Abstract
The activity of neurons in the posterior hypothalamus (PH) is thought to contribute to the production of wakefulness and electroencephalograph desynchronization. Inactivation of neuronal activity in this area is known to induce sleep. Most PH neurons decrease unit discharge during slow-wave sleep (SWS) relative to wake and rapid eye movement sleep. In the present study, we sought to examine potential sources of inhibition or disfacilitation underlying the reduction of PH unit activity during SWS in the cat. We employed the microdialysis technique in conjunction with high-performance liquid chromatography methods for the quantification of glutamate, glycine, and γ-aminobutyric acid (GABA) release. We found a selective increase in GABA release during SWS in the PH. Glutamate and glycine levels were unchanged across the sleep-wake cycle. Microinjection of the GABAA-receptor agonist muscimol, into the same areas from which microdialysis samples were collected, increased SWS time. Our studies support the hypothesis that GABA release in the posterior hypothalamus mediates inhibition of posterior hypothalamic neurons, thereby facilitating SWS.
Keywords: glutamate, glycine, microdialysis, cats, muscimol
It was suggested as early as 1918 that the posterior hypothalamus (PH) plays an important role in maintenance of the waking state. Von Economo (32) found that patients with lesions of the PH were hypersomnolent. Subsequently, it was shown that electrolytic or cytotoxic lesions of this area in rats and cats produced large increases in slow-wave sleep (SWS) (12,14,15). Reversible inactivation of cells in this area by the microinjection of the γ-aminobutyric acid (GABA) type A receptor agonist muscimol increases sleep time in intact cats and restores sleep in cats made insomniac by the administration of parachlorophenylalanine (PCPA) or preoptic area (POA) lesion (11, 21).
Anatomic and electrophysiological studies also support the hypothesis that the PH makes a significant contribution to the maintenance of electroencephalographic (EEG) arousal. The rat PH is the origin of direct projections to the cerebral cortex (22). Most neurons in this area of the cat exhibit their highest rates of firing during active wake and phasic rapid eye movement (REM) sleep (20, 27). Firing rates of most neurons in the PH are lowest during SWS. This pattern of activity resembles that of mesencephalic reticular neurons (MRF) (24, 27).
Recently, we demonstrated changes in GABA release opposite to the predominant state-related pattern of activity in the dorsal raphe (DR) and locus ceruleus (LC) nuclei of the cat (16, 17). GABA release in each of these areas was greatest during REM sleep, a time when noradrenergic and serotonergic units cease discharge (6,13,18). In this study, we sought to determine whether GABA, glutamate, and/or glycine concentrations in the PH fluctuate as a function of sleep-wake state. In vivo microdialysis in combination with high-performance liquid chromatography (HPLC) techniques was used to measure the release of these amino acid neurotransmitters in the PH as a function of sleep-wake state. To further characterize the action of GABA at our microdialysis sites in the PH, we performed microinjections of the GABAA-receptor agonist muscimol and determined its effect on sleep-wake state organization.
METHODS AND EXPERIMENTAL PROCEDURES
Aseptic surgery was performed on six cats using pentobarbital sodium anesthesia (35 mg/kg ip). Screw electrodes were placed in the posterior orbit and sensorimotor cortex for the recording of eye movements and EEG. Tripolar electrodes were implanted into the lateral geniculate nucleus for the recording of ponto-geniculo-occipital spikes. Flexible stainless steel wires were inserted into the neck musculature for the recording of electromyographic activity. Stainless steel guide cannulas (21-gauge thin wall) were stereotaxically implanted at sites 1 mm above the PH. Coordinates for the two sites were anterior 10, lateral 2.5, and ventral 2.5 (25).
Cats were maintained on a 12:12-h light-dark cycle with lights on between 7:00 a.m. and 7:00 p.m. At least 2 wk after surgery, animals were connected to a recording cable for polygraphic recording. A 50-mm concentric microdialysis probe with a 2-mm semipermeable membrane 0.27 mm in diameter was inserted to a position 3 mm beyond the tip of the guide. The molecular cutoff size of the dialysis membrane was 50 kDa; our probes have recovery rates between 12 and 17% in vitro. The probe was perfused at a rate of 2 μl/min with artificial cerebrospinal fluid (aCSF) of the following composition (in mM): 145 Na+, 2.7 K+, 1.0 Mg2+, 1.2 Ca2+, 152.1 CI−, and 2.0 Na2HPO4, pH 7.4. The delay between probe insertion and sample collection was 17 h. Microdialysis samples were then collected from immediately adjacent 4- to 10-min periods of SWS, REM sleep, quiet wake, and active wake. SWS and REM sleep were identified by standard criteria (28). During quiet wake, cats were lying down with raised head or in the sphinx posture. Active wake was elicited by engaging cats in play behavior using a string and consisted of continuous motor activity.
Sample collection was timed precisely by the use of an Eicom fraction collector incorporating a 10-min delay to account for the time for perfusate to travel from the tip of the probe to the outlet of the tubing. Samples were immediately frozen on dry ice and stored at −40°C for subsequent analysis.
