset. The increase in heart rate was highly consistent, occurring in 27 out of 30 monitored attacks (P < 0.01 sign test). In contrast, as shown in Figs. 4 and 5 and in the analysis of variance, blood pressure did not change consistently during this period.
FIG. 5.
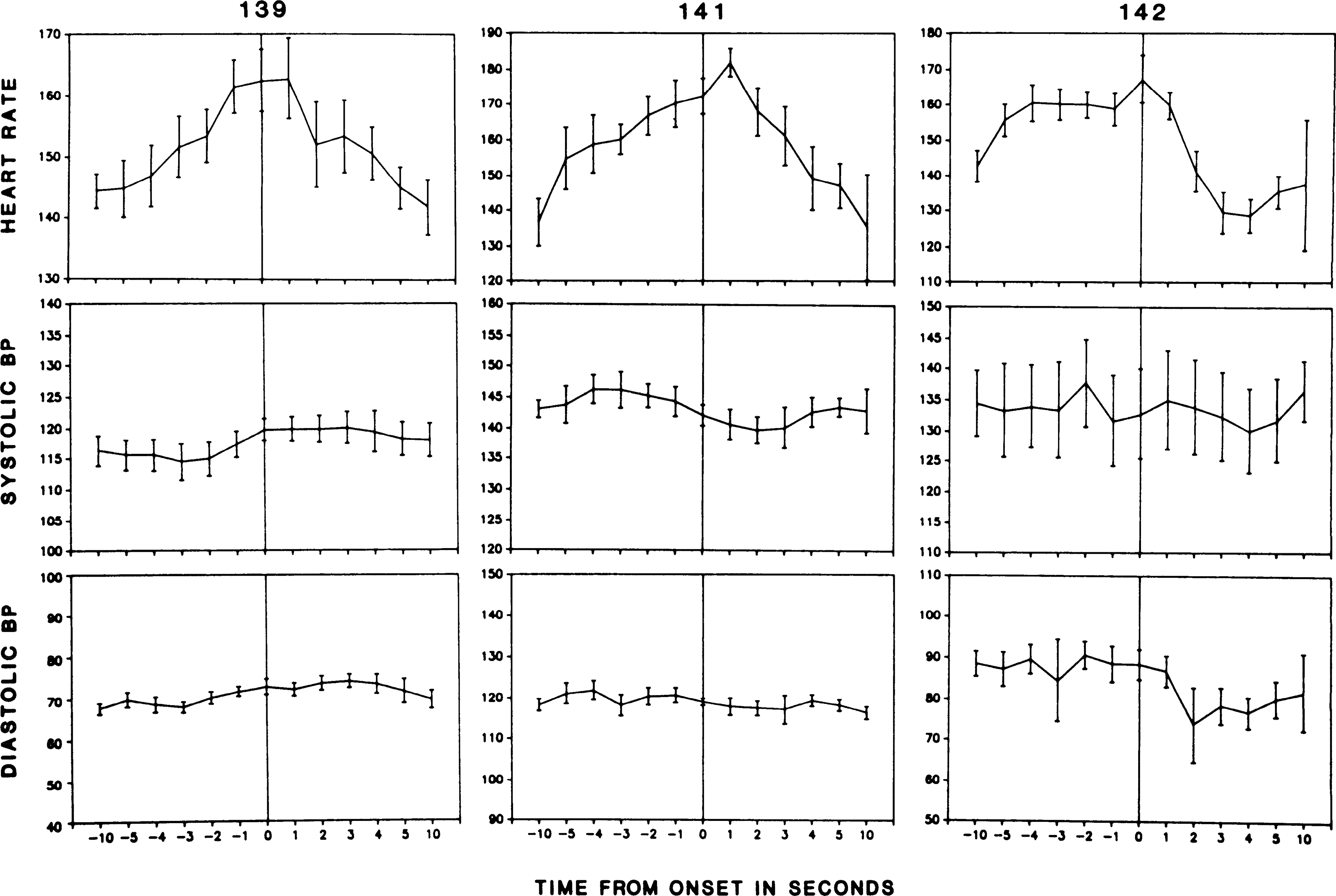
Time course of changes in heart rate and blood pressure (BP) during 10 s before and after cataplexy onset.
Triggering of cataplexy by elevation of blood pressure.