The content of the amino acids glutamate, glycine, and GABA in microdialysis samples was analyzed by HPLC with electrochemical detection using a system manufactured by Eicom. The system employed a 4.6 × 150-mm C18 separation column (Rainin) and a glassy-carbon electrode held at + 700 mV. Equal volumes of the collected samples were reacted with 5 μl of 4 mM o-pthaldialdehyde and 2-mercaptoethanol for 2 min before injection onto the separation column. The mobile phase flow rate was set at either 1.0 or 1.2 ml/min. The mobile phase consisted of 0.1 M sodium phosphate buffer with 35*% methanol at a pH between 6.06 and 6.14. The pH of the mobile phase was adjusted to obtain the best separation of the compounds of interest. Separation of the GABA peak was especially dependent on precise maintenance of the pH. The assay was able to detect 10 fmol of the amino acids glutamate, glycine, and GABA, with a signal-to-noise ratio of 3:1. Repeated assays of identical amino acid standard solutions were performed and indicated a peak height variability of <2%. The assay consistently showed a linear response of amino acid peak height to step increases of amino acid concentration between 10 fmol and 200 pmol in standard solutions. The volume of samples analyzed in this study ranged between 8 and 20 μl, corresponding to collection periods of 4–10 min. Neurotransmitter concentrations were calculated by comparing peak heights of glutamate, glycine, and GABA in microdialysis samples with peak heights of known concentrations of the same compounds analyzed on the same day.
Microdialysis samples were collected during 17 separate sleep-wake cycles. Within each of these cycles, individual samples were collected corresponding to periods of SWS, REM sleep, and wake that were immediately adjacent in time. Thus statistical comparisons were always made between samples collected using the same microdialysis probe over a period of ≤30 min. This design overcomes potential confounding factors caused by comparing samples collected at different intervals after implantation of the microdialysis probe because the recovery rate of microdialysis probes slowly decreases over the course of experiments. In addition, the potentially confounding effects of circadian temperature or hormone-release rhythms on neurotransmitter release is minimized by this design.
Muscimol (0.5 μg in 0.5 μl aCSF, 4.4 mM) was microinjected in five of the eight PH sites from which microdialysis samples had been collected. In each case, a period of at least 1 wk separated microdialysis and microinjection experiments. Microdialysis experiments always preceded microinjection experiments. One additional microinjection was made in a PH site not used for microdialysis collection. Microinjections were made at 9:00 a.m.; polygraphic recordings were made for 6 h after the administration of muscimol. The microinjection took place over the course of 1 min; the injection cannula was retained in place for an additional minute before removal. The injection cannula was positioned 1 mm beyond the tip of the guide cannula. Sham and vehicle (0.5 μl aCSF) microinjections made at each site were used as controls for analysis of the effects of muscimol administration. The order of sham, vehicle, and muscimol injections was randomized. Sham injections consisted of insertion of a dry injection cannula and mock injection over the same time course as vehicle and muscimol injections.
Animals were perfused under deep pentobarbital sodium anesthesia (50 mg/kg ip), and the brains were removed for histological verification of microdialysis collection sites. Fifty-micrometer brain stem slices from the PH were stained with neutral red to localize dialysis and microinjection sites.
RESULTS
Microdialysis samples were collected from a total of eight sites in five animals. Probe placements are summarized in Fig. 1. All microdialysis and muscimol microinjection sites were found to lie within posterior hypothalamic areas where muscimol microinjections have been shown to induce SWS (11). These areas also contain neurons exhibiting reduced activity during SWS compared with waking (20, 26).
Fig. 1.
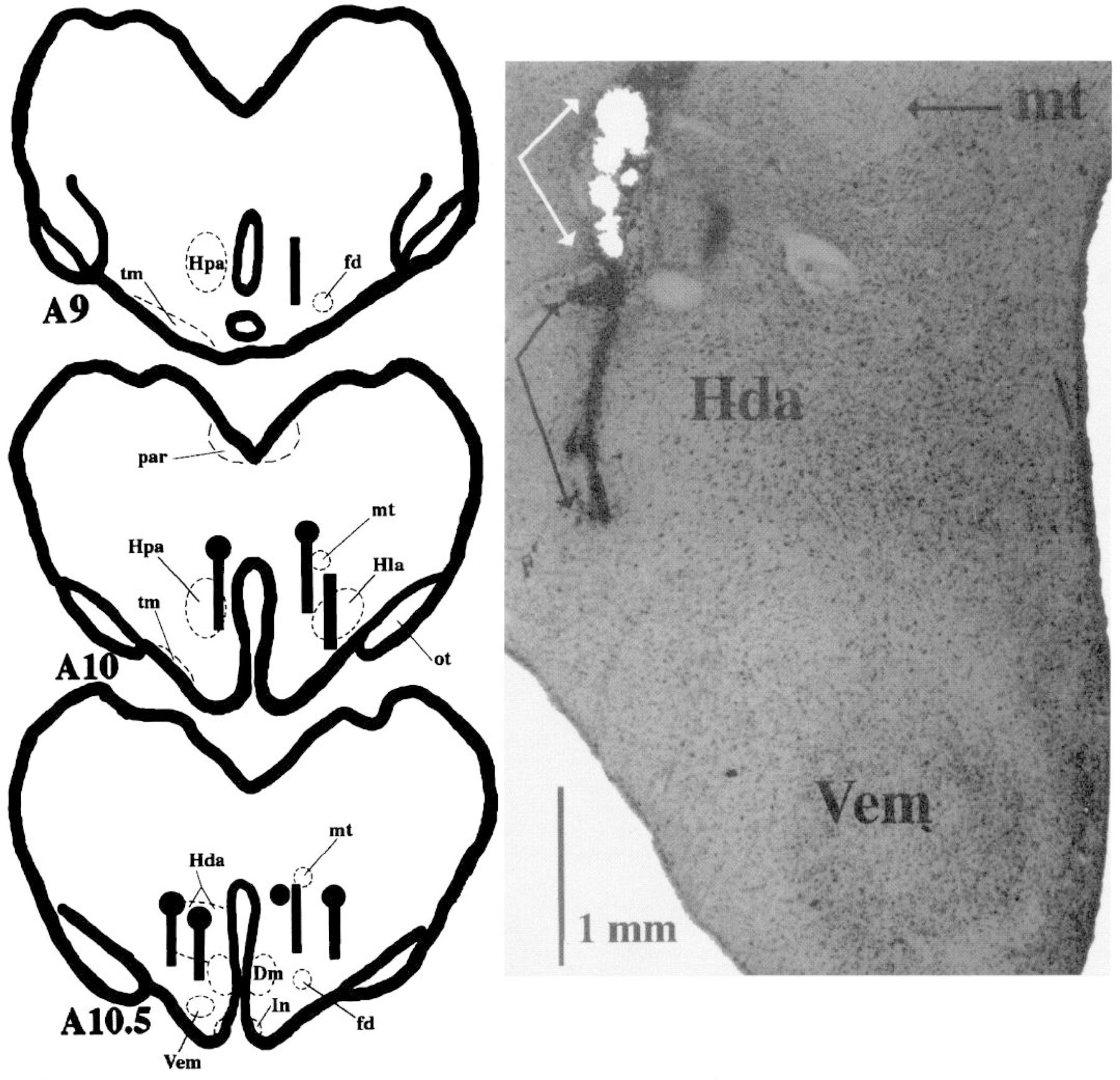
Summary of histologically verified posterior hypothalamic microdialysis and microinjection sites. Black bars represent the placement of microdialysis probe membrane in each of the experiments. Black circles represent muscimol microinjection sites. Black bars with black circles represent sites used in both microdialysis and microinjection studies. Photomicrograph (×20) corresponds to leftmost microinjection-microdialysis site depicted in schematic at anterior-posterior level (A) 10.5. White arrows in photomicrograph define area of microinjection. Black arrows define track left by microdialysis probe. Dm, dorsomedial nucleus; fd, descending column of the fornix; Hda, dorsal hypothalamic area; Hla, lateral hypothalamic area; Hpa, posterior hypothalamic area; In, infundibular nucleus; mt, mamillothalamic tract; ot, optic tract; tm, tuberomamillary nucleus; Vem, ventromedial nucleus.