Figure 6 shows the effect of norepinephrine injection on the occurrence of cataplexy in narcoleptic dogs 141, 142, and 148. Norepinephrine was injected at time 0 (t0) in dogs 141, 142, and 148. These three dogs had >l 60-s epoch with cataplexy during the 10-min active waking base-line period. Blood pressure elevation after NE administration was accompanied by an increase in cataplectic attacks, reflected in an increase in the percentage of “wake with cataplexy” episodes [sign test (30) P < 0.01, two tailed]. To observe the effect of decreased blood pressure on cataplexy, we administered SNP to two dogs (Fig. 7). This produced an abrupt blood pressure drop, accompanied by a marked increase in active waking. However, despite the increase in the active waking state out of which cataplexy normally arises, there was no increase in the occurrence of episodes of cataplexy. NE was also administered to two narcoleptic dogs that had infrequent spontaneous attacks (no cataplexy during the IO-min active waking base-line period) but in which cataplexy could be evoked by physostigmine administration (dogs 139 and 146). NE was without effect on cataplexy in these animals.
FIG. 6.
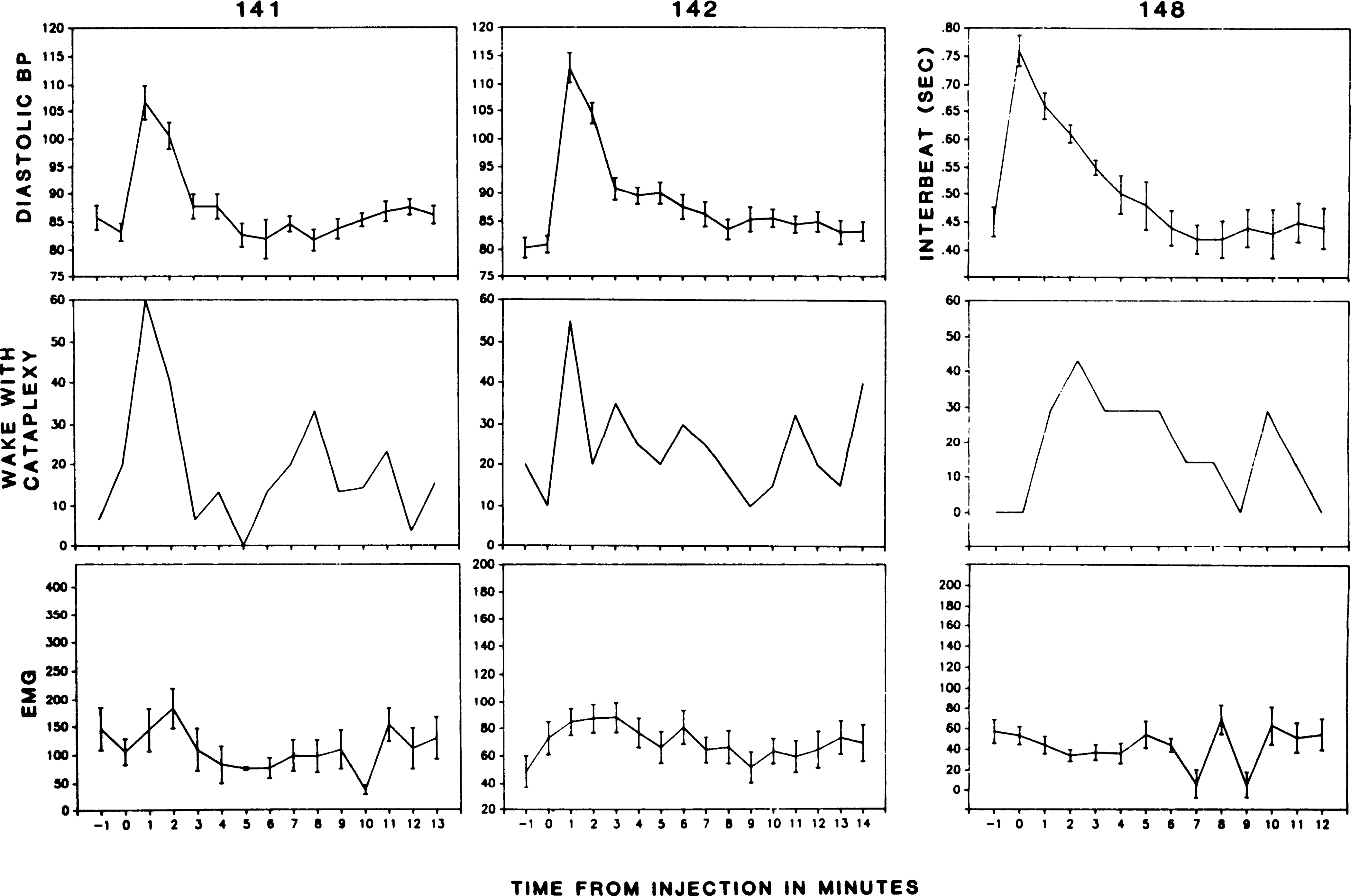
Effect of norepinephrine administration on frequency of cataplexy attacks. Blood pressure (BP) in mmHg. “Wake with cataplexy,” percentage of waking episodes in which one or more cataplectic attacks occurred; EMG, voltage of nuchal electromyogram. EMG reflects average muscle activity across each interval. Because intervals with short periods of cataplexy have considerable tone before and after attacks, average EMG activity does not mirror “wake with cataplexy” values.