It was possible to compare the release of GABA, glutamate, and glycine in active versus quiet wake in nine pairs of samples collected during adjacent time periods from five of the eight collection sites. There was no consistent difference in the release of each of these amino acid neurotransmitters in both waking states as determined by paired t-tests. Therefore, data from quiet wake and active wake samples for these sites were combined for comparison with SWS and REM sleep values.
The content of GABA was analyzed in samples from 17 individual sleep-wake cycles; the number of cycles collected at each of the eight sites varied between one and five. The content of GABA in samples collected during SWS was 8.67 ± 0.80 fmol/μl (Fig. 2). GABA release during SWS was greater than that during wake and REM sleep in 13 of the 17 cases (P < 0.05, sign test). Samples from the four sleep-wake cycles in which GABA release did not increase were collected from four different microdialysis sites. Figure 3 depicts GABA peaks in chromatograms corresponding to samples collected during wake, SWS, and REM sleep in an individual sleep-wake cycle. Figure 4 depicts GABA content in microdialysis samples from an individual site collected over the course of two consecutive sleep-wake cycles.
Fig. 2.
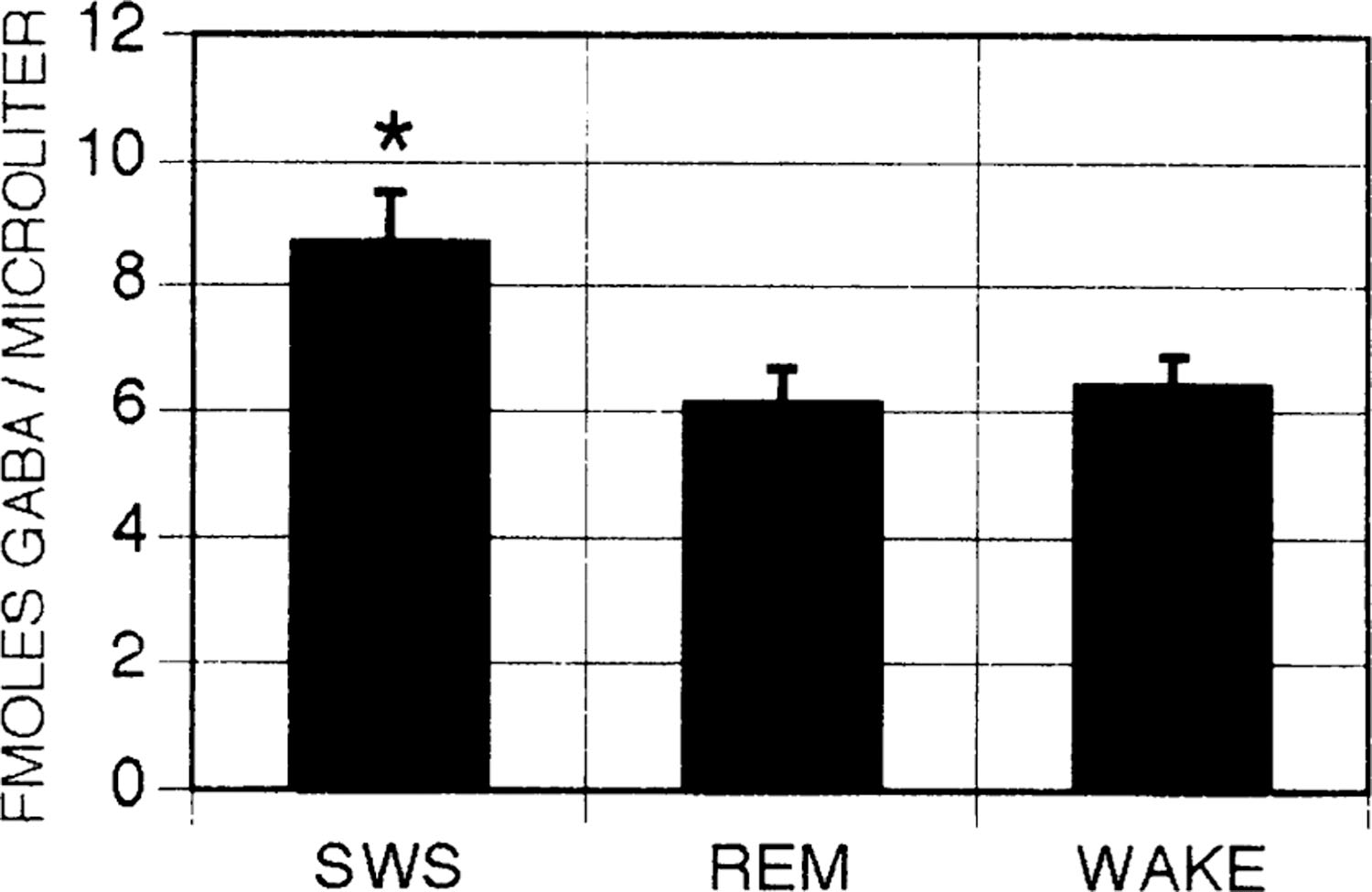
γ-Aminobutyric acid (GABA) release as a function of sleep-wake state. Average GABA concentrations in microdialysis samples collected in slow-wave sleep (SWS), rapid eye movement (REM) sleep, and wake. Error bars indicate SE; n = 17 for SWS, REM, and wake. *P < 0.05 analysis of variance with Newman-Keuls post hoc t-tests.
Fig. 3.
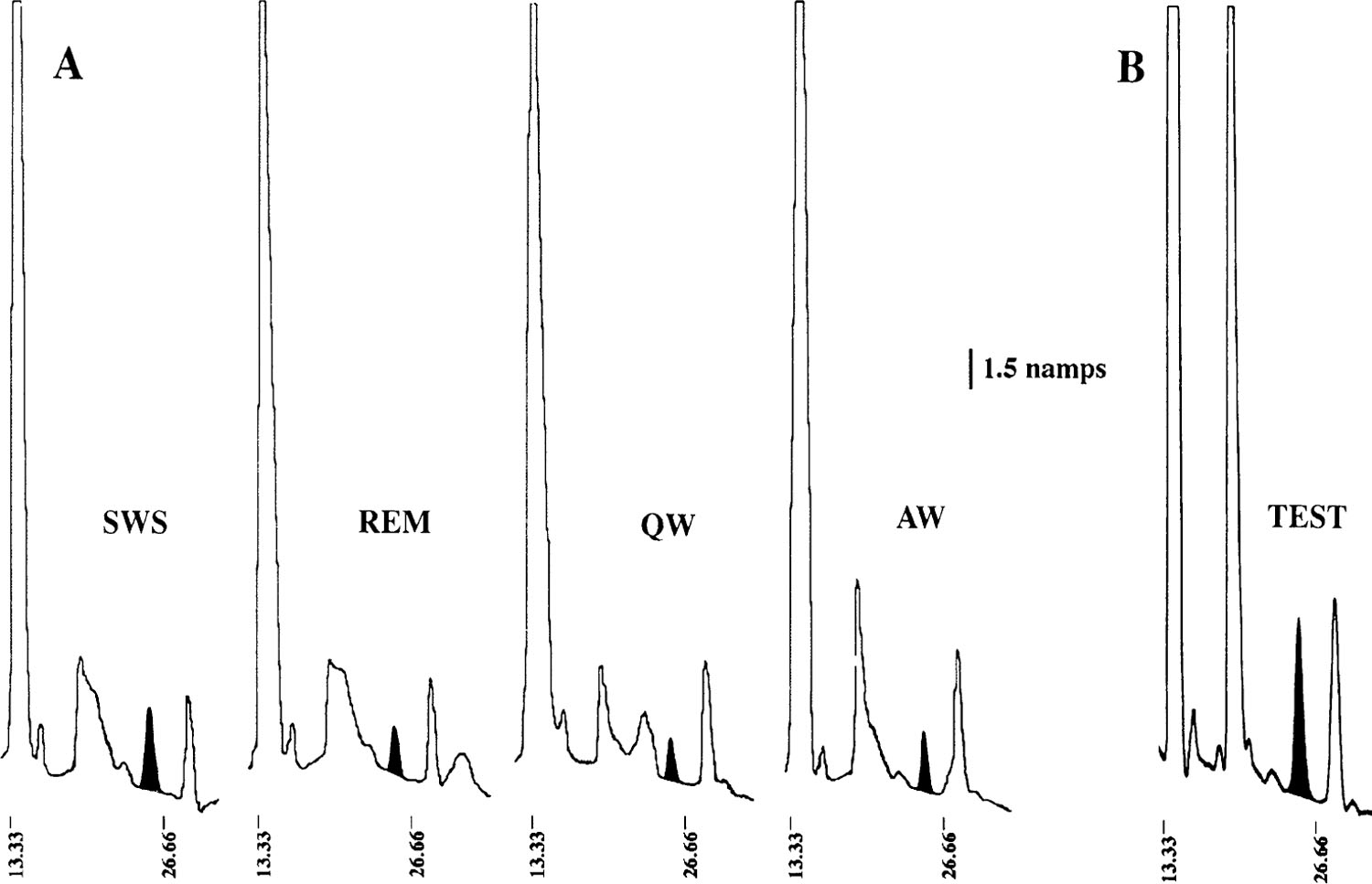
High-performance liquid chromatography and electrochemical detection analysis of GABA release. A: GABA peaks in microdialysis samples from a typical sleep-wake cycle. In each case, peak corresponding to GABA has been blackened. Note increase in height of GABA peak in SWS sample. B: analysis of test microdialysis sample collected from same microdialysis site as in A. Sample was spiked with 100 fmol of GABA to verify identification of GABA peak. QW, quiet waking; AW, active waking.
Fig. 4.
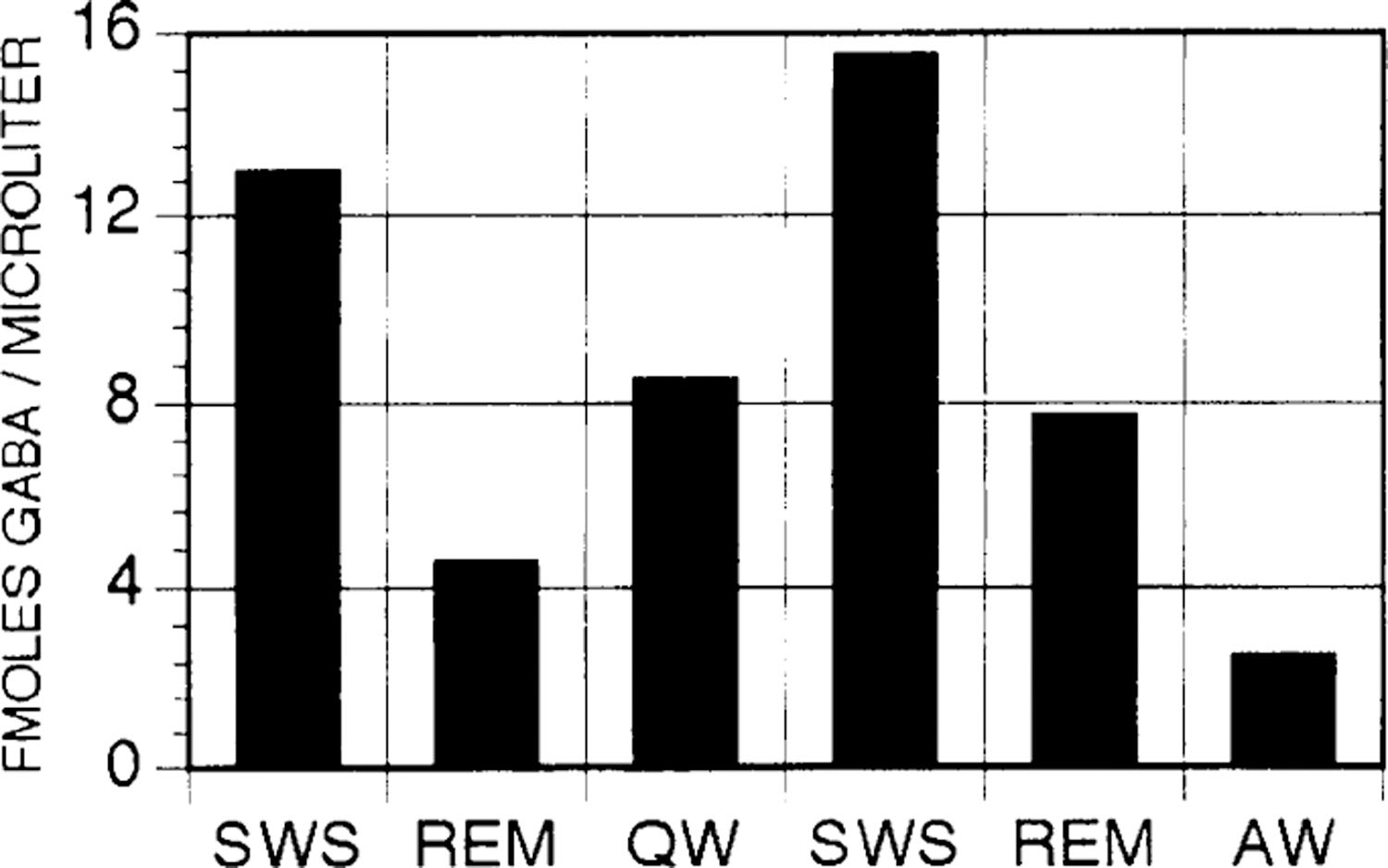
GABA content in microdialysis samples collected from a single microdialysis site over the course of 2 consecutive sleep-wake cycles.
No significant differences in glycine or glutamate release as a function of state were found. Glutamate release during SWS, REM sleep, and wake was 0.55 ± 0.05, 0.64 ± 0.08, and 0.50 ± 0.04 pmol/μl. Glycine release during SWS, REM sleep, and wake was 0.76 ± 0.09, 0.73 ± 0.04, and 0.72 ± 0.11 pmol/μl.