fig. 7.
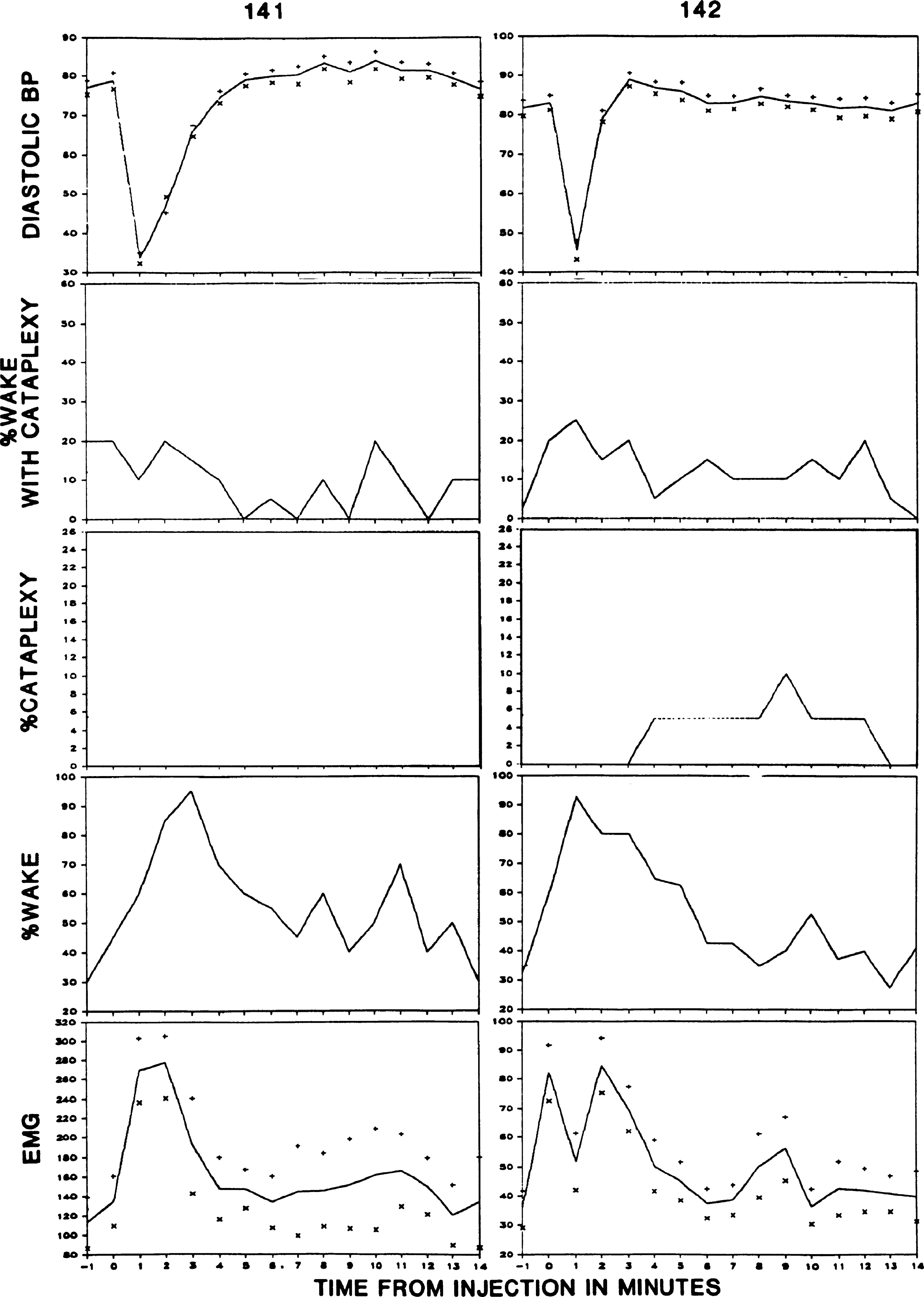
Effect of sodium nitroprusside administration on frequency of cataplexy attacks. % Wake with cataplexy, percentage of waking episodes in which one or more cataplectic attacks occurred; % Cataplexy, percentage of episodes in which cataplexy, defined as complete loss of nuchal muscle tone, occupied >50% of the 1-min epoch; % Wake, percentage of episodes with waking, uninterrupted by cataplexy.