A total of six microinjections of muscimol into the PH were made. In each case, sham and vehicle microinjections were also made for comparison. In five cases, the site of injection was the same as that previously used for microdialysis collection. Muscimol produced increases in SWS in each case (17% increase over combined sham and vehicle controls, P < 0.01, analysis of variance with Newman-Keuls post hoc t-tests). SWS increases were accompanied by decreases in wake and REM sleep (Fig. 5). It should be noted that the percentage of time spent in SWS in the control conditions was >50% over a 6-h period. Thus SWS time was increased over control amounts under conditions where the percentage of time spent in sleep was already quite high.
Fig. 5.
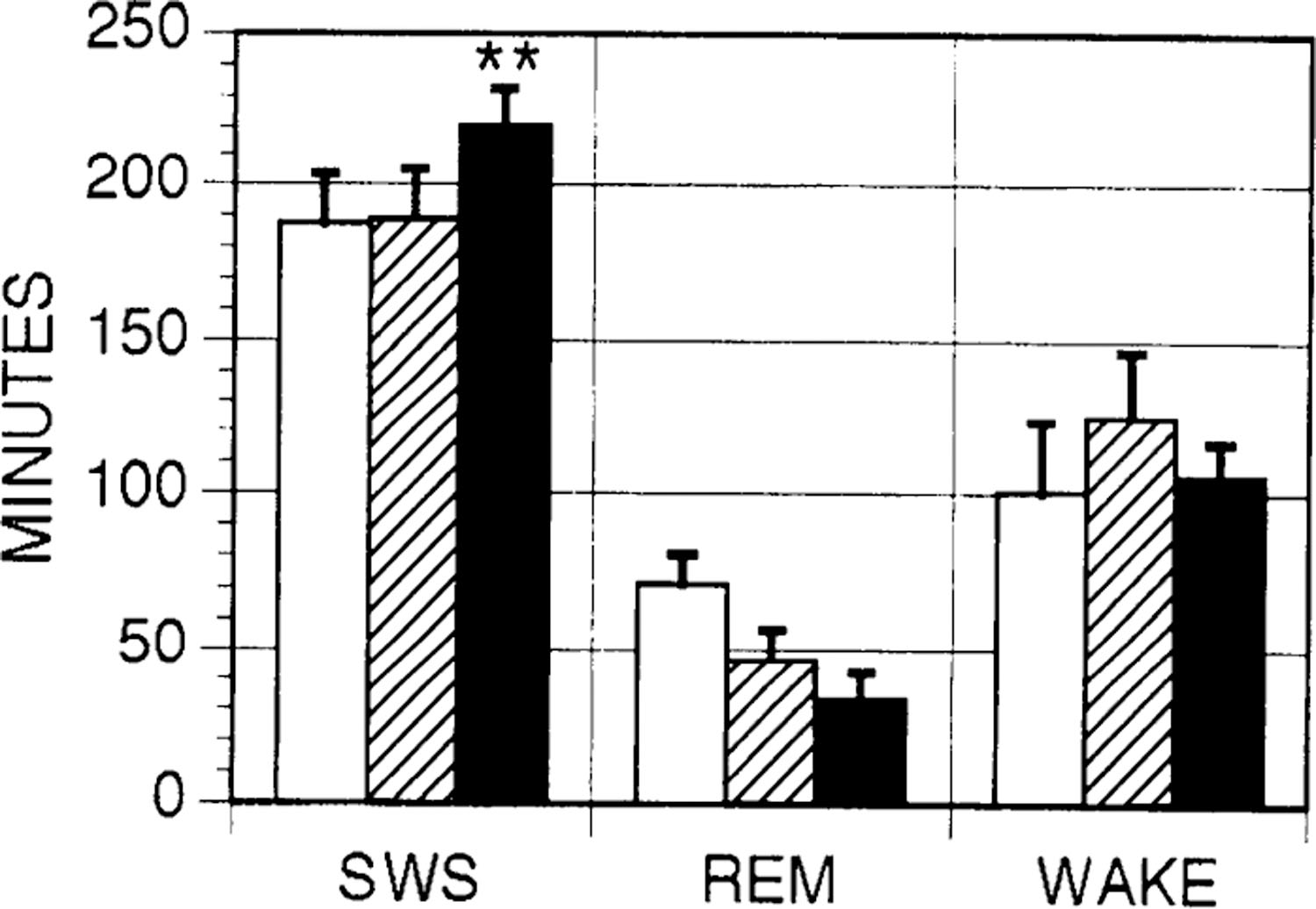
Effects of muscimol microinjection on sleep. Total time spent in SWS, REM sleep, and wake in 6 h after sham (open bars), vehicle control (hatched bars), and muscimol (filled bars) injections in posterior hypothalamus. Error bars indicate SE; n = 6 for each condition. **P < 0.01.
DISCUSSION
GABA release in the PH during SWS increased by 41 and 35% wake and REM sleep values, respectively. We hypothesize that this GABA release is responsible for the reduced discharge of PH waking and REM sleep-active cells in SWS. Furthermore, the data suggest that there is a population of GABAergic neurons projecting to this region that are maximally active during SWS. The increase in SWS time after muscimol microinjections corroborates the findings of others in suggesting that inhibition of posterior hypothalamic neurons is an important step in the production of synchronized cortical EEG and SWS and is sufficient to trigger sleep onset (11).