To raise blood pressure nonpharmacologically, a balloon-tipped catheter was implanted in the descending aorta in three dogs (dogs 141, 142, and 146). The occurrence of cataplexy increased significantly in this group during the period of inflation (sign test, P < 0.025, two tailed). Balloon inflation produced an increase in blood pressure. The maximal effect on cataplexy occurred 36–48 s after inflation. Figure 8 shows the increased blood pressure and subsequent cataplectic attack after balloon inflation. Figure 9 presents averaged time course data after inflation of the aortic balloon.
FIG. 8.
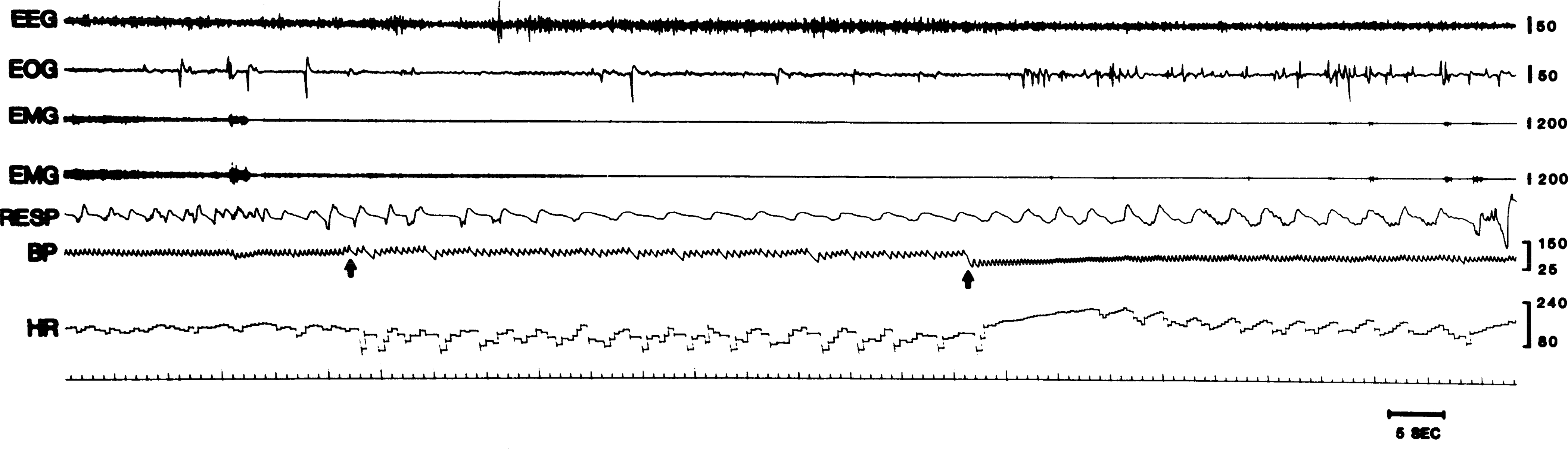
Triggering of cataplexy by obstruction of descending aorta. Top electromyogram (EMG) tracing is from right, and bottom trace is from left neck muscles. Respiration is from thoracic strain gauge (inspiration up). EEG, electroencephalogram; EOG, electrooculogram; BP, blood pressure; HR, heart rate. First arrow, time of inflation of aortic balloon; 2nd arrow, time of deflation of balloon.
FIG. 9.
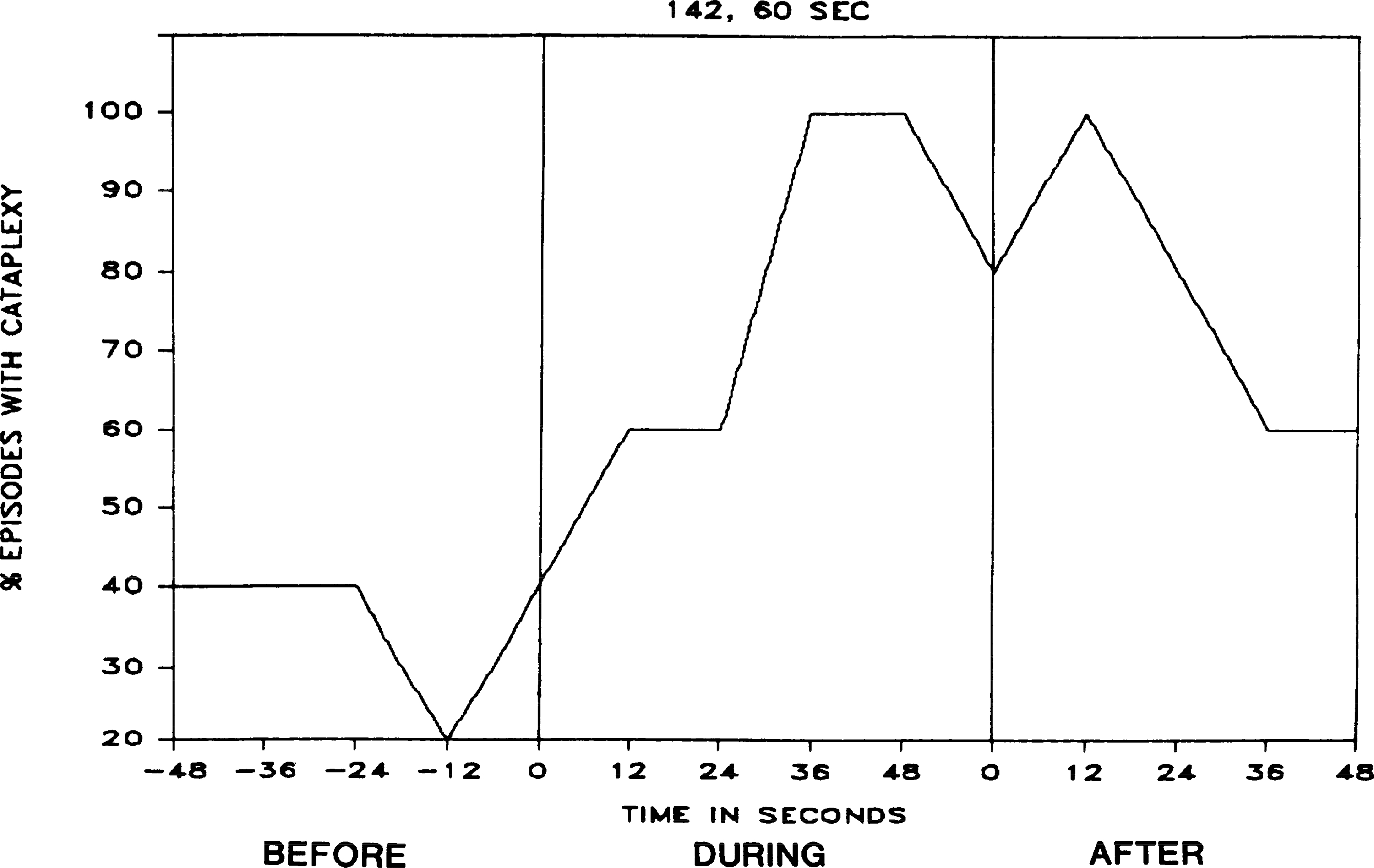
Percentage of episodes with cataplexy before, during, and after inflation of aortic balloon, as shown in Fig. 8.
DISCUSSION
We find that blood pressure increase, triggered pharmacologically or by obstruction of the descending aorta, can trigger cataplexy in narcoleptic dogs. Blood pressure decrease of corresponding magnitude does not increase and may decrease cataplexy. We also find that an extremely large and consistent increase in heart rate precedes spontaneous cataplexies. Results of an initial study of heart rate changes in human cataplexy were “inconclusive.” However, 8 of 14 attacks observed were preceded by a heart rate increase of >8 beats/min and three of these by an increase of ≥15 beats/min (11).