The lack of any change in glutamate release across the sleep-wake cycle suggests that disfacilitation of an excitatory glutamatergic PH input is unlikely to be a major factor underlying the decrease in PH unit activity linked to SWS. Glutamate and glycine release was also unchanged as a function of sleep-wake state in the LC and DR nuclei (16, 17). Glutamate and glycine levels in all states were ~100 times higher than GABA levels recovered from the same probes. In the absence of electrical, pharmacological, or mechanical stimulation, the content of glutamate and/or glycine release in microdialysis samples may better reflect the extracellular concentration of glutamate and glycine derived from metabolic processes. These findings may indicate that the microdialysis technique is not well suited to the resolution of relatively small increments in hypothalamic glutamate and/or glycine release that may occur in conjunction with changes in sleep-wake state.
The increased GABA release in the PH during SWS could result from the increased firing of GABA neurons found within the PH itself. Although a population of GABAergic neurons in the PH has been identified (9), unit recording studies have not identified neurons in this area that increase firing selectively during SWS.
Alternatively, increases in GABA concentration could derive from glial cells in the PH. Microdialysis studies in the striatum indicate that a significant percentage of GABA in microdialysis samples is not Na+ or Ca2+ dependent (2, 7). Although no mechanism has been established to account for alterations in glial cell release or reuptake of GABA over the relatively short time periods corresponding to the sleep-wake cycle in the cat, the possibility that glial cells contribute to state-related changes in extracellular amino acid concentrations cannot be discounted at this time.
Szymusiak and McGinty (29) found neurons in the basal forebrain of the cat that increase discharge during SWS. SWS-active neurons have also been found in the preoptic area of the cat (1, 8). Gritti et al. (5) demonstrated that GABAergic neurons of the rat POA and basal forebrain (BF) project to the PH. Recently, Fos immunolabeling studies have identified neurons in the POA that are activated in SWS and that project to the PH (23). It remains to be determined whether these projections are also present in the cat. Warming of the POA induces SWS in cats (19). Warm-sensitive neurons in the cat POA are also active during SWS (1). Krilowicz et al. (10) found that warming of the POA selectively inhibits the activity of PH waking -and REM sleep-active neurons. Finally, Lin et al. (11) found that insomnia induced by POA lesion is reversed by the activation of posterior hypothalamic GABAA receptors. Thus the present data are consistent with previous hypotheses (5,10) that some SWS-active neurons of the POA and BF are GABAergic and are responsible for the active inhibition of waking- and REM sleep-active neurons in the PH during SWS. The same mechanism may be responsible for the decrease in unit activity in the MRF because units in this area have similar state-related discharge patterns to neurons in the PH and because stimulation of the POA suppresses firing of MRF units (28).
The present finding builds on our previous findings in the DR and LC nuclei demonstrating that GABA release across the sleep-wake cycle is opposite that of the predominant state-related profile of unit discharge (16, 17). Together, these findings suggest that the production of both SWS and REM sleep is, in part, an active process mediated by GABAergic neurons. During sleep, we propose that GABA counteracts excitatory inputs in the DR and LC nuclei and PH through the opening of K+ and Cl− channels by activation of GABAB and GABAA receptors, respectively. Finally, the findings of this study and previous studies in the LC and DR nuclei suggest that at least two distinct populations of GABAergic neurons are maximally active, respectively, in SWS and REM sleep.
There are several explanations for the decrease of REM sleep time after the microinjection of muscimol. First, the activation of posterior hypothalamic neurons may be essential for the production of REM sleep. Such activation could be an essential component for EEG desynchronization during REM sleep, given the inactivity of serotonergic and noradrenergic neurons (6, 13, 18) that normally contribute to EEG desynchronization during wake (4, 31). Alternatively, GABAergic inhibition of hypothesized posterior hypothalamic thermoregulatory and/or cardiovascular control mechanisms could preclude the production of REM sleep (26).
Several investigators have suggested that the production of cortical EEG desynchronization is a function of the combined activity of several neuronal groups, each of which innervate large numbers of thalamic or cortical neurons. This concept is consistent with studies demonstrating that axon-sparing lesions of the PH, the MRF, and the pedunculopontine (PPN) nucleus have only transient effects on the production of EEG-desynchronized states (3, 33). The present findings also support this concept. GABAergic inhibition of posterior hypothalamic activity did not completely block the spontaneous generation of EEG desynchronization during REM sleep and waking. Rather, activation of the GABAA receptor in this region by muscimol microinjection merely increased SWS time. Thus it is likely that SWS time was increased by the inhibition of one of the several systems contributing to cortical activation. In this respect, it will be of special interest in the future to determine whether the decreased activity of neurons in the MRF and PPN during SWS is also accompanied by an increased release of GABA.
In conclusion, the present findings suggest that increased GABA release mediates inhibition of waking- and REM sleep-active neurons during SWS. In contrast, we previously found that GABA release in the LC and DR increases during REM sleep (16, 17). Together, these findings indicate that state-related profiles of GABA release are region-specific and add to evidence that GABA neurons are essential for the production of specific sleep-wake states.
Acknowledgments
This research was supported by the Medical Research Service of the Department of Veterans Affairs by Public Health Service (PHS) Grants NS-14610 and HL-41370, and by PHS predoctoral fellowship 5F31MH10451-02.