We do not find a consistent increase in blood pressure before spontaneously occurring cataplexies. Thus although induced increases in systemic blood pressure are sufficient to trigger cataplexy in narcoleptic dogs, and stimulation of the vagoaortic nerve triggers atonia in encéphale isolé cats (22, 23, 24, 27), blood pressure increases are not necessary for cataplexy, as had been hypothesized (27). Furthermore, blood pressure elevation produced increases in cataplexy occurrence only in our most severely affected subjects. These results suggest that baroreceptor activation is not the underlying mechanism triggering cataplexy. Our findings, however, do not exclude a role for baroreceptor activation as one route for the elicitation of cataplexy. Because blood pressure increase elicited by NE and aortic balloon inflation was accompanied by heart rate slowing, heart rate increase, while strongly correlated with onset of spontaneous cataplexy, does not appear to be critical. What then is the underlying variable?
Our work in acute decerebrate cats suggests one hypothesis; cerebral vasoconstriction leading to activation of central chemoreceptors may be responsible for the triggering of atonia (15). The evidence is as follows: Magoun and Rhines (17) first reported that stimulation of the medial medulla led to a complete loss of muscle tone. The only behavioral states in which a similar loss occurs are REM sleep and cataplexy. We found that blood pressure reduction rapidly blocked the loss of tone triggered by medullary stimulation (15). Preparations with low blood pressure had no change or an increase in tone in antigravity muscles after stimulation, whereas preparations with high pressure consistently had atonia after stimulation. In an attempt to discover the mechanism controlling the loss of muscle tone, we removed baroreceptor input and severed the spinal cord. This did not diminish the effect of blood pressure manipulations on atonia susceptibility, i.e., medullary stimulation continued to produce atonia and blood pressure reduction continued to rapidly block atonia. Thus, this work is consistent with our findings in the narcoleptic dogs, in that baroreceptor activation does not seem to be required for the triggering of cataplexy. Furthermore, the effects of blood pressure on atonia, in both decerebrate cats and narcoleptic dogs, is maximal several seconds after the peak blood pressure change, a delay well beyond that attributable to baroreceptor activation. A change in central chemoreceptor activity would be consistent with such a delay. pH changes at the level of the ventral medullary chemoreceptor have been shown to occur 5–7 s after changes in end tidal PCO2 (1). Blood pressure changes would affect central PCO2 by changing brain stem blood flow and respiratory rate (16, 34, 35). Pharmacologically induced changes in blood pressure have been shown to change brain stem blood flow and central CO2 levels with the direction of effects varying as a function of species and level of anesthesia (5, 6, 21, 26). Narcoleptics have reduced levels of brain stem blood flow in waking and increased blood flow at sleep onset (18). This change contrasts with the reduction in brain stem blood flow seen at sleep onset in normals. We hypothesize that both the heart rate changes reported here and the blood flow changes reported by Meyer et al. (18) represent a reflex response to increased central CO2. Hypercapnia is known to produce a centrally mediated heart rate increase (7). According to our hypothesis, an increased brain stem blood flow at cataplexy onset, similar to that known to occur at REM sleep onset (4, 13, 18), would serve to reverse the elevated central CO2 precipitating cataplexy. To test this hypothesis, direct measurements of central CO2 levels before and during cataplexy are necessary. An alternate hypothesis is that cataplexy occurs in response to a centrally elicited shift in the balance between sympathetic and parasympathetic activity, producing a relative sympathetic activation. This shift results in tachycardia, unaccompanied by blood pressure change. Cataplexy onset represents a neural reflex, mediated by intrinsic brain stem circuits, which reverses this imbalance. This reversal can be achieved by triggering REM sleep, since parasympathetic activity predominates in this state (3, 9, 25). We would hypothesize that a similar parasympathetic predominance characterizes cataplexy.
Our findings indicate that, from a cardiovascular standpoint, cataplexy can be subdivided into two very different states, the cataplexy onset period and the cataplexy period existing 10 or more seconds after onset. At and shortly after onset of the atonia of cataplexy, heart rates are higher than those seen in any sleep state and are equal to the maximum seen during waking. Heart rate and blood pressure decrease rapidly after cataplexy onset. No other “sleep” or transitional state is accompanied by a similar change in heart rate. In particular, REM sleep, the state in which muscle atonia normally arises, does not have any substantial heart rate change at or after onset (28).