REFERENCES
- 1.Alam MN, McGinty D, and Szymusiak R Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am. J. Physiol 269 (Regulatory Integrative Comp. Physiol. 38): R1240–R1249, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Campbell K, Kalen P, Lundberg C, Wictorin K, Rosengren E, and Bjorklund A Extracellular gamma-aminobutyric acid levels in the rat caudate-putamen: monitoring the neuronal and glial contribution by intracerebral microdialysis. Brain Res 614: 241–250, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Denoyer M, Sallanon M, Buda C, Kitahama K, and Jouvet M Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res 539: 287–303, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Foote S, Berridge C, Adams L, and Pineda J Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog. Brain Res 88: 521–532, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Gritti I, Mainville L, and Jones B Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J. Comp. Neurol 339: 251–268, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Hobson JA, McCarley RW, and Nelson JP Location and spike-train characteristics of cells in anterodorsal pons having selective decreases in firing rate during desynchronized sleep. J. Neurophysiol 50: 770–783, 1983. [DOI] [PubMed] [Google Scholar]
- 7.Hondo H, Nakahara T, Nakamura K, Hirano M, Uchimura H, and Tashiro N The effect of phencyclidine on the basal and high potassium evoked extracellular GABA levels in the striatum of freely-moving rats: an in vivo microdialysis study. Brain Res 671: 54–62, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Kaitin K Preoptic area unit activity during sleep and wakefulness in the cat. Exp. Neurol 83: 347–357, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Kitahama K, Sallanon M, Okamura H, Geffard M, and Jouvet M Cellules presetant une immunoreactive au GABA dans l’hypothalamus du chat. C. R. Acad. Sci. Paris, Serie III: 507–511, 1989. [PubMed] [Google Scholar]
- 10.Krilowicz BL, Szymusiak R, and McGinty D Regulation of posterior lateral hypothalamic arousal related neuronal discharge by preoptic anterior hypothalamic warming. Brain Res 668: 30–38, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Sakai K, Vanni-Mercier G, and Jouvet M A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res 479: 225–240, 1989. [DOI] [PubMed] [Google Scholar]
- 12.McGinty D Somnolence, recovery, and hyposomnia following ventromedial diencephalic lesions in the rat. Electroencephalogr. Clin. Neurophysiol 26: 70–79, 1969. [DOI] [PubMed] [Google Scholar]
- 13.McGinty D, and Harper R Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101: 569–575, 1976. [DOI] [PubMed] [Google Scholar]
- 14.Moruzzi G The sleep-waking cycle. Ergeh. Physiol 64: 1–165, 1972. [DOI] [PubMed] [Google Scholar]
- 15.Naquet R, Denavit M, and Albe-Fessard D Comparaison entre le rôle du subthalamus et celui des différentes structures bulbomésencéphaliques dans le maintien de la vigilance. Electroencephalogr. Clin. Neurophysiol 20: 149–164, 1966. [DOI] [PubMed] [Google Scholar]
- 16.Nitz D, and Siegel J GABA release in the locus coeruleus as a function of sleep/wake state. Soc. Neurosci. Abstr 496: 3, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nitz D, and Siegel J Inhibitory amino-acid neurotransmission in the dorsal raphe nucleus during sleep-wake states. Soc. Neurosci. Abstr 742: 13, 1993. [Google Scholar]
- 18.Reiner P Correlational analysis of central noradrenergic activity and sympathetic tone in behaving cats. Brain Res 378: 86–96, 1986. [DOI] [PubMed] [Google Scholar]
- 19.Roberts W, and Robinson T Relaxation and sleep induced by warming of the preoptic region and the anterior hypothalamus in cats. Exp. Neurol 25: 282–294, 1969. [DOI] [PubMed] [Google Scholar]
- 20.Sakai K, El Mansari M, Lin J, Zhang J, and Vanni-Mercier G The posterior hypothalamus in the regulation of wakefulness and paradoxical sleep. In: The Diencephalon and Sleep, edited by Mancia M and Marini G New York: Raven, 1990, p. 171–198. [Google Scholar]
- 21.Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, and Jouvet M Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience 12: 669–683, 1989. [DOI] [PubMed] [Google Scholar]
- 22.Saper C Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J. Comp. Neurol 237: 21–46, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Sherin JE, Shiromani PJ, McCarley RW, and Saper CB Activation of ventrolateral preoptic neurons during sleep. Science Wash. DC 271: 216–219, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Siegel JM Behavioral functions of the reticular formation. Brain Res. Rev 1: 69–105, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snider R, and Niemer W A Stereotaxic Atlas of the Cat Brain Chicago, IL: University of Chicago Press, 1961. [Google Scholar]
- 26.Stotz-Potter EH, Willis LR, and DiMicco JA Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J. Neurosci 16: 1173–1179, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szymusiak R, Iriye T, and McGinty D Sleep-waking discharge of neurons in the posterior lateral hypothalamic area of cats. Brain Res. Bull 23: 111–120, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Szymusiak R, and McGinty D Effects of basal forebrain stimulation on the waking discharge of neurons in the midbrain reticular formation of cats. Brain Res 498: 355–359, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Szymusiak R, and McGinty D Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res 370: 82–92, 1986. [DOI] [PubMed] [Google Scholar]
- 30.Ursin R, and Sterman B A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat Los Angeles, CA: University of California, 1981. [Google Scholar]
- 31.Vanderwolf C, Baker G, and Dickson C Serotonergic control of cerebral activity and behavior: models of dementia. Ann. NY Acad. Sci 600: 366–382, 1990. [DOI] [PubMed] [Google Scholar]
- 32.Von Economo C Die Encephalitis Lethargica Vienna Deuticke, 1918. [Google Scholar]
- 33.Webster HH, and Jones BE Neurotoxic lesions of the dorsolateral mesencephalic tegmentum cholinergic cell area in the cat: effects upon sleep-waking states. Brain Res 458: 285–302, 1988. [DOI] [PubMed] [Google Scholar]