Finally, our findings are consistent with behavioral data and the subjective reports of narcoleptics. In both narcoleptic dogs and humans, cataplexy is elicited by vigorous exercise and strong emotions. Common triggers of cataplexy are sudden anger, surprise, sexual activity, and embarrassment (10). All of these behaviors are associated with changes in cardiovascular function. Narcoleptics can often prevent cataplexy by anticipating strong emotions and the stimuli provoking these emotions. This successful coping strategy can be hypothesized to act by preventing the large cardiovascular changes and resulting changes in brain stem blood flow. which would otherwise be triggered. Studies of chemoreceptor activity and hemodynamics at cataplexy onset should provide further insights into the mechanisms controlling this state.
Acknowledgments
This study was supported by the Medical Research Service of the Veterans Administration, National Institutes of Health Grants NS-1640 and NS-23724, and the American Narcolepsy Association.
REFERENCES
- 1.Ahmad HR, and Loeschcke HH. Transient and steady state responses of pulmonary ventilation to the medullary extracellular pH after approximately rectangular changes in alveolar Pco2. Pfluegers Arch. 395: 285–292, 1982. [DOI] [PubMed] [Google Scholar]
- 2.Baker TL, and Dement WC. Canine narcolepsy-cataplexy syndrome: evidence for an inherited monoaminergic-cholinergic imbalance. In: Brain Mechanisms of Sleep, edited by Mcginty DJ, Drucker-Colin R, Morrison A, and Parmeggiani PL. New York: Raven, 1985, p. 199–234. [Google Scholar]
- 3.Baust W, Boehmke J, and Blossfeld U. Somato-sympathetic reflexes during natural sleep and wakefulness in unrestrained cats. Exp. Brain Res 12: 361–369, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Birzis L, and Tachibana S. Local cerebral impedance and blood flow during sleep and arousal. Exp. Neurol 9: 269–285, 1964. [DOI] [PubMed] [Google Scholar]
- 5.Colley PS, and Sivarajan M. Regional blood flow in dogs during halothane anesthesia and controlled hypotension produced by nitroprusside or nitroglycerin. Anesth. Analg 63: 503–510, 1984. [PubMed] [Google Scholar]
- 6.Conway RS, and Weiss HR. Effect of papaverine on regional cerebral blood flow and small vessel blood content. Eur. J. Pharmacol 68: 17–24, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Downing SE, Mitchell JH, and Wallace AG. Cardiovascular responses to ischemia, hypoxia, and hypercapnia of the central nervous system. Am. J. Physiol 204: 881–887, 1963. [Google Scholar]
- 8.Dreifuss FE, and Flynn DV. Narcolepsy in a horse (Letter). J. Am. Vet. Med. Assoc 184: 132, 1984. [PubMed] [Google Scholar]
- 9.Futuro-Neto HA, and Coote JH. Changes in sympathetic activity to heart and blood vessels during desynchronized sleep. Brain Res. 252: 259–268, 1982. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C Cataplexy. In: Narcolepsy, edited by Guilleminault C, Dement WC, and Passouant P. New York: Spectrum, 1976, p. 125–143. [Google Scholar]
- 11.Guilleminault C, Salva MAQ, Mancuso J, and Hayes B. Narcolepsy, cataplexy, heart rate, and blood pressure. Sleep 9: 222–226, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Hays WL Statistics for Psychologists. New York: Holt, Reinhart, and Winston, 1965. [Google Scholar]
- 13.Kawamura M, and Sawyer CH. Elevation in brain temperature during paradoxical sleep. Science Wash. DC 150: 912–913, 1965. [DOI] [PubMed] [Google Scholar]
- 14.Knecht CD, Oliver JE, Redding ER, Selcer R, and Johnson G. Narcolepsy in a dog and a cat. J. Am. Vet. Med. Assoc 162: 1052–1053, 1973. [PubMed] [Google Scholar]
- 15.Lai YY, Siegel JM, and Wilson WJ. Effect of blood pressure on changes in muscle tone produced by stimulation of the medial medulla. Am. J. Physiol 252 (Heart Circ. Physiol. 21): H1249–H1257, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu GP, Kaul DK, Baez S, and Orkin LR. Comparison of verapamil and nitroprusside hypotensive effects on brain microcirculation (Abstract). Anesthesiology 63: A90, 1985. [Google Scholar]
- 17.Magoun HW, and Rhines R. An inhibitory mechanism in the bulbar reticular formation. J. Neurophysiol 9: 165–171, 1946. [DOI] [PubMed] [Google Scholar]
- 18.Meyer JS, Ishikawa Y, Hata T, and Karacan I. Cerebral blood flow in normal and abnormal sleep and dreaming. Brain Cogn. 6: 266–294, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Mitler MM, Boyson BG, Campbell L, and Dement WC. Narcolepsy-cataplexy in a female dog. Exp. Neural 45: 332–340, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitler MM, Soave O, and Dement WC. Narcolepsy in seven dogs. J. Am. Vet. Med. Assoc 168: 1036–1038, 1976. [PubMed] [Google Scholar]
- 21.Pinard E, and Seylaz J. Intracerebral gas partial pressure changes under vasoactive drugs: a mass spectrometry study. Pfluegers Arch. 375: 25–30, 1978. [DOI] [PubMed] [Google Scholar]
- 22.Puizillout JJ, and Foutz AS. Vago-aortic nerve stimulation and REM sleep: evidence for a REM-triggering and a REM-maintenance factor. Brain Res. 111: 181–184, 1976. [DOI] [PubMed] [Google Scholar]
- 23.Puizillout JJ, and Foutz AS. Characteristics of the experimental reflex sleep induced by vago-aortic nerve stimulation. Electroencephalogr. Clin. Neurophysiol 42: 552–563, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Puizillout JJ, Ternaux JP, Foutz AS, and Fernandez G. Les stades de sommeil de la preparation “encephale isole.” I. Declenchement des pointes ponto-geniculo-occipitales et du sommeil phasique à ondes lentes. Role des noyaux du raphe. Electroencephalogr. Clin. Neurophysiol 37: 561–576, 1974. [DOI] [PubMed] [Google Scholar]
- 25.Reiner PB Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats. Bruin Res 378: 86–96, 1986. [DOI] [PubMed] [Google Scholar]
- 26.Rutland MD, Lee TY, Nimmon CC, Granowska M, and Britton KE. Measurement of the effects of a single dose of prazosin on the cerebral blood flow in hypertensive patients. Postgrad. Med. J 56: 818–822, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scrima L An etiology of narcolepsy-cataplexy and a proposed cataplexy neuromechanism. Int. J. Neurosci 15: 69–86, 198l. [DOI] [PubMed] [Google Scholar]
- 28.Siegel JM, Fahringer H, Tomaszewski KS, Kaitan K, Kilduff T, and Dement WC. Heart rate and blood pressure changes associated with cataplexy in canine narcolepsy. Sleep 9: 216–221, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel JM, Nienhuis R, and Tomaszewski KS. Rostra1 brainstem contributes to medullary inhibition of muscle tone. Brain Res. 268: 344–348, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel S Nonparametric Statistics. New York: McGraw-Hill, 1956. [Google Scholar]
- 31.Sprague JM, and Chambers WW. Regulation of posture in intact and decerebrate cat. I. Cerebellum, reticular formation, vestibular nuclei. J. Neurophysiol 16: 451–463, 1953. [DOI] [PubMed] [Google Scholar]
- 32.Strain GM, Olcott BM, Archer RM, and Mc-Clintock BK. Narcolepsy in a Brahman bull. J. Am. Vet. Med. Assoc 185: 538–541, 1984. [PubMed] [Google Scholar]
- 33.Sweeney CR, Hendricks JC, Beech J, and Morrison AR. Narcolepsy in a horse. J. Am. Vet. Med. Assoc 183: 126–128, 1983. [PubMed] [Google Scholar]
- 34.Trelease RB, Sieck GC, Marks JD, and Harper RM. Respiratory inhibition induced by transient hypertension during anesthesia and sleep states. Exp. Neural 90: 173–186, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Youmans WB, Murphy QR, Turner JK, Davis LD, Briggs DI, and Hoye AS. Activity of abdominal muscles elicited from the circulatory system. Am. J. Phys. Med 42: 1–70, 1963. [PubMed] [Google Scholar]


